Study of the Incorporation of Gel and Aloe vera Peel Extract in a Polymer Matrix Based on Polyvinylpyrrolidone
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining Biological Material
2.2. Obtaining of Peel extract and Aloe vera Gel
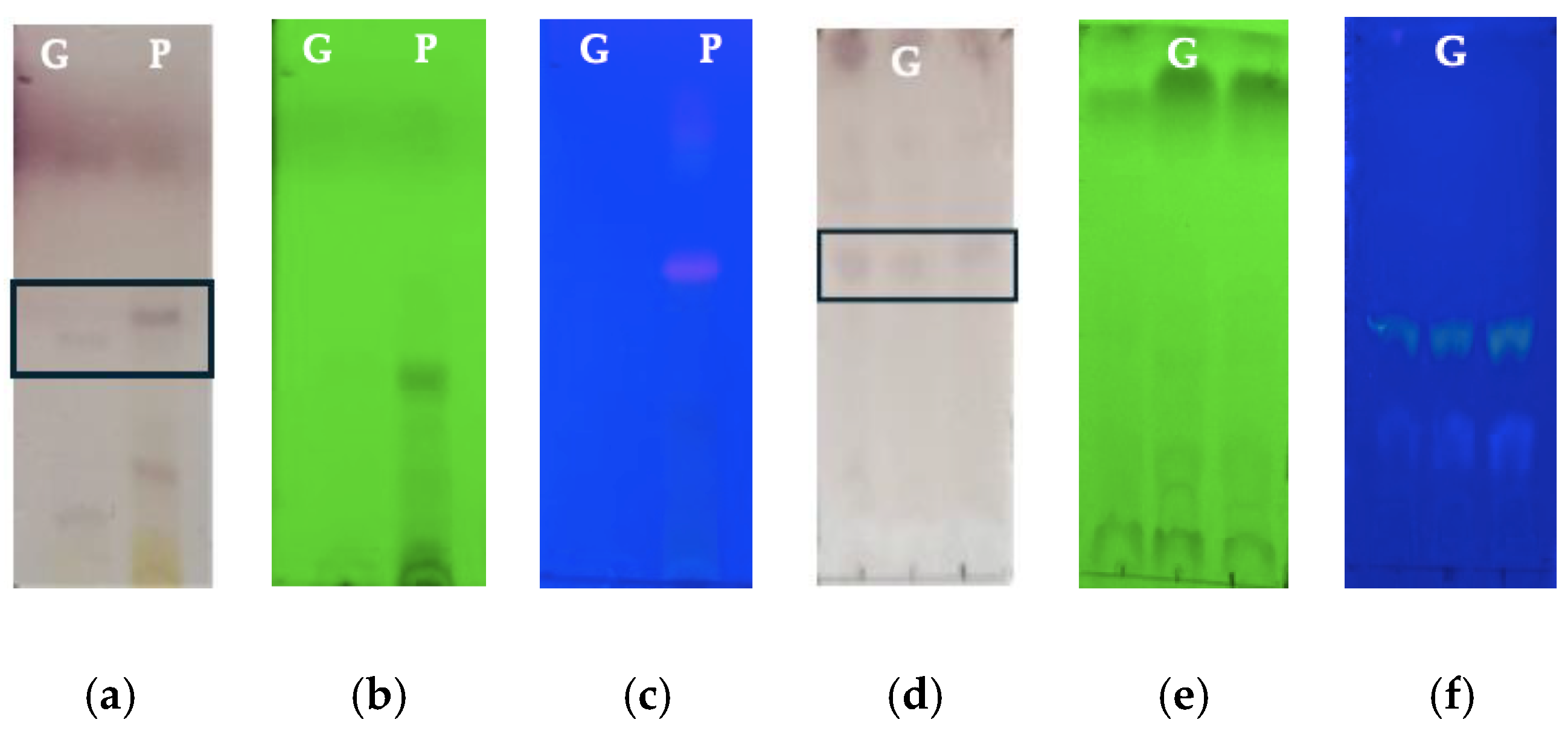
2.3. Thin-Layer Chromatography (TLC)
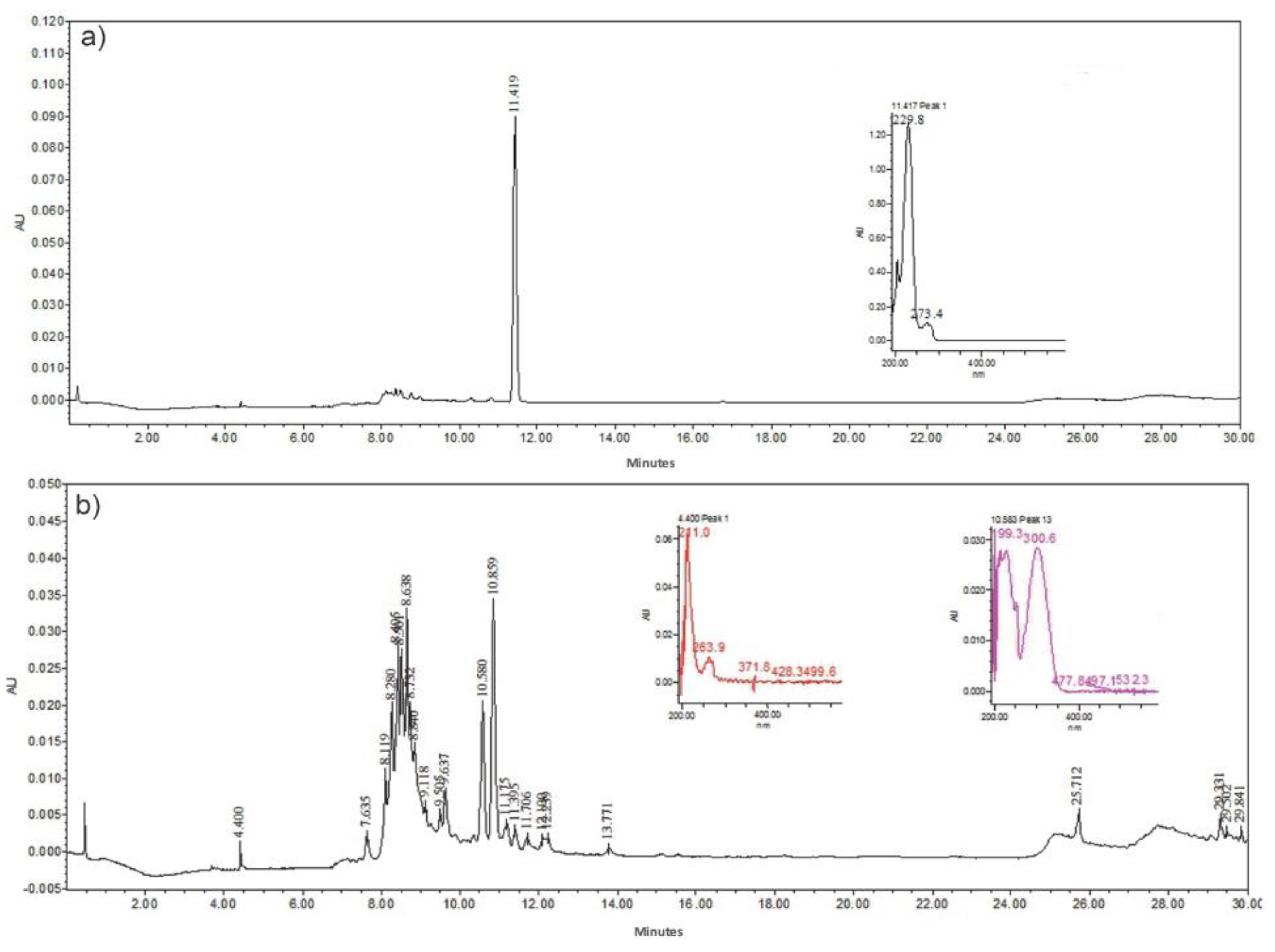
2.4. High-Performance Liquid Chromatography (HPLC)
2.5. Preparation of Electrospun Polyvinylpyrrolidone and Aloe vera Membranes
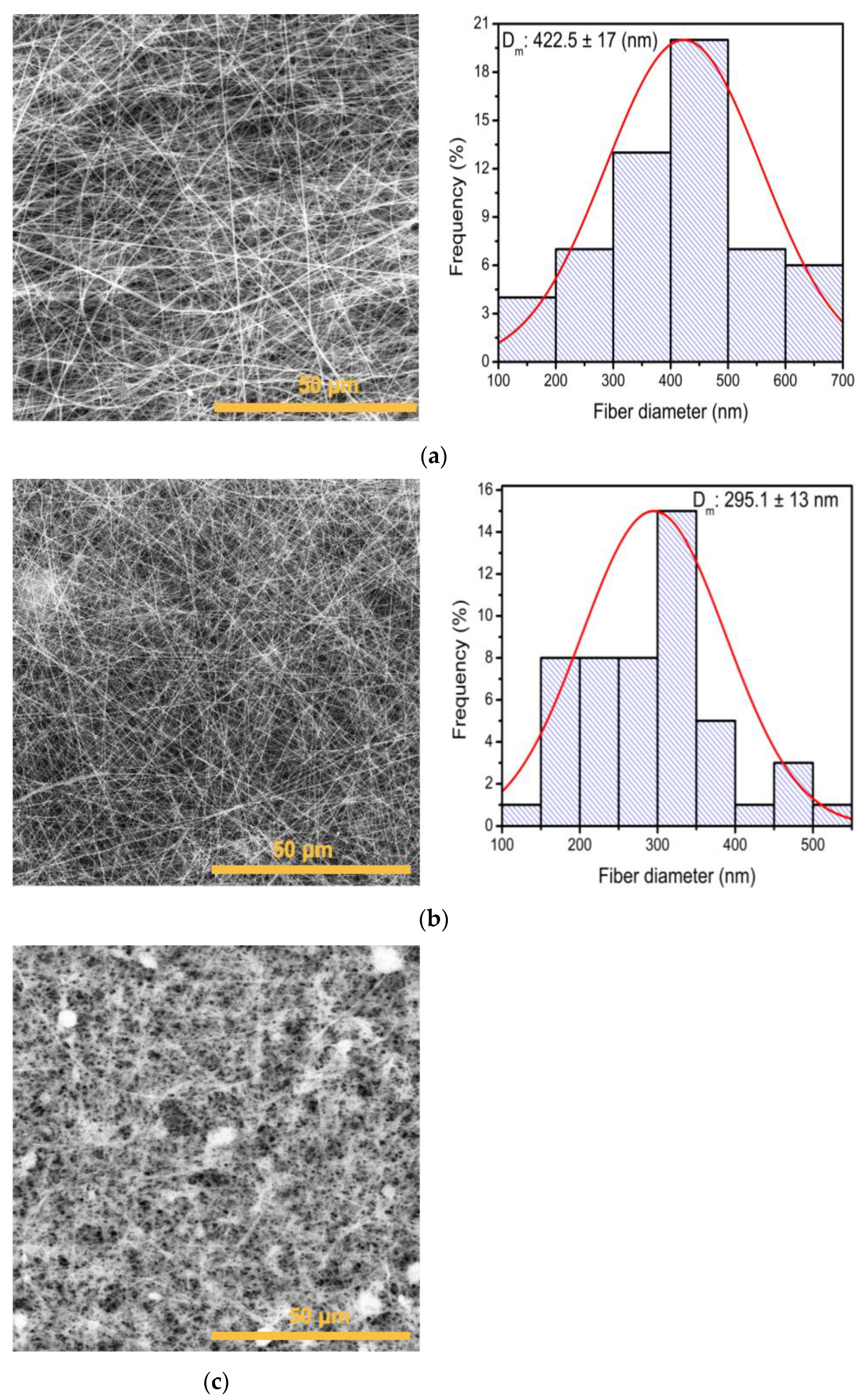
2.6. Scanning Electron Microscopy (SEM)
2.7. UV–Vis Spectrophotometry (UV–Vis)
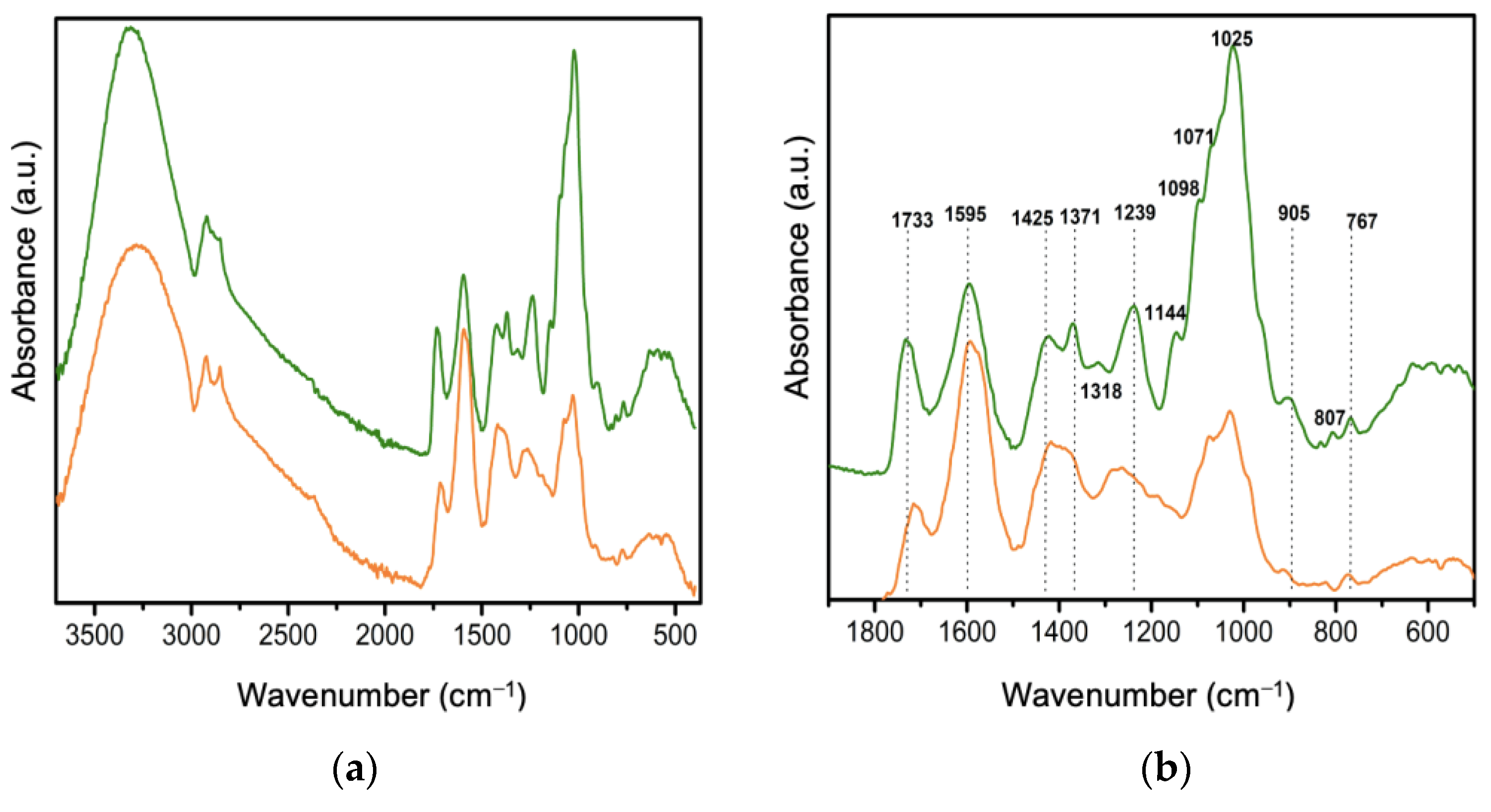
2.8. Fourier Transform Infrared Spectroscopy (FTIR)
3. Results and Discussion
3.1. Characterization of Aloe vera Components (Gel and Peel Extract)
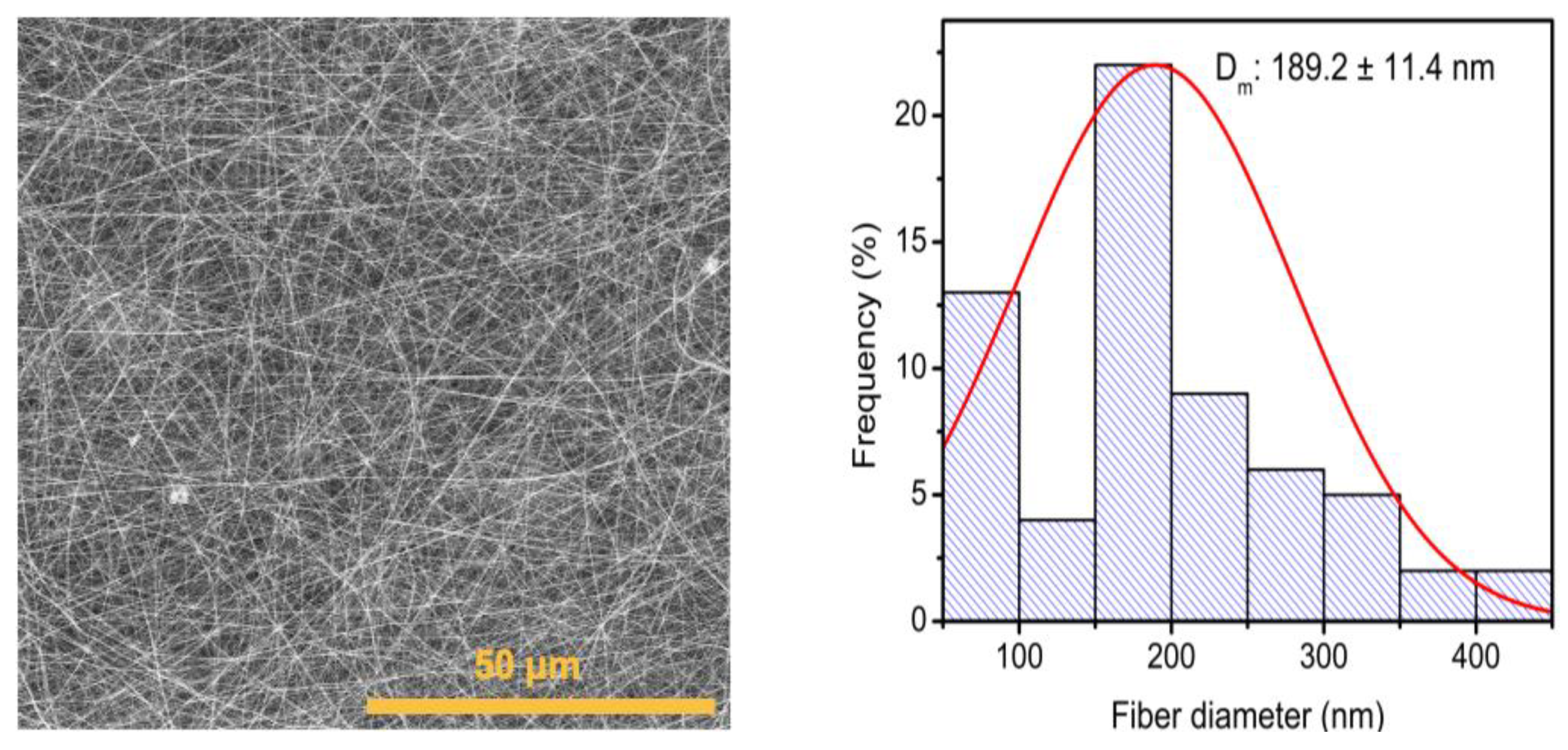
3.2. Evaluation of Electrospinning Conditions to Obtain PVP Membranes
3.3. Characterization of Electrospun Membranes from PVP/Av Gel by FTIR-ATR and UV–Vis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Reynolds, T. (Ed.) Aloe chemistry. In Aloes: The Genus Aloe, 1st ed.; CRC Press LLC: Boca Raton, FL, USA, 2004; Volume 38, pp. 39–74. [Google Scholar]
- Marimuthu, A.A.; Malar, R.J.J.; Beaulah, N.; Laju, R.S.; Anupriya, G.; Renola, J.J.E.T. Anti-bacterial and anti- fungal activity of Aloe vera gel extract. Int. J. Biomed. Adv. Res. 2012, 3, 184–187. [Google Scholar]
- Balaji, A.; Vellayappan, M.V.; John, A.A.; Subramanian, A.P.; Jaganathan, S.K.; SelvaKumar, M.; Faudzi, A.A.B.M.; Supriyanto, E.; Yusof, M. Biomaterials based nano-applications of Aloe vera and its perspective: A review. RSC Adv. 2015, 5, 86199–86213. [Google Scholar] [CrossRef]
- Park, M.K.; Park, J.H.; Kim, N.Y.; Shin, Y.G.; Choi, Y.S.; Lee, J.G.; Kim, K.H.; Lee, S.K. Analysis of 13 phenolic compounds in Aloe species by high performance liquid chromatography. Phytochem. Anal. 1998, 9, 186–191. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans (Ed.) Aloe vera. In Some Drugs and Herbal Products, 1st ed.; International Agency for Research on Cancer: Lyon, France, 2015; Volume 108, pp. 36–71. [Google Scholar]
- Soltanizadeh, N.; Ghiasi-Esfahani, H. Qualitative improvement of low meat beef burger using Aloe vera. Meat Sci. 2015, 99, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Radha, M.H.; Laxmipriya, N.P. Evaluation of biological properties and clinical effectiveness of Aloe vera: A systematic review. J. Tradit. Complement. Med. 2015, 5, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Kalirajan, C.; Dukle, A.; Nathanael, A.J.; Oh, T.H.; Manivasagam, G. A critical review on polymeric biomaterials for biomedical applications. Polymers 2021, 13, 3015. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, H.; Zhang, M.; Yu, D.G. Electrospun medicated nanofibers for wound healing: Review. Membranes 2021, 11, 770. [Google Scholar] [CrossRef]
- Dasari, A.; Quirós, J.; Herrero, B.; Boltes, K.; García-Calvo, E.; Rosal, R. Antifouling membranes prepared by electrospinning polylactic acid containing biocidal nanoparticles. J. Membr. Sci. 2012, 405, 134–140. [Google Scholar] [CrossRef]
- Huang, H.H.; Ni, X.P.; Loy, G.L.; Chew, C.H.; Tan, K.L.; Loh, F.C.; Deng, J.F.; Xu, G.Q. Photochemical formation of silver nanoparticles in poly(N-vinylpyrrolidone). Langmuir 1996, 12, 909–912. [Google Scholar] [CrossRef]
- Balamurugan, R.; Sundarrajan, S.; Ramakrishna, S. Recent trends in nanofibrous membranes and their suitability for air and water filtrations. Membranes 2011, 1, 232–248. [Google Scholar] [CrossRef]
- Kanani, A.G.; Bahrami, S.H. Review on electrospun nanofibers scaffold and biomedical applications. Trends Biomater. Artif. Organs. 2010, 24, 93–115. [Google Scholar]
- Abrigo, M.; McArthur, S.L.; Kingshott, P. Electrospun nanofibers as dressings for chronic wound care: Advances, challenges, and future prospects. Macromol. Biosci. 2014, 14, 772–792. [Google Scholar] [CrossRef] [PubMed]
- Kohli, N.; Sharma, V.; Brown, S.J.; García-Gareta, E. Synthetic Polymers for Skin Biomaterials. In Biomaterials for Skin Repair and Regeneration, 1st ed.; García-Gareta, E., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 125–149. [Google Scholar]
- Metwally, S.; Stachewicz, U. Surface potential and charges impact on cell responses on biomaterials interfaces for medical applications. Mater. Sci. Eng. 2019, 104, 109883. [Google Scholar] [CrossRef] [PubMed]
- Terzopoulou, Z.; Zamboulis, A.; Koumentakou, I.; Michailidou, G.; Noordam, M.J.; Bikiaris, D.N. Biocompatible synthetic polymers for tissue engineering purposes. Biomacromolecules 2022, 23, 1841–1863. [Google Scholar] [CrossRef]
- Kurakula, M.; Rao, G.S.N.K. Pharmaceutical assessment of polyvinylpyrrolidone (PVP): As excipient from conventional to controlled delivery systems with a spotlight on COVID-19 inhibition. J. Drug Deliv. Sci. Technol. 2020, 60, 102046. [Google Scholar] [CrossRef]
- Bühler, V. Polyvinylpyrrolidone Excipients for Pharmaceuticals: Povidone, Crospovidone and Copovidone, 1st ed.; Springer Science & Business Media: Berlin, Germany, 2005. [Google Scholar]
- Agnes-Mary, S.; Giri-Dev, V.R. Electrospun herbal nanofibrous wound dressings for skin tissue engineering. J. Text. Inst. 2014, 106, 886–895. [Google Scholar] [CrossRef]
- Moghaddam, A.B.; Shirvani, B.; Aroon, M.A.; Nazari, T. Physico-chemical properties of hybrid electrospun nanofibers containing polyvinylpyrrolidone (PVP), propolis and aloe vera. Mater. Res. Express 2018, 5, 125404. [Google Scholar] [CrossRef]
- Aghamohamadi, N.; Sanjani, N.; Majidi, R.; Y Nasrollahi, S. Preparation and characterization of Aloe vera acetate and electrospinning fibers as promising antibacterial properties materials. Mater. Sci. Eng. C 2019, 94, 445–452. [Google Scholar] [CrossRef]
- Sotelo, C.; Flores, C.; Bernal, A.; González, M.; Toledo, E.; Marquina, S.; Álvarez-Fitz, P.; Zamilpa, A. Chemical composition of Bessera elegans (Asparagaceae) flower extracts and their insecticidal effect against Melanaphis sacchari Zehntner (Hemiptera: Aphididae). S. Afr. J. Bot. 2023, 156, 186–191. [Google Scholar] [CrossRef]
- Wagner, H.; Bladt, S. Plant Drug Analysis: A Thin Layer Chromatography Atlas, 2nd ed.; Springer: Berlin, Germany, 1996. [Google Scholar]
- Ray, A.; Ghosh, S. Chemometrics for functional group distribution, and UV absorption potential of Aloe vera L. gel at different growth periods. Mater. Today Proc. 2018, 5, 22245–22253. [Google Scholar] [CrossRef]
- Elamthuruthy, A.T.; Shah, C.R.; Khan, T.A.; Tatke, P.A.; Gabhe, S.Y. Standardization of marketed Kumariasava—An Ayurvedic Aloe vera product. J. Pharm. Biomed. Anal. 2005, 37, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Añibarro-Ortega, M.; Pinela, J.; Ćirić, A.; Lopes, E.; Molina, A.K.; Calhelha, R.C.; Sokovi´c, M.; Ferreira, O.; Ferreira, I.C.F.R.; Barros, L. Extraction of Aloesin from Aloe vera Rind Using Alternative Green Solvents: Process Optimization and Biological Activity Assessment. Biology 2021, 10, 951. [Google Scholar] [CrossRef] [PubMed]
- Sayed, E.; Ezzat, S.Y.; Hawary, S. In vivo diabetic wound healing effect and HPLC-DAD-MS/MS profiling of the methanol extracts of eight Aloe species. Braz. J. Pharmacogn. 2016, 23, 352–363. [Google Scholar] [CrossRef]
- Keyhanian, S.; Stahl-Biskup, E. Phenolic constituents in dried flowers of Aloe vera (Aloe barbadensis) and their in vitro antioxidative capacity. Planta Med. 2007, 73, 599–602. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Carter, P.; Bhattarai, N. Aloe Vera for Tissue Engineering Applications. J. Funct. Biomater. 2017, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Nejatzadeh-Barandozi, F.; Enferadi, S.T. FT-IR study of the polysaccharides isolated from the skin juice, gel juice, and flower of Aloe vera tissues affected by fertilizer treatment. Org. Med. Chem. Lett. 2012, 2, 33. [Google Scholar] [CrossRef] [PubMed]
- Uslu, I.; Keskin, S.; Gül, A.; Karabulut, T.C.; Aksu, M.L. Preparation and properties of electrospun poly(vinyl alcohol) blended hybrid polymer with aloe vera and HPMC as wound dressing. Hacet. J. Biol. Chem. 2010, 38, 19–25. [Google Scholar]
- Sadiq, U.; Gill, H.; Chandrapala, J. Temperature and pH Stability of Anthraquinones from Native Aloe vera Gel, Spray-Dried and Freeze-Dried Aloe vera Powders during Storage. Foods. 2022, 11, 1613. [Google Scholar] [CrossRef] [PubMed]
- Minjares-Fuentes, R.; Femenia, A.; Comas-Serra, F.; Rosselló, C.; Rodríguez-González, V.M.; González-Laredo, R.F.; Gallegos-Infante, J.A.; Medina-Torres, L. Effect of different drying procedures on physicochemical properties and flow behavior of Aloe vera (Aloe barbadensis Miller) gel. LWT 2016, 74, 378–386. [Google Scholar] [CrossRef]
- Abbasi, M.S.A.; Tahir, M.A.; Meer, S. FTIR Spectroscopic study of aloe vera barbadensis Mill Buds. Asian J. Chem. Sci. 2020, 7, 1–6. [Google Scholar] [CrossRef]
- Prato, M.; Ávila, R.; Donquis, C.; Medina, E.; Reyes, R. Antraquinonas en Aloe vera barbadensis de zonas semiáridas de Falcón, Venezuela, como inhibidores de la corrosión. Rev. Multiciencias 2008, 8, 148–154. [Google Scholar]
- Paradossi, G.; Cavalieri, F.; Pizzoferrato, L.; Liquori, A.M. A physico-chemical study on the polysaccharide ulvan from hot water extraction of the macroalga Ulva. Int. J. Biol. Macromol. 1999, 25, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Kacurakova, M.; Capek, P.; Sasinkova, V.; Wellner, N.; Ebringerova, A. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Minjares, R.; Rodríguez, V.; González, R.; Eim, V.; González, M.; Femenia, A. Effect of different drying procedures on the bioactive polysaccharide acemannan from Aloe vera (Aloe barbadensis Miller). Carbohydr. Polym. 2017, 168, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.C.; Cui, C.Y.; Zhang, Y.; Chu, R.H. Separation, purification and structural analysis of polysaccharides from aloe. Chem. J. Chin. Univ. 2003, 24, 1189. [Google Scholar]
- Jettanacheawchankit, S.; Sasithanasate, S.; Sangvanich, P.; Banlunara, W.; Thunyakitpisal, P. Acemannan stimulates gingival fibroblast proliferation; expressions of keratinocyte growth factor-1, vascular endothelial growth factor, and type I collagen; and wound healing. J. Pharmacol. Sci. 2009, 109, 525–531. [Google Scholar] [CrossRef]
- Ahl, L.; Al-Husseini, N.; Al-Helle, S.; Staerk, D.; Grace, O.; Willats, W.; Mravec, J.; Jørgensen, B.; Rønsted, N. (Detection of Seasonal Variation in Aloe Polysaccharides Using Carbohydrate Detecting Microarrays. Front. Plant Sci. 2019, 10, 512. [Google Scholar] [CrossRef]
- Surjushe, A.; Vasani, R.; Saple, D.G. Aloe vera: A short review. Indian J. Dermatol. 2008, 53, 163–166. [Google Scholar] [CrossRef]
- Megelski, S.; Stephens, J.S.; Chase, D.B.; Rabolt, J.F. Micro and nanostructured surface morphology on electrospun polymer fibers. Macromolecules 2002, 35, 8456–8466. [Google Scholar] [CrossRef]
- Augustine, R.; Rehman, S.R.U.; Ahmed, R.; Zahid, A.A.; Sharifi, M.; Falahati, M.; Hasan, A. Electrospun chitosan membranes containing bioactive and therapeutic agents for enhanced wound healing. Int. J. Biol. Macromol. 2020, 156, 153–170. [Google Scholar] [CrossRef]
- Hikmawati, D.; Rohmadanik, A.R.; Putra, A.P.; Siswanto; Aminatun. The Effect of Aloe vera Extract Variation in Electrospun Polyvinyl Alcohol (PVA)-Aloe vera-Based Nanofiber Membrane. J. Phys. Conf. Ser. 2018, 1120, 12096. [Google Scholar] [CrossRef]
- Fayemi, O.E.; Ekennia, A.C.; Katata-Seru, L.; Ebokaiwe, A.P.; Ijomone, O.M.; Onwudiwe, D.C.; Ebenso, E.E. Antimicrobial and wound healing properties of polyacrylonitrile-moringa extract nanofibers. ACS Omega 2018, 3, 4791–4797. [Google Scholar] [CrossRef] [PubMed]
- Chinatangkul, N.; Tubtimsri, S.; Panchapornpon, D.; Akkaramongkolporn, P.; Limmatvapirat, C.; Limmatvapirat, S. Design and characterization of electrospun shellac-polyvinylpyrrolidone blended micro/nanofibres loaded with monolaurin for application in wound healing. Int. J. Pharm. 2019, 562, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Ravi, S.; Kabilar, P.; Velmurugan, S.; Kumar, R.A.; Gayathiri, M. Spectroscopy Studies on the Status of Aloin in Aloe vera and Commercial Samples. J. Exp. Sci. 2011, 2, 10–13. [Google Scholar]
- Gansukh, E.; Gopal, J.; Paul, D.; Muthu, M.; Kim, D.H.; Oh, J.W.; Chun, S. Ultrasound mediated accelerated anti-influenza activity of Aloe vera. Sci Rep. 2018, 8, 17782. [Google Scholar] [CrossRef]
- Ozsoy, N.; Can, A.; Yanardag, R.; Akev, N. Antioxidant activity of Smilax excelsa L. leaf extracts. Food Chem. 2008, 110, 571–583. [Google Scholar] [CrossRef]
- Behera, M.; Ram, S. Spectroscopy-based study on the interaction between gold nanoparticle and poly(vinylpyrrolidone) molecules in a non-hydrocolloid. Int. Nano Lett. 2013, 3, 17. [Google Scholar] [CrossRef]





| Membrane | Applied Voltage (kV) | Feed Rate (mL/h) |
|---|---|---|
| A | 15 | 0.2 |
| B | 15 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez Rafael, B.J.; Zaca Moran, O.; Delgado Macuil, R.J.; Martínez Gutiérrez, H.; García Juárez, M.; Lopez Gayou, V. Study of the Incorporation of Gel and Aloe vera Peel Extract in a Polymer Matrix Based on Polyvinylpyrrolidone. Polymers 2024, 16, 1998. https://doi.org/10.3390/polym16141998
Gutiérrez Rafael BJ, Zaca Moran O, Delgado Macuil RJ, Martínez Gutiérrez H, García Juárez M, Lopez Gayou V. Study of the Incorporation of Gel and Aloe vera Peel Extract in a Polymer Matrix Based on Polyvinylpyrrolidone. Polymers. 2024; 16(14):1998. https://doi.org/10.3390/polym16141998
Chicago/Turabian StyleGutiérrez Rafael, Britania Janet, Orlando Zaca Moran, Raúl Jacobo Delgado Macuil, Hugo Martínez Gutiérrez, Marcos García Juárez, and Valentin Lopez Gayou. 2024. "Study of the Incorporation of Gel and Aloe vera Peel Extract in a Polymer Matrix Based on Polyvinylpyrrolidone" Polymers 16, no. 14: 1998. https://doi.org/10.3390/polym16141998
APA StyleGutiérrez Rafael, B. J., Zaca Moran, O., Delgado Macuil, R. J., Martínez Gutiérrez, H., García Juárez, M., & Lopez Gayou, V. (2024). Study of the Incorporation of Gel and Aloe vera Peel Extract in a Polymer Matrix Based on Polyvinylpyrrolidone. Polymers, 16(14), 1998. https://doi.org/10.3390/polym16141998






