Injectable Carrageenan/Green Graphene Oxide Hydrogel: A Comprehensive Analysis of Mechanical, Rheological, and Biocompatibility Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of the Graphene Dispersions
2.2.1. Preparation of Graphene Dispersions
2.2.2. Thermogravimetric Analysis
2.2.3. Raman Spectroscopy of Graphene Dispersions
2.2.4. UV-Visible Spectroscopy
2.3. Hydrogel Preparation
2.4. Mechanical Properties
2.5. Scanning Electron Microscope (SEM)
2.6. X-ray Diffraction
2.7. Swelling Behavior
2.8. Rheological Properties
2.9. Cell Assays
Cell Cultures
2.10. Biocompatibility Assay
3. Results
3.1. Mechanical Properties
3.2. Scanning Electron Microscopy
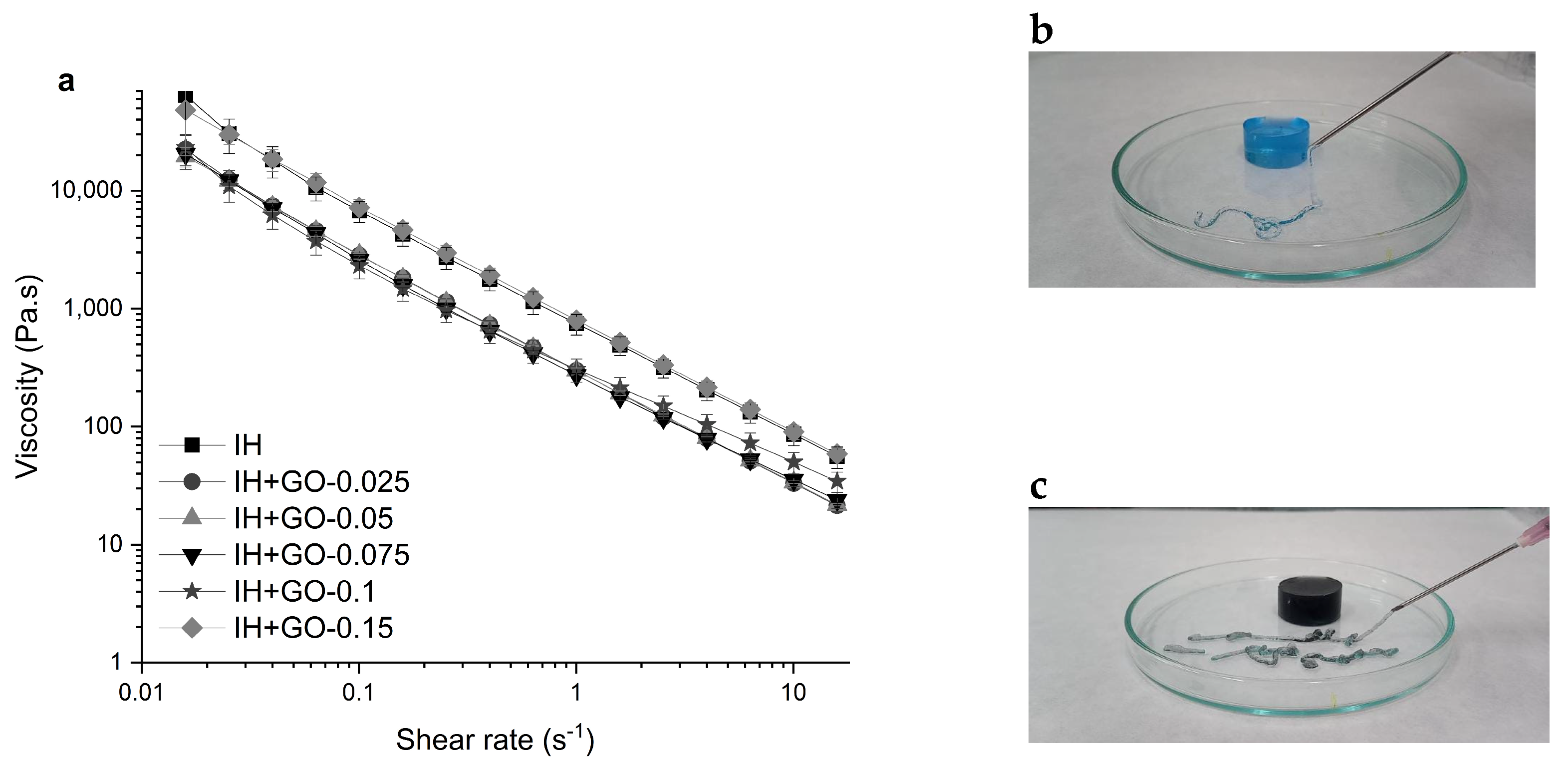
3.3. Rheological Properties
3.4. Swelling Behavior
3.5. Biocompatibility Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hernández-González, A.C.; Téllez-Jurado, L.; Rodríguez-Lorenzo, L.M. Alginate hydrogels for bone tissue engineering, from injectables to bioprinting: A review. Carbohydr. Polym. 2020, 229, 115514. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Ding, J. Injectable hydrogels as unique biomedical materials. Chem. Soc. Rev. 2008, 37, 1473–1481. [Google Scholar] [CrossRef]
- Fan, Z.; Cheng, P.; Gao, Y.; Wang, D.; Jia, G.; Zhang, P.; Prakash, S.; Wang, Z.; Han, J. Understanding the rheological properties of a novel composite salecan/gellan hydrogels. Food Hydrocoll. 2022, 123, 107162. [Google Scholar] [CrossRef]
- Gyles, D.A.; Castro, L.D.; Silva, J.O.C.; Ribeiro-Costa, R.M. A review of the designs and prominent biomedical advances of natural and synthetic hydrogel formulations. Eur. Polym. J. 2017, 88, 373–392. [Google Scholar] [CrossRef]
- Pettinelli, N.; Rodríguez-Llamazares, S.; Abella, V.; Barral, L.; Bouza, R.; Farrag, Y.; Lago, F. Entrapment of chitosan, pectin or κ-carrageenan within methacrylate based hydrogels: Effect on swelling and mechanical properties. Mater. Sci. Eng. C 2019, 96, 583–590. [Google Scholar] [CrossRef]
- Darge, H.F.; Andrgie, A.T.; Tsai, H.C.; Lai, J.Y. Polysaccharide and polypeptide based injectable thermo-sensitive hydrogels for local biomedical applications. Int. J. Biol. Macromol. 2019, 133, 545–563. [Google Scholar] [CrossRef]
- Graham, S.; Marina, P.F.; Blencowe, A. Thermoresponsive polysaccharides and their thermoreversible physical hydrogel networks. Carbohydr. Polym. 2019, 207, 143–159. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L. Carrageenan: A review. Vet. Med. 2013, 58, 187–205. [Google Scholar] [CrossRef]
- Barbeyron, T.; Michel, G.; Potin, P.; Henrissat, B.; Kloareg, B. ι-Carrageenases constitute a novel family of glycoside hydrolases, unrelated to that of κ-carrageenases. J. Biol. Chem. 2000, 275, 35499–35505. [Google Scholar] [CrossRef]
- Guan, J.; Li, L.; Mao, S. Applications of Carrageenan in Advanced Drug Delivery. In Seaweed Polysaccharides: Isolation, Biological and Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 283–303. [Google Scholar] [CrossRef]
- Evageliou, V.I.; Ryan, P.M.; Morris, E.R. Effect of monovalent cations on calcium-induced assemblies of kappa carrageenan. Food Hydrocoll. 2019, 86, 141–145. [Google Scholar] [CrossRef]
- Cao, Y.; Li, S.; Fang, Y.; Nishinari, K.; Phillips, G.O.; Lerbret, A.; Assifaoui, A. Specific binding of trivalent metal ions to λ-carrageenan. Int. J. Biol. Macromol. 2018, 109, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Running, C.A.; Falshaw, R.; Janaswamy, S. Trivalent iron induced gelation in lambda-carrageenan. Carbohydr. Polym. 2012, 87, 2735–2739. [Google Scholar] [CrossRef] [PubMed]
- Campo, V.L.; Kawano, D.F.; da Silva, D.B.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Yegappan, R.; Selvaprithiviraj, V.; Amirthalingam, S.; Jayakumar, R. Carrageenan based hydrogels for drug delivery, tissue engineering and wound healing. Carbohydr. Polym. 2018, 198, 385–400. [Google Scholar] [CrossRef]
- Bui, V.T.N.T.; Nguyen, B.T.; Nicolai, T.; Renou, F. Mixed iota and kappa carrageenan gels in the presence of both calcium and potassium ions. Carbohydr. Polym. 2019, 223, 115107. [Google Scholar] [CrossRef]
- Ngwabebhoh, F.A.; Patwa, R.; Zandraa, O.; Saha, N.; Saha, P. Preparation and characterization of injectable self-antibacterial gelatin/carrageenan/bacterial cellulose hydrogel scaffolds for wound healing application. J. Drug Deliv. Sci. Technol. 2021, 63, 102415. [Google Scholar] [CrossRef]
- Pettinelli, N.; Rodríguez-Llamazares, S.; Bouza, R.; Barral, L.; Feijoo-Bandín, S.; Lago, F. Carrageenan-based physically crosslinked injectable hydrogel for wound healing and tissue repairing applications. Int. J. Pharm. 2020, 589, 119828. [Google Scholar] [CrossRef] [PubMed]
- Kobyliukh, A.; Olszowska, K.; Godzierz, M.; Kordyka, A.; Kubacki, J.; Mamunya, Y.; Pusz, S.; Stoycheva, I.; Szeluga, U. Effect of graphene material structure and iron oxides deposition method on morphology and properties of graphene/iron oxide hybrids. Appl. Surf. Sci. 2022, 573, 151567. [Google Scholar] [CrossRef]
- Phiri, J.; Gane, P.; Maloney, T.C. General overview of graphene: Production, properties and application in polymer composites. Mater. Sci. Eng. B 2017, 215, 9–28. [Google Scholar] [CrossRef]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Chung, C.; Kim, Y.K.; Shin, D.; Ryoo, S.R.; Hong, B.H.; Min, D.H. Biomedical applications of graphene and graphene oxide. Acc. Chem. Res. 2013, 46, 2211–2224. [Google Scholar] [CrossRef]
- Paul, A.; Hasan, A.; al Kindi, H.; Gaharwar, A.K.; Rao, V.T.S.; Nikkhah, M.; Shin, S.R.; Krafft, D.; Dokmeci, M.R.; Shum-Tim, D.; et al. Injectable graphene oxide/hydrogel-based angiogenic gene delivery system for vasculogenesis and cardiac repair. ACS Nano 2014, 8, 8050–8062. [Google Scholar] [CrossRef]
- Shin, S.R.; Aghaei-Ghareh-Bolagh, B.; Gao, X.; Nikkhah, M.; Jung, S.M.; Dolatshahi-Pirouz, A.; Kim, S.B.; Kim, S.M.; Dokmeci, M.R.; Tang, X.; et al. Layer-by-layer assembly of 3D tissue constructs with functionalized graphene. Adv. Funct. Mater. 2014, 24, 6136–6144. [Google Scholar] [CrossRef]
- Vinothini, K.; Rajendran, N.K.; Munusamy, M.A.; Alarfaj, A.A.; Rajan, M. Development of biotin molecule targeted cancer cell drug delivery of doxorubicin loaded κ-carrageenan grafted graphene oxide nanocarrier. Mater. Sci. Eng. C 2019, 100, 676–687. [Google Scholar] [CrossRef]
- Mokhtari, H.; Kharaziha, M.; Karimzadeh, F.; Tavakoli, S. An injectable mechanically robust hydrogel of Kappa-carrageenan-dopamine functionalized graphene oxide for promoting cell growth. Carbohydr. Polym. 2019, 214, 234–249. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Raza, M.A.; Mehboob, H.; Kadir, M.R.A.; Razak, S.I.A.; Shah, S.A.; Iqbal, M.Z.; Amin, R. Development and: In vitro evaluation of κ-carrageenan based polymeric hybrid nanocomposite scaffolds for bone tissue engineering. RSC Adv. 2020, 10, 40529–40542. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, J.; Chen, F.; Hou, F.; Bai, D.; Xi, P.; Zeng, Z. Biomimetic and cell-mediated mineralization of hydroxyapatite by carrageenan functionalized graphene oxide. ACS Appl. Mater. Interfaces 2014, 6, 3132–3140. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, H.; Zhou, Q.; Liu, K.; Ma, W.; Fan, S. Biomass-derived carbon for supercapacitors electrodes—A review of recent advances. Inorg. Chem. Commun. 2023, 153, 110768. [Google Scholar] [CrossRef]
- Aparicio-Collado, J.L.; García-San-Martín, N.; Molina-Mateo, J.; Cabanilles, C.T.; Quiles, V.D.; Serrano-Aroca, A.; Serra, R.S.I. Electroactive calcium-alginate/polycaprolactone/reduced graphene oxide nanohybrid hydrogels for skeletal muscle tissue engineering. Colloids Surf. B Biointerfaces 2022, 214, 112455. [Google Scholar] [CrossRef]
- Biru, E.I.; Necolau, M.I.; Zainea, A.; Iovu, H. Graphene Oxide–Protein-Based Scaffolds for Tissue Engineering. Recent Advances and Applications. Polymers 2022, 14, 1032. [Google Scholar] [CrossRef]
- Salleh, A.; Mustafa, N.; Teow, Y.H.; Fatimah, M.N.; Khairudin, F.A.; Ahmad, I.; Fauzi, M.B. Dual-Layered Approach of Ovine Collagen-Gelatin/Cellulose Hybrid Biomatrix Containing Graphene Oxide-Silver Nanoparticles for Cutaneous Wound Healing: Fabrication, Physicochemical, Cytotoxicity and Antibacterial Characterisation. Biomedicines 2022, 10, 816. [Google Scholar] [CrossRef] [PubMed]
- Saharan, R.; Paliwal, S.K.; Tiwari, A.; Babu, M.A.; Tiwari, V.; Singh, R.; Beniwal, S.K.; Kumar, M.; Sharma, A.; Almalki, W.H.; et al. Beyond traditional hydrogels: The emergence of graphene oxide-based hydrogels in drug delivery. J. Drug Deliv. Sci. Technol. 2024, 94, 105506. [Google Scholar] [CrossRef]
- Lee, E.J.; Kang, E.; Kang, S.W.; Huh, K.M. Thermo-irreversible glycol chitosan/hyaluronic acid blend hydrogel for injectable tissue engineering. Carbohydr. Polym. 2020, 244, 116432. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Liu, L.; Chen, Q.; Zhang, Q.; Guo, G. Tough, Stretchable, Compressive Novel Polymer/Graphene Oxide Nanocomposite Hydrogels with Excellent Self-Healing Performance. ACS Appl. Mater. Interfaces 2017, 9, 38052–38061. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Wang, J.; Wang, T.; Gao, X.; Wan, Q.; Pei, X. Preparation and characterization of chitosan/β-glycerophosphate thermal-sensitive hydrogel reinforced by graphene oxide. Front. Chem. 2018, 6, 565. [Google Scholar] [CrossRef] [PubMed]
- Platero, E.; Fernandez, M.E.; Bonelli, P.R.; Cukierman, A.L. Graphene oxide/alginate beads as adsorbents: Influence of the load and the drying method on their physicochemical-mechanical properties and adsorptive performance. J. Colloid Interface Sci. 2017, 491, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Serafin, A.; Culebras, M.; Collins, M.N. Synthesis and evaluation of alginate, gelatin, and hyaluronic acid hybrid hydrogels for tissue engineering applications. Int. J. Biol. Macromol. 2023, 233, 123438. [Google Scholar] [CrossRef]
- Qian, B.; Yang, Q.; Wang, M.; Huang, S.; Jiang, C.; Shi, H.; Long, Q.; Zhou, M.; Zhao, Q.; Ye, X. Encapsulation of lyophilized platelet-rich fibrin in alginate-hyaluronic acid hydrogel as a novel vascularized substitution for myocardial infarction. Bioact. Mater. 2022, 7, 401–411. [Google Scholar] [CrossRef]
- Rahmani, S.; Olad, A.; Rahmani, Z. Preparation of self-healable nanocomposite hydrogel based on Gum Arabic/gelatin and graphene oxide: Study of drug delivery behavior. Polym. Bull. 2023, 80, 4117–4138. [Google Scholar] [CrossRef]
- García-Astrain, C.; Ahmed, I.; Kendziora, D.; Guaresti, O.; Eceiza, A.; Fruk, L.; Corcuera, M.A.; Gabilondo, N. Effect of maleimide-functionalized gold nanoparticles on hybrid biohydrogels properties. RSC Adv. 2015, 5, 50268–50277. [Google Scholar] [CrossRef]
- González, K.; García-Astrain, C.; Santamaria-Echart, A.; Ugarte, L.; Avérous, L.; Eceiza, A.; Gabilondo, N. Starch/graphene hydrogels via click chemistry with relevant electrical and antibacterial properties. Carbohydr. Polym. 2018, 202, 372–381. [Google Scholar] [CrossRef]
- Jannatamani, H.; Motamedzadegan, A.; Farsi, M.; Yousefi, H. Rheological properties of wood/bacterial cellulose and chitin nano-hydrogels as a function of concentration and their nano-films properties. IET Nanobiotechnol. 2022, 16, 158–169. [Google Scholar] [CrossRef]
- Stojkov, G.; Niyazov, Z.; Picchioni, F.; Bose, R.K. Relationship between structure and rheology of hydrogels for various applications. Gels 2021, 7, 255. [Google Scholar] [CrossRef]
- Feng, W.; Wang, Z. Shear-thinning and self-healing chitosan-graphene oxide hydrogel for hemostasis and wound healing. Carbohydr. Polym. 2022, 294, 119824. [Google Scholar] [CrossRef]
- Moncada, D.; Rico, M.; Montero, B.; Rodríguez-Llamazares, S.; Feijoo-Bandín, S.; Gualillo, O.; Lago, F.; Aragón-Herrera, A.; Salavagione, H.; Pettinelli, N.; et al. Injectable hybrid hydrogels physically crosslinked based on carrageenan and green graphene for tissue repair. Int. J. Biol. Macromol. 2023, 235, 123777. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Low, H.R.; Loi, X.Y.; Merel, L.; Mohd Cairul Iqbal, M.A. Fabrication and evaluation of bacterial nanocellulose/poly(acrylic acid)/graphene oxide composite hydrogel: Characterizations and biocompatibility studies for wound dressing. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 2140–2151. [Google Scholar] [CrossRef]
- Gaur, M.; Misra, C.; Yadav, A.B.; Swaroop, S.; Maolmhuaidh, F.; Bechelany, M.; Barhoum, A. Biomedical Applications of Carbon Nanomaterials: Fullerenes, Quantum Dots, Nanotubes, Nanofibers, and Graphene. Materials 2021, 14, 5978. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zeng, H.; Zeng, Z.; Zeng, Y.; Xie, T. Promising Graphene-Based Nanomaterials and Their Biomedical Applications and Potential Risks: A Comprehensive Review. ACS Biomater. Sci. Eng. 2021, 7, 5363–5396. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Hu, D.; Lin, C.T.; Li, J.; Lin, Y. Aptamer/graphene oxide nanocomplex for in situ molecular probing in living cells. J. Am. Chem. Soc. 2010, 132, 9274–9276. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, V.C.; Jachak, A.; Hurt, R.H.; Kane, A.B. Biological interactions of graphene-family nanomaterials: An interdisciplinary review. Chem. Res. Toxicol. 2012, 25, 15–34. [Google Scholar] [CrossRef]






| Sample Code | GO w/v (%) |
|---|---|
| IH control | 0 |
| IH+GO-0.01 | 0.01 |
| IH+GO-0.02 | 0.02 |
| IH+GO-0.025 | 0.025 |
| IH+GO-0.05 | 0.05 |
| IH+GO-0.075 | 0.075 |
| IH+GO-0.1 | 0.1 |
| IH+GO-0.15 | 0.15 |
| Sample Code | G′/G″ |
|---|---|
| IH | 9 |
| IH+GO-0.025 | 8 |
| IH+GO-0.05 | 7 |
| IH+GO-0.075 | 3 |
| IH+GO-0.01 | 1 |
| IH+GO-0.15 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moncada, D.; Bouza, R.; Rico, M.; Rodríguez-Llamazares, S.; Pettinelli, N.; Aragón-Herrera, A.; Feijóo-Bandín, S.; Gualillo, O.; Lago, F.; Farrag, Y.; et al. Injectable Carrageenan/Green Graphene Oxide Hydrogel: A Comprehensive Analysis of Mechanical, Rheological, and Biocompatibility Properties. Polymers 2024, 16, 2345. https://doi.org/10.3390/polym16162345
Moncada D, Bouza R, Rico M, Rodríguez-Llamazares S, Pettinelli N, Aragón-Herrera A, Feijóo-Bandín S, Gualillo O, Lago F, Farrag Y, et al. Injectable Carrageenan/Green Graphene Oxide Hydrogel: A Comprehensive Analysis of Mechanical, Rheological, and Biocompatibility Properties. Polymers. 2024; 16(16):2345. https://doi.org/10.3390/polym16162345
Chicago/Turabian StyleMoncada, Danny, Rebeca Bouza, Maite Rico, Saddys Rodríguez-Llamazares, Natalia Pettinelli, Alana Aragón-Herrera, Sandra Feijóo-Bandín, Oreste Gualillo, Francisca Lago, Yousof Farrag, and et al. 2024. "Injectable Carrageenan/Green Graphene Oxide Hydrogel: A Comprehensive Analysis of Mechanical, Rheological, and Biocompatibility Properties" Polymers 16, no. 16: 2345. https://doi.org/10.3390/polym16162345
APA StyleMoncada, D., Bouza, R., Rico, M., Rodríguez-Llamazares, S., Pettinelli, N., Aragón-Herrera, A., Feijóo-Bandín, S., Gualillo, O., Lago, F., Farrag, Y., & Salavagione, H. (2024). Injectable Carrageenan/Green Graphene Oxide Hydrogel: A Comprehensive Analysis of Mechanical, Rheological, and Biocompatibility Properties. Polymers, 16(16), 2345. https://doi.org/10.3390/polym16162345











