Preparation of 6-Amino-N-hydroxyhexanamide-Modified Porous Chelating Resin for Adsorption of Heavy Metal Ions
Abstract
1. Introduction
2. Experiments
2.1. Materials and Chemicals
2.2. Preparation of 6-Amino-N-Hydroxyhexanamide (6-AHHA)
2.3. Synthesis of D851-6-AHHA Resin
2.4. Analytical Methods
2.5. Bath Adsorption Experiments
2.6. Column Experiments
3. Results and Discussion
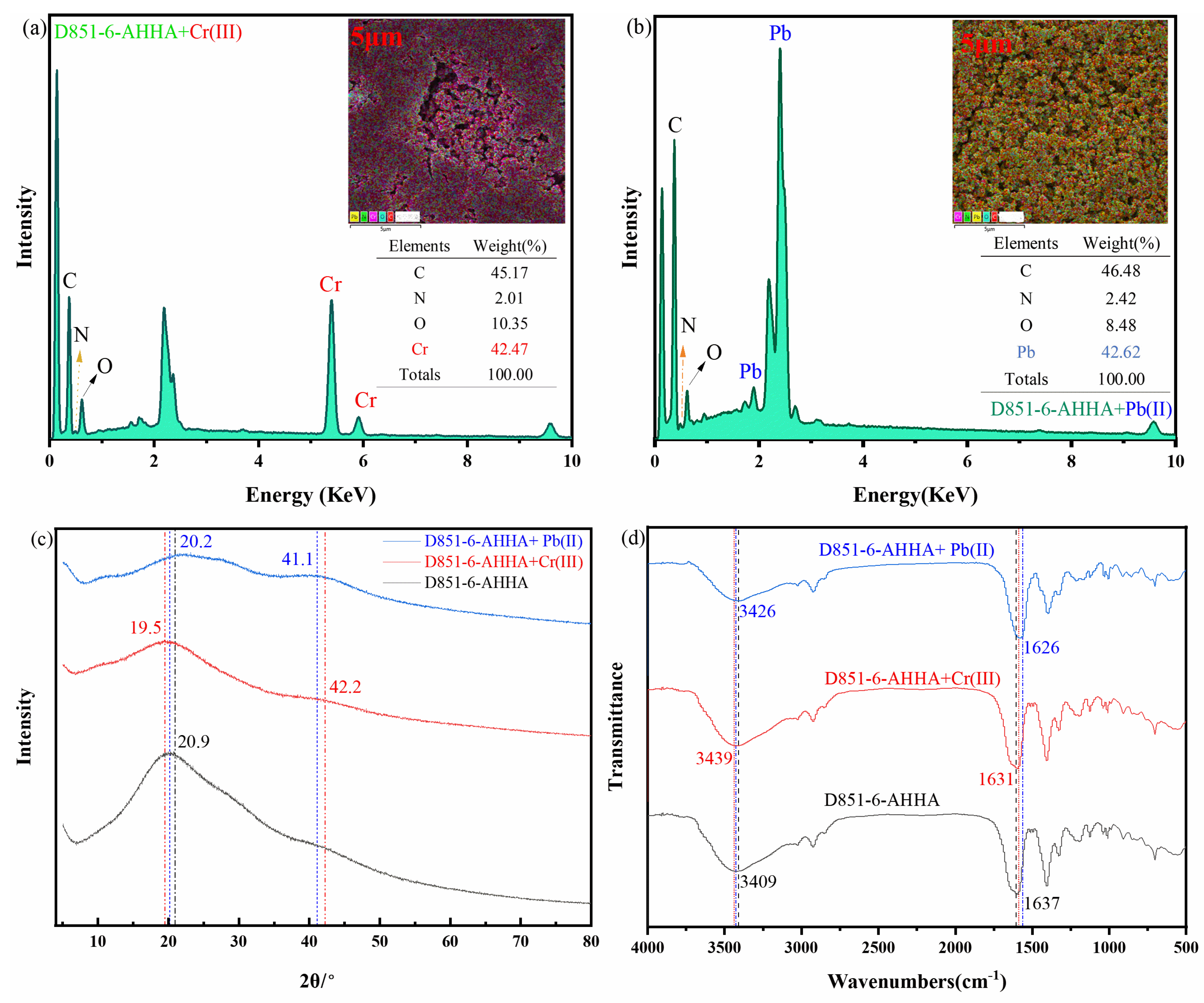
3.1. Characterization
3.2. Bath Adsorption Experiments
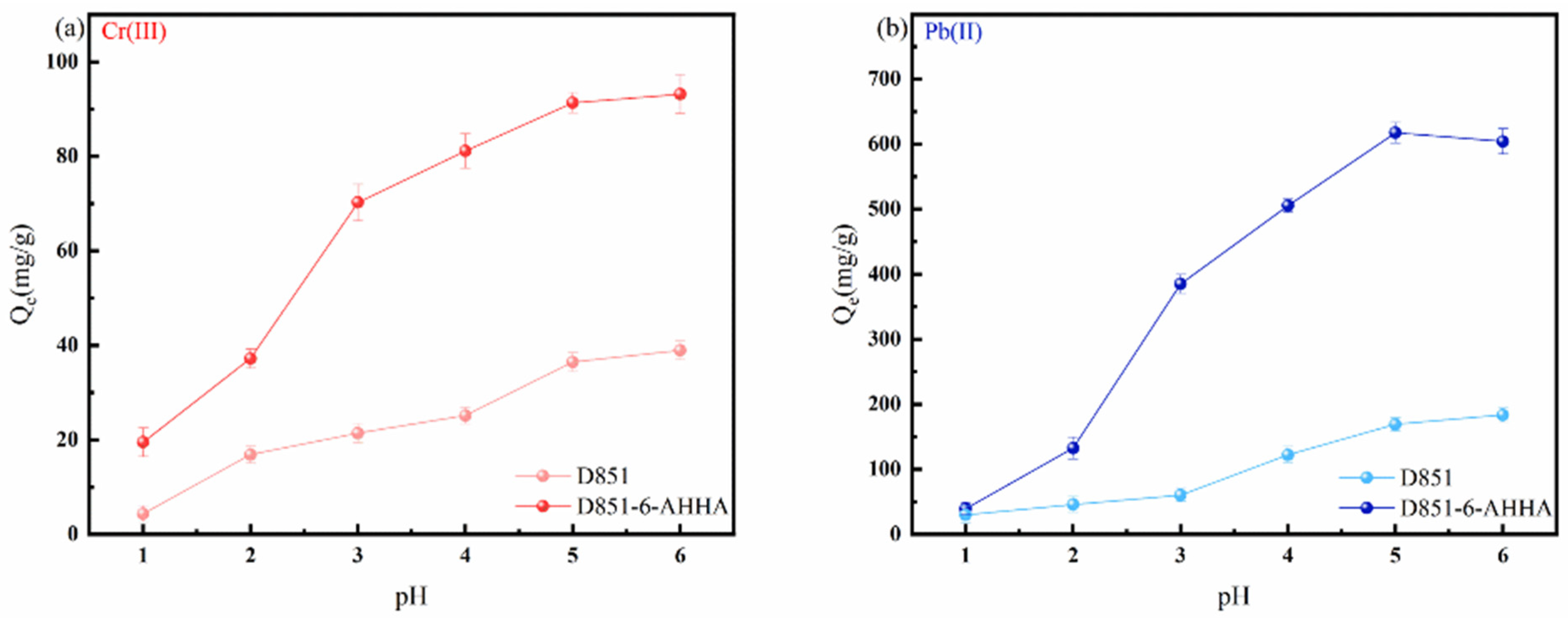
3.2.1. Effect of pH
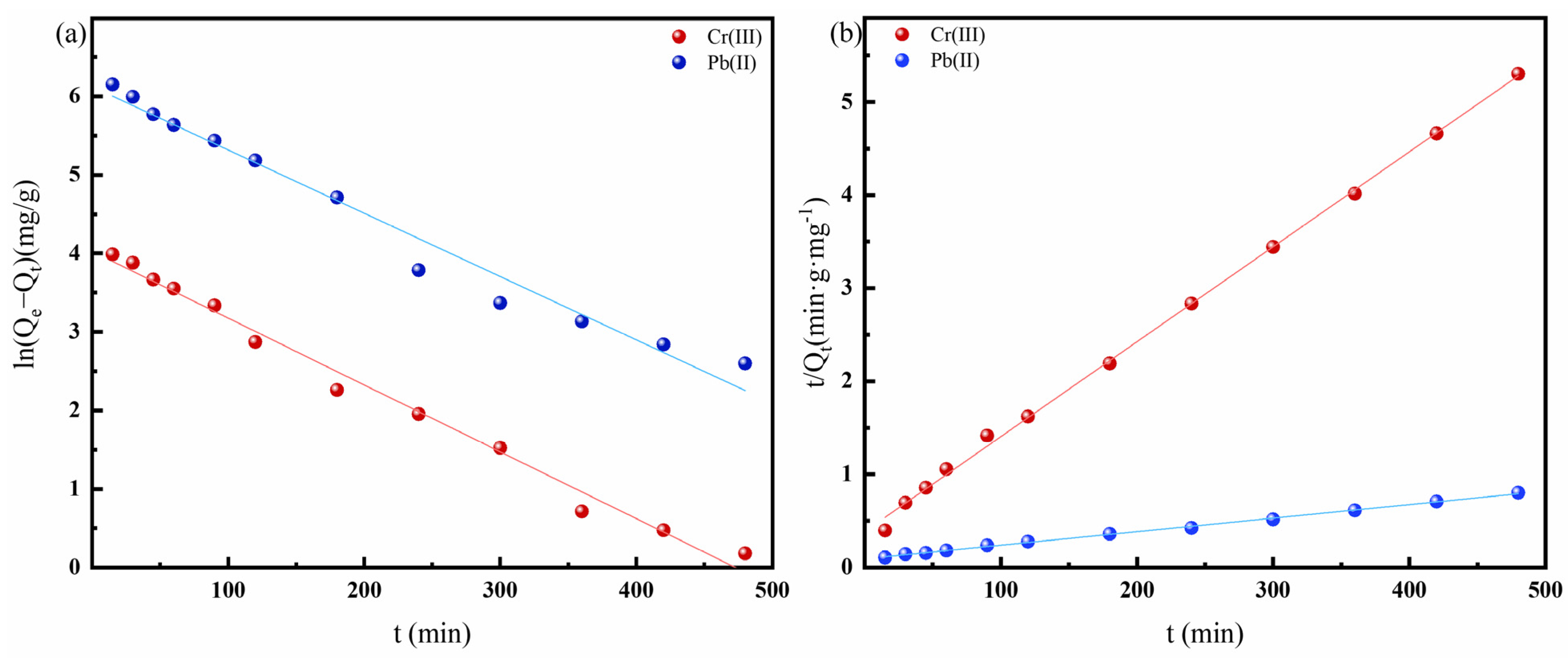
3.2.2. Adsorption Kinetics Study
3.2.3. Isotherm Study
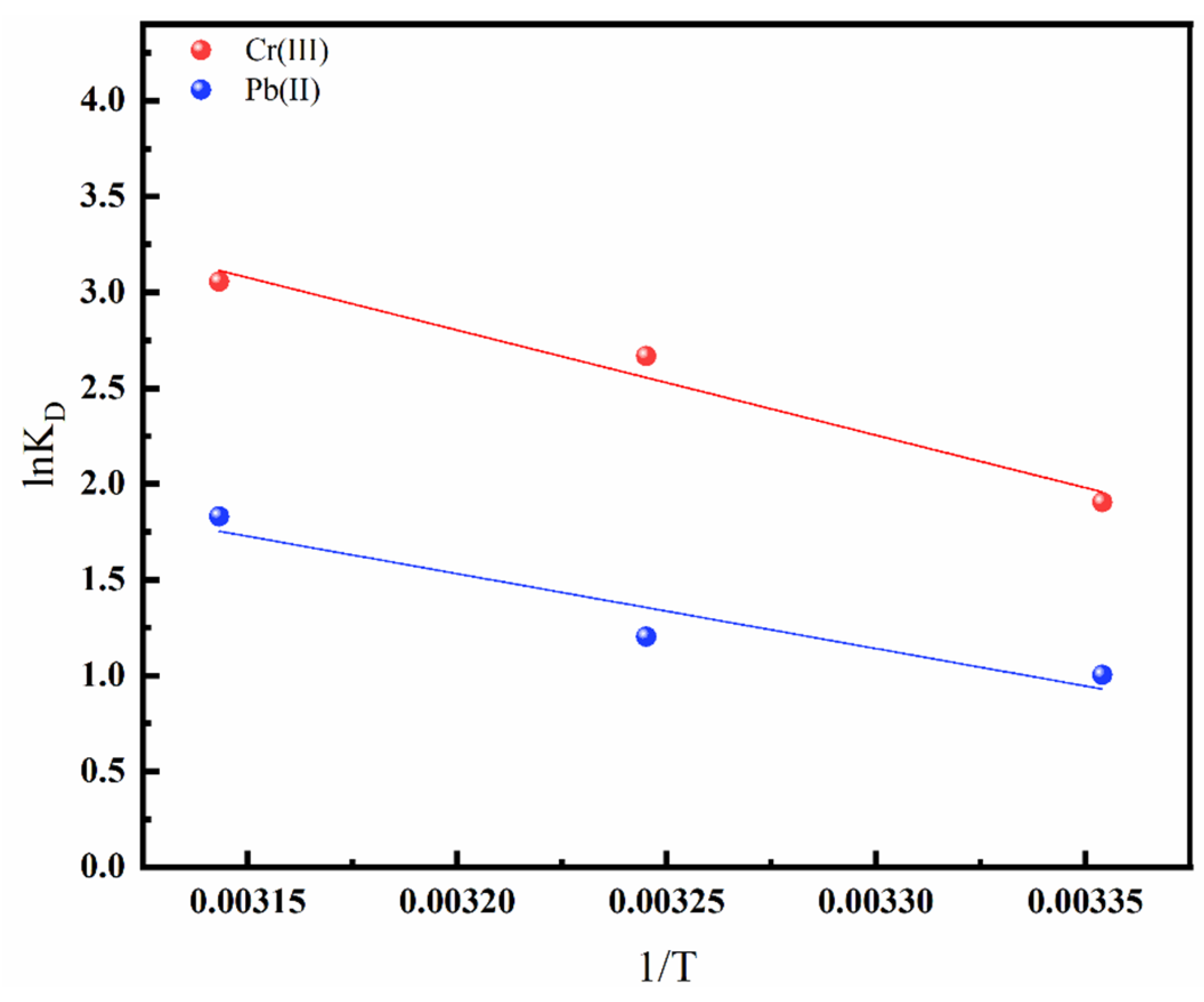
3.2.4. Adsorption Thermodynamics
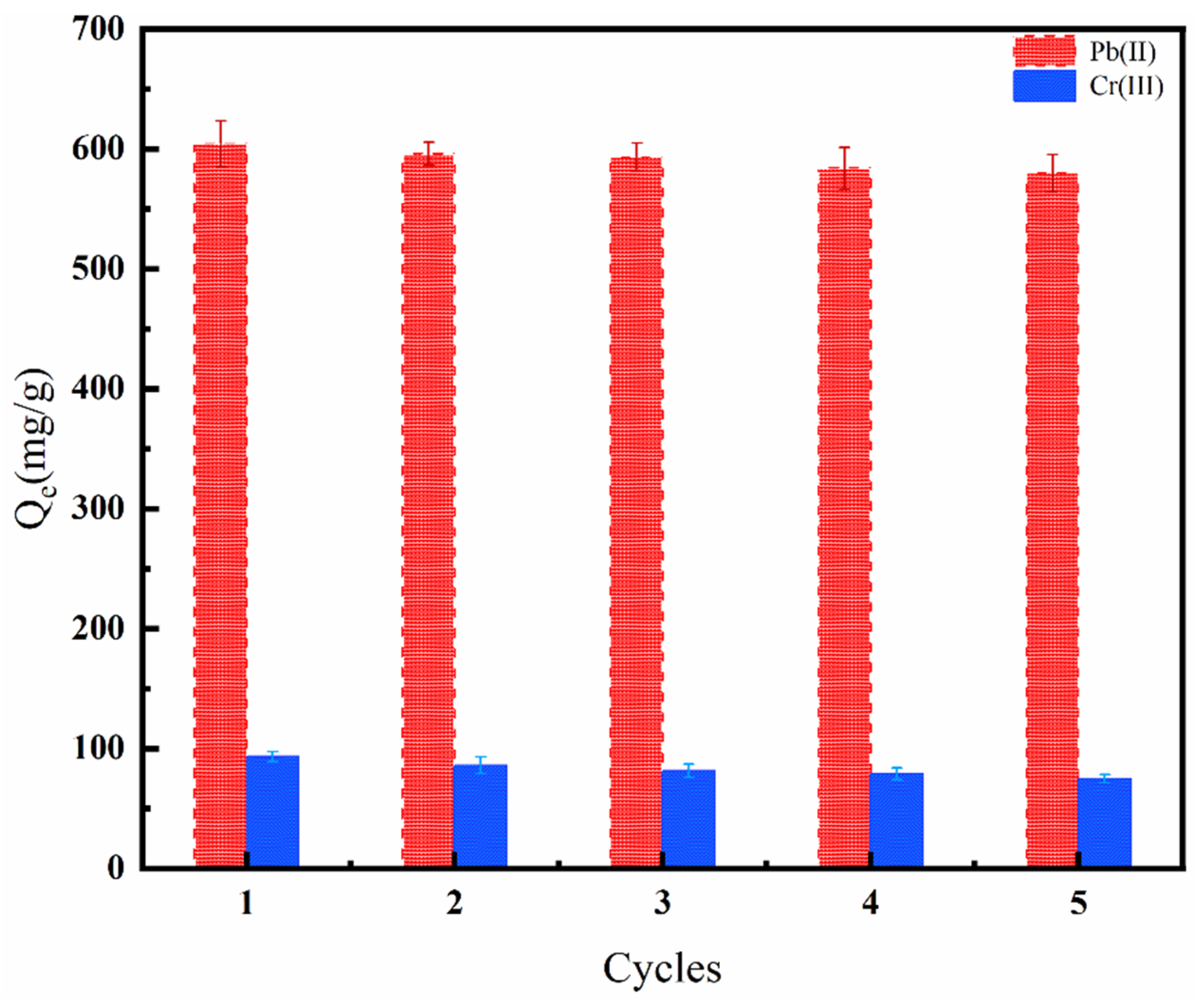
3.2.5. Reusability of the D851-6-AHHA Resin
3.2.6. Performance of D851-6-AHHA Resin in Fixed-Bed Post Systems
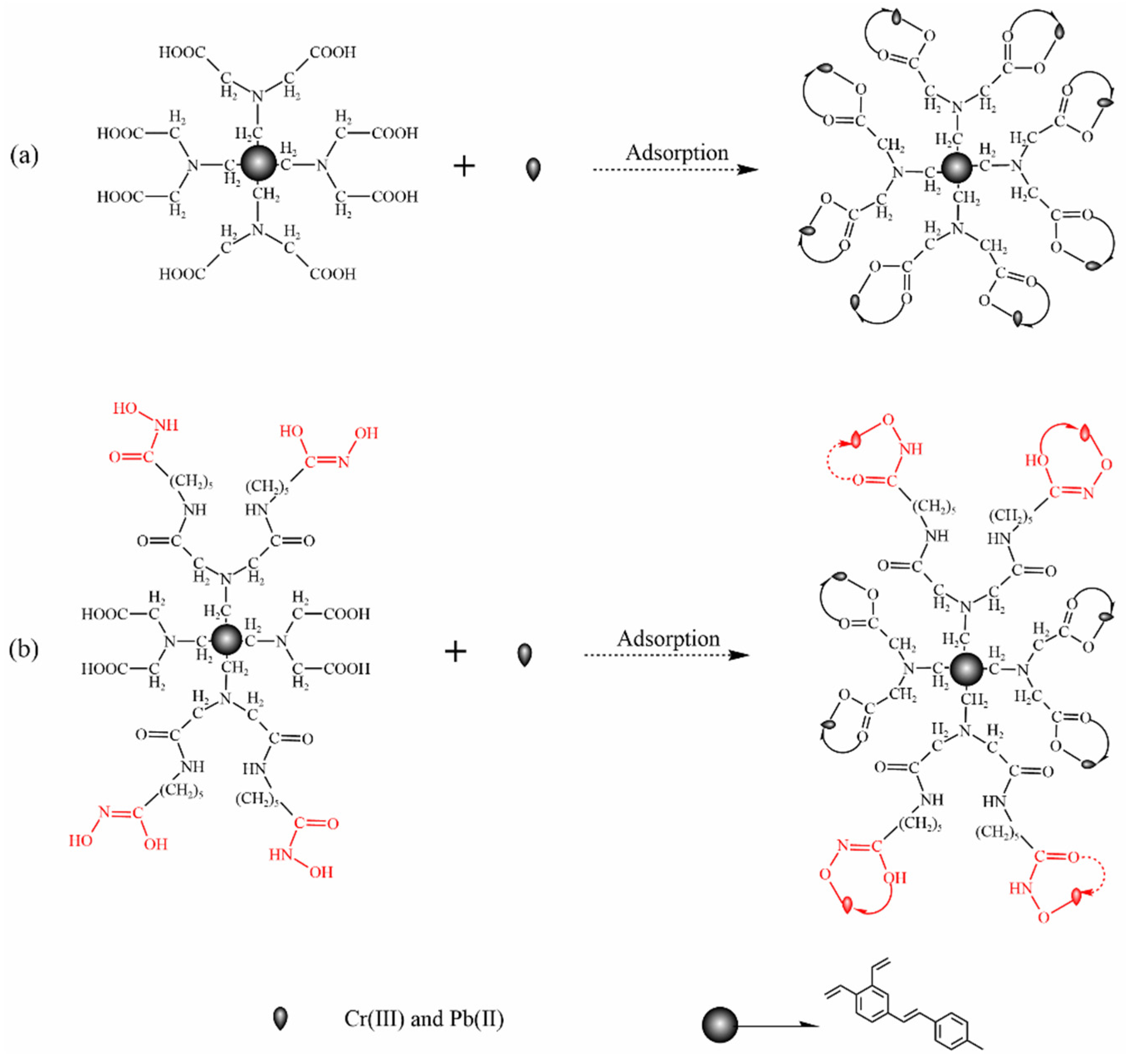
3.3. Adsorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abuhatab, S.; El-Qanni, A.; Al-Qalaq, H.; Hmoudah, M.; Al-Zerei, W. Effective adsorptive removal of Zn2+, Cu2+, and Cr3+ heavy metals from aqueous solutions using silica-based embedded with NiO and MgO nanoparticles. J. Environ. Manag. 2020, 268, 110713. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.L.; Auyoong, Y.L.; Hassan, K.; Farivar, F.; Tran, D.N.; Ma, J.; Losic, D. Multithiol functionalized graphene bio-sponge via photoinitiated thiol-ene click chemistry for efficient heavy metal ions adsorption. Chem. Eng. J. 2020, 395, 124965. [Google Scholar] [CrossRef]
- Kamal, S.; Yang, T.C.-K. Silver enriched silver phosphate microcubes as an efficient recyclable SERS substrate for the detection of heavy metal ions. J. Colloid Interface Sci. 2022, 605, 173–181. [Google Scholar] [CrossRef]
- Duan, W.; Xu, C.; Liu, Q.; Xu, J.; Weng, Z.; Zhang, X.; Basnet, T.B.; Dahal, M.; Gu, A. Levels of a mixture of heavy metals in blood and urine and all-cause, cardiovascular disease and cancer mortality: A population-based cohort study. Environ. Pollut. 2020, 263, 114630. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Zhang, Y.; Wang, H.; Tang, J.; Li, Y.; Wang, S. Removal of Cr(VI) from wastewater by artificial zeolite spheres loaded with nano Fe–Al bimetallic oxide in constructed wetland. Chemosphere 2020, 257, 127224. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Li, Z.; Qu, G.; Zhang, Y.; Wang, C.; Zeng, Y.; Chen, Y. The selective and sustainable separation of Cd(II) using C6MImT/[C6MIm] PF6 extractant. Ecotoxicol. Environ. Saf. 2023, 255, 114792. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.-S.; Jin, H.-T.; Talukder, M.; Ge, J.; Zhang, C.; Lv, M.-W.; Ismail, M.A.Y.; Li, J.-L. The protective effect of nnano-selenium against cadmium-induced cerebellar injury via the heat shock protein pathway in chicken. Food Chem. Toxicol. 2021, 154, 112332. [Google Scholar] [CrossRef]
- Mutlu, M.; Simsek, U.G.; Iflazoglu, S.; Yilmaz, A.; Karabulut, B.; Incili, C.A.; Cevik, A.; Incili, G.K.; Seven, P.T.; Mutlu, S.I. Potential effect dietary supplementation of calcium tetraborate in quails exposed to cadmium: Its impact on productive performance, oxidative stress, cecal microflora, and histopathological changes. Ecotoxicol. Environ. Saf. 2024, 270, 115883. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Ku, J.; Jung, J.; Park, Y.S.; Choi, G.H.; Hwang, S.S.; Lee, J.-H.; Lee, A.S. Ion-exchangeable and sorptive reinforced membranes for efficient electrochemical removal of heavy metal ions in wastewater. J. Clean. Prod. 2024, 438, 140779. [Google Scholar] [CrossRef]
- Buzukashvili, S.; Sommerville, R.; Hu, W.; Brooks, O.; Kökkılıç, O.; Ouzilleau, P.; Rowson, N.A.; Waters, K.E. Zeolite synthesis from coal fly ash and its application to heavy metals remediation from water contaminated with Pb, Cu, Zn and Ni ions. Miner. Eng. 2024, 209, 108619. [Google Scholar] [CrossRef]
- Chen, L.; Wu, K.; Zhang, M.; Liu, N.; Li, C.; Qin, J.; Zhao, Q.; Ye, Z. Synthesis of carbon disulfide modified chitosan resin and its adsorption properties for palladium(Ⅱ) in wastewater. Chem. Eng. J. 2023, 466, 143082. [Google Scholar] [CrossRef]
- Yuan, M.; Liu, D.; Shang, S.; Song, Z.; You, Q.; Huang, L.; Cui, S. A novel magnetic Fe3O4/cellulose nanofiber/polyethyleneimine/thiol-modified montmorillonite aerogel for efficient removal of heavy metal ions: Adsorption behavior and mechanism study. Int. J. Biol. Macromol. 2023, 253, 126634. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Han, Y.; Wu, X.; Wang, H.; Wang, S.; Zheng, J.; Ran, R.; Zhang, C. Review: Phytate modification serves as a novel adsorption strategy for the removal of heavy metal pollution in aqueous environments. J. Environ. Chem. Eng. 2023, 11, 111440. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Y.; Chen, H.; Lu, J.; Yu, G.; Möslang, M.; Zhou, Y. Superior adsorption capacity of functionalised straw adsorbent for dyes and heavy-metal ions. J. Hazard. Mater. 2020, 382, 121040. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-R.; Park, J.Y.; Park, E.Y. Effect of ethanol, phytic acid and citric acid treatment on the physicochemical and heavy metal adsorption properties of corn starch. Food Chem. 2024, 431, 137167. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.Z.N.; Salleh, W.N.W.; Ismail, A.F.; Yusof, N.; Yusop, M.Z.M.; Aziz, F. Adsorptive removal of heavy metal ions using graphene-based nanomaterials: Toxicity, roles of functional groups and mechanisms. Chemosphere 2020, 248, 126008. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Liu, Y.; Liu, Y. Adsorption of Ag(Ⅰ) on chelate resins containing N and S: Comparison and computational study with anionic resin. Sep. Purif. Technol. 2024, 339, 126600. [Google Scholar] [CrossRef]
- Duan, G.; Li, X.; Ma, X.; Zhong, W.; Wang, S. High-efficiency adsorption removal for Cu(II) and Ni(II) using a novel acylamino dihydroxamic acid chelating resin. Sci. Total Environ. 2023, 864, 160984. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.A.; Elkholy, S.S.; Morsi, R.E.; Elsabee, M.Z. Studies on adsorption behavior of Cu (II) and Cd (II) onto aminothiophene derivatives of Styrene Maleic anhydride copolymer. J. Taiwan Inst. Chem. Eng. 2016, 64, 325–335. [Google Scholar] [CrossRef]
- Keth, J.; Johann, T.; Frey, H. Hydroxamic Acid: An Underrated Moiety? Marrying Bioinorganic Chemistry and Polymer Science. Biomacromolecules 2020, 21, 2546–2556. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, K.; Wang, S.; Cao, Z.; Ma, X.; Zhong, H. Structural modification of hydroxamic acid collectors to enhance the flotation performance of malachite and associated mechanism. J. Mol. Liq. 2021, 344, 117959. [Google Scholar] [CrossRef]
- Cao, X.; Zhou, C.; Wang, S.; Man, R. Adsorption Properties for La(III), Ce(III), and Y(III) with Poly(6-acryloylamino-hexyl hydroxamic acid) Resin. Polymers 2020, 13, 3. [Google Scholar] [CrossRef]
- Duan, G.; Cao, Z.; Zhong, H.; Ma, X.; Wang, S. Highly efficient poly(6-acryloylamino-N-hydroxyhexanamide) resin for adsorption of heavy metal ions. J. Environ. Manag. 2022, 308, 114631. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Yang, X.; Li, W.; Liu, Y. Efficient removal and recovery of vanadium (IV and V) from high acidic waste water with resins D851 and D201: A comparative study. J. Water Process. Eng. 2022, 49, 103153. [Google Scholar] [CrossRef]
- Tsurko, E.S.; Feicht, P.; Nehm, F.; Ament, K.; Rosenfeldt, S.; Pietsch, I.; Roschmann, K.; Kalo, H.; Breu, J. Large Scale Self-Assembly of Smectic Nanocomposite Films by Doctor Blading versus Spray Coating: Impact of Crystal Quality on Barrier Properties. Macromolecules 2017, 50, 4344–4350. [Google Scholar] [CrossRef]
- Liu, Y.; Qiao, L.; Wang, A.; Li, Y.; Zhao, L.; Du, K. Tentacle-type poly(hydroxamic acid)-modified macroporous cellulose beads: Synthesis, characterization, and application for heavy metal ions adsorption. J. Chromatogr. A 2021, 1645, 462098. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Choi, H.-J. Persimmon leaf bio-waste for adsorptive removal of heavy metals from aqueous solution. J. Environ. Manag. 2018, 209, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Siyal, A.A.; Shamsuddin, M.R.; Khan, M.I.; Rabat, N.E.; Zulfiqar, M.; Man, Z.; Siame, J.; Azizli, K.A. A review on geopolymers as emerging materials for the adsorption of heavy metals and dyes. J. Environ. Manag. 2018, 224, 327–339. [Google Scholar] [CrossRef]
- Ma, P.; Yao, S.; Wang, Z.; Qi, F.; Liu, X. Preparation of nitrogen-doped hierarchical porous carbon aerogels from agricultural wastes for efficient pollution adsorption. Sep. Purif. Technol. 2023, 311, 123250. [Google Scholar] [CrossRef]
- Shakila, A.; Ravikumar, S.; Pandiyan, V.; Gaba, R. Influence of temperature on thermo physical properties of binary mixtures of ethyl acrylate and alkyl amines: An experimental and theoretical approach. J. Mol. Liq. 2018, 265, 544–555. [Google Scholar] [CrossRef]
- Deng, L.; Wang, S.; Zhong, H.; Liu, G. N-(6-(hydroxyamino)-6-oxohexyl) decanamide collector: Flotation performance and adsorption mechanism to diaspore. Appl. Surf. Sci. 2015, 347, 79–87. [Google Scholar] [CrossRef]
- Tang, T.; Tang, G.; Kou, S.; Zhao, J.; Culnane, L.F.; Zhang, Y. Experimental and DFT studies on the vibrational and electronic spectra of 2-(1H-Imidazo [4,5-ƒ][1,10]phenanthrolin-2-yl)phenol. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 117, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Li, S.; Zhou, G.; Wang, Y.; Dong, X.; Cao, X. Preparation and characterization of magnetic modified bone charcoal for removing Cu2+ ions from industrial and mining wastewater. J. Environ. Manag. 2021, 297, 113221. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xie, L.; Liu, G.; Zhong, H.; Wang, Y.; Zeng, H. Hetero-difunctional Reagent with Superior Flotation Performance to Chalcopyrite and the Associated Surface Interaction Mechanism. Langmuir 2019, 35, 4353–4363. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Zhang, C.; Liao, F.; Wang, Y.; Li, M.; Meng, L.; Jiang, J. Removal of methylene blue from aqueous solution with magnetite loaded multi-wall carbon nanotube: Kinetic, isotherm and mechanism analysis. J. Hazard. Mater. 2011, 198, 282–290. [Google Scholar] [CrossRef]
- Sarmah, D.; Karak, N. Double network hydrophobic starch based amphoteric hydrogel as an effective adsorbent for both cationic and anionic dyes. Carbohydr. Polym. 2020, 242, 116320. [Google Scholar] [CrossRef]
- Xiao, W.; Garba, Z.N.; Sun, S.; Lawan, I.; Wang, L.; Lin, M.; Yuan, Z. Preparation and evaluation of an effective activated carbon from white sugar for the adsorption of rhodamine B dye. J. Clean. Prod. 2020, 253, 119989. [Google Scholar] [CrossRef]
- Cai, L.; Ying, D.; Liang, X.; Zhu, M.; Lin, X.; Xu, Q.; Cai, Z.; Xu, X.; Zhang, L. A novel cationic polyelectrolyte microsphere for ultrafast and ultra-efficient removal of heavy metal ions and dyes. Chem. Eng. J. 2021, 410, 128404. [Google Scholar] [CrossRef]
- Qiao, L.; Zhao, L.; Du, K. Construction of hierarchically porous chitin microspheres via a novel Dual-template strategy for rapid and High-capacity removal of heavy metal ions. Chem. Eng. J. 2020, 393, 124818. [Google Scholar] [CrossRef]
- Mittal, H.; Al Alili, A.; Morajkar, P.P.; Alhassan, S.M. Graphene oxide crosslinked hydrogel nanocomposites of xanthan gum for the adsorption of crystal violet dye. J. Mol. Liq. 2021, 323, 115034. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Zhang, P.; Liu, X.; Han, L. Facile fabrication of magnetic bio-derived chars by co-mixing with Fe3O4 nanoparticles for effective Pb2+ adsorption: Properties and mechanism. J. Clean. Prod. 2020, 262, 121350. [Google Scholar] [CrossRef]
- Verma, M.; Lee, I.; Oh, J.; Kumar, V.; Kim, H. Synthesis of EDTA-functionalized graphene oxide-chitosan nanocomposite for simultaneous removal of inorganic and organic pollutants from complex wastewater. Chemosphere 2022, 287, 132385. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Z.; Yue, R.; Gao, F.; Ren, R.; Wei, J.; Wang, X.; Kong, Z. Rapid preparation of adsorbent based on mussel inspired chemistry and simultaneous removal of heavy metal ions in water. Chem. Eng. J. 2020, 383, 123107. [Google Scholar] [CrossRef]
- Huang, Q.-S.; Wu, W.; Wei, W.; Song, L.; Sun, J.; Ni, B.-J. Highly-efficient Pb2+ removal from water by novel K2W4O13 nanowires: Performance, mechanisms and DFT calculation. Chem. Eng. J. 2020, 381, 122632. [Google Scholar] [CrossRef]
- Jilal, I.; El Barkany, S.; Bahari, Z.; Sundman, O.; El Idrissi, A.; Abou-Salama, M.; Romane, A.; Zannagui, C.; Amhamdi, H. New quaternized cellulose based on hydroxyethyl cellulose (HEC) grafted EDTA: Synthesis, characterization and application for Pb (II) and Cu (II) removal. Carbohydr. Polym. 2018, 180, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Jena, K.K.; Mittal, H.; Wadi, V.S.; Mani, G.K.; Alhassan, S.M. Advanced TiO2–SiO2–Sulfur (Ti–Si–S) Nanohybrid Materials: Potential Adsorbent for the Remediation of Contaminated Wastewater. ACS Appl. Mater. Interfaces 2019, 11, 30247–30258. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, X.; Zhang, X.; Yang, Y.; Duan, J. A high-efficiency and plane-enhanced chitosan film for cefotaxime adsorption compared with chitosan particles in water. Chem. Eng. J. 2021, 413, 127494. [Google Scholar] [CrossRef]
- Cao, X.; Wang, Q.; Wang, S.; Man, R. Preparation of a Novel Polystyrene-Poly(hydroxamic Acid) Copolymer and Its Adsorption Properties for Rare Earth Metal Ions. Polymers 2020, 12, 1905. [Google Scholar] [CrossRef]
- Gode, F.; Pehlivan, E. Removal of chromium(III) from aqueous solutions using Lewatit S 100: The effect of pH, time, metal concentration and temperature. J. Hazard. Mater. 2006, 136, 330–337. [Google Scholar] [CrossRef]
- Hu, X.; Li, Y.; Wang, Y.; Li, X.; Li, H.; Liu, X.; Zhang, P. Adsorption kinetics, thermodynamics and isotherm of thiacalix[4]arene-loaded resin to heavy metal ions. Desalination 2010, 259, 76–83. [Google Scholar] [CrossRef]
- Kanwal, F.; Imran, M.; Mitu, L.; Rashid, Z.; Razzaq, H.; Ain, Q.U. Removal of Chromium(III) Using Synthetic Polymers, Copolymers and their Sulfonated Derivatives as Adsorbents. J. Chem. 2012, 9, 621–630. [Google Scholar] [CrossRef][Green Version]
- Kocaoba, S.; Akcin, G. Removal of chromium (III) and cadmium (II) from aqueous solutions. Desalination 2005, 180, 151–156. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Z.; Wang, C.; Ding, S.; Li, F.; Lin, H. Preparation of magnetic resin microspheres M-P(MMA-DVB-GMA) and the adsorption property to heavy metal ions. Appl. Surf. Sci. 2019, 496, 143708. [Google Scholar] [CrossRef]
- Ucarli, O.; Yayintas, O.T.; Engin, M.S.; Cay, S.; Saglikoglu, G.; Yilmaz, S. Investigation of Competitive and Noncompetitive Adsorption of Some Heavy Metals Ions on Leucodon sciuroides (Hedw.) Schwägr. Langmuir 2020, 36, 8265–8271. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhai, Y.; Yang, Y.; Yang, X.; Zhu, Z. Electrostatic assembly of superwetting porous nanofibrous membrane toward oil-in-water microemulsion separation. Chem. Eng. J. 2018, 354, 463–472. [Google Scholar] [CrossRef]
- Zhang, B.; Zhong, H. Determination of hydroxamic acids by direct spectrophotometry of colored complex in acidic solution. Research on Chemical Intermediates. Res. Chem. Intermediat. 2010, 36, 495–501. [Google Scholar] [CrossRef]
- Lima, E.C.; Hosseini-Bandegharaei, A.; Moreno-Piraján, J.C.; Anastopoulos, I. A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the Van’t Hoof equation for calculation of thermodynamic parameters of adsorption. J. Mol. Liq. 2018, 273, 425–434. [Google Scholar] [CrossRef]
- Haladu, S.A. Highly efficient adsorption of malachite green dye onto a cross-linked pH-responsive cycloterpolymer resin: Kinetic, equilibrium and thermodynamic studies. J. Mol. Liq. 2022, 357, 119115. [Google Scholar] [CrossRef]
- Guo, J.; Zhai, Z.; Wang, L.; Wang, Z.; Wu, J.; Zhang, B.; Zhang, J. Dynamic and thermodynamic mechanisms of TFA adsorption by particulate matter. Environ. Pollut. 2017, 225, 175–183. [Google Scholar] [CrossRef]
- Zhao, G.; Jiang, L.; He, Y.; Li, J.; Dong, H.; Wang, X.; Hu, W. Sulfonated Graphene for Persistent Aromatic Pollutant Management. Adv. Mater. 2011, 23, 3959–3963. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yu, M.; Wang, C.; Feng, J.; Yan, W. Insight into the Synergistic Effect on Selective Adsorption for Heavy Metal Ions by a Polypyrrole/TiO2 Composite. Langmuir 2018, 34, 10187–10196. [Google Scholar] [CrossRef]
- Zou, B.; Zhang, S.; Sun, P.; Ye, Z.; Zhao, Q.; Zhang, W.; Zhou, L. Preparation of a novel Poly-chloromethyl styrene chelating resin containing heterofluorenone pendant groups for the removal of Cu (II), Pb (II), and Ni (II) from wastewaters. Colloid Interface Sci. Commun. 2021, 40, 100349. [Google Scholar] [CrossRef]
- Kumar, P.A.; Chakraborty, S. Fixed-bed column study for hexavalent chromium removal and recovery by short-chain polyaniline synthesized on jute fiber. J. Hazard. Mater. 2009, 162, 1086–1098. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, M.; Gong, Y.; Zeng, E.Y. Efficient removal of mercury from simulated groundwater using thiol-modified graphene oxide/Fe–Mn composite in fixed-bed columns: Experimental performance and mathematical modeling. Sci. Total Environ. 2020, 714, 136636. [Google Scholar] [CrossRef] [PubMed]
- Bolar, S.; Shit, S.; Murmu, N.C.; Samanta, P.; Kuila, T. Activation Strategy of MoS2 as HER Electrocatalyst through Doping-Induced Lattice Strain, Band Gap Engineering, and Active Crystal Plane Design. ACS Appl. Mater. Interfaces 2021, 13, 765–780. [Google Scholar] [CrossRef]
- Ayalew, Z.M.; Zhang, X.; Guo, X.; Ullah, S.; Leng, S.; Luo, X.; Ma, N. Removal of Cu, Ni and Zn directly from acidic electroplating wastewater by Oligo-Ethyleneamine dithiocarbamate (OEDTC). Sep. Purif. Technol. 2020, 248, 117114. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, H.; Shao, T.; Zhao, X.; Peng, H.; Gong, Y.; Wan, H. Enhanced copper adsorption by DTPA-chitosan/alginate composite beads: Mechanism and application in simulated electroplating wastewater. Chem. Eng. J. 2018, 339, 322–333. [Google Scholar] [CrossRef]
- Yu, Y.; Hu, Z.; Chen, Z.; Yang, J.; Gao, H.; Chen, Z. Organically-modified magnesium silicate nanocomposites for high-performance heavy metal removal. RSC Adv. 2016, 6, 97523–97531. [Google Scholar] [CrossRef]
- Zong, E.; Huang, G.; Liu, X.; Lei, W.; Jiang, S.; Ma, Z.; Wang, J.; Song, P. A lignin-based nano-adsorbent for superfast and highly selective removal of phosphate. J. Mater. Chem. A 2018, 6, 9971–9983. [Google Scholar] [CrossRef]
- Zhang, M.; Song, L.; Jiang, H.; Li, S.; Shao, Y.; Yang, J.; Li, J. Biomass based hydrogel as an adsorbent for the fast removal of heavy metal ions from aqueous solutions. J. Mater. Chem. A 2017, 5, 3434–3446. [Google Scholar] [CrossRef]
- Yan, J.; Li, K. A magnetically recyclable polyampholyte hydrogel adsorbent functionalized with β-cyclodextrin and graphene oxide for cationic/anionic dyes and heavy metal ion wastewater remediation. Sep. Purif. Technol. 2021, 277, 119469. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, S.; Ma, X.; Zhong, H. Desulfurization in high-sulfur bauxite with a novel thioether-containing hydroxamic acid: Flotation behavior and separation mechanism. Sep. Purif. Technol. 2021, 275, 119147. [Google Scholar] [CrossRef]
- Ma, J.; Khan, M.A.; Xia, M.; Fu, C.; Zhu, S.; Chu, Y.; Lei, W.; Wang, F. Effective adsorption of heavy metal ions by sodium lignosulfonate reformed montmorillonite. Int. J. Biol. Macromol. 2019, 138, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Tang, L.; Zeng, G.; Zhu, Z.; Yan, M.; Zhou, Y.; Wang, J.; Liu, Y.; Wang, J. Insight into highly efficient simultaneous photocatalytic removal of Cr(VI) and 2,4-diclorophenol under visible light irradiation by phosphorus doped porous ultrathin g-C3N4 nanosheets from aqueous media: Performance and reaction mechanism. Appl. Catal. B Environ. 2017, 203, 343–354. [Google Scholar] [CrossRef]
- Green, B.P.; Renfrew, A.K.; Glenister, A.; Turner, P.; Hambley, T.W. The influence of the ancillary ligand on the potential of cobalt(iii) complexes to act as chaperones for hydroxamic acid-based drugs. Dalton Trans. 2017, 46, 15897–15907. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Liu, G.; Dong, Y. Probing the hydrophobic mechanism of N-[(3-hydroxyamino)-propoxy]-N-octyl dithiocarbamate toward bastnaesite flotation by in situ AFM, FTIR and XPS. J. Colloid Interface Sci. 2020, 572, 179–189. [Google Scholar] [CrossRef]
- Raymond, K.N.; Allred, B.E.; Sia, A.K. Coordination Chemistry of Microbial Iron Transport. Accounts Chem. Res. 2015, 48, 2496–2505. [Google Scholar] [CrossRef]
- Lin, G.; Wang, S.; Zhang, L.; Hu, T.; Peng, J.; Cheng, S.; Fu, L. Selective Adsorption of Ag+ on a New Cyanuric-Thiosemicarbazide Chelating Resin with High Capacity from Acid Solutions. Polymers 2017, 9, 568. [Google Scholar] [CrossRef] [PubMed]





| Adsorbent | Adsorbates | Qexp (mg/g) | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|---|---|
| Qcal (mg/g) | K1 (min−1) | R2 | Qcal (mg/g) | K2 (g∙mg−1∙min−1) | R2 | |||
| D851-6-AHHA | Cr(III) | 91.50 | 56.15 | 0.0085 | 0.9884 | 98.04 | 0.0003 | 0.9986 |
| Pb(II) | 611.92 | 456.88 | 0.9742 | 0.9742 | 689.66 | 0.00002 | 0.9980 | |
| Adsorbent | Adsorbates | T (K) | Langmuir Constants | Freundlich Constants | ||||
|---|---|---|---|---|---|---|---|---|
| Qm | KL | R2 | 1/n | KF | R2 | |||
| D851-6-AHHA | Cr(III) | 298.15 | 97.84 | 0.2733 | 0.9579 | 0.1805 | 37.6713 | 0.6539 |
| 308.15 | 98.23 | 0.6037 | 0.9916 | 0.1525 | 44.5854 | 0.6622 | ||
| 318.15 | 101.67 | 0.7263 | 0.9402 | 0.1228 | 53.1289 | 0.6899 | ||
| Pb(II) | 298.15 | 615.81 | 0.5754 | 0.9947 | 0.1823 | 214.1348 | 0.8342 | |
| 308.15 | 642.32 | 0.6218 | 0.9876 | 0.1827 | 221.5111 | 0.8306 | ||
| 318.15 | 636.07 | 1.4201 | 0.9935 | 0.1596 | 258.6712 | 0.7916 | ||
| Adsorbent | Adsorbates | ΔS (J/mol/K) | ΔH (KJ/mol) | R2 | ΔG (KJ/mol) | ||
|---|---|---|---|---|---|---|---|
| 298.15 K | 308.15 K | 318.15 K | |||||
| D851-6-AHHA | Cr(III) | 169.14 | 45.58 | 0.9727 | −4.85 | −6.54 | −8.23 |
| Pb(II) | 116.67 | 32.48 | 0.9073 | −2.30 | −3.47 | −4.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Wang, Z.; He, M.; Zhu, J. Preparation of 6-Amino-N-hydroxyhexanamide-Modified Porous Chelating Resin for Adsorption of Heavy Metal Ions. Polymers 2024, 16, 1966. https://doi.org/10.3390/polym16141966
Liu S, Wang Z, He M, Zhu J. Preparation of 6-Amino-N-hydroxyhexanamide-Modified Porous Chelating Resin for Adsorption of Heavy Metal Ions. Polymers. 2024; 16(14):1966. https://doi.org/10.3390/polym16141966
Chicago/Turabian StyleLiu, Shaomin, Zihan Wang, Mingyi He, and Jinglin Zhu. 2024. "Preparation of 6-Amino-N-hydroxyhexanamide-Modified Porous Chelating Resin for Adsorption of Heavy Metal Ions" Polymers 16, no. 14: 1966. https://doi.org/10.3390/polym16141966
APA StyleLiu, S., Wang, Z., He, M., & Zhu, J. (2024). Preparation of 6-Amino-N-hydroxyhexanamide-Modified Porous Chelating Resin for Adsorption of Heavy Metal Ions. Polymers, 16(14), 1966. https://doi.org/10.3390/polym16141966







