Self-Healing and Recyclable Polyurethane/Nanocellulose Elastomer Based on the Diels–Alder Reaction
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Materials
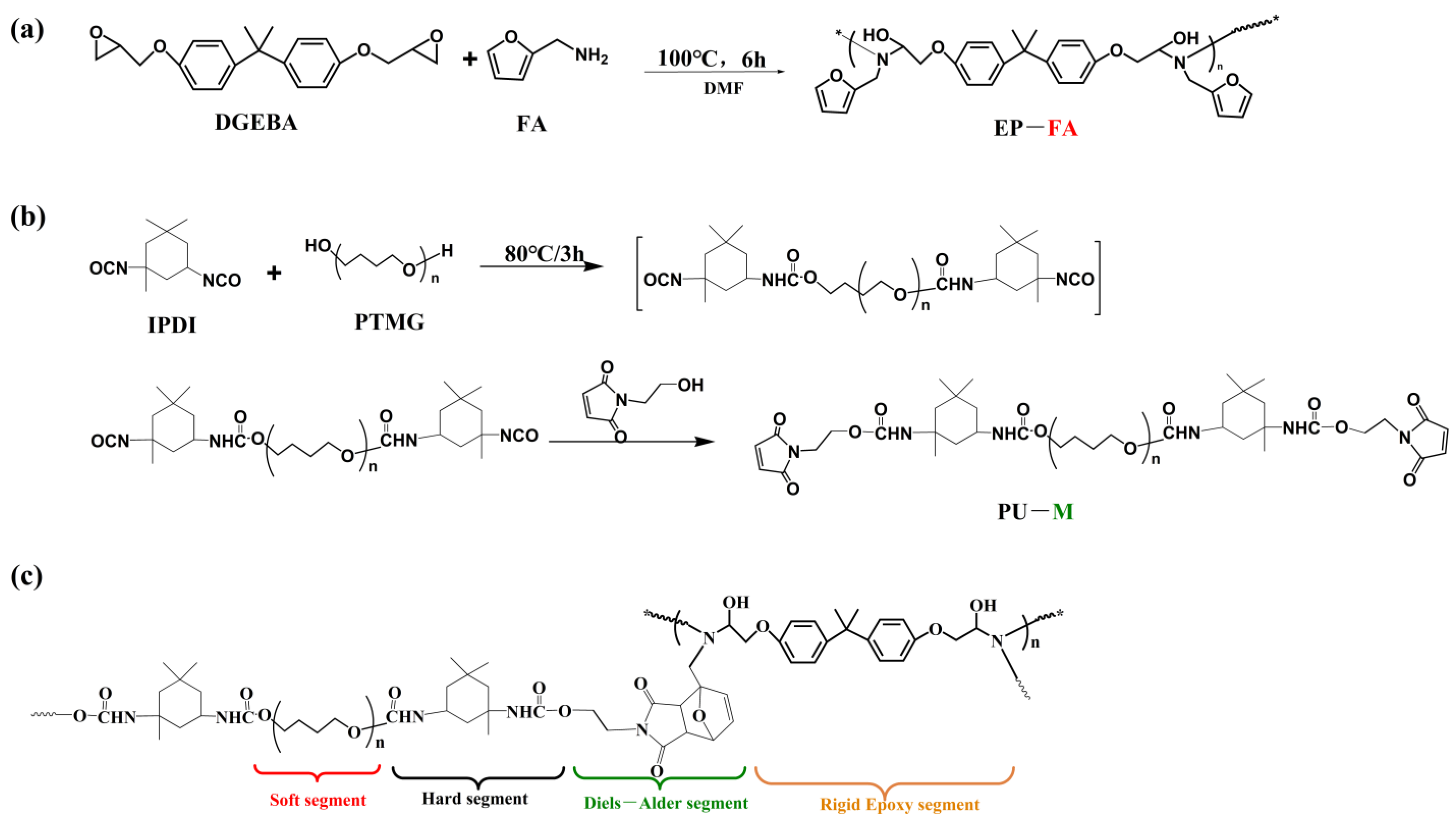
2.2.1. Synthesis of Furan-Pendent Epoxy
2.2.2. Synthesis of Maleimide End-Capped Polyurethane
2.2.3. Preparation of Diels–Alder Cross-Linked Elastomer and Composites
2.3. Characterization
3. Results and Discussion
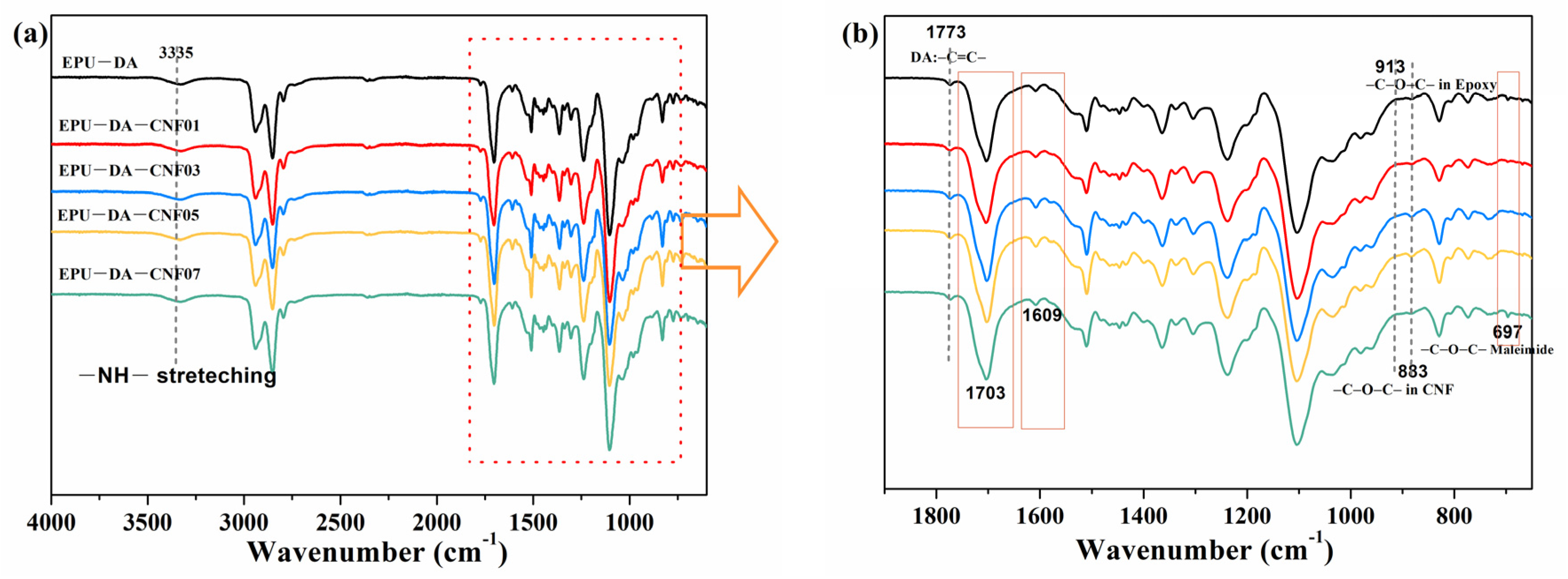
3.1. Synthesis and Characterization of Elastomer and Composites
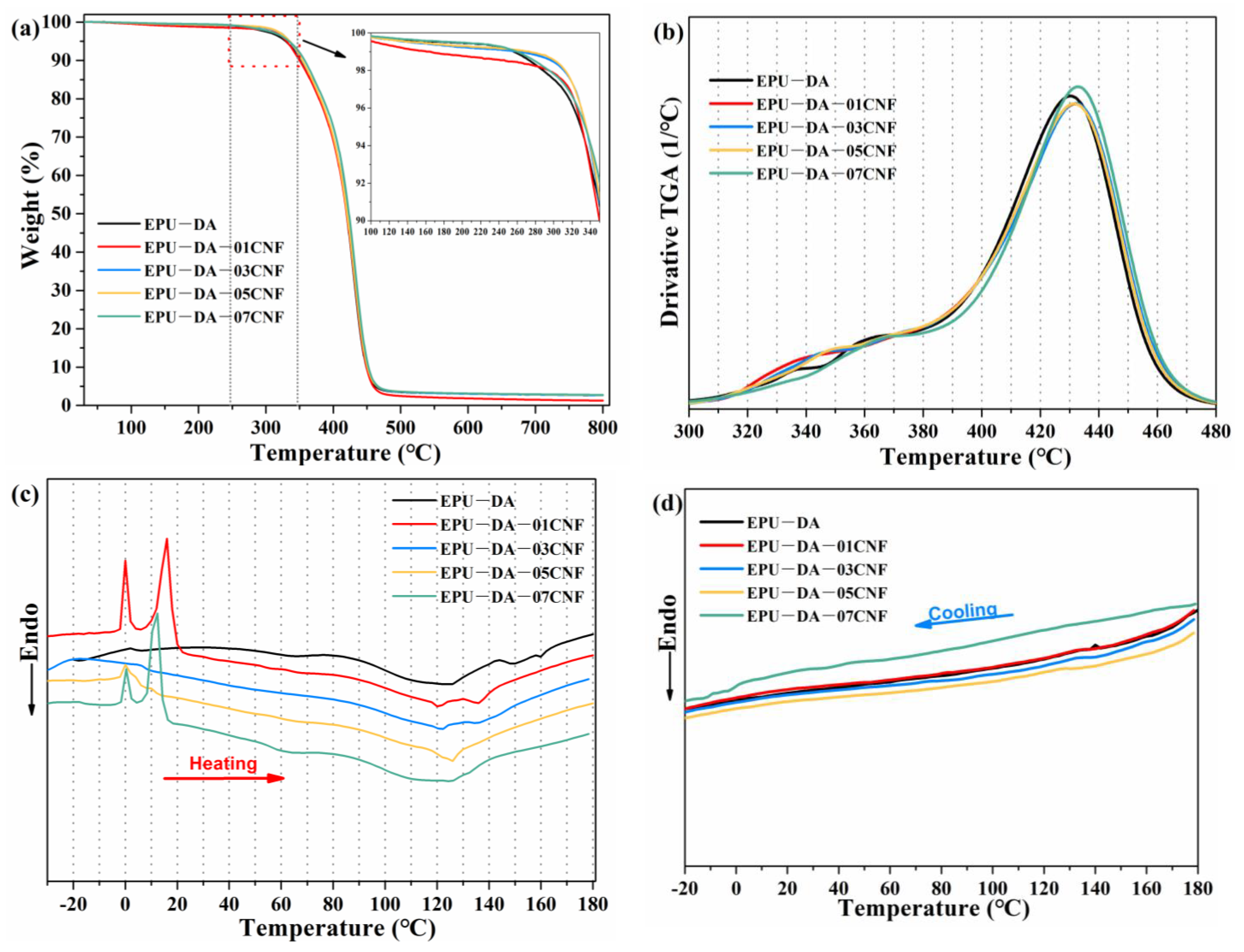
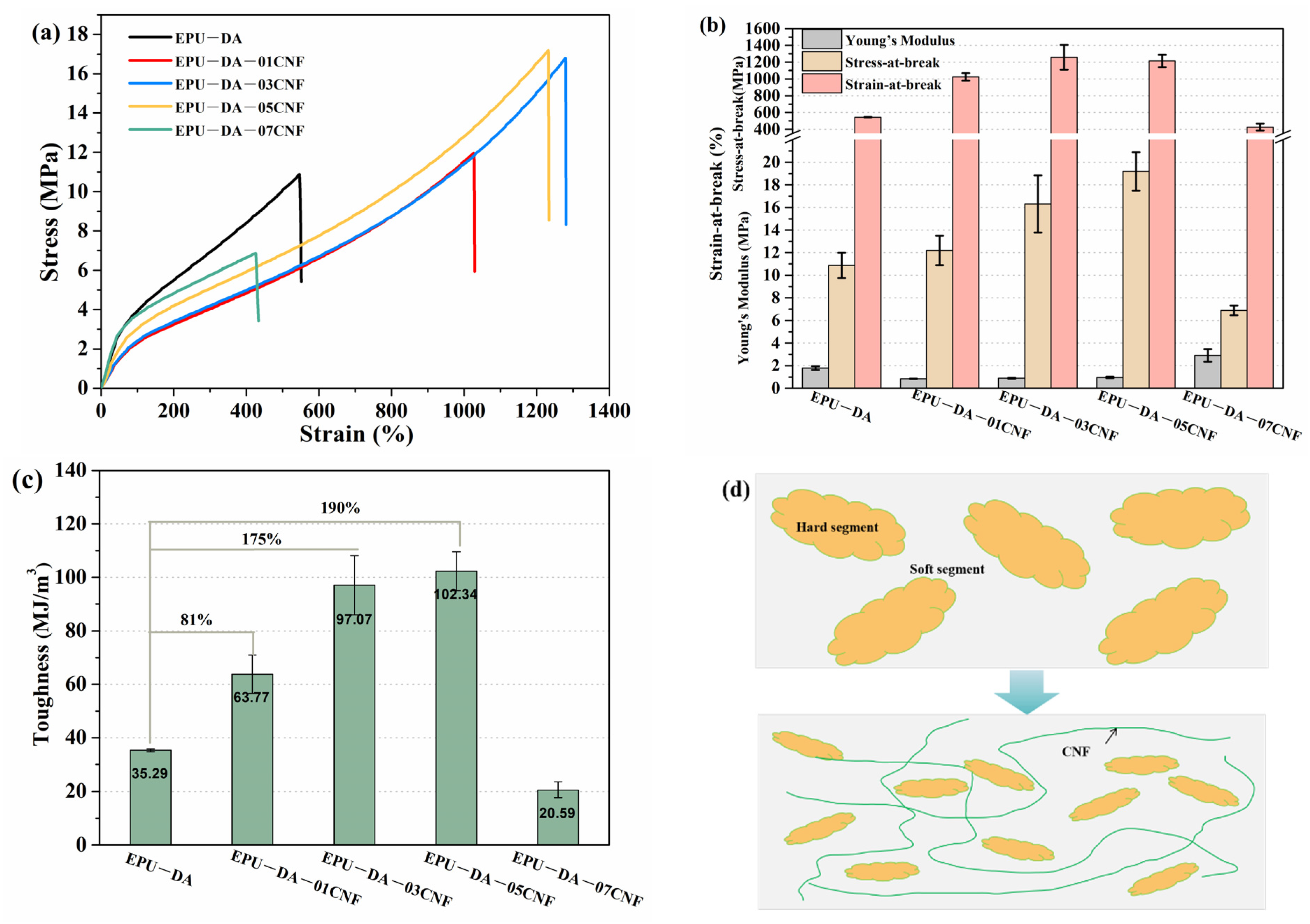
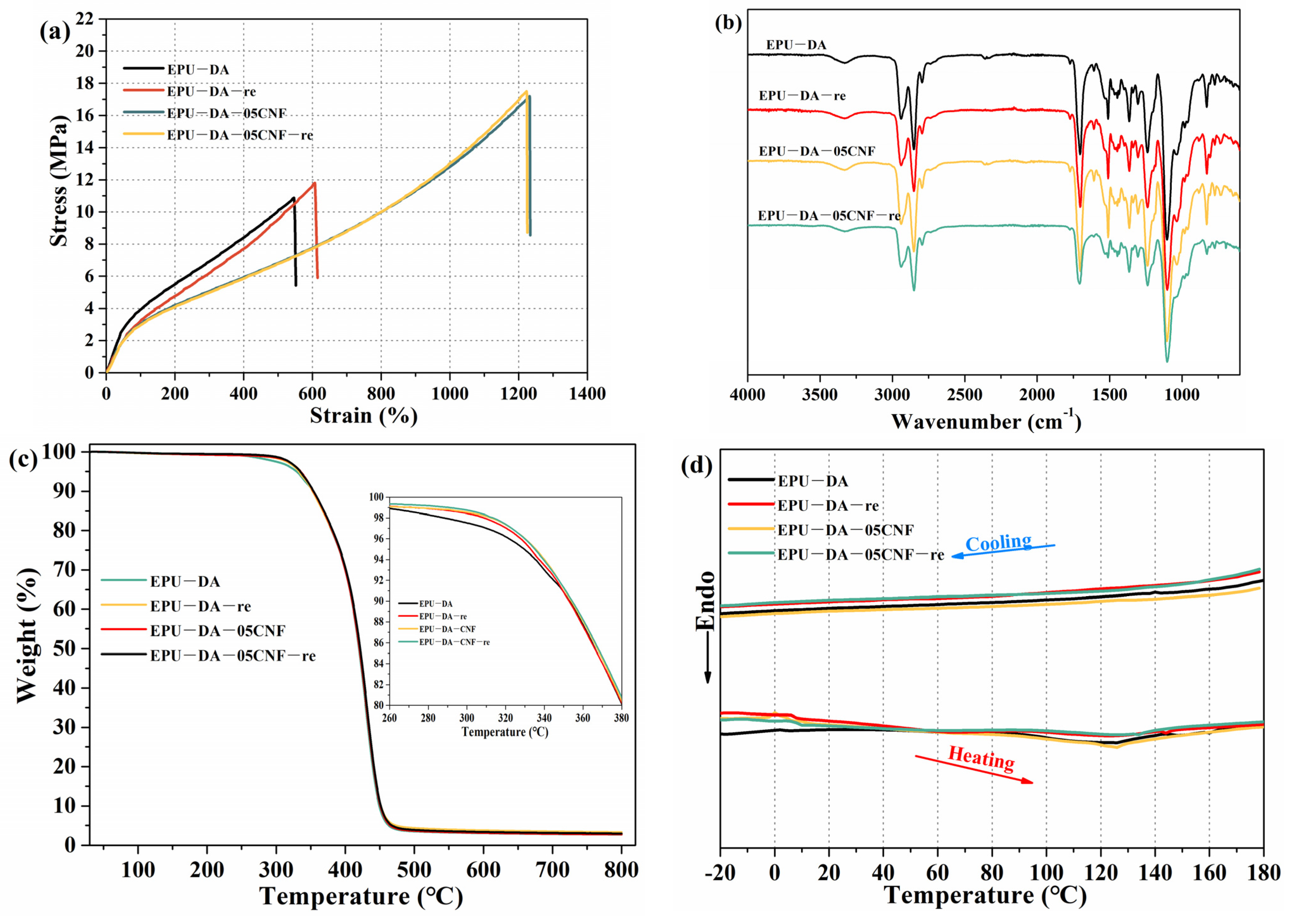
3.2. Mechanical and Thermal Recyclable Properties of Elastomer and Composites
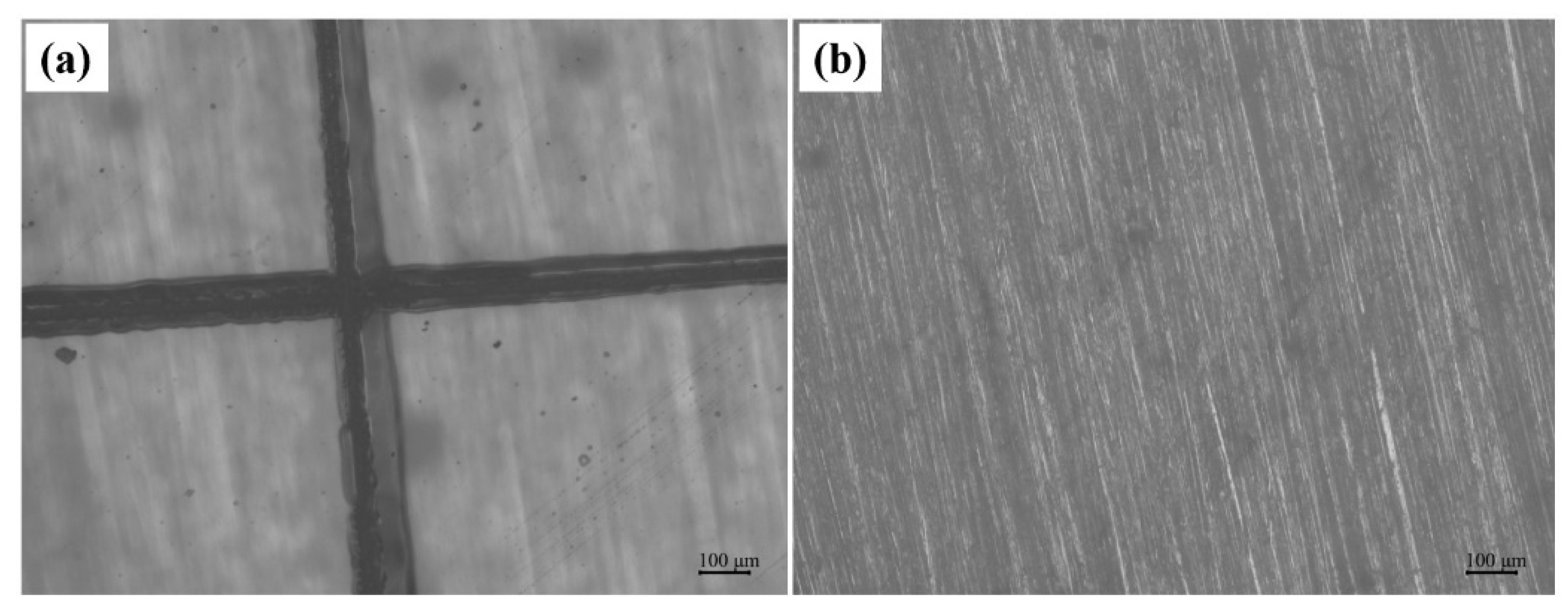
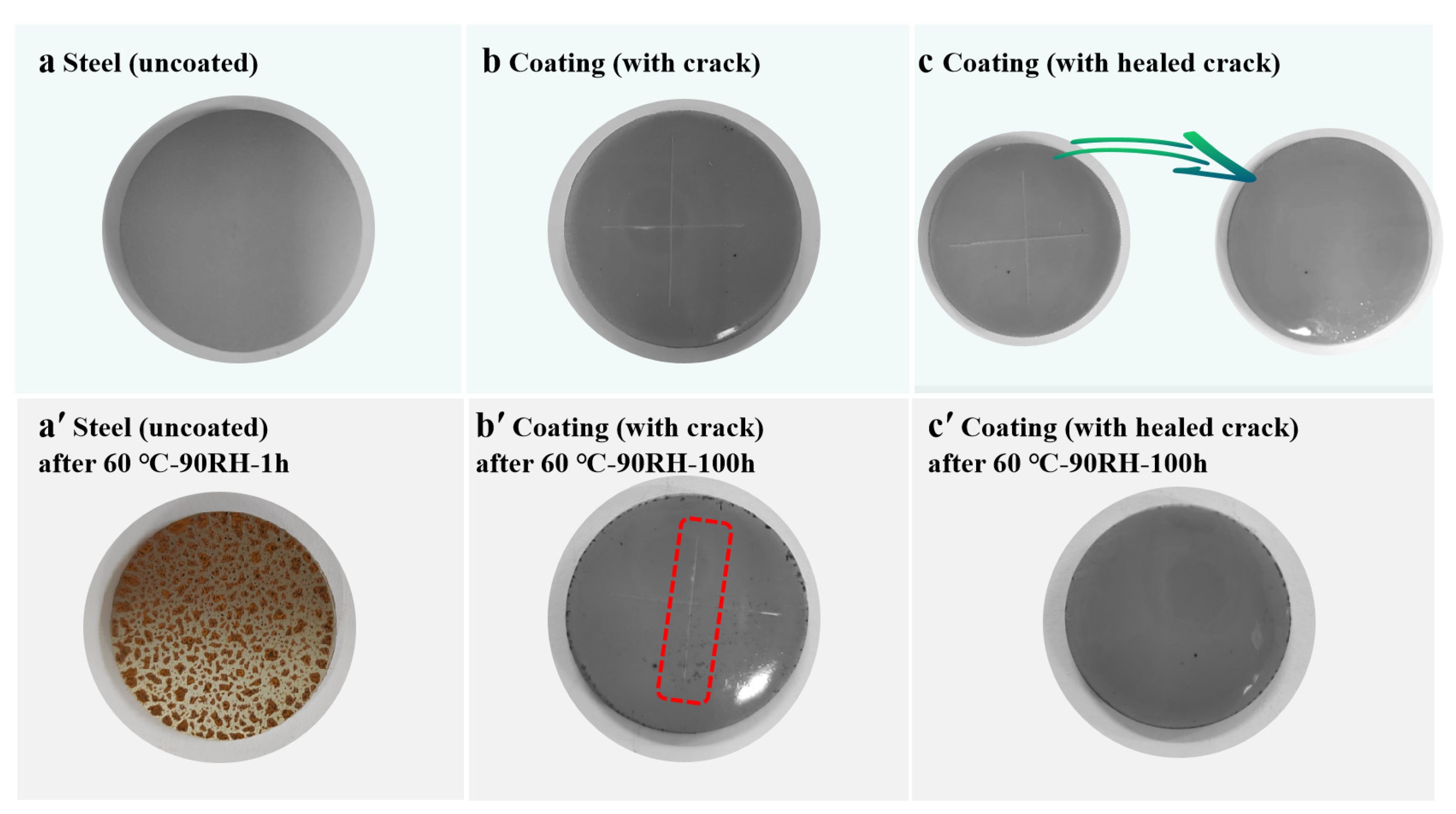
3.3. Self-Healing and Protecting Experiments of Elastomer Coating
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, D.; Chen, H.; Dai, Q.; Xiang, C.; Li, Y.; Xiong, X.; Zhou, Y.; Zhang, J. Stimuli-mild, robust, commercializable polyurethane-urea vitrimer elastomer via N, N′-diaryl urea crosslinking. Macromol. Chem. Phys. 2020, 221, 1900564. [Google Scholar] [CrossRef]
- Li, W.; Chang, Q.; Xiao, L.; Zhang, K.; Huang, J.; Wang, Y.; Chen, J.; Nie, X. Readily Recyclable, Auto-Catalyzed Tung Oil-Derived Vitrimers and Carbon Fiber-Reinforced Composites. ACS Appl. Polym. Mater. 2023, 5, 4498–4508. [Google Scholar] [CrossRef]
- Wang, S.; Chen, X.; Guo, L.; Wang, S.; Dong, F.; Liu, H.; Xu, X. A human muscle-inspired, high strength, good elastic recoverability, room-temperature self-healing, and recyclable polyurethane elastomer based on dynamic bonds. Compos. Sci. Technol. 2024, 248, 110457. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, M.; Xia, L.; Yang, X.; Shi, Y. Room-temperature self-healing polyurethane elastomers with high strength and superior self-healing efficiency based on aromatic disulfide-induced. Colloid. Surf. A 2024, 681, 132829. [Google Scholar] [CrossRef]
- Khan, A.; Wang, C.-F.; Kisannagar, R.R.; Chuang, W.-T.; Nhien, P.Q.; Mahmood, S.; Katiyar, M.; Gupta, D.; Wei, K.-H.; Lin, H.-C. Highly stretchable, tough, healable and mechanoresponsive polyurethane elastomers for flexible capacitor applications. J. Mater. Chem. A 2023, 11, 305–315. [Google Scholar] [CrossRef]
- Li, C.H.; Zuo, J.L. Self-healing polymers based on coordination bonds. Adv. Mater. 2020, 32, 1903762. [Google Scholar] [CrossRef]
- Shen, Z.; Chen, J.; Li, G.; Situ, G.; Ma, X.; Sha, Y.; Zhao, D.; Gu, Q.; Zhang, M.; Luo, Z. Mechanically robust self-repairing polyurea elastomers: The roles of hard segment content and ordered/disordered hydrogen-bonding arrays. Eur. Polym. J. 2022, 181, 111657. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, D.; Lu, F.; Yuan, W.; Xu, X.; Zhang, Q.; Liu, H.; Shao, Q.; Guo, Z.; Huang, Y. Multistimuli-responsive intrinsic self-healing epoxy resin constructed by host-guest interactions. Macromolecules 2018, 51, 5294–5303. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Li, T.T.; Wang, X.; Ren, H.T.; Lou, C.W.; Lin, J.H. Preparation and Properties of Polyaniline Self-Healing Conductive Materials Based on Hydrogen Bonding and Electrostatic Interaction Forces. ACS Appl. Polym. Mater. 2023, 5, 9585–9593. [Google Scholar] [CrossRef]
- Guimard, N.K.; Oehlenschlaeger, K.K.; Zhou, J.; Hilf, S.; Schmidt, F.G.; Barner-Kowollik, C. Current trends in the field of self-healing materials. Macromol. Chem. Phys. 2012, 213, 131–143. [Google Scholar] [CrossRef]
- An, Z.-W.; Xue, R.; Ye, K.; Zhao, H.; Liu, Y.; Li, P.; Chen, Z.-M.; Huang, C.-X.; Hu, G.-H. Recent advances in self-healing polyurethane based on dynamic covalent bonds combined with other self-healing methods. Nanoscale 2023, 15, 6505–6520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, M.; Zhou, J.; Sheng, Y.; Xu, M.; Jiang, X.; Ma, Y.; Lu, X. Preparation of room-temperature self-healing elastomers with high strength based on multiple dynamic bonds. Eur. Polym. J. 2021, 156, 110614. [Google Scholar] [CrossRef]
- Yang, S.; Wang, S.; Du, X.; Du, Z.; Cheng, X.; Wang, H. Mechanically robust self-healing and recyclable flame-retarded polyurethane elastomer based on thermoreversible crosslinking network and multiple hydrogen bonds. Chem. Eng. J. 2020, 391, 123544. [Google Scholar] [CrossRef]
- Zhu, X.; Han, K.; Li, C.; Wang, J.; Yuan, J.; Pan, Z.; Pan, M. Tough, Photoluminescent, Self-Healing Waterborne Polyurethane Elastomers Resulting from Synergistic Action of Multiple Dynamic Bonds. ACS Appl. Mater. Interfaces 2023, 15, 19414–19426. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, J.; Song, G.; Zhang, L.; Liu, Z.; Jing, X.; Luo, F.; Zhang, Y.; Zhang, Y.; Li, X. Self-healing polyurethane elastomers with high mechanical properties based on synergistically thermo-reversible and quadruple hydrogen bonds. ACS Appl. Polym. Mater. 2023, 5, 1302–1311. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, Y.; Chen, Y.; Li, W.; Chang, Q.; He, Z.; Zhu, Y.; Huang, J.; Nie, X. Construction of multiple sacrificial bonds between epoxy resin and tung oil-based modifier towards mechanical performance enhancement. Ind. Crop. Prod. 2024, 210, 118091. [Google Scholar] [CrossRef]
- Backes, E.H.; Harb, S.V.; Pinto, L.A.; de Moura, N.K.; de Melo Morgado, G.F.; Marini, J.; Passador, F.R.; Pessan, L.A. Thermoplastic polyurethanes: Synthesis, fabrication techniques, blends, composites, and applications. J. Mater. Sci. 2024, 59, 1123–1152. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, H.; Li, J.; Yang, J.; Chen, Z. Preparation and characterization of solvent-free anti-corrosion polyurethane-urea coatings. Surf. Interfaces 2023, 36, 102504. [Google Scholar] [CrossRef]
- Chen, B.; Liao, M.; Sun, J.; Shi, S. A novel biomass polyurethane-based composite coating with superior radiative cooling, anti-corrosion and recyclability for surface protection. Prog. Org. Coat. 2023, 174, 107250. [Google Scholar] [CrossRef]
- Duran, M.M.; Moro, G.; Zhang, Y.; Islam, A. 3D printing of silicone and polyurethane elastomers for medical device application: A review. Adv. Ind. Manuf. Eng. 2023, 7, 100125. [Google Scholar] [CrossRef]
- Gao, H.; Sun, Y.; Jian, J.; Dong, Y.; Liu, H. Study on mechanical properties and application in communication pole line engineering of glass fiber reinforced polyurethane composites (GFRP). Case Stud. Constr. Mater. 2023, 18, 01942. [Google Scholar] [CrossRef]
- Zhao, X.; Jin, R.; Niu, Z.; Gao, Y.; Hu, S. Fabrication of Polyurethane Elastomer/Hindered Phenol Composites with Tunable Damping Property. Int. J. Mol. Sci. 2023, 24, 4662. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhang, T.; Peng, H.; Ma, Z.; Zhang, M.; Lynch, M.; Toan, D.; Zhezhe, Z.; Yonghong, Z.; Song, P. Fire-retardant, anti-dripping, biodegradable and biobased polyurethane elastomers enabled by hydrogen-bonding with cellulose nanocrystals. Nano Res. 2024, 35, 2300286. [Google Scholar] [CrossRef]
- Xu, H.; Tu, J.; Li, H.; Ji, J.; Liang, L.; Tian, J.; Guo, X. Room-temperature self-healing, high ductility, recyclable polyurethane elastomer fabricated via asymmetric dynamic hard segments strategy combined with self-cleaning function application. Chem. Eng. J. 2023, 454, 140101. [Google Scholar] [CrossRef]
- Lai, Y.; Kuang, X.; Zhu, P.; Huang, M.; Dong, X.; Wang, D. Colorless, transparent, robust, and fast scratch-self-healing elastomers via a phase-locked dynamic bonds design. Adv. Mater. 2018, 30, 1802556. [Google Scholar] [CrossRef] [PubMed]
- Teng, Q.; Huang, Y.; Wu, H.; Li, W.; Wu, Q.; Wu, J. Self-healing polyurethane elastomer with ultra-high mechanical strength and enhanced thermal mechanical properties. Polymer 2024, 290, 126579. [Google Scholar] [CrossRef]
- Shao, J.; Dong, X.; Wang, D. Stretchable Self-Healing Plastic Polyurethane with Super-High Modulus by Local Phase-Lock Strategy. Macromol. Rapid Commun. 2023, 44, 2200299. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, J.; Li, H.; Gao, H.; Wu, M.; Wang, Z.; Wang, Z. Dual-hard phase structures make mechanically tough and autonomous self-healable polyurethane elastomers. Chem. Eng. J. 2023, 454, 140268. [Google Scholar] [CrossRef]
- Huang, J.; Wang, H.; Liu, W.; Huang, J.; Yang, D.; Qiu, X.; Zhao, L.; Hu, F.; Feng, Y. Solvent-free synthesis of high-performance polyurethane elastomer based on low-molecular-weight alkali lignin. Int. J. Biol. Macromol. 2023, 225, 1505–1516. [Google Scholar] [CrossRef]
- Wang, D.; Shan, Y.; Liu, L.; Diao, M.; Yao, J. Lignin demethylation enabled robust and reprocessable bio-polyurethane elastomer with adaptable cross-linking network. Ind. Crops Prod. 2024, 208, 117905. [Google Scholar] [CrossRef]
- Yang, W.; Zhu, Y.; Liu, T.; Puglia, D.; Kenny, J.M.; Xu, P.; Zhang, R.; Ma, P. Multiple Structure Reconstruction by Dual Dynamic Crosslinking Strategy Inducing Self-Reinforcing and Toughening the Polyurethane/Nanocellulose Elastomers. Adv. Funct. Mater. 2023, 33, 2213294. [Google Scholar] [CrossRef]
- Xi, P.; Wu, L.; Quan, F.; Xia, Y.; Fang, K.; Jiang, Y. Scalable nano building blocks of waterborne polyurethane and nanocellulose for tough and strong bioinspired nanocomposites by a self-healing and shape-retaining strategy. ACS Appl. Mater. Interfaces 2022, 14, 24787–24797. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.; Sodano, H.A. Self-healing polyurethanes with shape recovery. Adv. Funct. Mater. 2014, 24, 5261–5268. [Google Scholar] [CrossRef]
- Chen, T.; Fang, L.; Lu, C.; Xu, Z. Effects of Blended Reversible Epoxy Domains on Structures and Properties of Self-Healing/Shape-Memory Thermoplastic Polyurethane. Macromol Mater Eng. 2020, 305, 1900578. [Google Scholar] [CrossRef]
- Lin, C.; Ge, H.; Wang, T.; Huang, M.; Ying, P.; Zhang, P.; Wu, J.; Ren, S.; Levchenko, V. A self-healing and recyclable polyurethane/halloysite nanocomposite based on thermoreversible Diels-Alder reaction. Polymer 2020, 206, 122894. [Google Scholar] [CrossRef]
- Lin, C.; Ge, H.; Ying, P.; Wang, T.; Huang, M.; Zhang, P.; Yang, T.; Wu, J.; Levchenko, V. Synthesis and Properties of Dynamic Crosslinking Polyurethane/PEG Shape-Stable Phase Change Materials Based on the Diels-Alder Reaction. ACS Appl. Polym. Mater. 2023, 5, 4190–4198. [Google Scholar] [CrossRef]
- Qian, Z.; Xiao, Y.; Zhang, X.; Li, Q.; Wang, L.; Fu, F.; Diao, H.; Liu, X. Bio-based epoxy resins derived from diphenolic acid via amidation showing enhanced performance and unexpected autocatalytic effect on curing. Chem. Eng. J. 2022, 435, 135022. [Google Scholar] [CrossRef]
- Lanna, A.; Suklueng, M.; Kasagepongsan, C.; Suchat, S. Performance of novel engineered materials from epoxy resin with modified epoxidized natural rubber and nanocellulose or nanosilica. Adv. Polym. Tech. 2020, 2020, 2123836. [Google Scholar] [CrossRef]
- Li, X.; Yu, R.; He, Y.; Zhang, Y.; Yang, X.; Zhao, X.; Huang, W. Four-dimensional printing of shape memory polyurethanes with high strength and recyclability based on Diels-Alder chemistry. Polymer 2020, 200, 122532. [Google Scholar] [CrossRef]
- Kim, Y.; Huh, P.; Yoo, S.I. Mechanical Reinforcement of Thermoplastic Polyurethane Nanocomposites by Surface-Modified Nanocellulose. Macromol. Chem. Phys. 2023, 224, 2200383. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, Z.; Fu, X.; Wang, Y.; Yuan, A.; Lei, J. Effect of crystalline structure on water resistance of waterborne polyurethane. Eur. Polym. J. 2021, 157, 110647. [Google Scholar] [CrossRef]
- Amin, K.N.M.; Amiralian, N.; Annamalai, P.K.; Edwards, G.; Chaleat, C.; Martin, D.J. Scalable processing of thermoplastic polyurethane nanocomposites toughened with nanocellulose. Chem. Eng. J. 2016, 302, 406–416. [Google Scholar] [CrossRef]
- Xu, J.; Gao, F.; Wang, H.; Dai, R.; Dong, S.; Wang, H. Organic/inorganic hybrid waterborne polyurethane coatings with self-healing properties for anticorrosion application. Prog. Org. Coat. 2023, 174, 107244. [Google Scholar] [CrossRef]
- Myshkin, N.; Kovalev, A. Adhesion and surface forces in polymer tribology-A review. Friction 2018, 6, 143–155. [Google Scholar] [CrossRef]


| Samples | EP–FA/g | PU–M/g | CNF/g |
|---|---|---|---|
| EPU–DA | 3 | 12.68 | 0 |
| EPU–DA–01CNF | 3 | 12.68 | 0.01568 |
| EPU–DA–03CNF | 3 | 12.68 | 0.04704 |
| EPU–DA–05CNF | 3 | 12.68 | 0.0784 |
| EPU–DA–07CNF | 3 | 12.68 | 0.10976 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Lin, C.; Huang, M.; Ying, P.; Zhang, P.; Wu, J.; Wang, T.; Kovalev, A.; Myshkin, N.; Levchenko, V. Self-Healing and Recyclable Polyurethane/Nanocellulose Elastomer Based on the Diels–Alder Reaction. Polymers 2024, 16, 2029. https://doi.org/10.3390/polym16142029
Yang T, Lin C, Huang M, Ying P, Zhang P, Wu J, Wang T, Kovalev A, Myshkin N, Levchenko V. Self-Healing and Recyclable Polyurethane/Nanocellulose Elastomer Based on the Diels–Alder Reaction. Polymers. 2024; 16(14):2029. https://doi.org/10.3390/polym16142029
Chicago/Turabian StyleYang, Tao, Changhong Lin, Min Huang, Puyou Ying, Ping Zhang, Jianbo Wu, Tianle Wang, Alexander Kovalev, Nikolai Myshkin, and Vladimir Levchenko. 2024. "Self-Healing and Recyclable Polyurethane/Nanocellulose Elastomer Based on the Diels–Alder Reaction" Polymers 16, no. 14: 2029. https://doi.org/10.3390/polym16142029
APA StyleYang, T., Lin, C., Huang, M., Ying, P., Zhang, P., Wu, J., Wang, T., Kovalev, A., Myshkin, N., & Levchenko, V. (2024). Self-Healing and Recyclable Polyurethane/Nanocellulose Elastomer Based on the Diels–Alder Reaction. Polymers, 16(14), 2029. https://doi.org/10.3390/polym16142029






