Abstract
Heart problems are quite prevalent worldwide. Cardiomyocytes and stem cells are two examples of the cells and supporting matrix that are used in the integrated process of cardiac tissue regeneration. The objective is to create innovative materials that can effectively replace or repair damaged cardiac muscle. One of the most effective and appealing 3D/4D scaffolds for creating an appropriate milieu for damaged tissue growth and healing is hydrogel. In order to successfully regenerate heart tissue, bioactive and biocompatible hydrogels are required to preserve cells in the infarcted region and to bid support for the restoration of myocardial wall stress, cell survival and function. Heart tissue engineering uses a variety of hydrogels, such as natural or synthetic polymeric hydrogels. This article provides a quick overview of the various hydrogel types employed in cardiac tissue engineering. Their benefits and drawbacks are discussed. Hydrogel-based techniques for heart regeneration are also addressed, along with their clinical application and future in cardiac tissue engineering.
1. Introduction
The human heart is an incredible example of natural engineering where the contraction of the cardiac muscle pumps are nutrients and oxygen rich blood which flows throughout the body. The heart muscle receives blood flow via the coronary arteries (CA). In a matter of minutes, cardiac cell death brought on by CA constriction or obstruction results in myocardial infarction (MI). This causes the heart’s function to fail [1,2]. Collagen-containing scar tissue eventually fills the infarcted region in order to bear the increased pressure during the reduction cycle (systole). The heart’s ability to function fail when the scar tissue thins, leading to congestive heart failure (CHF). The leading cause of death globally is still cardiovascular diseases (CVDs), which include coronary heart disease, rheumatic heart disease, congenital heart disease, myocardial infarction (MI), and stroke [3,4,5,6].
Treatment involves transporting cells directly into the heart to promote cardiac rejuvenation and results in the integration of cells into the native heart to reform its muscle. There are several clinical studies being conducted regarding the implantation of stem cells in the heart in which a buffer is a widely utilized delivery method [7,8]. Very low quantities of oxygen and nutrients are triggered by coronary artery stenosis. Apoptotic cytokines and cell-toxic reactive oxygen species (ROS) are released concurrently with phagocytosis. A given cell’s ability to survive is limited by each of these elements [9,10]. A technique known as reformative cardiac tissue engineering, in which cells are given inside a supportive matrix, such as a scaffold or hydrogel, may increase the engraftment rate. The supporting matrix creates an environment that shields cells from the severe conditions of the MI heart and minimizes cell loss after injection. Furthermore, it serves as a mechanical support upon which to lower the increased wall stress brought on by the loss of healthy cardiac muscle cells, improving heart function. Because biomolecules may be easily added to the matrix to create a cell-friendly environment for improved regeneration, this method is also adaptable [11,12,13].
The World Health Organization (WHO) projects that the sum of CVD deaths will rise to 23.3 million by 2030, in spite of the discovery of innovative treatments and the implementation of risk-reducing measures [14]. As there are currently no viable treatments to entirely reestablish function of cardiovascular organ following injury, there is a high demand for the development of reformative techniques like stem-cell-based treatments and in vitro tissue engineering due to the shortage of organ donors. Currently, a variety of biomaterials can be employed to sustain tissue function, repair damaged components, or even stimulate tissue remodeling or regeneration. Metals, metal alloys, and naturally occurring and synthesized polymers are examples of these biomaterials [15,16,17,18]. Scaffold patches and hydrogels are the two primary materials that are in practice for cardiac tissue engineering. The focus of this study is on hydrogels that have a polymer matrix achieved via crosslinking and high-water content, and that are also insoluble in water. The modification and advancement of hydrogel-based biomaterials is one area of interest in the arena of reformative medicine. These materials may exhibit enhanced mechanical functioning or they may be bioactive, releasing growth factors, medicines, proteins, and extracellular matrix (ECM) components [19,20]. This review article delivers an outline of hydrogel-based biomaterials currently explored in the fields of cardiovascular tissue engineering and reformative medicine.
2. Types of Polymeric Hydrogels
There are mainly two types of polymeric hydrogels are being used for cardiac tissue engineering i.e., natural and synthetic. Polymeric hydrogels are primarily used because of their stimuli responsiveness, softness, and viscoelasticity [21,22,23,24,25]. Some of the examples that have been discussed here are in Figure 1. Additionally, Table 1 contains detailed literature on the polymers used to formulate hydrogels; these have various properties that have been compiled with regard to its application in cardiac tissue engineering.
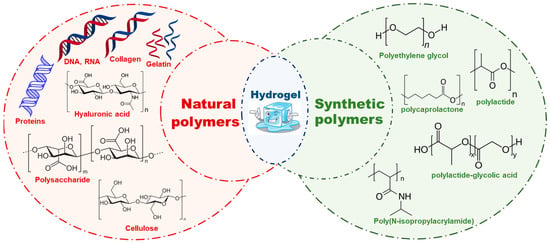
Figure 1.
Injectable hydrogels showing their types, such as natural polymers and synthetic polymers with few examples and their chemical structures.
- i.
- Natural Polymeric Hydrogels
Natural polymers, such as silk, wool, DNA, cellulose and proteins, arise in nature and are frequently water based. Collagen, gelatin, laminin, matrigel, hyaluronic acid, alginate, and chitosan are characteristic natural hydrogels, which, due to the identical structures in biological organisms, can reduce the prospect of immune response after in vivo implantation [26,27,28,29,30]. Some examples of natural polymeric hydrogels are discussed in this paper.
The majority of tissues’ mechanical strength is derived from collagen. The human body has a wide variety of collagen types, with type I collagen being the most widely distributed. Tissue engineering has made use of type I collagen because of its exceptional biocompatibility. Collagen can be dissolved in 0.3% glacial acetic acid solution to synthesize type I collagen hydrogel, and the mixture can subsequently be neutralized to a pH of 7.4. Furthermore, an acidic solution injection into an infarcted heart may cause a localized inflammatory response, though slow gelation time is a significant challenge during its use in cardiac tissue engineering [31,32]. To address this problem, Mirsadraee et al. produced a decellularized matrix containing collagen that is non-cytotoxic to human dermal fibroblasts [33,34]. Ott et al. have demonstrated that a decellularized rat heart may be utilized as a model by which to create a functioning heart via the introduction of smooth muscle cells (SMCs), endothelial cells (ECs), and cardiomyocytes [35]. Fibrinogen and thrombin act collectively during the hemostatic coagulation process with calcium catalysis to form fibrin gel, which is nontoxic and biodegradable. Consequently, it is a promising candidate for tissue engineering. The implantation of adult rats’ femoral arteries with hollow fibrin gel tubes filled with neonatal cardiomyocytes was reported by Birla et al. [36,37,38]. Rat cardiomyocytes were implanted in fibrin gel by Huang et al., who discovered that the cells’ contractility could be preserved for up to two months while maintaining normal pacing ability [39]. The fibrin/cardiomyocyte constructions were cultivated by Black et al. in conditions of radial contraction [40].
- ii.
- Synthetic polymeric hydrogels
Poly(ethylene glycol) (PEG), polylactide (PLA), polylactide-glycolic acid (PLGA), polycaprolactone (PCL), polyacrylamide (PAA), and polyurethane (PU) are examples of synthetic materials utilized in cardiac tissue engineering [41,42]. Some physical and chemical characteristics, for instance modulus, water affinity, and degradation rate, can be easily tailored to fulfill the needs of cardiac muscle tissue engineering and thus serve as an excellent candidate for tissue engineering [43,44]. However, one of the main issues with synthetic polymers is their possible cytotoxicity. Some of the widely used polymers in drug deliveries are PEG, PLA, and PLGA. Indeed, it has been previously established that several other polymers, such as PAA and PU, are non-toxic both in vitro and in vivo. Ring-opening polymerization of ethylene oxide produces PEG, a water-soluble polymer. It is biocompatible and widely used in drug delivery. Many authors have reported diacrylate-modified PEG polymerized via photo-polymerization in the presence of UV light to form PEG hydrogel. PEG gel has been extensively employed by way of a supporting matrix in each aspect of tissue engineering, including the liver, pancreas, bladder, skin, and cartilage, due to low protein adsorption and inert surface. Low protein affinity, however, does not help with cell adhesion. To improve cell adhesion, techniques including conjugating proteins or peptides that are sticky to cells and adding growth factors have been employed [40,45,46]. The 3D environment of cardiomyocyte–matrix interactions has been studied using PEG hydrogel [47,48]. Poly(2-hydroxyethyl methacrylate) (PHEMA) that has pendant hydroxyl groups is a hydrophilic polymer [49]. The cardiac tissue engineering field has made use of PHEMA hydrogel in the canine epicardium as reported by Walker et al. Here, they rooted poly(ethylene terephthalate) (PET) mesh-reinforced PHEMA gel [50,51]. Because peptides/proteins mimic the amide structure ability of PAA gels, they are promising candidates for cardiac tissue engineering. As with PEG hydrogels, crosslinking is desirable when seeking to fabricate PAA hydrogels. PAA gel has very unique properties and a thermo-responsive sol–gel transition, which allows PAA solution to remain liquid at lower temperature and a soft gel at human body temperature. These properties indicate them to be innovative injectable hydrogels that can be delivered in vivo in a liquid form through a needle before they become solid gels once in contact with warm tissue [52,53]. A widely used thermosensitive polymer, Poly(N-isopropylacrylamide) (PNIPAM), has LCST value 32 °C. At room temperature, PNIPAM is liquid, turning into gel at 37 °C. These features were explored by Okano et al. to form cell sheets for tissue engineering. Neonatal cardiomyocytes were cultivated on top of tissue culture plates coated with PNIPAM at 37 °C. The culture plate was cooled to room temperature and the cells were raised to form a monolayer cell sheet. After that, the cardiomyocyte monolayers were inserted into the heart that had been infarcted. Four weeks following implantation, there was a noticeable improvement in cardiac function [54,55].

Table 1.
Compiled literature on injectable polymeric hydrogels with various properties as well as on their application and concerns with regard to cardiac tissue engineering.
Table 1.
Compiled literature on injectable polymeric hydrogels with various properties as well as on their application and concerns with regard to cardiac tissue engineering.
| Hydrogel | Properties | Application | Concern | Ref. |
|---|---|---|---|---|
| Natural | ||||
| Collagen | Biocompatible, biodegradable |
|
| [28,31,56,57,58,59] |
| EHT | Electricity conductive |
|
| [60,61,62] |
| Fibrin | Biocompatible, biodegradable, availability |
|
| [63,64,65,66,67,68] |
| Matrigel | Closely resemble native structure |
|
| [69,70,71,72] |
| Alginate | Biocompatible, biodegradable, non-toxic, non-immunogenic, non-thrombogenic |
|
| [73,74,75,76,77,78] |
| PHA | Biocompatible, bioresorbable |
|
| [79,80,81,82,83] |
| Silk | Bioresorbable |
|
| [84,85,86,87,88,89,90,91] |
| Chitosan | Biocompatible |
|
| [92,93,94,95,96,97,98,99,100] |
| Synthetic | ||||
| Poly ethylene glycol (PEG) | Bio-inert, biocompatible, FDA approved for drug delivery. |
|
| [101,102,103,104,105,106,107,108,109] |
| Poly(2-hydroxyethyl methacrylate) (PHEMA) | Biocompatible and available for functionalization. |
|
| [110,111,112,113] |
| Polyamides | Biocompatible, bioresorbable. |
|
| [31,114] |
| Poly(N-isopropylacrylamide) (PNIPAM) | Biocompatible, non-toxic. |
|
| [115,116,117,118,119,120,121] |
| PEG-dimethacrylate (PEGDMA) | Biocompatible, Biodegradable. |
|
| [122] |
3. Properties of Injectable Hydrogels
3.1. Self-Healing Mechanism in Hydrogels
The unique properties of polymeric hydrogels are self-healing, a process which is driven from reversible chemical or physical linkages. The conductivity, fast adhesion, and stimulus responsiveness are intrinsic properties of self-healing hydrogels. More significantly, there should be sufficient mechanical toughness in the biological use of such hydrogels. The healing capacity of hydrogels influenced by a physical bond is demonstrable on reversible non-covalent interactions such as ionic or electrostatic interactions, hydrophobic interactions, host–guest bond interactions, and hydrogen bonds [123,124,125]. The hydrogel cross-linking bonding method is depicted in Figure 2. Zhang et al. described 36% w/v PVA-based hydrogels with excellent self-healing features. It is mainly hydroxyl groups in the polymer matrix that encourage hydrogen bond restructuring and enable the material to be self-healable [126]. Rumon et al. stated that graphene-oxide-functionalized polyacrylamide gel resulted in 70% healing due to richness in the -OH and -COOH groups, which experience physical bond formation with the polymer chain [127].
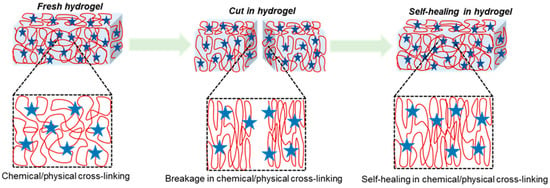
Figure 2.
Hydrogel formation via chemical or physical cross-linking between polymers, showing self-healing efficiency where cuts in hydrogel resulted in the breakage of chemical or physical cross-linking bonds, which enable self-healing via reforming bonds where red globular shows polymer chain and blue star represents cross-linking agents.
Mechanically strengthened polymeric gels can be made using irreversible covalent bonds. Additionally, the self-healing feature can be introduced via reversible crosslinked covalent bonds in dynamic chemistry, where a damaged or cracked polymeric chain network can be restored via reversible covalent bonds, for example, a disulfide bond, dynamic imine bond, or Diels–Alder reaction [54,128,129,130]. Self-healing hydrogels based on a dynamic imine bond have been reported by Zhang et al. to be used in biological applications [131]. The pH-dependent self-healing hydrogel was invented by Guo et al. Its self-healing efficacy is highly reliant on the pH of the solution, and it must reach a certain degree of healing efficiency in order to be employed in biomedical applications [132].
3.2. Gelation Time
For cardiac tissue engineering applications, injectable hydrogels should ideally undergo a quick phase transition from a liquid solution (when in a catheter before injection) to a gel state (after being injected into the targeted location) [133] as shown in Figure 3. The best possible cellular engraftment, hydrogel deployment, and retention of cells and/or biomolecules should all be achieved throughout the sol–gel transition [134,135]. In order to do this, an injectable hydrogel’s gelation kinetics should keep the substance in solution while it is still inside the catheter and then quickly, possibly in a matter of seconds, form a gel upon injection into the intended tissue [136]. Natural injectable hydrogels frequently exhibit delayed sol-to-gel transition rates (15 min to 24 h), which, when injected into cardiac tissue with a high concentration of vessels, may exacerbate the loss of cells and biomolecules because the semiliquid gel can be washed away. Slow gelation rates raise the possibility of disrupting normal blood flow and ultimately causing tissue necrosis, in addition to worries about the loss of therapeutic molecules and cells. Conversely, the gelation periods of synthetic injectable materials are usually substantially rapid, ranging from a few minutes to a few seconds [137].
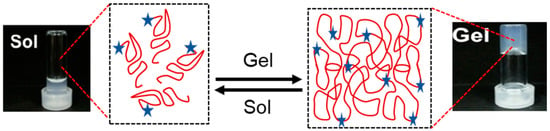
Figure 3.
Gelation time of hydrogel showing sol and gel quick transition in presence of stimuli red globular shows polymer chain and blue star represents cross-linking agents, sol vials show fluid liquid and gel vial shows soft gel formed.
3.3. Gelation Stimuli
Stimuli that are triggered on gelation include light, pH, temperature, and others shown in Figure 4 [138]. Thermoresponsive hydrogels have the advantage of not requiring UV light for crosslinking, which might cause endogenous oxidative damage to DNA, or other potentially irritating solutions, which could be less damaging to encapsulated cells. The formation of thermoresponsive hydrogels can occur through a variety of processes, including swelling behavior brought on by temperature changes. Because of its affinity for water, the hydrogel holds water and swells below its lower critical solution temperature (LCST). The hydrogel becomes increasingly hydrophobic above the LCST, and the swelling process terminates by forming a stable hydrogel. Triblock copolymers are composed of a hydrophilic–hydrophobic–hydrophilic backbone that undergoes sol-to-gel transition based on micelle formation due to an increase in temperature [139,140,141,142]. Injectable hydrogels can also go through the sol-to-gel transition by another well-liked gelation method called light-induced crosslinking or photopolymerization. A monomer’s polymerization or a hydrogel’s crosslinking can both be aided by light [143,144,145]. The use of pH as a trigger to cause hydrogel gelation has also been investigated [146,147,148]. Alimirzaei et al. reported on these pH-responsive hydrogels, developing a pH-sensitive chitosan hydrogel for the encapsulation of human adipose MSCs (hADSCs). When this hydrogel approaches physiological pH, a sol–gel transition occurs. These novel hydrogels represent a promising candidate, with suitable mechanical properties for cardiac tissue engineering [149].
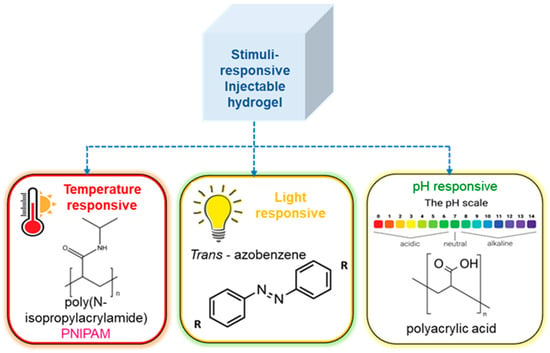
Figure 4.
Stimuli responsive gelation of hydrogel, showing the effect of various stimuli such as temperature, pH, light.
3.4. Mechanical Strength
Post myocardial infarction, alterations in the myocardial biomechanical microenvironment and cellular loss may result in unfavorable ventricular remodeling, such as ventricular dilatation and wall stress, which can ultimately cause myocardial dysfunction, fibrosis, and hypertrophy [150,151]. The myocardial experiences irreversible necrosis as a result of a series of events that damage the cell membrane and disturb the structure of the heart tissue, which sets off this maladaptive remodeling. In a perfect world, injectable hydrogels would provide the damaged myocardium with appropriate mechanical support to make up for the tissue damage. Even though a lot of injectable materials—like ECM hydrogels—have relatively soft mechanical properties that make it simple to inject them into a damaged myocardium, they are usually not strong enough to provide the injured cardiac tissue—which is constantly under strain and contraction—with sustained, continuous mechanical support [152,153]. Researchers have discovered that the mechanical strength of injectable hydrogels may be changed to resemble the mechanical strengths present in heart tissue by adjusting the quantity of polymer dissolved in aqueous solutions. An injectable PEG hydrogel with mechanical tailoring was created by Chow et al. who created and examined hydrogels with polymer concentrations of 5, 10, 20, and 30% w/v. The materials’ respective shear moduli were 0.8, 6.9, 17.2, and 35 kPa. The 10% and 20% w/v hydrogels were found to most nearly resemble the shear moduli of the infarcted (18 kPa) and normal (6 kPa) myocardium, respectively. They observed that, 10 weeks after injecting the 10% hydrogels into an MI rat model, the rats’ pathogenic ventricular remodeling was reduced in comparison with the controls [149,154].
3.5. Biocompatibility
Biocompatibility is a property of any medical material that is used in contact with a living body. The requirements for hydrogels are nontoxicity, an ability to be sterilized, and biocompatibility. Cytotoxicity assesses how the hydrogel affects the viability of cells. Assays like MTT, XTT, or live/dead staining can be used for this [155,156,157]. The breakdown products of biodegradable hydrogel must be non-toxic and readily absorbed or eliminated by the body. The loss of the protective microenvironment that would have supported the injured cardiac tissue, the time it takes for the encapsulated cells to become established and engrafted, and the therapeutic molecules that are locally required for their action, can all be problematic for matrices that degrade too quickly and may be cleared away soon after injection. The MMP family of proteases causes the majority of natural materials, including collagen, fibrin, and decellularized extracellular matrix, to degrade rapidly [158,159,160,161]. Wassenaar et al. introduced doxycycline, an MMP inhibitor, into the ECM in order to encourage comparatively slow degradation of the decellularized porcine ventricular myocardium. Two distinct techniques were used to do this, one included chemically cross-linking doxycycline to the hydrogel, and the other involved combining doxycycline with the hydrogel matrix in solution during production [162].
4. Hydrogels Functionalization
Grafting or functionalization of polymers is required to boost hydrogels’ mechanical strength. Hydrophobic or hydrophilic compounds can covalently link to various functional groups to modify hydrogels. This gives them intriguing qualities for use in biological applications [163]. Polymeric hydrogels can have their physical characteristics and solution behavior tuned by chemical modification, such as adding hydrophobic, acidic, basic, or other functionality to the polymer chain. Hydrogels have been chemically modified using a variety of techniques and can be used for specific purposes. Important examples of chemical modification of polymeric hydrogels include PEGylation, grafting of polymeric chains by polymerization, and formation of polymer–drug conjugates [164]. Figure 5 shows a schematic illustration of the hydrogels’ surface functionalization, with some examples of polymers and their cross-linking agents for various application in Table 2.
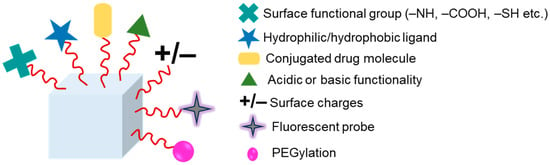
Figure 5.
Hydrogel functionalization via chemical linkages such as attaching surface functional group, hydrophilic/hydrophobic ligand, drug conjugation, acid or base functionality, introducing surface charges, fluorescent probe, PEGylation.
A robust approach for enhancing and boosting compatibility is grafting. Grafting is such a simple and efficient way to change the chemical composition of polymers. Many natural polymers, including cellulose, hyaluronic acid, starch, and chitosan, have been employed in grafting to enhance performance. By reacting with functional groups or polymerizing a monomer, a hydrophilic or hydrophobic polymeric moiety can be grafted on the polymeric chain’s backbone. This process is known as “grafting through”, “grafting on”, and “grafting from”. The former strategy has been explored the most among these [165,166]. Enzymatic synthesis or microwave grafting are two methods that are widely available. In grafting, the microwave irradiation method shortens the reaction time and uses less hazardous solvents. As a result, when compared with polymeric hydrogels grafted using the conventional approach, the hydrogels grafted using microwave technology have better qualities [167,168].

Table 2.
Examples of functionalized hydrogels and their cross-linking agents, showcasing their diverse applications in cardiovascular tissue engineering.
Table 2.
Examples of functionalized hydrogels and their cross-linking agents, showcasing their diverse applications in cardiovascular tissue engineering.
| Polymer | Cross-Linking Agent | Cross-Linking Type | Application | Ref. |
|---|---|---|---|---|
| Surface functionalization | ||||
| PVA | NH4OH, NaOH, CH3COOH | Thermal | Cardiac | [169] |
| Hydrophobic/hydrophilic ligand functionalization | ||||
| Dextran | Dextran bifunctionalized with methacrylate and aldehyde | Photo | Vascular | [170,171] |
| Conjugated drug molecule | ||||
| PEG, PVA | Cyclodextrin, doxorubicin | Host-Guest | Heart valve | [172,173] |
| Acidic and Basic functionality | ||||
| Gelatin | Hydroxyphenylpropionic acid | Photo-enzymatic | epicardium | [174] |
| Chondroitin sulfate | Furfuryl amine grafted chondroitin sulfate | Diels-Alder | myocardium | [175] |
| Surface charge | ||||
| Chitosan | lecithin | Thermal | Cardiac blood vessel | [176] |
| Fluorescence probe | ||||
| PNIPAM/Gelatin | PNIPAM-based copolymer, Thiol dye modified gelatin | Thermal and Michael addition | Cardiac patch | [177] |
| PEGylation | ||||
| PEG | Norbornene-terminated PEG | Michael addition | Vascular tissue engineering | [178] |
5. Hydrogels in Cardiovascular Tissue Engineering and Biomedical Application
The multidisciplinary zone of tissue engineering practices ideas from biology and engineering to produce biological tissue replacements that preserve, enhance, or restore function. Scaffolds are essential for polymers and tissue engineering. Natural and synthetic polymers are valuable resources for scaffolding. A characteristic approach is to immobilize cells in a 3D hydrogel. Numerous materials that are categorized based on gelation mechanism can be taken into consideration, based on the desirable gelation time, cell type, damage site, and desired period of disintegration along with their preclinical investigations [179,180]. Wang et al. used injections of brown adipose tissue-derived stem cells that had been fixed in a thermoresponsive chitosan gel to confirm cardiomyocyte differentiation and improve cell survival. Using appropriate catheters, in situ formation of hydrogels can be made with the benefit of less invasive delivery. With this method, catheter occlusion from early gelation must be prevented, and the hydrogel should develop as soon as the damaged area is injected [181]. Bencherif et al. provided a technique by which to create a preformulated hydrogel with shape memory effect that is still injectable in order to get over this issue. The foundation material in this case was alginate, which was metharcylated to enable subsequent radical polymerization. The preformulated alginate hydrogel, which is nanoporous and polymerized at −20 °C, may be inserted using a needle or catheter. When the shear force exerted is released, the hydrogel reverts to its initial shape [182]. Hydrogels can be loaded with bioactive substances or seeded with cells for use in the heart in order to promote stem cell migration or differentiation and, consequently, tissue regeneration, as shown in Figure 6. Though the process of developing injectable cell-loaded hydrogels yielded encouraging results, the number of cells that successfully differentiated into cardiomyocytes in these experiments was inadequate [14]. However, from a regulatory perspective, a cell-free approach is quite appealing, and, since it is not patient-specific, it can be produced in an off-the-shelf, up-scalable manner. Current in vitro and in vivo research has concentrated on the utilization of hydrogels that release drugs bio-actively. Bio-active compounds that are now in use and encapsulated include tiny molecules like RNA, prostaglandins, growth hormones, and other factors like bone and sonic hedgehog and bone morphogenetic protein-2 [183,184,185,186].
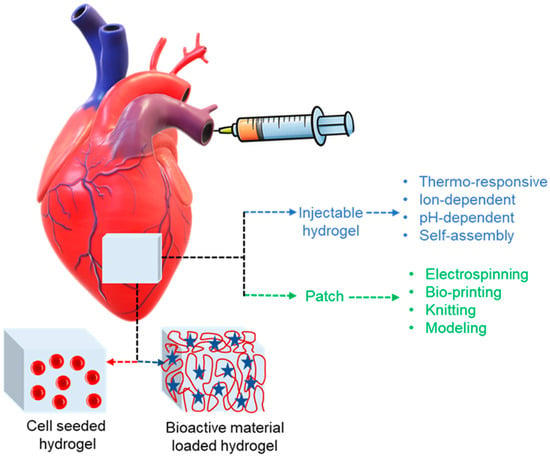
Figure 6.
Polymeric materials in cardiac applications with two major treatment approaches for heart injury. These are injectable hydrogels and cardiac patches functionalized with bioactive materials like RNA, small molecules, proteins, or they can be seeded with cells where red buttons represents cell, red globular indicate polymer chain and blue star shows bioactive material.
The flawless porous hydrogel scaffold is mechanically robust, biocompatible, biodegrades at a pace that does not release any toxins, has a surface shape that encourages cell interaction, serves as a template for the tissue, and promotes cell attachment, proliferation, and differentiation. The extracellular matrix (ECM) is an ordered network made up of different proteins and polysaccharides that gives cells structural support. Therefore, materials based on proteins and polysaccharides may have the benefit of promoting cell adhesion and activity [182,187]. Hydrogel composition has an influence on in vivo performance when building hydrogels for cardiac applications, where mechanical strength and stiffness are critical design factors. For instance, MI causes wall weakening, necessitating the use of a hydrogel to mechanically maintain the injured area. It should be remembered that excessively stiff hydrogels may result in diastolic dysfunctions. As a result, it is crucial to define suitable mechanical qualities. Furthermore, it is well known that all cells, including stem cells, react to their surroundings, and it has been demonstrated that hydrogel stiffness is a key factor in stem cell differentiation [188].
While injectable hydrogels are the subject of much research right now, there are alternative biomaterials-based approaches that can help with heart healing. Solid prefabricated scaffolds, often referred to as patches, can be placed epicardially into the injured heart, for instance. These scaffolds may be loaded with bioactive chemicals or seeded with cells, just like hydrogels, as shown in Figure 6. Not only may patches transfer cells or release bioactive compounds for medicinal purposes, but they can also lower dilation and offer mechanical support. However, in designing a cardiac patch, the following specifications must be taken into account: the patch should be strong mechanically and flexible at the same time. However, materials that are overly stiff might cause diastolic dysfunctions and result in non-contractile structures. Most of the time, it is intended that the patch degrades after sufficient remodeling [138,188].
Because gelatin is digested or denatured collagen, it has been extensively studied and used for the generation of widely applied semi-synthetic compounds that are extensively used, including gelatin methacryloyl and various biorthogonal derivatives. Despite missing biological binding motifs, alginate-based natural hydrogel derived from algae has been used because of its facile polymerization controlled by cations like Ca or Mg and because its adaptable mechanical characteristics. Hydrogels made from silk and its derivatives have also been created by adding electrically active particles or chemical groups that may be photo crosslinked. In order to determine if the added nanoparticles will encourage the development of myocardial tissue, researchers also used alginate-based cardiac patches with magnetically responsive nanoparticles. The patches were subjected to an external magnetic stimulation at a physiologically relevant frequency (5 Hz) [189,190,191].
Electrically conductive hydrogels have attracted much interest lately in the biomedical domain for tissue engineering and bioelectrode applications. Conductive hydrogels are often created by combining conductive elements with hydrophilic polymers, as traditional hydrogels are electrically insulating. Therefore, it is possible to construct conductive hydrogels that have mechanical and electrical characteristics that are comparable to those of healthy cardiac tissue. Electrically conductive biomaterials can stimulate the development of CMs in the infarcted heart and stop it from progressing to arrhythmia. They can also improve electrical coupling and cardiac contraction/relaxation functions. As a result, conductive hydrogels may be a useful tool for post-MI cardiac tissue healing by providing mechanical support for heart tissues and efficient electrical signal transmission throughout the myocardial. To create conductive hydrogels for cardiac tissue engineering, a variety of electrically conductive materials, such as conductive metals, polymers, and nanomaterials have been employed. Pre-polymers containing conductive materials can be crosslinked for in situ reactions, including photo-crosslinking, click chemistry, Schiff base reactions, and Michael reactions, in order to create injectable conductive hydrogels following intramyocardial or intrapericardial injection. Through in situ polymerization of tetraaniline-polyethylene glycol (TA-PEG) and thiolated hyaluronic acid (HA-SH) via Michael addition, an injectable conductive hydrogel was created by Wang et al. The infarcted heart was injected with adipose-derived stem cells (ADSCs) and lipofectamine nanocomplexes carrying plasmid DNA-eNOs (endothelial nitric oxide synthase) put into the gels. Electrocardiography, cardiography, and histological investigation revealed that they had improved structural and functional cardiac function along with elevated proangiogenic factors and enhanced expression of eNOs in the myocardium. Another intriguing use of conductive hydrogels for stimulating heart tissue regeneration is in conductive cardiac patches. A cardiac patch does not need invasion of the myocardial or pericardium, as a contrast with injection into the myocardium. Patches made of conductive hydrogel can offer a 2D electrical bypass around the epicardium. The common methods for attaching cardiac patches, including conductive hydrogels, to the epicardium include light irradiation, staples, or traditional surgical sutures. These methods may result in bleeding, occlusion of the blood supply, or increased inflammatory reactions [13,192,193].
Injectable hydrogels have the important role of stimulating angiogenesis, an essential component of heart regeneration. Hydrogel injections reduce pathologic remodeling and stimulate neoangiogenesis in the superficial myocardium. Using injectable hydrogels to create vascularized hearts and vascular analogs has various benefits. Furthermore, because of their networked porosity, hydrogels allow blood vessels to open and encourage vascularization in vivo. Hydrogels’ biological characteristics include their capacity to bind to and interact with cells, which aids in the vascularization processes. An injectable hydrogel not only thickens the ventricular wall but also improves the geometry of the left ventricle, supports cardiac tissue, and encourages the regeneration of cardiac tissue. In the interim, an injectable hydrogel may alleviate left ventricular dilatation and infarct expansion by lowering the pressure on the ventricular wall. Additionally, an injectable hydrogel has high safety and therapeutic efficacy and considerably improves heart function. It has been demonstrated that the recovery of cardiac function is associated with improvements in cardiomyocyte survival, repair, and myocardial pathological remodeling. The injectable hydrogel can save at-risk cardiac cells by delivering proangiogenic agents that promote angiogenesis, enhance local neovascularization, reduce inflammatory response, and rescue the ischemic myocardium by improving and optimizing the microenvironment in the damaged heart tissue. Overall, hydrogels have the potential to open up new avenues for the creation of vascular grafts and nanocarriers, which might improve the efficacy of the pharmacological therapy and revascularization strategies that are discussed here [29,193,194].
5.1. Hydrogel-Based Therapy
Collagen deposition is, from a pathology perspective, the final stage of remodeling following MI. If the hypotension in the myocardium is not alleviated, the ventricles will continue to dilate, ultimately leading to CHF. It has been suggested that injecting a supportive gel into the infarcted heart to relieve the high wall stress will stop this process. Ventricular dilation has been found to be attenuated by injection of PNIPAM-based copolymers. This shows that, by giving the infarcted region enough mechanical support, hydrogel-only treatment may be useful in postponing the cascade that results in CHF. Unfortunately, this treatment is merely passive and is unable to regenerate new myocardial due to a scarcity of cells [183,184,185,186].
5.2. Stem-Cell-Loaded Injectable Hydrogel-Based Delivery
Hydrogel-based cell therapy is a potential approach by which to create heart tissue. During injection, the viscous hydrogel keeps the cells in the intended location and the weakening heart wall is mechanically supported by the gel itself. The supplied cells may be able to survive and develop into cardiomyocytes in the gel environment in order to rebuild heart muscle in the interim. Hydrogels and biochemicals can be co-delivered to promote cell growth, survival, and differentiation. Adipose-derived stromal cells (ASCs), cardiomyocytes (CMs), epithelial cells (ECs), cardiac progenitor cells (CPCs), endothelial progenitor cells (EPCs), embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), and bone marrow-derived mesenchymal stem cells (BMMSCs) are just a few of the stem cells that have been the subject of research in recent years. Furthermore, there are several obstacles in the way of stem cells’ clinical use for heart regeneration because of their extremely low survival and retention rates in ischemic tissue. Stem-cell loaded injectable hydrogel offer stem cells the perfect milieu to preserve their stemness, which encourages their survival and development. It has been discovered that MSCs can help treat MI therapeutically. Alginate hydrogels have been shown in one study to improve MSC retention and impulse conduction in swine MI models. According to a different study, type I collagen and a new injectable thermosensitive hydrogel consisting of copolymerized N-isopropylacrylamide/acrylic acid/2-hydroxylethyl methacrylate-poly(e-caprolactone) might be used to retain MSCs. In the MI models, it has been demonstrated that this hydrogel greatly increases the transplanted MSCs’ survival, stimulates neovascularization, attenuates fibrosis, and further improves heart function [13,138,189,190,192].
5.3. Drug-Loaded Injectable Hydrogel-Based Delivery
Therapeutic drug-loaded injectable hydrogel can be injected to the human or animal body to improve safety and efficacy at the targeted tissue at a controlled rate and time. Hydrogels, with their moderate pore size for improved drug delivery and protection of pharmaceuticals against degradation in the body, have demonstrated considerable benefits over traditional approaches for attaining the required concentration of a medication in the body. They can also shorten the time it takes for the medicine to reach its target tissues and help with its sustainable release. Because of their biocompatibility and biodegradability, alginate, chitosan, cellulose, dextran, PLA, and PLGA can be used as potential candidates. The characteristics of polymers, target drugs and target tissues determine which hydrogel and drug is best for tissue engineering [163,164,165,166].
5.4. Cytokines, Nucleic Acids, Plasmids and Peptides
MI have been treated with a variety of cytokines. Growth factors, such as fibroblast growth factor-2 (FGF-2), hepatocyte growth factor (HGF), insulin-like growth factor 1 (IGF-1), nerve growth factor (NGF), platelet-derived growth factor (PDGF), transforming growth factor beta-1 (TGF-b1), and vascular endothelial growth factor 165 (VEGF165), are most frequently used to control cell fate and function during regeneration. However, rather than a burst release profile, the distribution of growth factors necessitates a moderate release pace. Hydrogels play a vital role in the release of growth factors, as they are able to maintain control over the release time. Furthermore, these substances have the capacity to improve growth factor stability and specificity. The majority of the research has shown intriguing findings that indicate that different hydrogels can provide an ideal platform for growth factors to release and facilitate the restoration of cardiac function after MI. All of these growth factors have already been applied to various hydrogels in the investigation of MI models. Therapeutic targets like small nucleic acids and plasmids can be conjugated to various hydrogels and have been used to release siRNA against MMP2. Following MI, MMP2 is in charge of cardiac remodeling. Hydrogels are broken down by proteases, releasing active siRNA that further reduced MMP2 in primary CFs. After four weeks in a rat model of MI, hydrogels releasing siMMP2 significantly increased ejection fraction (EF), stroke volume (SV), and cardiac output (CO) in comparison with hydrogels containing control siRNA. They also reduced myocardial thickness in the infarct area. Peptides can be delivered via hydrogels to keep heart function from declining in MI models. A chitosan–collagen-based hydrogel immobilized with pro-survival angiopoietin-1-derived peptide was reported to better reduce post-MI cardiac remodeling by injection into the peri-infarct/MI zone in rat models with MI than gel without peptide or gel with PBS groups. After two weeks, this hydrogel began to break down around the third week. Both scar thickness and cardiac dysfunction were markedly improved by the combination. Furthermore, after applying hydrogel, more CMs were found in the MI zone without triggering an inflammatory reaction [165,166,167,168,179].
5.5. Oxygen Delivery System
Following MI, cardiomyocytes are susceptible to cell death because of a deficiency of oxygen in the surrounding cardiac tissue. One possible strategy to preserve the ischemic heart cells and encourage heart healing is to introduce controlled oxygen release into the infarcted region. Because of the reduced blood flow in the ischemic region, current oxygen delivery systems are unable to adequately distribute oxygen into the infarcted area. Using hydrogels with quick gelation times and thermosensitive properties in combination with oxygen-releasing microspheres, some researchers have created a novel oxygen delivery method. It has been demonstrated to selectively diffuse to infarcted heart tissue and release oxygen on a regular basis. With 1% O2, the concentration of infarcted heart tissue, this system’s ability to provide oxygen continuously for four weeks greatly increased the survival of cardiac cells under ischemic circumstances. Following four weeks of this system’s treatment to infarcted hearts, there was a considerable drop in the number of macrophages and an improvement in cell survival. Additionally, myofibroblast density and collagen deposition were noticeably decreased, while angiogenesis was markedly enhanced, all of which contributed to a considerable improvement in heart function [13,192,193,194].
6. Three-Dimensional and Four-Dimensional Bioprinting as Recent Technology
For tissue engineering and regenerative medicine, three-dimensional (3D) printing is a new and exciting area. It has the ability to create 3D organs or tissues for in vivo practice. Moreover, the drug delivery effectiveness and safety may be evaluated using 3D-printed structures. Under the exact management of pre-manufactured computer software, the bio-ink used in 3D printing may change from a liquid phase into a solid form to produce functioning 3D architectures. The bio-ink comprises a variety of cell types and biomaterials [38,39,180]. Several cell types, such as endothelial cells, cardiomyocytes, fibroblast cells, mesenchymal cells, and smooth muscle cells, can be used for 3D bioprinting in heart regeneration. Three-dimensional bioprinting can also function as the heart’s natural ECM. An ideal material for bioprinting is easily tunable, has a specific viscosity, is able to change from a liquid to a solid state, be nontoxic, have good biocompatibility without triggering an immune response or inflaming the body, have the right mechanical qualities, be biodegradable, and have the right pore size [193,194]. For 3D bioprinting, biomaterials like alginate, agarose, chitosan, collagen, HA, and decellularized ECM can be employed. A more advanced printing technology is 4D bioprinting, which is far more sophisticated than 3D bioprinting. Four-dimensional bioprinting offers a dynamic means of generating structural and cell changes over time to build tissue architectures that mimic those seen in nature. Seeding 4D-bioprinted tissue into the required shape, for example, a tube that resembles a blood vessel, is one method of creating the 4D architecture. Stimulating tissues to aid in their self-assembly is another method [195,196,197,198,199]. In the future, this 4D bioprinting method will enable more innovative research and it shows promise for heart regeneration. Figure 7 shows the overall process engineering regarding use of hydrogels in cardiac tissue engineering.
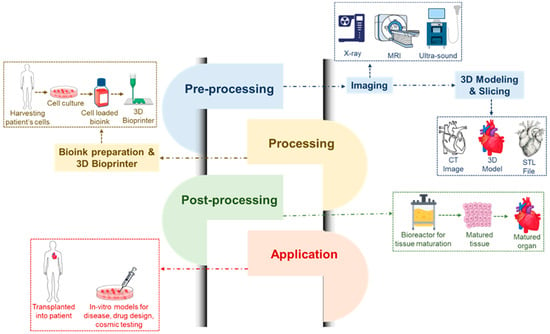
Figure 7.
Three-dimensional and four-dimensional bioprinting, showing process analysis where pre-processing techniques required imaging via X-ray, MRI and ultrasound followed by three-dimensional modeling and slicing with CT imaging, three-dimensional modeling and STL file capturing. The next step is processing data by bio-ink preparation and 3D printing, where steps are followed by harvesting the patient’s cell, collecting cell culture, putting them into bio-ink and finally printing them using a 3D printer. Afterwards, post-processing requires reaction in a bioreactor for collected cell tissue growth, the mature tissues are then collected and inserted into a mature organ. Finally, a 3D-printed materials are applied either in in vitro models development, drug design and cosmic testing or transplant into patient’s body.
Here, Table 3 shows the compiled literature regarding the process evolution for printing and fabrication using several injectable hydrogels. Some old techniques have several disadvantages, which result in the need to invent smart techniques to overcome them. Further research and clinical trials are ongoing for the development and scaleup of novel techniques which are cost-effective and useful for cardiac tissue engineering.

Table 3.
An overview of the benefits and drawbacks of printing and fabrication techniques for cardiac tissue engineering.
7. Challenges in Hydrogels Usage
Injectable hydrogels are gaining rapid attention in ECM analogs as a viable method of encapsulating and releasing various therapeutic drugs or cells. These hydrogels may also be used as functional cardiac tissue engineering tools, providing a therapeutic response for the replacement of damaged heart tissue. However, a few important problems remain to be addressed, including when to inject, how the injectable hydrogel materials biodegrade, and certain critical hydrogel characteristics for myocardial repair. Hydrogels biodegrade in the heart for a sufficient amount of time to continue serving the intended therapeutic purpose in the infected zones. In contrast, a shorter time frame is required to ensure that the material fully degrades before the disease site fully recovers and does not have any side effects [194,211]. More significantly, the simultaneous change in the hydrogel’s stiffness as a result of degradation may not coincide precisely with the myocardium’s time-dependent change in stiffness following myocardial infarction. The intended material must be able to offer continuous mechanical support in order to facilitate the progressive transfer of the cardiac load from an unhealthy tissue to a newly developed one, in order to solve this crucial issue. In light of the foregoing assumption, the hydrogel systems ought to be engineered to maintain their integrity in vivo for a minimum of one week, given that the majority of cell death transpires during the initial days following MI and should ultimately undergo total degradation after around six weeks. The hydrogel should be effectually injected as soon as MI occurs. This will give the myocardium mechanical support and allow for tissue healing, which is likely to be hindered by delayed injection after MI. As a result, the most often used injection timing in an animal model is right after MI; however, as patients typically receive therapy at least a few hours after MI, this method is not realistic in a clinical setting [212,213]. The standards used to assess the effectiveness of injectable hydrogel-based cardiac regeneration are not uniform. It is still unknown from currently conducted short-term studies in small animals which of these parameters represent the most important one for the patients’ lifespan, despite the fact that all of the well-documented typical parameters, such as wall thickness, left ventricular volume, shortening fraction, ejection fraction, and vascularization of the infarct region, are essential for myocardial regeneration [56,214,215]. From a translational perspective, some injectable hydrogels have a remarkable track record, but they also face several obstacles. These include the potential for complex synthesis procedures that are challenging to scale up, high costs, and toxicity to human tissue. Despite the high clinical esteem for PEG and PVA, carrier systems based on these pre-polymers are difficult to scale up due to their high cost and the cross-linkers necessary to prepare PVA hydrogels. Several hydrogels encounter obstacles on their journey to the clinic, and some of them fade away over time, without ever reaching a patient. Because of this, the majority of hydrogel carriers that have received clinical approval are based on old concepts that have been redesigned with a new idea. To determine the importance of these factors for effective myocardial regeneration and the patient’s lifetime, further research in a large animal model with longer-term follow-up monitoring is required. Furthermore, it is important to evaluate the injected hydrogel’s overall biosafety.
8. Conclusions and Future Perception
This review summarizes the state of the art in cardiac tissue engineering using hydrogels. The formulation of innovative functional biomaterials that can aid in the healing of damaged heart tissue following cardiovascular illnesses is essential for the efficient treatment of cardiovascular diseases nowadays. There is a lot of potential for using hydrogels in heart regeneration and repair following MI. The bioactivity, biocompatibility, and biodegradability of hydrogels means that it can serve as a potential candidate for the same. Because of their strong binding affinity, swelling ability, and absorption capacity, hydrogels are the perfect biomaterials for agent delivery systems and cardiac implants. The cross-linked spatial structure of hydrogels can be used to increase colonization resistance, prolonging their half-life in vivo and delaying fast breakdown. In the meantime, hydrogels can elevate electric conductivity benefits towards contraction. Hydrogels are utilized to give therapeutic medicines with controlled release while also giving structural support to an infected area. Hydrogels and encapsulated bioactive compounds work well together meaning that, as a consequence, both must operate at the same time for the benefit of heart regeneration. Clinical studies and commercial products are few in the field of heart regeneration medicine, so it is necessary to look into clinical viability further. In cardiovascular tissue engineering, injectable hydrogels and hydrogel-based cardiac patches are the most often utilized applications. While hydrogel-based cardiac patches decrease MI stress, which passively reduces ventricular expansion. Injectable hydrogels allow for the in-situ delivery of bioactive chemicals and cells to support in situ repair and regeneration. Cardiac patches made of hydrogel function as mechanically supporting biomaterials. The benefits of intelligent hydrogels increase the range of applications, improve the techniques of regulation, and improve the injectable hydrogels’ and hydrogel-based cardiac patches’ controllability. Nutrient transfer can be aided by the three/four-dimensional (3D/4D) network structure of hydrogels; however, diffusion is the primary method used to do this. In terms of the building of scaffolds that closely resemble native heart tissue, multi-material structures made using 3D/4D printing techniques and customized structures for individual patients have great potential for new, near-future advancements in cardiac tissue engineering.
In conclusion, hydrogels have a lot of potential for heart regeneration and repair following MI. Intelligent hydrogels and patches extend the spectrum of applications in cardiac tissue engineering by rearranging their structure via physiochemical changes in the surrounding medium. In cardiac tissue engineering, there are possibilities and problems that coexist and so it is necessary to further look into clinical viability. In the near future, hydrogels should provide enormous translational promise for heart regeneration and repair. Recent years have seen remarkable advancements in the field of cardiac tissue engineering, with hydrogels serving as scaffolding for medicinal compounds. Therefore, one of the main areas of focus for materials science and tissue engineering research in the upcoming decades will be the creation of innovative injectable hydrogels that may serve as soft scaffolds for medicinal chemicals.
Author Contributions
R.P. is medical student, responsible for the collection of literature and the initial arrangement of the draft. He conducted comprehensive searches of relevant medical databases and organized the initial findings into a structured draft format. D.P. is responsible for the in-depth analysis of the collected literature. She critically evaluated the sources and information, refined the draft by review and editing processes. All authors have read and approved the final manuscript, contributing to its conception, design, and overall development. All authors have read and agreed to the published version of the manuscript.
Funding
This work did not receive any specific grant from any funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
The authors acknowledge Banas Medical College and Research Institute, Palanpur, and School of Civil and Environmental Engineering, Cornell University, USA, for providing the data accessibility and internet facility.
Conflicts of Interest
The authors declare no conflicts of interest. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Monteiro, L.M.; Vasques-Nóvoa, F.; Ferreira, L.; Pinto-do-Ó, P.; Nascimento, D.S. Restoring Heart Function and Electrical Integrity: Closing the Circuit. NPJ Regen. Med. 2017, 2, 9. [Google Scholar] [CrossRef]
- Buckberg, G.D.; Nanda, N.C.; Nguyen, C.; Kocica, M.J. What Is the Heart? Anatomy, Function, Pathophysiology, and Misconceptions. J. Cardiovasc. Dev. Dis. 2018, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Xue, J.; Wang, G.; Diao, Q. Nanoparticle-Based Drug Delivery Systems for the Treatment of Cardiovascular Diseases. Front. Pharmacol. 2022, 13, 999404. [Google Scholar] [CrossRef] [PubMed]
- Horn, M.A.; Trafford, A.W. Aging and the Cardiac Collagen Matrix: Novel Mediators of Fibrotic Remodelling. J. Mol. Cell. Cardiol. 2016, 93, 175–185. [Google Scholar] [CrossRef]
- Nikolov, A.; Popovski, N. Extracellular Matrix in Heart Disease: Focus on Circulating Collagen Type I and III Derived Peptides as Biomarkers of Myocardial Fibrosis and Their Potential in the Prognosis of Heart Failure: A Concise Review. Metabolites 2022, 12, 297. [Google Scholar] [CrossRef]
- Tani, H.; Kobayashi, E.; Yagi, S.; Tanaka, K.; Kameda-Haga, K.; Shibata, S.; Moritoki, N.; Takatsuna, K.; Moriwaki, T.; Sekine, O. Heart-Derived Collagen Promotes Maturation of Engineered Heart Tissue. Biomaterials 2023, 299, 122174. [Google Scholar] [CrossRef]
- Hashimoto, H.; Olson, E.N.; Bassel-Duby, R. Therapeutic Approaches for Cardiac Regeneration and Repair. Nat. Rev. Cardiol. 2018, 15, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mignone, J.; MacLellan, W.R. Cardiac Regeneration and Stem Cells. Physiol. Rev. 2015, 95, 1189–1204. [Google Scholar] [CrossRef]
- Liu, J.; Han, X.; Zhang, T.; Tian, K.; Li, Z.; Luo, F. Reactive Oxygen Species (ROS) Scavenging Biomaterials for Anti-Inflammatory Diseases: From Mechanism to Therapy. J. Hematol. Oncol. 2023, 16, 116. [Google Scholar] [CrossRef]
- Kibel, A.; Lukinac, A.M.; Dambic, V.; Juric, I.; Selthofer-Relatic, K. Oxidative Stress in Ischemic Heart Disease. Oxidative Med. Cell. Longev. 2020, 6627144. [Google Scholar] [CrossRef]
- Xu, Q.; Xiao, Z.; Yang, Q.; Yu, T.; Deng, X.; Chen, N.; Huang, Y.; Wang, L.; Guo, J.; Wang, J. Hydrogel-Based Cardiac Repair and Regeneration Function in the Treatment of Myocardial Infarction. Mater. Today Bio 2024, 25, 100978. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhang, Z.; Luo, R.; Jiang, Q.; Yang, L.; Wang, Y. Advances in Injectable Hydrogel Strategies for Heart Failure Treatment. Adv. Healthc. Mater. 2023, 12, 2300029. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Liu, W. Functional Hydrogels for the Treatment of Myocardial Infarction. NPG Asia Mater. 2022, 14, 9. [Google Scholar] [CrossRef]
- Hinderer, S.; Brauchle, E.; Schenke-Layland, K. Generation and Assessment of Functional Biomaterial Scaffolds for Applications in Cardiovascular Tissue Engineering and Regenerative Medicine. Adv. Healthc. Mater. 2015, 4, 2326–2341. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Yang, Y.; Zhang, T.; Zhu, B.; Wei, G.; Li, H. Research Progress of Metal Biomaterials with Potential Applications as Cardiovascular Stents and Their Surface Treatment Methods to Improve Biocompatibility. Heliyon 2024, 10, e25515. [Google Scholar] [CrossRef] [PubMed]
- Moravej, M.; Mantovani, D. Biodegradable Metals for Cardiovascular Stent Application: Interests and New Opportunities. Int. J. Mol. Sci. 2011, 12, 4250–4270. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yu, X.; Cui, J.; Yu, F.; Liu, M.; Chen, Y.; Wu, J.; Sun, B.; Mo, X. Development of Biodegradable Polymeric Stents for the Treatment of Cardiovascular Diseases. Biomolecules 2022, 12, 1245. [Google Scholar] [CrossRef]
- Tetali, S.S.V.; Fricker, A.T.R.; van Domburg, Y.A.; Roy, I. Intelligent Biomaterials for Cardiovascular Applications. Curr. Opin. Biomed. Eng. 2023, 28, 100474. [Google Scholar] [CrossRef]
- Karam, M.; Fahs, D.; Maatouk, B.; Safi, B.; Jaffa, A.A.; Mhanna, R. Polymeric Nanoparticles in the Diagnosis and Treatment of Myocardial Infarction: Challenges and Future Prospects. Mater. Today Bio 2022, 14, 100249. [Google Scholar] [CrossRef]
- Leal, B.B.J.; Wakabayashi, N.; Oyama, K.; Kamiya, H.; Braghirolli, D.I.; Pranke, P. Vascular Tissue Engineering: Polymers and Methodologies for Small Caliber Vascular Grafts. Front. Cardiovasc. Med. 2021, 7, 592361. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/Stimuli-Responsive Hydrogels: Cutting-Edge Platforms for Tissue Engineering and Other Biomedical Applications. Mater. Today Bio 2022, 13, 100186. [Google Scholar] [CrossRef] [PubMed]
- Dhara, M. Polymer Hydrogels: Classification and Recent Advances. J. Macromol. Sci. Part A 2024, 61, 265–288. [Google Scholar] [CrossRef]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-Responsive Hydrogels: Theory, Modern Advances, and Applications. Mater. Sci. Eng. R Rep. 2015, 93, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Bisht, A.; Alexander, A.; Dave, V.; Sharma, S. Biomedical Applications of Hydrogels in Drug Delivery System: An Update. J. Drug Deliv. Sci. Technol. 2021, 66, 102914. [Google Scholar] [CrossRef]
- Volpi, M.; Paradiso, A.; Costantini, M.; Świȩszkowski, W. Hydrogel-Based Fiber Biofabrication Techniques for Skeletal Muscle Tissue Engineering. ACS Biomater. Sci. Eng. 2022, 8, 379–405. [Google Scholar] [CrossRef]
- Vieira, S.; da Silva Morais, A.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L. Natural-Based Hydrogels: From Processing to Applications. Encycl. Polym. Sci. Technol. 2002, 1–27. [Google Scholar] [CrossRef]
- Sionkowska, A. Collagen Blended with Natural Polymers: Recent Advances and Trends. Prog. Polym. Sci. 2021, 122, 101452. [Google Scholar] [CrossRef]
- Aazmi, A.; Zhang, D.; Mazzaglia, C.; Yu, M.; Wang, Z.; Yang, H.; Huang, Y.Y.S.; Ma, L. Biofabrication Methods for Reconstructing Extracellular Matrix Mimetics. Bioact. Mater. 2024, 31, 475–496. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, Y.; Zhang, J.; Liang, H.; Chen, X.; Tan, H. Natural Polymer-Based Hydrogels: From Polymer to Biomedical Applications. Pharmaceutics 2023, 15, 2514. [Google Scholar] [CrossRef]
- Majid, Q.A.; Fricker, A.T.R.; Gregory, D.A.; Davidenko, N.; Hernandez Cruz, O.; Jabbour, R.J.; Owen, T.J.; Basnett, P.; Lukasiewicz, B.; Stevens, M. Natural Biomaterials for Cardiac Tissue Engineering: A Highly Biocompatible Solution. Front. Cardiovasc. Med. 2020, 7, 554597. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guan, J. Hydrogels for Cardiac Tissue Engineering. Polymers 2011, 3, 740–761. [Google Scholar] [CrossRef]
- Mirsadraee, S.; Wilcox, H.E.; Korossis, S.A.; Kearney, J.N.; Watterson, K.G.; Fisher, J.; Ingham, E. Development and Characterization of an Acellular Human Pericardial Matrix for Tissue Engineering. Tissue Eng. 2006, 12, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Mirsadraee, S.; Wilcox, H.E.; Watterson, K.G.; Kearney, J.N.; Hunt, J.; Fisher, J.; Ingham, E. Biocompatibility of Acellular Human Pericardium. J. Surg. Res. 2007, 143, 407–414. [Google Scholar] [CrossRef]
- Ott, H.C.; Matthiesen, T.S.; Goh, S.-K.; Black, L.D.; Kren, S.M.; Netoff, T.I.; Taylor, D.A. Perfusion-Decellularized Matrix: Using Nature’s Platform to Engineer a Bioartificial Heart. Nat. Med. 2008, 14, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Birla, R.K.; Huang, Y.C.; Dennis, R.G. Development of a Novel Bioreactor for the Mechanical Loading of Tissue-Engineered Heart Muscle. Tissue Eng. 2007, 13, 2239–2248. [Google Scholar] [CrossRef] [PubMed]
- Hecker, L.; Khait, L.; Radnoti, D.; Birla, R. Development of a Microperfusion System for the Culture of Bioengineered Heart Muscle. ASAIO J. 2008, 54, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Birla, R.K.; Borschel, G.H.; Dennis, R.G.; Brown, D.L. Myocardial Engineering in Vivo: Formation and Characterization of Contractile, Vascularized Three-Dimensional Cardiac Tissue. Tissue Eng. 2005, 11, 803–813. [Google Scholar] [CrossRef]
- Huang, Y.; Khait, L.; Birla, R.K. Contractile Three-dimensional Bioengineered Heart Muscle for Myocardial Regeneration. J. Biomed. Mater. Res. A 2007, 80, 719–731. [Google Scholar] [CrossRef]
- Black, L.D., III; Meyers, J.D.; Weinbaum, J.S.; Shvelidze, Y.A.; Tranquillo, R.T. Cell-Induced Alignment Augments Twitch Force in Fibrin Gel–Based Engineered Myocardium via Gap Junction Modification. Tissue Eng. Part. A 2009, 15, 3099–3108. [Google Scholar] [CrossRef]
- Elango, J.; Zamora-Ledezma, C.; Maté-Sánchez de Val, J.E. Natural vs Synthetic Polymers: How Do They Communicate with Cells for Skin Regeneration—A Review. J. Compos. Sci. 2023, 7, 385. [Google Scholar] [CrossRef]
- Satchanska, G.; Davidova, S.; Petrov, P.D. Natural and Synthetic Polymers for Biomedical and Environmental Applications. Polymers 2024, 16, 1159. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xu, P.; Lei, B. Engineering Multifunctional Bioactive Citrate-Based Biomaterials for Tissue Engineering. Bioact. Mater. 2023, 19, 511–537. [Google Scholar] [CrossRef] [PubMed]
- Kalirajan, C.; Dukle, A.; Nathanael, A.J.; Oh, T.-H.; Manivasagam, G. A Critical Review on Polymeric Biomaterials for Biomedical Applications. Polymers 2021, 13, 3015. [Google Scholar] [CrossRef] [PubMed]
- Vasile, C.; Pamfil, D.; Stoleru, E.; Baican, M. New Developments in Medical Applications of Hybrid Hydrogels Containing Natural Polymers. Molecules 2020, 25, 1539. [Google Scholar] [CrossRef] [PubMed]
- Rahman, C.V.; Kuhn, G.; White, L.J.; Kirby, G.T.S.; Varghese, O.P.; McLaren, J.S.; Cox, H.C.; Rose, F.R.A.J.; Müller, R.; Hilborn, J. PLGA/PEG-hydrogel Composite Scaffolds with Controllable Mechanical Properties. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Andreopoulos, F.M.; Beckman, E.J.; Russell, A.J. Light-Induced Tailoring of PEG-Hydrogel Properties. Biomaterials 1998, 19, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Anseth, K.S. PEG Hydrogels for the Controlled Release of Biomolecules in Regenerative Medicine. Pharm. Res. 2009, 26, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Dalton, P.D.; Flynn, L.; Shoichet, M.S. Manufacture of Poly(2-Hydroxyethyl Methacrylate-Co-Methyl Methacrylate) Hydrogel Tubes for Use as Nerve Guidance Channels. Biomaterials 2002, 23, 3843–3851. [Google Scholar] [CrossRef]
- WALKER, A.S.; BLUE, M.A.; BRANDON, T.A.; EMMANUAL, J.; GUILBEAU, E.J. Performance of a Hydrogel Composite Pericardial Substitute After Long-Term Implant Studies. ASAIO J. 1992, 38, M550–M554. [Google Scholar] [CrossRef]
- Allder, M.A.; Guilbeau, E.J.; Brandon, T.A.; Walker, A.S.; Koeneman, J.B.; Fisk, R.L. A Hydrogel Pericardial Patch. ASAIO J. 1990, 36, M572–M574. [Google Scholar]
- Calixto, G.; Yoshii, A.C.; Rocha e Silva, H.; Stringhetti Ferreira Cury, B.; Chorilli, M. Polyacrylic Acid Polymers Hydrogels Intended to Topical Drug Delivery: Preparation and Characterization. Pharm. Dev. Technol. 2015, 20, 490–496. [Google Scholar] [CrossRef]
- Li, W.; Zhao, H.; Teasdale, P.R.; John, R.; Zhang, S. Synthesis and Characterisation of a Polyacrylamide–Polyacrylic Acid Copolymer Hydrogel for Environmental Analysis of Cu and Cd. React. Funct. Polym. 2002, 52, 31–41. [Google Scholar] [CrossRef]
- Miyagawa, S.; Sawa, Y.; Sakakida, S.; Taketani, S.; Kondoh, H.; Memon, I.A.; Imanishi, Y.; Shimizu, T.; Okano, T.; Matsuda, H. Tissue Cardiomyoplasty Using Bioengineered Contractile Cardiomyocyte Sheets to Repair Damaged Myocardium: Their Integration with Recipient Myocardium. Transplantation 2005, 80, 1586–1595. [Google Scholar] [CrossRef] [PubMed]
- Haq, M.A.; Su, Y.; Wang, D. Mechanical Properties of PNIPAM Based Hydrogels: A Review. Mater. Sci. Eng. C 2017, 70, 842–855. [Google Scholar] [CrossRef] [PubMed]
- Clift, C.L.; McLaughlin, S.; Muñoz, M.; Suuronen, E.J.; Rotstein, B.H.; Mehta, A.S.; Drake, R.R.; Alarcon, E.I.; Angel, P.M. Evaluation of Therapeutic Collagen-Based Biomaterials in the Infarcted Mouse Heart by Extracellular Matrix Targeted MALDI Imaging Mass Spectrometry. J. Am. Soc. Mass. Spectrom. 2021, 32, 2746–2754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kumar, P.; Lv, S.; Xiong, D.; Zhao, H.; Cai, Z.; Zhao, X. Recent Advances in 3D Bioprinting of Vascularized Tissues. Mater. Des. 2021, 199, 109398. [Google Scholar] [CrossRef]
- Sarrigiannidis, S.O.; Rey, J.M.; Dobre, O.; González-García, C.; Dalby, M.J.; Salmeron-Sanchez, M. A Tough Act to Follow: Collagen Hydrogel Modifications to Improve Mechanical and Growth Factor Loading Capabilities. Mater. Today Bio 2021, 10, 100098. [Google Scholar] [CrossRef]
- Antoine, E.E.; Vlachos, P.P.; Rylander, M.N. Review of Collagen I Hydrogels for Bioengineered Tissue Microenvironments: Characterization of Mechanics, Structure, and Transport. Tissue Eng. Part B Rev. 2014, 20, 683–696. [Google Scholar] [CrossRef]
- Schaaf, S.; Eder, A.; Vollert, I.; Stöhr, A.; Hansen, A.; Eschenhagen, T. Generation of Strip-Format Fibrin-Based Engineered Heart Tissue (EHT); Humana Press: New York, NY, USA, 2014; pp. 121–129. [Google Scholar]
- Kurashina, Y.; Fukada, K.; Itai, S.; Akizuki, S.; Sato, R.; Masuda, A.; Tani, H.; Fujita, J.; Fukuda, K.; Tohyama, S.; et al. Hydrogel-Sheathed HiPSC-Derived Heart Microtissue Enables Anchor-Free Contractile Force Measurement. Adv. Sci. 2023, 10, e2301831. [Google Scholar] [CrossRef]
- Goldfracht, I.; Efraim, Y.; Shinnawi, R.; Kovalev, E.; Huber, I.; Gepstein, A.; Arbel, G.; Shaheen, N.; Tiburcy, M.; Zimmermann, W.Z.; et al. Engineered Heart Tissue Models from HiPSC-Derived Cardiomyocytes and Cardiac ECM for Disease Modeling and Drug Testing Applications. Acta Biomater. 2019, 92, 145–159. [Google Scholar] [CrossRef]
- Pereira, R.V.S.; EzEldeen, M.; Ugarte-Berzal, E.; Martens, E.; Malengier-Devlies, B.; Vandooren, J.; Vranckx, J.J.; Matthys, P.; Opdenakker, G. Physiological Fibrin Hydrogel Modulates Immune Cells and Molecules and Accelerates Mouse Skin Wound Healing. Front. Immunol. 2023, 14, 1170153. [Google Scholar] [CrossRef]
- Wein, S.; Schemmer, C.; Al Enezy-Ulbrich, M.A.; Jung, S.A.; Rütten, S.; Kühnel, M.; Jonigk, D.; Jahnen-Dechent, W.; Pich, A.; Neuss, S. Fibrin-Based Hydrogels with Reactive Amphiphilic Copolymers for Mechanical Adjustments Allow for Capillary Formation in 2D and 3D Environments. Gels 2024, 10, 182. [Google Scholar] [CrossRef]
- Li, Y.; Meng, H.; Liu, Y.; Lee, B.P. Fibrin Gel as an Injectable Biodegradable Scaffold and Cell Carrier for Tissue Engineering. Sci. World J. 2015, 2015, 685690. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, J.; Malyaran, H.; Al Enezy-Ulbrich, M.A.; Jung, S.; Radermacher, C.; Buhl, E.M.; Pich, A.; Neuss, S. Assessment of Fibrin-Based Hydrogels Containing a Fibrin-Binding Peptide to Tune Mechanical Properties and Cell Responses. Macromol. Mater. Eng. 2023, 308, 2200678. [Google Scholar] [CrossRef]
- Yu, Z.; Li, H.; Xia, P.; Kong, W.; Chang, Y.; Fu, C.; Wang, K.; Yang, X.; Qi, Z. Application of Fibrin-Based Hydrogels for Nerve Protection and Regeneration after Spinal Cord Injury. J. Biol. Eng. 2020, 14, 22. [Google Scholar] [CrossRef]
- Janmey, P.A.; Winer, J.P.; Weisel, J.W. Fibrin Gels and Their Clinical and Bioengineering Applications. J. R. Soc. Interface 2009, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, M.T.; Zook, H.N.; Chigumba, D.N.; Johnstone, C.P.; Caldera, L.F.; Shih, H.-P.; Tirrell, D.A.; Ku, H.T. A Matrigel-Free Method for Culture of Pancreatic Endocrine-like Cells in Defined Protein-Based Hydrogels. Front. Bioeng. Biotechnol. 2023, 11, 1144209. [Google Scholar] [CrossRef] [PubMed]
- Tarassoli, S.P.; Jessop, Z.M.; Kyle, S.; Whitaker, I.S. Candidate Bioinks for 3D Bioprinting Soft Tissue. In 3D Bioprinting for Reconstructive Surgery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 145–172. [Google Scholar]
- Cavo, M.; Caria, M.; Pulsoni, I.; Beltrame, F.; Fato, M.; Scaglione, S. A New Cell-Laden 3D Alginate-Matrigel Hydrogel Resembles Human Breast Cancer Cell Malignant Morphology, Spread and Invasion Capability Observed “in Vivo”. Sci. Rep. 2018, 8, 5333. [Google Scholar] [CrossRef]
- Kim, S.; Min, S.; Choi, Y.S.; Jo, S.-H.; Jung, J.H.; Han, K.; Kim, J.; An, S.; Ji, Y.W.; Kim, Y.-G.; et al. Tissue Extracellular Matrix Hydrogels as Alternatives to Matrigel for Culturing Gastrointestinal Organoids. Nat. Commun. 2022, 13, 1692. [Google Scholar] [CrossRef]
- Nagaraja, K.; Rao, K.M.; Rao, K.S.V.K. Alginate-Based Hydrogels. In Plant and Algal Hydrogels for Drug Delivery and Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2021; pp. 357–393. [Google Scholar]
- Wang, L.; Zhang, H.J.; Liu, X.; Liu, Y.; Zhu, X.; Liu, X.; You, X. A Physically Cross-Linked Sodium Alginate–Gelatin Hydrogel with High Mechanical Strength. ACS Appl. Polym. Mater. 2021, 3, 3197–3205. [Google Scholar] [CrossRef]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Akbari, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-Based Hydrogels as Drug Delivery Vehicles in Cancer Treatment and Their Applications in Wound Dressing and 3D Bioprinting. J. Biol. Eng. 2020, 14, 8. [Google Scholar] [CrossRef]
- Savić Gajić, I.M.; Savić, I.M.; Svirčev, Z. Preparation and Characterization of Alginate Hydrogels with High Water-Retaining Capacity. Polymers 2023, 15, 2592. [Google Scholar] [CrossRef] [PubMed]
- Tomić, S.L.; Babić Radić, M.M.; Vuković, J.S.; Filipović, V.V.; Nikodinovic-Runic, J.; Vukomanović, M. Alginate-Based Hydrogels and Scaffolds for Biomedical Applications. Mar. Drugs 2023, 21, 177. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Townsend, J.M.; Sanders, M.E.; Kiyotake, E.A.; Detamore, M.S. Independent Control of Molecular Weight, Concentration, and Stiffness of Hyaluronic Acid Hydrogels. Biomed. Mater. 2022, 17, 065005. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Yang, K.; Qin, X.; Luo, R.; Wang, H.; Huang, R. Polyhydroxyalkanoates in Tissue Repair and Regeneration. Eng. Regen. 2022, 3, 24–40. [Google Scholar] [CrossRef]
- Brelle, L.; Faÿ, F.; Ozturk, T.; Didier, N.; Renard, E.; Langlois, V. Hydrogel Based on Polyhydroxyalkanoate Sulfonate: Control of the Swelling Rate by the Ionic Group Content. Biomacromolecules 2023, 24, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zhang, X.; Wu, Y.; Jie, J.; Wang, Z.; Chen, G.-Q.; Sun, J.; Wu, L.-P. Amphiphilic and Fatigue-Resistant Organohydrogels for Small-Diameter Vascular Grafts. Sci. Adv. 2022, 8, eabn5360. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Che, X.; Yu, L.; Jia, W.; Shen, R.; Chen, J.; Ma, Y.; Chen, G.-Q. Synthesis and Characterization of Polyhydroxyalkanoate Organo/Hydrogels. Biomacromolecules 2019, 20, 3303–3312. [Google Scholar] [CrossRef]
- Lyu, Y.; Liu, Y.; He, H.; Wang, H. Application of Silk-Fibroin-Based Hydrogels in Tissue Engineering. Gels 2023, 9, 431. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, S.; Kundu, S.C. Silk Protein-Based Hydrogels: Promising Advanced Materials for Biomedical Applications. Acta Biomater. 2016, 31, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Huang, X.; Luo, Y.; Zhu, R.; Zheng, M.; Yang, J.; Bai, S. Silk Fibroin-Based Tough Hydrogels with Strong Underwater Adhesion for Fast Hemostasis and Wound Sealing. Biomacromolecules 2023, 24, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Huang, R.; Huang, Y.; Yan, S.; Zhang, Q. Comparison of Silk Hydrogels Prepared via Different Methods. Polymers 2023, 15, 4419. [Google Scholar] [CrossRef] [PubMed]
- Egan, G.; Phuagkhaopong, S.; Matthew, S.A.L.; Connolly, P.; Seib, F.P. Impact of Silk Hydrogel Secondary Structure on Hydrogel Formation, Silk Leaching and in Vitro Response. Sci. Rep. 2022, 12, 3729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, D.; Zhang, Y.; Li, M.; Chai, R. Silk Fibroin Hydrogels for Biomedical Applications. Smart Med. 2022, 1, e20220011. [Google Scholar] [CrossRef]
- Madappura, A.P.; Madduri, S. A Comprehensive Review of Silk-Fibroin Hydrogels for Cell and Drug Delivery Applications in Tissue Engineering and Regenerative Medicine. Comput. Struct. Biotechnol. J. 2023, 21, 4868–4886. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zuo, B. Functional Silk Fibroin Hydrogels: Preparation, Properties and Applications. J. Mater. Chem. B 2021, 9, 1238–1258. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Yang, F.; Guo, Z. The Chitosan Hydrogels: From Structure to Function. New J. Chem. 2018, 42, 17162–17180. [Google Scholar] [CrossRef]
- Farasati Far, B.; Omrani, M.; Naimi Jamal, M.R.; Javanshir, S. Multi-Responsive Chitosan-Based Hydrogels for Controlled Release of Vincristine. Commun. Chem. 2023, 6, 28. [Google Scholar] [CrossRef]
- Carpa, R.; Farkas, A.; Dobrota, C.; Butiuc-Keul, A. Double-Network Chitosan-Based Hydrogels with Improved Mechanical, Conductive, Antimicrobial, and Antibiofouling Properties. Gels 2023, 9, 278. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A Functional Chitosan-Based Hydrogel as a Wound Dressing and Drug Delivery System in the Treatment of Wound Healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, A.; Farooq, M.A.; Parveen, A. Thermosensitive Chitosan-Based Injectable Hydrogel as an Efficient Anticancer Drug Carrier. ACS Omega 2020, 5, 20450–20460. [Google Scholar] [CrossRef] [PubMed]
- Thirupathi, K.; Raorane, C.J.; Ramkumar, V.; Ulagesan, S.; Santhamoorthy, M.; Raj, V.; Krishnakumar, G.S.; Phan, T.T.V.; Kim, S.-C. Update on Chitosan-Based Hydrogels: Preparation, Characterization, and Its Antimicrobial and Antibiofilm Applications. Gels 2022, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Shariatinia, Z.; Jalali, A.M. Chitosan-Based Hydrogels: Preparation, Properties and Applications. Int. J. Biol. Macromol. 2018, 115, 194–220. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Qiu, P.; Wang, Y.; Ren, P.; Liu, J.; Zhao, J.; Gou, D. Chitosan-Based Hydrogels: From Preparation to Applications, a Review. Food Chem. X 2024, 21, 101095. [Google Scholar] [CrossRef] [PubMed]
- Taokaew, S.; Kaewkong, W.; Kriangkrai, W. Recent Development of Functional Chitosan-Based Hydrogels for Pharmaceutical and Biomedical Applications. Gels 2023, 9, 277. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Tyson, C.; Szyniec, A.; Agro, S.; Tavakol, T.N.; Harmon, A.; Lampkins, D.; Pearson, L.; Dumas, J.E.; Taite, L.J. Bioactive Polyurethane–Poly(Ethylene Glycol) Diacrylate Hydrogels for Applications in Tissue Engineering. Gels 2024, 10, 108. [Google Scholar] [CrossRef]
- Wakabayashi, R.; Ramadhan, W.; Moriyama, K.; Goto, M.; Kamiya, N. Poly(Ethylene Glycol)-Based Biofunctional Hydrogels Mediated by Peroxidase-Catalyzed Cross-Linking Reactions. Polym. J. 2020, 52, 899–911. [Google Scholar] [CrossRef]
- Lee, S.; Tong, X.; Yang, F. The Effects of Varying Poly(Ethylene Glycol) Hydrogel Crosslinking Density and the Crosslinking Mechanism on Protein Accumulation in Three-Dimensional Hydrogels. Acta Biomater. 2014, 10, 4167–4174. [Google Scholar] [CrossRef]
- Endres, K.J.; Dilla, R.A.; Becker, M.L.; Wesdemiotis, C. Poly(Ethylene Glycol) Hydrogel Crosslinking Chemistries Identified via Atmospheric Solids Analysis Probe Mass Spectrometry. Macromolecules 2021, 54, 7754–7764. [Google Scholar] [CrossRef]
- Lee, S.; Tong, X.; Yang, F. Effects of the Poly(Ethylene Glycol) Hydrogel Crosslinking Mechanism on Protein Release. Biomater. Sci. 2016, 4, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Bakaic, E.; Smeets, N.M.B.; Hoare, T. Injectable Hydrogels Based on Poly(Ethylene Glycol) and Derivatives as Functional Biomaterials. RSC Adv. 2015, 5, 35469–35486. [Google Scholar] [CrossRef]
- Wang, Z.; Ye, Q.; Yu, S.; Akhavan, B. Poly Ethylene Glycol (PEG)-Based Hydrogels for Drug Delivery in Cancer Therapy: A Comprehensive Review. Adv. Healthc. Mater. 2023, 12, 2300105. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J. Bioactive Modification of Poly(Ethylene Glycol) Hydrogels for Tissue Engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef]
- Sun, S.; Cui, Y.; Yuan, B.; Dou, M.; Wang, G.; Xu, H.; Wang, J.; Yin, W.; Wu, D.; Peng, C. Drug Delivery Systems Based on Polyethylene Glycol Hydrogels for Enhanced Bone Regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1117647. [Google Scholar] [CrossRef]
- Sharma, M.B.; Kap, Ö.; Abdelmohsen, H.A.M.; Ashton, M.D.; Harper, G.R.; Firlak, M.; Aaltonen, J.E.; Bolland, K.A.; Bragg, R.; Deeley, S.; et al. Poly(2-Hydroxyethyl Methacrylate) Hydrogel-Based Microneedles for Metformin Release. Glob. Chall. 2023, 7, 2300002. [Google Scholar] [CrossRef]
- Ouyang, T.; Su, S.; Deng, H.; Liu, Y.; Cui, L.; Rong, J.; Zhao, J. Superhydrophilic Poly(2-Hydroxyethyl Methacrylate) Hydrogel with Nanosilica Covalent Coating: A Promising Contact Lens Material for Resisting Tear Protein Deposition and Bacterial Adhesion. ACS Biomater. Sci. Eng. 2023, 9, 5653–5665. [Google Scholar] [CrossRef]
- Tuancharoensri, N.; Sonjan, S.; Promkrainit, S.; Daengmankhong, J.; Phimnuan, P.; Mahasaranon, S.; Jongjitwimol, J.; Charoensit, P.; Ross, G.M.; Viennet, C.; et al. Porous Poly(2-Hydroxyethyl Methacrylate) Hydrogel Scaffolds for Tissue Engineering: Influence of Crosslinking Systems and Silk Sericin Concentration on Scaffold Properties. Polymers 2023, 15, 4052. [Google Scholar] [CrossRef]
- Zare, M.; Bigham, A.; Zare, M.; Luo, H.; Rezvani Ghomi, E.; Ramakrishna, S. PHEMA: An Overview for Biomedical Applications. Int. J. Mol. Sci. 2021, 22, 6376. [Google Scholar] [CrossRef]
- Ho, D.K.; Nguyen, D.T.; Thambi, T.; Lee, D.S.; Huynh, D.P. Polyamide-Based PH and Temperature-Responsive Hydrogels: Synthesis and Physicochemical Characterization. J. Polym. Res. 2019, 26, 7. [Google Scholar] [CrossRef]
- Lanzalaco, S.; Armelin, E. Poly(N-Isopropylacrylamide) and Copolymers: A Review on Recent Progresses in Biomedical Applications. Gels 2017, 3, 36. [Google Scholar] [CrossRef] [PubMed]
- Santhamoorthy, M.; Vy Phan, T.T.; Ramkumar, V.; Raorane, C.J.; Thirupathi, K.; Kim, S.-C. Thermo-Sensitive Poly (N-Isopropylacrylamide-Co-Polyacrylamide) Hydrogel for PH-Responsive Therapeutic Delivery. Polymers 2022, 14, 4128. [Google Scholar] [CrossRef]
- Feng, H.; Tang, N.; An, M.; Guo, R.; Ma, D.; Yu, X.; Zang, J.; Yang, N. Thermally-Responsive Hydrogels Poly(N-Isopropylacrylamide) as the Thermal Switch. J. Phys. Chem. C 2019, 123, 31003–31010. [Google Scholar] [CrossRef]
- Lanzalaco, S.; Mingot, J.; Torras, J.; Alemán, C.; Armelin, E. Recent Advances in Poly(N-isopropylacrylamide) Hydrogels and Derivatives as Promising Materials for Biomedical and Engineering Emerging Applications. Adv. Eng. Mater. 2023, 25, 2201303. [Google Scholar] [CrossRef]
- Sanzari, I.; Buratti, E.; Huang, R.; Tusan, C.G.; Dinelli, F.; Evans, N.D.; Prodromakis, T.; Bertoldo, M. Poly(N-Isopropylacrylamide) Based Thin Microgel Films for Use in Cell Culture Applications. Sci. Rep. 2020, 10, 6126. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Wang, L.; Yang, X.; Feng, Y.; Li, Y.; Feng, W. Poly(N-Isopropylacrylamide)-Based Smart Hydrogels: Design, Properties and Applications. Prog. Mater. Sci. 2021, 115, 100702. [Google Scholar] [CrossRef]
- Ansari, M.J.; Rajendran, R.R.; Mohanto, S.; Agarwal, U.; Panda, K.; Dhotre, K.; Manne, R.; Deepak, A.; Zafar, A.; Yasir, M.; et al. Poly(N-Isopropylacrylamide)-Based Hydrogels for Biomedical Applications: A Review of the State-of-the-Art. Gels 2022, 8, 454. [Google Scholar] [CrossRef] [PubMed]
- Hakim Khalili, M.; Panchal, V.; Dulebo, A.; Hawi, S.; Zhang, R.; Wilson, S.; Dossi, E.; Goel, S.; Impey, S.A.; Aria, A.I. Mechanical Behavior of 3D Printed Poly(Ethylene Glycol) Diacrylate Hydrogels in Hydrated Conditions Investigated Using Atomic Force Microscopy. ACS Appl. Polym. Mater. 2023, 5, 3034–3042. [Google Scholar] [CrossRef]
- Dong, R.; Zhao, X.; Guo, B.; Ma, P.X. Self-Healing Conductive Injectable Hydrogels with Antibacterial Activity as Cell Delivery Carrier for Cardiac Cell Therapy. ACS Appl. Mater. Interfaces 2016, 8, 17138–17150. [Google Scholar] [CrossRef]
- Taylor, D.L.; in het Panhuis, M. Self-Healing Hydrogels. Adv. Mater. 2016, 28, 9060–9093. [Google Scholar] [CrossRef] [PubMed]
- Bastings, M.M.C.; Koudstaal, S.; Kieltyka, R.E.; Nakano, Y.; Pape, A.C.H.; Feyen, D.A.M.; van Slochteren, F.J.; Doevendans, P.A.; Sluijter, J.P.G.; Meijer, E.W.; et al. A Fast PH-Switchable and Self-Healing Supramolecular Hydrogel Carrier for Guided, Local Catheter Injection in the Infarcted Myocardium. Adv. Healthc. Mater. 2014, 3, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xia, H.; Zhao, Y. Poly(Vinyl Alcohol) Hydrogel Can Autonomously Self-Heal. ACS Macro Lett. 2012, 1, 1233–1236. [Google Scholar] [CrossRef] [PubMed]
- Rumon, M.H.; Sarkar, S.D.; Uddin, M.; Alam, M.; Karobi, S.N.; Ayfar, A.; Azam, S.; Roy, C.K. Graphene Oxide Based Crosslinker for Simultaneous Enhancement of Mechanical Toughness and Self-Healing Capability of Conventional Hydrogels. RSC Adv. 2022, 12, 7453–7463. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, X.; Zhang, J.; Shen, S.; Yin, W.; Ye, G.; Wang, L.; Hou, H.; Qiu, X. A Tunable Self-Healing Ionic Hydrogel with Microscopic Homogeneous Conductivity as a Cardiac Patch for Myocardial Infarction Repair. Biomaterials 2021, 273, 120811. [Google Scholar] [CrossRef] [PubMed]
- Phadke, A.; Zhang, C.; Arman, B.; Hsu, C.-C.; Mashelkar, R.A.; Lele, A.K.; Tauber, M.J.; Arya, G.; Varghese, S. Rapid Self-Healing Hydrogels. Proc. Natl. Acad. Sci. USA 2012, 109, 4383–4388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Liu, Y.; Qin, D.; Sun, M.; Wang, T.; Chen, X. Research Status of Self-Healing Hydrogel for Wound Management: A Review. Int. J. Biol. Macromol. 2020, 164, 2108–2123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Chen, Y.; Deng, Y.; Ngai, T.; Wang, C. Dynamic Supramolecular Hydrogels: Regulating Hydrogel Properties through Self-Complementary Quadruple Hydrogen Bonds and Thermo-Switch. ACS Macro Lett. 2017, 6, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. PH-Responsive Self-Healing Injectable Hydrogel Based on N-Carboxyethyl Chitosan for Hepatocellular Carcinoma Therapy. Acta Biomater. 2017, 58, 168–180. [Google Scholar] [CrossRef]
- Peña, B.; Laughter, M.; Jett, S.; Rowland, T.J.; Taylor, M.R.G.; Mestroni, L.; Park, D. Injectable Hydrogels for Cardiac Tissue Engineering. Macromol. Biosci. 2018, 18, e1800079. [Google Scholar] [CrossRef]
- Innocenzi, P. Understanding Sol–Gel Transition through a Picture. A Short Tutorial. J. Solgel Sci. Technol. 2020, 94, 544–550. [Google Scholar] [CrossRef]
- Sakai, T.; Katashima, T.; Matsushita, T.; Chung, U. Sol-Gel Transition Behavior near Critical Concentration and Connectivity. Polym. J. 2016, 48, 629–634. [Google Scholar] [CrossRef]
- Hong, L.T.A.; Kim, Y.-M.; Park, H.H.; Hwang, D.H.; Cui, Y.; Lee, E.M.; Yahn, S.; Lee, J.K.; Song, S.-C.; Kim, B.G. An Injectable Hydrogel Enhances Tissue Repair after Spinal Cord Injury by Promoting Extracellular Matrix Remodeling. Nat. Commun. 2017, 8, 533. [Google Scholar] [CrossRef]
- Li, Z.; Guo, X.; Matsushita, S.; Guan, J. Differentiation of Cardiosphere-Derived Cells into a Mature Cardiac Lineage Using Biodegradable Poly(N-Isopropylacrylamide) Hydrogels. Biomaterials 2011, 32, 3220–3232. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Hu, J.; Wang, J.; Zhang, J.; Wang, L.; Zhang, C. The Role of Hydrogel in Cardiac Repair and Regeneration for Myocardial Infarction: Recent Advances and Future Perspectives. Bioengineering 2023, 10, 165. [Google Scholar] [CrossRef] [PubMed]
- Miclotte, M.P.J.; Varlas, S.; Reynolds, C.D.; Rashid, B.; Chapman, E.; O’Reilly, R.K. Thermoresponsive Block Copolymer Core–Shell Nanoparticles with Tunable Flow Behavior in Porous Media. ACS Appl. Mater. Interfaces 2022, 14, 54182–54193. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Sakikawa, N.; Miyata, T. Thermo-Responsive Gels That Absorb Moisture and Ooze Water. Nat. Commun. 2018, 9, 2315. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, S.; Damiri, F.; Zehravi, M.; Joshi, R.; Kapare, H.; Prajapati, M.K.; Munot, N.; Berrada, M.; Giram, P.S.; Rojekar, S.; et al. Functional Thermoresponsive Hydrogel Molecule to Material Design for Biomedical Applications. Polymers 2022, 14, 3126. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.; Kan, C. Thermoresponsive Hydrogels and Their Biomedical Applications: Special Insight into Their Applications in Textile Based Transdermal Therapy. Polymers 2018, 10, 480. [Google Scholar] [CrossRef]
- Lee, J.; Ku, K.H.; Kim, J.; Lee, Y.J.; Jang, S.G.; Kim, B.J. Light-Responsive, Shape-Switchable Block Copolymer Particles. J. Am. Chem. Soc. 2019, 141, 15348–15355. [Google Scholar] [CrossRef]
- Schmalz, A.; Schmalz, H.; Müller, A.H.E. Smart Hydrogels Based on Responsive Star-Block Copolymers. Soft Matter 2012, 8, 9436–9445. [Google Scholar] [CrossRef]
- Xing, Y.; Zeng, B.; Yang, W. Light Responsive Hydrogels for Controlled Drug Delivery. Front. Bioeng. Biotechnol. 2022, 10, 1075670. [Google Scholar] [CrossRef] [PubMed]
- Ghauri, Z.H.; Islam, A.; Qadir, M.A.; Gull, N.; Haider, B.; Khan, R.U.; Riaz, T. Development and Evaluation of PH-Sensitive Biodegradable Ternary Blended Hydrogel Films (Chitosan/Guar Gum/PVP) for Drug Delivery Application. Sci. Rep. 2021, 11, 21255. [Google Scholar] [CrossRef]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.; Selvanathan, V.; Sonsudin, F.; Abouloula, C. PH Sensitive Hydrogels in Drug Delivery: Brief History, Properties, Swelling, and Release Mechanism, Material Selection and Applications. Polymers 2017, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Vegad, U.; Patel, M.; Khunt, D.; Zupančič, O.; Chauhan, S.; Paudel, A. PH Stimuli-Responsive Hydrogels from Non-Cellulosic Biopolymers for Drug Delivery. Front. Bioeng. Biotechnol. 2023, 11, 1270364. [Google Scholar] [CrossRef] [PubMed]
- Awada, H.K.; Long, D.W.; Wang, Z.; Hwang, M.P.; Kim, K.; Wang, Y. A Single Injection of Protein-Loaded Coacervate-Gel Significantly Improves Cardiac Function Post Infarction. Biomaterials 2017, 125, 65–80. [Google Scholar] [CrossRef]
- Li, W.; Wang, D.; Yang, W.; Song, Y. Compressive Mechanical Properties and Microstructure of PVA–HA Hydrogels for Cartilage Repair. RSC Adv. 2016, 6, 20166–20172. [Google Scholar] [CrossRef]
- Li, L.; Lu, N.; Zhang, H.; Gao, Y.; Jiang, D.; Wang, S.; Wang, G. Designing High Mechanical Strength and Rapid-Recovery Hydrogel Electrolyte Based on the Inspiration of the Mechanical Unfolding Behavior of a Protein. Chem. Mater. 2023, 35, 8733–8744. [Google Scholar] [CrossRef]
- Ning, X.; Huang, J.; Yimuhan, A.; Yuan, N.; Chen, C.; Lin, D. Research Advances in Mechanical Properties and Applications of Dual Network Hydrogels. Int. J. Mol. Sci. 2022, 23, 15757. [Google Scholar] [CrossRef]
- Hu, X.; Liang, R.; Li, J.; Liu, Z.; Sun, G. Mechanically Strong Hydrogels Achieved by Designing Homogeneous Network Structure. Mater. Des. 2019, 163, 107547. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Xue, X.; Hu, Y.; Deng, Y.; Su, J. Recent Advances in Design of Functional Biocompatible Hydrogels for Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2009432. [Google Scholar] [CrossRef]
- Kłosiński, K.K.; Wach, R.A.; Girek-Bąk, M.K.; Rokita, B.; Kołat, D.; Kałuzińska-Kołat, Ż.; Kłosińska, B.; Duda, Ł.; Pasieka, Z.W. Biocompatibility and Mechanical Properties of Carboxymethyl Chitosan Hydrogels. Polymers 2022, 15, 144. [Google Scholar] [CrossRef]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current Hydrogel Advances in Physicochemical and Biological Response-Driven Biomedical Application Diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef]
- IKADA, Y. Biocompatibility of Hydrogels. In Gels Handbook; Elsevier: Amsterdam, The Netherlands, 2001; pp. 388–407. [Google Scholar]
- Hong, Y.; Lin, Z.; Yang, Y.; Jiang, T.; Shang, J.; Luo, Z. Biocompatible Conductive Hydrogels: Applications in the Field of Biomedicine. Int. J. Mol. Sci. 2022, 23, 4578. [Google Scholar] [CrossRef]
- Sánchez-Cid, P.; Jiménez-Rosado, M.; Rubio-Valle, J.F.; Romero, A.; Ostos, F.J.; Rafii-El-Idrissi Benhnia, M.; Perez-Puyana, V. Biocompatible and Thermoresistant Hydrogels Based on Collagen and Chitosan. Polymers 2022, 14, 272. [Google Scholar] [CrossRef]
- SANNINO, A.; MADAGHIELE, M.; AMBROSIO, L. Biocompatibility and Other Properties of Hydrogels in Regenerative Medicine. In Cellular Response to Biomaterials; Elsevier: Amsterdam, The Netherlands, 2009; pp. 114–135. [Google Scholar]
- Wassenaar, J.W.; Braden, R.L.; Osborn, K.G.; Christman, K.L. Modulating in Vivo Degradation Rate of Injectable Extracellular Matrix Hydrogels. J. Mater. Chem. B 2016, 4, 2794–2802. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, Y.; Xu, F.-J. Versatile Functionalization of Polysaccharides via Polymer Grafts: From Design to Biomedical Applications. Acc. Chem. Res. 2017, 50, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Kuperkar, K.; Atanase, L.; Bahadur, A.; Crivei, I.; Bahadur, P. Degradable Polymeric Bio(Nano)Materials and Their Biomedical Applications: A Comprehensive Overview and Recent Updates. Polymers 2024, 16, 206. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Kuperkar, K.; Yusa, S.; Bahadur, P. Nanoscale Self-Assemblies from Amphiphilic Block Copolymers as Proficient Templates in Drug Delivery. Drugs Drug Candidates 2023, 2, 898–922. [Google Scholar] [CrossRef]
- Kuperkar, K.; Patel, D.; Atanase, L.I.; Bahadur, P. Amphiphilic Block Copolymers: Their Structures, and Self-Assembly to Polymeric Micelles and Polymersomes as Drug Delivery Vehicles. Polymers 2022, 14, 4702. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Qin, J.; Sun, M.; Yan, G.; Tang, R. PH-Sensitive, Dynamic Graft Polymer Micelles via Simple Synthesis for Enhanced Chemotherapeutic Efficacy. J. Biomater. Appl. 2019, 34, 1059–1070. [Google Scholar] [CrossRef] [PubMed]
- Atanase, L.I.; Desbrieres, J.; Riess, G. Micellization of Synthetic and Polysaccharides-Based Graft Copolymers in Aqueous Media. Prog. Polym. Sci. 2017, 73, 32–60. [Google Scholar] [CrossRef]
- Ren, T.; Gan, J.; Zhou, L.; Chen, H. Physically Crosslinked Hydrogels Based on Poly (Vinyl Alcohol) and Fish Gelatin for Wound Dressing Application: Fabrication and Characterization. Polymers 2020, 12, 1729. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chan-Park, M.B. Hydrogel Based on Interpenetrating Polymer Networks of Dextran and Gelatin for Vascular Tissue Engineering. Biomaterials 2009, 30, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Z.; Shi, T.; Zhao, P.; An, K.; Lin, C.; Liu, H. Injectable Dextran Hydrogels Fabricated by Metal-Free Click Chemistry for Cartilage Tissue Engineering. Mater. Sci. Eng. C 2017, 73, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Domiński, A.; Konieczny, T.; Kurcok, P. α-Cyclodextrin-Based Polypseudorotaxane Hydrogels. Materials 2019, 13, 133. [Google Scholar] [CrossRef] [PubMed]
- Cavalieri, F.; Chiessi, E.; Villa, R.; Viganò, L.; Zaffaroni, N.; Telling, M.F.; Paradossi, G. Novel PVA-Based Hydrogel Microparticles for Doxorubicin Delivery. Biomacromolecules 2008, 9, 1967–1973. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Peh, G.S.L.; Ang, H.-P.; Lwin, N.C.; Adnan, K.; Mehta, J.S.; Tan, W.S.; Yim, E.K.F. Sequentially-Crosslinked Bioactive Hydrogels as Nano-Patterned Substrates with Customizable Stiffness and Degradation for Corneal Tissue Engineering Applications. Biomaterials 2017, 120, 139–154. [Google Scholar] [CrossRef]
- Bai, X.; Lü, S.; Cao, Z.; Ni, B.; Wang, X.; Ning, P.; Ma, D.; Wei, H.; Liu, M. Dual Crosslinked Chondroitin Sulfate Injectable Hydrogel Formed via Continuous Diels-Alder (DA) Click Chemistry for Bone Repair. Carbohydr. Polym. 2017, 166, 123–130. [Google Scholar] [CrossRef]
- Pacelli, S.; Rampetsreiter, K.; Modaresi, S.; Subham, S.; Chakravarti, A.R.; Lohfeld, S.; Detamore, M.S.; Paul, A. Fabrication of a Double-Cross-Linked Interpenetrating Polymeric Network (IPN) Hydrogel Surface Modified with Polydopamine to Modulate the Osteogenic Differentiation of Adipose-Derived Stem Cells. ACS Appl. Mater. Interfaces 2018, 10, 24955–24962. [Google Scholar] [CrossRef]
- Navaei, A.; Truong, D.; Heffernan, J.; Cutts, J.; Brafman, D.; Sirianni, R.W.; Vernon, B.; Nikkhah, M. PNIPAAm-Based Biohybrid Injectable Hydrogel for Cardiac Tissue Engineering. Acta Biomater. 2016, 32, 10–23. [Google Scholar] [CrossRef]
- Mahadevaiah, S.; Robinson, K.G.; Kharkar, P.M.; Kiick, K.L.; Akins, R.E. Decreasing Matrix Modulus of PEG Hydrogels Induces a Vascular Phenotype in Human Cord Blood Stem Cells. Biomaterials 2015, 62, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Politakos, N. Block Copolymers in 3D/4D Printing: Advances and Applications as Biomaterials. Polymers 2023, 15, 322. [Google Scholar] [CrossRef] [PubMed]
- Gardin, C.; Ferroni, L.; Latremouille, C.; Chachques, J.C.; Mitrečić, D.; Zavan, B. Recent Applications of Three Dimensional Printing in Cardiovascular Medicine. Cells 2020, 9, 742. [Google Scholar] [CrossRef]
- Wang, H.; Shi, J.; Wang, Y.; Yin, Y.; Wang, L.; Liu, J.; Liu, Z.; Duan, C.; Zhu, P.; Wang, C. Promotion of Cardiac Differentiation of Brown Adipose Derived Stem Cells by Chitosan Hydrogel for Repair after Myocardial Infarction. Biomaterials 2014, 35, 3986–3998. [Google Scholar] [CrossRef]
- Bencherif, S.A.; Sands, R.W.; Bhatta, D.; Arany, P.; Verbeke, C.S.; Edwards, D.A.; Mooney, D.J. Injectable Preformed Scaffolds with Shape-Memory Properties. Proc. Natl. Acad. Sci. USA 2012, 109, 19590–19595. [Google Scholar] [CrossRef]
- Veerubhotla, K.; Lee, C.H. Design of Biodegradable 3D-Printed Cardiovascular Stent. Bioprinting 2022, 26, e00204. [Google Scholar] [CrossRef]
- Bracaglia, L.G.; Messina, M.; Winston, S.; Kuo, C.-Y.; Lerman, M.; Fisher, J.P. 3D Printed Pericardium Hydrogels To Promote Wound Healing in Vascular Applications. Biomacromolecules 2017, 18, 3802–3811. [Google Scholar] [CrossRef]
- Mosadegh, B.; Xiong, G.; Dunham, S.; Min, J.K. Current Progress in 3D Printing for Cardiovascular Tissue Engineering. Biomed. Mater. 2015, 10, 034002. [Google Scholar] [CrossRef]
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv. Sci. 2019, 6, 1900344. [Google Scholar] [CrossRef] [PubMed]
- Rosellini, E.; Zhang, Y.S.; Migliori, B.; Barbani, N.; Lazzeri, L.; Shin, S.R.; Dokmeci, M.R.; Cascone, M.G. Protein/Polysaccharide-Based Scaffolds Mimicking Native Extracellular Matrix for Cardiac Tissue Engineering Applications. J. Biomed. Mater. Res. A 2018, 106, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kim, M.C.; Lee, J.Y. Nanomaterial-Based Electrically Conductive Hydrogels for Cardiac Tissue Repair. Int. J. Nanomed. 2022, 17, 6181–6200. [Google Scholar] [CrossRef] [PubMed]
- Saludas, L.; Pascual-Gil, S.; Prósper, F.; Garbayo, E.; Blanco-Prieto, M. Hydrogel Based Approaches for Cardiac Tissue Engineering. Int. J. Pharm. 2017, 523, 454–475. [Google Scholar] [CrossRef] [PubMed]
- Yu, X. Application of Hydrogels in Cardiac Regeneration. Cardiol. Ther. 2023, 12, 637–674. [Google Scholar] [CrossRef]
- Alagarsamy, K.N.; Yan, W.; Srivastava, A.; Desiderio, V.; Dhingra, S. Application of Injectable Hydrogels for Cardiac Stem Cell Therapy and Tissue Engineering. Rev. Cardiovasc. Med. 2019, 20, 221–230. [Google Scholar] [CrossRef]
- Vetter, V.C.; Bouten, C.V.C.; van der Pol, A. Hydrogels for Cardiac Restorative Support: Relevance of Gelation Mechanisms for Prospective Clinical Use. Curr. Heart Fail. Rep. 2023, 20, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Bao, M.; Nie, Y. Extracellular Matrix–Based Biomaterials for Cardiac Regeneration and Repair. Heart Fail. Rev. 2021, 26, 1231–1248. [Google Scholar] [CrossRef]
- Zheng, Z.; Tan, Y.; Li, Y.; Liu, Y.; Yi, G.; Yu, C.-Y.; Wei, H. Biotherapeutic-Loaded Injectable Hydrogels as a Synergistic Strategy to Support Myocardial Repair after Myocardial Infarction. J. Control. Release 2021, 335, 216–236. [Google Scholar] [CrossRef]
- Cui, H.; Liu, C.; Esworthy, T.; Huang, Y.; Yu, Z.; Zhou, X.; San, H.; Lee, S.; Hann, S.Y.; Boehm, M.; et al. 4D Physiologically Adaptable Cardiac Patch: A 4-Month in Vivo Study for the Treatment of Myocardial Infarction. Sci. Adv. 2024, 6, eabb5067. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, H.; Esworthy, T.; Mei, D.; Wang, Y.; Zhang, L.G. Emerging 4D Printing Strategies for Next-Generation Tissue Regeneration and Medical Devices. Adv. Mater. 2022, 34, 2109198. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, H.; Wang, Y.; Xu, C.; Esworthy, T.J.; Hann, S.Y.; Boehm, M.; Shen, Y.-L.; Mei, D.; Zhang, L.G. 4D Printed Cardiac Construct with Aligned Myofibers and Adjustable Curvature for Myocardial Regeneration. ACS Appl. Mater. Interfaces 2021, 13, 12746–12758. [Google Scholar] [CrossRef] [PubMed]
- Serpooshan, V.; Hu, J.B.; Chirikian, O.; Hu, D.A.; Mahmoudi, M.; Wu, S.M. Chapter 8—4D Printing of Actuating Cardiac Tissue. In 3D Printing Applications in Cardiovascular Medicine; Al’Aref, S.J., Mosadegh, B., Dunham, S., Min, J.K., Eds.; Academic Press: Boston, MA, USA, 2018; pp. 153–162. ISBN 978-0-12-803917-5. [Google Scholar]
- Zhou, Z.; Tang, W.; Yang, J.; Fan, C. Application of 4D Printing and Bioprinting in Cardiovascular Tissue Engineering. Biomater. Sci. 2023, 11, 6403–6420. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Park, H.-J.; Kim, S.-W.; Kim, H.; Park, J.Y.; Na, S.J.; Kim, H.J.; Park, M.N.; Choi, S.H.; Park, S.H.; et al. 3D Printed Complex Tissue Construct Using Stem Cell-Laden Decellularized Extracellular Matrix Bioinks for Cardiac Repair. Biomaterials 2017, 112, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Hsiao, B.S.; Chu, B. Functional Electrospun Nanofibrous Scaffolds for Biomedical Applications. Adv. Drug Deliv. Rev. 2007, 59, 1392–1412. [Google Scholar] [CrossRef] [PubMed]
- Akbari, M.; Tamayol, A.; Bagherifard, S.; Serex, L.; Mostafalu, P.; Faramarzi, N.; Mohammadi, M.H.; Khademhosseini, A. Textile Technologies and Tissue Engineering: A Path Toward Organ Weaving. Adv. Healthc. Mater. 2016, 5, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A Review on Stereolithography and Its Applications in Biomedical Engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef] [PubMed]
- Mazzoli, A. Selective Laser Sintering in Biomedical Engineering. Med. Biol. Eng. Comput. 2013, 51, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.D.; Dalton, P.D.; Hutmacher, D.W. Direct Writing By Way of Melt Electrospinning. Adv. Mater. 2011, 23, 5651–5657. [Google Scholar] [CrossRef]
- Moroni, L.; de Wijn, J.R.; van Blitterswijk, C.A. 3D Fiber-Deposited Scaffolds for Tissue Engineering: Influence of Pores Geometry and Architecture on Dynamic Mechanical Properties. Biomaterials 2006, 27, 974–985. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Arneri, A.; Bersini, S.; Shin, S.-R.; Zhu, K.; Goli-Malekabadi, Z.; Aleman, J.; Colosi, C.; Busignani, F.; Dell’Erba, V.; et al. Bioprinting 3D Microfibrous Scaffolds for Engineering Endothelialized Myocardium and Heart-on-a-Chip. Biomaterials 2016, 110, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Sun, X.-Z.; Parmenter, C.D.J.; Fay, M.W.; Smith, E.F.; Rance, G.A.; He, Y.; Zhang, F.; Liu, Y.; Irvine, D.; et al. Additive Manufacture of Complex 3D Au-Containing Nanocomposites by Simultaneous Two-Photon Polymerisation and Photoreduction. Sci. Rep. 2017, 7, 17150. [Google Scholar] [CrossRef] [PubMed]
- Gaebel, R.; Ma, N.; Liu, J.; Guan, J.; Koch, L.; Klopsch, C.; Gruene, M.; Toelk, A.; Wang, W.; Mark, P.; et al. Patterning Human Stem Cells and Endothelial Cells with Laser Printing for Cardiac Regeneration. Biomaterials 2011, 32, 9218–9230. [Google Scholar] [CrossRef] [PubMed]
- Koch, L.; Kuhn, S.; Sorg, H.; Gruene, M.; Schlie, S.; Gaebel, R.; Polchow, B.; Reimers, K.; Stoelting, S.; Ma, N.; et al. Laser Printing of Skin Cells and Human Stem Cells. Tissue Eng. Part C Methods 2010, 16, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Ferrini, A.; Stevens, M.M.; Sattler, S.; Rosenthal, N. Toward Regeneration of the Heart: Bioengineering Strategies for Immunomodulation. Front. Cardiovasc. Med. 2019, 6, 26. [Google Scholar] [CrossRef]
- Traverse, J.H.; Henry, T.D.; Dib, N.; Patel, A.N.; Pepine, C.; Schaer, G.L.; DeQuach, J.A.; Kinsey, A.M.; Chamberlin, P.; Christman, K.L. First-in-Man Study of a Cardiac Extracellular Matrix Hydrogel in Early and Late Myocardial Infarction Patients. JACC Basic Transl. Sci. 2019, 4, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Frey, N.; Linke, A.; Süselbeck, T.; Müller-Ehmsen, J.; Vermeersch, P.; Schoors, D.; Rosenberg, M.; Bea, F.; Tuvia, S.; Leor, J. Intracoronary Delivery of Injectable Bioabsorbable Scaffold (IK-5001) to Treat Left Ventricular Remodeling After ST-Elevation Myocardial Infarction. Circ. Cardiovasc. Interv. 2014, 7, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.J.; Hong, S.; Fang, Y.H.; Song, M.; Son, K.H.; Son, H.S.; Kim, S.K.; Sun, K.; Park, Y. Differential Regeneration of Myocardial Infarction Depending on the Progression of Disease and the Composition of Biomimetic Hydrogel. J. Biosci. Bioeng. 2014, 118, 461–468. [Google Scholar] [CrossRef]
- Lee, L.C.; Zhihong, Z.; Hinson, A.; Guccione, J.M. Reduction in Left Ventricular Wall Stress and Improvement in Function in Failing Hearts Using Algisyl-LVR. J. Vis. Exp. 2013, 74, e50096. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).