A Systematic Review on the Generation of Organic Structures through Additive Manufacturing Techniques
Abstract
1. Introduction
2. Methods
- Phase 1: Identification of the research questions: Determine which will be the questions to be answered for this systematic review. The questions should be able to gather the main ideas of this study, arriving at a synthesis of the current state of the art about this review seeks to satisfy.
- Phase 2: Definition of the search strategy: Find the keywords that best represent the main ideas raised in the research questions. In addition, this phase determines how to use logical operators to achieve a more precise search.
- Phase 3: Definition of the inclusion and exclusion criteria: Establish the limits of the search by defining the conditions that must be met by all items to be considered in the review.
- Phase 4: Screening of the primary studies: Eliminate from the study the articles that do not meet the criteria defined in the previous phase.
- Phase 5: Data extraction: Define the data to be extracted from the resulting articles, synthesized in the Results section, and used to answer the research questions.
2.1. Identification of the Research Question
- Research Question 1 (RQ1): “What are the main types of mesoscopic structures for the generation of porous structures?”
- Research Question 2 (RQ2): “What are the most common methods used to generate stochastic porous structures?”
- Research Question 3 (RQ3)—Regarding Research Question 2: “What are the 3D printing methods used to generate non-regular structures?”
- Research Question 4 (RQ4): “What proposals exist for the generation of self-generating stochastic structures?”
2.2. Definition of the Search Strategy
“(TITLE-ABS-KEY (“bone*”) OR TITLE-ABS-KEY (“porous material”) OR TITLE-ABS-KEY (foam*) OR TITLE-ABS-KEY (open-cell) OR TITLE-ABS-KEY (“porous structure*”) OR TITLE-ABS-KEY (“complex structure*”)) AND (TITLE-ABS-KEY (“additive manufacturing”) OR TITLE-ABS-KEY (bioprint*) OR TITLE-ABS-KEY (“3d printing”) OR TITLE-ABS-KEY (fdm) OR TITLE-ABS-KEY (“geometric modeling”)) AND (TITLE-ABS-KEY (“BONE-LIKE”) OR TITLE-ABS-KEY (“no* lattice”) OR TITLE-ABS-KEY (“organic structure”) OR TITLE-ABS-KEY (“stochastic”) OR TITLE-ABS-KEY (“no* regular”) OR TITLE-ABS-KEY (“modeling approach”) OR TITLE-ABS-KEY (“no* parametric design”) OR TITLE-ABS-KEY (“network-based”) OR TITLE-ABS-KEY (“graph-based”) OR TITLE-ABS-KEY (“inhomogeneous porous structure*”) OR TITLE-ABS-KEY (“irregular internal morphology”)) AND PUBYEAR > 2013”.
2.3. Definition of the Inclusion and Exclusion Criteria
2.4. Screening of the Primary Studies
2.5. Data Extraction
3. Results
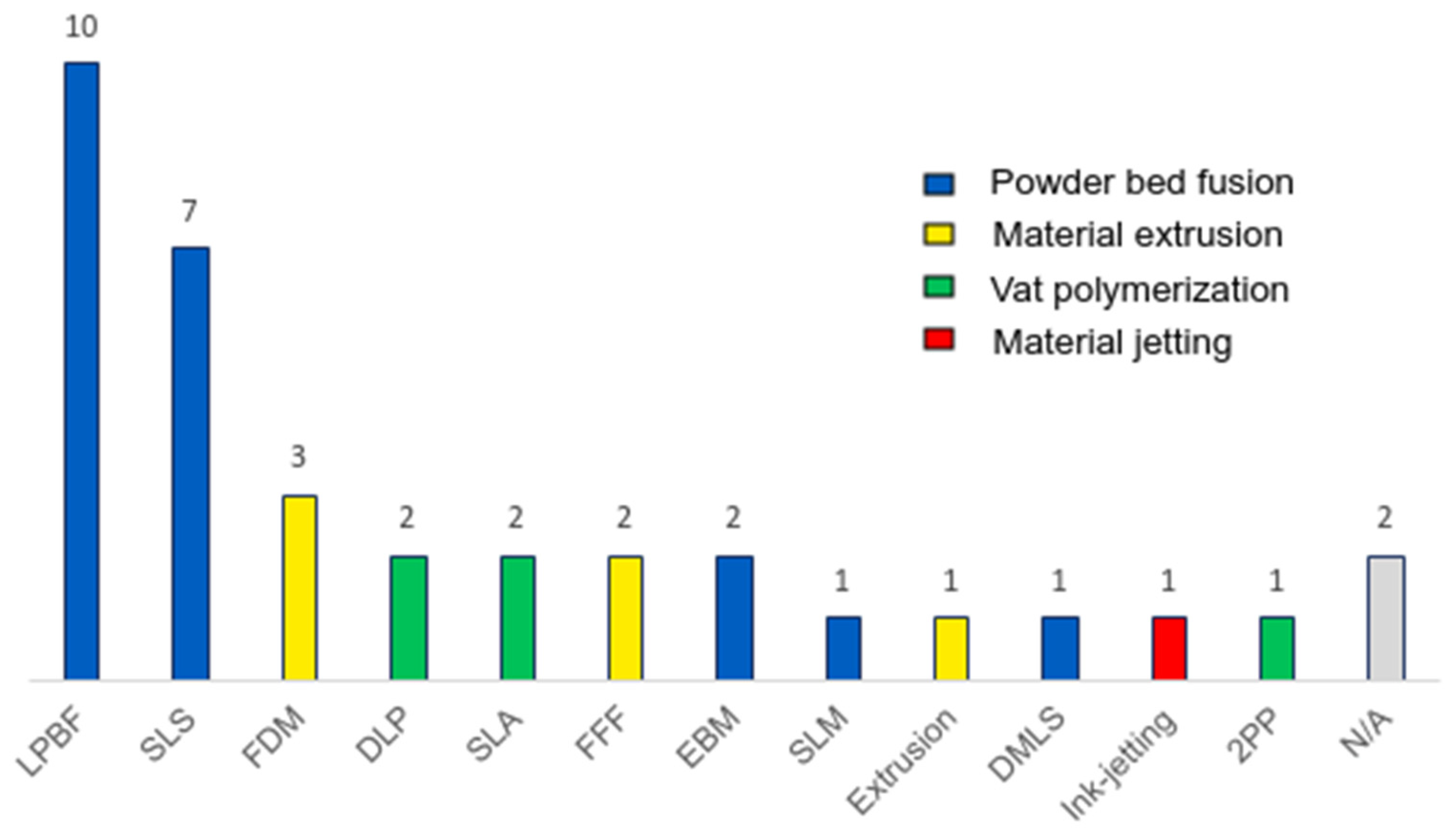
3.1. Printing Method
3.2. Structure
3.3. Three-Dimensional Model Generation
3.4. Self-Supporting Structures
3.5. Overview
4. Discussion
4.1. Answer to RQ1: What Are the Main Types of Mesoscopic Structures for the Generation of Porous Structures?
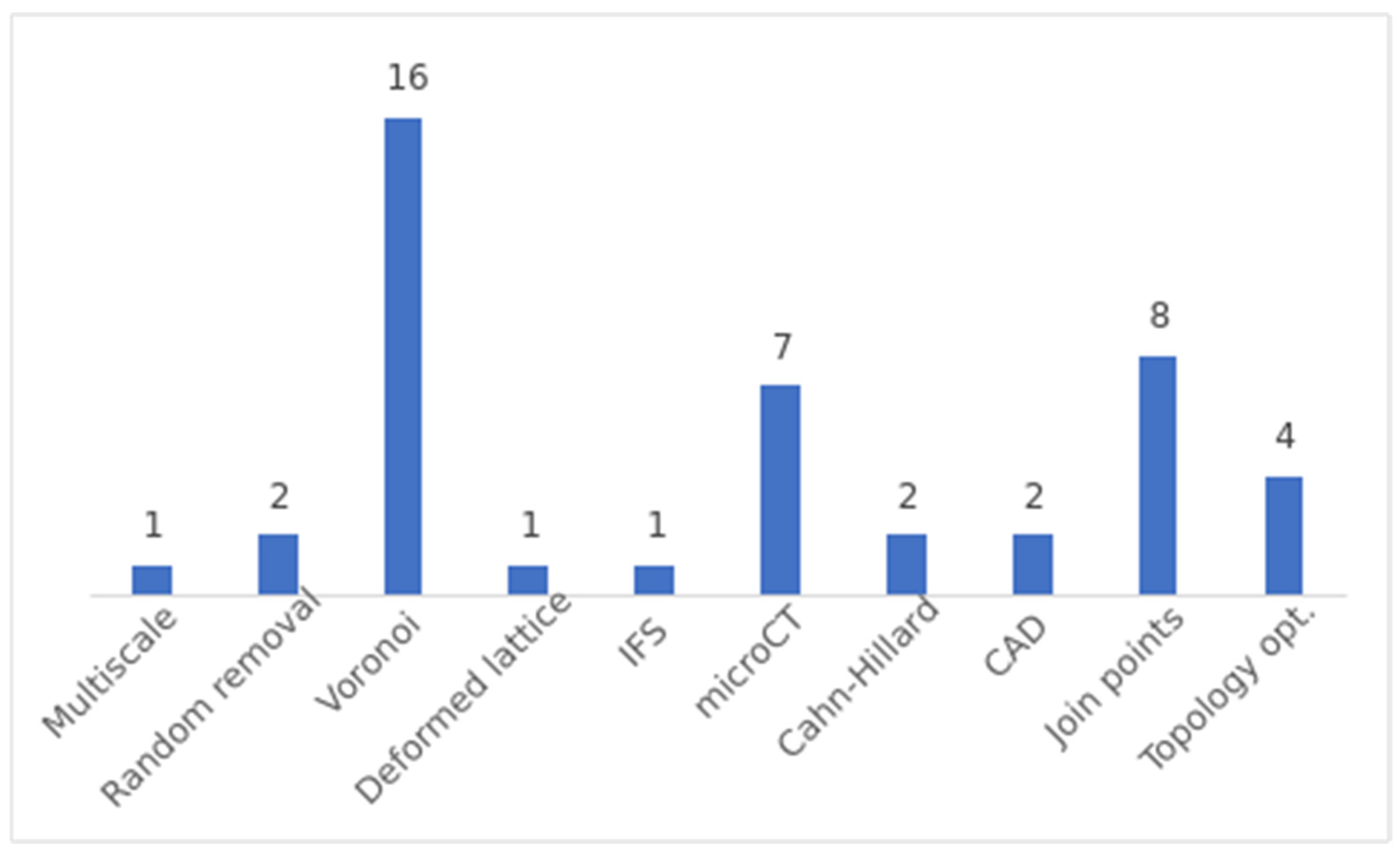
4.2. Answer to RQ2: What Are the Most Common Methods Used to Generate Stochastic Porous Structures?
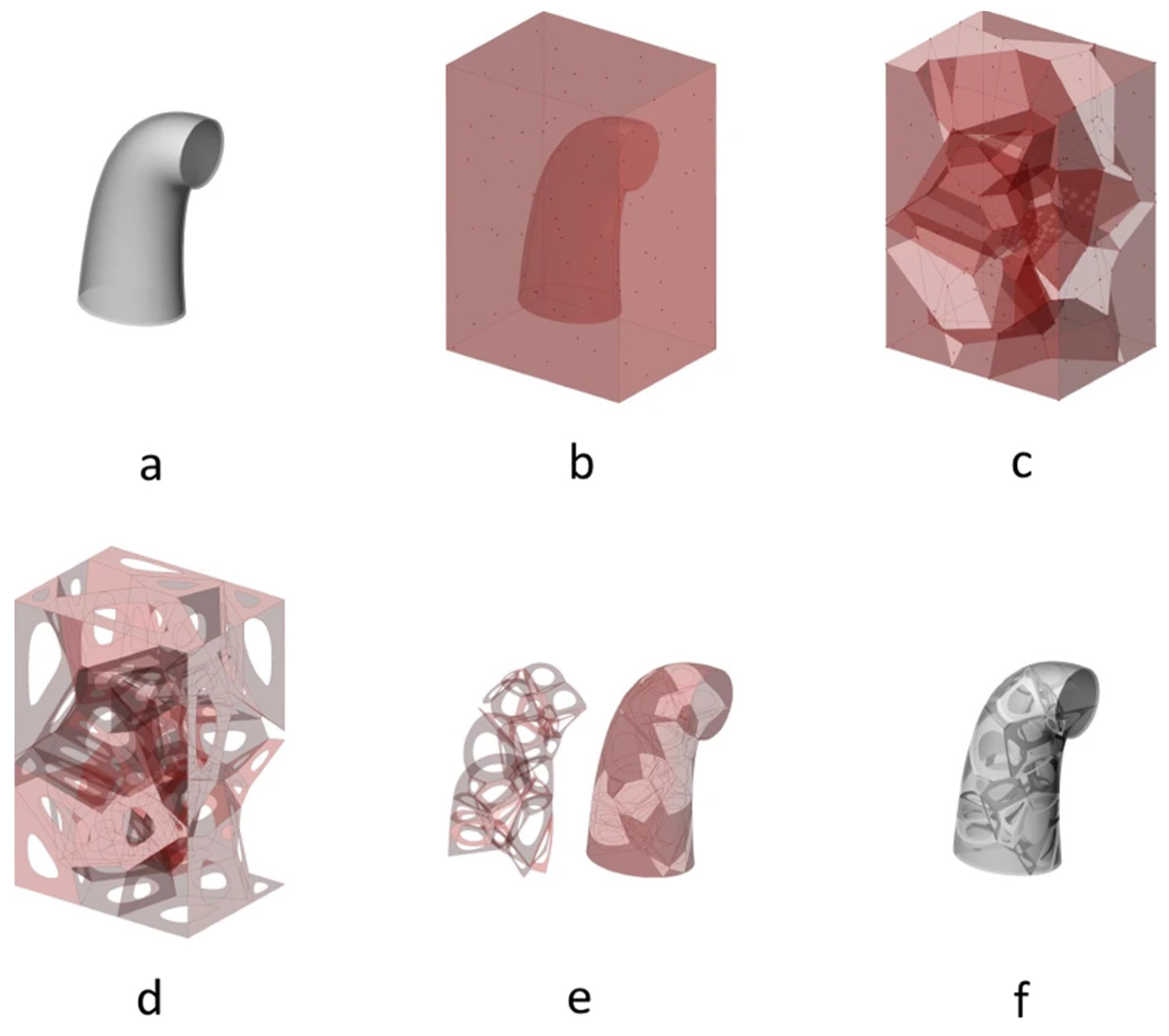
- Voronoi-based Approaches: Voronoi spaces are commonly used to generate stochastic structures. The general procedure involves filling a design space with random points or seeds and generating Voronoi spaces from these seeds. The resulting structure is formed by the intersection of the different Voronoi spaces. The specific method for generating the seeds can vary, including random placement in a larger space or using information extracted from bone µCT.
- Join Points: This method is used to generate beam-based structures. The first step is common to all variants and consists of generating random points, also called nodes, in a desired volume. These nodes are then joined according to certain criteria. Generally, the objective of this criterion is to control the nodal connection or the geometry between connections.
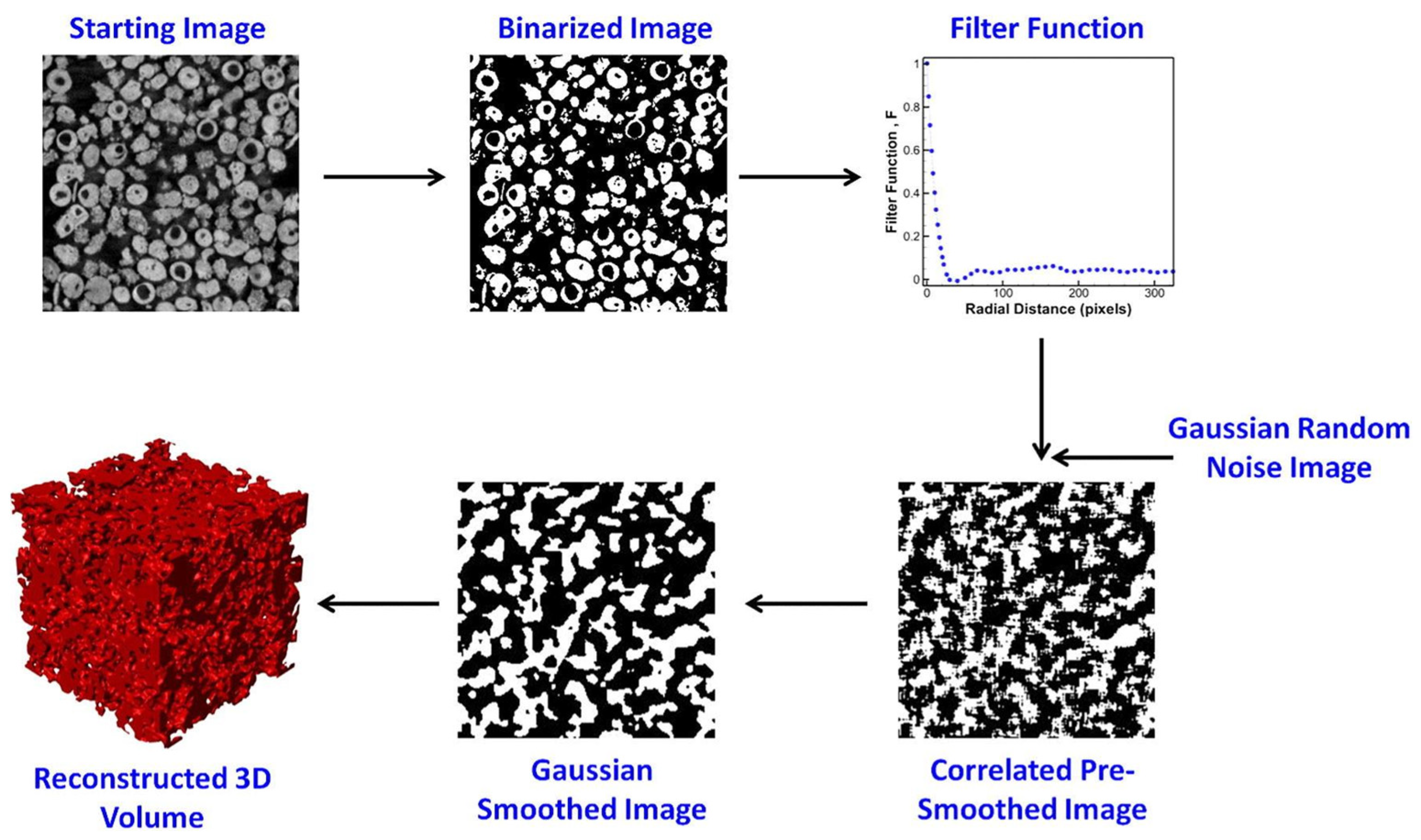
- µCT Scanning: µCT scanning is employed to generate porous structures from existing structures. This method involves scanning a porous structure to obtain its 3D CAD model, which can be used for further analysis and fabrication. Various types of structures, such as polyurethane sponges, cancellous bone scaffolds, human femoral heads, aluminium open-cell foams, realistic trabecular structures and randomly packed beds of glass beads, have been scanned and utilized to generate porous structures.
- Topological Optimization: Topological optimization algorithms based on principal stresses are used to obtain stiffness-optimized porous structures. These algorithms modify the structure based on stress distribution to achieve optimal mechanical properties. This is the unique method proposed to generate self-supported structures.
4.3. Answer to RQ3: Regarding RQ2, What Are the 3D Printing Methods Used to Generate Non-Regular Structures?
4.4. Answer to RQ4. What Proposals Exist for the Generation of Self-Generating Stochastic Structures?
- Topological Optimization: This method is employed by Wu et al. [54] and Martínez et al. [71]. The initial solid structure is subjected to different loads, and topological optimization based on principal stresses is performed. Regions experiencing lower stresses are eliminated to optimize the structure. However, these structures can exhibit anisotropic behaviour due to their dependency on the applied loads. Wu et al. [54] introduce anisotropic filters to mitigate this anisotropy.
- Voronoi Tessellation: Two articles, Alsheghri et al. [130] and Abdullahi et al. [129], utilize Voronoi tessellation to generate stochastic structures. Alsheghri et al. [130] start with an initial structure based on node connectivity data extracted from bone µCT. An iterative optimization process is then conducted by removing the least stressed edges until a convergence criterion is met. In contrast, Abdullahi et al. [129] define a desired volume and pore size and perform Voronoi tessellation to create a stochastic structure that satisfies the design parameters.
- Random Point Generation: The article by Zhang et al. [128] adopts a method where random points are generated within a desired volume. These points are connected following a predefined connection criterion. Coulomb repulsion and Hooke attraction forces are calculated for each node, and node positions are updated until equilibrium is reached, and the optimization process converges.
5. Conclusions
6. Future Directions
- Design self-supported stochastic patterns: The results show a lack of research in the development of algorithms capable of generating self-supported stochastic structures that can serve as bone scaffolds. It is true, however, that these scaffolds could be useful in fields other than bioprinting in applications such as thermal dissipation or vibration absorption.
- Development of AM techniques for bioprinting: Although many studies analysed generate porous structures that serve as scaffolds, many use printing methods that are not currently compatible with living cells (Table 8). However, under certain conditions, they can be biocompatible. For example, the development of photoinitiators that are not aggressive to cells or use less energetic wavelengths could make many vat polymerisation methods compatible with bioprinting.
- Hybrid manufacturing techniques: While there are printing methodologies that could be modified to be biocompatible, there are others, such as PBF, that by their nature could not. But that should not exclude these methods from the field of bioprinting as they could be combined with other methods to promote biocompatibility. It may be interesting to explore the possibilities offered by hybrid printing, where one method prints the scaffold, which provides mechanical strength to the structure, and another method prints the bio-inks, where the living cells could be embedded. This would solve the lack of mechanical strength that biocompatible materials have.
- Artificial intelligence for designing porous structures: The use of artificial intelligence in the articles has been considered during the data extraction process. No work has been found that uses AI to generate porous structures. Exploring the capabilities of generative AI to generate this type of structures could be a new methodological approach to obtain, in a simpler way, organic structures more like those present in nature.
Author Contributions
Funding
Conflicts of Interest
References
- Yazdanpanah, Z.; Johnston, J.D.; Cooper, D.M.L.; Chen, X. 3D Bioprinted Scaffolds for Bone Tissue Engineering: State-of-the-Art and Emerging Technologies. Front. Bioeng. Biotechnol. 2022, 10, 824156. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Herrera, J.A.; Garcia-Aznar, J.M.; Doblare, M. A Mathematical Model for Bone Tissue Regeneration inside a Specific Type of Scaffold. Biomech. Model. Mechanobiol. 2008, 7, 355–366. [Google Scholar] [CrossRef]
- Adachi, T.; Osako, Y.; Tanaka, M.; Hojo, M.; Hollister, S.J. Framework for Optimal Design of Porous Scaffold Microstructure by Computational Simulation of Bone Regeneration. Biomaterials 2006, 27, 3964–3972. [Google Scholar] [CrossRef]
- Zadpoor, A.A. Bone Tissue Regeneration: The Role of Scaffold Geometry. Biomater. Sci. 2015, 3, 231–245. [Google Scholar] [CrossRef]
- He, J.; Chen, G.; Liu, M.; Xu, Z.; Chen, H.; Yang, L.; Lv, Y. Scaffold Strategies for Modulating Immune Microenvironment during Bone Regeneration. Mater. Sci. Eng. C 2020, 108, 110411. [Google Scholar] [CrossRef]
- Stevens, B.; Yang, Y.; Mohandas, A.; Stucker, B.; Nguyen, K.T. A Review of Materials, Fabrication Methods, and Strategies Used to Enhance Bone Regeneration in Engineered Bone Tissues. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 85, 573–582. [Google Scholar] [CrossRef]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.T. Porous Scaffolds for Bone Regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D Biomaterial Scaffolds and Osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Mantila Roosa, S.M.; Kemppainen, J.M.; Moffitt, E.N.; Krebsbach, P.H.; Hollister, S.J. The Pore Size of Polycaprolactone Scaffolds Has Limited Influence on Bone Regeneration in an In Vivo Model. J. Biomed. Mater. Res. A 2010, 92, 359–368. [Google Scholar] [CrossRef]
- Sugawara, Y.; Kamioka, H.; Honjo, T.; Tezuka, K.I.; Takano-Yamamoto, T. Three-Dimensional Reconstruction of Chick Calvarial Osteocytes and Their Cell Processes Using Confocal Microscopy. Bone 2005, 36, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone Regenerative Medicine: Classic Options, Novel Strategies, and Future Directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Qamar Tanveer, M.; Mishra, G.; Mishra, S.; Sharma, R. Effect of Infill Pattern and Infill Density on Mechanical Behaviour of FDM 3D Printed Parts- a Current Review. Mater. Today Proc. 2022, 62, 100–108. [Google Scholar] [CrossRef]
- Bishop, E.S.; Mostafa, S.; Pakvasa, M.; Luu, H.H.; Lee, M.J.; Wolf, J.M.; Ameer, G.A.; He, T.C.; Reid, R.R. 3-D Bioprinting Technologies in Tissue Engineering and Regenerative Medicine: Current and Future Trends. Genes Dis. 2017, 4, 185–195. [Google Scholar] [CrossRef]
- Jagota, A.; Bennison, S.J. Spring-Network and Finite-Element Models for Elasticity and Fracture. In Non-Linearity and Breakdown in Soft Condensed Matter: Proceedings of a Workshop Held at Calcutta, India 1–9 December 1993; Springer: Berlin/Heidelberg, Germany, 1994. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; Clark, J.; et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Patterson, B.M.; Kuettner, L.; Shear, T.; Henderson, K.; Herman, M.J.; Ionita, A.; Chawla, N.; Williams, J.; Sun, T.; Fezzaa, K.; et al. Synchrotron CT Imaging of Lattice Structures with Engineered Defects. J. Mater. Sci. 2020, 55, 11353–11366. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Liang, H.; Liu, Y.; Bai, J.; Wang, M. Digital Light Processing (DLP) of Nano Biphasic Calcium Phosphate Bioceramic for Making Bone Tissue Engineering Scaffolds. Ceram. Int. 2022, 48, 27681–27692. [Google Scholar] [CrossRef]
- Baino, F.; Schwentenwein, M.; Verné, E. Modelling the Mechanical Properties of Hydroxyapatite Scaffolds Produced by Digital Light Processing-Based Vat Photopolymerization†. Ceramics 2022, 5, 593–600. [Google Scholar] [CrossRef]
- Baino, F.; Magnaterra, G.; Fiume, E.; Schiavi, A.; Tofan, L.P.; Schwentenwein, M.; Verné, E. Digital Light Processing Stereolithography of Hydroxyapatite Scaffolds with Bone-like Architecture, Permeability, and Mechanical Properties. J. Am. Ceram. Soc. 2022, 105, 1648–1657. [Google Scholar] [CrossRef]
- Wang, J.; Tang, Y.; Cao, Q.; Wu, Y.; Wang, Y.; Yuan, B.; Li, X.; Zhou, Y.; Chen, X.; Zhu, X.; et al. Fabrication and Biological Evaluation of 3D-Printed Calcium Phosphate Ceramic Scaffolds with Distinct Macroporous Geometries through Digital Light Processing Technology. Regen. Biomater. 2022, 9, rbac005. [Google Scholar] [CrossRef]
- Shin, C.S.; Chang, Y.C. Fabrication and Compressive Behavior of a Micro-lattice Composite by High Resolution Dlp Stereolithography. Polymers 2021, 13, 785. [Google Scholar] [CrossRef]
- Deshmukh, S.; Ronge, H.; Ramamoorthy, S. Design of Periodic Foam Structures for Acoustic Applications: Concept, Parametric Study and Experimental Validation. Mater. Des. 2019, 175, 107830. [Google Scholar] [CrossRef]
- Schmidt, J.; Altun, A.A.; Schwentenwein, M.; Colombo, P. Complex Mullite Structures Fabricated via Digital Light Processing of a Preceramic Polysiloxane with Active Alumina Fillers. J. Eur. Ceram. Soc. 2019, 39, 1336–1343. [Google Scholar] [CrossRef]
- Bauer, J.; Schroer, A.; Schwaiger, R.; Tesari, I.; Lange, C.; Valdevit, L.; Kraft, O. Push-to-Pull Tensile Testing of Ultra-Strong Nanoscale Ceramic-Polymer Composites Made by Additive Manufacturing. Extrem. Mech. Lett. 2015, 3, 105–112. [Google Scholar] [CrossRef]
- Zhang, D.; Xiao, J.; Moorlag, C.; Guo, Q.; Yang, J. Development of Ultralight, Super-Elastic, Hierarchical Metallic Meta-Structures with I3DP Technology. Nanotechnology 2017, 28, 455708. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zou, B.; Wang, X.; Zhou, X.; Yang, G.; Lai, Q.; Zhao, Y. SLA-3d Printed Building and Characteristics of GelMA/HAP Biomaterials with Gradient Porous Structure. J. Mech. Behav. Biomed. Mater. 2024, 155. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Gao, Y.; Yang, X.; Tian, C.; Yang, Z.; Zhang, J. Relationship between Topological Structures and Mechanical Properties of Artificially Architected SiC Cellular Ceramics: Experimental and Numerical Study. J. Eur. Ceram. Soc. 2023, 43, 4263–4276. [Google Scholar] [CrossRef]
- Xu, Y.; Pan, H.; Wang, R.; Du, Q.; Lu, L. New Families of Triply Periodic Minimal Surface-like Shell Lattices. Addit. Manuf. 2023, 77, 103779. [Google Scholar] [CrossRef]
- Chen, E.; Luan, S.; Gaitanaros, S. On the Strength of Brittle Foams with Uniform and Gradient Densities. Extrem. Mech. Lett. 2022, 51, 101598. [Google Scholar] [CrossRef]
- Drücker, S.; Lüdeker, J.K.; Blecken, M.; Kurt, A.; Betz, K.; Kriegesmann, B.; Fiedler, B. Probabilistic Analysis of Additively Manufactured Polymer Lattice Structures. Mater. Des. 2022, 213, 110300. [Google Scholar] [CrossRef]
- Liu, B.; Cao, W.; Zhang, L.; Jiang, K.; Lu, P. A Design Method of Voronoi Porous Structures with Graded Relative Elasticity Distribution for Functionally Gradient Porous Materials. Int. J. Mech. Mater. Des. 2021, 17, 863–883. [Google Scholar] [CrossRef]
- Seetoh, I.P.; Liu, X.; Markandan, K.; Zhen, L.; Lai, C.Q. Strength and Energy Absorption Characteristics of Ti6Al4V Auxetic 3D Anti-Tetrachiral Metamaterials. Mech. Mater. 2021, 156, 103811. [Google Scholar] [CrossRef]
- Seetoh, I.; Markandan, K.; Lai, C.Q. Effect of Reinforcement Bending on the Elastic Properties of Interpenetrating Phase Composites. Mech. Mater. 2019, 136, 103071. [Google Scholar] [CrossRef]
- Thavasiappan, K.; Sivakumar Venkatesan, M.; Ariffuddeen, M.; Ponnuchamy, O.; Ravichandran, N.; Murugesan, G. Design, Analysis, Fabrication and Testing of PC Porous Scaffolds Using Rapid Prototyping in Clinical Applications. Biomedicine 2019, 39, 339–345. [Google Scholar] [CrossRef]
- Carbajo, J.; Molina, J.M.; Kim, S.; Maiorano, L.P.; Mosanenzadeh, S.G.; Fang, N.X. Design of Replicated Open-Pore Aluminium Cellular Materials with a Non-Stochastic Structure for Sound Absorption Applications. Met. Mater. Int. 2022, 29, 1007–1018. [Google Scholar] [CrossRef]
- Polley, C.; Distler, T.; Scheufler, C.; Detsch, R.; Lund, H.; Springer, A.; Schneidereit, D.; Friedrich, O.; Boccaccini, A.R.; Seitz, H. 3D Printing of Piezoelectric and Bioactive Barium Titanate-Bioactive Glass Scaffolds for Bone Tissue Engineering. Mater. Today Bio 2023, 21, 100719. [Google Scholar] [CrossRef] [PubMed]
- Snelling, D.A.; Williams, C.B.; Suchicital, C.T.A.; Druschitz, A.P. Binder Jetting Advanced Ceramics for Metal-Ceramic Composite Structures. Int. J. Adv. Manuf. Technol. 2017, 92, 531–545. [Google Scholar] [CrossRef]
- Laskowska, D.; Bałasz, B.; Kaczorowski, W.; Grabarczyk, J.; Svobodova, L.; Szatkiewicz, T.; Mitura, K. The DLC Coating on 316L Stainless Steel Stochastic Voronoi Tessellation Structures Obtained by Binder Jetting Additive Manufacturing for Potential Biomedical Applications. Coatings 2022, 12, 1373. [Google Scholar] [CrossRef]
- Burden, E.; Oh, Y.; Mummareddy, B.; Negro, D.; Cortes, P.; Du Plessis, A.; MacDonald, E.; Adams, J.; Li, F.; Rojas, R. Unit Cell Estimation of Volumetrically-Varying Permittivity in Additively-Manufactured Ceramic Lattices with X-ray Computed Tomography. Mater. Des. 2021, 210, 110032. [Google Scholar] [CrossRef]
- Pinto, I.N.; da Silva Medeiros, F.; Nunes, F.C.; de Sousa, R.G.; Albergaria, J.D.S.; Santos, J.P.F. Additive Manufacturing of 3D Networks Based on Poly(Lactic Acid) and Amine Functionalized Graphene Oxide as a Platform for Scaffolds. J. Appl. Polym. Sci. 2024, 141, e55396. [Google Scholar] [CrossRef]
- Dadi, H.; Sarimalla, R.; Adapa, R.K. Design and 3D Printing of Non-Stochastic Polylactic Acid Structures for Biomedical Applications. Asia-Pac. J. Sci. Technol. 2023, 28, APST–28. [Google Scholar]
- Zenobi, E.; Merco, M.; Mochi, F.; Ruspi, J.; Pecci, R.; Marchese, R.; Convertino, A.; Lisi, A.; Del Gaudio, C.; Ledda, M. Tailoring the Microarchitectures of 3D Printed Bone-like Scaffolds for Tissue Engineering Applications. Bioengineering 2023, 10, 567. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wu, Z.; Wu, Y.; Zhong, Z.; Hong, Y. Construction of the Gypsum-Coated Scaffolds for In Situ Bone Regeneration. ACS Appl. Mater. Interfaces 2021, 13, 31527–31541. [Google Scholar] [CrossRef]
- Mancuso, E.; Shah, L.; Jindal, S.; Serenelli, C.; Tsikriteas, Z.M.; Khanbareh, H.; Tirella, A. Additively Manufactured BaTiO3 Composite Scaffolds: A Novel Strategy for Load Bearing Bone Tissue Engineering Applications. Mater. Sci. Eng. C 2021, 126, 112192. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Zahedi, S.A.; Ismail, S.O. Mechanical Performances of Hip Implant Design and Fabrication with PEEK Composite. Polymer 2021, 227, 112192. [Google Scholar] [CrossRef]
- Muallah, D.; Sembdner, P.; Holtzhausen, S.; Meissner, H.; Hutsky, A.; Ellmann, D.; Assmann, A.; Schulz, M.C.; Lauer, G.; Kroschwald, L.M. Adapting the Pore Size of Individual, 3D-printed Cpc Scaffolds in Maxillofacial Surgery. J. Clin. Med. 2021, 10, 2654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, L.; Song, P.; Pei, X.; Sun, H.; Wu, L.; Zhou, C.; Wang, K.; Fan, Y.; Zhang, X. 3D Printed Bone Tissue Regenerative PLA/HA Scaffolds with Comprehensive Performance Optimizations. Mater. Des. 2021, 201, 109490. [Google Scholar] [CrossRef]
- Qu, H.; Han, Z.; Chen, Z.; Tang, L.; Gao, C.; Liu, K.; Pan, H.; Fu, H.; Ruan, C. Fractal Design Boosts Extrusion-Based 3D Printing of Bone-Mimicking Radial-Gradient Scaffolds. Research 2021, 2021, 9892689. [Google Scholar] [CrossRef]
- Ambekar, R.S.; Mohanty, I.; Kishore, S.; Das, R.; Pal, V.; Kushwaha, B.; Roy, A.K.; Kumar Kar, S.; Tiwary, C.S. Atomic Scale Structure Inspired 3D-Printed Porous Structures with Tunable Mechanical Response. Adv. Eng. Mater. 2021, 23, 2001428. [Google Scholar] [CrossRef]
- Kou, T.; Wang, S.; Shi, R.; Zhang, T.; Chiovoloni, S.; Lu, J.Q.; Chen, W.; Worsley, M.A.; Wood, B.C.; Baker, S.E.; et al. Periodic Porous 3D Electrodes Mitigate Gas Bubble Traffic during Alkaline Water Electrolysis at High Current Densities. Adv. Energy Mater. 2020, 10, 2002955. [Google Scholar] [CrossRef]
- Maiti, A.; Small, W.; Lewicki, J.P.; Chinn, S.C.; Wilson, T.S.; Saab, A.P. Age-Aware Constitutive Materials Model for a 3D Printed Polymeric Foam. Sci. Rep. 2019, 9, 15923. [Google Scholar] [CrossRef]
- Fairag, R.; Rosenzweig, D.; Luis, J.; Garcialuna, R.; Weber, M.H.; Haglund, L. 3D-Printed Polylactic Acid (PLA) Scaffolds Promote Bone-like Matrix Deposition In-Vitro. ACS Appl. Mater. Interfaces 2019, 11, 15306–15315. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.H.; Lin, Y.H.; Shie, M.Y.; Lin, C.P. Effects of Bone Morphogenic Protein-2 Loaded on the 3D-Printed MesoCS Scaffolds. J. Formos. Med. Assoc. 2018, 117, 879–887. [Google Scholar] [CrossRef]
- Wu, J.; Aage, N.; Westermann, R.; Sigmund, O. Infill Optimization for Additive Manufacturing—Approaching Bone-like Porous Structures. IEEE Trans. Vis. Comput. Graph. 2016, 24, 1127–1140. [Google Scholar] [CrossRef]
- Holmes, B.; Bulusu, K.; Plesniak, M.; Zhang, L.G. A Synergistic Approach to the Design, Fabrication and Evaluation of 3D Printed Micro and Nano Featured Scaffolds for Vascularized Bone Tissue Repair. Nanotechnology 2016, 27, 064001. [Google Scholar] [CrossRef]
- Khennab, A.; Benhizia, A. Design and Evaluation of Compressive Properties of 3D-Printed PLA Stochastic Open-Cell Foam. Int. J. Crashworthiness 2023, 29, 468–481. [Google Scholar] [CrossRef]
- Sadeghzade, M.; Gharehbaghi, H.; Toozandehjani, H.; Farrokhabadi, A. Experimental Study of Energy Absorption Capability in the Lattice Structures Based on the Octagonal Bipyramid Unit Cell. J. Braz. Soc. Mech. Sci. Eng. 2023, 45, 460. [Google Scholar] [CrossRef]
- Sivakumar, N.K.; Palaniyappan, S.; Sekar, V.; Alodhayb, A.; Braim, M. An Optimization Approach for Studying the Effect of Lattice Unit Cell’s Design-Based Factors on Additively Manufactured Poly Methyl Methacrylate Cranio-Implant. J. Mech. Behav. Biomed. Mater. 2023, 141, 105791. [Google Scholar] [CrossRef] [PubMed]
- Noroozi, R.; Tatar, F.; Zolfagharian, A.; Brighenti, R.; Shamekhi, M.A.; Rastgoo, A.; Hadi, A.; Bodaghi, M. Additively Manufactured Multi-Morphology Bone-like Porous Scaffolds: Experiments and Micro-Computed Tomography-Based Finite Element Modeling Approaches. Int. J. Bioprint. 2022, 8, 40–53. [Google Scholar] [CrossRef]
- Santiago, C.C.; Yelamanchi, B.; Diosdado De la Peña, J.A.; Lamb, J.; Roguski, K.; Turzyński, F.; Faruqui, R.; Choo, K.; Du Plessis, A.; Sillani, F.; et al. Thermoplastic Extrusion Additive Manufacturing of High-Performance Carbon Fiber Peek Lattices. Crystals 2021, 11, 1453. [Google Scholar] [CrossRef]
- Tripathi, Y.; Shukla, M.; Bhatt, A.D. Idealization through Interactive Modeling and Experimental Assessment of 3D-Printed Gyroid for Trabecular Bone Scaffold. Proc. Inst. Mech. Eng. H 2021, 235, 1025–1034. [Google Scholar] [CrossRef]
- Gupta, D.; Vashisth, P.; Bellare, J. Multiscale Porosity in a 3D Printed Gellan–Gelatin Composite for Bone Tissue Engineering. Biomed. Mater. 2021, 16, 034103. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, V.H.; Rawson, S.D.; Puga, H.; Meireles, J.; Withers, P.J. Additive Manufacturing Assisted Investment Casting: A Low-Cost Method to Fabricate Periodic Metallic Cellular Lattices. Addit. Manuf. 2020, 33, 101085. [Google Scholar] [CrossRef]
- Murab, S.; Gruber, S.M.S.; Lin, C.Y.J.; Whitlock, P. Elucidation of Bio-Inspired Hydroxyapatie Crystallization on Oxygen-Plasma Modified 3D Printed Poly-Caprolactone Scaffolds. Mater. Sci. Eng. C 2020, 109, 110529. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, M.; Zhu, Y.; Li, X.; Wang, L.; Hu, J. A Novel Optimization Design Method of Additive Manufacturing Oriented Porous Structures and Experimental Validation. Mater. Des. 2019, 163, 107550. [Google Scholar] [CrossRef]
- Ahlfeld, T.; Cubo-Mateo, N.; Cometta, S.; Guduric, V.; Vater, C.; Bernhardt, A.; Akkineni, A.R.; Lode, A.; Gelinsky, M. A Novel Plasma-Based Bioink Stimulates Cell Proliferation and Differentiation in Bioprinted, Mineralized Constructs. ACS Appl. Mater. Interfaces 2020, 12, 12557–12572. [Google Scholar] [CrossRef]
- Ge, C.; Priyadarshini, L.; Cormier, D.; Pan, L.; Tuber, J. A Preliminary Study of Cushion Properties of a 3D Printed Thermoplastic Polyurethane Kelvin Foam. Packag. Technol. Sci. 2018, 31, 361–368. [Google Scholar] [CrossRef]
- Bai, C.; Franchin, G.; Elsayed, H.; Zaggia, A.; Conte, L.; Li, H.; Colombo, P. High-Porosity Geopolymer Foams with Tailored Porosity for Thermal Insulation and Wastewater Treatment. J. Mater. Res. 2017, 32, 3251–3259. [Google Scholar] [CrossRef]
- Rajasekharan, A.K.; Bordes, R.; Sandström, C.; Ekh, M.; Andersson, M. Hierarchical and Heterogeneous Bioinspired Composites—Merging Molecular Self-Assembly with Additive Manufacturing. Small 2017, 13, 1700550. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Lee, S.J.; Jo, H.H.; Lee, J.H.; Kim, W.D.; Lee, J.Y.; Park, S.A. Fabrication and Characterization of 3D-Printed Bone-like β-Tricalcium Phosphate/Polycaprolactone Scaffolds for Dental Tissue Engineering. J. Ind. Eng. Chem. 2017, 46, 175–181. [Google Scholar] [CrossRef]
- Martínez, J.; Song, H.; Dumas, J.; Lefebvre, S. Orthotropic K-Nearest Foams for Additive Manufacturing. ACM Trans. Graph. 2017, 36, 1–12. [Google Scholar] [CrossRef]
- Montalbano, G.; Borciani, G.; Cerqueni, G.; Licini, C.; Banche-Niclot, F.; Janner, D.; Sola, S.; Fiorilli, S.; Mattioli-Belmonte, M.; Ciapetti, G.; et al. Collagen Hybrid Formulations for the 3d Printing of Nanostructured Bone Scaffolds: An Optimized Genipin-Crosslinking Strategy. Nanomaterials 2020, 10, 1681. [Google Scholar] [CrossRef] [PubMed]
- Montalbano, G.; Molino, G.; Fiorilli, S.; Vitale-Brovarone, C. Synthesis and Incorporation of Rod-like Nano-Hydroxyapatite into Type I Collagen Matrix: A Hybrid Formulation for 3D Printing of Bone Scaffolds. J. Eur. Ceram. Soc. 2020, 40, 3689–3697. [Google Scholar] [CrossRef]
- Tariverdian, T.; Behnamghader, A.; Brouki Milan, P.; Barzegar-Bafrooei, H.; Mozafari, M. 3D-Printed Barium Strontium Titanate-Based Piezoelectric Scaffolds for Bone Tissue Engineering. Ceram. Int. 2019, 45, 14029–14038. [Google Scholar] [CrossRef]
- Fotsing, E.R.; Dubourg, A.; Ross, A.; Mardjono, J. Acoustic Properties of a Periodic Micro-Structures Obtained by Additive Manufacturing. Appl. Acoust. 2019, 148, 322–331. [Google Scholar] [CrossRef]
- Muerza-Cascante, M.L.; Shokoohmand, A.; Khosrotehrani, K.; Haylock, D.; Dalton, P.D.; Hutmacher, D.W.; Loessner, D. Endosteal-like Extracellular Matrix Expression on Melt Electrospun Written Scaffolds. Acta Biomater. 2017, 52, 145–158. [Google Scholar] [CrossRef]
- Berger, M.B.; Jacobs, T.W.; Boyan, B.D.; Schwartz, Z. Hot Isostatic Pressure Treatment of 3D Printed Ti6Al4V Alters Surface Modifications and Cellular Response. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 1262–1273. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, A.; Ekade, P.; Krishnan, S. Experimental Investigation of Heat Transfer and Fluid Flow in Octet-Truss Lattice Geometry. Int. J. Therm. Sci. 2019, 143, 64–75. [Google Scholar] [CrossRef]
- Araya, M.; Jaskari, M.; Rautio, T.; Guillén, T.; Järvenpää, A. Assessing the Compressive and Tensile Properties of TPMS-Gyroid and Stochastic Ti64 Lattice Structures: A Study on Laser Powder Bed Fusion Manufacturing for Biomedical Implants. J. Sci. Adv. Mater. Devices 2024, 9, 100663. [Google Scholar] [CrossRef]
- Singh, S.; Roy, R.; Kumar Meena, V.; Vashisth, P.; Kalyanasundaram, D. Nanotextured and Drug Loaded Neovius Ti6Al4V ELI Scaffolds with Osteogenesis and Anti-Cancer Potential. Mater. Des. 2024, 237, 112570. [Google Scholar] [CrossRef]
- Voyiadjis, G.Z.; Znemah, R.A.; Wood, P. Microstructure and Geometry Effects on the Compressive Behavior of LPBF- Manufactured Inconel 718 Honeycomb Structures. J. Mater. Res. Technol. 2023, 24, 1562–1578. [Google Scholar] [CrossRef]
- Novak, N.; Al-Ketan, O.; Mauko, A.; Yilmaz, Y.E.; Krstulović-Opara, L.; Tanaka, S.; Hokamoto, K.; Rowshan, R.; Al-Rub, R.A.; Vesenjak, M.; et al. Impact Loading of Additively Manufactured Metallic Stochastic Sheet-Based Cellular Material. Int. J. Impact. Eng. 2023, 174, 104527. [Google Scholar] [CrossRef]
- Wang, Y.T.; Chang, C.M.; Liu, P.H.; Lin, C.L. Feasibility Evaluation of a New Lattice for Porous Surface Design in Additive Manufacturing Medical Implants under Interfacial Tensile Bonded Testing. Addit. Manuf. 2023, 66, 103455. [Google Scholar] [CrossRef]
- Kohli, N.; Theodoridis, K.; Hall, T.A.G.; Sanz-Pena, I.; Gaboriau, D.C.A.; van Arkel, R.J. Bioreactor Analyses of Tissue Ingrowth, Ongrowth and Remodelling around Implants: An Alternative to Live Animal Testing. Front. Bioeng. Biotechnol. 2023, 11, 1054391. [Google Scholar] [CrossRef]
- Kechagias, S.; Theodoridis, K.; Broomfield, J.; Malpartida-Cardenas, K.; Reid, R.; Georgiou, P.; van Arkel, R.J.; Jeffers, J.R.T. The Effect of Nodal Connectivity and Strut Density within Stochastic Titanium Scaffolds on Osteogenesis. Front. Bioeng. Biotechnol. 2023, 11, 1305936. [Google Scholar] [CrossRef]
- Zhang, T.; liu, F.; Deng, X.; Zhao, M.; Zhou, H.; Zhang, D.Z. Experimental Study on the Thermal Storage Performance of Phase Change Materials Embedded with Additively Manufactured Triply Periodic Minimal Surface Architected Lattices. Int. J. Heat Mass. Transf. 2022, 199, 123452. [Google Scholar] [CrossRef]
- Liu, H.; Gu, D.; Yang, J.; Shi, K.; Yuan, L. Laser Powder Bed Fusion of Node-Reinforced Hybrid Lattice Structure Inspired by Crystal Microstructure: Structural Feature Sensitivity and Mechanical Performance. Mater. Sci. Eng. A 2022, 858, 144048. [Google Scholar] [CrossRef]
- Longhitano, G.A.; Machado, L.M.R.; Jardini, A.L.; Baldin, E.K.; Santos, P.B.; Filho, R.M.; de Fraga Malfatti, C.; de Carvalho Zavaglia, C.A. Fracture Behavior under Compression Loading of Surface-Cleaned Metallic Lattice Structures. Int. J. Adv. Manuf. Technol. 2022, 121, 3309–3321. [Google Scholar] [CrossRef]
- Kechagias, S.; Oosterbeek, R.N.; Munford, M.J.; Ghouse, S.; Jeffers, J.R.T. Controlling the Mechanical Behaviour of Stochastic Lattice Structures: The Key Role of Nodal Connectivity. Addit. Manuf. 2022, 54, 102730. [Google Scholar] [CrossRef]
- Gu, Y.; Wei, L.; Zhang, Z.; Van Dessel, J.; Driesen, R.B.; Lambrichts, I.; Jacobs, R.; Tian, L.; Sun, Y.; Liu, Y.; et al. BMP-2 Incorporated Biomimetic CaP Coating Functionalized 3D Printed Ti6Al4V Scaffold Induces Ectopic Bone Formation in a Dog Model. Mater. Des. 2022, 215, 110443. [Google Scholar] [CrossRef]
- Chu, S.; Guilleminot, J.; Kelly, C.; Abar, B.; Gall, K. Stochastic Modeling and Identification of Material Parameters on Structures Produced by Additive Manufacturing. Comput. Methods Appl. Mech. Eng. 2021, 387, 114166. [Google Scholar] [CrossRef]
- Hossain, U.; Ghouse, S.; Nai, K.; Jeffers, J.R. Controlling and Testing Anisotropy in Additively Manufactured Stochastic Structures. Addit. Manuf. 2021, 39, 101849. [Google Scholar] [CrossRef]
- Matheson, K.E.; Cross, K.K.; Nowell, M.M.; Spear, A.D. A Multiscale Comparison of Stochastic Open-Cell Aluminum Foam Produced via Conventional and Additive-Manufacturing Routes. Mater. Sci. Eng. A 2017, 707, 181–192. [Google Scholar] [CrossRef]
- Ghouse, S.; Babu, S.; Van Arkel, R.J.; Nai, K.; Hooper, P.A.; Jeffers, J.R.T. The Influence of Laser Parameters and Scanning Strategies on the Mechanical Properties of a Stochastic Porous Material. Mater. Des. 2017, 131, 498–508. [Google Scholar] [CrossRef]
- Mappa, T.A.; Chu, S.F.; Hung, K.S.; Saito, T.; Ruslin, M.; Lan, W.C.; Kuo, H.H.; Cho, Y.C.; Hsieh, C.C.; Huang, J.; et al. An Innovative Three-Dimensional Printed Titanium Implant with a Biomimetic Structure Design for Promoting Osseointegration Potential. Mater. Des. 2024, 238, 112692. [Google Scholar] [CrossRef]
- Li, J.; Tian, C.; Xia, R. Enhanced Compressive Mechanical Properties in Stochastic Bicontinuous Porous Structures. Eng. Fract. Mech. 2023, 282, 109192. [Google Scholar] [CrossRef]
- Wang, E.; Chen, C.; Zhang, G.; Luo, Q.; Li, Q.; Sun, G. Multiaxial Mechanical Characterization of Additively Manufactured Open-Cell Kelvin Foams. Compos. Struct. 2023, 305, 116505. [Google Scholar] [CrossRef]
- Colamartino, I.; Anghileri, M.; Boniardi, M. Investigation of the Compressive Properties of Three-Dimensional Voronoi Reticula. Int. J. Solids Struct. 2023, 284, 112501. [Google Scholar] [CrossRef]
- Kanwar, S.; Vijayavenkataraman, S. 3D Printable Bone-Mimicking Functionally Gradient Stochastic Scaffolds for Tissue Engineering and Bone Implant Applications. Mater. Des. 2022, 223, 111199. [Google Scholar] [CrossRef]
- Shuai, C.; Yang, W.; Feng, P.; Peng, S.; Pan, H. Accelerated Degradation of HAP/PLLA Bone Scaffold by PGA Blending Facilitates Bioactivity and Osteoconductivity. Bioact. Mater. 2021, 6, 490–502. [Google Scholar] [CrossRef]
- Vlad, M.D.; Fernández Aguado, E.; Gómez González, S.; Ivanov, I.C.; Şindilar, E.V.; Poeată, I.; Iencean, A.Ş.; Butnaru, M.; Avădănei, E.R.; López López, J. Novel Titanium-Apatite Hybrid Scaffolds with Spongy Bone-like Micro Architecture Intended for Spinal Application: In Vitro and In Vivo Study. Mater. Sci. Eng. C 2020, 110, 110658. [Google Scholar] [CrossRef]
- Yang, N.; Wang, S.; Gao, L.; Men, Y.; Zhang, C. Building Implicit-Surface-Based Composite Porous Architectures. Compos. Struct. 2017, 173, 35–43. [Google Scholar] [CrossRef]
- Lausic, A.T.; Bird, A.G.; Steeves, C.A.; Hibbard, G.D. Scale-Dependent Failure of Stereolithographic Polymer Microtrusses in Three-Point Bending. J. Compos. Mater. 2016, 50, 1739–1749. [Google Scholar] [CrossRef]
- Zhu, W.; Holmes, B.; Glazer, R.I.; Zhang, L.G. 3D Printed Nanocomposite Matrix for the Study of Breast Cancer Bone Metastasis. Nanomedicine 2016, 12, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Gao, L.; Zhou, K. Simple Method to Generate and Fabricate Stochastic Porous Scaffolds. Mater. Sci. Eng. C 2015, 56, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Cerardi, A.; Caneri, M.; Meneghello, R.; Concheri, G.; Ricotta, M. Mechanical Characterization of Polyamide Cellular Structures Fabricated Using Selective Laser Sintering Technologies. Mater. Des. 2013, 46, 910–915. [Google Scholar] [CrossRef]
- Wang, P.; Yang, F.; Lu, G.; Bian, Y.; Zhang, S.; Zheng, B.; Fan, H. Anisotropic Compression Behaviors of Bio-Inspired Modified Body-Centered Cubic Lattices Validated by Additive Manufacturing. Compos. B Eng. 2022, 234, 109724. [Google Scholar] [CrossRef]
- Loginov, Y.N.; Koptyug, A.; Popov, V.V.; Belikov, S.V.; Mukanov, G.; Golodnov, A.I.; Stepanov, S.I. Compression Deformation and Fracture Behavior of Additively Manufactured Ti–6Al–4V Cellular Structures. Int. J. Lightweight Mater. Manuf. 2022, 5, 126–135. [Google Scholar] [CrossRef]
- Zhao, D.; Liang, H.; Han, C.; Li, J.; Liu, J.; Zhou, K.; Yang, C.; Wei, Q. 3D Printing of a Titanium-Tantalum Gyroid Scaffold with Superb Elastic Admissible Strain, Bioactivity and in-Situ Bone Regeneration Capability. Addit. Manuf. 2021, 47, 102223. [Google Scholar] [CrossRef]
- Cvrček, L.; Denk, F.; Čejka, Z. Comparison of 3D Printed Trabecular Structure with Porous Plasma Spray: A Method Based on Mapping the Local Modulus of Elasticity. Mater. Res. Express 2020, 7, 075403. [Google Scholar] [CrossRef]
- Soro, N.; Attar, H.; Wu, X.; Dargusch, M.S. Investigation of the Structure and Mechanical Properties of Additively Manufactured Ti-6Al-4V Biomedical Scaffolds Designed with a Schwartz Primitive Unit-Cell. Mater. Sci. Eng. A 2019, 745, 195–202. [Google Scholar] [CrossRef]
- Hedayati, R.; Hosseini-Toudeshky, H.; Sadighi, M.; Mohammadi-Aghdam, M.; Zadpoor, A.A. Multiscale Modeling of Fatigue Crack Propagation in Additively Manufactured Porous Biomaterials. Int. J. Fatigue 2018, 113, 416–427. [Google Scholar] [CrossRef]
- Gorguluarslan, R.M.; Choi, S.K.; Saldana, C.J. Uncertainty Quantification and Validation of 3D Lattice Scaffolds for Computer-Aided Biomedical Applications. J. Mech. Behav. Biomed. Mater. 2017, 71, 428–440. [Google Scholar] [CrossRef]
- Simoneau, C.; Brailovski, V.; Terriault, P. Design, Manufacture and Tensile Properties of Stochastic Porous Metallic Structures. Mech. Mater. 2016, 94, 26–37. [Google Scholar] [CrossRef]
- Phuoc, H.D.; Hoang, P.N.; Yang, S.; Fraser, D.; Nguyen, V.T. Osseointegrability of 3D-Printed Porous Titanium Alloy Implant on Tibial Shaft Bone Defect in Rabbit Model. PLoS ONE 2023, 18, e0282457. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Liu, M.; Shi, D.; Hu, N.; Feng, W. Mechanical Properties and In Vivo Assessment of Electron Beam Melted Porous Structures for Orthopedic Applications. Metals 2023, 13, 1034. [Google Scholar] [CrossRef]
- Wang, P.; Li, X.; Luo, S.; Nai, M.L.S.; Ding, J.; Wei, J. Additively Manufactured Heterogeneously Porous Metallic Bone with Biostructural Functions and Bone-like Mechanical Properties. J. Mater. Sci. Technol. 2021, 62, 173–179. [Google Scholar] [CrossRef]
- Wang, P.; Li, X.; Jiang, Y.; Nai, M.L.S.; Ding, J.; Wei, J. Electron Beam Melted Heterogeneously Porous Microlattices for Metallic Bone Applications: Design and Investigations of Boundary and Edge Effects. Addit. Manuf. 2020, 36, 101566. [Google Scholar] [CrossRef]
- Hernández-Nava, E.; Smith, C.J.; Derguti, F.; Tammas-Williams, S.; Léonard, F.; Withers, P.J.; Todd, I.; Goodall, R. The Effect of Density and Feature Size on Mechanical Properties of Isostructural Metallic Foams Produced by Additive Manufacturing. Acta Mater. 2015, 85, 387–395. [Google Scholar] [CrossRef]
- Habib, F.N.; Iovenitti, P.; Masood, S.H.; Nikzad, M. Fabrication of Polymeric Lattice Structures for Optimum Energy Absorption Using Multi Jet Fusion Technology. Mater. Des. 2018, 155, 86–98. [Google Scholar] [CrossRef]
- Ullah, A.M.M.S.; D’addona, D.M.; Seto, Y.; Yonehara, S.; Kubo, A. Utilizing Fractals for Modeling and 3d Printing of Porous Structures. Fractal Fract. 2021, 5, 40. [Google Scholar] [CrossRef]
- Sharif Ullah, A.M.M. Design for Additive Manufacturing of Porous Structures Using Stochastic Point-Cloud: A Pragmatic Approach. Comput. Aided Des. Appl. 2018, 15, 138–146. [Google Scholar] [CrossRef]
- Jetté, B.; Brailovski, V.; Dumas, M.; Simoneau, C.; Terriault, P. Femoral Stem Incorporating a Diamond Cubic Lattice Structure: Design, Manufacture and Testing. J. Mech. Behav. Biomed. Mater. 2018, 77, 58–72. [Google Scholar] [CrossRef] [PubMed]
- du Plessis, A.; Razavi, S.M.J.; Benedetti, M.; Murchio, S.; Leary, M.; Watson, M.; Bhate, D.; Berto, F. Properties and Applications of Additively Manufactured Metallic Cellular Materials: A Review. Prog. Mater. Sci. 2022, 125, 100918. [Google Scholar] [CrossRef]
- Yang, N.; Tian, Y.; Zhang, D. Novel Real Function Based Method to Construct Heterogeneous Porous Scaffolds and Additive Manufacturing for Use in Medical Engineering. Med. Eng. Phys. 2015, 37, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Piros, A.; Trautmann, L. Creating Interior Support Structures with Lightweight Voronoi Scaffold. Int. J. Interact. Des. Manuf. 2023, 17, 93–101. [Google Scholar] [CrossRef]
- Bici, M.; Campana, F.; Mancini, E.; Pilone, D.; Sasso, M. Mesoscale Modeling of Aluminum Foams for Fea of Scattering Effects Due to Cell Distribution. Comput. Aided Des. Appl. 2021, 18, 1296–1305. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, Z.; Zhou, Y.; Guo, Y. A Novel Complex Network-Based Modeling Method for Heterogeneous Product Design. Clust. Comput. 2019, 22, 7861–7872. [Google Scholar] [CrossRef]
- Abdullahi, H.S.; Liang, Y.; Gao, S. Predicting the Elastic Properties of Closed-Cell Aluminum Foams: A Mesoscopic Geometric Modeling Approach. SN Appl. Sci. 2019, 1, 380. [Google Scholar] [CrossRef]
- Alsheghri, A.; Reznikov, N.; Piché, N.; McKee, M.D.; Tamimi, F.; Song, J. Optimization of 3D Network Topology for Bioinspired Design of Stiff and Lightweight Bone-like Structures. Mater. Sci. Eng. C 2021, 123, 112010. [Google Scholar] [CrossRef]
- Gómez, S.; Vlad, M.D.; López, J.; Fernández, E. Design and Properties of 3D Scaffolds for Bone Tissue Engineering. Acta Biomater. 2016, 42, 341–350. [Google Scholar] [CrossRef]
- Homberg, U.; Baum, D.; Prohaska, S.; Günster, J.; Krauß-Schüler, S. Adapting Trabecular Structures for 3D Printing: An Image Processing Approach Based on ΜcT Data. Biomed. Phys. Eng. Express 2017, 3, 035027. [Google Scholar] [CrossRef]
- Bodla, K.K.; Garimella, S.V.; Murthy, J.Y. 3D Reconstruction and Design of Porous Media from Thin Sections. Int. J. Heat Mass. Transf. 2014, 73, 250–264. [Google Scholar] [CrossRef]
- Long, K.; Chen, Z.; Zhang, C.; Yang, X.; Saeed, N. An Aggregation-free Local Volume Fraction Formulation for Topological Design of Porous Structure. Materials 2021, 14, 5726. [Google Scholar] [CrossRef]
- Rahmat, N.; Kadkhodapour, J.; Arbabtafti, M. Mechanical Characterization of Additively Manufactured Orthopedic Cellular Implants: Case Study on Different Cell Types and Effect of Defects. Phys. Mesomech. 2023, 26, 443–458. [Google Scholar] [CrossRef]




| Cell | Location | Size | Function |
|---|---|---|---|
| OSTEOCYTES | Cortical bone | 7–15 µm | Coordinates the bone remodelling process at all levels |
| OSTEOBLAST | Periosteum, Endosteum | 20–30 µm | Bone tissue formation and maintenance |
| OSTEOCLAST | Trabecular surface, cortical endosteum | 100 µm | Bone resorption |
| LIMITING CELLS | Endosteum surface | 1–2 µm | Activates bone remodelling |
| Keywords (“AND”) | Results |
|---|---|
| AM, bone | 5713 |
| AM, bone, bioprinting | 31 |
| AM, bone, bioprinting, auto generative | 0 |
| AM, bone, bioprinting, organic structure | 0 |
| AM, bone, bioprinting, non-lattice | 0 |
| AM, bone, bioprinting, microextrusion | 1 |
| AM, bone, bioprinting, 3D | 29 |
| AM, bone, bioprinting, generative design | 0 |
| AM, bioprinting | 150 |
| AM, bioprinting, microextrusion | 3 |
| AM, bioprinting, microextrusion, bone | 1 |
| AM, organic structures | 10 |
| Non-lattice, bioprinting | 0 |
| Non-lattice, AM | 19 |
| Lattice, bioprinting | 71 |
| Category | Keywords |
|---|---|
| Organic structure | Bone-mimicking, foam, open-cell, porous structure, complex structure. |
| 3D printing | Additive Manufacturing, bioprinting, 3D printing, FDM, geometric modelling. |
| Non-regular | Bone-like, no-lattice, non-regular, modelling approach, non-parametric design, network-based, graph-based, inhomogeneous structure, irregular internal morphology. |
| Inclusion | Exclusion |
|---|---|
| IC1: Scientific articles. IC2: 3D porous structures are designed. | EC1: Abstracts, reviews or conference proceedings. EC2: Porous structures are not designed. EC3: Porous structures are not generated by the authors. |
| Category | Field | Research Question | Extraction Rate |
|---|---|---|---|
| Reference information |
| - | - |
| Manufacturing |
| RQ3 RQ3 RQ3 RQ2 - | 100% 98.4% 97.7% 100% 100% |
| Infill design |
| RQ1 RQ1 RQ2 | 100% 100% 100% |
| Procedure |
| RQ2 and RQ4 RQ2 and RQ4 RQ2 and RQ4 RQ4 RQ4 RQ4 | 100% 62% 100% 100% 100% 100% |
| Family | Method | Articles |
|---|---|---|
| Vat polymerization (20) | Twp-Photon Polymerization (2PP) (1) | [16] |
| Digital Light Processing (DLP) (7) | [17,18,19,20,21,22,23] | |
| Direct Laser Writing (DLW) (1) | [24] | |
| Initiator Integrated 3D printing (i3DP) (1) | [25] | |
| Stereolithography (SLA) (9) | [26,27,28,29,30,31,32,33,34] | |
| Projection micro stereolithography (PµSC) (1) | [35] | |
| Material jetting (4) | Binder jetting (2) | [36,37] |
| Ink-jetting (2) | [38,39] | |
| Material extrusion (37) | Fused Deposition Modelling (FDM) (16) | [40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55] |
| Fused Filament Fabrication (FFF) (10) | [56,57,58,59,60,61,62,63,64,65] | |
| Extrusion (6) | [66,67,68,69,70,71] | |
| µExtrusion (4) | [72,73,74,75] | |
| Melt Electrowriting (MEW) (1) | [76] | |
| Powder bed fusion (44) | Direct Metal Laser Sintering (DMLS) (2) | [77,78] |
| Laser Powder Bed Fusion (LPBF) (16) | [79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94] | |
| Selective Laser Sintering (SLS) (12) | [95,96,97,98,99,100,101,102,103,104,105,106] | |
| Selective Laser Melting (SLM) (8) | [107,108,109,110,111,112,113,114] | |
| Electron Beam Melting (EBM) (5) | [115,116,117,118,119] | |
| Multi Jet Fusion (MJF) (1) | [120] | |
| Not specified (2) | [121,122] |
| Method | Description | Advantages | Disadvantages |
|---|---|---|---|
| Vat polymerization | Uses a UV light source to cure and solidify a photopolymer resin layer by layer. | Ideal for structures with fine features. High detail and smooth surfaces. | Restricted to photopolymer resins. Intricate post-processing. |
| Material jetting | Deposits droplets of photopolymer material layer by layer, which are then cured using UV light. | Material versatility. Efficient for producing complex structures relatively quickly. | Parts may require additional post-processing. Lower resolution and rougher finish. |
| Material extrusion | Involves pushing material through a heated nozzle to form layers | Low-cost production. Ease of use. | May require additional support structures. Rougher surface finish. |
| Powder bed fusion | Uses a laser or electron beam to fuse powdered material layer by layer. | Exceptional mechanical properties. No additional support structures needed. | Significant post-processing required. Very high cost. |
| Method | Biocompatibility |
|---|---|
| Vat polymerization | Potentially harmful to living cells when using UV light and cytotoxic photoinitiators for curing and solidifying processes. |
| Material jetting | The conditions that the living cells are exposed to during the ejection can be harmful and incompatible with living cells. |
| Material extrusion | Suitable for printing living cells as it operates at room or body temperature. Can use hydrogels and bio-inks that provide a supportive environment for cell growth and differentiation. High-viscosity materials require high pressures to extrude, which can be harmful for the living cells. |
| Powder bed fusion | The high temperatures used in fusing powders are incompatible with living cells. The powder used is not compatible with cell culture. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernadi-Forteza, A.; Mallon, M.; Velasco-Gallego, C.; Cubo-Mateo, N. A Systematic Review on the Generation of Organic Structures through Additive Manufacturing Techniques. Polymers 2024, 16, 2027. https://doi.org/10.3390/polym16142027
Bernadi-Forteza A, Mallon M, Velasco-Gallego C, Cubo-Mateo N. A Systematic Review on the Generation of Organic Structures through Additive Manufacturing Techniques. Polymers. 2024; 16(14):2027. https://doi.org/10.3390/polym16142027
Chicago/Turabian StyleBernadi-Forteza, Alex, Michael Mallon, Christian Velasco-Gallego, and Nieves Cubo-Mateo. 2024. "A Systematic Review on the Generation of Organic Structures through Additive Manufacturing Techniques" Polymers 16, no. 14: 2027. https://doi.org/10.3390/polym16142027
APA StyleBernadi-Forteza, A., Mallon, M., Velasco-Gallego, C., & Cubo-Mateo, N. (2024). A Systematic Review on the Generation of Organic Structures through Additive Manufacturing Techniques. Polymers, 16(14), 2027. https://doi.org/10.3390/polym16142027









