Abstract
Thermo-responsive, biocompatible polyurethane (PU) with shape memory properties is highly desirable for biomedical applications. An innovative approach to producing wound closure strips using shape memory polymers (SMPs) is of significant interest. In this work, PU composed of polycaprolactone (PCL) and 1,4-butanediol (BDO) was synthesized using two-step polymerization. Palm oil (PO) was added to PU for enhancing the Young’s modulus of the PU beyond the set criterion of 130 MPa. It was found that PU had the ability to crystallize at room temperature and the segments of individual PCL and BDO polyurethanes crystallized separately. The crystalline domains and hard segment of PU greatly affected the tensile properties. The reduction of crystalline domains by the addition of PO and deformation at the higher melting temperature of the crystalline PCL polyurethane phase improved the shape fixity and shape recovery ratios. The new irreversible phase, raised from the permanent deformation upon stretching at the between melting temperature of the crystalline PCL and BDO polyurethanes of 70 °C, resulted in a decrease in shape fixity ratio after the first thermomechanical stretching–recovering cycles. The demonstration of PU as a wound closure strip showed its efficiency and potential until the surgical wound healed.
1. Introduction
SMPs are a type of smart polymer that has become a significant category of responsive materials for medical implants and biomedical and engineering applications [1,2,3,4]. The key feature of SMPs is their ability to be programmed into a temporary shape by deformation at the programming temperature (Tp) and then return to their original shape when exposed to external stimuli such as heat, electricity, enzymes, etc. [4,5]. Thermo-responsive SMPs typically consist of two phases: a thermally reversible phase (e.g., hydrogen bonds and glassy-amorphous domains) that maintains a temporary shape and an irreversible phase (e.g., chemical/physical cross-links and crystalline domains) that enables the recovery of the original shape [4,6]. The reversible phase is responsible for fixing the temporary shape after sample deformation by cooling and can be reversed by heating across the shape-recovery temperature (Tr) [4,7,8]. The permanent network or irreversible phase stores the elastic energy that drives the shape recovery upon stimulation and determines the original shape [4,6]. For polymer materials, the glass transition temperature (Tg) of the amorphous segment or melting temperature (Tm) of crystalline domains can be used as the Tr [4]. These properties allow SMPs to be used in many applications, including orthodontic wires [9], orthodontic aligners [7], smart plaster [10], smart splints [3], sutures [4], and wound closure strips [4].
Recent advancements in SMPs hold promise for use as wound closure strips. Wound closure is a critical step in wound healing, as bringing the tissues surrounding a surgical wound into close proximity facilitates efficient healing, reducing the risk of infection [11]. Currently, various methods, such as sutures or surgical staples, are employed for wound closure [12]. However, while these methods are effective, they may also result in increased pain and/or additional scarring [13]. This drawback has spurred research into alternative products, including adhesive-based solutions like glue wounds [14] or Steri-strip [15]. Nevertheless, these alternatives present challenges such as application difficulties and limitations in closing certain types of wounds, rendering them unable to address all scenarios [16,17].
For wound closure applications, the designed wound closure strip must be tough and chemically safe to avoid causing skin irritation. More importantly, the smart strip needs to effectively bring the skin together without losing its shape. In other words, from the material property point of view, the Young’s modulus of the strip must be greater than that of the skin. This modulus is required to ensure that the strip maintains its shape while pulling the two sides of the wound together. For wound closure applications, the smart strip needs to effectively bring the skin together without losing its shape, similar to that of a suture. Greenwald et al. [18] suggested that the mechanical properties of monofilament sutures (Maxon or PDS), which are currently popular for surgical wound closure, include a Young’s modulus of 130 MPa. This modulus is greater than that of human skin and is more than sufficient to hold surgical wounds together.
For the thermo-responsive SMP wound closure strip, the Tr is also important. Heat is applied on the strip to recover its primary shape. At Tr, the strip shrinks, providing the force necessary to pull the skin around the wound together. Ideally, the Tr should be slightly above the physiological temperature. The risk of thermal injury for most mammalian tissues depends on both temperature and the duration of exposure. It was reported that tissue underwent irreversible damage after 60 min of exposure at 43 °C. The same level of damage occurred with 30 min of exposure at 44 °C or 15 min at 45 °C, indicating that the heating time halved for each degree increase in temperature [19]. Therefore, enhancing the modulus of PU-based SMPs to meet the requirements for wound closure with a low Tr close to human temperature, the body core temperature of 36–38 °C [19], is a crucial attribute to consider for achieving practical application.
PU is a promising candidate for meeting these requirements due to its adaptable mechanical properties, which can be tailored to fit specific applications [1,20,21]. In an early application [22], a PU-based SMP was employed for a custom-designed spoon handle intended for the physically handicapped. In this use case, the spoon handle was heated and shaped to match an individual hand shape, and then its shape was fixed at room temperature to provide a comfortable and personalized fit. PU is composed of linear soft and hard segments linking with urethane bonds, formed by the reaction of a diisocyanate hard segment with a polyol soft segment [23,24,25]. The properties of PU-based SMPs largely depend on the structure, shape, and crystallization of hard and soft segments. The symmetrical diisocyanate tends to form a crystallizable structure [25]. Ahmad, M. et al. [24] synthesized various PU-based SMPs by utilizing five different polyols as soft segments and two different diisocyanate structures (liner aromatic bent structure of 4,4′-diphenylmethane diisocyanate (MDI) and cycloaliphatic isophorone diisocyanate (IPDI)) as hard segments. A higher MDI content results in SMPUs with enhanced shape-memory properties. Because IPDI had short-range conformational motions and restricted segmental movements of soft-segment chains, increasing IPDI content led to a weaker shape-memory effect while the fixity rate increased. The Tr of the SMPUs can be adjusted between 30 and 45 °C by altering the MDI-to-IPDI molar ratio, making them suitable for medical device applications due to their proximity to body temperature. In the literature, it was found that manipulating the hard-segment contents in the PU-based SMPs played a crucial role in physical characteristics and their shape memory properties [6,26]. A high degree of crosslinking is essential for producing PU-based SMPs with good thermomechanical properties and shape-memory effects [24]. Kim, B.K. et al. [6] reported that, as a major component was the soft segment, the Young’s modulus and tensile strength decreased as the block lengths increased. In addition, the low content of the hard segment was detrimental to shape recovery. The BDO hard segment was used to synthesize PU-based SMPs with good mechanical and shape memory properties [6,26]. Lee, B.S. et al. [26] revealed that maximum stress, tensile modulus, and elongation of PU-based SMPs increased significantly as the BDO hard-segment content moved beyond 30 wt%. In addition, the high shape recovery of around 80–95% was achieved at 30–45 wt% of hard-segment contents.
PCL is a soft semi-crystalline polymer with biodegradable and biocompatible properties, having low Tg and Tm at around −60 and 60 °C [27], respectively. Thus, this material exhibits flexible and rubber-like characteristics at room temperature, and it is applicable for thermal stimulation of shape recovery close to human temperature. PU-based SMPs composed of PCL soft segments and BDO hard segments demonstrated a shape recovery of over 94%, with a wide Tr ranging from 40 to 65 °C. However, the shape fixity is highly dependent on both the hard-segment content and the temperature programming [28]. Their crystallinities, mechanical properties, and shape memory properties varied depending on the molecular weight of PCL and the molar ratio of soft and hard segments [28,29].
It is well known that PU comprises block copolymers composed of polyester or polyether segments connected by urethane linkages (–NHCOO–), which are formed by the reaction between isocyanate (–N=C=O) and hydroxyl (–OH) groups [30]. Several research studies have explored the use of palm oil (PO) for PU preparation [20,31,32]. The addition of PO facilitates the promotion of urethane bond formation [31], influencing the creation of hard segments, which increases both the Young’s modulus and elongation as well as achieves good shape fixity and shape recovery ratios [20,32]. However, PO primarily consists of triglycerides (Tri-Gs), making it necessary to introduce hydroxyl groups to the Tri-G molecule for urethane reactions [33]. PO-based polyols can be synthesized using different methods [30,34,35,36,37]. Examples of palm oil applications in the synthesis of polyols for the preparation of rigid PU can be found in the literature [30,32,34,35,36]. Such polyols are usually obtained by transesterification using various agents, such as diethanolamine (DEA) [38,39] and dibutyltin dilaurate (DBTDL) [20,40].
This work aimed to synthesize PU-based SMPs composed of PCL soft segments and BDO hard segments. The Young’s modulus of 130 MPa—derived from the linear elastic mechanical properties of synthetic suture wires [18,41]—was selected for the criterion of the thermo-responsive wound closure strip in this study. The effect of PO hard-segment contents on the thermal, mechanical, and shape memory properties of PU was characterized, and the proof of concept for the use of PU-based SMPs in thermo-responsive wound closure strip application was demonstrated. Our findings suggest that PU-based SMPs with the addition of PO hold significant promise for improving wound closure techniques, potentially leading to better clinical outcomes.
2. Materials and Methods
2.1. Materials
The following chemicals were acquired from Sigma-Aldrich (St. Louis, MO, USA): PCL with an average molecular weight (Mn) of 2000 g/mol, 4,4′-methylenebis (cyclohexyl isocyanate) (HMDI) with a purity of 90% and a variety of isomers, BDO with a purity of 99%, and DBTDL with a purity of 95%. PO, an edible palm oil commercially available in the Thailand market, was a product from Lam Soon Public Co., Ltd. (Bangkok, Thailand), with a specific gravity of 0.9202 g/cm3 [37]. All the chemicals were used as received. In addition, an artificial 3-layer human skin model (model number SP01) was purchased from Shawn Science Manufacturing (Atlanta, GA, USA).
2.2. Synthesis of PU and Sample Preparation
PU was synthesized via a standard two-step pre-polymer method in the absence of organic solvent. The chemical reaction for the synthesis of PU is described elsewhere [42,43,44,45,46]. In the first step, the pre-polymer was prepared by adding the PCL as a soft-segment precursor and HMDI as a coupling agent in a round-bottom flask, heating to the temperature of 110 °C in a pre-heated silicone oil bath, and stirring using a magnetic stirrer at a speed of 190 rpm for 2.5 h. After that, PO as a hard segment was introduced into the mixture and mixed for a further 2 h. The reaction was carried out under the vacuum condition to remove the H2O molecules as a by-product. In the second step, the pre-polymer was then reacted with BDO as a hard segment and one drop of DBTDL as the catalyst for a further 20 min. The molar ratio of HMDI and BDO was fixed, whereas the molar ratio of PCL and PO was varied. Table 1 summarizes the molar ratio of all reagents and the sample code name and hard segment content (HSC). The HSC was calculated from the following equation [28]:
where , , , and are the molecular weights of each component and , , and are the mole numbers of the components in the PU molecule.

Table 1.
The formulation, sample code name, and HSC of PU.
For the mechanical, thermal, and shape memory tests, the resulting PU was hot-pressed into dumbbell-shaped specimens with a thickness of 0.5 mm, according to ASTM D638-14 [47], in a compression molding (LabTech Model LP20-B, Samutprakarn, Thailand) at 150 °C under the pressure of 15 MPa for 5 min. The rectangular-shaped strip with a dimension of 5 × 20 mm was cut from the narrow section of a dumbbell-shaped specimen to demonstrate the wound closure strip application. All samples were kept at room temperature before characterization.
2.3. Characterizations
The Fourier-transform infrared spectroscopy (FT-IR) (BRUKER, VERTEX 70, Billerica, MA, USA) analysis was performed in a transmission mode to verify the success of the PU synthesis. The FT-IR spectra of the PU specimen were recorded with a resolution of 4 cm−1 and scan number of 64 in the range of 4000 to 500 cm−1 for each measurement. The thermal behaviours of PU were determined using Differential Scanning Calorimetry (DSC) (METTLER TOLEDO, DSC3, Columbus, OH, USA). The sample of around 5 mg was cut and sealed in an aluminium pan. The sample was equilibrated at −100 °C for 2 min before heating to 120 °C with the heating rate of 5 °C/min under 50 mL/min N2 flow. The Tg, Tm, and melting enthalpy () of PU were acquired from the first DSC heating scan to relate to the mechanical properties and shape memory characteristic of PU. The crystallinity percentage of the PCL phase in PU was calculated by the following Equation [48]:
where the is the weight fraction of the PCL phase in the PU, and is the 100% melting enthalpy of PCL crystals, having a value of 140 J/g [49].
The X-ray diffraction (XRD) (Bruker, D8 Advance, Billerica, MA, USA) measurement was conducted at room temperature using a Cu-Kα X-ray source. The diffraction pattern was recorded over a 2θ range from 10° to 80°. Thermogravimetric analysis (TGA) (Mettler Toledo, TGA/DSC1, Columbus, OH, USA) was employed to assess the thermal stability and decomposition temperature of the PU. The samples were heated from 35 to 500 °C with a heating rate of 10 °C/min under a N2 flow rate of 100 mL/min. The tensile properties were evaluated using a universal testing machine (UTM) (Instron 5565, Northwood, MA, USA) at a crosshead speed of 50 mm/min with a 5 kN load cell according to ASTM D638 [47]. The average value from 5 samples was reported.
To evaluate the shape memory characteristics of the PU, the shape deformation was analyzed using the UTM coupled with a temperature-controlled chamber (Instron, 3119-406, Norwood, MA, USA). As shown in Figure 1, a dumbbell-shaped specimen was measured with the original length as the primary shape (step 1), denoted as . Then, the specimen was equilibrated at various Tp ranging from 40 to 80 °C inside the temperature-controlled chamber for 3 min before stretching to a specified length of 10 mm at a rate of 50 mm/min to form a temporary shape (step 2), denoted as . Subsequently, the specimen was rapidly cooled using cool pads for 1 min while the force was being maintained. Next, the specimen was removed from the clamps, conditioned in a cooler box (~0 °C) for 12 h, and the final length was measured (step 3), denoted as (). After that, the specimen was immersed in warm water with the corresponding Tr for 1 min. The changes in length of the specimen were measured, denoted as (). The shape fixity ratio () and the shape recovery ratio () were calculated using the following equation:
where , , , and are the stain length of the material at the initial length, after stretching to 10 mm at Tp, after cooling for 12 h, and after recovery [8], respectively. At least 5 replications were averaged and reported.
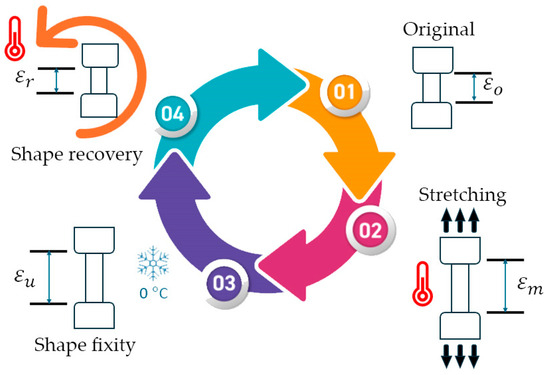
Figure 1.
The thermomechanical program for the shape memory test of PU.
The proof-of-concept experiment for the smart wound closure strip performance on artificial human skin was designed based on information acquired from the finite element (FE) model from the literature [50,51,52,53]. A small open wound with a dimension of 10 mm in length and 4 mm in depth was created, as shown in Figure 2a. In order to demonstrate the ability of the PU wound closure strip to retain its shape when subjected to pulling forces from artificial human skin after undergoing shape recovery, PU-based shape memory polymers (SMPs) with a Young’s modulus of 130 MPa and above were utilized. A pre-stretched specimen at 70 °C with 10 mm was attached to the artificial human skin across the middle of the wound opening area. It was then heated with a hot-air dryer (HRHD01BK, HAN RIVER, Shenzhen Yunuo Supply Chain Co., Ltd., Shenzhen, China), which was set to heating level 3 for 30 s. The experimental setup is shown in Figure 2b. The air temperature monitored using a thermocouple at the surface of the artificial human skin was around 47 ± 0.3 °C. After recovery, it was kept in a room with a controlled temperature of 25 °C for 15 days to simulate the duration required for the wound healing process. The performance of the wound closure strip was assessed by capturing a sequence of photos both during and after the recovery process, and the distance between the wound edges was measured using the ImageJ program. The result was documented as the width of the wound opening at various intervals of observation. A minimum of 5 replications were taken, and an average value was reported.
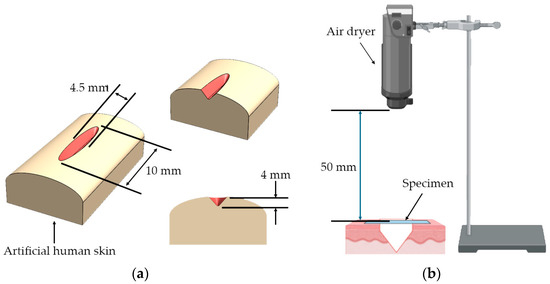
Figure 2.
(a) Wound cutting dimension on artificial human skin and (b) the setup for the shape recovery of wound closure strip made from PU.
3. Results and Discussion
3.1. FT-IR Analysis
FTIR spectroscopy was employed to identify the functional groups present in PCL, HMDI, and PU molecules. Figure 3 illustrates their FT-IR spectra. In accordance with the literature, the spectrum of PCL demonstrates distinct absorption peaks at the wavenumbers of 1720 and 1220 cm−1, which correspond to the stretching of C=O and C–O–C bonds, respectively [45,54,55]. Additionally, the vibrations of –CH2 groups were seen as symmetric stretching at 2929 cm−1 and asymmetric stretching at 2850 cm−1 [55]. The FT-IR spectra of PU with various amounts of added PO revealed the characteristic absorption peaks of PCL, as previously mentioned, along with the identification of a newly emerging amine functional group. The peak detected at 3290 cm−1 corresponds to the stretching vibration of the –NH group in the urethane linkage. The peaks seen at 1680, 1520, and 1230 cm−1 can be ascribed to the amide I, amide II, and amide III vibrations, respectively, as documented in references [54,55,56]. There were no absorption peaks at 2256 cm−1 in any of the FT-IR spectra of PU, indicating the absence of the carbamate group (–N=C=O) of HMDI. Additionally, it was noted that the intensity of the peak at 1680 cm−1 corresponding to the amide I was significantly associated with the existence of chemical bonds formed between the isocyanate group and PO. The intensity of amide I at 1680 cm−1 tended to increase over the peak intensity of the C=O bond at 1720 cm−1 when the PO content increased, as clearly seen for PU20 to PU40. Comparable FT-IR spectra of PCL-based PU have been documented in various research studies [43,45,54,55].
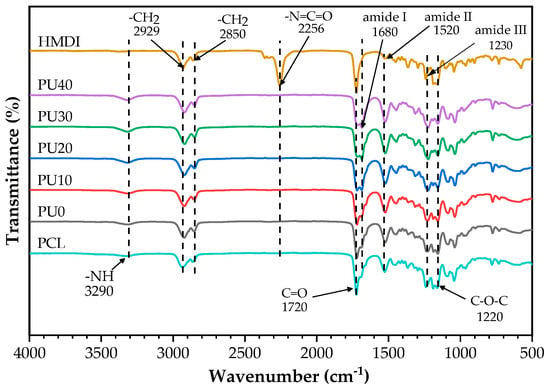
Figure 3.
FTIR spectra of PCL, HMDI, and PU.
3.2. DSC Analysis
Figure 4 shows the first DSC heating scan of PU with various PO contents; all DSC parameters are summarized in Table 2. Of course, the crystallization of all PU samples was completed during the sample preparation process due to the absence of the cold-crystallization behavior upon the DSC heating scan. The lower Tm at around 50–54 °C was attributed to the melting of the crystalline polycaprolactone-polyurethane (PCL-PU) phase, and the higher Tm at about 74–80 °C belonged to the crystalline BDO polyurethane (BDO-PU) phase. The literature reported that the Tms of PCL-PU was in the range of 40–60 °C [24,28,29]. Ping, P. et al. [29] revealed that the PCL-PU with the Mn of the PCL precursor lower than 2000 g/mol was non-crystalline and absent of the melting peak upon DSC heating scan. With the Mn of PCL above 2000 g/mol, PCL-PU could crystallize and exhibit a Tm of around 53.3 °C. These results are in agreement with the findings reported in this work, where the Mn of PCL was 2000 g/mol and the Tm of the PCL-PU phase was around 53 °C. In addition, the literature reported that the melting of BDO-PU occurred at high Tm [26]. Thus, it was confirmed that the lower and higher Tm corresponded to the melting of crystalline PCL-PU and crystalline BDO-PU phases, respectively. Moreover, the two melting peaks were observed for all PU samples, indicating that the PCL-PU phase and BDO-PU phase crystallized separately due to the different structural regularities and thermodynamic incompatibility.
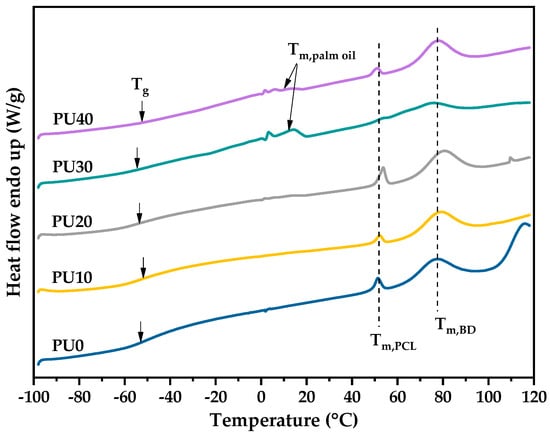
Figure 4.
The 1st DSC heating thermogram of PU.

Table 2.
The DSC parameters of PU.
Furthermore, a broad and small melting endotherm at the temperature range from 4 to 15 °C was attributed to the melting of PO [57,58] which was prominently observed at the higher PO content. The Tm of PO was not seen in PU10 due to the low PO content. Initial signs of PO melting began to appear in PU20, albeit with minimal deviations from the baseline. The distinct peak associated with PO melting became clearly observable starting from PU30 and above. This characteristic peak is indicative of the presence and influence of palm oil in the formulation. The addition of PO had insignificant effects on the Tgs of PU due to it being far below room temperature. However, it was found to influence the crystallization ability and degree of crystallinity determined from the of both PCL-PU and BDO-PU phases. The %Xc of the PCL-PU phase was negligible (less than 1%) due to it being a minor phase (Table 1). As seen in Table 2, the of the BDO-PU phase for PU10 decreased as compared to the PU0 sample without the addition of PO, indicating the interruption of crystallization from PO. With increasing of the PO content, the clear Tm of PO indicated the phase separation. Thus, the crystallization ability of PU was enhanced, resulting in the increase of the of the BDO-PU phase. However, PU30 exhibited small for both PCL-PU and BDO-PU phases, showing a lower crystallinity of PCL-PU and BDO-PU phases.
3.3. XRD Analysis
XRD analysis was used to evaluate the crystalline state of PU samples. As shown in Figure 5, all PU samples presented a broad crystalline peak with an amorphous diffraction pattern in the XRD curves, indicating the crystalline structure developed during the sample preparation process. The crystalline diffraction peaks at 18.5°, 30°, and 43° were attributed to the characteristic pattern of the crystalline BDO-PU phase. A similar XRD diffraction pattern was found for the palm kernel oil-based PU [20]. Normally, PCL as a semi-crystalline polymer exhibits a sharp distinct diffraction peak at 21.6° and a small-intensity sharp diffraction peak at 24.1°, corresponding to the (110) and (200) lattice planes of the PCL [54,59], respectively. As the PCL precursor formed PCL-PU, the intensities of both crystalline peaks became broadened and decreased in magnitude [48]. This was because the hard segments in PU have higher polarity from isocyanate groups, short chain length, and molecular asymmetry, unlike the soft segments [48,60]. Consequently, the asymmetrical hard segment was difficult to crystallize and exhibited a lower crystallinity. However, in this case, BDO was a symmetric molecule and capable of forming a crystalline structure. Therefore, a broad diffraction peak crystalline PCL-PU phase could be overlapped with a broad diffraction pattern of the crystalline BDO-PU phase. A similar diffraction pattern of PCL and BO-based PU was observed by others [20,54]. The diffraction intensity of the crystalline PCL-PU phase reduced as the PO content increased due to the portion of PCL soft segments being reduced. This observation was inconsistent with the increase in peak intensity of the amide I group at 1680 cm−1 from the FTIR analysis (Figure 3). The PO phase could not crystallize because the samples were kept and measured at room temperature above the Tm of PO (4–15 °C) thereby resulting in a liquid phase and acting as a chain extender. The intensity of crystalline peaks of the BD-PU phase seemed to experience a negligible effect from the PO. Thus, there was a co-existence of the crystalline and amorphous phases in the PU samples. In addition, all peak positions of all crystalline diffraction patterns were located at the same 2θ angle. This evidence indicated that the crystal structure of the crystalline PCL-PU and BDO-PU did not change and confirmed the DSC results that both phases crystallized separately and that PO was not crystalline.
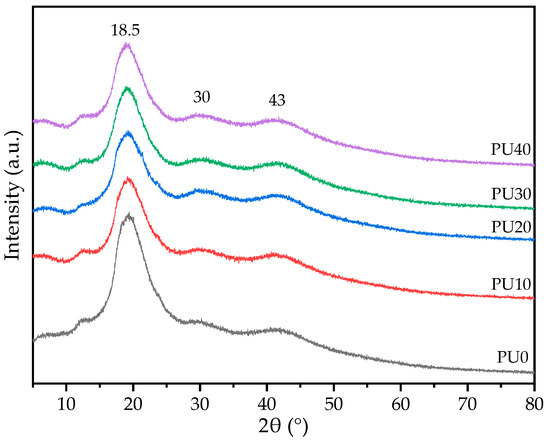
Figure 5.
The XRD patterns showing the intensity of the crystalline diffraction peaks of PU with different PO contents.
3.4. Tensile Behaviors
The tensile results of PU are summarized in Table 3. As the Tg of PU was far below room temperature, the PU samples were in a rubbery state. Without the addition of PO, PU0 exhibited ductile behavior, having a low Young’s modulus of 88.6 ± 3.20 × 10−2 MPa and low tensile strength of 10.15 ± 2.09 MPa, with the elongation at break of nearly 114%. By adding a small amount of PO, PU10 showed a lower Young’s modulus than that of PU0, while the elongation was maintained. With increasing the PO contents, the Young’s modulus increased and showed the maximum at 327.60 ± 2.80 × 10−2 MPa for the PU30 sample. Further increase of the PO content (PU40) resulted in a slight decrease of the Young’s modulus. However, the tensile strength and elongation were improved by the addition of PO.

Table 3.
The tensile testing results.
The literature reported that the symmetrically hard segment could form the crystalline structure and affect the tensile properties [23]. In this case, the PCL-PU soft segment had a very low degree of crystallinity (~1%), and the coupling agent HMDI hard segment was asymmetric. Thus, the tensile properties of PU were influenced by the crystallinity of the BDO-PU phase and the HSC in the PU molecular chains (Table 1). The addition of PO had two contrasting effects on the tensile properties. DSC and XRD measurements indicated that the crystallinity of the BDO-PU phase decreases with the addition of PO, leading to a reduction in Young’s modulus and tensile strength for the PU10 sample. Although the crystallinity reduced, the Young’s modulus and tensile strength appeared to increase and reached their maximum in the PU30 sample. This outcome was due to the increase in HSC from the higher PO content through the filler-like effect, which played an important role in enhancing the mechanical properties of the PU, resulting in a stiffer material [23,46]. On the other hand, the reduction in crystallinity after the incorporation of PO improved the elongation of PU.
3.5. Shape Memory Properties
The PU-based SMPs underwent testing for their shape memory properties through two processes. The first process involved assessing the temperature effect to ascertain the effective operating temperature for shape memory. Additionally, the shape recovery of PU was tested to establish a temperature suitable for further assessing the functionality of the wound closure strip. In the second process, a repeatable cycle testing procedure was employed to identify the point at which the PU-based SMPs achieved a stable state. This stable state was determined to be the most suitable point for simulating wound closure strip testing.
3.5.1. Effect of Temperature on Shape Memory Properties of PU
At room temperature, a crystalline portion was observed in PU as confirmed by DSC and XRD measurements. The crystalline domains of PCL-PU and BDO-PU as well as physical crosslinks or entangled chains served as an irreversible phase and controlled the shape memory effects in this situation. On the other hand, the amorphous PCL-PU phase, amorphous BDO-PU phase, PO, and un-crystallizable HMDI phase acted as a reversible phase upon the deformation and recovery processes. Five deformation temperatures of 40, 50, 60, 70, and 80 °C were chosen from the lower Tm of the PCL-PU phase, peak maximum Tm of the PCL-PU phase, greater Tm of PCL-PU phase, broad-based melting endotherm of the BDO-PU phase, and peak maximum Tm of the BDO-PU phase (Figure 4), respectively.
Figure 6 shows the effect of Tp on the shape fixity and shape recovery ratios of PU at the corresponding Tp. At the temperature lower than the Tm of the PCL-PU phase (Tp = 40 °C), the shape fixity ratio of PU0 without the addition of PO around 82% was lower than that of the PU with the addition of PO, as shown in Figure 6a. A similar result was observed at the Tp of 50 °C, but the shape fixity ratio of PU0 slightly improved to 86%. Compared to the Tp above the Tm of the PCL-PU phase (Tp = 60–80 °C), all PU samples showed a high shape fixity ratio, maintaining a value of around 95–98%, with minimal variation observed between samples with and without the addition of PO. Herein, at a temperature lower than the Tm of the PCL-PU phase (Tp = 40 °C), both crystalline PCL-PU and BOD-PU domains existed. In combination with the low Tp, once the PU sample deformed, the high elastic energy stored in the crystalline domain forced the temporary shape to restore its original shape and resulted in a low shape fixity ratio. The overall crystalline domains of PU were reduced by the addition of PO. Thus, the shape fixity improved. In a similar manner, at the Tp above the Tm of the PCL-PU phase (Tp > 50 °C), the elastic energy storage was derived from what the crystalline PCL-PU domains had lost, making the polymer chains more flexible and mobile. Consequently, the temporary shape could be effectively maintained, resulting in a high shape fixity ratio.
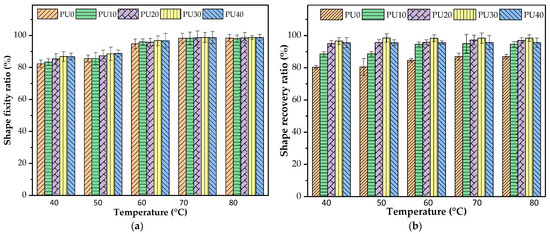
Figure 6.
(a) Effect of temperature on shape fixity ratio of PU and (b) the recovery ability of PU at the corresponding Tp.
As seen in Figure 6b, the shape recovery ratio for all PU samples appeared to be independent of Tp. The addition of PO had a more significant effect on the shape recovery ability of PU. Regarding the Tp and Tr below the Tm of the PCL-PU phase (Tp = 40 °C), the existing crystalline domains of both PCL-PU and BDO-PU phases could be permanently deformed, and the irreversible deformation took place upon stretching, resulting in the low shape recovery in the PU0 sample. As the crystalline domains decreased with the addition of PO, the shape recovery of PU increased. Furthermore, when the crystalline domains of the PCL-PU phase melted (Tp > 50 °C), the increase in mobility allowed the material to be deformed more easily. The stress could then be transferred to the hard segment and stored. Clearly, with higher PO content, the shape recovery ratio increased due to the rise in HSC.
From the above results, both the crystalline domain and HSC had influences on the shape memory properties of the PU. The high crystalline domains showed a negative effect on both shape fixity and shape recovery. By decreasing the overall crystalline domain by stretching at the temperature above the Tm of the PCL-PU phase, the hard segment played the dominant role in enhancing the shape memory properties. Within the Tp range of 60–80 °C, the shape fixity ratio was maximized and still retained the temporary shape effectively, with a desirable shape recovery ability. The Tp and Tr at 70 °C were selected for a study of the repeatability of PU.
3.5.2. Repeatability Study of PU-Based SMPs
The repeatability of PU-based SMPs was evaluated to determine the stability of shape memory parameters over multiple cycles and to ensure consistent functional behavior after the initial cycles. Many SMPs exhibited an improvement in shape recovery after the first deformation cycle [61,62,63]. This effect is well known as the “training effect” [64] and “cyclic hardening” [65].
However, as seen in Figure 7, the shape fixity ratio of all PU samples decreased and then remained stable after the first cycle of the repeatability test, while the shape recovery remained almost constant along with the five thermomechanical stretching-recovering cycles. In this case, the Tp of 70 °C was in the broad-based melting endotherm region of the BDO-PU phase. A number of crystalline domains of the BDO-PU phase existed in the PU sample. As aforementioned, the deformation that arose from the crystalline domains could cause permanent deformation upon stretching and consequently contributed to forming a new irreversible phase structure in the first deformation cycle, resulting in a greater HSC and higher elastic energy storage. Consequently, the shape fixity ratio was reduced in a second deformation cycle. The stable shape fixity ratio of the second to fifth deformation cycle indicated no occurrence of a new irreversible phase. Thus, the shape fixity ratio after the involuntary generation of greater HSC was around 69–75%. A similar observation was revealed in other PU-based SMP systems [66].
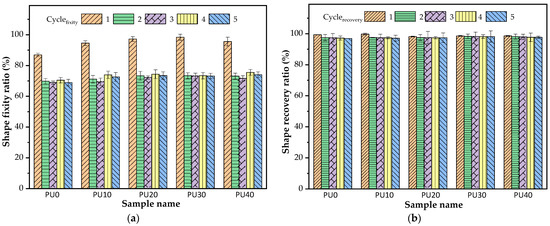
Figure 7.
(a) Shape fixity ratio and (b) shape recovery ratio of PU at Tp and Tr of 70 °C under 5 thermomechanical stretching–recovering cycles.
3.6. Proof-of-Concept of Thermo-Responsive Wound Closure Strip Application
As aforementioned, the criterion of Young’s modulus in this work was set as 130 MPa. From tensile results, PU20, PU30, and PU40 had Young’s modulus values of 155.60 ± 6.22 × 10−2, 327.60 ± 2.80 × 10−2, and 235.60 ± 0.82 × 10−2 MPa, respectively. The PU20 and PU30 samples were selected to demonstrate a thermo-responsive wound closure application due to the lowest value occurring at higher the criterion and the maximum value achieved among the PU samples, respectively. Figure 8 illustrates the wound dehiscence on artificial human skin, where the wound typically displays separation on either side. The specimen was positioned in the center of the wound on the artificial human skin. It was secured with pins at both ends to ensure it adhered to the artificial skin. Employing the principle of shape memory polymer recovery, the wound closure strip pulled the separated wound edges together, promoting closure. Figure 9 demonstrates the capability of using PU-based SMPs as wound closure strips. Both sides of the wound were effectively brought together for healing following the recovery of the PU-based SMPs to their primary shape. It was observed that the wound edges separated from each other after a few days due to the tension from the artificial human skin. The distance between the two sides of the separated wound edge is shown in Figure 10. The results indicated that, using PU20, the center distance of the wound edges began to separate on the first day and tended to increase over time, resulting in the appearance of a channel at the top and bottom parts of the wound dehiscence. Conversely, PU30, which has the highest Young’s modulus, displayed a small wound channel after 5 days, slightly increasing to 0.03 mm by day 15. The top and bottom parts, which were not attached to the wound closure strip, began to separate after 3 days. The results demonstrated that PU30 with a high Young’s modulus had a greater efficiency in maintaining wound closure than that of PU20. This suggested that thermo-responsive PU-based SMPs have the potential to be utilized to cover wounds until the surgical wounds heal.

Figure 8.
Digital image of (a) wound dehiscence on artificial human skin and (b) wound dehiscence with the wound closure strip made from PU.
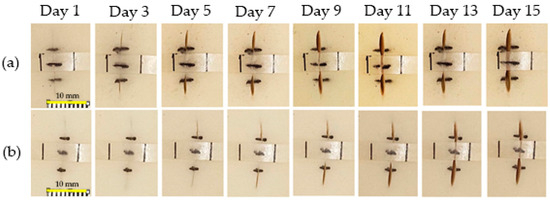
Figure 9.
Digital image presentation of a wound closure strip made from (a) PU20 and (b) PU30 for maintaining the closure of wound dehiscence.
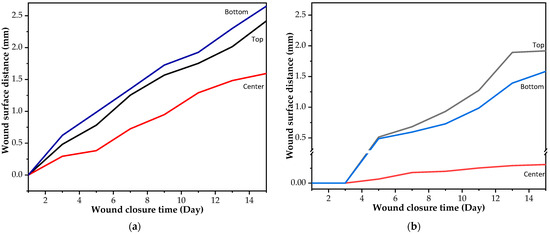
Figure 10.
(a) Wound closure strip made of PU20 and (b) wound closure strip made of PU30.
From our point of view, a single wound closure strip made of PU-based SMPs with a Young’s modulus slightly above 130 MPa was not effective during the wound healing process. PU-based SMPs with a higher Young’s modulus had a significant impact on resisting the pulling force. Future research will focus more on balancing the Young’s modulus, mechanical properties, and shape memory properties of PU. The aspects of bio-adhesion between the PU surface and the artificial skin, as well as the sterilization process, were not addressed here. In the future, it will be essential to use a sterilization technique such as hydrogen peroxide, rather than relying on temperature, to maintain the temporary shape of the PU wound closure strip. Additionally, using bio-adhesives instead of pins in practical applications would be a favorable choice due to their improved ability to adhere to the skin and their non-destructive effect on live cells.
4. Conclusions
In conclusion, the aim of enhancing the tensile modulus of PU-based SMPs was achieved by adjusting the ratio of PO to PCL from 0 to 40 molar ratio, targeting a Young’s modulus of 130 MPa for wound closure applications. The successful incorporation of palm oil into the PU-based SMP structure was confirmed by FTIR analysis. Mechanical testing demonstrated that the target modulus was achieved by incorporating PO, starting from PU20. A significant increase in Young’s modulus was observed, from 88.60 ± 3.20 × 102 MPa at PU0 to 327.60 ± 2.80 × 102 MPa at PU30, indicating a nearly 2.7-fold increase due to PO, attributed to its filler-like effect and increased hard-segment content.
Furthermore, the shape fixity and recovery properties of PU-based samples were found to be influenced by the Tp and hard-segment content, both of which increased with PO content. In the proof-of-concept experiment, effective wound closure without gaps on the first day was demonstrated by PU20 and PU30 wound closure strips. By day 15, PU20 exhibited a 1.5 mm increase in wound size, whereas PU30 showed only a 0.03 mm increase, highlighting PU30’s approximately 50-fold superior wound-holding ability over PU20.
While promising results were achieved in this study, challenges remain. The relatively high recovery temperature of the samples may require careful adjustment to optimize practical applications alongside mechanical properties. Furthermore, investigation into the cytotoxicity of PO-modified PU-based SMPs is crucial for ensuring their safety and suitability in medical applications. Addressing these challenges will be pivotal for advancing smart wound closure strips based on PO-modified PU-based SMPs.
Author Contributions
Conceptualization, S.K., W.T. and T.T.; methodology, S.K., W.T., N.S. and T.T.; formal analysis, S.K., T.K., N.S. and T.T.; investigation, S.K. and T.K.; data curation, S.K.; visualization, S.K.; validation, T.K., W.T., N.S. and T.T.; writing—original draft preparation, S.K. and T.K.; supervision, N.S. and T.T.; writing—review and editing, N.S. and T.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Center of Excellence in Biomechanics Medicine and Thailand Science Research and Innovation (TSRI), National Science, Research and Innovation Fund (NRSF) (NRIIS number 160344).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
The authors are grateful to Suranaree University of Technology (SUT), Thailand Science Research and Innovation (TSRI), the National Science, Research and Innovation Fund (NSRF) (NRIIS number 160344), Center of Excellence in Biomechanics Medicine, and the Research Center for Biocomposite Materials for the Medical Industry and Agricultural and Food Industry for the financial support. The first author expresses his gratitude to Mullika Sungsanit for the One Research One Graduate scholarship (OROG) provided by Suranaree University of Technology.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Peterson, G.I.; Dobrynin, A.V.; Becker, M.L. α-Amino Acid-Based Poly(Ester urea)s as Multishape Memory Polymers for Biomedical Applications. ACS Macro Lett. 2016, 5, 1176–1179. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.G.; Sandstroröm, R.; Miyazaki, S. Shape-memory materials and hybrid composites for smart systems: Part I Shape-memory materials. J. Mater. Sci. 1998, 33, 3743–3762. [Google Scholar] [CrossRef]
- Sokolowski, W.; Metcalfe, A.; Hayashi, S.; Yahia, L.H.; Raymond, J. Medical applications of shape memory polymers. Biomed. Mater. 2007, 2, S23. [Google Scholar] [CrossRef] [PubMed]
- Peterson, G.I.; Dobrynin, A.V.; Becker, M.L. Biodegradable Shape Memory Polymers in Medicine. Adv. Healthc. Mater. 2017, 6, 1700694. [Google Scholar] [CrossRef]
- Meng, H.; Hu, J. A Brief Review of Stimulus-active Polymers Responsive to Thermal, Light, Magnetic, Electric, and Water/Solvent Stimuli. J. Intell. Mater. Syst. Struct. 2010, 21, 859–885. [Google Scholar] [CrossRef]
- Kim, B.K.; Shin, Y.J.; Cho, S.M.; Jeong, H.M. Shape-memory behavior of segmented polyurethanes with an amorphous reversible phase: The effect of block length and content. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 2652–2657. [Google Scholar] [CrossRef]
- Elshazly, T.M.; Keilig, L.; Alkabani, Y.; Ghoneima, A.; Abuzayda, M.; Talaat, S.; Bourauel, C.P. Primary Evaluation of Shape Recovery of Orthodontic Aligners Fabricated from Shape Memory Polymer (A Typodont Study). Dent. J. 2021, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Barik, S.K.; Rao, P.S.; Mohanty, R. A review on initiation, structure and advance utilization of shape memory polymer. J. Adv. Sci. Res. 2019, 10, 131–138. [Google Scholar]
- Jung, Y.C.; Cho, J.W. Application of shape memory polyurethane in orthodontic. J. Mater. Sci. Mater. Med. 2010, 21, 2881–2886. [Google Scholar] [CrossRef]
- Jeewantha, J.; Jayalath, S.; Emmanuel, C.; Herath, M.; Forster, E.; Islam, M.; Leng, J.; Epaarachchi, J. Shape memory polymer smart plaster for orthopaedic treatments. Smart Mater. Struct. 2022, 31, 115016. [Google Scholar] [CrossRef]
- Valerie, E.-J. Essential Microbiology for Wound Care; OUP Oxford: Oxford, UK, 2016. [Google Scholar]
- CHAPTER 14–Tissue Adhesives and Alternative Wound Closure. In Wounds and Lacerations, 3rd ed.; Trott, A.T., Ed.; Mosby: Philadelphia, Pennsylvania, 2005; pp. 209–222. [Google Scholar]
- Simcock, J.W.; Armitage, J.; Dixon, L.; MacFarlane, K.; Robertson, G.M.; Frizelle, F.A. Skin closure after laparotomy with staples or sutures: A study of the mature scar. ANZ J. Surg. 2014, 84, 656–659. [Google Scholar] [CrossRef] [PubMed]
- Shagan, A.; Zhang, W.; Mehta, M.; Levi, S.; Kohane, D.; Mizrahi, B. Hot Glue Gun Releasing Biocompatible Tissue Adhesive. Adv. Funct. Mater. 2019, 30, 1900998. [Google Scholar] [CrossRef]
- van de Gevel, D.F.D.; Hamad, M.A.S.; Elenbaas, T.W.O.; Ostertag, J.U.; Schönberger, J.P.A.M. Is the use of Steri-Strip™ S for wound closure after coronary artery bypass grafting better than intracuticular suture? Interact. CardioVascular Thorac. Surg. 2010, 10, 561–564. [Google Scholar] [CrossRef]
- Taira, B.R.; Singer, A.J.; Rooney, J.; Steinhauff, N.T.; Zimmerman, T. An In-Vivo Study of the Wound-Bursting Strengths of Octyl-Cyanoacrylate, Butyl-Cyanoacrylate, and Surgical Tape in Rats. J. Emerg. Med. 2010, 38, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.Y.; Yun, H.G.; Park, I.Y. n-Butyl-2-cyanoacrylate tissue adhesive (Histoacryl) vs. subcuticular sutures for skin closure of Pfannenstiel incisions following cesarean delivery. PLoS ONE 2018, 13, e0202074. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, D.; Shumway, S.; Albear, P.; Gottlieb, L. Mechanical comparison of 10 suture materials before and after in vivo incubation. J. Surg. Res. 1994, 56, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, L.S.; Dewhirst, M.W.; Repacholi, M.; Kheifets, L. Summary, conclusions and recommendations: Adverse temperature levels in the human body. Int. J. Hyperth. 2003, 19, 373–384. [Google Scholar] [CrossRef]
- Saad, N.M.; Zubir, S.A. Palm Kernel Oil Polyol-based Polyurethane as Shape Memory Material: Effect of Polyol Molar Ratio. J. Phys. Sci. 2019, 30, 77–89. [Google Scholar] [CrossRef]
- Xia, Y.; Larock, R.C. Vegetable oil-based polymeric materials: Synthesis, properties, and applications. Green Chem. 2010, 12, 1893–1909. [Google Scholar] [CrossRef]
- Tobushi, H.; Hayashi, S.; Kojima, S. Mechanical Properties of Shape Memory Polymer of Polyurethane Series: Basic Characteristics of Stress-Strain-Temperature Relationship. JSME Int. J. Ser. 1 Solid Mech. Strength Mater. 1992, 35, 296–302. [Google Scholar] [CrossRef]
- Tantisuwanno, C.; Jain, T.; Tseng, Y.-M.; Joy, A. Pendant Amines in the Hard or Soft Segments of PCL-Polyurethanes Have Contrasting Effects on the Mechanical and Surface Properties. Macromolecules 2024, 57, 4448–4459. [Google Scholar] [CrossRef]
- Ahmad, M.; Luo, J.; Xu, B.; Purnawali, H.; King, P.J.; Chalker, P.R.; Fu, Y.; Huang, W.; Miraftab, M. Synthesis and Characterization of Polyurethane-Based Shape-Memory Polymers for Tailored Tg around Body Temperature for Medical Applications. Macromol. Chem. Phys. 2011, 212, 592–602. [Google Scholar] [CrossRef]
- Kalita, H. Shape Memory Polymers: Theory and Application; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2018. [Google Scholar]
- Lee, B.S.; Chun, B.C.; Chung, Y.-C.; Sul, K.I.; Cho, J.W. Structure and Thermomechanical Properties of Polyurethane Block Copolymers with Shape Memory Effect. Macromolecules 2001, 34, 6431–6437. [Google Scholar] [CrossRef]
- Wang, H.; Domingos, M.; Scenini, F. Advanced mechanical and thermal characterization of 3D bioextruded poly(e-caprolactone)-based composites. Rapid Prototyp. J. 2018, 24, 731–738. [Google Scholar] [CrossRef]
- Eyvazzadeh Kalajahi, A.; Rezaei, M.; Abbasi, F. Preparation, characterization, and thermo-mechanical properties of poly (ε-caprolactone)-piperazine-based polyurethane-urea shape memory polymers. J. Mater. Sci. 2016, 51, 4379–4389. [Google Scholar] [CrossRef]
- Ping, P.; Wang, W.; Chen, X.; Jing, X. Poly(ε-caprolactone) Polyurethane and Its Shape-Memory Property. Biomacromolecules 2005, 6, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, S.N.S.; Jusoh, M.A.; Jasim, S.E.; Gambang, P. A Review: Method of Preparing Palm Oil Based Polyurethane. In Proceedings of the National Conference for Postgraduate University Malaysia Pahang, Pekan, Malaysia, 24–25 September 2016; pp. 655–662. [Google Scholar]
- Stavila, E.; Yuliati, F.; Adharis, A.; Laksmono, J.A.; Iqbal, M. Recent advances in synthesis of polymers based on palm oil and its fatty acids. RSC Adv. 2023, 13, 14747–14775. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, H.; Prociak, A. Influence of Palm Oil-Based Polyol on the Properties of Flexible Polyurethane Foams. J. Polym. Environ. 2012, 20, 438–445. [Google Scholar] [CrossRef]
- Tanaka, R.; Hirose, S.; Hatakeyama, H. Preparation and characterization of polyurethane foams using a palm oil-based polyol. Bioresour. Technol. 2008, 99, 3810–3816. [Google Scholar] [CrossRef]
- Zhang, C. Plant Oil-based Polyurethanes. In Green Chemistry and Green Materials from Plant Oils and Natural Acids; Liu, Z., Kraus, G., Eds.; Royal Society of Chemistry: London, UK, 2023; Volume 83. [Google Scholar]
- Kaikade, D.S.; Sabnis, A.S. Recent Advances in Polyurethane Coatings and Adhesives Derived from Vegetable Oil-Based Polyols. J. Polym. Environ. 2023, 31, 4583–4605. [Google Scholar] [CrossRef]
- Garrison, T.F.; Kessler, M.R. 3-Plant Oil-Based Polyurethanes. In Bio-Based Plant Oil Polymers and Composites; Madbouly, S.A., Zhang, C., Kessler, M.R., Eds.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 37–54. [Google Scholar]
- Lumcharoen, D.; Saravari, O. Preparation and characterization of flexible polyurethane foams from palm oil-based polyol. Adv. Mater. Res. 2014, 911, 352–356. [Google Scholar] [CrossRef]
- Lee, C.S.; Ooi, T.L.; Chuah, C.H.; Ahmad, S. Rigid Polyurethane Foam Production from Palm Oil-Based Epoxidized Diethanolamides. J. Am. Oil Chem. Soc. 2007, 84, 1161–1167. [Google Scholar] [CrossRef]
- Badri, K.; Othman, Z.; Ahmad, S. Rigid polyurethane foams from oil palm resources. J. Mater. Sci. 2004, 39, 5541–5542. [Google Scholar] [CrossRef]
- Brito, Y.C.; Ferreira, D.A.C.; Fragoso, D.M.d.A.; Mendes, P.R.; Oliveira, C.M.J.d.; Meneghetti, M.R.; Meneghetti, S.M.P. Simultaneous conversion of triacylglycerides and fatty acids into fatty acid methyl esters using organometallic tin(IV) compounds as catalysts. Appl. Catal. A Gen. 2012, 443–444, 202–206. [Google Scholar] [CrossRef]
- Chanda, A.; Unnikrishnan, V. A Realistic 3D Computational Model of the Closure of Skin Wound with Interrupted Sutures. J. Mech. Med. Biol. 2017, 17, 1750025. [Google Scholar] [CrossRef]
- Khan, F.; Valere, S.; Fuhrmann, S.; Arrighi, V.; Bradley, M. Synthesis and cellular compatibility of multi-block biodegradable poly(ε-caprolactone)-based polyurethanes. J. Mater. Chem. B 2013, 1, 2590–2600. [Google Scholar] [CrossRef]
- Eftekhari, B.S.; Karkhaneh, A.; Alizadeh, A. Physically targeted intravenous polyurethane nanoparticles for controlled release of atorvastatin calcium. Iran. Biomed. J. 2017, 21, 369. [Google Scholar]
- Rubino, L.; Torrisi, G.; Brambilla, L.; Rubino, L.; Ortenzi, M.A.; Galimberti, M.; Barbera, V. Polyhydroxylated Nanosized Graphite as Multifunctional Building Block for Polyurethanes. Polymers 2022, 14, 1159. [Google Scholar] [CrossRef]
- Binti, K.; Haji Badri, K.; Maisara, S.; Binti, S.; Shahrom, R.; Hao, L.; Baderuliksan, N.; Norzali, A.; Sien, W. FTIR spectroscopy analysis of the prepolymerization of palm-based polyurethane. Solid State Sci. Technol. 2010, 18, 1–8. [Google Scholar]
- Yeoh, F.H.; Lee, C.S.; Kang, Y.B.; Wong, S.F.; Cheng, S.F.; Ng, W.S. Production of Biodegradable Palm Oil-Based Polyurethane as Potential Biomaterial for Biomedical Applications. Polymers 2020, 12, 1842. [Google Scholar] [CrossRef]
- International, A. ASTM D638-14; Standard Test Method for Tensile Properties of Plastics. ASTM International: West Conshohocken, PA, YSA, 2015.
- Sabahi, N.; Roohani, I.; Wang, C.H.; Farajzadeh, E.; Li, X. Thermoplastic polyurethane-based shape memory polymers with potential biomedical application: The effect of TPU soft-segment on shape memory effect and cytocompatibility. Polymer 2023, 283, 126189. [Google Scholar] [CrossRef]
- Crescenzi, V.; Manzini, G.; Calzolari, G.; Borri, C. Thermodynamics of fusion of poly-β-propiolactone and poly-ϵ-caprolactone. comparative analysis of the melting of aliphatic polylactone and polyester chains. Eur. Polym. J. 1972, 8, 449–463. [Google Scholar] [CrossRef]
- Capek, L.; Jacquet, E.; Dzan, L.; Simunek, A. The analysis of forces needed for the suturing of elliptical skin wounds. Med. Biol. Eng. Comput. 2012, 50, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Larrabee, W.F., Jr. A finite element model of skin deformation. I. Biomechanics of skin and soft tissue: A review. Laryngoscope 1986, 96, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Larrabee, W.F., Jr.; Sutton, D. A finite element model of skin deformation. II. An experimental model of skin deformation. Laryngoscope 1986, 96, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Larrabee, W.F.; Galt, J.A. A finite element model of skin deformation. III. The finite element model. Laryngoscope 2009, 96, 413–419. [Google Scholar] [CrossRef]
- Kandi, R.; Pandey, P.M.; Majood, M.; Mohanty, S. Fabrication and characterization of customized tubular scaffolds for tracheal tissue engineering by using solvent based 3D printing on predefined template. Rapid Prototyp. J. 2021, 27, 421–428. [Google Scholar] [CrossRef]
- Han, J.; Chen, B.; Ye, L.; Zhang, A.-y.; Zhang, J.; Feng, Z.-g. Synthesis and characterization of biodegradable polyurethane based on poly(ε-caprolactone) and L-lysine ethyl ester diisocyanate. Front. Mater. Sci. China 2009, 3, 25–32. [Google Scholar] [CrossRef]
- Tan, C.; Tirri, T.; Wilen, C.-E. Investigation on the Influence of Chain Extenders on the Performance of One-Component Moisture-Curable Polyurethane Adhesives. Polymers 2017, 9, 184. [Google Scholar] [CrossRef] [PubMed]
- Berger, K.G.; Siew, W.L.; Oh, F.C.H. Factors affecting slip melting point of palm oil products. J. Am. Oil Chem. Soc. 1982, 59, 244–249. [Google Scholar] [CrossRef]
- Tan, C.P.; Che Man, Y.B. Differential scanning calorimetric analysis of palm oil, palm oil based products and coconut oil: Effects of scanning rate variation. Food Chem. 2002, 76, 89–102. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Wang, J.; Li, C. Preparation and characterization of poly(ɛ-caprolactone) nonwoven mats via melt electrospinning. Polymer 2012, 53, 248–253. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, F.; Wu, Y. Robust Stretchable Thermoplastic Polyurethanes with Long Soft Segments and Steric Semisymmetric Hard Segments. Ind. Eng. Chem. Res. 2020, 59, 4483–4492. [Google Scholar] [CrossRef]
- Staszczak, M.; Nabavian Kalat, M.; Golasiński, K.M.; Urbański, L.; Takeda, K.; Matsui, R.; Pieczyska, E.A. Characterization of Polyurethane Shape Memory Polymer and Determination of Shape Fixity and Shape Recovery in Subsequent Thermomechanical Cycles. Polymers 2022, 14, 4775. [Google Scholar] [CrossRef] [PubMed]
- Tobushi, H.; Shimada, D.; Hayashi, S.; Endo, M. Shape fixity and shape recovery of polyurethane shape-memory polymer foams. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2003, 217, 135–143. [Google Scholar] [CrossRef]
- Pringpromsuk, S.; Xia, H.; Ni, Q.-Q. Multifunctional stimuli-responsive shape memory polyurethane gels for soft actuators. Sens. Actuators A Phys. 2020, 313, 112207. [Google Scholar] [CrossRef]
- Tobushi, H.; Hashimoto, T.; Ito, N.; Hayashi, S.; Yamada, E. Shape Fixity and Shape Recovery in a Film of Shape Memory Polymer of Polyurethane Series. J. Intell. Mater. Syst. Struct. 1998, 9, 127–136. [Google Scholar] [CrossRef]
- Lendlein, A.; Kelch, S. Shape-Memory Polymers. Angew. Chem. Int. Ed. 2002, 41, 2034–2057. [Google Scholar] [CrossRef]
- Ohki, T.; Ni, Q.-Q.; Ohsako, N.; Iwamoto, M. Mechanical and shape memory behavior of composites with shape memory polymer. Compos. Part A Appl. Sci. Manuf. 2004, 35, 1065–1073. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).