Synthesis and Characterization of ZnO and TiO2 Hybrid Coatings for Textile UV Anti-Aging Protection
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Textile Materials
2.2. Preparation of Sols and Hybrid Materials
2.3. Instrumental Methods
3. Results
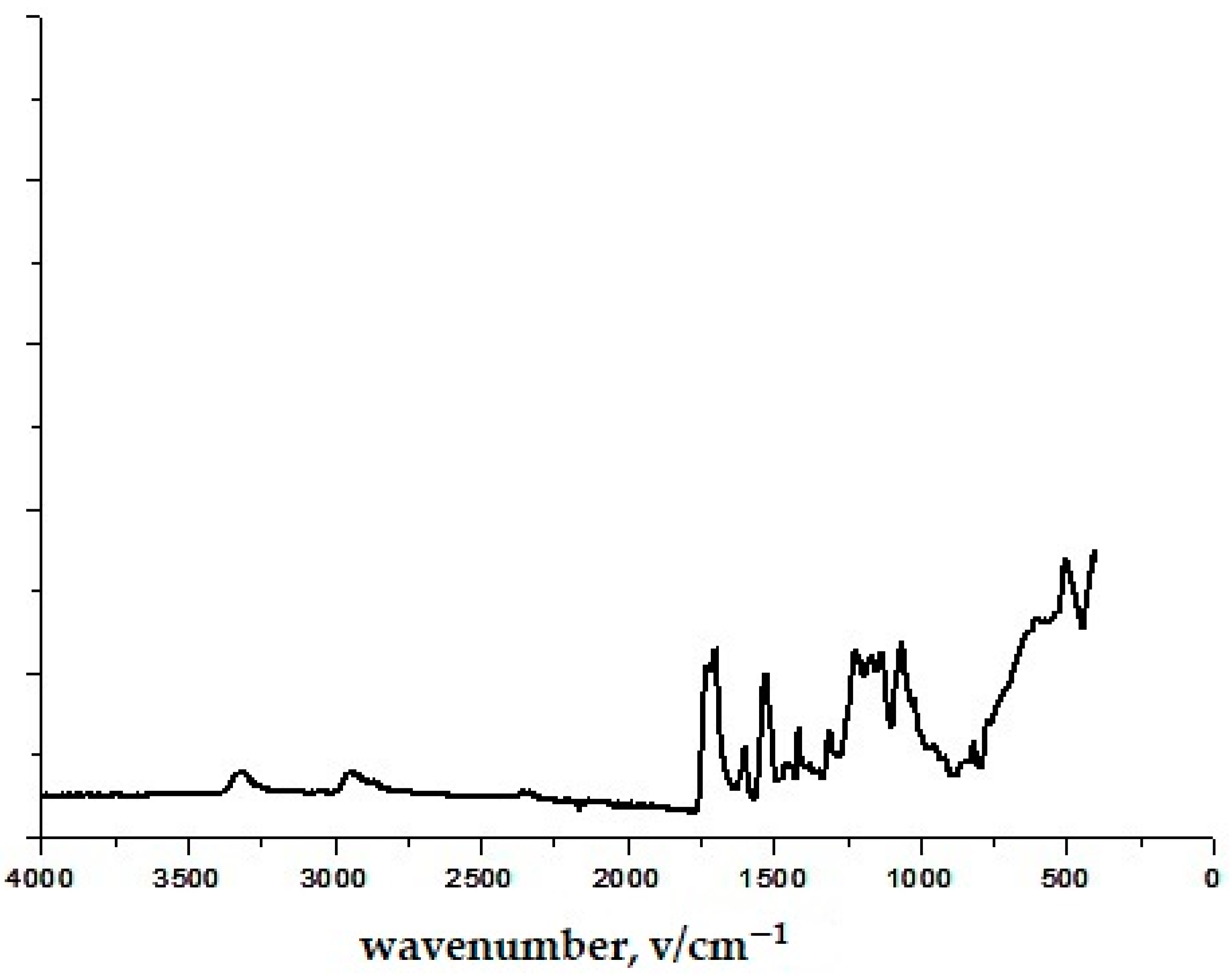
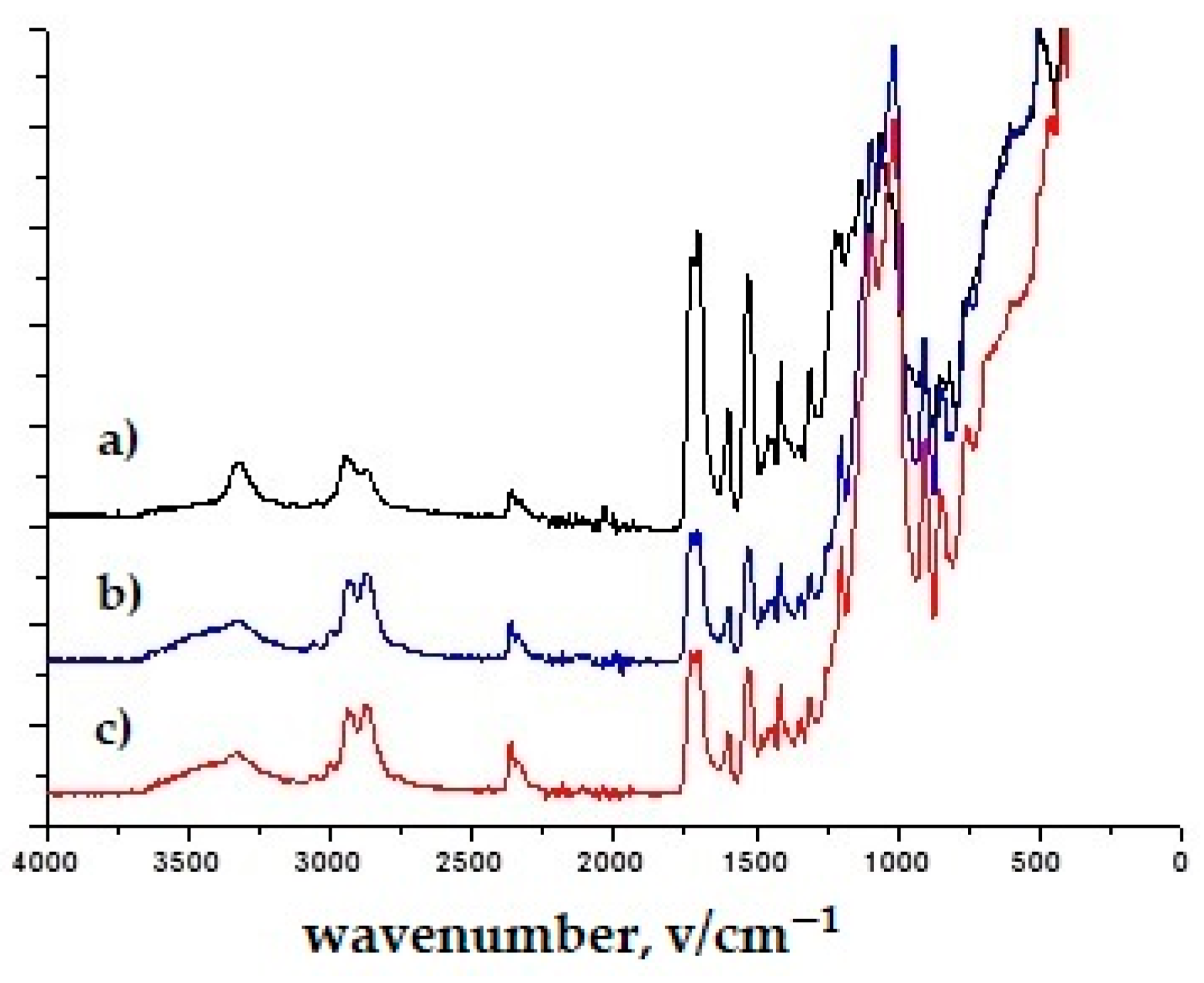
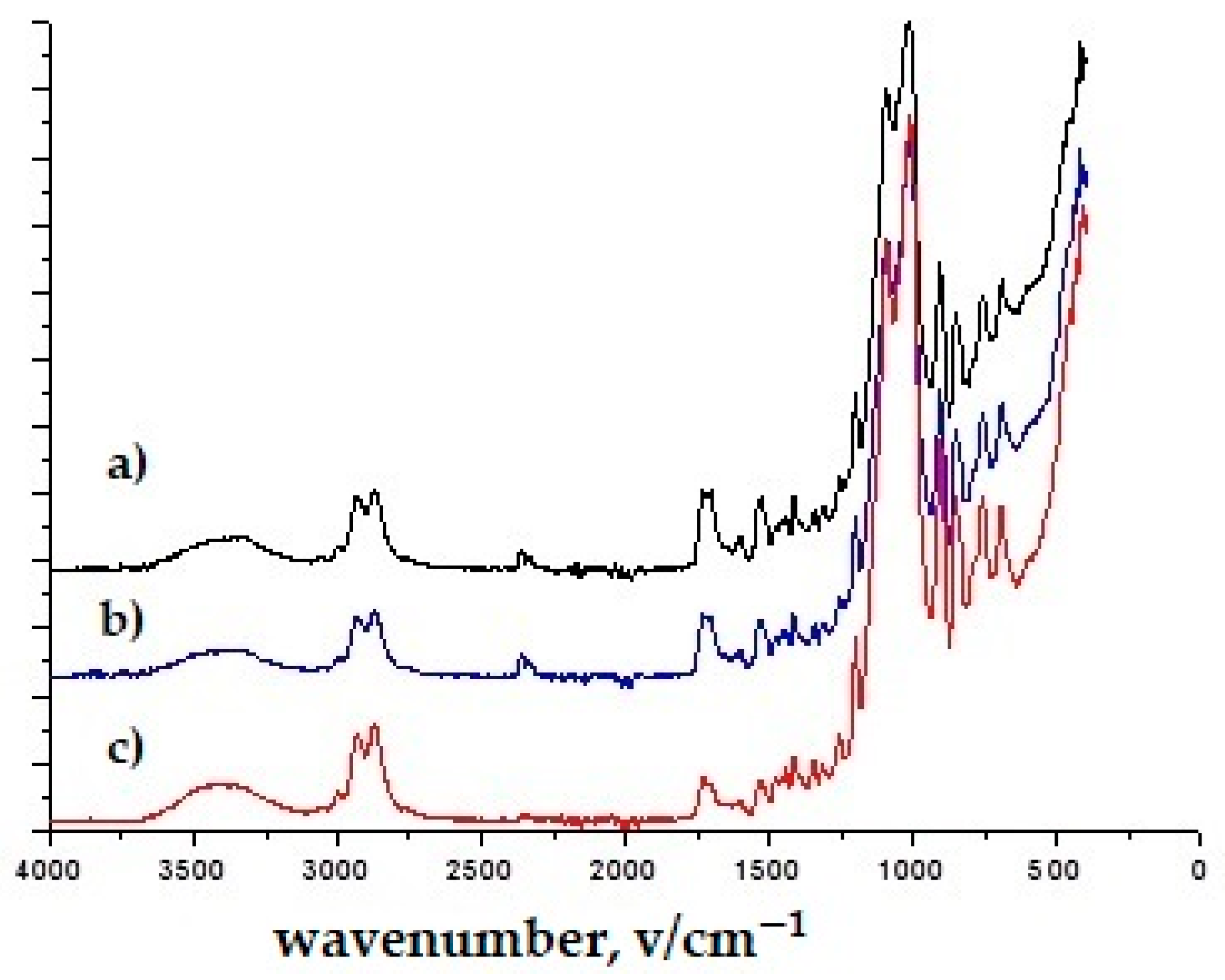
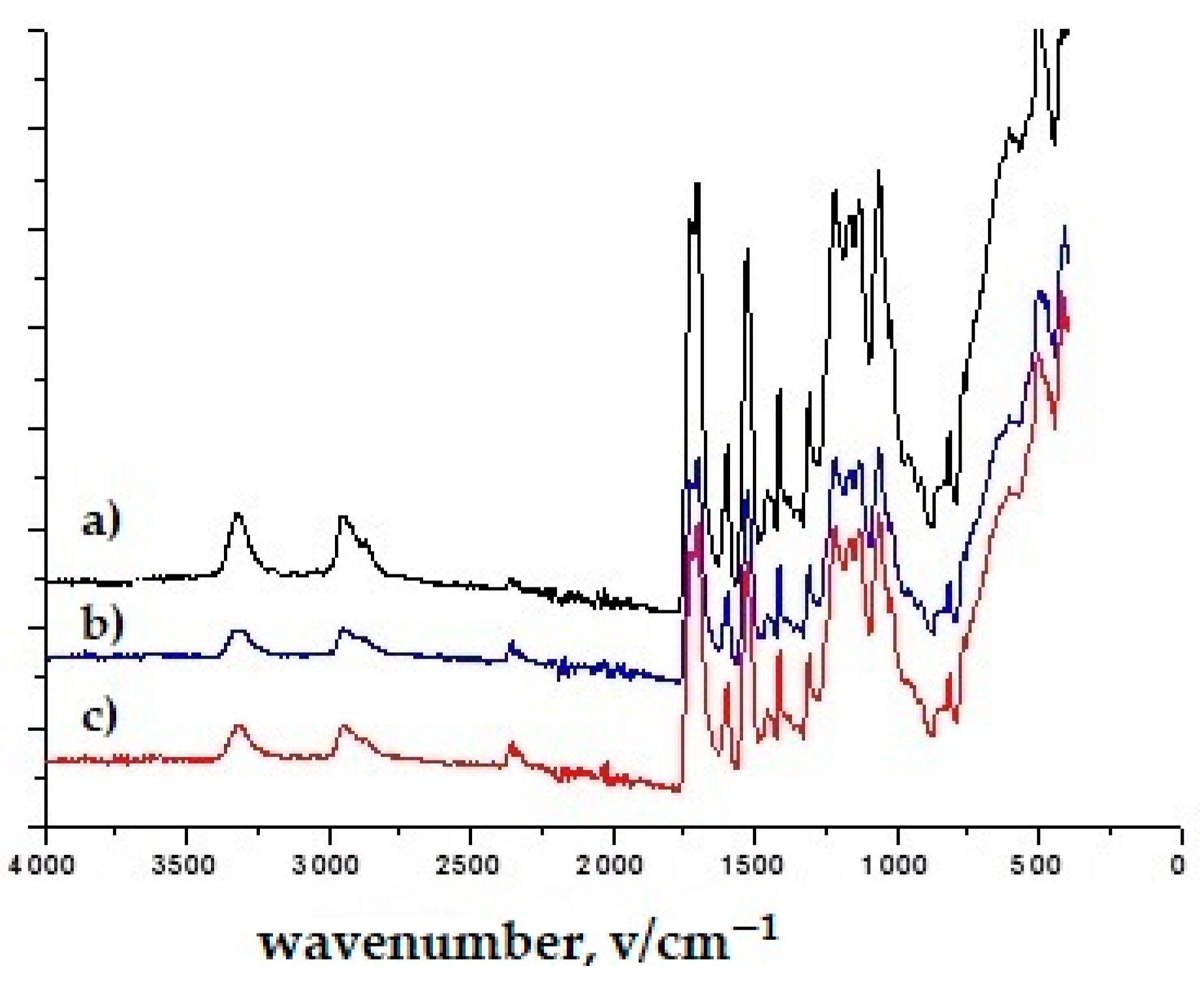
3.1. Results of Infrared Spectroscopy
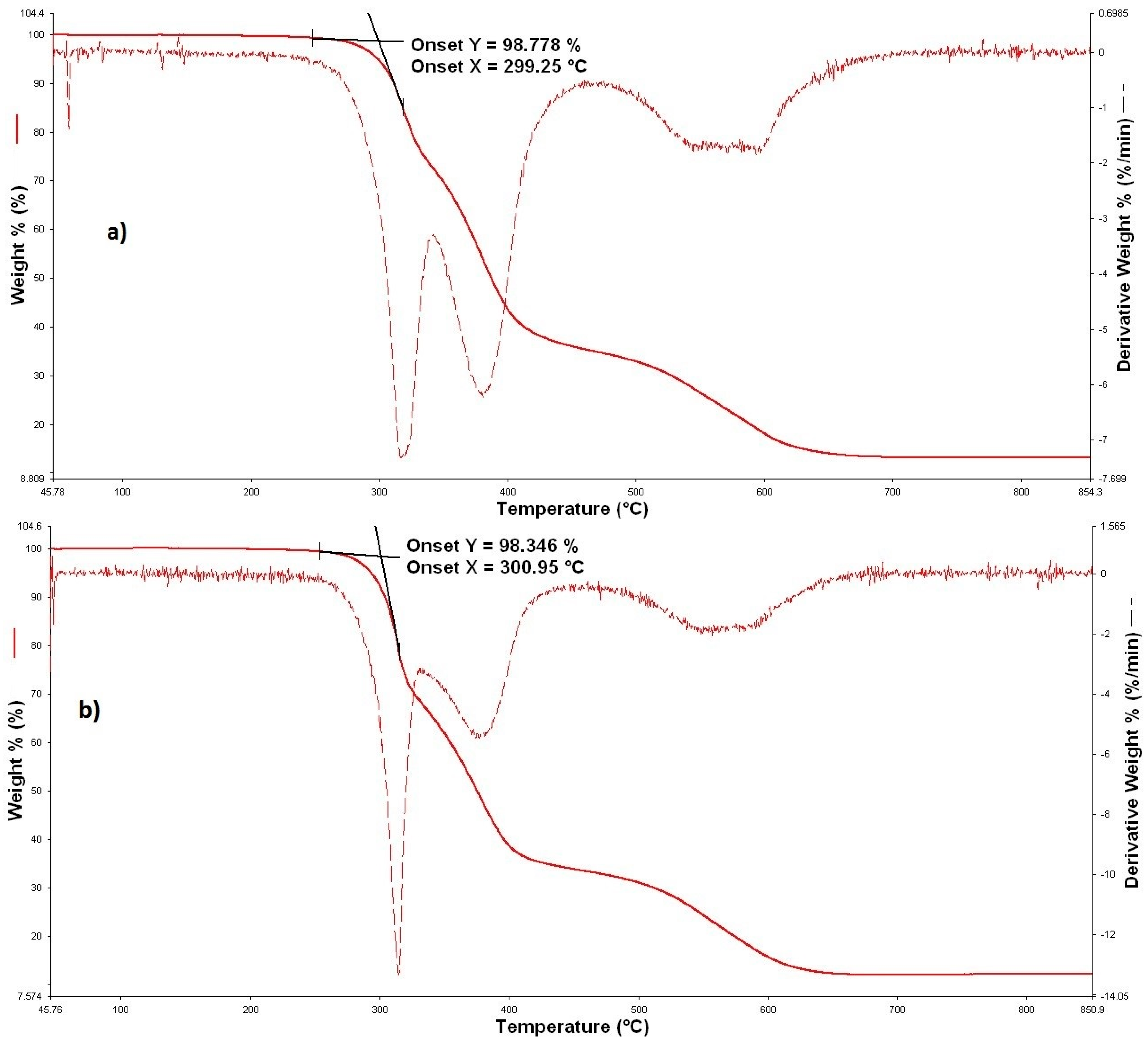
3.2. Thermal Properties
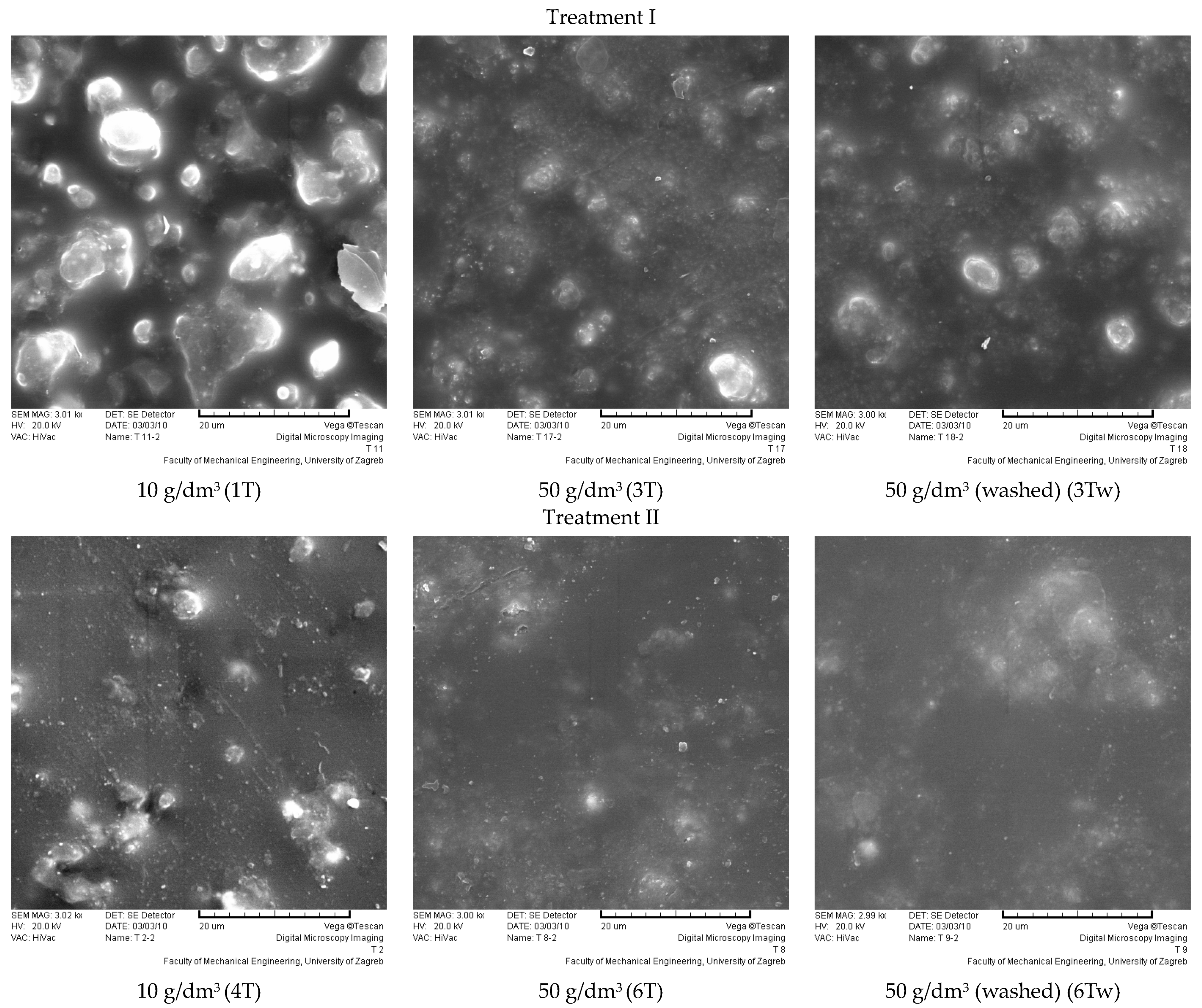
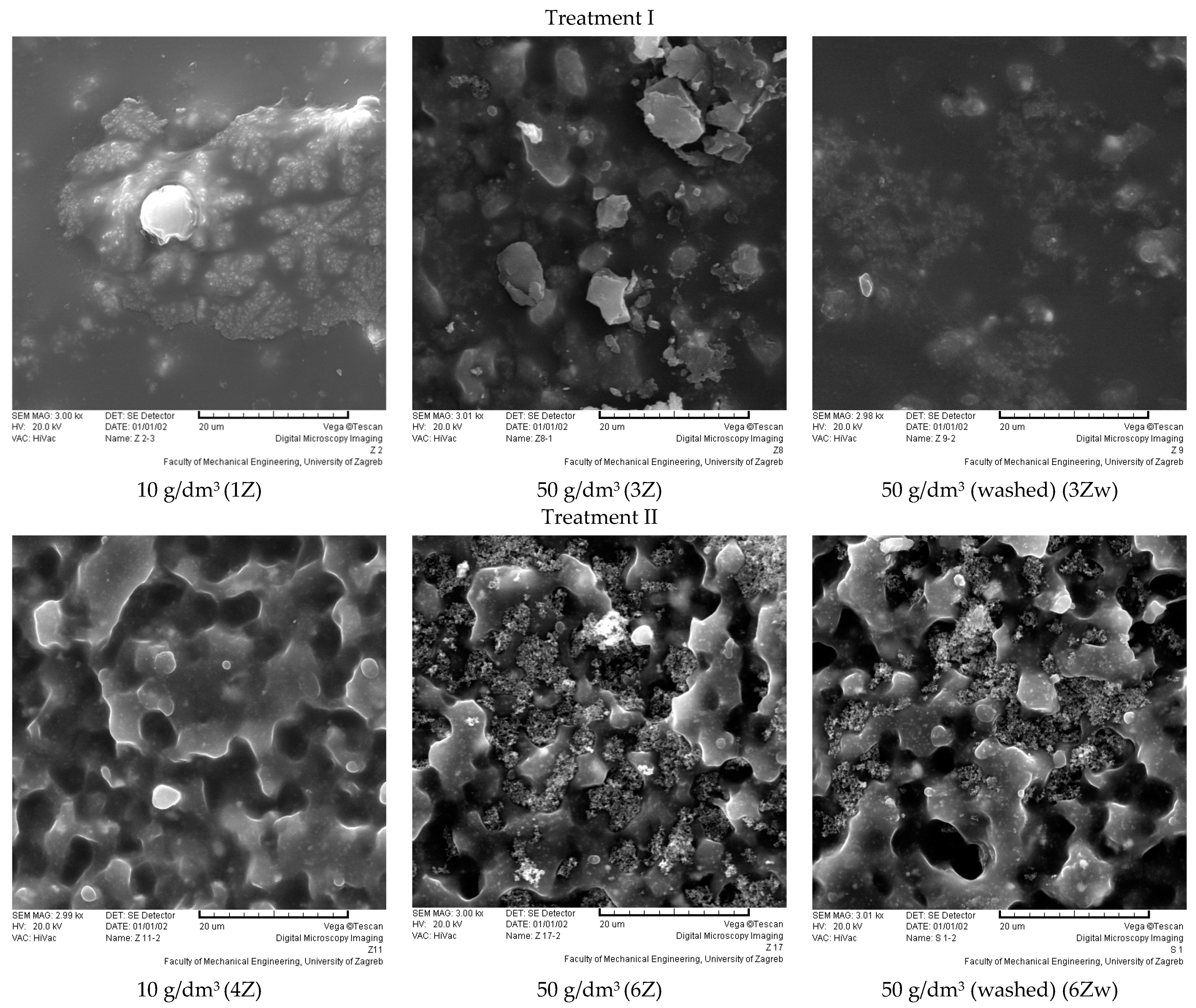
3.3. Results of the Surface Structure and Morphological Characteristics
3.4. Results of Color Fastness
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Davies, P.; Evrard, G. Accelerated ageing of polyurethanes for marine applications. Polym. Degrad. Stab. 2007, 92, 1455–1464. [Google Scholar] [CrossRef]
- Li, G.; Duan, B.; Leng, G.; Liu, J.; Zhang, T.; Lu, Z.; Wang, S.; Qu, J. Preparation of Yellowing-Resistant Waterborne Polyurethane Modified with Disulfide Bonds. Molecules 2024, 29, 2099. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, M.; Krasowska, K.; Heimowska, A.; Steinka, I.; Janik, H. Degradation of polyurethanes in sea water. Polym. Degrad. Stab. 2002, 76, 233–239. [Google Scholar] [CrossRef]
- Aquino, F.; Coutinho, F.M.; Sheldrake, T.H.; Clevelario, J.D.; Pires, F.D. Study of the ageing of polyurethanes in general applications for UV radiation. In Proceedings of the Materials Science Anais do 10o Congresso Brasileiro de Polímeros—Foz do Iguaçu, São Carlos, Brazil, 13–17 October 2009; Available online: https://api.semanticscholar.org/CorpusID:115143325 (accessed on 7 July 2024).
- Mayer-Trzaskowska, P.; Robakowska, M.; Gierz, Ł.; Pach, J.; Mazur, E. Observation of the Effect of Aging on the Structural Changes of Polyurethane/Polyurea Coatings. Polymer 2024, 16, 23. [Google Scholar] [CrossRef] [PubMed]
- Potočić Matković, V.M.; Skenderi, Z. Influence of Modification on the Structural and Tensile Properties of Polyurethane Coated Knitted Fabrics after Natural Weathering. Fibres Text. East. Eur. 2017, 25, 89–94. [Google Scholar] [CrossRef]
- Costantini, R.; Nodari, L.; La Nasa, J.; Modugno, F.; Bonasera, L.; Rago, S.; Zoleo, A.; Legnaioli, S.; Tomasin, P. Preserving the Ephemeral: A Micro-Invasive Study on a Set of Polyurethane Scenic Objects from the 1960s and 1970s. Polymers 2023, 15, 2111. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.G.; Osborn, J.C.; Kober, E.M.; Schoonover, J.R. Effects of hydrolysis-induced molecular weight changes on the phase separation of a polyester polyurethane. Polym. Degrad. Stab. 2006, 91, 3360–3370. [Google Scholar] [CrossRef]
- Somogyi Škoc, M.; Macan, J.; Pezelj, E. Modification of polyurethane-coated fabrics by sol-gel thin films. J. Appl. Polym. Sci. 2014, 131, 39914-1–39914-13. [Google Scholar] [CrossRef]
- Mahltig, B.; Textor, T. Nanosols and Textiles; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2008. [Google Scholar]
- Puoci, F.; Saturnino, C.; Trovato, V.; Iacopetta, D.; Piperopoulos, E.; Triolo, C.; Bonomo, M.G.; Drommi, D.; Parisi, O.I.; Milone, C.; et al. Sol–Gel Treatment of Textiles for the Entrapping of an Antioxidant/Anti-Inflammatory Molecule: Functional Coating Morphological Characterization and Drug Release Evaluation. Appl. Sci. 2020, 10, 2287. [Google Scholar] [CrossRef]
- Yaman, N. Preparation and flammability properties of hybrid materials containing phosphorous compounds via sol-gel process. Fibers Polym. 2009, 10, 413–418. [Google Scholar] [CrossRef]
- Zeljko, M.; Ocelić Bulatović, V.; Špada, V.; Blagojević, S.L. Environmentally Friendly UV-Protective Polyacrylate/TiO2 Nanocoatings. Polymers 2021, 13, 2609. [Google Scholar] [CrossRef] [PubMed]
- Hanif, M.A.; Shin, H.; Chun, D.; Kim, H.G.; Kwac, L.K.; Han, S.-W.; Kang, S.-S.; Kim, Y.S. Development of Highly Ultraviolet-Protective Polypropylene/TiO2 Nonwoven Fiber. J. Compos. Sci. 2024, 8, 86. [Google Scholar] [CrossRef]
- Mahltig, B.; Fiedler, D.; Fischer, A.; Simon, P. Antimicrobial coatings on textiles-modification of sol-gel layers with organic and inorganic biocides. J. Sol-Gel Sci. Technol. 2010, 55, 269–277. [Google Scholar] [CrossRef]
- Farouk, A.; Textor, T.; Schollmeyer, E.; Tarbuk, A.; Grancarić, A.M. Sol-gel derived inorganic-anorganic hybrid polymers filled with ZnO nanoparticles as ultraviolet protection finish for textiles. AUTEX Res. J. 2009, 9, 114–120. [Google Scholar] [CrossRef]
- Mahltig, B.; Knittel, D.; Schollmeyer, E.; Böttcher, H. Incorporation of Triarylmethane Dyes into Sol-Gel Matrices Deposited on Textiles. J. Sol-Gel Sci. Technol. 2004, 31, 293–297. [Google Scholar] [CrossRef]
- Mahtlig, B.; Böttcher, H.; Rauch, K.; Dieckmann, U.; Nitsche, R.; Fritz, T. Optimized UV protecting coatings by combination of organic and inorganic UV absorbers. Thin Solid Film 2005, 485, 108–114. [Google Scholar] [CrossRef]
- Foksowicz-Flaczyk, J.; Walentowska, J.; Przybylak, M.; Maciejewski, H. Multifunctional durable properties of textile materials modified by biocidal agents in the sol-gel process. Surf. Coat. Technol. 2016, 304, 160–166. [Google Scholar] [CrossRef]
- Berendjchi, A.; Khajavi, R.; Yazdanshenas, M.E. Fabrication of superhydrophobic and antibacterial surface on cotton fabric by doped silica-based sols with nanoparticles of copper. Nanoscale Res. Lett. 2011, 6, 594. [Google Scholar] [CrossRef]
- Tarimala, S.; Kothari, N.; Abidi, N.; Hequet, E.; Fralick, J.; Dai, L.L. New Approach to Antibacterial Treatment of Cotton Fabric with Silver Nanoparticle–Doped Silica Using Sol–Gel Process. J. Appl. Polym. Sci. 2006, 101, 2938–2943. [Google Scholar] [CrossRef]
- Shaker, S.; Zafarian, S.; Chakra, C.S.; Rao, K.V. Preparation and characterization of magnetite nanoparticles by sol-gel method for water treatment. Int. J. Innov. Res. Sci. Eng. Technol. 2013, 2, 2969–2973. [Google Scholar]
- Vihodceva, S.; Kukle, S. Thin Coatings on the Raw Cotton Textile Deposited by the Sol-Gel Method. Mater. Sci. Text. Cloth. Technol. 2012, 7, 69–73. [Google Scholar]
- Khan, M.Z.; Taghavian, H.; Fijalkowski, M.; Militky, J.; Tomkova, B.; Venkataraman, M.; Adach, K. Effect of microwave power on bactericidal and UV protection properties of the ZnO nanorods grown cotton fabrics. Colloids Surf. A Physicochem. Eng. Asp. 2023, 664, 131135. [Google Scholar] [CrossRef]
- Saleemi, S.; Malik, S.A.; Syed, U.; Tanwari, A. Investigation of Wash Durability of Silica Nanoparticle Coated 100% Cotton Reactive Dyed Fabric Treated by Sol-Gel Technique. J. Eng. Fibers Fabr. 2014, 9, 16–24. [Google Scholar] [CrossRef]
- Montarsolo, A.; Periolatto, M.; Zerbola, M.; Mossotti, R.; Ferrero, F. Hydrophobic sol-gel finishing for textiles: Improvement by plasma pre-treatment. Text. Res. J. 2013, 83, 1190–1200. [Google Scholar] [CrossRef]
- Pellegrini, C.; Duluard, S.; Gressier, M.; Turq, V.; Ansart, F.; Menu, M.-J. Development of Multifunctional Hybrid Coatings (Mechanically Resistant and Hydrophobic) Using Methyltrimethoxysilane–Diethoxydimethylsilane–Tetraethoxysilane Mixed Systems. Materials 2024, 17, 368. [Google Scholar] [CrossRef] [PubMed]
- Malucelli, G. Sol-Gel and Layer-by-Layer Coatings for Flame-Retardant Cotton Fabrics: Recent Advances. Coatings 2020, 10, 333. [Google Scholar] [CrossRef]
- Periolatto, M.; Ferrero, F. Cotton and polyester surface modification by methacrylic silane and fluorinated alkoxysilane via sol–gel and UV-curing coupled process. Surf. Coat. Technol. 2015, 271, 165–173. [Google Scholar] [CrossRef]
- Raditoiu, A.; Raditoiu, V.; Raduly, M.F.; Gabor, A.R.; Frone, A.N.; Grapin, M.; Anastasescu, M. Cellulose Fabrics Functionalized with Sol–Gel Photocatalytic Coatings Based on Iron (III) Phthalocyanine Tetracarboxylic Acids–TiO2–Silica Hybrids. Gels 2023, 9, 860. [Google Scholar] [CrossRef] [PubMed]
- Martinaga Pintarić, L.; Somogi Škoc, M.; Ljoljić Bilić, V.; Pokrovac, I.; Kosalec, I.; Rezić, I. Synthesis, Modification and Characterization of Antimicrobial Textile Surface Containing ZnO Nanoparticles. Polymers 2020, 12, 1210. [Google Scholar] [CrossRef]
- Onar, N.; Mete, G.; Aksit, A.; Kutlu, B.; Celik, E. Water- and Oil-Repellency Properties of Cotton Fabric Treated with Silane, Zr, Ti based Nanosols. Int. J. Text. Sci. 2015, 4, 84–96. [Google Scholar] [CrossRef]
- Rezić, I.; Somogyi Škoc, M.; Majdak, M.; Jurić, S.; Stracenski, K.S.; Vinceković, M. Functionalization of Polymer Surface with Antimicrobial Microcapsules. Polymers 2022, 14, 1961. [Google Scholar] [CrossRef] [PubMed]
- Matějka, L.; Dukh, O.; Brus, J.; Simonsick, W.J., Jr.; Meissner, B. Cage-like structure formation during sol–gel polymerization of glycidyloxypropyltrimethoxysilane. J. Non-Cryst. Solid 2000, 270, 34–47. [Google Scholar] [CrossRef]
- Somogyi Škoc, M.; Pezelj, E. Properties and Preparation of Resistant Polyurethane-coated Fabrics to Ageing by Sol-Gel Thin Films. In Proceedings of the Conference Proceedings International Symposium on New Frontiers in Fiber Materials Science, Charleston, SC, USA, 11–13 October 2011. [Google Scholar]
- Test Method: SATRA TM352: Distinguishing between Types of Polyurethane. Available online: https://www.satra.com/test_methods/detail.php?id=263 (accessed on 19 January 2024).
- ISO 2286-2:2016; Rubber- or Plastics-Coated Fabrics—Determination of Roll Characteristics, Part 2: Methods for Determination of Total Mass per Unit Area, Mass per Unit Area of Coating and Mass per Unit Area of Substrate. International Organization for Standardization: Geneva, Switzerland, 2016.
- Agresti, A.; Di Giacomo, F.; Pescetelli, S.; Di Carlo, A. Scalable deposition techniques for large-area perovskite photovoltaic technology: A multi-perspective review. Nano Energy 2024, 122, 109317. [Google Scholar] [CrossRef]
- ISO 6330:2021; Textiles—Domestic Washing and Drying Procedures for Textile Testing. International Organization for Standardization: Geneva, Switzerland, 2021.
- ISO 105-B02:2014; Textiles—Tests for Colour Fastness, Part B02: Colour Fastness to Artificial Light: Xenon Arc Fading Lamp Test. International Organization for Standardization: Geneva, Switzerland, 2014.
- Clift, S.M.; Grimminger, J.; Muha, K. New Polyisocyanurate Catalysts for Rigid Polyurethane Foams. Air Prod. Chem. 1994, 112, 10–15. [Google Scholar]
- Smith, B.C. Infrared Spectroscopy of Polymers XIII: Polyurethanes. Spectroscopy 2023, 38, 14–16. [Google Scholar] [CrossRef]
- Gabardo, R.S.; de Carvalho Cotre, D.S.; Lis Arias, M.J.; Moisés, M.P.; Martins Ferreira, B.T.; Samulewski, R.B.; Hinestroza, J.P.; Bezerra, F.M. Surface Modification of Polyester Fabrics by Ozone and Its Effect on Coloration Using Disperse Dyes. Materials 2021, 14, 3492. [Google Scholar] [CrossRef] [PubMed]
- Godnjavec, J.; Znoj, B.; Vince, J.; Steinbucher, M.; Venturini, P. Stabilization of rutile TiO2 nanoparticles with GLYMO in polyacrylic clear coating. Mater. Technol. 2012, 46, 19–24. [Google Scholar]
- Uysal, I.; Severcan, F.; Evis, Z. Characterization by Fourier transform infrared spectroscopy of hydroxyapatite co-doped with zinc and fluoride. Ceram. Int. 2013, 39, 7727–7733. [Google Scholar] [CrossRef]
- Berendjchi, A.; Khajavi, R.; Yazdanshenas, M.E. Application of Nanosols in Textile Industry. Int. J. Green Nanotechnol. 2013, 1, 1–7. [Google Scholar] [CrossRef]
- Periyasamy, A.P.; Venkataraman, M.; Kremenakova, D.; Militky, J.; Zhou, Y. Progress in Sol-Gel Technology for the Coatings of Fabrics. Materials 2020, 13, 1838. [Google Scholar] [CrossRef]
- Rabiei, H.; Farhang Dehghan, S.; Montazer, M.; Khaloo, S.S.; Koozekonan, A.G. UV protection properties of workwear fabrics coated with TiO2 nanoparticles. Front. Public Health 2022, 10, 929095. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S. Nanomaterials for UV protective textiles. J. Ind. Text. 2022, 51 (Suppl. S4), 5592S–5621S. [Google Scholar] [CrossRef]
- Sfameni, S.; Hadhri, M.; Rando, G.; Drommi, D.; Rosace, G.; Trovato, V.; Plutino, M.R. Inorganic Finishing for Textile Fabrics: Recent Advances in Wear-Resistant, UV Protection and Antimicrobial Treatments. Inorganics 2023, 11, 19. [Google Scholar] [CrossRef]
- Sasani Ghamsari, M.; Alamdari, S.; Han, W.; Park, H.H. Impact of nanostructured thin ZnO film in ultraviolet protection. Int. J. Nanomed. 2016, 12, 207–216. [Google Scholar] [CrossRef] [PubMed]




| Treatments | |||||
|---|---|---|---|---|---|
| ↙ | ↘ | ||||
| Treatment I GLYMO hydrolized with pure water GLYMO:water ratio of 1:3 | Treatment II GLYMO hydrolized with catalyst (water, ethanol 1.6 mol/L, hydrochloric acid 0.1 mol/L) GLYMO:water ratio of 1:1.5 | ||||
| ↙ + TiO2 (10 g/dm3) | ↓ + TiO2 (20 g/dm3) | ↘ + TiO2 (50 g/dm3) | ↙ + TiO2 (10 g/dm3) | ↓ + TiO2 (20 g/dm3) | ↘ + TiO2 (50 g/dm3) |
| ↓ continuous magnetic stirring 20 ± 2 °C processing time 1 h | |||||
| ↓ samples 5 × 5 cm in size → dip-coated Teflon pans drawing speed of 0.5 mm/s, 1 mm/s, 1.5 mm/s, 2 mm/s | |||||
| ↓ modified samples left to gel at room temperature for 24 h dried at 100 °C for 1 h | |||||
| ↓ FITR-ATR SEM-EDS TGA Xenon arc lamp test (EN ISO 105-B02) → colour fastness (blue wool scales and Spectraflash SF 300) modified samples with the highest mass concentration → durability during washing (EN ISO 6330) | |||||
| Code | Mass Concentration | Treatment |
|---|---|---|
| 0 | / | unmodified |
| I | / | Treatment I |
| II | / | Treatment II |
| 1T | 10 g/dm3 | Treatment I + TiO2 |
| 2T | 20 g/dm3 | Treatment I + TiO2 |
| 3T | 50 g/dm3 | Treatment I + TiO2 |
| 3Tw | 50 g/dm3 | Treatment I + TiO2—washed |
| 4T | 10 g/dm3 | Treatment II + TiO2 |
| 5T | 20 g/dm3 | Treatment II + TiO2 |
| 6T | 50 g/dm3 | Treatment II + TiO2 |
| 6Tw | 50 g/dm3 | Treatment II + TiO2—washed |
| 1Z | 10 g/dm3 | Treatment I + ZnO |
| 2Z | 20 g/dm3 | Treatment I + ZnO |
| 3Z | 50 g/dm3 | Treatment I + ZnO |
| 3Zw | 50 g/dm3 | Treatment I + ZnO—washed |
| 4Z | 10 g/dm3 | Treatment II + ZnO |
| 5Z | 20 g/dm3 | Treatment II + ZnO |
| 6Z | 50 g/dm3 | Treatment II + ZnO |
| 6Zw | 50 g/dm3 | Treatment II + ZnO—washed |
| Treatment | tonset (°C) | tmax,1 (°C) | tmax,2 (°C) | Residue (%) | ||
|---|---|---|---|---|---|---|
| Unmodified | 296.45 | 310 | / | 11 | ||
| TiO2 | Treatment I | 50 g/dm3 (3T) | 302.83 | 318 | 381 | 14 |
| 50 g/dm3—washed (3Tw) | 301.96 | 315 | / | 12 | ||
| Treatment II | 50 g/dm3 (6T) | 305.00 | 317 | / | 12 | |
| 50 g/dm3—washed (6Tw) | 306.47 | 319 | / | 11 | ||
| ZnO | Treatment I | 50 g/dm3 (3Z) | 300.09 | 330 | / | 15 |
| 50 g/dm3—washed (3Zw) | 306.25 | 328 | / | 14 | ||
| Treatment II | 50 g/dm3 (6Z) | 295.64 | 319 | / | 16 | |
| 50 g/dm3—washed (6Zw) | 299.05 | 319 | / | 14 | ||
| Sample | Element | (Weight%) | (Atomic%) | |
|---|---|---|---|---|
| Unmodified (0) | C | 59.11 | 68.15 | |
| O | 33.04 | 28.60 | ||
| Al | 4.39 | 2.25 | ||
| Ti | 3.46 | 1.00 | ||
| Treatment I | C | 55.35 | 64.97 | |
| O | 35.18 | 31.00 | ||
| Al | 2.15 | 1.12 | ||
| Si | 3.63 | 1.82 | ||
| Ti | 3.70 | 1.09 | ||
| Treatment II | C | 47.30 | 58.74 | |
| O | 33.65 | 31.37 | ||
| Si | 17.50 | 9.29 | ||
| Cl | 1.05 | 0.44 | ||
| Ti | 0.51 | 0.16 | ||
| ZnO Treatment I | 50 g/dm3 (3Z) | C | 45.21 | 56.85 |
| O | 41.92 | 39.58 | ||
| Si | 1.06 | 0.57 | ||
| Ti | 3.26 | 1.03 | ||
| Zn | 8.56 | 1.98 | ||
| 50 g/dm3 washed (3Zw) | C | 52.73 | 61.62 | |
| O | 39.01 | 34.22 | ||
| Al | 7.43 | 3.71 | ||
| Si | 0.35 | 0.21 | ||
| Cl | 0.34 | 0.18 | ||
| Ti | 0.13 | 0.05 | ||
| Zn | 0 | 0 | ||
| TiO2 Treatment II | 50 g/dm3 (6T) | C | 43.52 | 55.35 |
| O | 36.78 | 35.12 | ||
| Si | 14.43 | 7.85 | ||
| Ti | 5.27 | 1.68 | ||
| 50 g/dm3 washed (6Tw) | C | 44.05 | 54.96 | |
| O | 39.73 | 37.22 | ||
| Si | 12.24 | 6.53 | ||
| Cl | 0.48 | 0.20 | ||
| Ti | 3.50 | 1.09 | ||
| Treatment/Mass Concentration of ZnO, Drawing Speed | Change in Color | ||||
|---|---|---|---|---|---|
| Treatment I | Treatment II | ||||
| Gray Scale | ΔE | Gray Scale | ΔE | ||
| 10 g/dm3 | 0.5 mm/s | 6 | 3.16 | 6 | 3.76 |
| 1 mm/s | 6 | 3.61 | 6 | 4.22 | |
| 1.5 mm/s | 6 | 3.82 | 6 | 4.34 | |
| 2 mm/s | 6 | 3.66 | 6 | 4.13 | |
| 1 mm/s, washed | 6 | 3.90 | 6 | 4.00 | |
| 20 g/dm3 | 1 mm/s | 7 | 3.05 | 6 | 4.17 |
| 1 mm/s, washed | 7 | 2.74 | 6 | 3.72 | |
| 50 g/dm3 | 1 mm/s | 7 | 3.30 | 6 | 3.79 |
| 1 mm/s, washed | 7 | 1.86 | 6 | 3.38 | |
| Unmodified (producer additive): 4–5 (gray scale); 6.79 (ΔE) | |||||
| Treatment/Mass Concentration of ZnO, Drawing Speed | Change in Color | ||||
|---|---|---|---|---|---|
| Treatment I | Treatment II | ||||
| Gray Scale | ΔE | Gray Scale | ΔE | ||
| 10 g/dm3 | 0.5 mm/s | 6 | 3.70 | 6 | 3.67 |
| 1 mm/s | 6 | 3.68 | 6 | 3.95 | |
| 1.5 mm/s | 6 | 3.66 | 6 | 4.31 | |
| 2 mm/s | 6 | 3.81 | 6 | 4.41 | |
| 1 mm/s, washed | 6 | 3.41 | 6 | 3.76 | |
| 20 g/dm3 | 1 mm/s | 7 | 3.40 | 6 | 4.09 |
| 1 mm/s, washed | 7 | 2.92 | 6 | 3.38 | |
| 50 g/dm3 | 1 mm/s | 7 | 3.01 | 7 | 3.56 |
| 1 mm/s, washed | 7 | 1.51 | 7 | 2.25 | |
| Unmodified (producer additive): 4–5 (gray scale); 6.79 (ΔE) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somogyi Škoc, M.; Macan, J.; Jakovljević, S.; Rezić, I. Synthesis and Characterization of ZnO and TiO2 Hybrid Coatings for Textile UV Anti-Aging Protection. Polymers 2024, 16, 2001. https://doi.org/10.3390/polym16142001
Somogyi Škoc M, Macan J, Jakovljević S, Rezić I. Synthesis and Characterization of ZnO and TiO2 Hybrid Coatings for Textile UV Anti-Aging Protection. Polymers. 2024; 16(14):2001. https://doi.org/10.3390/polym16142001
Chicago/Turabian StyleSomogyi Škoc, Maja, Jelena Macan, Suzana Jakovljević, and Iva Rezić. 2024. "Synthesis and Characterization of ZnO and TiO2 Hybrid Coatings for Textile UV Anti-Aging Protection" Polymers 16, no. 14: 2001. https://doi.org/10.3390/polym16142001
APA StyleSomogyi Škoc, M., Macan, J., Jakovljević, S., & Rezić, I. (2024). Synthesis and Characterization of ZnO and TiO2 Hybrid Coatings for Textile UV Anti-Aging Protection. Polymers, 16(14), 2001. https://doi.org/10.3390/polym16142001








