Incorporation of Nanostructural Hydroxyapatite and Curcumin Extract from Curcuma longa L. Rhizome into Polylactide to Obtain Green Composite
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydroxyapatite Synthesis
2.3. Hydroxyapatite Modification with Turmeric Extract
2.4. Composites Preparation
2.5. Characterization Methods
3. Results and Discussion
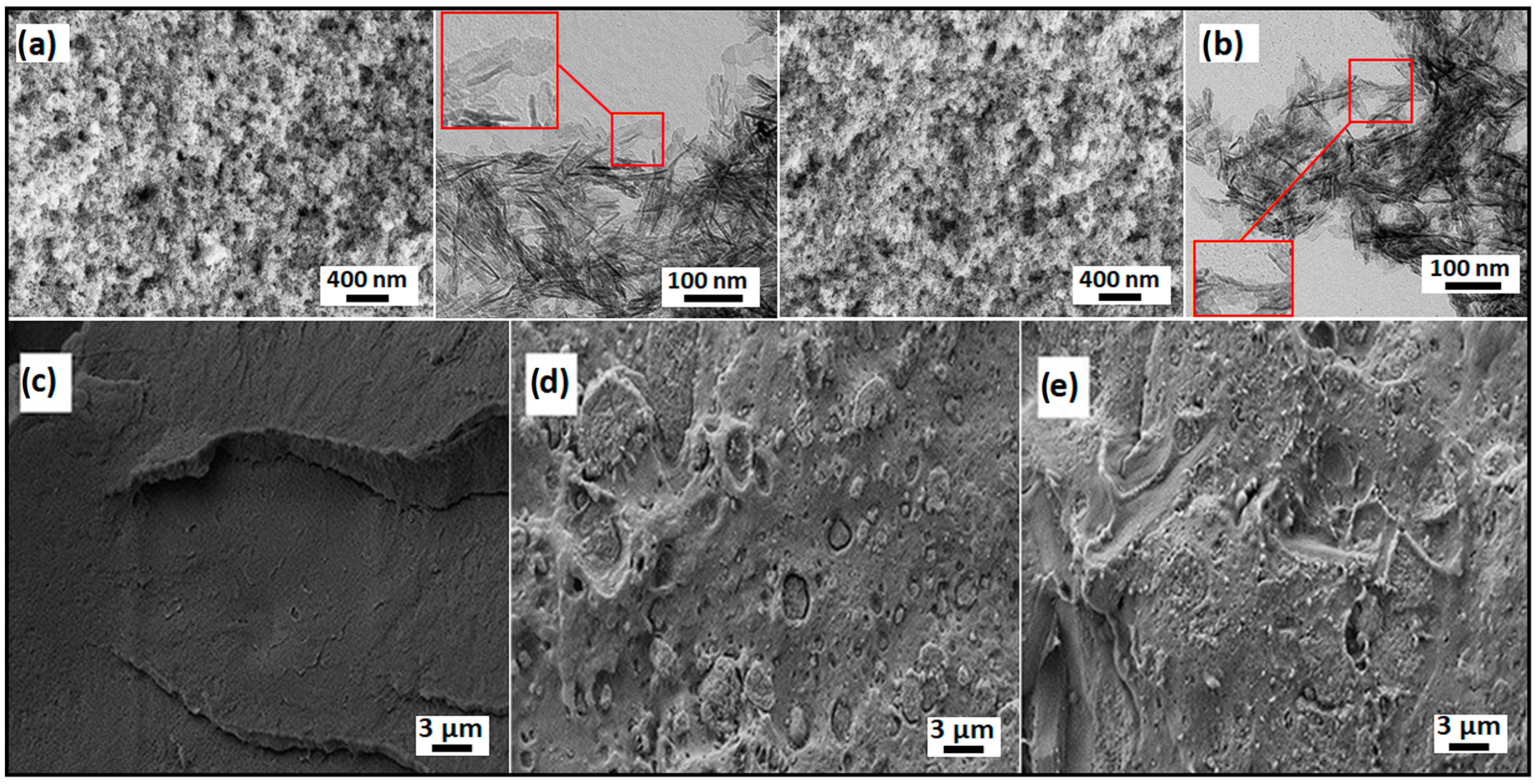
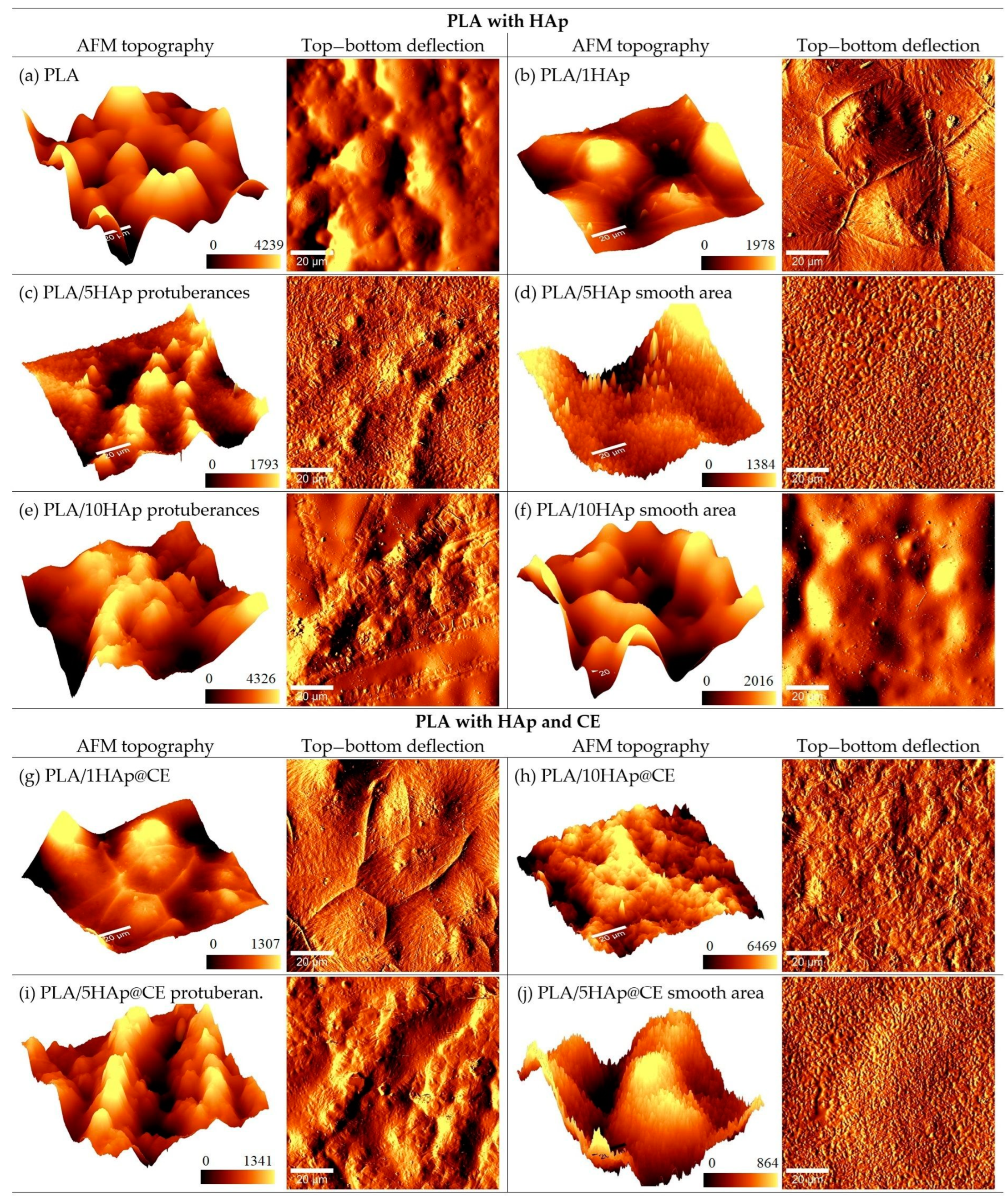
3.1. Morphology Studies
3.2. Spectral and Optical Properties
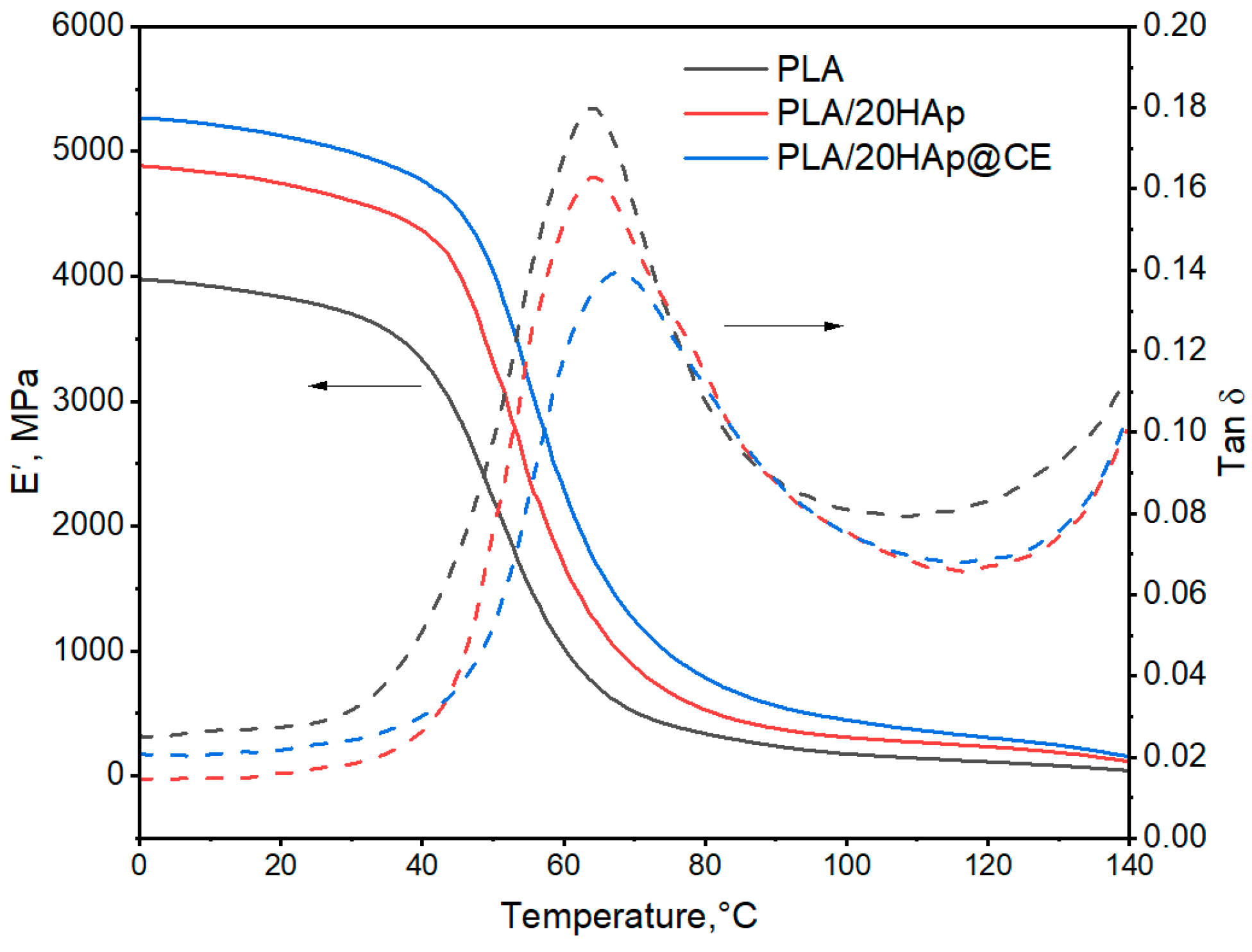
3.3. Thermal Stability
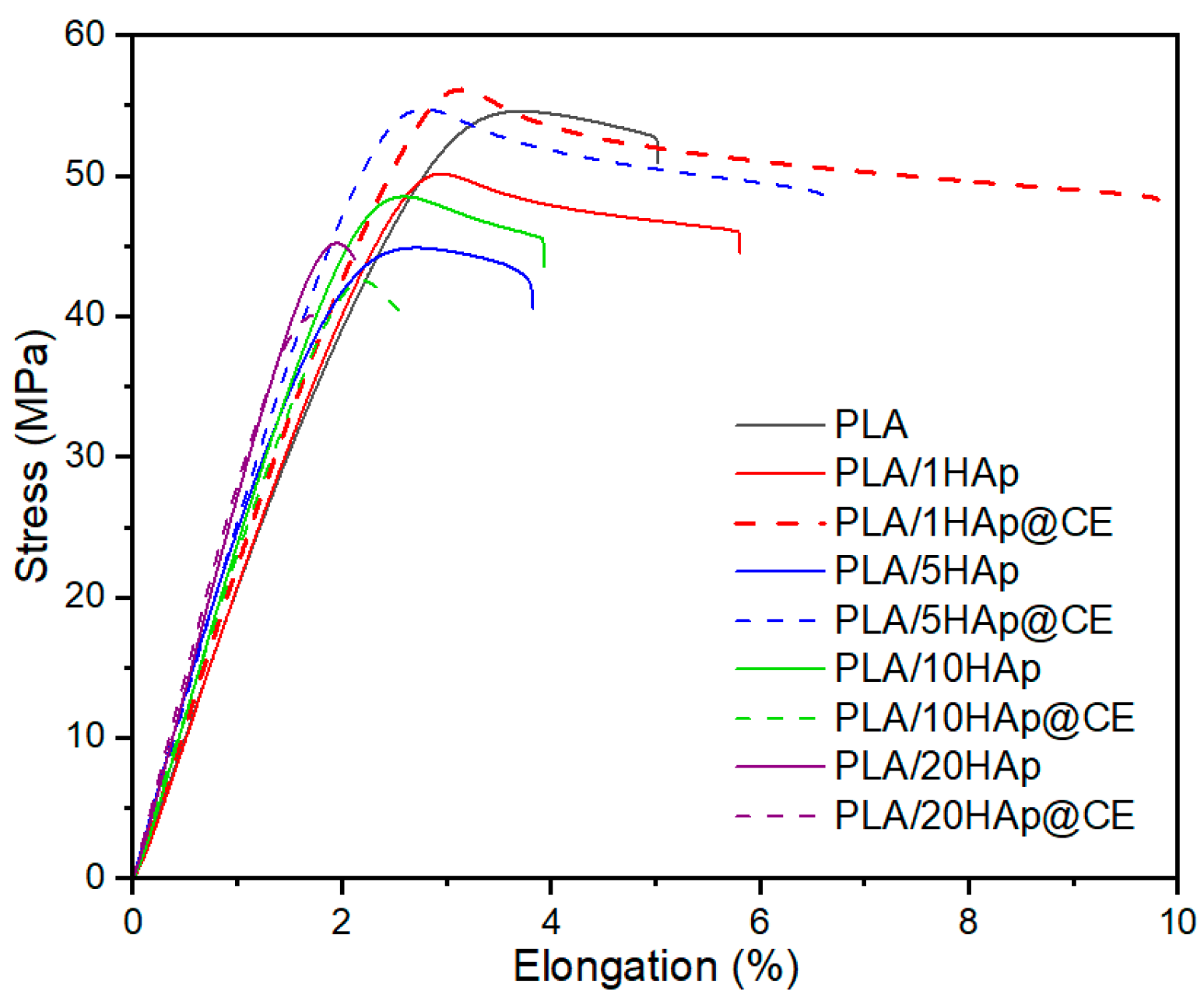
3.4. Mechanical Studies
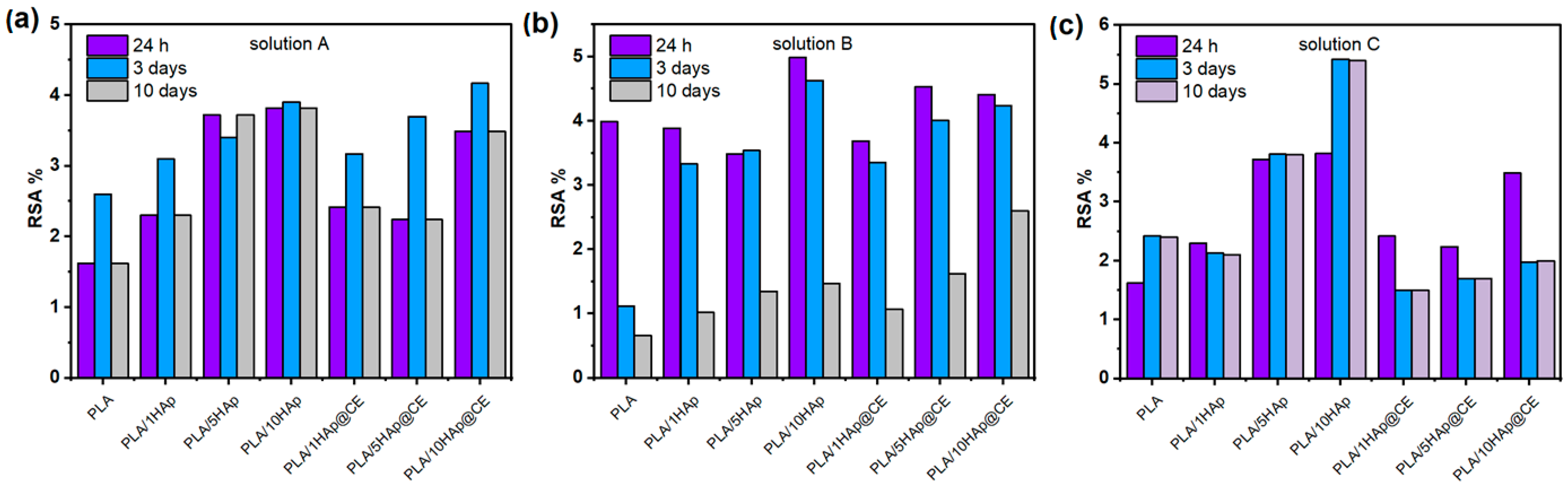
3.5. Capacity to Scavenge the Free Radicals
3.6. Contact Angle Studies
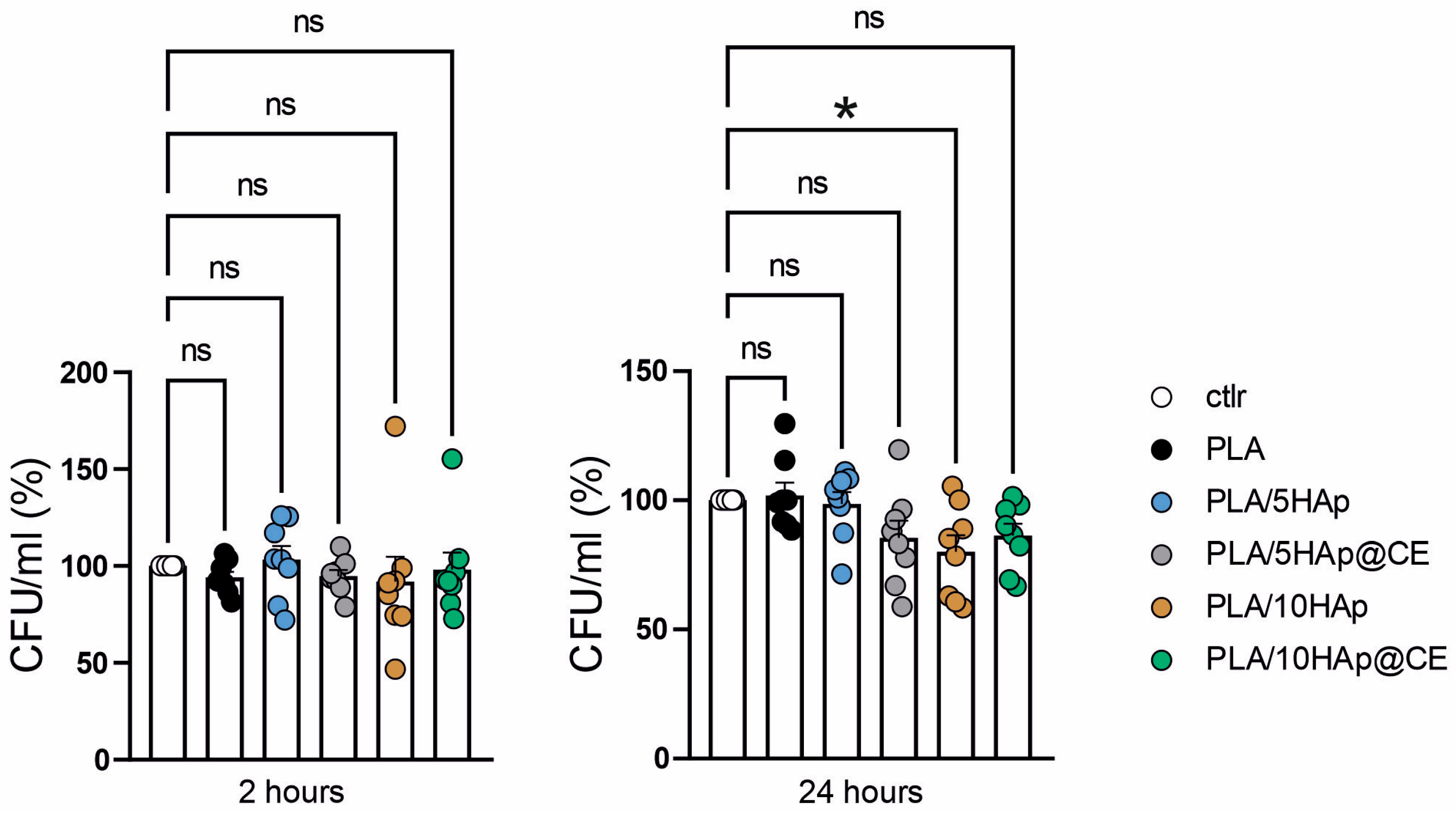
3.7. Antimicrobial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Iannace, S.; Park, C.B. BIOFOAMS: Science and Applications of Bio-Based Cellular and Porous Materials; CRC Press: Boca Raton, FL, USA, 2015; ISBN 9781466561809. [Google Scholar]
- Qi, X.; Pang, X.; Wu, K. Applications of Polylactide and Its Copolymers in Medical Device Fields. Zhongguo Yi Liao Qi Xie Za Zhi 2014, 38. [Google Scholar]
- Kost, B.; Basko, M.; Bednarek, M.; Socka, M.; Kopka, B.; Łapienis, G.; Biela, T.; Kubisa, P.; Brzeziński, M. The Influence of the Functional End Groups on the Properties of Polylactide-Based Materials. Prog. Polym. Sci. 2022, 130, 101556. [Google Scholar] [CrossRef]
- de Oliveira, J.; Vandenberghe, L.P.S.; Zawadzki, S.F.; Rodrigues, C.; de Carvalho, J.C.; Soccol, C.R. Production and Application of Polylactides. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 633–653. [Google Scholar]
- Biela, T.; Kubisa, P.; Lapienis, G. Main Chain Modified Polylactides. Methods of Synthesis and Applications. Polym. Rev. 2024, 64, 939–979. [Google Scholar] [CrossRef]
- Castellón, S.M.; Standau, T.; Altstädt, V.; Bonten, C. Effect of the Chemical Modification on the Thermal and Rheological Properties of Different Polylactides for Foaming; AIP Publishing: Melville, NY, USA, 2020; p. 020068. [Google Scholar]
- Hussain, M.; Khan, S.M.; Shafiq, M.; Abbas, N. A Review on PLA-Based Biodegradable Materials for Biomedical Applications. Giant 2024, 18, 100261. [Google Scholar] [CrossRef]
- Ramanadha Reddy, S.; Venkatachalapathi, N. A Review on Characteristic Variation in PLA Material with a Combination of Various Nano Composites. Mater. Today Proc. 2023; in press. [Google Scholar] [CrossRef]
- Trivedi, A.K.; Gupta, M.K.; Singh, H. PLA Based Biocomposites for Sustainable Products: A Review. Adv. Ind. Eng. Polym. Res. 2023, 6, 382–395. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Heim, H.P.; Feldmann, M. Impact Modified PLA-Hydroxyapatite Composites—Thermo-Mechanical Properties. Compos. Part A Appl. Sci. Manuf. 2018, 107, 326–333. [Google Scholar] [CrossRef]
- Li, J.; Lu, X.L.; Zheng, Y.F. Effect of Surface Modified Hydroxyapatite on the Tensile Property Improvement of HA/PLA Composite. Appl. Surf. Sci. 2008, 255, 494–497. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Heim, H.P.; Feldmann, M. Effects of Surface Modification on Dispersion, Mechanical, Thermal and Dynamic Mechanical Properties of Injection Molded PLA-Hydroxyapatite Composites. Compos. Part A Appl. Sci. Manuf. 2017, 103, 96–105. [Google Scholar] [CrossRef]
- Esposito Corcione, C.; Gervaso, F.; Scalera, F.; Padmanabhan, S.K.; Madaghiele, M.; Montagna, F.; Sannino, A.; Licciulli, A.; Maffezzoli, A. Highly Loaded Hydroxyapatite Microsphere/PLA Porous Scaffolds Obtained by Fused Deposition Modelling. Ceram. Int. 2019, 45, 2803–2810. [Google Scholar] [CrossRef]
- Mondal, S.; Nguyen, T.P.; Pham, V.H.; Hoang, G.; Manivasagan, P.; Kim, M.H.; Nam, S.Y.; Oh, J. Hydroxyapatite Nano Bioceramics Optimized 3D Printed Poly Lactic Acid Scaffold for Bone Tissue Engineering Application. Ceram. Int. 2020, 46, 3443–3455. [Google Scholar] [CrossRef]
- Duraisamy, R. Biocompatibility and Osseointegration of Nanohydroxyapatite. Int. J. Dent. Oral Sci. 2021, 8, 4136–4139. [Google Scholar] [CrossRef]
- Ghiasi, B.; Sefidbakht, Y.; Rezaei, M. Hydroxyapatite for Biomedicine and Drug Delivery. In Nanomaterials for Advanced Biological Applications; Springer: Cham, Switzerland, 2019; pp. 85–120. [Google Scholar]
- Gomes, D.S.; Santos, A.M.C.; Neves, G.A.; Menezes, R.R. A Brief Review on Hydroxyapatite Production and Use in Biomedicine. Cerâmica 2019, 65, 282–302. [Google Scholar] [CrossRef]
- Kusuma, H.H.; Sifah, L.; Anggita, S.S. The Characterization of Hydroxyapatite from Blood Clam Shells and Eggs Shells: Shyntesis by Hydrothermal Method. J. Phys. Conf. Ser. 2021, 1918, 022040. [Google Scholar] [CrossRef]
- Mazumder, S.; Nayak, A.K.; Ara, T.J.; Hasnain, M.S. Hydroxyapatite Composites for Dentistry. In Applications of Nanocomposite Materials in Dentistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 123–143. [Google Scholar]
- Huang, Z.; Wan, Y.; Peng, M.; Yang, Z.; Luo, H. Incorporating Nanoplate-like Hydroxyapatite into Polylactide for Biomimetic Nanocomposites via Direct Melt Intercalation. Compos. Sci. Technol. 2020, 185, 107903. [Google Scholar] [CrossRef]
- Park, Y.; Cho, C.-H. Effects of Additives on the Mechanical and Thermal Properties of Epoxy-Based Nanocomposites Produced Using Sonication. Korean J. Chem. Eng. 2016, 33, 1938–1941. [Google Scholar] [CrossRef]
- Tan, J.; Wu, M.; Li, Y.; Peng, J.; Xiong, Y. Bio-Inspired Hydroxyapatite/Gelatin Transparent Nanocomposites. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2024, 39, 298–308. [Google Scholar] [CrossRef]
- Wan, Y.; Wu, C.; Xiong, G.; Zuo, G.; Jin, J.; Ren, K.; Zhu, Y.; Wang, Z.; Luo, H. Mechanical Properties and Cytotoxicity of Nanoplate-like Hydroxyapatite/Polylactide Nanocomposites Prepared by Intercalation Technique. J. Mech. Behav. Biomed. Mater. 2015, 47, 29–37. [Google Scholar] [CrossRef]
- Ma, P.; Li, T.; Wu, W.; Shi, D.; Duan, F.; Bai, H.; Dong, W.; Chen, M. Novel Poly(Xylitol Sebacate)/Hydroxyapatite Bio-Nanocomposites via One-Step Synthesis. Polym. Degrad. Stab. 2014, 110, 50–55. [Google Scholar] [CrossRef]
- Pielichowska, K. Thermooxidative Degradation of Polyoxymethylene Homo- and Copolymer Nanocomposites with Hydroxyapatite: Kinetic and Thermoanalytical Study. Thermochim. Acta 2015, 600, 7–19. [Google Scholar] [CrossRef]
- Zaharescu, T.; Tardei, C.; Râpă, M.; Iordoc, M. Size Particle Effects on the Thermal Stability of Poly(Lactic Acid)/Hydroxyapatite Hybrids for Biodegradable Package. Ceram. Int. 2020, 46, 7288–7297. [Google Scholar] [CrossRef]
- Simionescu, B.C.; Drobota, M.; Timpu, D.; Vasiliu, T.; Constantinescu, C.A.; Rebleanu, D.; Calin, M.; David, G. Biopolymers/Poly(ε-Caprolactone)/Polyethylenimine Functionalized Nano-Hydroxyapatite Hybrid Cryogel: Synthesis, Characterization and Application in Gene Delivery. Mater. Sci. Eng. C 2017, 81, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Morawska, M.; Krasowska, K. Degradability of Polylactide Films by Commercial Microbiological Preparations for Household Composters. Pol. J. Chem. Technol. 2017, 19, 44–48. [Google Scholar] [CrossRef][Green Version]
- Andrzejewska, A.; Mazurkiewicz, A.; Ligaj, B. Investigation of Mechanical Properties the Polylactide in Function Its Degradation Rate. IOP Conf. Ser. Mater. Sci. Eng. 2018, 393, 012033. [Google Scholar] [CrossRef]
- Yang, H.; Park, S.-Y.; Park, S.B.; Park, J.; Hwang, S.Y. Flexible Poly(Lactic Acid) Suitable for Deep Freeze Packaging Synthesized Using Lactic Acid-Based Ester Precursors. Polym. Test. 2023, 127, 108197. [Google Scholar] [CrossRef]
- Widiastuti, I. Polylactide Nanocomposites for Packaging Materials: A Review; AIP Publishing: Melville, NY, USA, 2016; p. 030020. [Google Scholar]
- Capra, P.; Briasco, B.; Sorrenti, M.; Catenacci, L.; Sachet, M.; Perugini, P. Preliminary Evaluation of Packaging-content Interactions: Mechanical and Physicochemical Characterization of Polylactide Bottles. J. Appl. Polym. Sci. 2014, 131, 40067. [Google Scholar] [CrossRef]
- Thomas, J.; Patil, R.S.; Patil, M.; John, J. Addressing the Sustainability Conundrums and Challenges within the Polymer Value Chain. Sustainability 2023, 15, 15758. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Preparation of Bioactive Functional Poly(Lactic Acid)/Curcumin Composite Film for Food Packaging Application. Int. J. Biol. Macromol. 2020, 162, 1780–1789. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-H.; Loo, C.-Y.; Rohanizadeh, R. Functionalizing the Surface of Hydroxyapatite Drug Carrier with Carboxylic Acid Groups to Modulate the Loading and Release of Curcumin Nanoparticles. Mater. Sci. Eng. C 2019, 99, 929–939. [Google Scholar] [CrossRef]
- Lee, W.-H.; Rohanizadeh, R.; Loo, C.-Y. In Situ Functionalizing Calcium Phosphate Biomaterials with Curcumin for the Prevention of Bacterial Biofilm Infections. Colloids Surf. B Biointerfaces 2021, 206, 111938. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Wilczewski, S.; Lewandowski, J.; Majkowska-Pilip, A.; Żelechowska-Matysiak, K.; Nieciecka, D.; Studziński, W.; Olusegun, S.J.; Syczewski, M.; Giersig, M.; et al. 5-Fluorouracil and Curcuminoids Extract from Curcuma longa L. Loaded into Nanohydroxyapatite as a Drug Delivery Carrier for SKOV-3 and HepG2 Cancer Cells Treatment. Ceram. Int. 2023, 49, 25775–25787. [Google Scholar] [CrossRef]
- Xidaki, D.; Agrafioti, P.; Diomatari, D.; Kaminari, A.; Tsalavoutas-Psarras, E.; Alexiou, P.; Psycharis, V.; Tsilibary, E.; Silvestros, S.; Sagnou, M. Synthesis of Hydroxyapatite, β-Tricalcium Phosphate and Biphasic Calcium Phosphate Particles to Act as Local Delivery Carriers of Curcumin: Loading, Release and In Vitro Studies. Materials 2018, 11, 595. [Google Scholar] [CrossRef] [PubMed]
- Wilczewski, S.; Skórczewska, K.; Tomaszewska, J.; Lewandowski, K.; Studziński, W.; Osial, M.; Jenczyk, P.; Grzywacz, H.; Domańska, A. Curcuma longa L. Rhizome Extract as a Poly(Vinyl Chloride)/Graphene Nanocomposite Green Modifier. Molecules 2022, 27, 8081. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Osial, M.; Wilczewski, S.; Szulc, J.; Nguyen, H.D.; Nguyen, T.K.O.; Skórczewska, K.; Majkowska-Pilip, A.; Żelechowska-Matysiak, K.; Nieciecka, D.; Pregowska, A.; et al. Nanohydroxyapatite Loaded with 5-Fluorouracil and Calendula officinalis L. Plant Extract Rich in Myo-Inositols for Treatment of Ovarian Cancer Cells. Coatings 2023, 13, 1944. [Google Scholar] [CrossRef]
- Aworinde, A.K.; Adeosun, S.O.; Oyawale, F.A.; Akinlabi, E.T.; Akinlabi, S.A. Comparative Effects of Organic and Inorganic Bio-Fillers on the Hydrophobicity of Polylactic Acid. Results Eng. 2020, 5, 100098. [Google Scholar] [CrossRef]
- Li, D.; Jiang, Y.; Lv, S.; Liu, X.; Gu, J.; Chen, Q.; Zhang, Y. Preparation of Plasticized Poly (Lactic Acid) and Its Influence on the Properties of Composite Materials. PLoS ONE 2018, 13, e0193520. [Google Scholar] [CrossRef] [PubMed]
- Singla, P.; Mehta, R.; Berek, D.; Upadhyay, S.N. Microwave Assisted Synthesis of Poly(Lactic Acid) and Its Characterization Using Size Exclusion Chromatography. J. Macromol. Sci. Part A 2012, 49, 963–970. [Google Scholar] [CrossRef]
- Torres-Huerta, A.M.; Del Angel-López, D.; Domínguez-Crespo, M.A.; Palma-Ramírez, D.; Perales-Castro, M.E.; Flores-Vela, A. Morphological and Mechanical Properties Dependence of PLA Amount in PET Matrix Processed by Single-Screw Extrusion. Polym. Technol. Eng. 2016, 55, 672–683. [Google Scholar] [CrossRef]
- Marin, E.; Rondinella, A.; Bin Idrus, D.M.; Lanzutti, A.; de Leitenburg, C.; Danielis, M.; Zhu, W.; Xu, H.; Pezzotti, G. Non-Destructive Spectroscopic Diagnostic Tools for the Assessment of the Mechanical Strength of 3D-Printed PLA. Polym. Degrad. Stab. 2023, 216, 110506. [Google Scholar] [CrossRef]
- Mohan, P.R.K.; Sreelakshmi, G.; Muraleedharan, C.V.; Joseph, R. Water Soluble Complexes of Curcumin with Cyclodextrins: Characterization by FT-Raman Spectroscopy. Vib. Spectrosc. 2012, 62, 77–84. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An Overview of Polylactides as Packaging Materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef]
- Kim, I.; Viswanathan, K.; Kasi, G.; Sadeghi, K.; Thanakkasaranee, S.; Seo, J. Poly(Lactic Acid)/ZnO Bionanocomposite Films with Positively Charged ZnO as Potential Antimicrobial Food Packaging Materials. Polymers 2019, 11, 1427. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, R.; Shi, H.; Li, X.; Li, Y.; Taha, A.; Xu, C. Protective Effect of Curcumin against Ultraviolet A Irradiation-induced Photoaging in Human Dermal Fibroblasts. Mol. Med. Rep. 2018, 17, 7227–7237. [Google Scholar] [CrossRef]
- Li, H.; Gao, A.; Jiang, N.; Liu, Q.; Liang, B.; Li, R.; Zhang, E.; Li, Z.; Zhu, H. Protective Effect of Curcumin against Acute Ultraviolet B Irradiation-induced Photo-damage. Photochem. Photobiol. 2016, 92, 808–815. [Google Scholar] [CrossRef]
- Bauer, L.; Rogina, A.; Ivanković, M.; Ivanković, H. Medical-Grade Poly(Lactic Acid)/Hydroxyapatite Composite Films: Thermal and In Vitro Degradation Properties. Polymers 2023, 15, 1512. [Google Scholar] [CrossRef]
- Kervran, M.; Vagner, C.; Cochez, M.; Ponçot, M.; Saeb, M.R.; Vahabi, H. Thermal Degradation of Polylactic Acid (PLA)/Polyhydroxybutyrate (PHB) Blends: A Systematic Review. Polym. Degrad. Stab. 2022, 201, 109995. [Google Scholar] [CrossRef]
- Aydemir, D.; Gardner, D.J. Biopolymer Blends of Polyhydroxybutyrate and Polylactic Acid Reinforced with Cellulose Nanofibrils. Carbohydr. Polym. 2020, 250, 116867. [Google Scholar] [CrossRef]
- Tazibt, N.; Kaci, M.; Dehouche, N.; Ragoubi, M.; Atanase, L.I. Effect of Filler Content on the Morphology and Physical Properties of Poly(Lactic Acid)-Hydroxyapatite Composites. Materials 2023, 16, 809. [Google Scholar] [CrossRef]
- Motloung, M.P.; Mofokeng, T.G.; Ray, S.S. Viscoelastic, Thermal, and Mechanical Properties of Melt-Processed Poly (ε-Caprolactone) (Pcl)/Hydroxyapatite (Hap) Composites. Materials 2022, 15, 104. [Google Scholar] [CrossRef]
- Zhu, J.; Abeykoon, C.; Karim, N. Investigation into the Effects of Fillers in Polymer Processing. Int. J. Light. Mater. Manuf. 2021, 4, 370–382. [Google Scholar] [CrossRef]
- Athanasoulia, I.-G.I.; Christoforidis, M.N.; Korres, D.M.; Tarantili, P.A. The Effect of Hydroxyapatite Nanoparticles on Crystallization and Thermomechanical Properties of PLLA Matrix. Pure Appl. Chem. 2017, 89, 125–140. [Google Scholar] [CrossRef]
- Jandas, P.J.; Mohanty, S.; Nayak, S.K. Thermal Properties and Cold Crystallization Kinetics of Surface-Treated Banana Fiber (BF)-Reinforced Poly(Lactic Acid) (PLA) Nanocomposites. J. Therm. Anal. Calorim. 2013, 114, 1265–1278. [Google Scholar] [CrossRef]
- Backes, E.; Pires, L.; Costa, L.; Passador, F.; Pessan, L. Analysis of the Degradation During Melt Processing of PLA/Biosilicate® Composites. J. Compos. Sci. 2019, 3, 52. [Google Scholar] [CrossRef]
- Meng, X.; Gong, W.; Chen, W.; Shi, Y.; Sheng, Y.; Zhu, S.; Xin, Z. Isothermal and Non-Isothermal Crystallization of Isotactic Polypropylene in the Presence of an α Nucleating Agent and Zeolite 13X. Thermochim. Acta 2018, 667, 9–18. [Google Scholar] [CrossRef]
- Barczewski, M.; Mysiukiewicz, O.; Andrzejewski, J.; Matykiewicz, D.; Skórczewska, K.; Lewandowski, K.; Jakubowicz, M.; Aniśko, J.; Gapiński, B.; Sałasińska, K.; et al. BioXpulTM—Technology for Manufacturing PLA-Based Biocomposites with Increased Thermomechanical Stability. Manuf. Lett. 2023, 35, 43–47. [Google Scholar] [CrossRef]
- Fortunati, E.; Armentano, I.; Iannoni, A.; Kenny, J.M. Development and Thermal Behaviour of Ternary PLA Matrix Composites. Polym. Degrad. Stab. 2010, 95, 2200–2206. [Google Scholar] [CrossRef]
- Pölöskei, K.; Csézi, G.; Hajba, S.; Tábi, T. Investigation of the Thermoformability of Various D-Lactide Content Poly(Lactic Acid) Films by Ball Burst Test. Polym. Eng. Sci. 2020, 60, 1266–1277. [Google Scholar] [CrossRef]
- Maniglia, B.C.; de Paula, R.L.; Domingos, J.R.; Tapia-Blácido, D.R. Turmeric Dye Extraction Residue for Use in Bioactive Film Production: Optimization of Turmeric Film Plasticized with Glycerol. LWT—Food Sci. Technol. 2015, 64, 1187–1195. [Google Scholar] [CrossRef]
- Rodrigues, F.M.S.; Tavares, I.; Aroso, R.T.; Dias, L.D.; Domingos, C.V.; de Faria, C.M.G.; Piccirillo, G.; Maria, T.M.R.; Carrilho, R.M.B.; Bagnato, V.S.; et al. Photoantibacterial Poly(Vinyl)Chloride Films Applying Curcumin Derivatives as Bio-Based Plasticizers and Photosensitizers. Molecules 2023, 28, 2209. [Google Scholar] [CrossRef]
- Saltos, J.A.; Shi, W.; Mancuso, A.; Sun, C.; Park, T.; Averick, N.; Punia, K.; Fata, J.; Raja, K. Curcumin-Derived Green Plasticizers for Poly(Vinyl) Chloride. RSC Adv. 2014, 4, 54725–54728. [Google Scholar] [CrossRef]
- Baskaran, M.; Hashim, R.; Sulaiman, O.; Awalludin, M.F.; Sudesh, K.; Arai, T.; Kosugi, A. Properties of Particleboard Manufactured from Oil Palm Trunk Waste Using Polylactic Acid as a Natural Binder. Waste Biomass Valorization 2019, 10, 179–186. [Google Scholar] [CrossRef]
- Maiza, M.; Benaniba, M.T.; Quintard, G.; Massardier-Nageotte, V. Biobased Additive Plasticizing Polylactic Acid (PLA). Polímeros 2015, 25, 581–590. [Google Scholar] [CrossRef]
- Zhang, T.; Cai, W.; Chu, F.; Zhou, F.; Liang, S.; Ma, C.; Hu, Y. Hydroxyapatite/Polyurea Nanocomposite: Preparation and Multiple Performance Enhancements. Compos. Part A Appl. Sci. Manuf. 2020, 128, 105681. [Google Scholar] [CrossRef]
- Cvek, M.; Paul, U.C.; Zia, J.; Mancini, G.; Sedlarik, V.; Athanassiou, A. Biodegradable Films of PLA/PPC and Curcumin as Packaging Materials and Smart Indicators of Food Spoilage. ACS Appl. Mater. Interfaces 2022, 14, 14654–14667. [Google Scholar] [CrossRef] [PubMed]
- Subbuvel, M.; Kavan, P. Preparation and Characterization of Polylactic Acid/Fenugreek Essential Oil/Curcumin Composite Films for Food Packaging Applications. Int. J. Biol. Macromol. 2022, 194, 470–483. [Google Scholar] [CrossRef] [PubMed]
- Ak, T.; Gülçin, İ. Antioxidant and Radical Scavenging Properties of Curcumin. Chem. Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Iñiguez-Franco, F.; Auras, R.; Burgess, G.; Holmes, D.; Fang, X.; Rubino, M.; Soto-Valdez, H. Concurrent Solvent Induced Crystallization and Hydrolytic Degradation of PLA by Water-Ethanol Solutions. Polymer 2016, 99, 315–323. [Google Scholar] [CrossRef]
- Diez-Escudero, A.; Espanol, M.; Ginebra, M.-P. High-Aspect-Ratio Nanostructured Hydroxyapatite: Towards New Functionalities for a Classical Material. Chem. Sci. 2024, 15, 55–76. [Google Scholar] [CrossRef]
- Nakade, K.; Jindai, K.; Sagawa, T.; Kojima, H.; Shimizu, T.; Shingubara, S.; Ito, T. Adhesion and Bactericidal Properties of a Wettability-Controlled Artificial Nanostructure. ACS Appl. Nano Mater. 2018, 1, 5736–5741. [Google Scholar] [CrossRef]
- Moazzami Goudarzi, Z.; Behzad, T.; Sheykhzadeh, A. Effect of Hydrophobically Modified Extracted Starch Nanocrystal on the Properties of LDPE/Thermoplastic Starch (TPS)/PE-g-MA Nanocomposite. J. Appl. Polym. Sci. 2022, 139, 51490. [Google Scholar] [CrossRef]




| Sample | Tonset, °C | TDTG, °C |
|---|---|---|
| PLA | 339.0 | 358.4 |
| PLA/1HAp | 344.3 | 363.5 |
| PLA/5HAp | 343.5 | 361.8 |
| PLA/10HAp | 341.4 | 361.3 |
| PLA/20HAp | 337.8 | 359.4 |
| PLA/1HAp@CE | 342.9 | 359.2 |
| PLA/5HAp@CE | 343.7 | 362.0 |
| PLA/10HAp@CE | 342.2 | 361.6 |
| PLA/20HAp@CE | 338.7 | 359.8 |
| Sample | TgMID, °C | TgINFL, °C | ΔHcc, J g−1 | ΔHm, J g−1 | Tt, °C | K, % |
|---|---|---|---|---|---|---|
| PLA | 59.3 | 60.3 | 14.12 | 17.27 | 150.9 | 3.4 |
| PLA/1HAp | 58.9 | 60.5 | 13.46 | 16.82 | 150.3 | 3.6 |
| PLA/5HAp | 59.3 | 60.5 | 4.42 | 7.24 | 150.4 | 3.2 |
| PLA/10HAp | 59.1 | 60.6 | 0.92 | 3.70 | 150.3 | 3.3 |
| PLA/20HAp | 59.2 | 60.2 | 0.05 | 1.15 | 149.8 | 1.5 |
| PLA/1HAp@CE | 58.5 | 59.8 | 19.0 | 22.21 | 149.7 | 3.5 |
| PLA/5HAp@CE | 59.6 | 60.7 | 3.62 | 5.82 | 150.7 | 2.5 |
| PLA/10HAp@CE | 59.0 | 60.6 | 1.60 | 3.80 | 150.1 | 2.7 |
| PLA/20HAp@CE | 58.3 | 59.7 | 0 | 1.61 | 149.8 | 2.1 |
| Filler Content, wt.% | E′, MPa | Tg, °C | ||
|---|---|---|---|---|
| 0 °C | 25 °C | E’onset | tanδ | |
| PLA | 3923 | 3754 | 40.2 | 64.3 |
| PLA/1HAp | 4084 | 3885 | 39.8 | 63.0 |
| PLA/5HAp | 4101 | 3946 | 41.4 | 65.6 |
| PLA/10HAp | 4718 | 4494 | 41.6 | 63.5 |
| PLA/20HAp | 4885 | 4707 | 43.6 | 64.4 |
| PLA/1HAp@CE | 4211 | 4039 | 40.0 | 63.0 |
| PLA/5HAp@CE | 4331 | 4105 | 38.7 | 61.8 |
| PLA/10HAp@CE | 4611 | 4357 | 37.0 | 60.7 |
| PLA/20HAp@CE | 5149 | 4969 | 44.6 | 66.3 |
| Filler Content, wt.% | HAp | HAp@CE | ||||
|---|---|---|---|---|---|---|
| Et, MPa | σm, MPa | σm, % | Et, MPa | σm, MPa | σm, % | |
| 0 | 2200 ± 132 | 54.7 ± 1.5 | 3.2 ± 0.5 | 2200 ± 132 | 54.7 ± 1.5 | 3.2 ± 0.5 |
| 1 | 2120 ± 21 | 49.9 ± 0.8 | 2.9 ± 0.02 | 2160 ± 35 | 55.5 ± 0.8 | 3.2 ± 0.1 |
| 5 | 2610 ± 32 | 44.6 ± 0.4 | 2.6 ± 0.2 | 2490 ± 56 | 54.9 ± 0.4 | 5.8 ± 0.9 |
| 10 | 2390 ± 65 | 48.2 ± 3.0 | 2.6 ± 0.1 | 2250 ± 72 | 43.2 ± 5.8 | 2.5 ± 0.3 |
| 20 | 2560 ± 110 | 45.2 ± 1.6 | 2.0 ± 0.1 | 2890 ± 75 | 40.9 ± 1.7 | 2.1 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osial, M.; Wilczewski, S.; Godlewska, U.; Skórczewska, K.; Hilus, J.; Szulc, J.; Roszkiewicz, A.; Dąbrowska, A.; Moazzami Goudarzi, Z.; Lewandowski, K.; et al. Incorporation of Nanostructural Hydroxyapatite and Curcumin Extract from Curcuma longa L. Rhizome into Polylactide to Obtain Green Composite. Polymers 2024, 16, 2169. https://doi.org/10.3390/polym16152169
Osial M, Wilczewski S, Godlewska U, Skórczewska K, Hilus J, Szulc J, Roszkiewicz A, Dąbrowska A, Moazzami Goudarzi Z, Lewandowski K, et al. Incorporation of Nanostructural Hydroxyapatite and Curcumin Extract from Curcuma longa L. Rhizome into Polylactide to Obtain Green Composite. Polymers. 2024; 16(15):2169. https://doi.org/10.3390/polym16152169
Chicago/Turabian StyleOsial, Magdalena, Sławomir Wilczewski, Urszula Godlewska, Katarzyna Skórczewska, Jakub Hilus, Joanna Szulc, Agata Roszkiewicz, Agnieszka Dąbrowska, Zahra Moazzami Goudarzi, Krzysztof Lewandowski, and et al. 2024. "Incorporation of Nanostructural Hydroxyapatite and Curcumin Extract from Curcuma longa L. Rhizome into Polylactide to Obtain Green Composite" Polymers 16, no. 15: 2169. https://doi.org/10.3390/polym16152169
APA StyleOsial, M., Wilczewski, S., Godlewska, U., Skórczewska, K., Hilus, J., Szulc, J., Roszkiewicz, A., Dąbrowska, A., Moazzami Goudarzi, Z., Lewandowski, K., Wypych, T. P., Nguyen, P. T., Sumara, G., & Giersig, M. (2024). Incorporation of Nanostructural Hydroxyapatite and Curcumin Extract from Curcuma longa L. Rhizome into Polylactide to Obtain Green Composite. Polymers, 16(15), 2169. https://doi.org/10.3390/polym16152169








