Sustainability in the Development of Natural Pigment-Based Colour Masterbatches and Their Application in Biopolymers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure
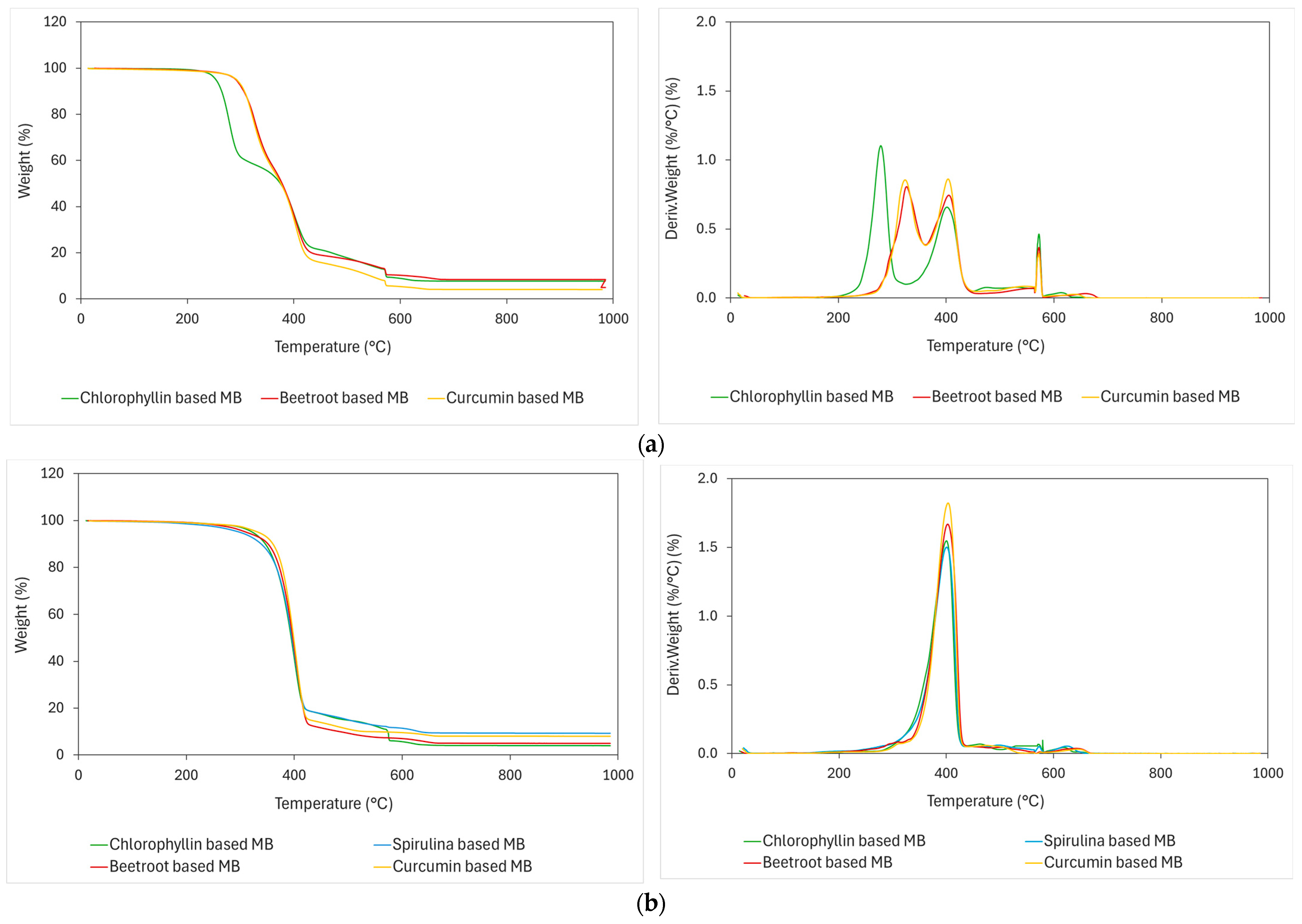
2.2.1. Thermal Analysis
2.2.2. Preparation of Natural Pigment-Based MBs
2.2.3. Melt Flow Rate (MFR)
2.2.4. Injection Moulding
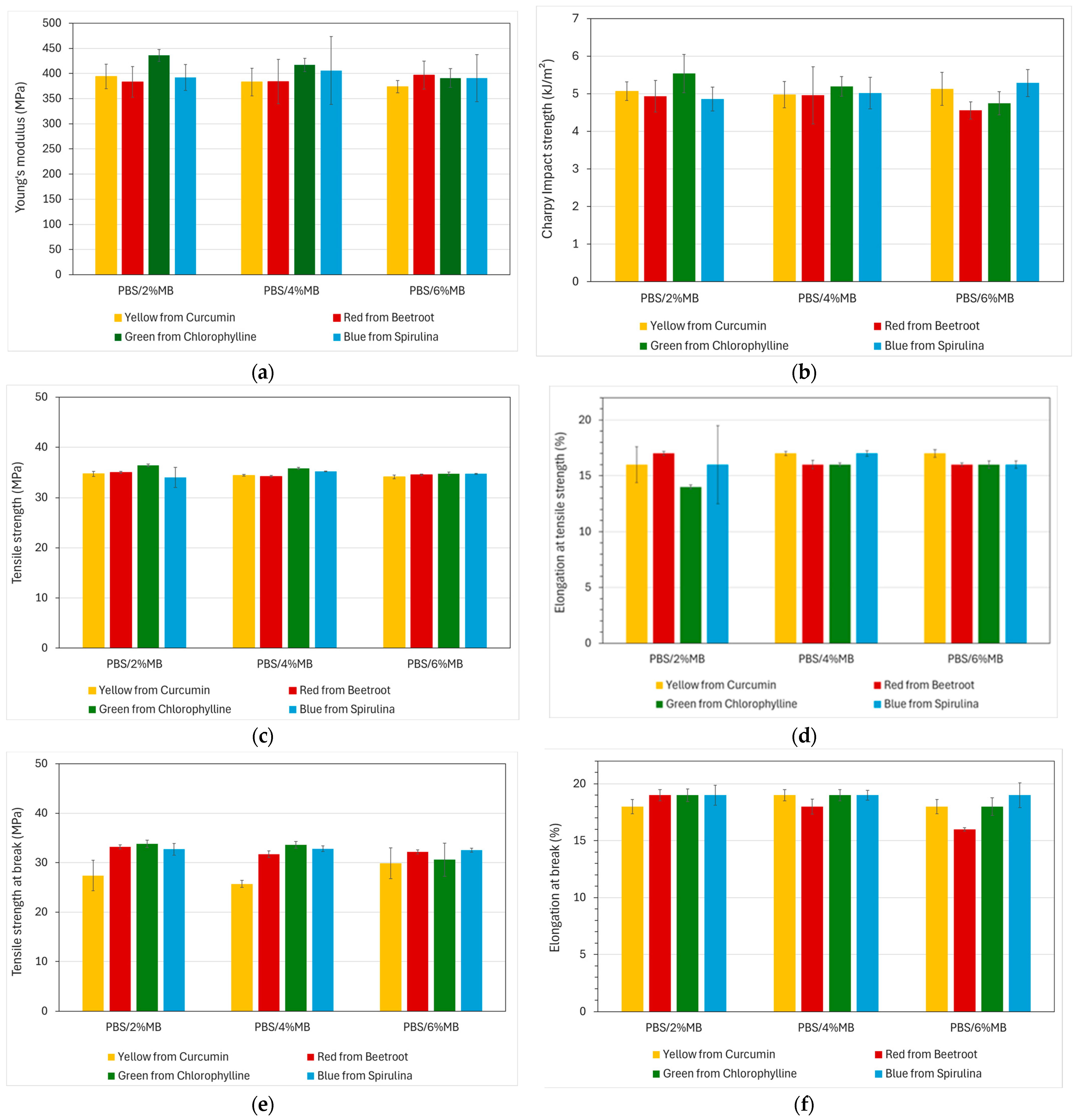
2.2.5. Mechanical Properties
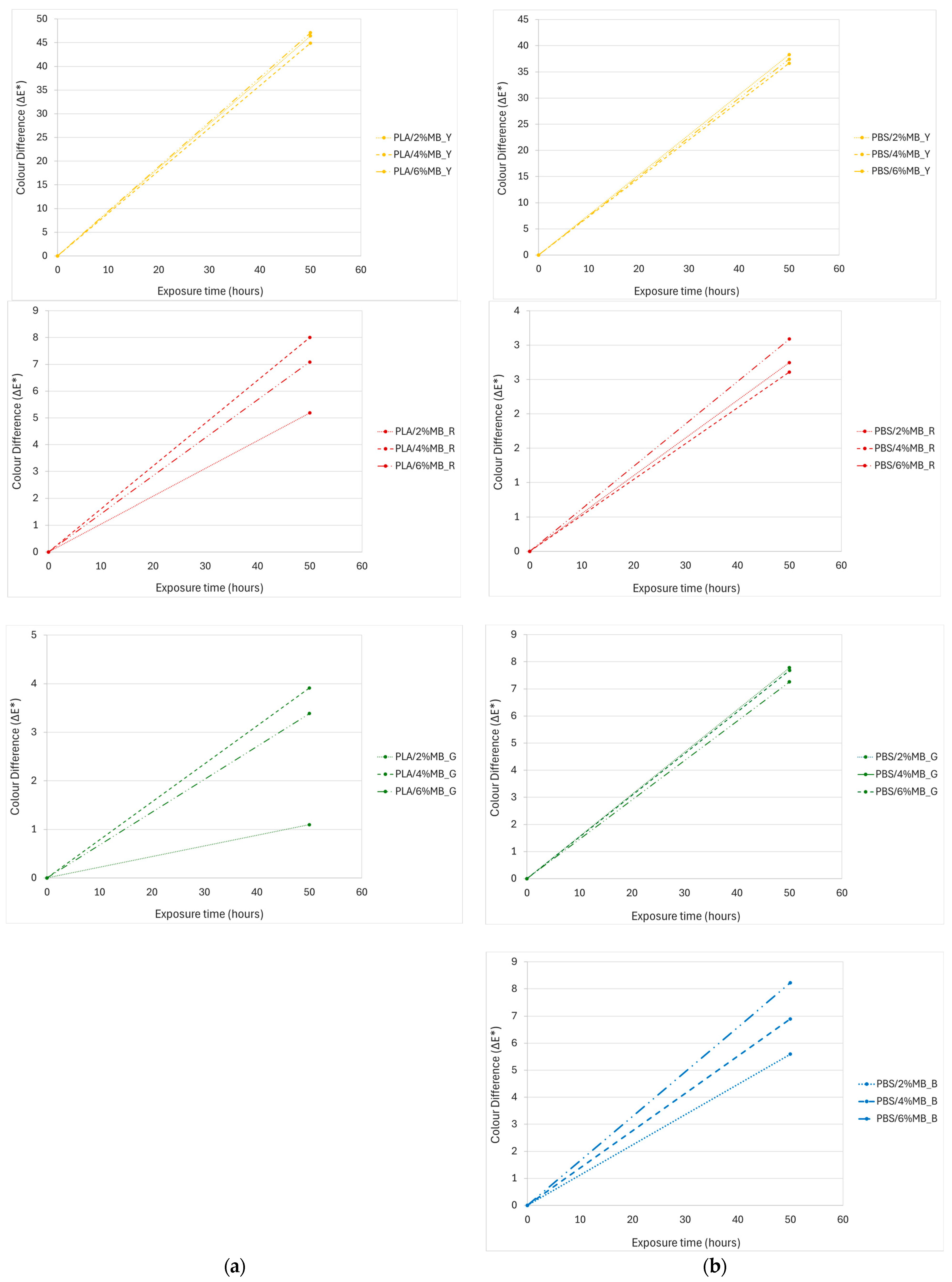
2.2.6. Ageing Test
2.2.7. Colour Measurements
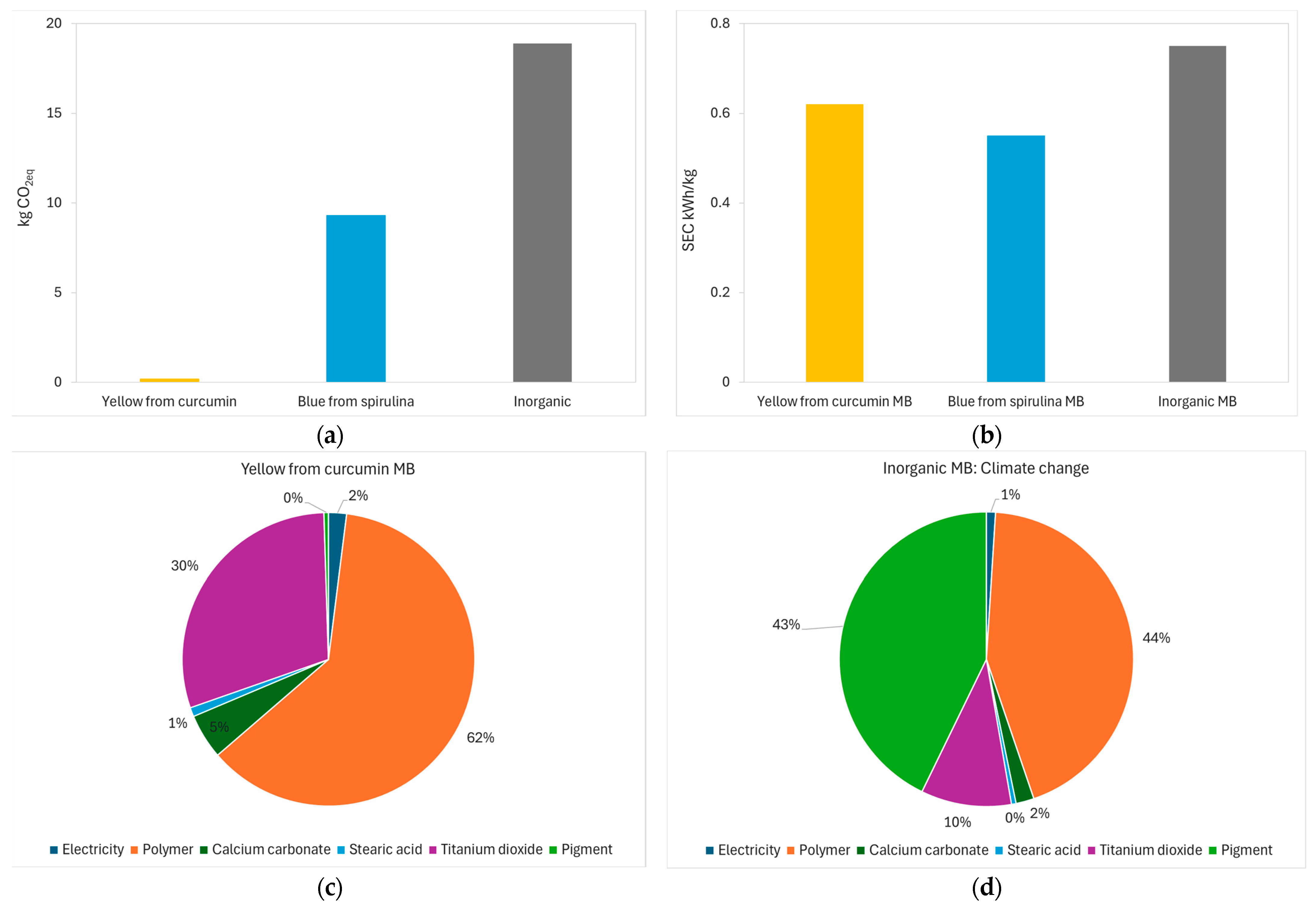
2.2.8. Environmental Concerns
3. Results
3.1. Development of Natural Pigment-Based MBs
3.2. Characterization of Natural Pigment-Based MBs
3.3. Characterization of the Injected Specimens with Natural Pigment-Based MBs
3.4. Mechanical Properties
3.5. Ageing Test of Injected Samples
3.6. Environmental Concerns
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- European Bioplastics. Bioplastics Market Development Update 2023; European Bioplastics e.V.: Berlin, Germany, 2023. [Google Scholar]
- Ibáñez-García, A.; Martínez-García, A.; Ferrándiz-Bou, S. Recyclability analysis of starch thermoplastic/almond shell biocomposite. Polymers 2021, 13, 1159. [Google Scholar] [CrossRef] [PubMed]
- AGarcía, I.; García, A.M.; Bou, S.F. Study of the influence of the almond shell variety onthe mechanical properties of starch-basedpolymer biocomposites. Polymers 2020, 12, 2049. [Google Scholar] [CrossRef]
- Zafar, M.F.; Siddiqui, M.A. ScienceDirect Raw natural fiber reinforced polystyrene composites: Effect of fiber size and loading. Mater. Today Proc. 2018, 5, 5908–5917. [Google Scholar] [CrossRef]
- Azwa, Z.N.; Yousif, B.F.; Manalo, A.C.; Karunasena, W. A review on the degradability of polymeric composites based on natural fibres. Mater. Des. 2013, 47, 424–442. [Google Scholar] [CrossRef]
- Al-Oqla, F.M.; Sapuan, S.M. Natural fiber reinforced polymer composites in industrial applications: Feasibility of date palm fibers for sustainable automotive industry. J. Clean. Prod. 2014, 66, 347–354. [Google Scholar] [CrossRef]
- Ray, D.; Sain, S. Plant fibre reinforcements. In Biocomposites for High-Performance Applications: Current Barriers and Future Needs towards Industrial Development; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 1–21. [Google Scholar] [CrossRef]
- Zhang, C.; Garrison, T.F.; Madbouly, S.A.; Kessler, M.R. Recent advances in vegetable oil-based polymers and their composites. Prog. Polym. Sci. 2017, 71, 91–143. [Google Scholar] [CrossRef]
- Balart, J.F.; Fombuena, V.; Fenollar, O.; Boronat, T.; Sánchez-Nacher, L. Processing and characterization of high environmental efficiency composites based on PLA and hazelnut shell flour (HSF) with biobased plasticizers derived from epoxidized linseed oil (ELO). Compos. Part B Eng. 2016, 86, 168–177. [Google Scholar] [CrossRef]
- Orue, A.; Eceiza, A.; Arbelaiz, A. Preparation and characterization of poly(lactic acid) plasticized with vegetable oils and reinforced with sisal fibers. Ind. Crop. Prod. 2018, 112, 170–180. [Google Scholar] [CrossRef]
- Chieng, B.W.; Ibrahim, N.A.; Then, Y.Y.; Loo, Y.Y. Epoxidized vegetable oils plasticized poly(lactic acid) biocomposites: Mechanical, thermal and morphology properties. Molecules 2014, 19, 16024–16038. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Montanes, N.; Garcia-Garcia, D.; Carbonell-Verdu, A.; Balart, R.; Torres-Giner, S. Effect of different compatibilizers on injection-molded green composite pieces based on polylactide filled with almond shell flour. Compos. Part B Eng. 2018, 147, 76–85. [Google Scholar] [CrossRef]
- Liminana, P.; Garcia-Sanoguera, D.; Quiles-Carrillo, L.; Balart, R.; Montanes, N. Development and characterization of environmentally friendly composites from poly(butylene succinate) (PBS) and almond shell flour with different compatibilizers. Compos. Part B Eng. 2018, 144, 153–162. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Ferri, J.M.; Montanes, N.; Lopez-Martinez, J.; Balart, R. Plasticization effects of epoxidized vegetable oils on mechanical properties of poly(3-hydroxybutyrate). Polym. Int. 2016, 65, 1157–1164. [Google Scholar] [CrossRef]
- Carbonell-Verdu, A.; Samper, M.D.; Garcia-Garcia, D.; Sanchez-Nacher, L.; Balart, R. Plasticization effect of epoxidized cottonseed oil (ECSO) on poly(lactic acid). Ind. Crops Prod. 2017, 104, 278–286. [Google Scholar] [CrossRef]
- Samarth, N.B.; Mahanwar, P.A. Modified Vegetable Oil Based Additives as a Future Polymeric Material—Review. Open J. Org. Polym. Mater. 2015, 5, 53012. [Google Scholar] [CrossRef]
- Ruellan, A.; Guinault, A.; Sollogoub, C.; Chollet, G.; Ait-Mada, A.; Ducruet, V.; Domenek, S. Industrial vegetable oil by-products increase the ductility of polylactide. Express Polym. Lett. 2015, 9, 1087–1103. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Fenollar, O.; Fombuena, V.; Lopez-Martinez, J.; Balart, R. Improvement of Mechanical Ductile Properties of Poly(3-hydroxybutyrate) by Using Vegetable Oil Derivatives. Macromol. Mater. Eng. 2017, 302, 1600330. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhang, L.; Ma, S.; Yang, Y.; Zhang, C.; Tang, Z.; Zhu, J. Effect of castor oil enrichment layer produced by reaction on the properties of PLA/HDI-g-starch blends. Carbohydr. Polym. 2013, 94, 235–243. [Google Scholar] [CrossRef]
- Bond-Tee, Y.; Talib, R.; Abdan, K.; Ling, N.; Kadir, R.; Faezah, K. Comparative study of chemical, mechanical, thermal and barrier properties of Poly(lactic acid) plasticized with epoxidized soybean oil and epoxidized palm oil. BioResources 2016, 11, 1518–1540. [Google Scholar]
- Choi, J.S.; Park, W.H. Effect of biodegradable plasticizers on thermal and mechanical properties of poly(3-hydroxybutyrate). Polym. Test. 2004, 23, 455–460. [Google Scholar] [CrossRef]
- Di Salvo, E.; Vecchio, G.L.; De Pasquale, R.; De Maria, L.; Tardugno, R.; Vadalà, R.; Cicero, N. Natural Pigments Production and Their Application in Food, Health and Other Industries. Nutrients 2023, 15, 1923. [Google Scholar] [CrossRef]
- Ndwandwe, B.K.; Malinga, S.P.; Kayitesi, E.; Dlamini, B.C. Recent developments in the application of natural pigments as pH-sensitive food freshness indicators in biopolymer-based smart packaging: Challenges and opportunities. Int. J. Food Sci. Technol. 2024, 59, 2148–2161. [Google Scholar] [CrossRef]
- Elsahida, K.; Fauzi, A.M.; Sailah, I.; Siregar, I.Z. Sustainability of the use of natural dyes in the textile industry. IOP Conf. Ser. Earth Environ. Sci. 2019, 399, 012065. [Google Scholar] [CrossRef]
- Šabarić, I.; Sutlović, A.; Filipčić, J.; Karin, F. Contribution of Plant Transfer Printing to Sustainable Fashion. Sustainability 2024, 16, 4361. [Google Scholar] [CrossRef]
- Mahmud, M.H.; Raihan, M.T.; Shakhik, M.T.Z.; Khan, F.T.; Islam, M.T. Dragon Fruit (Hylocereus polyrhizus): A Green Colorant for Cotton Fabric. Colorants 2023, 2, 230–244. [Google Scholar] [CrossRef]
- Szadkowski, B.; Kuśmierek, M.; Śliwka-Kaszyńska, M.; Marzec, A. Structure and Stability Characterization of Natural Lake Pigments Made from Plant Extracts and Their Potential Application in Polymer Composites for Packaging Materials. Materials 2022, 15, 4608. [Google Scholar] [CrossRef]
- Morilla, M.J.; Ghosal, K.; Romero, E.L. More Than Pigments: The Potential of Astaxanthin and Bacterioruberin-Based Nanomedicines. Pharmaceutics 2023, 15, 1828. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; de la Lastra, J.M.P.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Polyphenols as Antioxidant/Pro-Oxidant Compounds and Donors of Reducing Species: Relationship with Human Antioxidant Metabolism. Processes 2023, 11, 2771. [Google Scholar] [CrossRef]
- Borchani, K.E.; Carrot, C.; Jaziri, M. Biocomposites of Alfa fibers dispersed in the Mater-Bi® type bioplastic: Morphology, mechanical and thermal properties. Compos. Part A Appl. Sci. Manuf. 2015, 78, 371–379. [Google Scholar] [CrossRef]
- ISO 1133-1:2011; Plastics—Determination of the Melt Mass-Flow Rate (MFR) and Melt Volume-Flow Rate (MVR) of Thermoplastics—Part 1: Standard Method. International Organization for Standardization: Geneva, Switzerland, 2011.
- ISO 527-1:2019; Plastics—Determination of Tensile Propertiespart 1: General Principles. International Organization for Standardization: Geneva, Switzerland, 2019.
- ISO 179-1:2023; Plastics—Determination of Charpy Impact Properties Part 1: Non-Instrumented Impact Test. International Organization for Standardization: Geneva, Switzerland, 2023.
- ISO 4892-2:2013; Plastics—Methods of Exposure to Laboratory Light Sources Part 2: Xenon-Arc lamps. International Organization for Standardization: Geneva, Switzerland, 2013.
- Mokrzycki, W.; Tatol, M. Colour difference ∆E—A survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- ISO 14040:2006; Environmental Management—Life Cycle Assessment—Principles and Framework. International Organization for Standardization: Geneva, Switzerland, 2006.
- ISO 14044:2006; Environmental Management—Life Cycle Assessment—Requirements and Guidelines. International Organization for Standardization: Geneva, Switzerland, 2006.
- Oliquino-Abasolo, A.; Zamora, O.B. Agro-environmental sustainability of conventional and organic vegetable production systems in Tayabas, Quezon, Philippines. J. Environ. Sci. Manag. 2016, 19, 58–71. [Google Scholar] [CrossRef]
- Wakte, P.S.; Sachin, B.S.; Patil, A.A.; Mohato, D.M.; Band, T.H.; Shinde, D.B. Optimization of microwave, ultra-sonic and supercritical carbon dioxide assisted extraction techniques for curcumin from Curcuma longa. Sep. Purif. Technol. 2011, 79, 50–55. [Google Scholar] [CrossRef]
- Quintero, C.D.; Ventura, A.; Lépine, O.; Pruvost, J. Eco-design of spirulina solar cultivation: Key aspects to reduce environmental impacts using Life Cycle Assessment. J. Clean. Prod. 2021, 299, 126741. [Google Scholar] [CrossRef]
- Rathoure, A.K.; Aggarwal, S.G. Manufacturing and Mass Balance of Copper Phthalocyanine (CPC) Blue and Green Pigments. J. Drug Discov. Dev. 2018, 2, 18–23. [Google Scholar]
- EN 15804:2012+A2:2020; Sustainability of Construction Works—Environmental Product Declarations—Core Rules for the Product Category of Construction Products. International Organization for Standardization: Geneva, Switzerland, 2020.
- Tomčíková, Z.; Krivoš, Š.; Hrbáľ, F.; Rerková, D. Preparation, Characterization and Color Performance of PLA Photoluminescent Fibres. Fibres Text. 2021, 8, 100–105. [Google Scholar]
- Balakrishnan, N.K.; Siebert, S.; Richter, C.; Groten, R.; Seide, G. Effect of Colorants and Process Parameters on the Properties of Dope-Dyed Polylactic Acid Multifilament Yarns. Polymers 2022, 14, 5021. [Google Scholar] [CrossRef] [PubMed]
- Fajraoui, A.; Ben Nasr, J.; Lacoste, C.; Ben Amar, M.; Dony, P.; Odof, S.; El Halouani, F. Coloration of the polylactic acid with the natural dye extracted from acacia cyanophylla flowers. Polym. Test. 2019, 78, 105988. [Google Scholar] [CrossRef]





| Property | Beograde INJ038 | BioPBSFZ71PM |
|---|---|---|
| Melting temperature (°C) | 165 | 115 |
| Melt flow rate (g/10 min) | 17 | 22 |
| Density (g/cm3) | 1.20 | 1.26 |
| Property | Extract SP-180 | Extract 95%P | Extract RBS-100 | Extract 95 HPD-P1 | |
|---|---|---|---|---|---|
| Origin | Spirulina | Curcumin | Beetroot | Chlorophyllin | |
| Colour | Blue | Yellow | Red | Green | |
| Particle size (µm) | |||||
| D10 | 10.3 | 1.46 | 8.04 | 1.6 | |
| D50 | 24.5 | 9.3 | 33.1 | 5.42 | |
| D90 | 44.9 | 35.1 | 69.3 | 22.9 | |
| Purity (%) | 98 | 90–100 | 95–100 | 95 | |
| Heavy metals (ppm) | As < 3, Pb < 5, Hg < 1, Cd < 1 | As < 3, Pb < 10, Hg < 1, Cd < 1 | As < 1, Pb < 1, Hg < 1, Cd < 1 | As < 3, Pb < 5, Hg < 1, Cd < 1 | |
| Property | Dogbones Based on Beograde INJ038 | Dogbones Based on BioPBSFZ71PM |
|---|---|---|
| Barrel profile (°C) | 190-190-180-170-35 | 160-160-150-140-35 |
| Mould temperature (°C) | 25 | 25 |
| Injection speed (mm/s) | 60 | 60 |
| Pack pressure (bar) | 600 | 400 |
| Pack time (s) | 10 | 15 |
| Back pressure (bar) | 50 | 50 |
| Cooling time (s) | 40 | 40 |
| Samples | TOnset (°C) | TMax (°C) | Residual Weight (%) |
|---|---|---|---|
| Curcumin | 244.19 | 305.62 | 0 |
| Beetroot | 266.57 | 304.93 | 1.42 |
| Chlorophyllin | 257.66 | 298.77 | 0 |
| Spirulina | 228.50 | 303.83 | 0 |
| PLA INJ038 | 325.07 | 343.49 and 408.53 | 0 |
| BioPBSFZ71PM | 366.50 | 402.84 | 0 |
| Samples | TOnset (°C) | TMax (°C) | Residual Weight (%) |
|---|---|---|---|
| Curcumin-based MBPLA | 299.77 | 323.68 (TMax1) and 403.52 (TMax2) | 4.104 |
| Beetroot-based MBPLA | 300.43 | 326.51 (TMax1) and 404.95 (TMax2) | 8.424 |
| Chlorophyllin-based MBPLA | 258.35 | 326.51 (TMax1) and 404.95 (TMax2) | 7.749 |
| Curcumin-based MBPBS | 369.57 | 403.27 | 8.038 |
| Beetroot-based MBPBS | 366.41 | 402.82 | 4.999 |
| Chlorophyllin-based MBPBS | 356.62 | 400.65 | 9.339 |
| Spirulina-based MBPBS | 356.62 | 400.24 | 4.028 |
| Samples | MFR at 190 °C/2.16 kg (g/10 min) |
|---|---|
| As-received PLA | 50.00 ± 4.91 |
| Curcumin-based MBPLA | 49.36 ± 1.62 |
| Beetroot-based MBPLA | 49.18 ± 10.56 |
| Chlorophyllin-based MBPLA | 48.24 ± 28.19 |
| As-received PBS | 23.47 ± 0.15 |
| Curcumin-based MBPBS | 22.39 ± 5.02 |
| Beetroot-based MBPBS | 22.71 ± 3.92 |
| Chlorophyllin-based MBPBS | 22.27 ± 4.78 |
| Spirulina-based MBPBS | 22.80 ± 2.56 |
| Samples | ||||
|---|---|---|---|---|
| PLA/2%MB_Y | 75.75 | −9.57 | 65.32 | --- |
| PLA/4%MB_Y | 76.26 | −9.52 | 66.56 | 1.34 |
| PLA/6%MB_Y | 75.60 | −6.18 | 67.38 | 3.97 |
| PLA/2%MB_R | 59.61 | 6.55 | 3.58 | --- |
| PLA/4%MB_R | 60.46 | 7.29 | 4.11 | 1.25 |
| PLA/6%MB_R | 63.66 | 9.92 | 6.16 | 5.87 |
| PLA/2%MB_G | 29.31 | −4.96 | 0.89 | --- |
| PLA/4%MB_G | 29.96 | −5.00 | 0.65 | 2.61 |
| PLA/6%MB_G | 29.82 | −4.97 | 1.08 | 4.09 |
| PBS/2%MB_Y | 66.18 | 5.46 | 56.85 | --- |
| PBS/4%MB_Y | 68.18 | 6.24 | 58.75 | 2.87 |
| PBS/6%MB_Y | 68.00 | 7.19 | 59.57 | 3.70 |
| PBS/2%MB_R | 79.11 | 9.64 | 6.61 | --- |
| PBS/4%MB_R | 78.05 | 12.86 | 7.29 | 3.46 |
| PBS/6%MB_R | 77.52 | 13.86 | 7.32 | 4.57 |
| PBS/2%MB_G | 43.82 | −9.32 | 2.83 | --- |
| PBS/4%MB_G | 41.35 | −9.04 | 2.03 | 3.88 |
| PBS/6%MB_G | 39.94 | −8.65 | 1.72 | 5.05 |
| PBS/2%MB_B | 76.35 | −3.65 | −2.41 | --- |
| PBS/4%MB_B | 74.38 | −2.91 | −5.67 | 3.88 |
| PBS/6%MB_B | 73.24 | −3.37 | −6.38 | 5.05 |
| Samples | Young’s Modulus (MPa) | σM (MPa) | ƐM (%) | σR (MPa) | ƐR (%) | Charpy Impact Strength (kJ/m²) |
|---|---|---|---|---|---|---|
| As-received PLA | 1710 ± 20.2 | 65.6 ± 0.3 | 3.8 ± 0.1 | 59.3 ± 2.4 | 4.5 ± 0.5 | 18.21 ± 1.5 |
| PLA/2%MB_Y | 1730 ± 18.1 | 65.8 ± 0.9 | 3.9 ± 0.1 | 60.9 ± 5.1 | 4.6 ± 0.6 | 19.02 ± 2.1 |
| PLA/4%MB_Y | 1690 ± 43.6 | 65.1 ± 0.5 | 3.9 ± 0.03 | 55.3 ± 1.8 | 5.2 ± 0.6 | 24.96 ± 2.7 |
| PLA/6%MB_Y | 1670 ± 19.9 | 64.0 ± 0.3 | 3.9 ± 0.03 | 59.9 ± 3.6 | 4.3 ± 0.5 | 26.12 ± 2.8 |
| PLA/2%MB_R | 1680 ± 41.2 | 66.7 ± 0.9 | 3.9 ± 0.04 | 63.0 ± 3.9 | 4.5 ± 0.5 | 14.98 ± 0.8 |
| PLA/4%MB_R | 1670 ± 58.5 | 64.8 ± 0.3 | 3.8 ± 0.1 | 61.1 ± 3.6 | 4.4 ± 0.7 | 14.38 ± 1.0 |
| PLA/6%MB_R | 1640 ± 23.3 | 63.7 ± 0.4 | 3.8 ± 0.02 | 58.2 ± 1.3 | 4.5 ± 0.3 | 14.15 ± 1.1 |
| PLA/2%MB_G | 1660 ± 36.4 | 62.1 ± 0.4 | 3.7 ± 0.04 | 53.3 ± 1.3 | 5.0 ± 0.3 | 17.79 ± 2.2 |
| PLA/4%MB_G | 1610 ± 29.4 | 65.3 ± 0.4 | 3.8 ± 0.4 | 54.2 ± 3.1 | 4.9 ± 1.0 | 17.76 ± 1.9 |
| PLA/6%MB_G | 1650 ± 48.0 | 64.9 ± 0.3 | 3.3 ± 0.3 | 53.8 ± 3.7 | 4.2 ± 0.3 | 19.02 ± 2.4 |
| Samples | Young’s Modulus (MPa) | σM (MPa) | ƐM (%) | σR (MPa) | ƐR (%) | Charpy Impact Strength (kJ/m²) |
|---|---|---|---|---|---|---|
| As-received PBS | 392 ± 17.2 | 34.5 ± 0.2 | 16 ± 0.7 | 26.5 ± 2.1 | 18 ± 0.5 | 5.17 ± 0.18 |
| PBS/2%MB_Y | 394 ± 24.3 | 34.7 ± 0.5 | 16 ± 1.60 | 27.4 ± 3.1 | 18 ± 0.6 | 5.07 ± 0.25 |
| PBS/4%MB_Y | 383 ± 27.2 | 34.4 ± 0.2 | 17 ± 0.20 | 25.7 ± 0.7 | 19 ± 0.5 | 4.98 ± 0.35 |
| PBS/6%MB_Y | 374 ± 12.3 | 34.1 ± 0.4 | 17 ± 0.33 | 29.9 ± 3.1 | 18 ± 0.6 | 5.13 ± 0.44 |
| PBS/2%MB_R | 383 ± 30.5 | 35.0 ± 0.2 | 17 ± 0.20 | 33.2 ± 0.4 | 19 ± 0.5 | 4.94 ± 0.42 |
| PBS/4%MB_R | 384 ± 43.9 | 34.2 ± 0.2 | 16 ± 0.38 | 31.7 ± 0.7 | 18 ± 0.7 | 4.96 ± 0.76 |
| PBS/6%MB_R | 397 ± 28.0 | 34.5 ± 0.2 | 16 ± 0.14 | 32.2 ± 0.4 | 16 ± 0.1 | 4.56 ± 0.23 |
| PBS/2%MB_B | 392 ± 25.7 | 34.0 ± 2.0 | 14 ± 3.50 | 32.7 ± 1.2 | 19 ± 0.9 | 4.86 ± 0.32 |
| PBS/4%MB_B | 406 ± 67.6 | 35.2 ± 0.1 | 16 ± 0.25 | 32.8 ± 0.58 | 19 ± 0.4 | 5.02 ± 0.42 |
| PBS/6%MB_B | 391 ± 46.9 | 34.7 ± 0.1 | 16 ± 0.34 | 32.5 ± 0.393 | 19 ± 1.1 | 5.29 ± 0.36 |
| PBS/2%MB_G | 436 ± 11.7 | 36.4 ± 0.3 | 16 ± 0.19 | 33.8 ± 0.733 | 19 ± 0.6 | 5.54 ± 0.51 |
| PBS/4%MB_G | 417 ± 13.2 | 35.8 ± 0.2 | 17 ± 0.14 | 33.6 ± 0.7 | 19 ± 0.5 | 5.20 ± 0.26 |
| PBS/6%MB_G | 391 ± 18.8 | 34.7 ± 0.4 | 16 ± 0.33 | 30.6 ± 3.4 | 18 ± 0.8 | 4.75 ± 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibáñez-García, A.; Berbegal-Pina, R.; Vidal, R.; Martínez-García, A. Sustainability in the Development of Natural Pigment-Based Colour Masterbatches and Their Application in Biopolymers. Polymers 2024, 16, 2116. https://doi.org/10.3390/polym16152116
Ibáñez-García A, Berbegal-Pina R, Vidal R, Martínez-García A. Sustainability in the Development of Natural Pigment-Based Colour Masterbatches and Their Application in Biopolymers. Polymers. 2024; 16(15):2116. https://doi.org/10.3390/polym16152116
Chicago/Turabian StyleIbáñez-García, Ana, Raquel Berbegal-Pina, Rosario Vidal, and Asunción Martínez-García. 2024. "Sustainability in the Development of Natural Pigment-Based Colour Masterbatches and Their Application in Biopolymers" Polymers 16, no. 15: 2116. https://doi.org/10.3390/polym16152116
APA StyleIbáñez-García, A., Berbegal-Pina, R., Vidal, R., & Martínez-García, A. (2024). Sustainability in the Development of Natural Pigment-Based Colour Masterbatches and Their Application in Biopolymers. Polymers, 16(15), 2116. https://doi.org/10.3390/polym16152116









