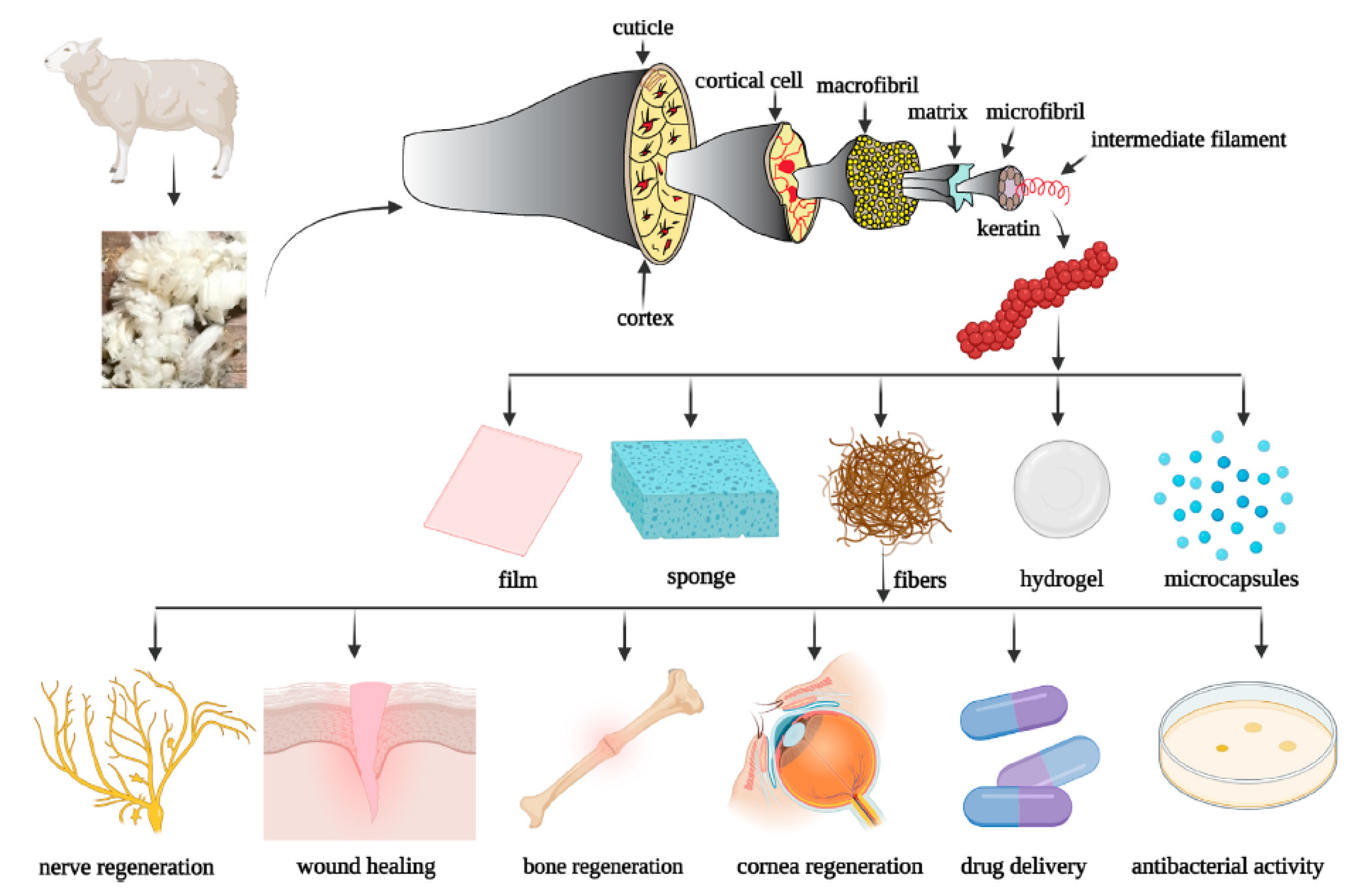
Keratin from Animal By-Products: Structure, Characterization, Extraction and Application—A Review
Abstract
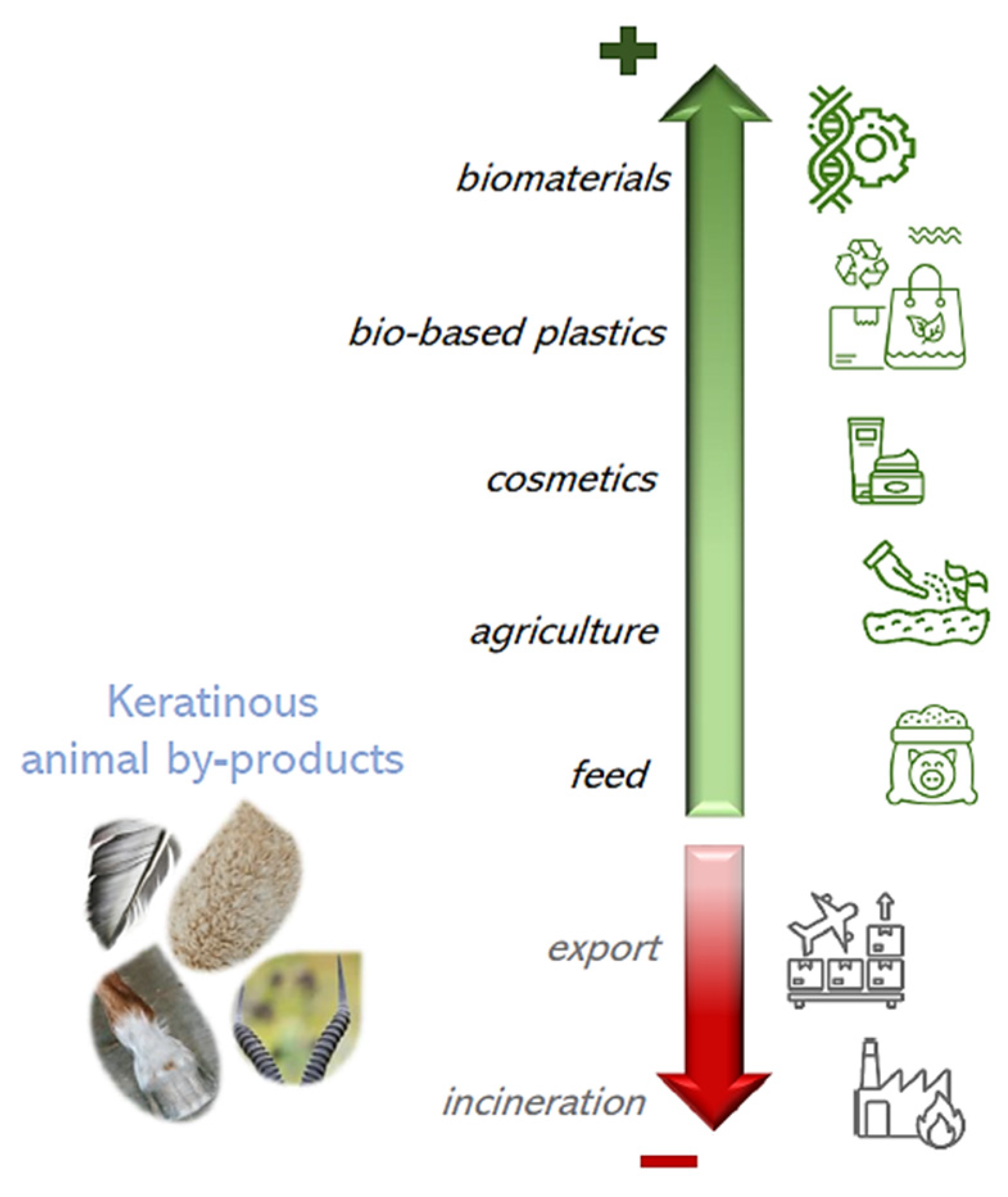
1. Introduction
2. Keratin Structure
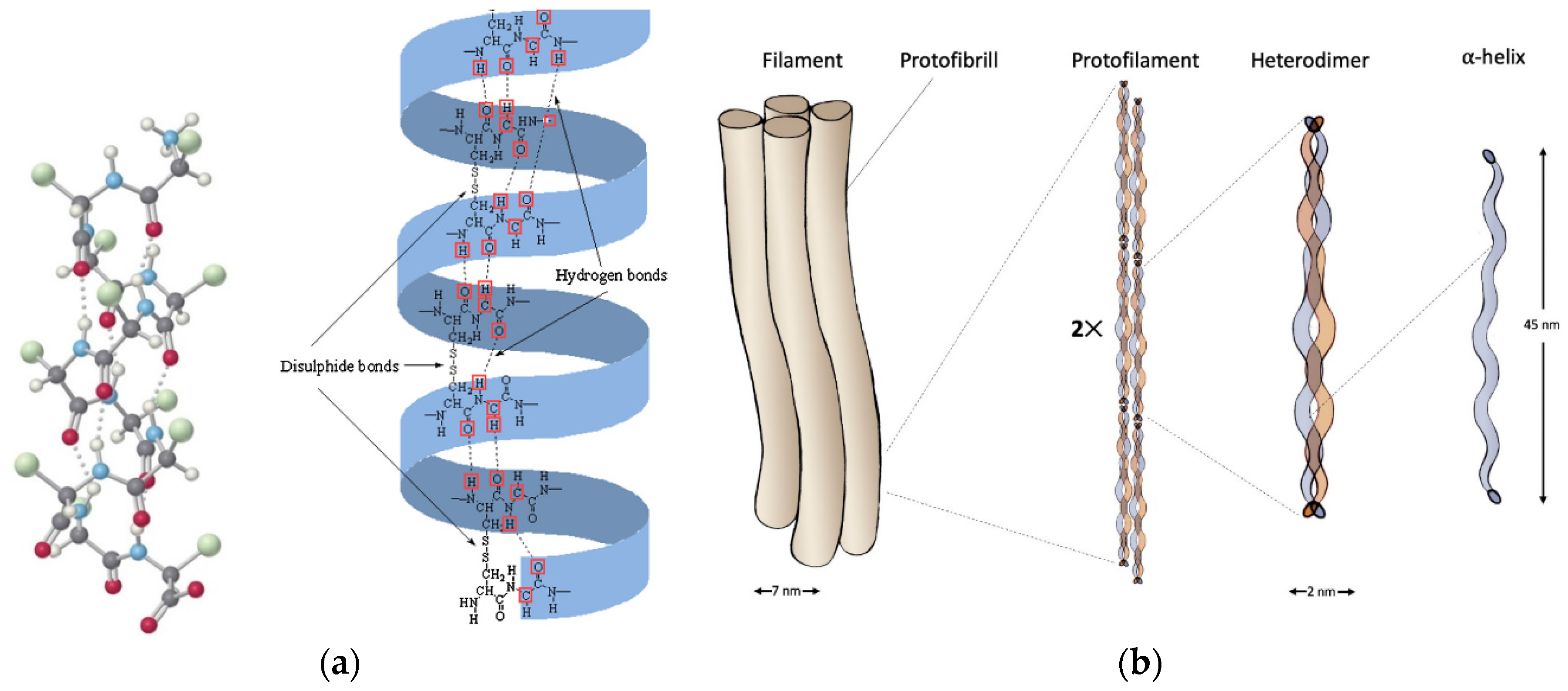
2.1. α-Keratin
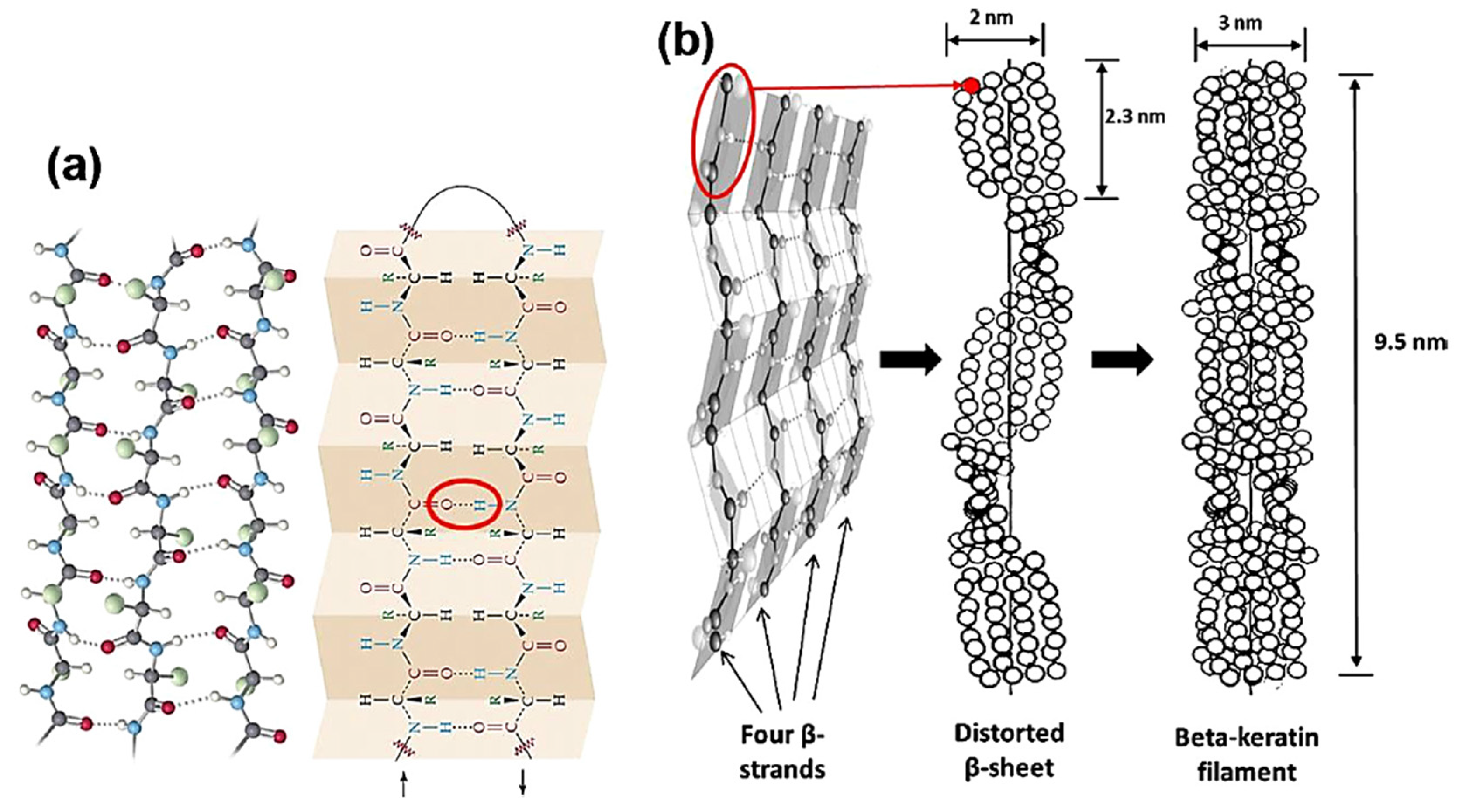
2.2. β-Keratin
| Feature | α-Keratin | β-Keratin |
|---|---|---|
| Common structure | Filament matrix: the filaments are embedded into an amorphous matrix | |
| Occurrence | Wool, hair, stratum corneum, fingernails, horns, hooves, quills | Feathers, beaks, and claws; reptilian scales; turtle carapaces and plastron |
| Type of filaments and diameter | Intermediate filaments (IFs), 7 nm | Beta-keratin filaments, 3–4 nm |
| Constituting proteins | The IFs can be several kinds of low-sulfur proteins while the matrix is made of high-sulfur and high-glycine–tyrosine proteins | The filament and the matrix are incorporated into one single protein |
| Synthesis | In the beginning, IFs (low-sulfur) are synthesized; as the cell approaches maturation, matrix proteins (high-sulfur) are produced between the IFs and after the synthesis takes place concomitantly | There are no different synthesis stages; filaments and matrix increase in a coordinated way; the mechanism of aggregation is not known in detail |
| Molecular unit (MU) | Dimer | Distorted pleated sheet |
| Protofilament molecular mass | 40–68 kDa | 10–22 kDa |
| Number of residues in the MU | 33–35 for the helical zone, 136 for the non-helical zone | 34 for the pleated sheet, 59–168 for the non-sheet zone |
| Mechanical properties | α-keratin has lower stiffness than β-keratin | |
| α-helix changes into β-pleated sheet under tension | ||
| Young’s modulus for α- and β-keratin decreases with an increase in humidity | ||
| Mineralization with calcium can harden both keratins | ||
| Two-phase model: crystalline and water-resistant IFs in an amorphous matrix that is modeled as an elastomer that can interact with water | Crystalline filaments wound by an amorphous matrix. (only a few studies are available) | |
| Amino Acid (mol%) | Feathers (Whole) (β-Keratin) | Feather (Rachis) (β-Keratin) | Wool (Sheep) (α-Keratin) | Horn (Sheep) (α-Keratin) | Hoof (Sheep) (α-Keratin) | Bristles (Pig) (α-Keratin) |
|---|---|---|---|---|---|---|
| Alanine | 3.60 | 8.7 | 5.20 | 5.90 | 6.37 | 4.90 |
| Arginine | 5.40 | 3.8 | 6.24 | 6.68 | 7.16 | 7.65 |
| Aspartic acid | 4.70 | 5.6 | 5.93 | 7.80 | 8.39 | 6.05 |
| half-Cystine | 7.70 | 7.8 | 13.10 | 6.24 | 5.66 | 10.75 * |
| Glutamic acid | 7.70 | 6.9 | 11.10 | 12.90 | 13.70 | 12.55 |
| Glycine | 6.20 | 13.7 | 8.56 | 11.10 | 9.10 | 9.25 |
| Histidine | nr | 0.2 | 0.79 | 1.33 | 0.94 | nr |
| Isoleucine | 4.30 | 3.2 | 2.98 | 3.31 | 3.56 | 3.15 |
| Leucine | 7.00 | 8.3 | 7.20 | 9.13 | 9.51 | 6.95 |
| Lysine | 0.60 | 0.6 | 2.66 | 3.76 | 3.96 | 2.60 |
| Methionine | 1.30 | 0.1 | 0.54 | 0.81 | 0.80 | 0.65 |
| Phenylalanine | 4.20 | 3.1 | 2.48 | 2.64 | 2.65 | 2.30 |
| Proline | 8.70 | 9.8 | 6.60 | 3.83 | 3.99 | 7.15 |
| Serine | 9.30 | 14.1 | 10.80 | 9.56 | 9.54 | 11.30 |
| Threonine | 3.50 | 4.1 | 6.53 | 4.78 | 4.95 | 6.95 |
| Tyrosine | 1.95 | 1.4 | 3.78 | 5.00 | 4.03 | 3.85 |
| Valine | 6.94 | 7.8 | 5.68 | 5.21 | 5.66 | 4.85 |
| [2] | [6] | [23] | [23] | [23] | [24] |
2.3. Other Keratin Classifications
2.4. Structure of Keratinous Livestock By-Products
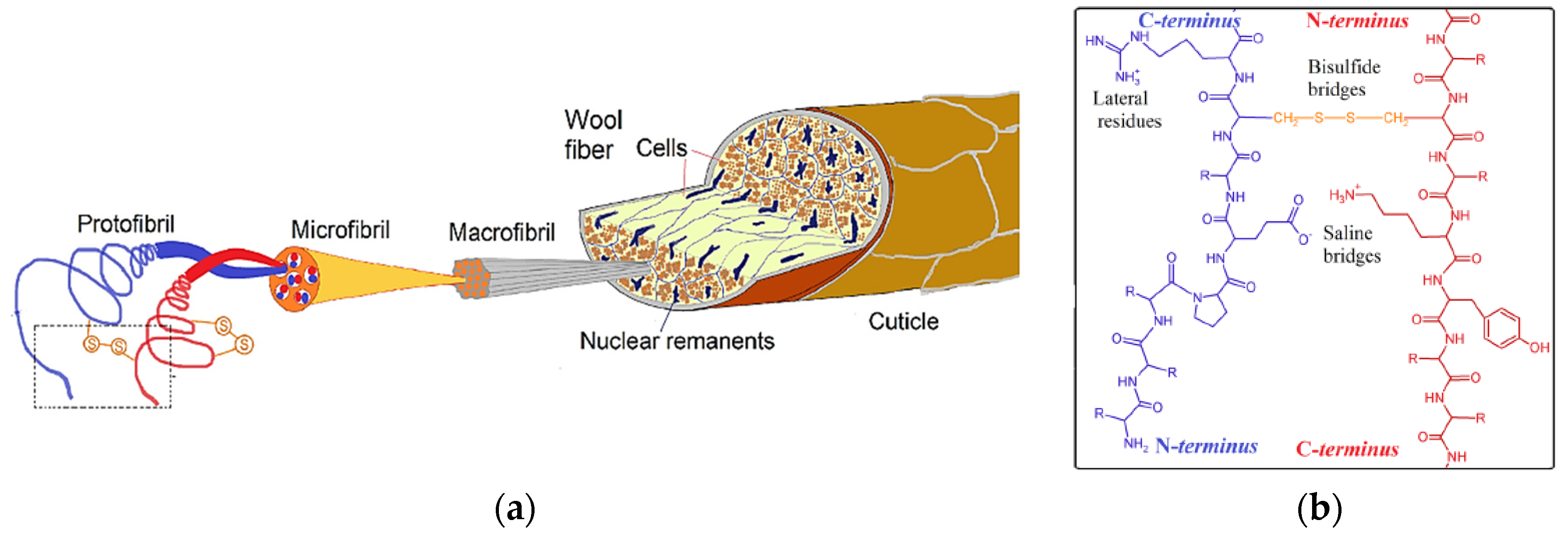
2.4.1. Wool Keratin
2.4.2. Feather Keratin
| Amino Acid (mol%) | White | White | Black | White Crossed Strains |
|---|---|---|---|---|
| Alanine | 4.01 | 3.90 | 4.33 | 2.90 |
| Arginine | 6.16 | 6.58 | 5.10 | 6.80 |
| Aspartic acid | 5.23 | 6.15 | 5.20 | 4.20 |
| half-Cystine | 7.16 | 7.60 | 10.54 | 6.60 |
| Glutamic acid | 8.76 | 10.34 | 7.75 | 8.20 |
| Glycine | 6.31 | 6.87 | 6.80 | 5.20 |
| Histidine | 0.40 | 0.52 | 0.32 | 0.20 |
| Isoleucine | 4.28 | 4.78 | 3.22 | 3.90 |
| Leucine | 7.38 | 7.75 | 6.86 | 5.70 |
| Lysine | 1.11 | 1.69 | 0.80 | 1.60 |
| Methionine | 0.25 | 0.57 | 0.19 | 0.70 |
| Phenylalanine | 4.40 | 4.52 | 3.93 | 3.50 |
| Proline | 8.84 | 9.37 | 7.67 | nr |
| Serine | 8.93 | 11.44 | 8.91 | nr |
| Threonine | 3.77 | 4.66 | 3.51 | 3.50 |
| Tryptophan | 0.97 | 2.17 | 0.94 | nr |
| Tyrosine | 2.44 | nr | 2.12 | nr |
| Valine | 6.12 | 6.30 | 6.19 | 5.30 |
| [36] | [37] | [36] | [30] |
2.4.3. Hoof and Horn Keratin
2.4.4. Pig Bristles
3. Characterization of Keratin and Keratinous Tissue Structure
3.1. Spectroscopy Techniques
3.1.1. Fourier Transform Infrared Spectroscopy (FTIR)
3.1.2. Terahertz Spectroscopy
3.2. Microscopy Techniques
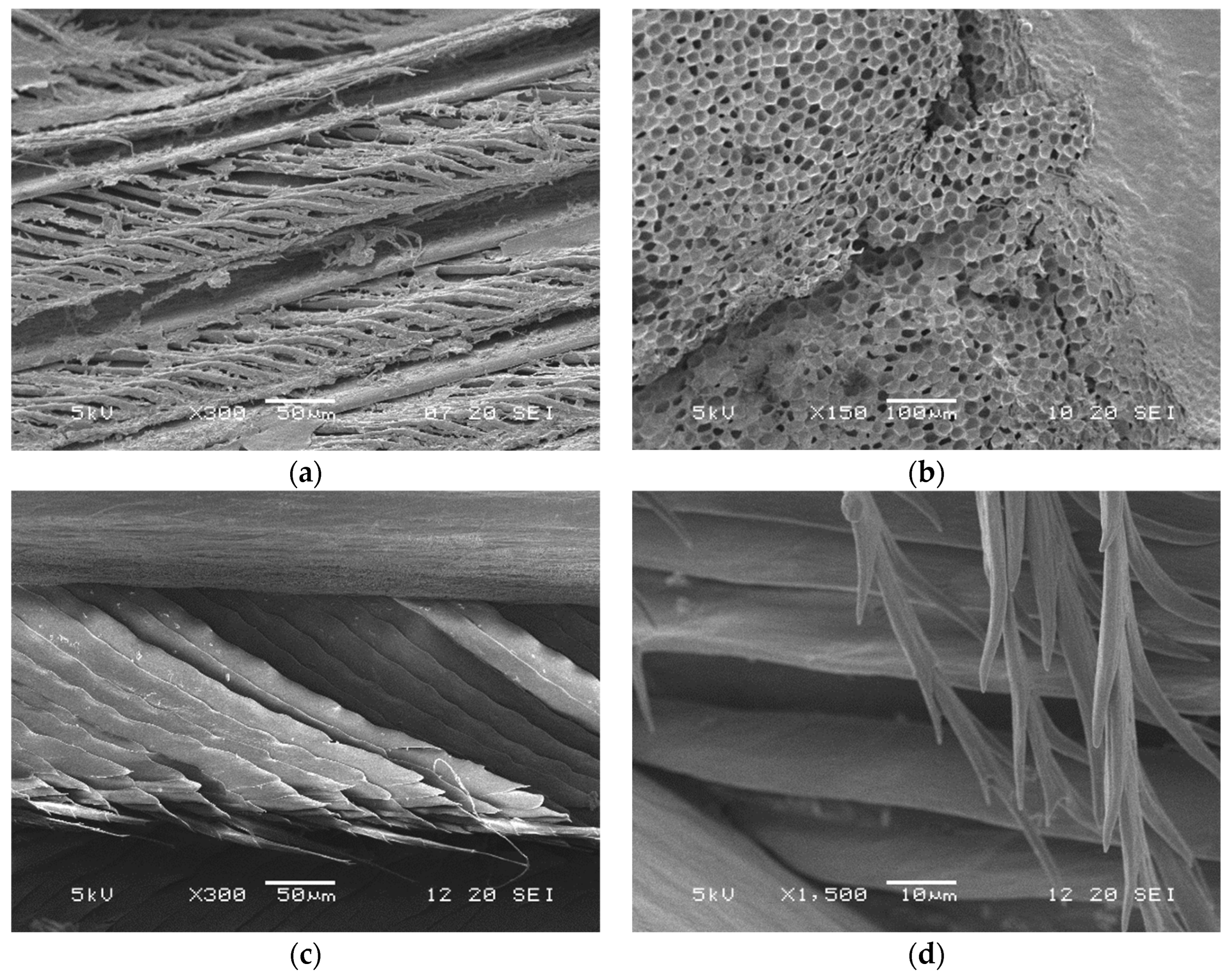
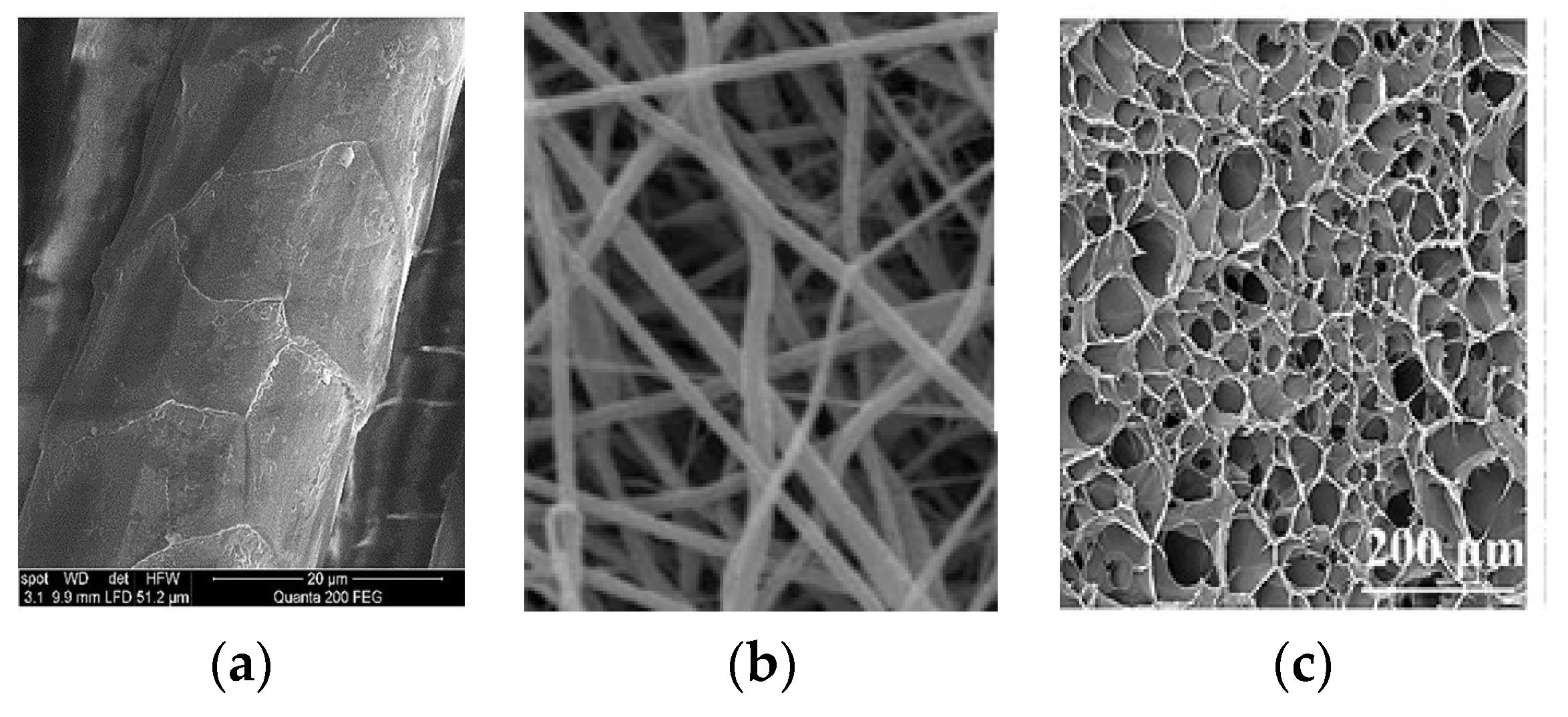
3.2.1. Scanning Electron Microscopy (SEM)
3.2.2. Transmission Electron Microscopy (TEM)
3.2.3. Second-Harmonic Generation (SHG) Microscopy
3.3. X-ray-Based Techniques
3.3.1. X-ray Diffraction (XRD)
3.3.2. Small-Angle/Wide-Angle X-ray Scattering
3.3.3. Micro-Computed Tomography (μCT)
3.4. Thermal Analysis and Calorimetry
3.4.1. Differential Scanning Calorimetry (DSC)
3.4.2. Thermogravimetric Analysis (TGA)
3.5. Nuclear Magnetic Resonance (NMR) Spectroscopy
4. Methods for Extraction of Keratin from Livestock By-Products
4.1. Chemical Methods
4.1.1. Acidic Hydrolysis
4.1.2. Alkaline Hydrolysis
4.1.3. Oxidation
4.1.4. Reduction
4.1.5. Ionic Liquids (ILs)
4.2. Biological Methods
4.2.1. Microbial Methods
4.2.2. Enzymatic Hydrolysis
4.3. Novel Methods
| Keratin Source | Extraction Technique | Extraction Conditions | Product/Application | Reference |
|---|---|---|---|---|
| Mixture of keratinous tissues | Hydrothermal pre-treatment followed by microbial hydrolysis with fungi | 50–55% humidity, 170–180 °C, 60 s pre-treatment; 4 g of pre-treated material incubated with keratinase extracted from Acremonium chrysogenium, ratio E:S 1:4, 1–8, in 1 L reactor, 55 °C with continuous stirring, for 1–6 h | Low-molecular-weight keratin hydrolysates and free amino acids with 100% bioavailability | [123] |
| Chicken feathers | Reducing agent | Chicken feathers treated with calcium hydroxide; temperature of 50–150 °C, duration of 0–300 min, and varying raw material concentration followed by centrifuge separation | The final product is rich in soluble amino acids and polypeptides and can be used for animal feed; it can be a potential protein source for ruminants | [124] |
| Alkaline enzymatic hydrolysis | Pre-heating of ground chicken feathers by boiling water, followed by addition of lipolytic enzymes and pH adjustment; 24 h stirring followed by the addition of 0.3% w/w KOH in water for alkaline hydrolysis; separation through filtration | Preparation of water emulsions for cosmetic application, containing 2, 4, and 6% by weight of keratin hydrolysates for dermal use | [125] | |
| Alkaline hydrolysis | Defatted and milled chicken feathers into NaOH aqueous solution at different concentrations in a wide range of pH, temperature, and reaction time | Purified keratin hydrolysates with biochemical properties; bio-adhesives | [99] | |
| Alkaline extraction | Washed, dried, and defatted chicken feathers by soaking in ether for 24 h; extraction of keratin from 50 g of feathers in 1 L of 1 M NaOH for 24 h, followed by stirring for 5 h at 50 °C; centrifugation at 10,000 rpm to remove biomass waste | Nitrogen source and pH regulator for microbial culture in the production of lactic acid bacteria from date pulp waste by fermentation | [126] | |
| Reduction by L-cysteine | Cleaned chicken feathers in 8 M urea and L-cysteine, 1:17 liquor ratio; pH 10.5 using 50% w/w NaOH; 12 h stirring at 70 °C; 10,000 rpm, 20 min centrifugation and filtration | Keratin fibers with potential application in the biomedical field for tissue engineering and drug delivery; high yield or recovery, 60% | [127] | |
| Enzymatic digestion with keratinase | Cibenza IND900 keratinase from B. licheniformis, 1 g in 30 mL phosphate buffer saline, pH 9; 1:30 to 1:30,000 dilution tested; 1 g feathers added to 3 mL of each dilution, 45 °C, 12 h reaction time | Recovery of glucocorticoids from feathers and other non-protein analytes from keratinous tissues | [128] | |
| Duck feathers | Ionic liquid | Feathers immersed in 8 M urea, 4 mM 1,4–dithiothreitol, or 8 mM cysteine, 1:17 liquor ratio, pH 8, 70 °C, 12 h. Oven-drying and pulverization | Keratin filaments with increased ductility with respect to natural feathers; 60% extraction yield | [129] |
| Imidazole ionic liquid | Different ratios of ionic liquid, feathers, Na2SO3, and water; separation of solid keratin from liquid by filtration | Keratin hydrolysate with a dissolution rate of 96.7% and extraction yield of ca. 75% | [112] | |
| Turkey feathers | Ionic liquids assisted by ultrasounds | 0.5 g of cleaned feathers in 20 mL ionic liquid; sonication at 20 kHz and varying powers, 120, 200, and 280 W, 130 °C; followed by mechanically stirring until complete feather dissolution in the solvent | Biodegradable films and other applications in materials; increased thermal stability of regenerated keratin compared to raw feathers; higher yield of recovery and lower extraction times when compared to conventional methods | [130] |
| Enzymatic treatment with alkaline keratinase produced from the Aspergillus sp. DHE7 | 20 g of feathers pre-treated with 1 L dimethyl-sulfoxide, heated at 100 °C for 2 h; 8000 g, 10 min, 4 °C centrifugation to collect the precipitate; 1 mL enzyme solution to 1 mL keratin solution (1% in 50 mM Tris-HCl, pH 8); incubation at 50 °C for 30 min, reaction stopped with 15% trichloroacetic acid; centrifugation to collect the supernatant | Culture media for Aspergillus sp. DHE7, which has potential applications in laundry detergents, biocatalysts, production of keratin hydrolysates for feed use | [131] | |
| Feather mix from poultry industry | Hydrolysis with microbial keratinases | Keratinase purification from Bacillus genus (B. licheniformis, B. subtilis, B. pumilus) and fungi (Microsporum fulvum, Paecilomyces marquandii). Complete solubilization of feathers achieved by incubation for 6 h, from 45 to 60 °C | Keratin-derived polypeptide chains that can be used to improve feed formulations, production of organic soil fertilizers and bioactive peptides with anti-hypertensive and antidiabetic capacity | [132] |
| Wool keratin | Reduction with L-cysteine | Wool fibers placed in a mixture of aqueous solution of 8 M urea and 0.165 M L-cysteine; pH adjusted at 10.5 with NaOH, followed by shaking at 75 °C for 5 h | Keratin powder with higher β-sheet, lower α-helix, and lower disordered structure contents than native wool | [28] |
| Moderate hydrolysis by keratinase | Wool immersion in a water solution at pH 10 and stirred for 1 h at 65 °C, followed by addition of keratinase under continuous stirring at 50 °C for 48 h, centrifugation, and freeze-drying | Biomedical materials, accelerated wool healing | [118] | |
| Sheep wool | Ionic liquids assisted by ultrasounds | Wool fibers washed in 1:1 v/v hexane and dichloromethane; 0.5 g of wool dried and cut into small pieces added to 10 mL of ionic liquids; ultrasonication at 130 kW, 50 Hz for 15 min; 4000 rpm, 15 min centrifugation at room temperature to collect the precipitate | High-molecular-weight keratin hydrolysates (37–75 kDa); innovative extraction technique with the potential for large-scale application | [133] |
| Merino wool | Multiple techniques (alkali hydrolysis, sulfitolysis, reduction, oxidation, ionic liquid) | Reduction: dried and defatted wool treated with urea and 2-mercaptoethanol; Sulfitolysis: wool treated with a mixture of urea and sodium metabisulfite; Alkali hydrolysis: wool treated with 2% w/w NaOH; Oxidation: wool oxidized with 2% w/v peracetic acid for 12 h at 25 °C; Ionic liquid: wool dissolution in 1-butyl-3-methylimidazolium chloride (BMIM) | Biomedical products without toxicity on fibroblast cells | [134] |
| Ionic liquid | Wool fibers cleaned with ether, cut into small pieces, and immersed into ionic liquid at a ratio of 1:6 w/w, at 120, 150, and 180 °C for 30 min. Distillation of the hot mixture and precipitation of water-insoluble keratin; 4000 rpm, 15 min centrifugation to remove the ionic liquid | High-molecular-weight keratin hydrolysates (35–75 kDa) for the production of stretchable keratin films/sheets | [135] | |
| Thermal treatment and electrospinning | Cleaned and ether-defatted wool fibers, cut in millimeter pieces, treated with 8 M urea, 0.5 M Na2S2O5, pH 6.5 adjusted with 5 M NaOH, 1:20 liquor ratio, 2 h reaction time under shaking, 65 °C; filtration and dialysis against distilled water; freeze-drying to obtain pure keratin powder, followed by electrospinning | Keratin hydrolysates of 11–60 kDa molecular weight; pure keratin nanofibers; novel thermal stabilization to enhance thermal and water stability of the obtained pure keratin extract | [136] | |
| Pig bristles | Enzymatic digestion and degradation by B. cereus (B5esz) under several conditions | Condition 1: thermo-chemical pre-treatment followed by enzymatic digestion; Condition 2: enzymatic digestion of untreated feathers in the presence of sulfite; Condition 3: thermo-chemical pre-treatment followed by microbial degradation | Solutions rich in branched amino acids. Biodegradation of bristles with B. cereus culture, instead of B5esz alone, resulted in a more complex peptide composition | [137] |
| Two-step pre-treatment followed by microbial digestion with bacteria | Bristle cleaning; culture of B. cereus PMC2849 on cleaned bristles followed by hydrolysis of 10 g of pig bristles in 250 mL distilled water and 50 mL broth of keratinase extracted from B. cereus PCM 2849; autoclavation of the mixture in a sodium sulfite solution (1 g of bristles in 100 mL) | Free amino acid mixture rich in branched residues, for non-feed application | [138] | |
| Thermal pre-treatment followed by microbial digestion with fungi cultivated with a novel fermentation technique | Chopped and thermally pre-treated bristles at 150 °C, 600 kPa, 20 min, dried and cut into 1.4 mm size; two-stage fermentation process by A. keratiniphila D2, in the 28–88 °C temperature range and 5–11 pH range | Keratin small peptides and free amino acids with high pepsin digestibility, 95%, with potential application in animal feed; high yield of recovery, 73% | [139] | |
| Thermal hydrolysis | Two heating steps: (i) swelling and denaturation of the keratin network, (ii) cleavage of the disulfide bonds. 20 g of washed and dried hog hair in 1 L deionized water and in stirring conditions; 3°C/min heating rate up to vapor generation, then from 100 to 220 °C to break S-S bonds | High-molecular-weight keratin hydrolysates (20–100 kDa) and a wide range of weight distribution; high yield (ca. 70%) and comparable to chemical processes; compared to soybean meals, on dry matter, the extracted hydrolysates can provide twice as much essential amino acid content | [140] | |
| Bovine hoofs | Reduction | Defatted hooves treated with 7 M urea, sodium lauryl sulfate, and 2-mercaptoethanol, under shaking for 12 h at 60 °C and pH 7 | Production of a biocompatible material for cellular attachment and biomedical applications | [141] |
5. Application of Keratin from Animal By-Products
5.1. Bio-Based Plastics
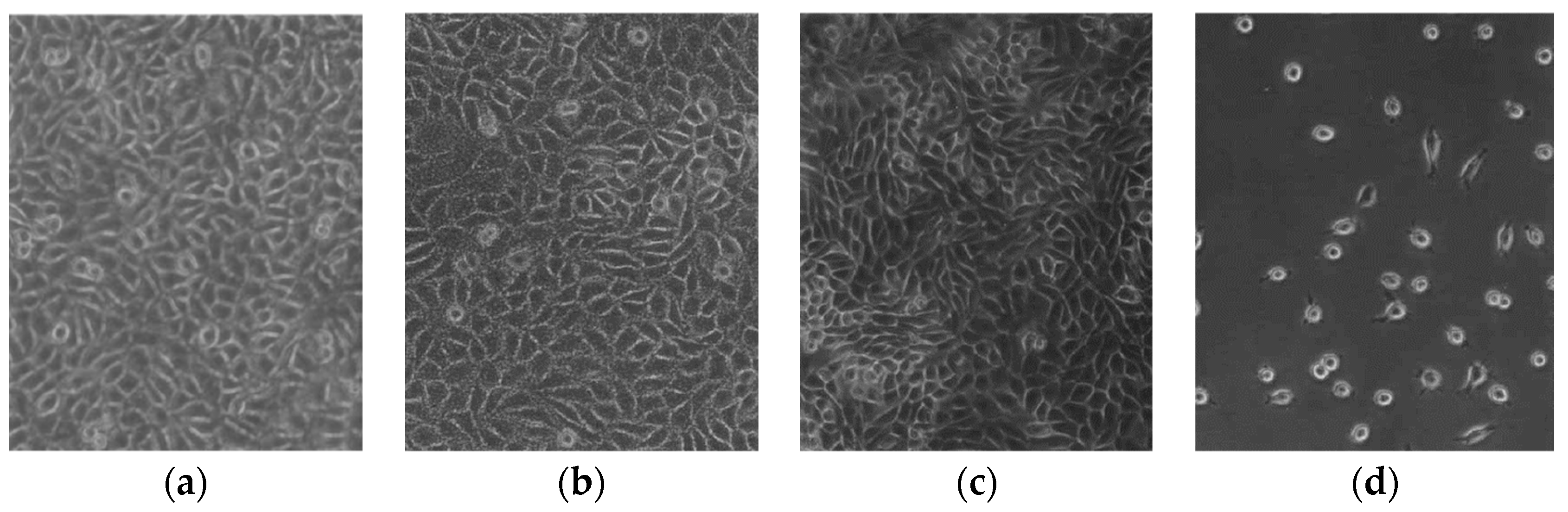
5.2. Biomedical Domain
5.3. Biosorbents
5.4. Biofertilizers
5.5. Cosmetics
5.6. Animal Feed
5.7. Energy Devices
6. Environmental and Economic Impact of Keratinous Animal By-Products and of Their Valorization
7. Future Trends
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, H.; Gao, S.; Li, Y.; Xu, H.J.; Li, W.; Wang, J.; Zhang, Y. Valorization of livestock keratin waste: Application in agricultural fields. Int. J. Environ. Res. Public Health 2022, 19, 6681. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, V.; Anton, M.; Santé-Lhoutellier, V. The “sisters” α-helices of collagen, elastin and keratin recovered from animal by-products: Functionality, bioactivity and trends of application. Trends Food Sci. Technol. 2016, 51, 65–75. [Google Scholar] [CrossRef]
- Timorshina, S.; Popova, E.; Osmolovskiy, A. Sustainable applications of animal waste proteins. Polymers 2022, 14, 1601. [Google Scholar] [CrossRef] [PubMed]
- Vineis, C.; Varesano, A.; Varchi, G.; Aluigi, A. Extraction and characterization of keratin from different biomasses. In Keratin as a Protein Biopolymer: Extraction from Waste Biomass and Applications; Sharma, S., Kumar, A., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 35–76. [Google Scholar] [CrossRef]
- Sarma, A. Biological importance and pharmaceutical significance of keratin: A review. Int. J. Biol. Macromol. 2022, 219, 395–413. [Google Scholar] [CrossRef]
- Wang, B.; Yang, W.; McKittrick, J.; Meyers, M.A. Keratin: Structure, mechanical properties, occurrence in biological organisms, and efforts at bioinspiration. Prog. Mater. Sci. 2016, 76, 229–318. [Google Scholar] [CrossRef]
- Er Rafik, M.; Doucet, J.; Briki, F. The intermediate filament architecture as determined by X-ray diffraction modeling of hard alpha-keratin. Biophys. J. 2004, 86, 3893–3904. [Google Scholar] [CrossRef] [PubMed]
- Donato, R.K.; Mija, A. Keratin associations with synthetic, biosynthetic and natural polymers: An extensive review. Polymers 2019, 12, 32. [Google Scholar] [CrossRef]
- Feroz, S.; Muhammad, N.; Ratnayake, J.; Dias, G. Keratin-Based materials for biomedical applications. Bioact. Mater. 2020, 5, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Market and Market. Bioplastics & Biopolymers Markets by Products Type (Biodegradable, Non-Biodegradable/Bio-Based), End-Use Industry (Packaging, Consumer Goods, Textiles, Agriculture & Horticulture, AnT, Coatings & Adhesives), & Region-global Forecast. 2024. Available online: https://www.marketsandmarkets.com/ (accessed on 7 June 2024).
- Ali, M.F.; Hossain, M.S.; Moin, T.S.; Ahmed, S.; Chowdhury, A.M.S. Utilization of waste chicken feather for the preparation of eco-friendly and sustainable composite. Clean. Eng. Technol. 2021, 4, 100190. [Google Scholar] [CrossRef]
- Shelyapina, M.G.; Rodríguez-Iznaga, I.; Petranovskii, V. Materials for CO2, SOx, and NOx emission reduction. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Kharissova, O.V., Torres-Martínez, L.M., Kharisov, B.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 2429–2458. [Google Scholar] [CrossRef]
- Fraser, R.D.B.; Macrae, T.P. Molecular structure and mechanical properties of keratins. Symp. Soc. Exp. Biol. 1980, 34, 211–246. [Google Scholar]
- Peng, Z.; Zhang, J.; Du, G.; Chen, J. Keratin waste recycling based on microbial degradation: Mechanisms and prospects. ACS Sustain. Chem. Eng. 2019, 7, 9727–9736. [Google Scholar] [CrossRef]
- Korniłłowicz-Kowalska, T.; Bohacz, J. Biodegradation of keratin waste: Theory and practical aspects. Waste Manag. 2011, 31, 1689–1701. [Google Scholar] [CrossRef] [PubMed]
- Toni, M.; Dalla Valle, L.; Alibardi, L. Hard (Beta-)keratins in the epidermis of reptiles: composition, sequence, and molecular organization. J. Proteome Res. 2007, 6, 3377–3392. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.-C.; Buehler, M.J. Structure and mechanical properties of human trichocyte keratin intermediate filament protein. Biomacromolecules 2012, 13, 3522–3532. [Google Scholar] [CrossRef]
- Fraser, R.D.B.; Macrae, T.P.; Sparrow, L.G.; Parry, D.A.D. Disulphide bonding in α-keratin. Int. J. Biol. Macromol. 1988, 10, 106–112. [Google Scholar] [CrossRef]
- Greenwold, M.J.; Bao, W.; Jarvis, E.D.; Hu, H.; Li, C.; Gilbert, M.T.P.; Zhang, G.; Sawyer, R.H. Dynamic evolution of the alpha (α) and beta (β) keratins has accompanied integument diversification and the adaptation of birds into novel lifestyles. BMC Evol. Biol. 2014, 14, 249. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Prajapati, B.P.; Kango, N.; Dey, K.K. A comprehensive and comparative study of the internal structure and dynamics of natural β-keratin and regenerated β-keratin by solid state NMR spectroscopy. Solid State Nucl. Magn. Reson. 2019, 101, 1–11. [Google Scholar] [CrossRef]
- Parry, D.A.D. Structure and topology of the linkers in the conserved lepidosaur β-keratin chain with four 34-residue repeats support an interfilament role for the central linker. J. Struct. Biol. 2020, 212, 107599. [Google Scholar] [CrossRef]
- Greenwold, M.J.; Sawyer, R.H. Molecular evolution and expression of archosaurian β-keratins: Diversification and expansion of archosaurian β-keratins and the origin of feather β-keratins. J. Exp. Zool. Part B Mol. Dev. Evol. 2013, 320, 393–405. [Google Scholar] [CrossRef]
- Marshall, R.C.; Gillespie, J.M. The keratin proteins of wool, horn and hoof from sheep. Aust. J. Biol. Sci. 1977, 30, 389–400. [Google Scholar] [CrossRef]
- Steinert, P.M.; Rogers, G.E. Characterization of the proteins of guinea-pig hair and hair-follicle tissue. Biochem. J. 1973, 135, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Rouse, J.G.; Van Dyke, M.E. A review of keratin-based biomaterials for biomedical applications. Materials 2010, 3, 999–1014. [Google Scholar] [CrossRef]
- Sheep Producers Australia. Sheep Sustainability Framework. 2023. Available online: https://www.sheepsustainabilityframework.com.au/globalassets/sheep-sustainability/media/sheep-sustainability-framework-2023-annual-report_updated-nov-23_web.pdf (accessed on 17 March 2024).
- Cardamone, J.M. Investigating the microstructure of keratin extracted from wool: Peptide sequence (MALDI-TOF/TOF) and protein conformation (FTIR). J. Mol. Struct. 2010, 969, 97–105. [Google Scholar] [CrossRef]
- Wang, K.; Li, R.; Ma, J.; Jian, Y.; Che, J. Extracting keratin from wool by using L-cysteine. Green Chem. 2016, 18, 476–481. [Google Scholar] [CrossRef]
- Fernández-d’Arlas, B. Tough and functional cross-linked bioplastics from sheep wool keratin. Sci. Rep. 2019, 9, 14810. [Google Scholar] [CrossRef] [PubMed]
- Bhari, R.; Kaur, M.; Sarup Singh, R. Chicken feather waste hydrolysate as a superior biofertilizer in agroindustry. Curr. Microbiol. 2021, 78, 2212–2230. [Google Scholar] [CrossRef] [PubMed]
- Henchion, M.; McCarthy, M.; Zimmermann, J.; Troy, D.J. International comparisons, domestic influences and where to next? The case of Irish meat consumption. Meat Sci. 2022, 193, 108921. [Google Scholar] [CrossRef]
- Fraser, R.D.B.; Parry, D.A.D. The structural basis of the filament-matrix texture in the avian/reptilian group of hard β-keratins. J. Struct. Biol. 2011, 173, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.; Yang, Y. Structure and properties of chicken feather barbs as natural protein fibers. J. Environ. Polym. Degrad. 2007, 15, 81–87. [Google Scholar] [CrossRef]
- Martínez-Hernández, A.; Velasco-Santos, C.; Icaza-Herrera, M.; Castaño, V. Microstructural characterisation of keratin fibres from chicken feathers. Int. J. Environ. Pollut. 2005, 23, 162–178. [Google Scholar] [CrossRef]
- Alahyaribeik, S.; Ullah, A. Methods of keratin extraction from poultry feathers and their effects on antioxidant activity of extracted keratin. Int. J. Biol. Macromol. 2020, 148, 449–456. [Google Scholar] [CrossRef]
- Yusuf, I.; Garba, L.; Shehu, M.A.; Oyiza, A.M.; Kabir, M.R.; Haruna, M. Selective biodegradation of recalcitrant black chicken feathers by a newly isolated thermotolerant bacterium Pseudochrobactrum sp. IY-BUK1 for enhanced production of keratinase and protein-rich hydrolysates. Int. Microbiol. 2020, 23, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Stilborn, H.; Moran, E.; Gous, R.; Harrison, M. Effect of age on feather amino acid content in two broiler strain crosses and sexes. J. Appl. Poult. Res. 1977, 6, 205–209. [Google Scholar] [CrossRef]
- Lazarus, B.S.; Chadha, C.; Velasco-Hogan, A.; Barbosa, J.D.V.; Jasiuk, I.; Meyers, M.A. Engineering with keratin: A functional material and a source of bioinspiration. iScience 2021, 24, 102798. [Google Scholar] [CrossRef] [PubMed]
- EUROSTAT. Agricultural Production—Livestock and Meat. 2023. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?oldid=427096#Meat_production (accessed on 7 June 2024).
- Kakkar, P.; Balaraman, M.; Shanmugam, G. Transient structures of keratins from hoof and horn influence their self association and supramolecular assemblies. Int. J. Biol. Macromol. 2016, 93, 172–178. [Google Scholar] [CrossRef]
- Gonzalo, M.; Jespersen, C.M.; Jensen, K.; Støier, S.; Meinert, L. Pig bristles—An underestimated biomass resource. In Proceedings of the 62th International Congress of Meat Science and Technology (ICoMST), Bangkok, Thailand, 14–19 August 2016. [Google Scholar]
- Riffel, A.; Lucas, F.; Heeb, P.; Brandelli, A. Characterization of a new keratinolytic bacterium that completely degrades native feather keratin. Arch. Microbiol. 2003, 179, 258–265. [Google Scholar] [CrossRef]
- Zuliani, A.; Muñoz-Batista, M.J.; Luque, R. Microwave-assisted valorization of pig bristles: Towards visible light photocatalytic Chalcocite composites. Green Chem. 2018, 20, 3001–3007. [Google Scholar] [CrossRef]
- Mohan, N.H.; Debnath, S.; Mahapatra, R.K.; Nayak, L.K.; Baruah, S.; Das, A.; Banik, S.; Tamuli, M.K. Tensile properties of hair fibres obtained from different breeds of pigs. Biosyst. Eng. 2014, 119, 35–43. [Google Scholar] [CrossRef]
- Georgiadis, M.; Müller, R.; Schneider, P. Techniques to assess bone ultrastructure organization: Orientation and arrangement of mineralized collagen fibrils. J. R. Soc. Interface 2016, 13, 20160088. [Google Scholar] [CrossRef]
- Falconer, R.J.; Markelz, A.G. Terahertz spectroscopic analysis of peptides and proteins. J. Infrared Millim. Terahertz Waves 2012, 33, 973–988. [Google Scholar] [CrossRef]
- Krimm, S.; Bandekar, J. Vibrational spectroscopy and conformation of peptides, polypeptides, and proteins. In Advances in Protein Chemistry; Anfinsen, C.B., Edsall, J.T., Richards, F.M., Eds.; Academic Press: Cambridge, MA, USA, 1986; Volume 8, pp. 181–364. [Google Scholar] [CrossRef]
- Bunaciu, A.A.; UdriŞTioiu, E.; Aboul-Enein, H. X-Ray Diffraction: Instrumentation and Applications. Crit. Rev. Anal. Chem. 2015, 45, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Millard, A.C.; Campagnola, P.J.; Mohler, W.; Lewis, A.; Loew, L.M. Second harmonic imaging microscopy. Methods Enzymol. 2003, 361, 47–69. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Apkarian, R.; Wang, Z.L.; Joy, D. Fundamentals of Scanning Electron Microscopy (SEM). In Scanning Microscopy for Nanotechnology: Techniques and Applications; Zhou, W., Wang, Z.L., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–40. [Google Scholar] [CrossRef]
- Gill, P.; Moghadam, T.T.; Ranjbar, B. Differential scanning calorimetry techniques: Applications in biology and nanoscience. J. Biomol. Tech. 2010, 21, 167–193. [Google Scholar] [PubMed]
- Haines, P.J.; Reading, M.; Wilburn, F.W. Differential Thermal Analysis and Differential Scanning Calorimetry. In Handbook of Thermal Analysis and Calorimetry; Brown, M.E., Ed.; Elsevier Science B.V.: Amsterdam, The Netherlands, 1998; pp. 279–361. [Google Scholar] [CrossRef]
- Wagner, M. Thermal Analysis in Practice: Collected Applications; Mettler-Toledo: Greifensee, Switzerland, 2009. [Google Scholar]
- Yang, H.; Yang, S.; Kong, J.; Dong, A.; Yu, S. Obtaining information about protein secondary structures in aqueous solution using Fourier transform IR spectroscopy. Nat. Protoc. 2015, 10, 382–396. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Pelton, J.T.; McLean, L.R. Spectroscopic methods for analysis of protein secondary structure. Anal. Biochem. 2000, 277, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Cebi, N.; Dogan, C.E.; Mese, A.E.; Ozdemir, D.; Arıcı, M.; Sagdic, O. A rapid ATR-FTIR spectroscopic method for classification of gelatin gummy candies in relation to the gelatin source. Food Chem. 2019, 277, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.Y.; Huang, P.Y.; Lee, Y.C.; Ng, C.S. Analysis and comparison of protein secondary structures in the rachis of avian flight feathers. PeerJ 2022, 10, e12919. [Google Scholar] [CrossRef] [PubMed]
- Mohan, N.; Choudhury, M.; Ammayappan, L.; Pathak, P.; Chakraborty, S.; Thomas, R.; Debnath, S.; Paul, M.; Sarma, D. Characterization of secondary structure of pig hair fiber using Fourier-transform Infrared Spectroscopy. J. Nat. Fibers 2022, 19, 4223–4235. [Google Scholar] [CrossRef]
- Fattinger, C.; Grischkowsky, D. Terahertz beams. Appl. Phys. Lett. 1989, 54, 490–492. [Google Scholar] [CrossRef]
- Beard, M.C.; Turner, G.M.; Schmuttenmaer, C.A. Terahertz Spectroscopy. J. Phys. Chem. B 2002, 106, 7146–7159. [Google Scholar] [CrossRef]
- Falconer, R.J.; Zakaria, H.A.; Fan, Y.Y.; Bradley, A.P.; Middelberg, A.P. Far-infrared spectroscopy of protein higher-order structures. Appl. Spectrosc. 2010, 64, 1259–1264. [Google Scholar] [CrossRef]
- Molloy, J.F.; Naftaly, M. Wool textile identification by terahertz spectroscopy. J. Text. Inst. 2014, 105, 794–798. [Google Scholar] [CrossRef]
- Cardamone, J.; Nunez, A.; Garcia, R.; Aldema-Ramos, M. Characterizing wool keratin. Res. Lett. Mater. Sci. 2009, 2009, 147175. [Google Scholar] [CrossRef]
- Welu, T.K.; Beyan, S.M.; Balakrishnan, S.; Admassu, H. Chicken feathers based keratin extraction process data analysis using response surface-box-behnken design method and characterization of keratin product. Curr. Appl. Sci. Technol. 2020, 20, 163–177. [Google Scholar] [CrossRef]
- Inkson, B.J. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) for materials characterization. In Materials Characterization Using Nondestructive Evaluation (NDE) Methods; Hübschen, G., Altpeter, I., Tschuncky, R., Herrmann, H.-G., Eds.; Woodhead Publishing: Sawston, UK, 2016; pp. 17–43. [Google Scholar] [CrossRef]
- Tang, C.Y.; Yang, Z. Transmission Electron Microscopy (TEM). In Membrane Characterization; Hilal, N., Ismail, A.F., Matsuura, T., Oatley-Radcliffe, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 145–159. [Google Scholar] [CrossRef]
- Saravanan, S.; Sameera, D.K.; Moorthi, A.; Selvamurugan, N. Chitosan scaffolds containing chicken feather keratin nanoparticles for bone tissue engineering. Int. J. Biol. Macromol. 2013, 62, 481–486. [Google Scholar] [CrossRef]
- Wang, B.; Meyers, M.A. Seagull feather shaft: Correlation between structure and mechanical response. Acta Biomater. 2017, 48, 270–288. [Google Scholar] [CrossRef]
- Campagnola, P.J.; Loew, L.M. Second-harmonic imaging microscopy for visualizing biomolecular arrays in cells, tissues and organisms. Nat. Biotechnol. 2003, 21, 1356–1360. [Google Scholar] [CrossRef]
- Campagnola, P.J.; Millard, A.C.; Terasaki, M.; Hoppe, P.E.; Malone, C.J.; Mohler, W.A. Three-dimensional high-resolution second-harmonic generation imaging of endogenous structural proteins in biological tissues. Biophys. J. 2002, 82, 493–508. [Google Scholar] [CrossRef]
- van Huizen, L.M.G.; Kuzmin, N.V.; Barbé, E.; van der Velde, S.; te Velde, E.A.; Groot, M.L. Second and third harmonic generation microscopy visualizes key structural components in fresh unprocessed healthy human breast tissue. J. Biophotonics 2019, 12, e201800297. [Google Scholar] [CrossRef]
- Chen, J.; Zhuo, S.; Luo, T.; Jiang, X.; Zhao, J. Spectral characteristics of autofluorescence and second harmonic generation from ex vivo human skin induced by femtosecond laser and visible lasers. Scanning 2006, 28, 319–326. [Google Scholar] [CrossRef]
- Whittig, L.D.; Allardice, W.R. X-ray Diffraction Techniques. In Methods of Soil Analysis Part 1. Physical and Mineralogical Methods; Black, C.A., Ed.; American Society of Agronomy—Soil Science Society of America: Madison, WI, USA, 1986; pp. 331–362. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Zhang, J.; Zhang, Y.; Han, Y.; Hu, J.; Li, Y. Synthesis and characterization of wool keratin/hydroxyapatite nanocomposite. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 896–902. [Google Scholar] [CrossRef]
- Al-Ashwal, B.; Gupta, A.; Bala Husain, M. Characterization of dehydrated keratin protein extracted from chicken feather. IOP Conf. Ser. Mater. Sci. Eng. 2019, 702, 012033. [Google Scholar] [CrossRef]
- Allec, N.; Choi, M.; Yesupriya, N.; Szychowski, B.; White, M.R.; Kann, M.G.; Garcin, E.D.; Daniel, M.-C.; Badano, A. Small-angle X-ray scattering method to characterize molecular interactions: Proof of concept. Sci. Rep. 2015, 5, 12085. [Google Scholar] [CrossRef]
- Mastroianni, A.J.; Sivak, D.A.; Geissler, P.L.; Alivisatos, A.P. Probing the conformational distributions of subpersistence length DNA. Biophys. J. 2009, 97, 1408–1417. [Google Scholar] [CrossRef]
- Saranathan, V.; Forster, J.; Noh, H.; Liew, S.F.; Mochrie, S.; Cao, H.; Dufresne, E.; Prum, R. Structure and optical function of amorphous photonic nanostructures from avian feather barbs: A comparative small angle X-ray scattering (SAXS) analysis of 230 bird species. J. R. Soc. Interface 2012, 9, 2563–2580. [Google Scholar] [CrossRef] [PubMed]
- Guntoro, P.I.; Ghorbani, Y.; Koch, P.-H.; Rosenkranz, J. X-ray microcomputed tomography (µCT) for mineral characterization: A review of data analysis methods. Minerals 2019, 9, 183. [Google Scholar] [CrossRef]
- Vásárhelyi, L.; Kónya, Z.; Kukovecz, Á.; Vajtai, R. Microcomputed tomography–based characterization of advanced materials: A review. Mater. Today Adv. 2020, 8, 100084. [Google Scholar] [CrossRef]
- Laurent, C.; Palmer, C.; Boardman, R.; Dyke, G.; Cook, R.B. Nanomechanical properties of bird feather rachises: Exploring naturally occurring fibre reinforced laminar composites. J. R. Soc. Interface 2014, 11, 20140961. [Google Scholar] [CrossRef]
- Laurent, C.; Ahmed, S.; Boardman, R.; Cook, R.; Dyke, G.; Palmer, C.; Schneider, P.; Roland, D.E.K. Imaging techniques for observing laminar geometry in the feather shaft cortex. J. Microsc. 2020, 277, 154–159. [Google Scholar] [CrossRef]
- Wen, J.; Arthur, K.; Chemmalil, L.; Muzammil, S.; Gabrielson, J.; Jiang, Y. Applications of Differential Scanning Calorimetry for thermal stability analysis of proteins: Qualification of DSC. J. Pharm. Sci. 2012, 101, 955–964. [Google Scholar] [CrossRef]
- de Castro Lima, C.R.R.; Machado, L.D.B.; Velasco, M.V.R.; do Rosário Matos, J. DSC measurements applied to hair studies. J. Therm. Anal. Calorim. 2018, 132, 1429–1437. [Google Scholar] [CrossRef]
- Cao, J.; Joko, K.; Cook, J.R. DSC studies of the melting behavior of α-form crystallites in wool keratin. Text. Res. J. 1997, 67, 117–123. [Google Scholar] [CrossRef]
- Cao, J. Melting study of the α-form crystallites in human hair keratin by DSC. Thermochim. Acta 1999, 335, 5–9. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mohanty, K. Pyrolysis kinetics and thermal behavior of waste sawdust biomass using thermogravimetric analysis. Bioresour. Technol. 2018, 251, 63–74. [Google Scholar] [CrossRef]
- Yang, J.; Hedin, N. Advances of lab-scale analytical methods for solidification/stabilization technologies. In Low Carbon Stabilization and Solidification of Hazardous Wastes; Tsang, D.C.W., Wang, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 483–495. [Google Scholar] [CrossRef]
- Ramakrishnan, N.; Sharma, S.; Gupta, A.; Alashwal, B.Y. Keratin based bioplastic film from chicken feathers and its characterization. Int. J. Biol. Macromol. 2018, 111, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Bingol, K.; Bruschweiler-Li, L.; Li, D.; Zhang, B.; Xie, M.; Brüschweiler, R. Emerging new strategies for successful metabolite identification in metabolomics. Bioanalysis 2016, 8, 557–573. [Google Scholar] [CrossRef]
- Markley, J.L.; Brüschweiler, R.; Edison, A.S.; Eghbalnia, H.R.; Powers, R.; Raftery, D.; Wishart, D.S. The future of NMR-based metabolomics. Curr. Opin. Biotechnol. 2017, 43, 34–40. [Google Scholar] [CrossRef]
- Emwas, A.-H.; Roy, R.; McKay, R.T.; Tenori, L.; Saccenti, E.; Gowda, G.A.N.; Raftery, D.; Alahmari, F.; Jaremko, L.; Jaremko, M.; et al. NMR spectroscopy for metabolomics research. Metabolites 2019, 9, 123. [Google Scholar] [CrossRef]
- Igumenova, T.I.; Frederick, K.K.; Wand, A.J. Characterization of the fast dynamics of protein amino acid side chains using NMR relaxation in solution. Chem. Rev. 2006, 106, 1672–1699. [Google Scholar] [CrossRef] [PubMed]
- Kricheldorf, H.R.; Müller, D. Secondary structure of peptides 16th. Characterization of proteins by means of 13C NMR CP/MAS spectroscopy. Colloid Polym. Sci. 1984, 262, 856–861. [Google Scholar] [CrossRef]
- Yee, A.; Chang, X.; Pineda-Lucena, A.; Wu, B.; Semesi, A.; Le, B.; Ramelot, T.; Lee, G.M.; Bhattacharyya, S.; Gutierrez, P.; et al. An NMR approach to structural proteomics. Proc. Natl. Acad. Sci. USA 2002, 99, 1825–1830. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.C.; Narkevicius, A.; Chow, W.Y.; Reid, D.G.; Rajan, R.; Brooks, R.A.; Green, M.; Duer, M.J. Solid state NMR of isotope labelled murine fur: A powerful tool to study atomic level keratin structure and treatment effects. J. Biomol. NMR 2016, 66, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Gupta, A.; Chik, S.; Kee, C.G.; Mistry, B.M.; Kim, D.H.; Sharma, G. Characterization of keratin microparticles from feather biomass with potent antioxidant and anticancer activities. Int. J. Biol. Macromol. 2017, 104 Pt A, 189–196. [Google Scholar] [CrossRef]
- Fagbemi, O.D.; Sithole, B.; Tesfaye, T. Optimization of keratin protein extraction from waste chicken feathers using hybrid pre-treatment techniques. Sustain. Chem. Pharm. 2020, 17, 100267. [Google Scholar] [CrossRef]
- Esparza, Y.; Bandara, N.; Ullah, A.; Wu, J. Hydrogels from feather keratin show higher viscoelastic properties and cell proliferation than those from hair and wool keratins. Mater. Sci. Eng. C. 2018, 90, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Chilakamarry, C.R.; Mahmood, S.; Saffe, S.; Arifin, M.A.B.; Gupta, A.; Sikkandar, M.Y.; Begum, S.S.; Narasaiah, B. Extraction and application of keratin from natural resources: A review. 3 Biotech 2021, 11, 220. [Google Scholar] [CrossRef]
- Dąbrowska, M.; Sommer, A.; Sinkiewicz, I.; Taraszkiewicz, A.; Staroszczyk, H. An optimal designed experiment for the alkaline hydrolysis of feather keratin. Environ. Sci. Pollut. Res. Int. 2022, 29, 24145–24154. [Google Scholar] [CrossRef]
- Alexander, P.; Earland, C. Structure of wool fibres; isolation of an alpha and beta-protein in wool. Nature 1950, 166, 396–397. [Google Scholar] [CrossRef]
- de Guzman, R.C.; Merrill, M.R.; Richter, J.R.; Hamzi, R.I.; Greengauz-Roberts, O.K.; Van Dyke, M.E. Mechanical and biological properties of keratose biomaterials. Biomaterials 2011, 32, 8205–8217. [Google Scholar] [CrossRef]
- Earland, C.; Knight, C.S. Studies on the structure of keratin: I. The analysis of fractions isolated from wool oxidized with peracetic acid. Biochim. Biophys. Acta 1955, 17, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Strasheim, A.; Buijs, K. An infra-red study of the oxidation of the disulphide bond in wool. Biochim. Biophys. Acta 1961, 47, 538–541. [Google Scholar] [CrossRef]
- Shavandi, A.; Bekhit, A.E.-D.A.; Carne, A.; Bekhit, A. Evaluation of keratin extraction from wool by chemical methods for bio-polymer application. J. Bioact. Compat. Polym. 2016, 32, 163–177. [Google Scholar] [CrossRef]
- Schrooyen, P.M.; Dijkstra, P.J.; Oberthür, R.C.; Bantjes, A.; Feijen, J. Partially carboxymethylated feather keratins. 2. Thermal and mechanical properties of films. J. Agric. Food Chem. 2001, 49, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.T.; Yang, G.; Yang, X.X.; Cao, Z.J.; Zhou, M.H. Preparation of regenerated keratin sponge from waste feathers by a simple method and its potential use for oil adsorption. Environ. Sci. Pollut. Res. Int. 2014, 21, 5730–5736. [Google Scholar] [CrossRef]
- Singh, S.K.; Savoy, A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Xie, H.; Li, S.; Zhang, S. Ionic liquids as novel solvents for the dissolution and blending of wool keratin fibers. Green Chem. 2005, 7, 606–608. [Google Scholar] [CrossRef]
- Ji, Y.; Chen, J.; Lv, J.; Li, Z.; Xing, L.; Ding, S. Extraction of keratin with ionic liquids from poultry feather. Sep. Purif. Technol. 2014, 132, 577–583. [Google Scholar] [CrossRef]
- Sharma, S.; Gupta, A. Sustainable management of keratin waste biomass: Applications and future perspectives. Braz. Arch. Biol. Technol. 2016, 59, e16150684. [Google Scholar] [CrossRef]
- Tamreihao, K.; Devi, L.J.; Khunjamayum, R.; Mukherjee, S.; Ashem, R.S.; Ningthoujam, D.S. Biofertilizing potential of feather hydrolysate produced by indigenous keratinolytic Amycolatopsis sp. MBRL 40 for rice cultivation under field conditions. Biocatal. Agric. Biotechnol. 2017, 10, 317–320. [Google Scholar] [CrossRef]
- Shestakova, A.; Timorshina, S.; Osmolovskiy, A. Biodegradation of keratin-rich husbandry waste as a path to sustainable agriculture. Sustainability 2021, 13, 8691. [Google Scholar] [CrossRef]
- Kunert, J. Physiology of keratinophilic fungi. Rev. Iberoam. Micol. 2000, 17, 77–85. [Google Scholar]
- Sinkiewicz, I.; Staroszczyk, H.; Sommer, A. Solubilization of keratins and functional properties of their isolates and hydrolysates. J. Food Biochem. 2018, 42, e12494. [Google Scholar] [CrossRef]
- Eslahi, N.; Dadashian, F.; Nejad, N.H. An investigation on keratin extraction from wool and feather waste by enzymatic hydrolysis. Prep. Biochem. Biotechnol. 2013, 43, 624–648. [Google Scholar] [CrossRef]
- Su, C.; Gong, J.-S.; Ye, J.-P.; He, J.-M.; Li, R.-Y.; Jiang, M.; Geng, Y.; Zhang, Y.; Chen, J.-H.; Xu, Z.-H.; et al. Enzymatic extraction of bioactive and self-assembling wool keratin for biomedical applications. Macromol. Biosci. 2020, 20, 2000073. [Google Scholar] [CrossRef]
- Chao, S.-J.; Chung, K.-H.; Lai, Y.-F.; Lai, Y.-K.; Chang, S.-H. Keratin particles generated from rapid hydrolysis of waste feathers with green DES/KOH: Efficient adsorption of fluoroquinolone antibiotic and its reuse. Int. J. Biol. Macromol. 2021, 173, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, W.; Yang, R. Steam flash explosion assisted dissolution of keratin from feathers. ACS Sustain. Chem. Eng. 2015, 3, 2036–2042. [Google Scholar] [CrossRef]
- Zoccola, M.; Aluigi, A.; Patrucco, A.; Vineis, C.; Forlini, F.; Locatelli, P.; Sacchi, M.C.; Tonin, C. Microwave-assisted chemical-free hydrolysis of wool keratin. Text. Res. J. 2012, 82, 2006–2018. [Google Scholar] [CrossRef]
- Eremeev, N.L.; Nikolaev, I.V.; Keruchen’ko, I.D.; Stepanova, E.V.; Satrutdinov, A.D.; Zinov’ev, S.V.; Ismailova, D.; Khotchenkov, V.P.; Tsurikova, N.V.; Sinitsyn, A.P.; et al. Enzymatic hydrolysis of keratin-containing stock for obtaining protein hydrolysates. Appl. Biochem. Microbiol. 2009, 45, 717–724. [Google Scholar] [CrossRef]
- Coward-Kelly, G.; Chang, V.; Agbogbo, F.; Holtzapple, M. Lime treatment of keratinous materials for the generation of highly digestible animal feed: 1. Chicken feathers. Bioresour. Technol. 2006, 97, 1337–1343. [Google Scholar] [CrossRef]
- Mokrejs, P.; Hutta, M.; Pavlackova, J.; Egner, P. Preparation of keratin hydrolysate from chicken feathers and its application in cosmetics. J. Vis. Exp. 2017, 129, 56254. [Google Scholar] [CrossRef]
- Ahmad, A.; Othman, I.; Tardy, B.L.; Hasan, S.W.; Banat, F. Enhanced lactic acid production with indigenous microbiota from date pulp waste and keratin protein hydrolysate from chicken feather waste. Bioresour. Technol. Rep. 2022, 18, 101089. [Google Scholar] [CrossRef]
- Ma, B.; Qiao, X.; Hou, X.; Yang, Y. Pure keratin membrane and fibers from chicken feather. Int. J. Biol. Macromol. 2016, 89, 614–621. [Google Scholar] [CrossRef]
- Alba, A.C.; Strauch, T.A.; Keisler, D.H.; Wells, K.D.; Kesler, D.C. Using a keratinase to degrade chicken feathers for improved extraction of glucocorticoids. Gen. Comp. Endocrinol. 2019, 270, 35–40. [Google Scholar] [CrossRef]
- Mi, X.; Li, W.; Xu, H.; Mu, B.; Chang, Y.; Yang, Y. Transferring feather wastes to ductile keratin filaments towards a sustainable poultry industry. Waste Manag. 2020, 115, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Azmi, N.A.; Idris, A.; Yusof, N.S.M. Ultrasonic technology for value added products from feather keratin. Ultrason. Sonochem. 2018, 47, 99–107. [Google Scholar] [CrossRef]
- El-Ghonemy, D.H.; Ali, T.H. Effective bioconversion of feather-waste keratin by thermo-surfactant stable alkaline keratinase produced from Aspergillus sp. DHE7 with promising biotechnological application in detergent formulations. Biocatal. Agric. Biotechnol. 2021, 35, 102052. [Google Scholar] [CrossRef]
- Brandelli, A.; Sala, L.; Kalil, S.J. Microbial enzymes for bioconversion of poultry waste into added-value products. Food Res. Int. 2015, 73, 3–12. [Google Scholar] [CrossRef]
- Feroz, S.; Muhammad, N.; Dias, G.; Alsaiari, M.A. Extraction of keratin from sheep wool fibres using aqueous ionic liquids assisted probe sonication technology. J. Mol. Liq. 2022, 350, 118595. [Google Scholar] [CrossRef]
- Shavandi, A.; Carne, A.; Bekhit, A.A.; Bekhit, A.E.-D.A. An improved method for solubilisation of wool keratin using peracetic acid. J. Environ. Chem. Eng. 2017, 5, 1977–1984. [Google Scholar] [CrossRef]
- Ghosh, A.; Clerens, S.; Deb-Choudhury, S.; Dyer, J.M. Thermal effects of ionic liquid dissolution on the structures and properties of regenerated wool keratin. Polym. Degrad. Stab. 2014, 108, 108–115. [Google Scholar] [CrossRef]
- Aluigi, A.; Zoccola, M.; Vineis, C.; Tonin, C.; Ferrero, F.; Canetti, M. Study on the structure and properties of wool keratin regenerated from formic acid. Int. J. Biol. Macromol. 2007, 41, 266–273. [Google Scholar] [CrossRef]
- Łaba, W.; Kopeć, W.; Chorążyk, D.; Kancelista, A.; Piegza, M.; Malik, K. Biodegradation of pretreated pig bristles by Bacillus cereus B5esz. Int. Biodeterior. Biodegrad. 2015, 100, 116–123. [Google Scholar] [CrossRef]
- Łaba, W.; Chorążyk, D.; Pudło, A.; Trojan-Piegza, J.; Piegza, M.; Kancelista, A.; Kurzawa, A.; Żuk, I.; Kopeć, W. Enzymatic degradation of pretreated pig bristles with crude keratinase of Bacillus cereus PCM 2849. Waste Biomass Valorization 2017, 8, 527–537. [Google Scholar] [CrossRef]
- Falco, F.C.; Espersen, R.; Svensson, B.; Gernaey, K.V.; Eliasson Lantz, A. An integrated strategy for the effective production of bristle protein hydrolysate by the keratinolytic filamentous bacterium Amycolatopsis keratiniphila D2. Waste Manag. 2019, 89, 94–102. [Google Scholar] [CrossRef]
- Tasaki, K. A novel thermal hydrolysis process for extraction of keratin from hog hair for commercial applications. Waste Manag. 2020, 104, 33–41. [Google Scholar] [CrossRef]
- Kakkar, P.; Madhan, B.; Shanmugam, G. Extraction and characterization of keratin from bovine hoof: A potential material for biomedical applications. SpringerPlus 2014, 3, 596. [Google Scholar] [CrossRef]
- Spierling, S.; Knüpffer, E.; Behnsen, H.; Mudersbach, M.; Krieg, H.; Springer, S.; Albrecht, S.; Herrmann, C.; Endres, H.J. Bio-based plastics—A review of environmental, social and economic impact assessments. J. Clean. Prod. 2018, 185, 476–491. [Google Scholar] [CrossRef]
- European Bioplastics. Bioplastics Market Update 2022. Available online: https://docs.european-bioplastics.org/publications/Report_Bioplastics%20Market%20Data_2022_short_version.pdf (accessed on 7 June 2024).
- Alshehhi, J.R.M.H.; Wanasingha, N.; Balu, R.; Mata, J.; Shah, K.; Dutta, N.K.; Choudhury, N.R. 3D-Printable sustainable bioplastics from gluten and keratin. Gels 2024, 10, 136. [Google Scholar] [CrossRef]
- John, M.W.M. Geotextiles; Chapman and Hall: New York, NY, USA, 1987; p. 2. [Google Scholar]
- Vadillo, J.; Montes, S.; Grande, H.J.; Verstichel, S.; Almqvist, J.; Wrześniewska-Tosik, K. Enhanced biodegradability in soil of chicken feather by steam explosion for potential application in agricultural biodegradable plastics. Polymers 2023, 15, 3701. [Google Scholar] [CrossRef]
- Shubha, A.; Sharmita, G.; Anita, L. Production and characterization of human hair keratin bioplastic films with novel plasticizers. Sci. Rep. 2024, 14, 1186. [Google Scholar] [CrossRef]
- Costa, F.; Silva, R.; Boccaccini, A.R. Fibrous protein-based biomaterials (silk, keratin, elastin, and resilin proteins) for tissue regeneration and repair. In Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair; Barbosa, M.A., Martins, M.C.L., Eds.; Woodhead Publishing: Sawston, UK, 2008; pp. 175–204. [Google Scholar] [CrossRef]
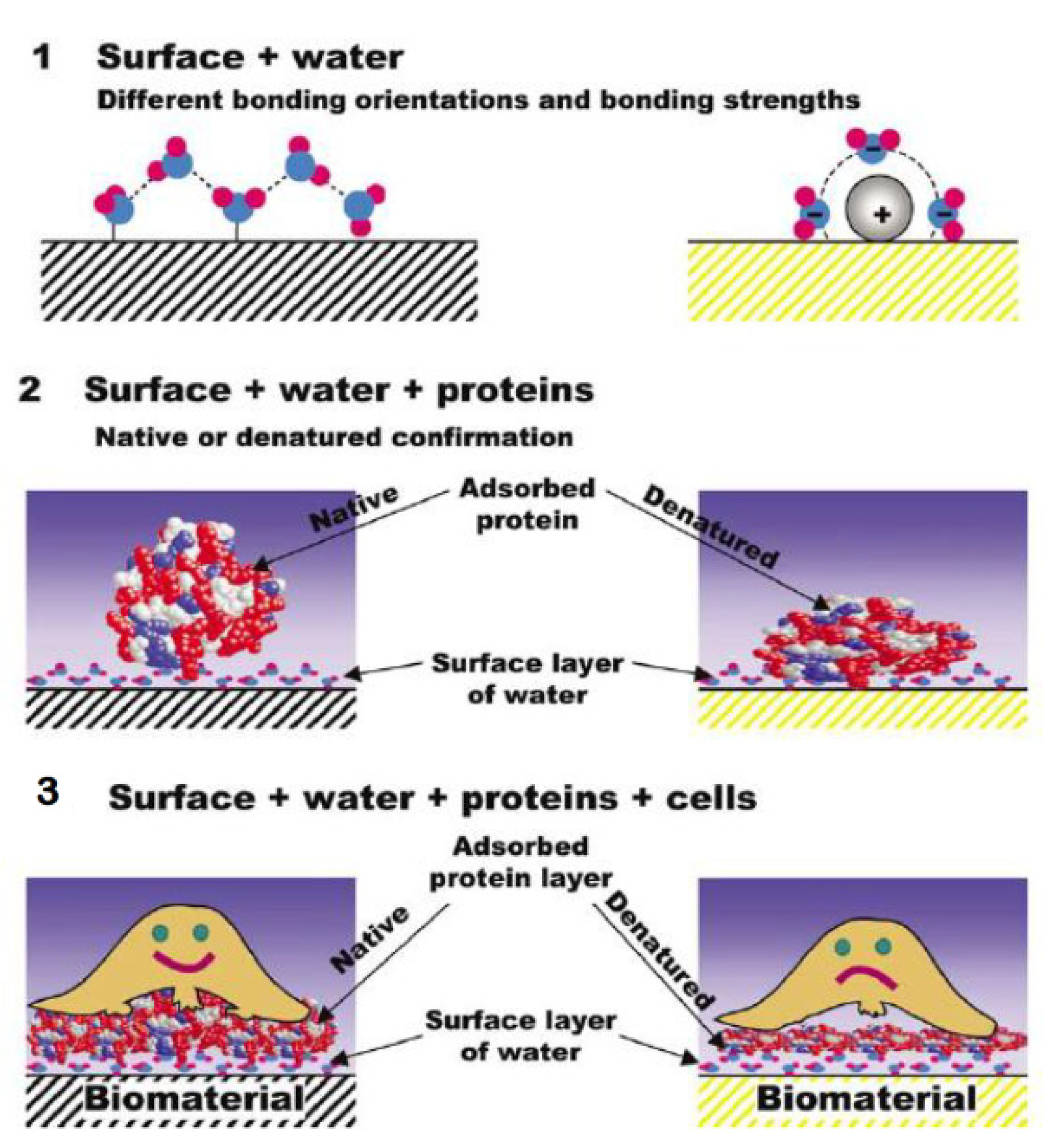
- Kasemo, B. Biological surface science. Surf. Sci. 2002, 500, 656–677. [Google Scholar] [CrossRef]
- Patrucco, A.; Visai, L.; Fassina, L.; Magenes, G.; Tonin, C. Keratin-based matrices from wool fibers and human hair. In Materials for Biomedical Engineering; Grumezescu, V., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 375–403. [Google Scholar] [CrossRef]
- Ranjit, E.; Stephen, H.; George, R.; Sharma, A.; Love, R.M. Biofunctional approaches of wool-based keratin for tissue engineering. J. Sci. Adv. Mater. Devices 2002, 7, 100398. [Google Scholar] [CrossRef]
- Belukhina, O.; Milasiene, D.; Ivanauskas, R. Investigation of the possibilities of wool fiber surface modification with copper selenide. Materials 2021, 14, 1648. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ramirez, D.O.; Vineis, C.; Cruz-Maya, I.; Tonetti, C.; Guarino, V.; Varesano, A. Wool keratin nanofibers for bioinspired and sustainable use in biomedical field. J. Funct. Biomater. 2023, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Meng, R.; Qing, R.; Li, W.; Wang, Z.; Hou, Y.; Deng, J.; Pu, W.; Gao, Z.; Wang, B.; et al. Bioinspired robust keratin hydrogels for biomedical applications. Nano Lett. 2022, 22, 8835–8844. [Google Scholar] [CrossRef] [PubMed]
- de Guzman, R.C.; Saul, J.M.; Ellenburg, M.D.; Merrill, M.R.; Coan, H.B.; Smith, T.L.; Van Dyke, M.E. Bone regeneration with BMP-2 delivered from keratose scaffolds. Biomaterials 2013, 34, 1644–1656. [Google Scholar] [CrossRef]
- Borrelli, M.; Witt, J.; Roth, M.; Reichl, S.; Bradenbrink, P.; Shoppe, M.; Schrader, S.; Geerling, G. Keratin films for ocular surface reconstruction: Wound healing in an in-vivo model. Exp. Eye Res. 2023, 227, 109356. [Google Scholar] [CrossRef]
- Ferroni, C.; Varchi, G. Keratin-based nanoparticles as drug delivery carriers. Appl. Sci. 2021, 11, 9417. [Google Scholar] [CrossRef]
- Tanabe, T.; Okitsu, N.; Tachibana, A.; Yamauchi, K. Preparation and characterization of keratin–chitosan composite film. Biomaterials 2002, 23, 817–825. [Google Scholar] [CrossRef]
- Donner, M.W.; Arshad, M.; Ullah, A.; Siddique, T. Unravelled keratin-derived biopolymers as novel biosorbents for the simultaneous removal of multiple trace metals from industrial wastewater. Sci. Total Environ. 2019, 647, 1539–1546. [Google Scholar] [CrossRef]
- Saha, S.; Zubair, M.; Khosa, M.A.; Song, S.; Ullah, A. Keratin and chitosan biosorbents for wastewater treatment: A review. J. Polym. Environ. 2019, 27, 1389–1403. [Google Scholar] [CrossRef]
- Petek, B.; Marinšek Logar, R. Management of waste sheep wool as valuable organic substrate in European Union countries. J. Mater. Cycles Waste Manag. 2021, 23, 44–54. [Google Scholar] [CrossRef]
- Li, Q. Perspectives on converting keratin-containing wastes into biofertilizers for sustainable agriculture. Front. Microbiol. 2022, 13, 918262. [Google Scholar] [CrossRef]
- Zainuddin, N.; Keni, M.F.; Ibrahim, S.A.S.; Masri, M.M.M. Effect of integrated biofertilizers with chemical fertilizers on the oil palm growth and soil microbial diversity. Biocatal. Agric. Biotechnol. 2022, 39, 102237. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef]
- Mokrejs, P.; Hutta, M.; Pavlackova, J.; Egner, P.; Benicek, L. The cosmetic and dermatological potential of keratin hydrolysate. J. Cosmet. Dermatol. 2017, 16, 21–27. [Google Scholar] [CrossRef]
- Burnett, C.L.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety assessment of keratin and keratin-derived ingredients as used in cosmetics. Int. J. Toxicol. 2021, 40 (Suppl. S2), 36S–51S. [Google Scholar] [CrossRef]
- Lusiana; Reichl, S.; Müller-Goymann, C.C. Keratin film made of human hair as a nail plate model for studying drug permeation. Eur. J. Pharm. Biopharm. 2011, 78, 432–440. [Google Scholar] [CrossRef]
- Ramírez Mileo, R.; Martí Gelabert, M.; Garay Peral, I.; Manich Bou, A.M.; Parra Juez, J.L.; Coderch Negra, L. Ceramides extracted from wool: Supercritical extraction processes. Text. Res. J. 2009, 79, 721–727. [Google Scholar] [CrossRef]
- Dalev, P.G. Utilisation of waste feathers from poultry slaughter for production of a protein concentrate. Bioresour. Technol. 1994, 48, 265–267. [Google Scholar] [CrossRef]
- Dalev, P.; Ivanov, I.; Liubomirova, A. Enzymic modification of feather keratin hydrolysates with lysine aimed at increasing the biological value. J. Sci. Food Agric. 1997, 73, 242–244. [Google Scholar] [CrossRef]
- Dias, G.J.; Haththotuwa, T.N.; Rowlands, D.S.; Gram, M.; Bekhit, A.E.-D.A. Wool keratin—A novel dietary protein source: Nutritional value and toxicological assessment. Food Chem. 2022, 383, 132436. [Google Scholar] [CrossRef] [PubMed]
- Das, R.S.; Tiwari, B.K.; Chemat, F.; Garcia-Vaquero, M. Impact of ultrasound processing on alternative protein systems: Protein extraction, nutritional effects and associated challenges. Ultrason. Sonochem. 2022, 91, 106234. [Google Scholar]
- Soon, W.L.; Peydayesh, M.; de Wild, T.; Donat, F.; Saran, R.; Müller, C.R.; Gubler, L.; Mezzenga, R.; Miserez, A. Renewable energy from livestock waste valorization: Amyloid-based feather keratin fuel cells. ACS Appl. Mater. Interfaces 2023, 15, 47049–47057. [Google Scholar] [CrossRef] [PubMed]
- Baumbgarther, M.; Hartmann, F.; Drack, M.; Preninger, D.; Wirthl, D.; Gerstmayr, R.; Lehner, L.; Mao, G.; Pruckner, R.; Demchyshyn, S.; et al. Resilient yet entirely degradable gelatin-based biogels for soft robots and electronics. Nat. Mater. 2020, 19, 1102–1109. [Google Scholar] [CrossRef]
- Scherhaufer, S.; Moates, G.; Hartikainen, H.; Waldron, K.; Obersteiner, G. Environmental impacts of food waste in Europe. Waste Manag. 2018, 77, 98–113. [Google Scholar] [CrossRef]
- OECD/FAO. Meat. In OECD-FAO Agricultural Outlook 2023–2032; OECD Publishing: Paris, France, 2023; Chapter 6; pp. 184–206. [Google Scholar] [CrossRef]
- Colantoni, A.; Recchia, L.; Bernabei, G.; Cardarelli, M.; Rouphael, Y.; Colla, G. Analyzing the environmental impact of chemically-produced protein hydrolysate from leather waste vs. enzymatically-produced protein hydrolysate from legume grains. Agriculture 2017, 7, 62. [Google Scholar] [CrossRef]
- Cruz-Casas, D.E.; Aguilar, C.N.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R.; Chávez-González, M.L.; Flores-Gallegos, A.C. Enzymatic hydrolysis and microbial fermentation: The most favorable biotechnological methods for the release of bioactive peptides. Food Chem. 2021, 23, 100047. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, M. Ionic Liquids toxicity—Benefits and threats. Int. J. Mol. Sci. 2020, 21, 6267. [Google Scholar] [CrossRef]
- Zheng, J.; Suh, S. Strategies to reduce the global carbon footprint of plastics. Nat. Clim. Chang. 2019, 9, 374–378. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banasaz, S.; Ferraro, V. Keratin from Animal By-Products: Structure, Characterization, Extraction and Application—A Review. Polymers 2024, 16, 1999. https://doi.org/10.3390/polym16141999
Banasaz S, Ferraro V. Keratin from Animal By-Products: Structure, Characterization, Extraction and Application—A Review. Polymers. 2024; 16(14):1999. https://doi.org/10.3390/polym16141999
Chicago/Turabian StyleBanasaz, Shahin, and Vincenza Ferraro. 2024. "Keratin from Animal By-Products: Structure, Characterization, Extraction and Application—A Review" Polymers 16, no. 14: 1999. https://doi.org/10.3390/polym16141999
APA StyleBanasaz, S., & Ferraro, V. (2024). Keratin from Animal By-Products: Structure, Characterization, Extraction and Application—A Review. Polymers, 16(14), 1999. https://doi.org/10.3390/polym16141999





