Optimized Design of Material Preparation for Cotton Linters-Based Carbon Black Dispersion Stabilizers Based on Response Surface Methodology
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Drugs and Instruments
2.2. Synthesis of CMC-AMPS-AM Hyperdispersan
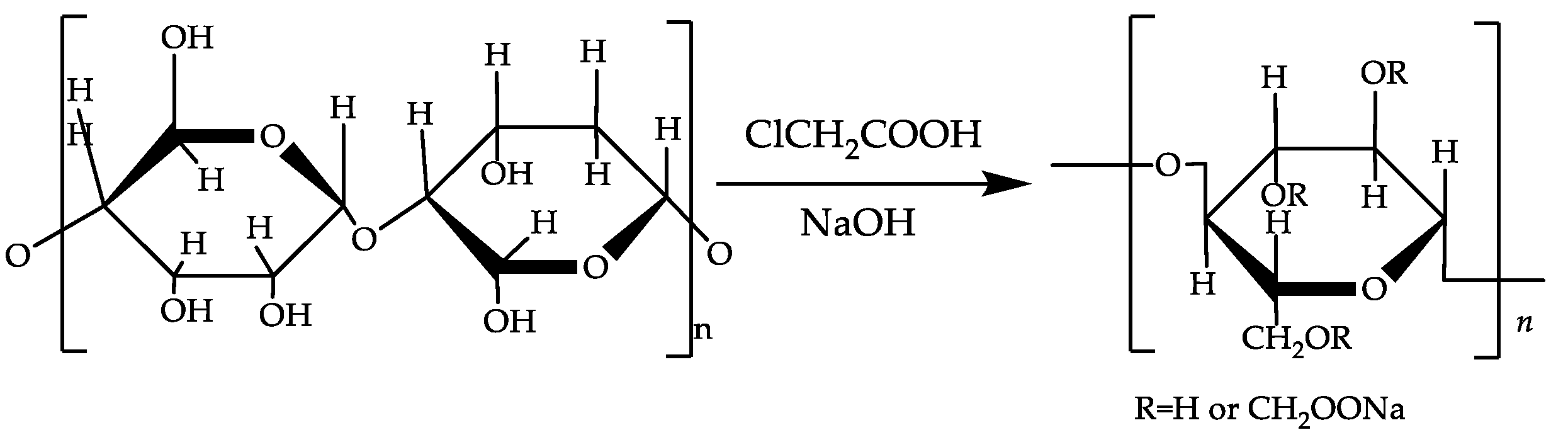
2.2.1. The Mechanism of Cotton Linters Staple Cellulose Modification
2.2.2. Sample Preparation
2.3. Conversion Rate Calculation
2.4. Response Surface Optimisation Experimental Design
2.5. Description
3. Results
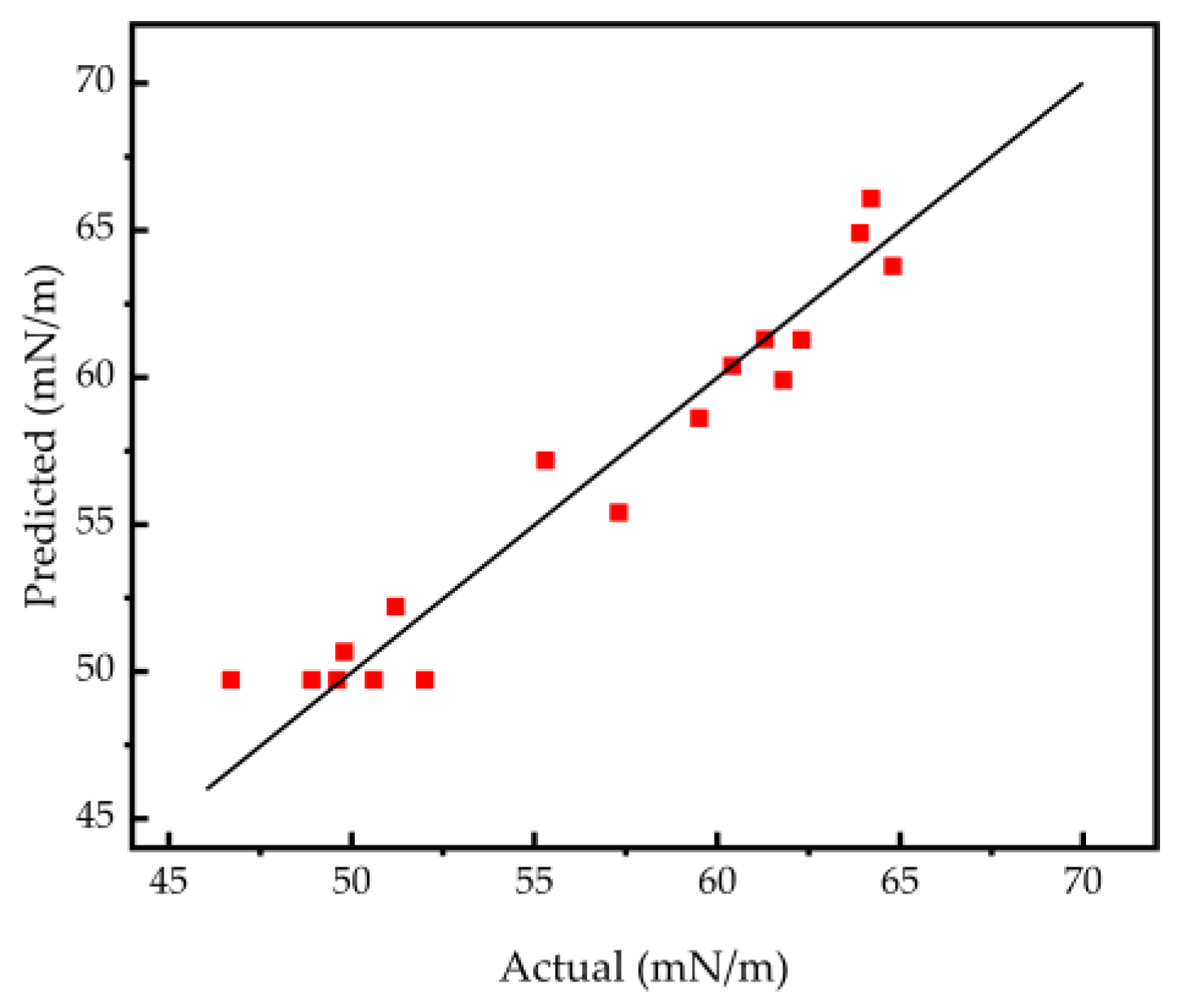
3.1. Response Surface Modelling and Analysis of Variance (ANOVA)
3.2. Response Surface 3D Graph
3.3. Carbon Black Dispersion Stability Performance
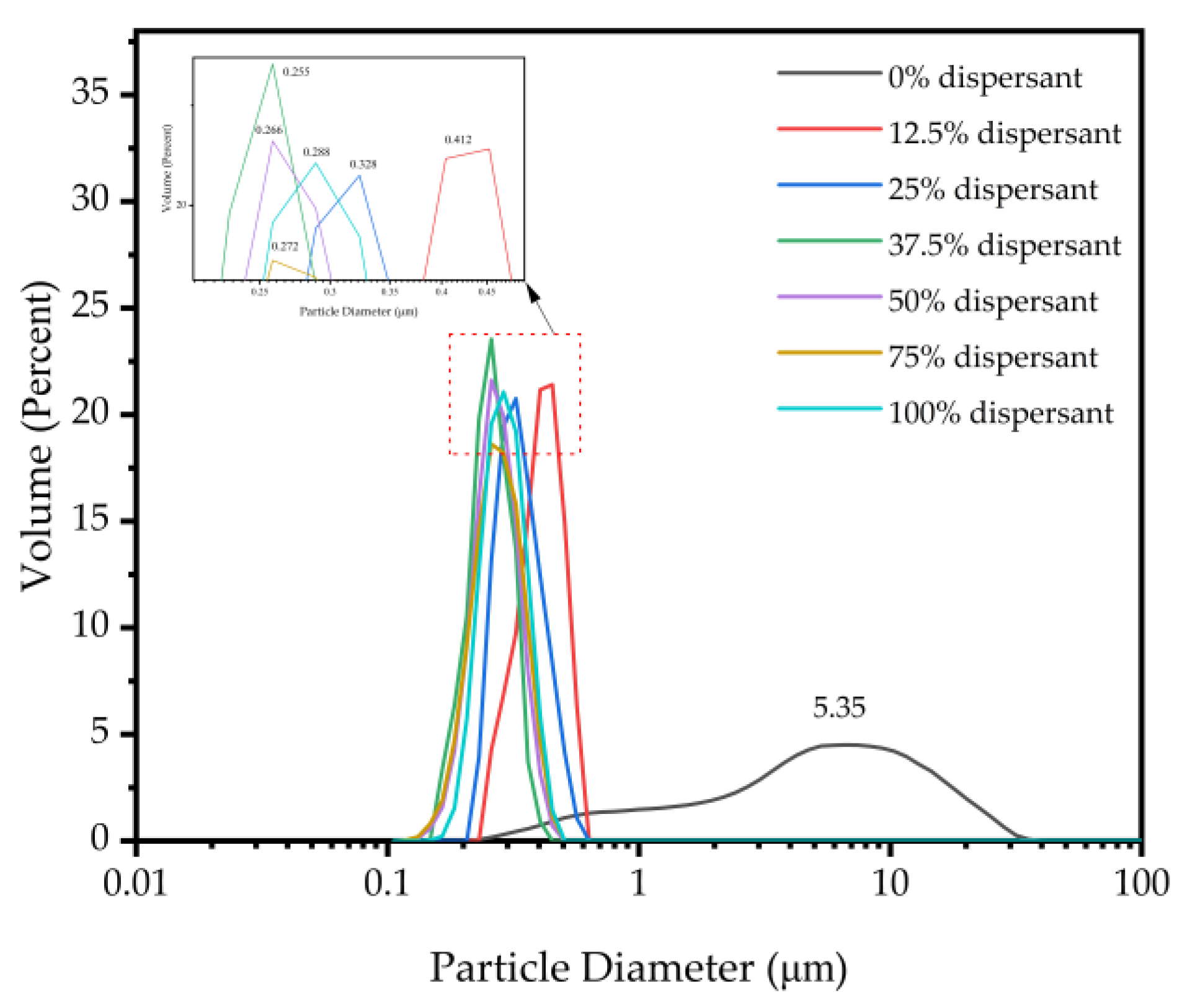
3.3.1. Effect of Dispersant Concentration
3.3.2. Zeta Potential Analysis
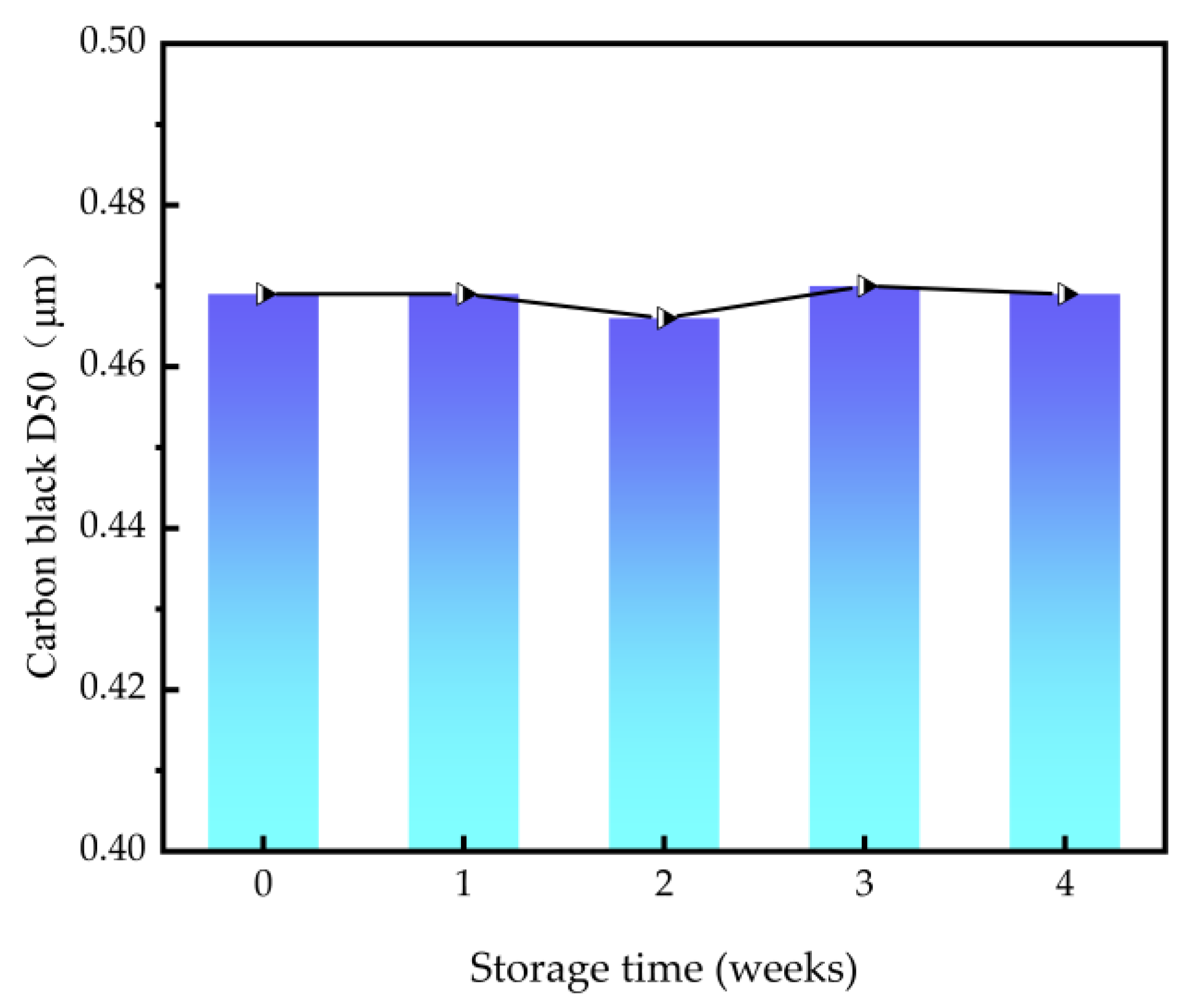
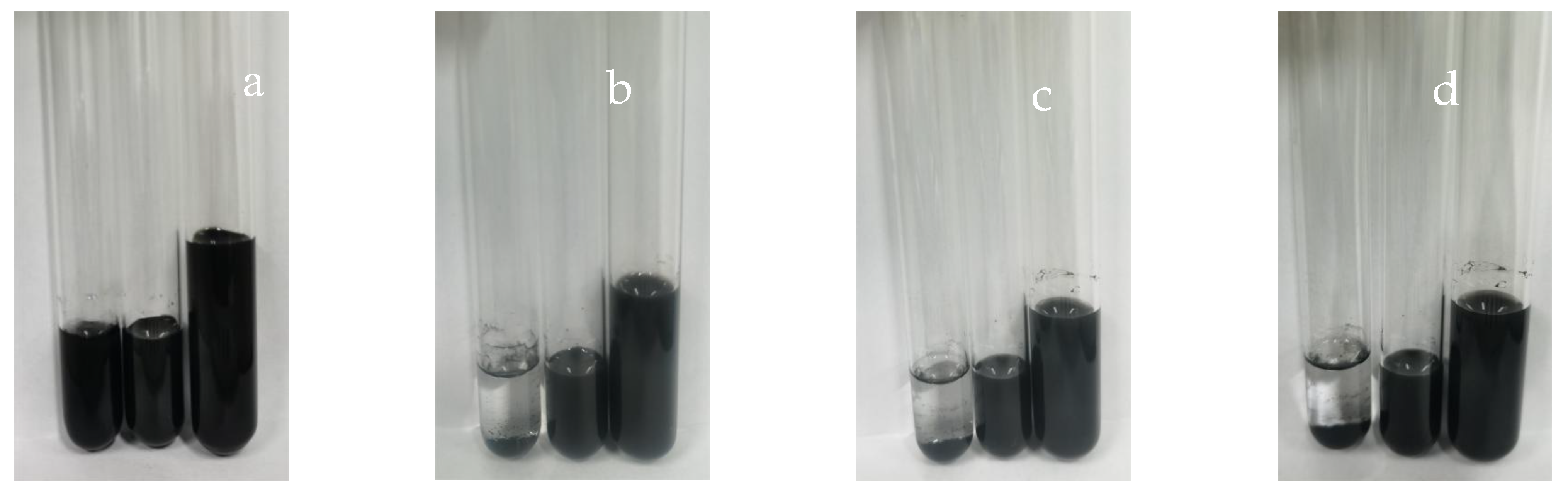
3.3.3. Stability of Carbon Black Slurry
3.3.4. Scanning Electron Microscopy (SEM) Analysis
3.4. Characterisation of Dispersant Materials
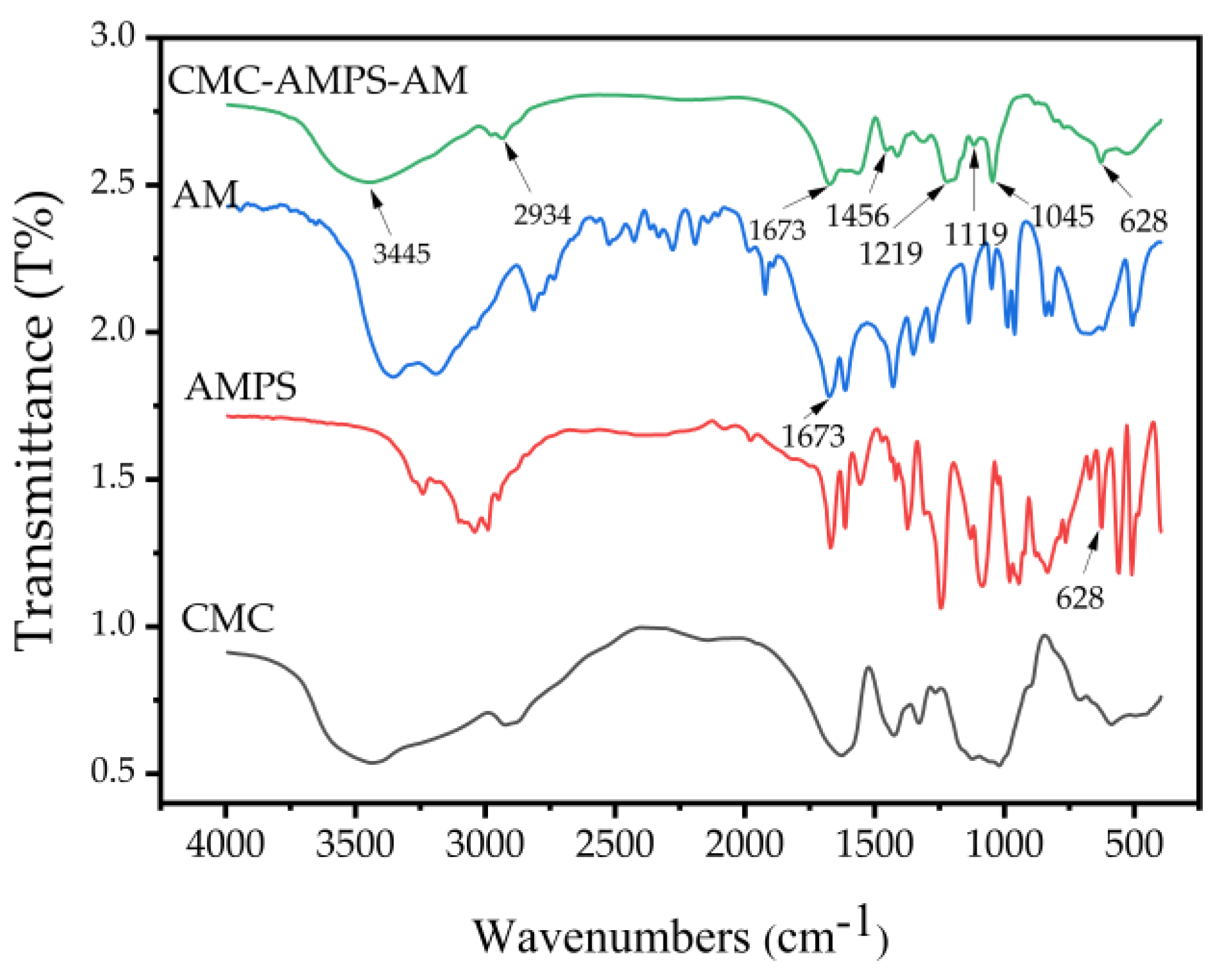
3.4.1. Infrared Spectroscopy Test (FTIR) Analysis
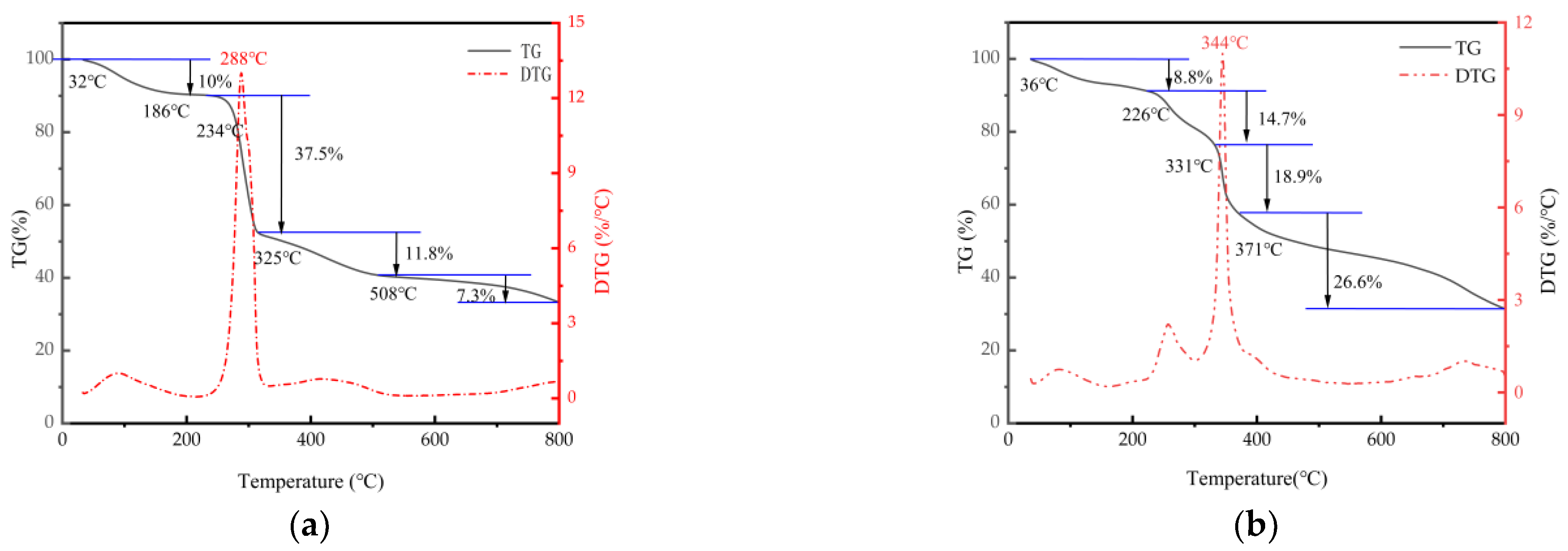
3.4.2. Thermogravimetric (DT) Analysis
3.4.3. X-ray Diffraction (XRD) Analysis
3.4.4. Molecular Weight Testing
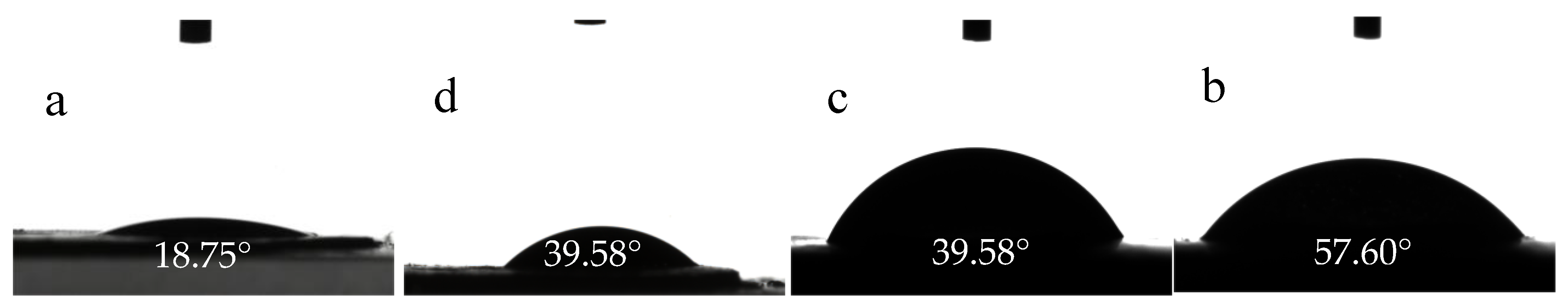
3.4.5. Contact Angle Analysis
3.5. Dissemination Mechanisms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, J.; Yan, W.; Chen, Z.; Liu, Y.; Hong, S. Effect of nano-carbon black content on wetting phenomenon of molten steel and alumina–carbon ceramic filter substrates. J. Iron Steel Res. Int. 2024, 1–14. [Google Scholar] [CrossRef]
- Pu, Z.; Fan, X.; Su, J.; Zhu, M.; Jiang, Z. Aqueous dispersing mechanism study of nonionic polymeric dispersant for organic pigments. Colloid Polym. Sci. 2022, 300, 167–176. [Google Scholar] [CrossRef]
- Lin, Q.; Gao, M.; Chang, J.; Ma, H. Adsorption properties of crosslinking carboxymethyl cellulose grafting dimethyldiallylammonium chloride for cationic and anionic dyes. Carbohydr. Polym. 2016, 151, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.H.; Pazhouhanfar, Y.; Delbari, S.A.; Shaddel, S.; Babapoor, A.; Mohammadpourderakhshi, Y.; Van Le, Q.; Shokouhimehr, M.; Asl, M.S.; Namini, A.S. Beneficial role of carbon black on the properties of TiC ceramics. Ceram. Int. 2020, 46, 23544–23555. [Google Scholar] [CrossRef]
- Goh, Y.; Lauro, S.; Barber, S.T.; Williams, S.A.; Trabold, T.A. Cleaner production of flexographic ink by substituting carbon black with biochar. J. Clean. Prod. 2021, 324, 129262. [Google Scholar] [CrossRef]
- Chollakup, R.; Suethao, S.; Suwanruji, P.; Boonyarit, J.; Smitthipong, W. Mechanical properties and dissipation energy of carbon black/rubber composites. Compos. Adv. Mater. 2021, 30, 26349833211005476. [Google Scholar] [CrossRef]
- Ji, C.; Zhang, Q.; Jing, Z.; Liu, Y.; Han, D.; Wang, J.; Zhang, W.; Sang, S. Highly sensitive wearable flexible pressure sensor based on conductive carbon black/sponge. IEEE Trans. Electron Devices 2021, 68, 5198–5203. [Google Scholar] [CrossRef]
- Du, W.; Liu, J.; Li, Z. High self-dispersibility carbon black particles prepared via hydroxylation and urethane chains encapsulation for enhancing properties of waterborne polyurethane composite films. Colloids Surf. A Physicochem. Eng. Asp. 2018, 543, 46–55. [Google Scholar] [CrossRef]
- Ali, M.; Lin, L.; Cartridge, D. High electrical conductivity waterborne dispersions of carbon black pigment. Progr. Org. Coat. 2019, 129, 199–208. [Google Scholar] [CrossRef]
- Jansen, J.; Kraus, G. New Methods for Estimating Dispersibility of Carbon Blacks in Rubber. Rubber Chem. Technol. 1980, 53, 48–65. [Google Scholar] [CrossRef]
- Ghose, T. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Wei, S.; Wu, X.; Li, X. Solubility analysis of nano particles, cellulose crystalline region and cellulose molecule, and the impact study of crystalline region on properties of cellulose insulating paper. Mol. Simul. 2021, 47, 1522–1529. [Google Scholar] [CrossRef]
- Srivastava, A.; Mandal, P.; Kumar, R. Solid state thermal degradation behaviour of graft copolymers of carboxymethyl cellulose with vinyl monomers. Int. J. Biol. Macromol. 2016, 87, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Lu, Y.; Feng, K.; Zhang, C.; Feng, X.; Zhao, Y.; Chen, L. Preparation of the self-accelerating photocatalytic self-cleaning carboxymethyl cellulose sodium-based hydrogel for removing cationic dyes. Int. J. Biol. Macromol. 2023, 250, 125891. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lv, J.; Li, D.; Wang, L. Rapid fabrication of TiO2@ carboxymethyl cellulose coatings capable of shielding UV, antifog and delaying support aging. Carbohydr. Polym. 2017, 169, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Su, B.; Wang, G.; Meng, Q.; Wang, J.; Zhang, C. Terahertz absorption characteristics of the sodium carboxymethyl cellulose colloid based on microfluidic technology. Int. J. Opt. 2021, 2021, 5555325. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, G.; Wang, Y.; Qin, X.; Lu, Y.; Wu, Z.; Zhang, C.; Xu, L.; Han, J.; Yang, S. l-Arginine carboxymethyl cellulose hydrogel releasing nitric oxide to improve wound healing. Eur. Polym. J. 2023, 189, 111940. [Google Scholar] [CrossRef]
- Magdassi, S.; Bassa, A.; Vinetsky, Y.; Kamyshny, A. Silver nanoparticles as pigments for water-based ink-jet inks. Chem. Mater. 2003, 15, 2208–2217. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, Y. Dispersion characterizations and adhesion properties of epoxy composites reinforced by carboxymethyl cellulose surface treated carbon nanotubes. Powder Technol. 2022, 404, 117505. [Google Scholar] [CrossRef]
- He, X.; Wu, S.; Fu, D.; Ni, J. Preparation of sodium carboxymethyl cellulose from paper sludge. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2009, 84, 427–434. [Google Scholar] [CrossRef]
- Ismail, N.; Bono, A.; Valintinus, A.; Nilus, S.; Chng, L. Optimization of reaction conditions for preparing carboxymethyl cellulose. J. Appl. Sci. 2010, 10, 2530–2536. [Google Scholar] [CrossRef][Green Version]
- Saputra, A.H.; Qadhayna, L.; Pitaloka, A.B. Synthesis and characterization of carboxymethyl cellulose (CMC) from water hyacinth using ethanol-isobutyl alcohol mixture as the solvents. Int. J. Chem. Eng. Appl. 2014, 5, 36. [Google Scholar] [CrossRef]
- Batelaan, J.; Van Ginkel, C.; Balk, F. Carboxymethylcellulose (cmc). In Detergents; Springer: Berlin/Heidelberg, Germany, 1992; pp. 329–336. [Google Scholar]
- Liu, Y.; Wan, X.; Xiang, X.; Li, Y.; Wu, S. Preparation of nano zero-valent iron-microfibrillated cellulose and its application in papermaking wastewater advanced treatment. China Pulp Pap. 2015, 34, 21–26. [Google Scholar]
- Zhang, H.; Lou, T.; Wang, X. Synthesis and properties of xanthan gum-acrylamide-carboxymethyl cellulose ternary anionic flocculants. J. Water Process Eng. 2023, 56, 104455. [Google Scholar] [CrossRef]
- Mao, J.; Guanhua, N.; Yuhang, X.; Hui, W.; Zhao, L.; Zhenyang, W. Modeling and optimization of mechanical properties of drilling sealing materials based on response surface method. J. Clean. Prod. 2022, 377, 134452. [Google Scholar] [CrossRef]
- Ofgea, N.M.; Tura, A.M.; Fanta, G.M. Activated carbon from H3PO4-activated Moringa Stenopetale Seed Husk for removal of methylene blue: Optimization using the response surface method (RSM). Environ. Sustain. Indic. 2022, 16, 100214. [Google Scholar] [CrossRef]
- Subramanian, S.; Øye, G. Aqueous carbon black dispersions stabilized by sodium lignosulfonates. Colloid Polym. Sci. 2021, 299, 1223–1236. [Google Scholar] [CrossRef]
- Jiang, S.; Bai, J.; Ying, X.; Han, J.; Zhu, K.; Mao, L. Synthesis and performance investigation of carbon black hyperdispersant IMD. J. Coat. Technol. Res. 2023, 20, 1529–1539. [Google Scholar] [CrossRef]
- Lee, P.T.; Chiu, C.-W.; Chang, L.-Y.; Chou, P.-Y.; Lee, T.-M.; Chang, T.-Y.; Wu, M.-T.; Cheng, W.-Y.; Kuo, S.-W.; Lin, J.-J. Tailoring pigment dispersants with polyisobutylene twin-tail structures for electrowetting display application. ACS Appl. Mater. Interfaces 2014, 6, 14345–14352. [Google Scholar] [CrossRef] [PubMed]
- Ramaye, Y.; Dabrio, M.; Roebben, G.; Kestens, V. Development and validation of optical methods for zeta potential determination of silica and polystyrene particles in aqueous suspensions. Materials 2021, 14, 290. [Google Scholar] [CrossRef]
- Fathi, A.; Hatami, K.; Grady, B.P. Effect of carbon black structure on low-strain conductivity of polypropylene and low-density polyethylene composites. Polym. Eng. Sci. 2012, 52, 549–556. [Google Scholar] [CrossRef]
- Liu, A.; Tian, H.; Ju, X.; Wang, W.; Han, P.; Li, W. In-situ growth of layered double hydroxides nanosheet arrays on graphite fiber as highly dispersed nanofillers for polymer coating with excellent anticorrosion performances. J. Taiwan Instit. Chem. Eng. 2019, 104, 330–340. [Google Scholar] [CrossRef]
- Muranaka, M.; Yoshizawa, H.; Ono, T. Design of polylactide-grafted copolymeric stabilizer for dispersion polymerization of D, L-lactide. Colloid Polym. Sci. 2009, 287, 525–532. [Google Scholar] [CrossRef]
- Pirrung, F.O.; Quednau, P.H.; Auschra, C. Wetting and dispersing agents. Chimia 2002, 56, 170. [Google Scholar] [CrossRef]
- Huo, J.H.; Peng, Z.G.; Ye, Z.b.; Feng, Q.; Zheng, Y.; Zhang, J.; Liu, X. Preparation, characterization, and investigation of poly (AMPS/AM/SSS) on application performance of water-based drilling fluid. J. Appl. Polym. Sci. 2018, 135, 46510. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, G.; Li, Y. Preparation of chitosan/polyacrylamide/graphene oxide composite membranes and study of their methylene blue adsorption properties. Materials 2020, 13, 4407. [Google Scholar] [CrossRef]
- Kukobat, R.; Hayashi, T.; Matsuda, T.; Sunaga, M.; Futamura, R.; Sakai, T.; Kaneko, K. Essential role of viscosity of SWCNT inks in homogeneous conducting film formation. Langmuir 2016, 32, 6909–6916. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yu, Y.; Xiong, D.; Qi, Z.; Fu, S.; Yu, X. Effects of chemical dispersant on the surface properties of kaolin and aggregation with spilled oil. Environ. Sci. Pollut. Res. 2022, 29, 30496–30506. [Google Scholar] [CrossRef]
- Kang, Y.; Zhu, A. Synthesis of SMA-PEG hyperdispersant and its stabilization mechanism for carbon black. J. Appl. Polym. Sci. 2023, 140, e54001. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, Y.; Sun, Y.; Xiong, Z.; Qi, C.; Zhang, Y.; Liu, Y. Robust superhydrophobic surface based on multiple hybrid coatings for application in corrosion protection. ACS Appl. Mater. Interfaces 2019, 11, 6512–6526. [Google Scholar] [CrossRef]
- Xiang, L.; Guo, Z.; Yang, L.; Qin, Y.; Chen, Z.; Wang, T.; Sun, W.; Wang, C. Modification of crystal growth of NaA zeolite with steric hindrance agents for removing ammonium ion from aqueous solution. J. Ind. Eng. Chem. 2024, 132, 326–334. [Google Scholar] [CrossRef]
- Shuai, W.; Wang, S.; Sun, T.; Yin, H.; Zu, Y.; Yao, G.; Li, Z.; Qi, Z.; Zhong, M. Improving the steric hindrance effect of linear sulfonated acetone–formaldehyde dispersant and its performance in coal–water slurry. RSC Adv. 2022, 12, 35508–35516. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.K.A.; Xianjun, H. Colloidal stability mechanism of copper nanomaterials modified by bis (2-ethylhexyl) phosphate dispersed in polyalphaolefin oil as green nanolubricants. J. Colloid Interface Sci. 2020, 578, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, K.; Sakai, E.; Daimon, M.; Kitahara, A. Role of steric hindrance in the performance of superplasticizers for concrete. J. Am. Ceram. Soc. 1997, 80, 2667–2671. [Google Scholar] [CrossRef]
- Zhao, H.; Deng, M.; Tang, M. Synthesis, characterization and dispersing mechanism of aminosulfonate-phenol-formaldehyde superplasticizer for the cement particles. Polym. Compos. 2018, 39, 2250–2258. [Google Scholar] [CrossRef]

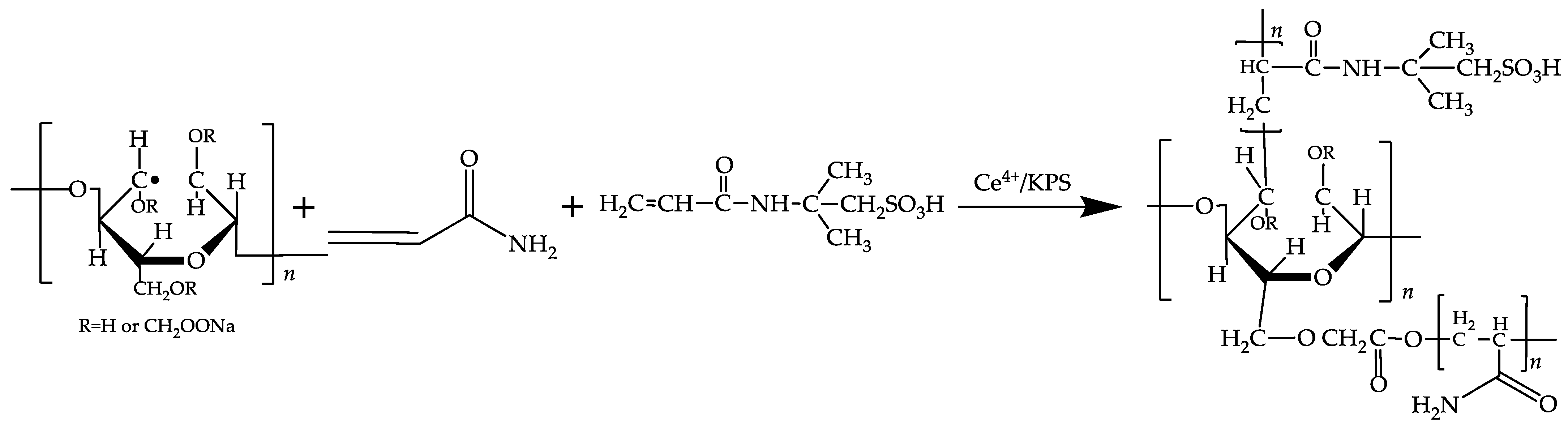






| Component Name | Cellulose | Pectin | Wax | Lignin | Ash Content |
|---|---|---|---|---|---|
| content/% | >95 | 0.5 | 1 | 1~2 | 1 |
| Level | Considerations | ||
|---|---|---|---|
| A:AMPS (g) | B:AM (g) | C:KPS (g) | |
| −1 | 1 | 2 | 0 |
| 0 | 3 | 3 | 0.075 |
| 1 | 5 | 4 | 0.150 |
| Std | AMPS | AM | KPS | Surface Tension of Solution (mN/m) |
|---|---|---|---|---|
| 16 | 0 | 0 | 0 | 48.9 |
| 15 | 0 | 0 | 0 | 52.0 |
| 4 | 1 | 1 | 0 | 55.3 |
| 17 | 0 | 0 | 0 | 49.6 |
| 5 | −1 | 0 | −1 | 60.4 |
| 7 | −1 | 0 | 1 | 49.8 |
| 9 | 0 | −1 | −1 | 64.2 |
| 3 | −1 | 1 | 0 | 51.2 |
| 1 | −1 | −1 | 0 | 61.8 |
| 14 | 0 | 0 | 0 | 50.6 |
| 2 | 1 | −1 | 0 | 64.8 |
| 12 | 0 | 1 | 1 | 57.3 |
| 6 | 1 | 0 | −1 | 59.5 |
| 10 | 0 | 1 | −1 | 62.3 |
| 8 | 1 | 0 | 1 | 61.3 |
| 13 | 0 | 0 | 0 | 47.5 |
| 11 | 0 | −1 | 1 | 63.9 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 576.94 | 9 | 64.10 | 14.26 | 0.0010 | Significant |
| A-AMPS | 39.16 | 1 | 39.16 | 8.71 | 0.0214 | |
| B-AM | 102.25 | 1 | 102.25 | 22.74 | 0.0020 | |
| C-KPS | 24.85 | 1 | 24.85 | 5.53 | 0.0510 | |
| AB | 0.3025 | 1 | 0.3025 | 0.0673 | 0.8028 | |
| AC | 38.44 | 1 | 38.44 | 8.55 | 0.0222 | |
| BC | 5.52 | 1 | 5.52 | 1.23 | 0.3043 | |
| A² | 20.19 | 1 | 20.19 | 4.49 | 0.0718 | |
| B² | 170.58 | 1 | 170.58 | 37.94 | 0.0005 | |
| C² | 143.60 | 1 | 143.60 | 31.94 | 0.0008 | |
| Residual | 31.47 | 7 | 4.50 | |||
| Lack of Fit | 19.88 | 3 | 6.63 | 2.29 | 0.2205 | not Significant |
| Pure Error | 11.59 | 4 | 2.90 | |||
| Cor Total | 608.41 | 16 |
| Specimens | N (%) | C (%) | H (%) | S(%) |
|---|---|---|---|---|
| CMC-AMPS-AM | 4.77 | 33.67 | 5.46 | 2.69 |
| Dispersed Materials | D50 Particle Size of Carbon Black (μm) | Reference |
|---|---|---|
| DP-3537 | 1.80 | [28] |
| IMD | 0.275 | [29] |
| PIB | 0.100 | [30] |
| BYK-192 | 0.785 | [29] |
| Specimens | Mn | Mw | PDI |
|---|---|---|---|
| CMC-AMPS-AM | 148159 | 278299 | 1.878381 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, X.; Yang, X.; Hu, C.; Ding, C. Optimized Design of Material Preparation for Cotton Linters-Based Carbon Black Dispersion Stabilizers Based on Response Surface Methodology. Polymers 2024, 16, 1964. https://doi.org/10.3390/polym16141964
An X, Yang X, Hu C, Ding C. Optimized Design of Material Preparation for Cotton Linters-Based Carbon Black Dispersion Stabilizers Based on Response Surface Methodology. Polymers. 2024; 16(14):1964. https://doi.org/10.3390/polym16141964
Chicago/Turabian StyleAn, Xiongfei, Xupeng Yang, Canming Hu, and Chengli Ding. 2024. "Optimized Design of Material Preparation for Cotton Linters-Based Carbon Black Dispersion Stabilizers Based on Response Surface Methodology" Polymers 16, no. 14: 1964. https://doi.org/10.3390/polym16141964
APA StyleAn, X., Yang, X., Hu, C., & Ding, C. (2024). Optimized Design of Material Preparation for Cotton Linters-Based Carbon Black Dispersion Stabilizers Based on Response Surface Methodology. Polymers, 16(14), 1964. https://doi.org/10.3390/polym16141964






