Abstract
Porous membrane technology has garnered significant attention in the fields of separation and biology due to its remarkable contributions to green chemistry and sustainable development. The porous membranes fabricated from polylactic acid (PLA) possess numerous advantages, including a low relative density, a high specific surface area, biodegradability, and excellent biocompatibility. As a result, they exhibit promising prospects for various applications, such as oil–water separation, tissue engineering, and drug release. This paper provides an overview of recent research advancements in the fabrication of PLA membranes using electrospinning, the breath-figure method, and the phase separation method. Firstly, the principles of each method are elucidated from the perspective of pore formation. The correlation between the relevant parameters and pore structure is discussed and summarized, subsequently followed by a comparative analysis of the advantages and limitations of each method. Subsequently, this article presents the diverse applications of porous PLA membranes in tissue engineering, oil–water separation, and other fields. The current challenges faced by these membranes, however, encompass inadequate mechanical strength, limited production efficiency, and the complexity of pore structure control. Suggestions for enhancement, as well as future prospects, are provided accordingly.
1. Introduction
In recent years, there has been rapid progress in the development of high-performance porous membrane materials, leading to an increased utilization of these membranes. As a result, there is a growing demand for enhanced membrane functionality, emphasizing the significance of creating porous membranes that demonstrate exceptional performance. When selecting porous membrane materials, a wide array of options is available. Presently, commonly employed separation membrane materials mainly consist of petroleum-derived polymers such as polyvinylidene fluoride (PVDF) [1,2], polypropylene (PP) [3,4], polysulfone (PSF) [5,6], and polyether sulfone (PES) [7,8]. However, these petroleum-based polymers pose challenges in terms of post-use degradation and have the potential to contribute to environmental pollution. PLA is a linear thermoplastic biodegradable polyester that can be directly synthesized through the polymerization reaction of lactic acid which can be derived from renewable plant resources [9]. Table 1 provides the advantages and limitations of PLA porous membranes.

Table 1.
Advantages and limitations of PLA porous membranes.
The PLA membrane can undergo complete degradation by microorganisms in the natural environment, making it a renewable and environmentally friendly material for various applications. Additionally, it exhibits excellent strength, processability and favorable biocompatibility, rendering it highly promising for tissue engineering [16,17,18], vascular transplantation [19,20,21], drug carrier [22,23,24], and other fields [15,25,26,27]. Zhong et al. [28] utilized PLA, nano-SiO2 and a mixture of good and poor solvents to fabricate superhydrophobic PLA film with nano/microstructured morphology using the non-solvent-induced phase separation (NIPS) method. The maximum water contact angle achieved was 164° ± 2.3°. The high hydrophobicity of the membrane holds significant potential in the realm of oil-water separation Liu et al. [29] immobilized a polydopamine (PDA) biological coating and silver nanoparticles (AgNPs) onto the membrane surface, followed by hydrophobic treatment with fluorinated mercaptan, resulting in the successful construction of a hierarchical rough structure of PLA. The water contact angle on the PLA membrane surface reached 158.6°, achieving a maximum permeate flux of 2664 L/(m2·h). Moreover, the separation efficiency for oil-water mixtures and water-in-oil emulsions exceeded 98.4%, demonstrating excellent stability and antibacterial properties. Hu et al. [30] utilized electrospinning technology to fabricate a composite nanofiber scaffold consisting of PLA, hydroxyapatite (HA), and polydopamine (PDA). Their findings revealed that the incorporation of HA-PDA particles significantly enhanced the hydrophilicity of the scaffold material. Moreover, compared to pure PLA nanofibers, the PLA/HA/PDA composite nanofiber scaffolds exhibited notably improved cell adhesion rate and survival rate. Consequently, these biocompatible (PLA)/HA/PDA composite nanofibers hold great potential as scaffolds for bone tissue engineering. Zhou et al. [31] employed electrospinning technology to fabricate a three-dimensional network structure of PLA/Polypyrrole (PPy) nanofiber scaffolds. In vitro experiments have demonstrated that the scaffold exhibits exceptional biocompatibility with human umbilical cord mesenchymal stem cells as well as Schwann cells, thereby enhancing their adhesion behavior. The PLA/graphene oxide (Go) drug-loaded nanofibers with different structures (single-axial and co-axial structure) were prepared by the electrospinning method, as demonstrated by Mao et al. [32]. A comparison of the drug release properties between PLA/Go nanofibers with different structures revealed that co-axial structure PLA/Go nanofibers exhibit enhanced stability and durability in drug release performance, effectively preventing sudden drug release. Consequently, these nanofibers hold significant potential for broad applications in the field of controlled drug release. Buscemi et al. [33] connected α,β-poly(N-2-hydroxyethyl)-D,L-aspartamide (PHEA) with PLA and blended it with polycaprolactone (PCL) to formulate an electrospinning solution. Subsequently, the electrospinning technology was employed to prepare a vascular stent exhibiting excellent mechanical strength and elasticity, along with favorable biocompatibility.single-axial and co-axial structure. Scientific research reports on the properties and potential applications of PLA porous membranes have consistently increased over the past three decades, as depicted in Figure 1.
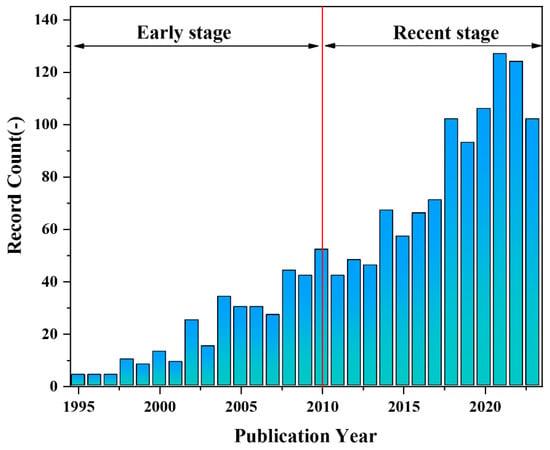
Figure 1.
The count evolution of publications obtained from the “Web of Science” database using “PLA porous membrane Paper & Patent” as key title terms.
The incorporation of biodegradable PLA as a substrate for crafting porous membrane materials not only overcomes challenges related to molding but also facilitates swift degradation by microorganisms into carbon dioxide and water upon disposal. This effectively addresses environmental pollution concerns, establishing it as a pivotal focus in research. The investigation and implementation of porous PLA membranes in tissue engineering and oil-water separation are poised to propel the progress of sustainable industry chains within the polymer materials domain. As a result, this confers notable research significance and practical value to the preparation of such membranes.
In this section, we propose the utilization of PLA, a biodegradable material, as one of the solutions in response to environmental pollution caused by petroleum-based polymers. A comprehensive analysis is provided on the origin, properties, and primary application domains of PLA materials
2. Preparation Method of PLA Porous Membranes
The current techniques employed for fabricating PLA porous membranes include electrospinning, breath figure technology, and phase separation technology. In this section, a succinct discussion is provided on the diverse techniques employed in the preparation of PLA porous membranes, placing particular emphasis on conducting a comparative analysis of their respective advantages and limitations.
2.1. Electrospinning
Electrospinning stands out as one of the most frequently employed techniques for producing PLA fiber membranes. The primary preparatory steps involve formulating a PLA spinning solution and subsequently injecting it into the spinning machine. The fundamental principle is to enhance the surface area of the spinning solution in order to induce an electric current through a high-voltage electric field. This leads to multiple stretching and splitting of the spinning solution during the injection process, as it shifts along a spiral path towards the receiving device for solidification into nanofibers. The overlapping of numerous fibers results in the formation of a porous film [34,35,36]. The schematic in Figure 2 illustrates the electrospinning process. By adjusting various electrospinning factors, such as voltage, spinneret diameter, working distance between the spinneret and receiving substrate, and other polymer parameters specific to PLA electrospinning, the manipulation of the structure and properties of PLA nanofibers becomes feasible [37,38]. Table 1 provides a summary of electrospinning parameters and pore sizes for different PLA based polymers.
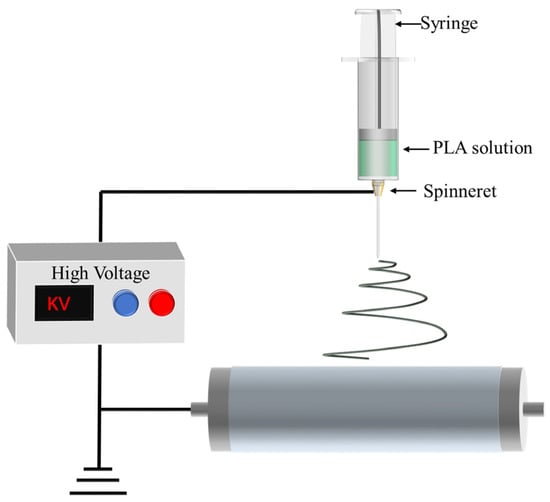
Figure 2.
Process diagram of preparing a PLA porous membrane by electrospinning technology.
In addition to the conventional nonwoven structure of PLA nanofibers, special functional PLA nanofibers can be prepared based on various polymer parameters and process conditions of PLA electrospinning. Examples include porous structure PLA nanofibers [39,40,41], and shell-core structure PLA nanofibers [42,43,44]. These unique structures possess characteristics that find application in diverse fields, garnering significant attention from researchers worldwide. This section introduces the porous structure and shell-core structure of PLA nanofibers.
2.1.1. PLA Nanofibers with Porous Structure
The high porosity and specific surface area of PLA nanofibers confer novel properties upon nanofiber materials [39,45]. Consequently, the electrospinning method for producing PLA nanofibers with a porous structure has captured significant attention from researchers. The choice of solvent during the electrospinning process plays a pivotal role in achieving a porous structure, with highly volatile solvents in the spinning solution contributing to the formation of a porous surface on the fibers.
Yang et al. [41] employed chloroform as a solvent in the electrospinning process to fabricate PLA nanofibers, observing the development of porous structures on nanofibers with diameters ranging from 100 to 400 nm. These porous structures notably enhanced the hydrophobicity of the PLA nanofibers. Tian et al. [39] immersed PLA electrospun nanofibers in acetone at 4 °C for 20 h, resulting in a highly porous structure on the nanofiber surface and an increase in specific surface area from 18.4 m2/g before immersion to 137.7 m2/g. Additionally, solid-state phase separation can be employed to produce PLA nanofibers with a multi-pore structure. Bognitzki et al. [40] crafted composite nanofibers of PLA/polyvinylpyrrolidone (PVP) using the electrospinning technique, followed by the selective removal of PVP in water, which process resulted in the formation of porous PLA nanofibers suitable for applications as adsorption materials.
2.1.2. PLA Nanofibers with Shell-Core Structure
The core/shell structure fiber is a composite fiber distinguished by its double or even multi-layered architecture, where the core and shell comprise components with distinct structures and physical properties. The fabrication process of PLA nanofibers with a shell-core structure not only incorporates the advantages of large specific surface area, high porosity, and small diameter typical of nanofiber materials but also facilitates the production of multifunctional nanofiber materials through the inclusion of an additional component [43,44].
Zhong et al. [42] produced a core-shell nanofiber material using Bletilla striata polysaccharide (BSP)-Polyvinyl alcohol (PVA)-PLA, as depicted in Figure 3 to illustrate the design strategy of the nanofibers.The formation of the core-shell structure was successfully confirmed through Confocal laser scanning microscopy (CLSM) and Fourier transform infrared spectroscopy (FTIR). Rosmarinic acid (RA)-BSP-PVA@PLA exhibits excellent biocompatibility. Moreover, the incorporation of BSP and RA significantly enhances the mechanical properties of coaxial fibers, possibly due to an increased number of intermolecular hydrogen bonds. Chen et al. [43] created core-shell porous nanofibers loaded with drugs using coaxial electrospinning and non-solvent-induced phase separation, employing polycaprolactone/PLA as the materials. In comparison to porous nanofibers prepared by single-axis electrospinning, these coaxial porous nanofibers exhibit improved control over drug release rates while promoting it. Tian et al. [44] utilizing nerve growth factor (NGF) and Silk Fibroin (SF) as the core layer and PLA as the shell layer, through coaxial electrospinning, prepared composite nanofibers with a shell structure for use as cell scaffold materials. The results demonstrate that the scaffold not only slowly releases NGF and prolongs NGF viability, but also promotes adhesion and proliferation of rat cells, crucial for advancements in the field of biological medicine. Table 2 provides the effects of different polymer types, solvent types, voltages, and tip-Collector Distance on the pore structure.
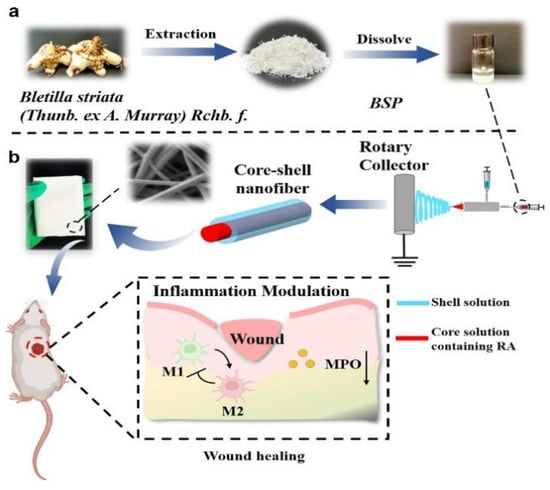
Figure 3.
RA-BSP-PVA-PLA coaxial nano-fiber design process and schematic diagram to promote wound healing [42]. (a) Preparation and application of RA-BSP-PVA@PLA nanofibers, extraction and preparation of BSP. (b) Schematic diagram of preparation of coaxial nanofibers and their promotion of wound healing.

Table 2.
Electrospinning for PLA-based polymer film formation.
Electrospinning can be utilized for the large-scale production of porous PLA membranes. However, it exhibits low production efficiency, a wide distribution of pore sizes, relatively high production costs, and suboptimal mechanical properties.
2.2. Breath-Figure Method
The phenomenon of Breath-Figure (BF) refers to the formation of water mist on solid or liquid surfaces, which was initially discovered and reported by Francois as the self-assembly of fine-scale polymer honeycomb structures facilitated by water [57]. The low surface temperature of a solid or liquid induces the release of heat from water vapor in the air, leading to its condensation on the surface. Due to the growth rate and mutual forces, organized arrangements of water droplets develop, acting as templates for surrounding solid material to solidify into regular patterns. Figure 4 illustrates the fabrication process of PLA porous membranes utilizing this technique.
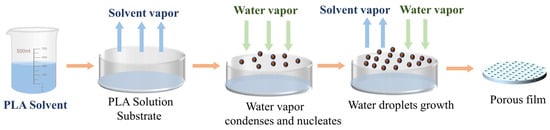
Figure 4.
Process diagram of preparing a PLA porous membrane by BF method.
The BF method provides the advantage of not requiring additional template removal while allowing simultaneous morphological construction and functional modification in a single step. This imparts convenience and efficiency compared to other methods for preparing porous films. The preparation conditions exhibit a linear relationship with the regular morphology and size, enabling easy control of pore sizes [58,59,60]. Consequently, an increasing number of researchers are employing the BF self-assembly technique to fabricate mesoporous films, finding extensive applications in fields such as bioengineering, optical devices, and sensing [61,62,63,64]. Preuksarattanawut et al. [65] successfully prepared a cellular-patterned porous biodegradable PLA membrane using the BF method. Their investigation considered the impact of initial polymer concentration in the solution, solvent type, and relative humidity of the airtight chamber on the pore size of the membranes. The findings suggest that a highly ordered porous membrane with an average pore size of 23.29 ± 4.55 μm can be achieved by employing a high PLA concentration (10% wt PLA) in dichloromethane at a relative humidity range of 80–85%.
However, directly preparing ordered porous films of PLA only with PLA by BF is challenging. In general, it is necessary to chemically or physically modify the PLA, for example, through chemical copolymerization. Copolymerization proves to be the simplest and most effective method for synthesizing new PLA composite materials. Li et al. [58] successfully synthesized structurally controllable polymethylene (PM)/poly (D, L-lactide) diblock copolymers (PM-b-PLA). These block copolymers, synthesized by combining the living polymerization of ylides with the ring opening polymerization of D, L-lactide, can be prepared into ordered porous films through the BF method. Although the PLA homopolymer film prepared by the BF method did not exhibit a regular porous structure, the film prepared by PM-b-PLA2 displayed a higher ordered honeycomb-like porous structure compared to the PLA homopolymer film, with a pore size of approximately 4.8 µm. The presence of PM segments reduces the hydrophilicity of PM-b-PLA2 and its solubility in dichloromethane, facilitating the formation of ordered porous structures. Bertrand et al. [59] successfully synthesized PLA-b-PS diblock copolymers via controlled polymerization, creating a block copolymer (BCP) capable of forming cylindrical structures. By combining the rapid solvent evaporation BF method with additional nanoscale self-assembly of PLA-b-PS diblock copolymers, they prepared a hierarchically porous polymer film with a highly regular honeycomb-like microporous structure.
Blending proves to be an effective method for modifying PLA and preparing porous membranes. Chen et al. [60] fabricated relatively ordered microporous structures using the BF method and introduced hydrophobic silica nanoparticles to construct micro/nanostructured surfaces with low surface energy. As depicted in Figure 5, a superhydrophobic PLA film exhibiting high water contact angle (WCA) of 150.2° and low sliding angle of 1.4° could be achieved when the nanoparticle content reached 12 mg/mL. Moreover, owing to the exceptional crystallinity and stability of silica particles, the PLA film exhibited remarkable self-cleaning properties as well as resistance against heat, acid, and water. This study offers a straightforward approach with industrial potential for manufacturing PLA materials with unique wettability properties and exceptional mechanical characteristics, suitable for applications in self-cleaning and moisture-proof packaging. Guo et al. [66] fabricated PLLA (poly(L-lactide))/Carbon Quantum Dots (CQD) porous membranes using a CQD-assisted static breathing method, wherein agricultural waste was transformed into high-value CQDs. The excellent dispersion of CQDs in water significantly enhanced the stability of water droplets on the surface of the PLA solution, facilitating the creation of a porous structure. Moreover, CQD demonstrated good degradability towards PLA. This study offers cost-effectiveness, ease of operation, and high versatility, effectively addressing limitations such as high humidity, air flow, and amphiphilic surfactants. Table 3 summarizes the effects of different polymer types, relative humidity levels, and solvent types on pore structure.
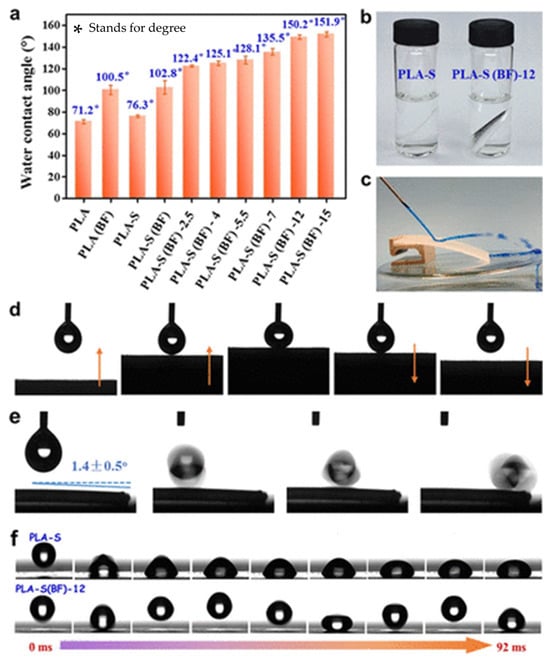
Figure 5.
(a) WCA of the oriented PLA films before and after surface modification. (b) Digital photograph of PLA-S and PLA-S(BF)-12 samples immersed in water. (c) Water jet bouncing off from the PLA-S(BF)-12 film surface. (d) Low-adhesive surface of the PLA-S(BF)-12 film. (e) Rolling of the water droplet on the PLA-S(BF)-12 film with a small tilt angle. (f) Droplet pinning and rebounding behavior when released from a height of 0.5 cm toward PLA-S and PLA-S(BF)-12 films [60]. (PLA-S stands for thin film prepared by method casting-thermal stretching; PLA-S(BF) stands for thin films that have been surface modified by the BF method and FAS-SiO2; 12 stands for 12 mg/mL of FAS-SiO2 suspensions; FAS-SiO2, fluorinated silica nanoparticles).

Table 3.
BF for PLA-based polymer film formation.
2.3. Phase Inversion Method (PI)
The phase inversion (PI) method, initially proposed by Leob et al. in 1964, currently stands as the most widely employed technique for fabricating polymer porous membranes [73]. Owing to its versatility and scalability, the phase inversion method has emerged as the predominant approach due to its simplicity and adaptable production scales. Based on the disparities in the thermodynamic conditions of polymer solutions, and there are four primary methods for phase inversion: thermally induced phase separation (TIPS), non-solvent-induced phase separation (NIPS), vapor-induced phase separation, and solvent evaporation phase inversion method [74,75,76]. Among these, the most frequently employed phase inversion techniques are TIPS and NIPS. Vapor-induced phase separation method is commonly utilized for preparing honeycomb block copolymer films, requiring specific polymer-solvent combinations. In contrast, the solvent evaporation phase inversion method is often chosen for producing polymer due to its prolonged film-forming duration and comparatively compact film structure. TIPS and NIPS are described in detail below.
2.3.1. Thermally Induced Phase Separation
The TIPS process involves the creation of a homogeneous and stable casting film solution at high temperatures, where a polymer and a diluent with a high boiling point are combined. The homogeneous casting film liquid undergoes phase separation and polymer solidification, leading to the extraction of the diluent and the formation of a porous film [77,78,79,80,81,82,83]. Figure 6 provides a schematic diagram of the TIPS process.
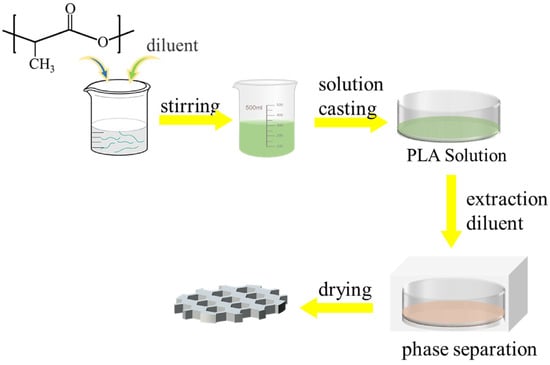
Figure 6.
Schematic diagram of the film formation process of porous PLA prepared by TIPS method.
The phase diagram serves as a fundamental tool for investigating the thermodynamic state of polymer solutions, enabling us to determine the suitability of a homogeneous polymer solution in a solvent for film formation [84]. A comprehensive phase diagram illustrating the TIPS method is presented in Figure 7.
The liquid-liquid separation region and solid-liquid separation region are demarcated by dynamic crystallization boundaries. The point of intersection between the binodal curve and the dynamic crystallization boundary is known as the monotectic point, which signifies the critical polymer concentration that distinguishes liquid-liquid phase separation from solid-liquid phase separation in the system. The polymer concentration for cooling paths 1, 2, and 3 is lower than the concentration at the monotectic point, resulting in liquid-liquid phase separation and leading to the formation of a bicontinuous film structure. The concentration of the polymer in path 4, however, surpasses the critical monotectic point concentration, leading to a phase separation between solid and liquid phases. As a result, a distinct spherical accumulation structure becomes visibly apparent in the solidified film. The absence of liquid-liquid phase separation is indicated [85]. Generally, as the polymer content gradually increases in the casting liquid system, the resulting membrane structure becomes more densely packed with reduced porosity and pore size [86].
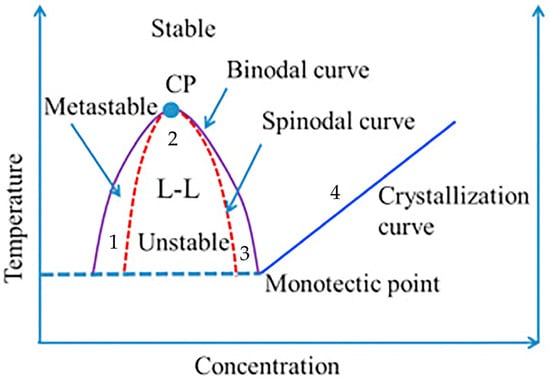
Figure 7.
Thermally induced phase separation binary phase diagram [86]. The abbreviation CP refers to the critical temperature.
The porosity morphology of the membrane in the TIPS method for porous membrane fabrication is influenced by the thermodynamic state of the solution. Modifying freezing temperature, solution composition, and concentration can effectively alter the porous morphology [87]. Tanaka et al. [88] initially fabricated PLLA microfiltration membranes using a thermally induced phase separation technique in 2004. They conducted an investigation on the impact of various cooling rates on the morphology of porous membranes, as depicted in Figure 8, and observed that higher cooling rates tend to generate anisotropic pore structures, whereas lower cooling rates lead to pore structures closer to isotropic.The presence of anisotropic pore structures can significantly enhance the permeation flux of microfiltration membranes. Fariba et al. [89] conducted a study on porous PLLA membranes using liquid-liquid and TIPS procedures to investigate the impact of quenching time, temperature, and film thickness on morphology, water absorption and mechanical properties. The findings suggest that an elevated freezing temperature can increase membrane porosity due to a reduced cooling rate and solvent nucleation rate within the specified thickness. Hou et al. [14] successfully fabricated a novel porous PLA film through blade coating and thermally induced phase separation. The leaf vein-like-oriented pores were obtained by enhancing the crystallization capacity of the stereoscopic composite crystals. The films exhibited an elongation at break of 45.5% and a porosity of 90.1%. Furthermore, the enhanced crystallinity significantly enhances the thermal resistance of the film Table 4 summarizes the preparation parameters of PLA membranes fabricated via the TIPS method.
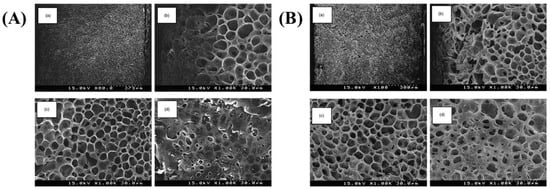
Figure 8.
Morphology of different cooling rates (A). Water from 80 to 0 °C. (B) Water from 50 to 0 °C. (a) Overview; (b) near the top side; (c) near the center; (d) the top surface [88].

Table 4.
Preparation parameters of PLA membranes fabricated via TIPS method.
2.3.2. Non-Solvent-Induced Phase Separation
The NIPS technique is widely employed in the field of phase inversion membrane technology. NIPS typically occurs at ambient temperature through the immersion of a homogeneous casting polymer solution into a non-solvent. The mass transfer between the solvent and non-solvent at the phase interface of the casting solution results in phase separation, polymer solidification, and the formation of a porous membrane. Figure 9 provides a schematic illustration of the non-solvent phase separation process.
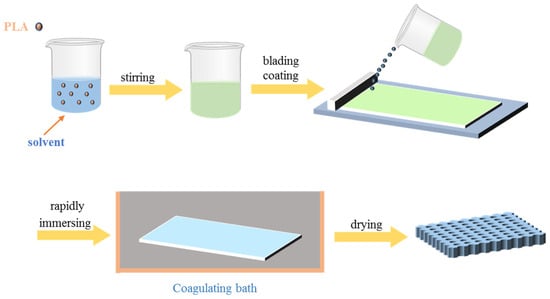
Figure 9.
Schematic diagram of the film formation process of porous PLA prepared by NIPS method.
The investigation of the thermodynamic phase diagram of the film-forming system facilitates the analysis and prediction of its film formation behavior [101]. The film formation process of the non-solvent phase separation method can be elucidated by referring to the ternary phase diagram illustrated in Figure 10. By manipulating the thermodynamic state and exerting control over phase transformation, precise manipulation of the film formation structure can be achieved. The immersion process of the primary film of polymer solution composed of B into a non-solvent bath may involve two distinct paths, namely path 1 and path 2. The path 1 involves passing through the two-node line to access the two-phase region, resulting in liquid-liquid phase separation and ultimately obtaining a membrane with a porous surface. The polymer solution undergoes a glass transition and directly enters the glass state for path 2, without traversing the two-node line. The film surface undergoes a transformation into a uniformly dense structure in this state.
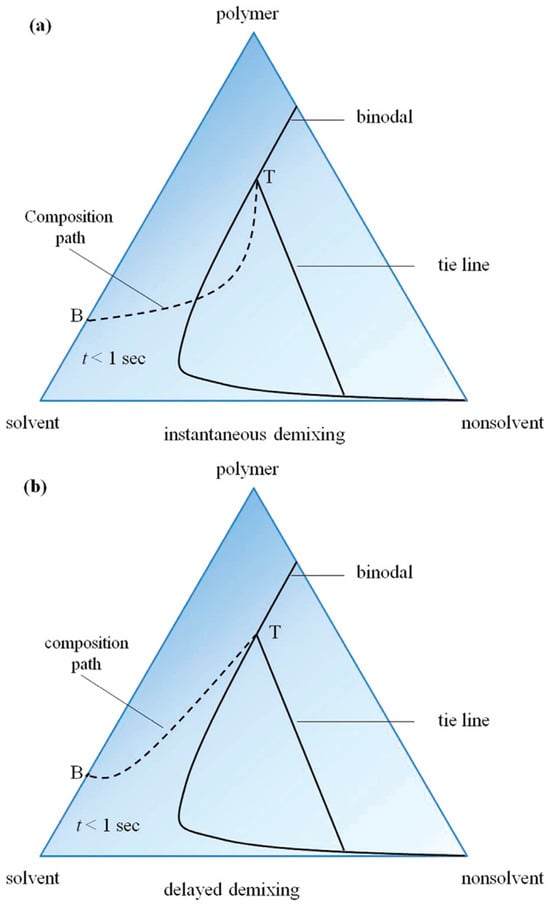
Figure 10.
Non-solvent phase separation process: (a) path 1, (b) path 2 [102].
The microstructure of PLA film is determined by the competitive outcome of phase separation, as dictated by the NIPS film forming mechanism, which is significantly influenced by the solvent/non-solvent system. Xing et al. [75] prepared PLA membranes by utilizing a coagulation bath consisting of a mixture of ethanol and water in varying proportions. They subsequently investigated the influence of the components within the coagulation bath on membrane structure, crystallinity, and porosity. The spongy structure was significantly influenced by the moisture content in the coagulation bath, with an observed disruption of the homogeneous spongy structure as the moisture content increased. However, accurately controlling the morphology of the PLA membrane proved challenging due to the difficulty in controlling the exchange rate between the solvent and non-solvent during the phase transition. To address this limitation, Hu et al. [103] proposed a novel approach to regulate the rate of solvent-to-non-solvent exchange by transforming non-solvent water from a continuous liquid phase into liquid microdroplets using an ultrasonic generator. In their study, the involvement of non-solvent states in the induced phase transition occurred in the form of microspheres, attenuating the exchange rate between solvent and non-solvent during the phase transition process and prolonging the curing time of the film. Moriya et al. [104] investigated the impact of different solvent types and PEG additives on film properties, finding that utilizing dimethyl sulfone as a solvent maintained the low viscosity of the casting film solution while resulting in a prepared film with excellent water permeability and ultrafiltration separation performance. The resulting membrane exhibited a membrane flux of 882 L/(m2·h·atm) and retained 80% of the bovine serum albumin (BSA) when employing 10% PEG. Table 5 summarizes the solvent and non-solvent preparation of PLA membranes by NIPS method and adds the necessary additional explanations in the comment column.
The concentration of the polymer in the casting solution will impact the morphology of the final film, as it is responsible for forming the membrane matrix [102]. The membrane porosity tends to decrease as the polymer concentration increases [105]. Additionally, when the polymer concentration is low, the casting liquid exhibits a reduced viscosity and enhanced fluidity. Consequently, the resulting film demonstrates excellent surface flatness and uniform thickness; however, it also possesses elevated porosity during film formation and compromised mechanical strength. The PLA solution with a mass percentage of 6%~20% (w/w) was prepared by Elas et al. [105] using the NIPS method. The impact of polymer concentration on the porous structure of the membrane was observed. As depicted in Figure 11, an increase in the mass percentage of PLA in the polymer solution resulted in a reduction in the number and average diameter of pores on the bottom surface, as well as a decrease in membrane porosity. The average pore size exhibits a significant decrease, particularly when the concentration of PLA solution exceeds 10% (w/w).
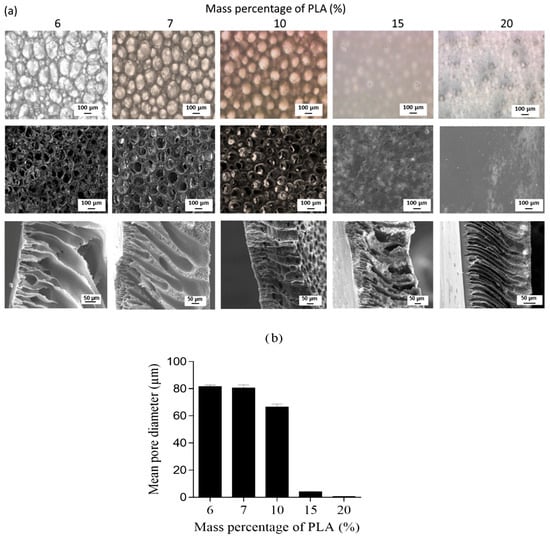
Figure 11.
(a) Optical microscopy (top) and SEM (middle) views of the porous bottom surface and SEM views of the cross section (bottom) of PLA membranes. (b) Mean diameter (μm) of macropores open on the bottom surface of the membrane [105].

Table 5.
Preparation parameters of PLA membranes fabricated via NIPS method.
Table 5.
Preparation parameters of PLA membranes fabricated via NIPS method.
| Species | Comments | |
|---|---|---|
| Solvent | DCM [106,107,108,109] | Solvent should be compatible with coagulation media. |
| NMP [11,13,110], | ||
| DMAc [111,112], | ||
| Acetic acid [113], | ||
| DMF [105,114,115,116], | ||
| DMSO [117] | ||
| 1,4-dioxane [75,103] | ||
| Non-solvent | Ethanol [109], | Due to cost considerations, non-solvents of PLA are often water. |
| Hexane [107], | ||
| Water [11,13,106,110,112,113,114,115,116,117,118], | ||
| NMP [108] |
In summary, membranes can be prepared through methods such as electrospinning, breath figure method, thermally induced phase separation, and nonsolvent-induced phase separation. A comparison of these four methods is summarized in Table 6.

Table 6.
Comparison of the various PLA porous membranes prepared by different methods.
3. Applications
The excellent degradation properties and biocompatibility of PLA porous membranes have led to their wide applications in various fields, such as oil-water separation, tissue engineering, and drug delivery [120,121]. The following section primarily discusses the application of PLA porous membranes in the fields of tissue engineering and oil-water separation. The application scope of PLA porous membrane is depicted comprehensively in Figure 12.
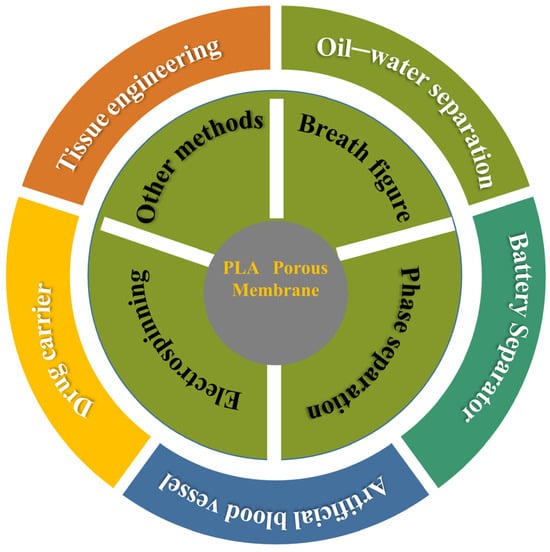
Figure 12.
The universal application of PLA porous membranes prepared by different methods.
3.1. Tissue Engineering
PLA possesses outstanding biocompatibility and biodegradability, and its degradation products are not significantly toxic to cells. It is recognized as a Food and Drug Administration (FDA) certified biomaterial for tissue engineering applications. PLA and its composite materials are currently widely utilized as biomaterials in tissue engineering. The interconnected network pore structure of PLA porous membranes demonstrates superior material transfer efficiency, providing ample internal space to support cell division and growth. Additionally, it promotes the flow and transport of nutrients and oxygen gas, along with the efficient removal of superfluous waste [122,123]. Pinto et al. [124] developed a composite membrane comprising PLA and graphene-based materials, specifically graphene oxide (GO) and graphene nanoplatelets (GNP), for tissue engineering applications. The incorporation of GO onto the membrane surface generated an appropriate surface topography and enhanced hydrophilicity, promoting cell adhesion and proliferation. The inclusion of GNP facilitated accelerated tissue regeneration while concurrently reducing thrombosis occurrence and postoperative complications. Li et al. [125] utilized the -COOH moiety of colorless 3-aminopropyltriethoxysilane (APTES) to react with the -NH2 group of heparin, immobilizing it onto the surface of PLA membrane through multiple self-condensation processes, including surface adhesion, polycondensation, and multilayer interaction. This approach effectively facilitates the surface heparinization of PLA porous membrane, enhancing its blood compatibility. Promnil et al. [126] successfully fabricated PLA/Silk Fibroin (SF) nanofiber scaffolds via the electrospinning technique. Their research findings indicate that viscosity plays a pivotal role in determining the ability, morphology, and size of fibers. The average fiber diameter increases proportionally with the increased viscosity of the solution. Three essential parameters influencing solution viscosity include concentration, structure, and molecular weight of PLA. Wang et al. [127] developed and fabricated a novel biocompatible PLA scaffold with a directional porous structure for bone tissue engineering using an ice template and the phase inversion technique. They discovered that the directional scaffold containing 8% of the material exhibited exceptional mechanical properties while maintaining a longitudinally connected porous structure with porosity ranging from 82% to 98%. This enhanced architecture facilitates efficient nutrient and cell penetration. Table 7 provides a summary of the applications of different PLA porous membranes in tissue engineering.

Table 7.
Applications of different PLA porous membranes in tissue engineering.
3.2. Oil–Water Separation
The severity of water pollution has been progressively escalating in recent years as a result of recurrent oil spills and the substantial release of industrial oily wastewater, thereby placing a considerable strain on the environment [135]. Conventional treatment methods include filtration, direct combustion, centrifugation, and electrochemical techniques [136,137,138,139]. The filtration method has gained widespread popularity due to its straightforward operation, exceptional efficiency, and cost-effectiveness. However, the majority of current membrane materials are non-degradable, posing a significant risk of secondary pollution. The incorporation of degradable polymers can effectively address this issue, rendering it imperative to opt for such materials in the fabrication of environmentally friendly oil-water separation materials.
PLA membranes exhibit high porosity and inherent hydrophobic properties, rendering them suitable for multiple recycling cycles following appropriate treatment. Consequently, the initial PLA oil-water separation material possesses superhydrophobic, superlipophilic, and biodegradable characteristics, making it an environmentally friendly separation material with significant developmental potential that can be fabricated through phase separation, electrospinning, and other techniques. Zhou et al. [140] successfully fabricated a hierarchical rough structure by depositing a titanium dioxide (TiO2) layer and methyltrichlorosilane (MTS) onto PLA nanofibers. The resulting film exhibited stable superhydrophobicity with a water contact angle of 157.4° ± 0.9° and demonstrated excellent resistance to water adhesion across different pH values. Moreover, the film displayed remarkable oil-water separation efficiency, achieving over 95% separation for various oils, making it highly suitable for efficient oil-water separation. Mo et al. [25] fabricated a hydrophobic PLA/carbon nanotubes (PLA/CNTs) composite fiber membrane via electrospinning, wherein the introduction and crystallization modification of carbon nanotubes resulted in a low polarity, rough, and porous surface that enhanced its hydrophobicity and oil-water selectivity. The prepared fiber film exhibited an oil absorption capacity of 114.01 g/g, a contact angle of water in air at 134.2°, and a contact angle of water under oil at 157.3°, indicating high separation efficiency for oil-water mixtures and making it a promising candidate for applications in oil-water separation. Xin et al. [141] utilized a simple slow solvent-evaporation induced precipitation method to fabricate porous PLA material with adjustable microstructure, and discovered that compared to solvent-casting bulk PLLA film, the porous PLA material exhibited higher contact angle (121°) and porosity (48.4%). Consequently, PLA porous material holds promising prospects in the field of oil-water separation. The oil-water separation performance of PLA-based materials is summarized in Table 8.

Table 8.
Summary of oil–water separation for PLA porous membranes.
3.3. Other Applications
3.3.1. Porous Polymer Electrolytes
Advancements in portable electronic devices, wearable technology, electric vehicles, and energy storage systems are driving increasing demands for battery performance [27,152]. Lithium-ion batteries offer numerous advantages, such as a high operating voltage, an extended cycle life, minimal self-discharge, absence of memory effect, and more [152,153]. The separator plays a crucial role in lithium-ion batteries, serving two primary functions. Firstly, it acts as a physical barrier between the positive and negative electrodes, effectively prevent short circuits and ensure the battery’s safe operation. Secondly, it facilitates efficient ion transfer during both charging and discharging processes, guaranteeing the battery’s normal functionality. Therefore, extensive research on separator-related matters is imperative for advancing towards the next generation of high-performance battery systems since the separator significantly influences both safety and ion transfer in batteries.
To mitigate the environmental impact of polymers used in energy storage systems, Barbosa et al. [27] proposed a lithium-ion battery membrane based on poly-L-lactic acid (PLLA). They utilized thermally-induced phase separation to fabricate PLLA membranes with varying polymer mass concentrations (8% to 12%). The microstructure and morphology of the membrane were found to be influenced by the polymer concentration, leading to the emergence of macroscopic cavities as the concentration increased. Moreover, changes in polymer concentration minimally affected the thermal properties and dimensions of the membrane. In terms of battery performance, the PLLA membrane with an 8% concentration exhibited a discharge capacity of 23 mAh/g at a 1C rate, while the PLLA membranes with concentrations of 10% and 12% demonstrated capacities of 93 mAh/g and 82 mAh/g, respectively. This study compared the efficiency of this PLLA separator with other natural polymers and commercial separators, highlighting its suitability for battery applications. MCC with high porosity and hydrophilicity was incorporated into the PLA/PBS biocomposite membrane through non-solvent phase separation, as reported by Thiangtham et al. The findings demonstrated that the composite membrane containing 5% MCC exhibited superior electrolyte infiltration and absorption rates, along with enhanced ionic conductivity (2.06 mS/cm) and reduced interface impedance (1115 Ω) [116]. Additionally, Thiangtham et al. [15] utilized phase inversion technique to blend sulfonated cellulose (SC) with PLA/PBS composites for the fabrication of lithium-ion battery separators with varying SC content. The incorporation of SC enhances the thermal stability and wettability of the biomembrane. Figure 13 illustrates a comparison in electrochemical performance between a battery assembled with a PLA/PBS/SC separator and one using Celgard 2400 separator. The biomembrane separators exhibit superior reversible capacity, enhanced rate performance, and excellent cycle stability at a low cost while also being environmentally friendly.
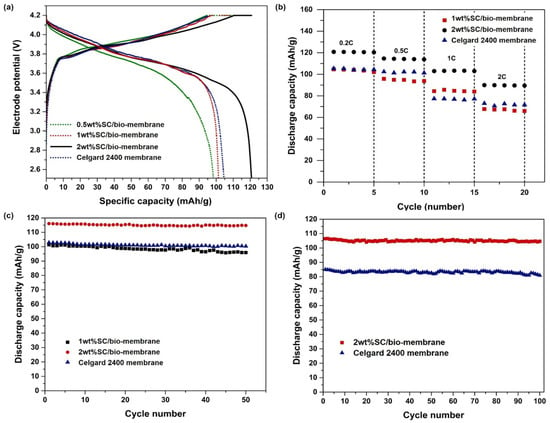
Figure 13.
(a) Initial-cycle charge–discharge curves, (b) C-rate performance of cells with various membranes, and discharged cell cycling performance at (c) the 0.5C rate and (d) 1C rate [15].
3.3.2. Drug Delivery
The future prospects of polymeric materials in drug delivery technologies appear to be more promising compared to other materials such as nanocrystals and metal-organic frameworks [22]. The compatibility of PLA with human tissues is exceptional, and it undergoes gradual degradation in the body, ultimately converting into carbon dioxide and water. Moreover, the intermediate product of PLA is lactic acid (LA), a normal sugar metabolite that does not accumulate in vital human organs [13].
The influence of solvent ratio (DCM/acetone) on the pore structure and antibacterial activity of fiber membranes was investigated by Seo et al. [24] using the liquid-liquid phase separation technique. The study revealed that increasing the DCM amount from 50% to 70% in the polymer-solvent system resulted in an increase in average fiber diameter from 1.87 μm to 2.43 μm, leading to the formation of a more interconnected structure with numerous pores. This interconnected porous structure facilitates maximum gentamicin adsorption, enabling slow and sustained drug release, thereby optimizing patient treatment and providing long-term utility. In order to achieve effective control over drug release, Ju et al. [37] employed electrospinning and phase separation techniques for the preparation of porous PLA fiber films. The release behavior of ketoprofen was investigated at different temperatures, revealing a relatively high release rate at 37 °C.
3.3.3. Artificial Blood Vessel
The recognition of cardiovascular disease as one of the leading causes of rising global mortality highlights its significant impact on individual health and lives, posing a formidable threat [154]. A comprehensive treatment plan for cardiovascular disease includes adherence to a nutritious diet, regular physical activity, and appropriate medication. In addition, certain patients may find relief through various chemical or physical interventions such as anticoagulant therapy for blood clot management or the surgical placement of a stent [155]. PLA has achieved significant advancements in the field of artificial blood vessels in recent years.
Pyzik et al. [156] investigated the use of heparin-modified bilayer PCL and PLA-based scaffolds for minor vessel tissue engineering. The findings suggest that the combination of thermally induced phase separation and electrospinning yields asymmetric scaffolds with enhanced mechanical properties. The discharge of heparin from the stent was confirmed by both direct and indirect tests. The endothelial cell culture test showed that the human aortic endothelial cell lines (HAEC) activity of the heparin-modified scaffolds was higher with longer culture time. Therefore, the design and composition of the proposed stent have prospective applications in small vessel tissue engineering. The surface of PLA was modified using radio frequency plasma surface activation with perfluorinated compounds by Khalifehzadeh et al. [157], resulting in a reduction in thrombus formation and platelet reactivity, thereby enhancing its blood compatibility. The electrospinning technique was employed by LeyvaVerduzco et al. [158] to fabricate a tubular membrane composed of a low cytotoxic blend of PLA and collagen. Additionally, the cell viability assessment conducted on Human Umbilical Vein Endothelial Cells (HUVECs) demonstrated that the blended samples exhibited enhanced cellular activity with minimal toxicity.
The PLA porous membrane has gained extensive utilization due to its biodegradability, non-toxic nature, biocompatibility, and renewability. This section primarily focuses on its key applications in tissue engineering and oil-water separation, followed by a comprehensive exploration of its diverse applications in other domains.
4. Summary and Prospect
The latest advancements and applications of the main methods for fabricating PLA porous membranes are comprehensively reviewed in this paper. Firstly, the performance of PLA as a porous membrane material is meticulously summarized from the perspective of porous polymer membranes, highlighting its distinct advantages over petroleum-based polymers, such as exceptional biocompatibility and biodegradability. Subsequently, we discussed the principles behind different methods and summarized the performance of PLA porous membranes and their latest research progress under various preparation methods. Additionally, through a comparative analysis of three methods, electrospinning and phase separation were found to be the most commonly used techniques for fabricating PLA porous membranes. Finally, based on the unique properties of PLA porous membranes, we primarily elucidated their diverse applications in tissue engineering, oil-water separation.
Although some progress has been made in the development of PLA porous membranes in recent years, numerous challenges still persist. Firstly, the mechanical properties of PLA porous membranes are generally subpar due to their high pore structure; thus, enhancing their mechanical properties holds significant importance. Modification technology is anticipated to enhance the mechanical properties of porous membranes. PLA modification can be achieved through three approaches: copolymerization modification, where other flexible molecular chain segments are introduced into the main chain of PLA; blending modification, which involves blending PLA with other ductile polymer materials; and plasticizing modification, wherein small molecules or low molecular weight plasticizers are mixed with PLA to improve its toughness. Secondly, considering the large specific surface area and high reactivity of nanoparticles, incorporating nanoparticles enables rapid establishment of reactions with PLA molecules to form new groups and enhance their mechanical properties. In addition, when selecting materials to enhance the performance of PLA porous film, it is necessary to consider factors such as biodegradability, environmental friendliness, economic feasibility, and biocompatibility in order to improve the mechanical properties of the film while maintaining its excellent degradability and biocompatibility.
Furthermore, the utilization of a solvent in the fabrication process of porous membranes poses potential risks to human health. Therefore, it is imperative to prioritize the development of an environmentally-friendly alternative solvent. There are two aspects to consider: 1. Consider the utilization of low-toxic or non-toxic solvents, such as triethyl phosphate (TEP) and ethyl lactate; however, it is essential to take into account the solvent’s ability to dissolve PLA. 2. Ionic liquids can also be regarded as a viable alternative for substituting toxic solvents due to their advantageous characteristics of low volatility, environmental friendliness, and excellent structural tunability [159,160,161].
Thirdly, the incorporation of SC crystals can ameliorate the issue of slow crystallization and enhance the degree of crystallinity, thereby augmenting the thermal stability of PLA films. However, there is currently limited research in this area, necessitating further discussions on the impact of different preparation methods on sc crystals as well as their pore structure size and distribution.
Finally, the structure and size distribution of pores are particularly important for the application of porous membranes. Despite being influenced by various factors, achieving nanoscale pore structures remains a formidable challenge. Therefore, it is imperative to optimize the preparation process and enhance material structure for the fabrication of nanostructured porous membranes, thereby broadening their range of applications. The current literature predominantly employs a single processing method for the preparation of PLA porous membranes, which severely restricts the diversity of membrane structures and limits their potential applications. It is imperative to integrate multiple processing techniques in order to explore the fabrication of membrane materials with enhanced structure and performance. Furthermore, in order to better fulfill the demands of large-scale production, it is imperative to optimize processing equipment, enhance production efficiency, and expand the application scope of PLA porous film. As more researchers delve into PLA porous membranes, we are confident that these issues will be resolved and the potential applications for PLA porous membranes will continue to broaden.
Author Contributions
Conceptualization, J.Z.; methodology, J.Y. and J.Z.; validation, X.P.; investigation, J.Y., J.Z. and X.L.; resources, J.Y.; writing—original draft, J.Z., J.Y. and X.L.; writing—review and editing, Z.S. and W.X.; supervision, J.Y.; project administration, J.Y.; funding acquisition, J.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the financial support of the National Natural Science Foundation of China (51903083) and the Natural Science Foundation of the Education Department of Hunan Province (18B291).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, C.; Cheng, S.; Li, H.; Zuo, D. Effect of different concentrations of spraying chitosan solution on structure and properties of PVDF porous membrane. Colloid. Polym. Sci. 2021, 299, 797–805. [Google Scholar] [CrossRef]
- Wei, N.; Li, Z.; Li, Q.; Yang, E.; Xu, R.; Song, X.; Sun, J.; Dou, C.; Tian, J.; Cui, H. Scalable and low-cost fabrication of hydrophobic PVDF/WS2 porous membrane for highly efficient solar steam generation. J. Colloid Interface Sci. 2021, 588, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, X.; Pei, C.; Dong, W.; Yao, Y. Hydrophilic modification of polypropylene membrane via tannic and titanium complexation for high-efficiency oil/water emulsion separation driven by self-gravity. Polym. Eng. Sci. 2022, 62, 2131–2142. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, L.; Xue, J.; Xue, Q. Preparation of micro-nano particles modified discarded face-mask by a versatile thermocompression modification approach and its application to emulsion separation. Sep. Sci. Technol. 2023, 58, 695–703. [Google Scholar] [CrossRef]
- Dong, C.; Xu, X.; Zhang, J.; Wang, H.; Xiang, Y.; Zhu, H.; Forsyth, M.; Lu, S. Proton transport of porous triazole-grafted polysulfone membranes for high temperature polymer electrolyte membrane fuel cell. Int. J. Hydrogen Energy 2022, 47, 8492–8501. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, F.; Zhang, S.; Zhang, Y.; Liu, S. Preparation of Nonstoichiometric Silica with Multi-Active Groups and Effect of Its Doping on Polysulfone Membrane Capabilities. Sep. Sci. Technol. 2012, 47, 2311–2319. [Google Scholar] [CrossRef]
- Zhang, S.-C.; Liu, T.; Wang, Y.-J. Porous and single-skinned polyethersulfone membranes support the growth of HepG2 cells: A potential biomaterial for bioartificial liver systems. J. Biomater. Appl. 2012, 27, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Yang, Y.; Wang, X.; Wang, Q.; Wang, S.; Gao, X.; Gao, C. Harvesting Microalgae Biomass Using Sulfonated Polyethersulfone (SPES)/PES Porous Membranes in Forward Osmosis Processes. J. Ocean Univ. China 2020, 19, 1345–1352. [Google Scholar] [CrossRef]
- Sakdaronnarong, C.; Srimarut, N.; Lucknakhul, N.; Na-songkla, N.; Jonglertjunya, W. Two-step acid and alkaline ethanolysis/alkaline peroxide fractionation of sugarcane bagasse and rice straw for production of polylactic acid precursor. Biochem. Eng. J. 2014, 85, 49–62. [Google Scholar] [CrossRef]
- Fan, Y.; Miao, X.; Hou, C.; Wang, J.; Lin, J.; Bian, F. High tensile performance of PLA fiber-reinforced PCL composite via a synergistic process of strain and crystallization. Polymers 2023, 270, 125778. [Google Scholar] [CrossRef]
- Gao, A.L.; Zhao, Y.Q.; Yang, Q.; Fu, Y.Y.; Xue, L.X. Facile preparation of patterned petal-like PLA surfaces with tunable water micro-droplet adhesion properties based on stereo-complex co-crystallization from non-solvent induced phase separation processes. J. Mater. Chem. 2016, 4, 12058–12064. [Google Scholar] [CrossRef]
- Shebi, A.; Lisa, S. Evaluation of biocompatibility and bactericidal activity of hierarchically porous PLA-TiO2 nanocomposite films fabricated by breath-figure method. Mater. Chem. Phys. 2019, 230, 308–318. [Google Scholar] [CrossRef]
- Su, Y.Z.; Zhao, Y.Q.; Zheng, W.G.; Yu, H.W.; Liu, Y.F.; Xu, L.Q. Asymmetric Sc-PLA Membrane with Multi-scale Microstructures: Wettability, Antifouling, and Oil-Water Separation. ACS Appl. Mater. Interfaces 2020, 12, 55520–55526. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Jia, H.; Pan, Y.; Liu, C.; Shen, C.; Liu, X. Porous poly (l-lactide)/poly (d-lactide) blend film with enhanced flexibility and heat resistance via constructing a regularly oriented pore structure. Macromolecules 2023, 56, 7606–7616. [Google Scholar] [CrossRef]
- Thiangtham, S.; Saito, N.; Manuspiya, H. Asymmetric Porous and Highly Hydrophilic Sulfonated Cellulose/Biomembrane Functioning as a Separator in a Lithium-Ion Battery. ACS Appl. Energy Mater. 2022, 5, 6206–6218. [Google Scholar] [CrossRef]
- Bakhshi, R.; Mohammadi-Zerankeshi, M.; Mehrabi-Dehdezi, M.; Alizadeh, R.; Labbaf, S.; Abachi, P. Additive manufacturing of PLA-Mg composite scaffolds for hard tissue engineering applications. J. Mech. Behav. Biomed. Mater. 2023, 138, 105655. [Google Scholar] [CrossRef]
- Osorio-Arciniega, R.; Garcia-Hipolito, M.; Alvarez-Fregoso, O.; Alvarez-Perez, M.A. Composite Fiber Spun Mat Synthesis and In Vitro Biocompatibility for Guide Tissue Engineering. Molecules 2021, 26, 7597. [Google Scholar] [CrossRef]
- Heydari, P.; Parham, S.; Kharazi, A.Z.; Javanmard, S.H.; Asgary, S. In Vitro Comparison Study of Plasma Treated Bilayer PGS/PCL and PGS/PLA Scaffolds for Vascular Tissue Engineering. Fibers Polym. 2022, 23, 2384–2393. [Google Scholar] [CrossRef]
- Mokhtari, N.; Kharazi, A.Z. Blood compatibility and cell response improvement of poly glycerol sebacate/poly lactic acid scaffold for vascular graft applications. J. Biomed. Mater. Res. Part A 2021, 109, 2673–2684. [Google Scholar] [CrossRef]
- Zhang, F.; Bambharoliya, T.; Xie, Y.; Liu, L.; Celik, H.; Wang, L.; Akkus, O.; King, M.W. A hybrid vascular graft harnessing the superior mechanical properties of synthetic fibers and the biological performance of collagen filaments. Mater. Sci. Eng. C 2021, 118, 111418. [Google Scholar] [CrossRef]
- Banitaba, S.N.; Gharehaghaji, A.A.; Jeddi, A.A.A. Fabrication and characterization of hollow electrospun PLA structure through a modified electrospinning method applicable as vascular graft. Bull. Mater. Sci. 2021, 44, 158. [Google Scholar] [CrossRef]
- Ebrahimifar, M.; Taherimehr, M. Evaluation of in-vitro drug release of polyvinylcyclohexane carbonate as a CO2-derived degradable polymer blended with PLA and PCL as drug carriers. J. Drug Delivery Sci. Technol. 2021, 63, 102491. [Google Scholar] [CrossRef]
- Jeong, H.; Lim, H.; Lee, D.Y.; Song, Y.-S.; Kim, B.-Y. Preparation and drug release behavior of nifedipine-loaded poly(lactic acid)/polyethylene glycol microcapsules. J. Nanosci. Nanotechnol. 2021, 21, 3735–3741. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.H.; Lee, K.E.; Yanilmaz, M.; Kim, J. Exploring the diverse morphology of porous poly(Lactic Acid) fibers for developing long-term controlled antibiotic delivery systems. Pharmaceutic 2022, 14, 1272. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Wang, Y.; Lin, J.; Ke, Y.; Zhou, C.; Wang, J.; Wen, J.; Gan, F.; Wang, L.; Ma, C. Polylactic acid/multi-wall carbon nanotubes composite fibrous membrane and their applications in oil-water separation. Surf. Interfaces 2023, 39, 102908. [Google Scholar] [CrossRef]
- Zhang, D.; Jin, X.-Z.; Huang, T.; Zhang, N.; Qi, X.; Yang, J.-h.; Zhou, Z.; Wang, Y. Electrospun fibrous membranes with dual-scaled porous structure: Super hydrophobicity, super lipophilicity, excellent water adhesion, and anti-icing for highly efficient oil adsorption/separation. ACS Appl. Mater. Interfaces 2019, 11, 5073–5083. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.; Reizabal, A.; Correia, D.; Fidalgo-Marijuan, A.; Gonçalves, R.; Silva, M.; Lanceros-Mendez, S.; Costa, C. Lithium-ion battery separator membranes based on poly (L-lactic acid) biopolymer. Mater. Today Energy 2020, 18, 100494. [Google Scholar] [CrossRef]
- Zhong, L.; Gong, X. Phase separation-induced superhydrophobic polylactic acid films. Soft Matter 2019, 15, 9500–9506. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yuan, W. A hierarchical functionalized biodegradable PLA electrospun nanofibrous membrane with superhydrophobicity and antibacterial properties for oil/water separation. J. Chem. 2018, 42, 17615–17624. [Google Scholar] [CrossRef]
- Hu, S.; Wu, J.; Cui, Z.; Si, J.; Wang, Q.; Peng, X. Study on the mechanical and thermal properties of polylactic acid/hydroxyapatite@ polydopamine composite nanofibers for tissue engineering. J. Appl. Polym. Sci. 2020, 137, 49077. [Google Scholar] [CrossRef]
- Zhou, J.-f.; Wang, Y.-g.; Cheng, L.; Wu, Z.; Sun, X.-d.; Peng, J. Preparation of polypyrrole-embedded electrospun poly (lactic acid) nanofibrous scaffolds for nerve tissue engineering. Neural Regener. Res. 2016, 11, 1644–1652. [Google Scholar] [CrossRef]
- Mao, Z.; Li, J.; Huang, W.; Jiang, H.; Zimba, B.L.; Chen, L.; Wan, J.; Wu, Q. Preparation of poly (lactic acid)/graphene oxide nanofiber membranes with different structures by electrospinning for drug delivery. RSC Adv. 2018, 8, 16619–16625. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, S.; Palumbo, V.D.; Maffongelli, A.; Fazzotta, S.; Palumbo, F.S.; Licciardi, M.; Fiorica, C.; Puleio, R.; Cassata, G.; Fiorello, L.; et al. Electrospun PHEA-PLA/PCL Scaffold for Vascular Regeneration: A Preliminary in Vivo Evaluation. Trans. Proc. 2017, 49, 716–721. [Google Scholar] [CrossRef]
- Afifi, A.M.; Nakajima, H.; Yamane, H.; Kimura, Y.; Nakano, S. Fabrication of Aligned Poly (l-lactide) Fibers by Electrospinning and Drawing. Macromol. Mater. Eng. 2009, 294, 658–665. [Google Scholar] [CrossRef]
- Samatham, R.; Kim, K. Electric current as a control variable in the electrospinning process. Polym. Eng. Sci. 2006, 46, 954–959. [Google Scholar] [CrossRef]
- Moon, S.; Choi, J.; Farris, R.J. Highly porous polyacrylonitrile/polystyrene nanofibers by electrospinning. Fibers Polym. 2008, 9, 276–280. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, I.H. Controlled release of ketoprofen from electrospun porous polylactic acid (PLA) nanofibers. J. Polym. Res. 2011, 18, 1287–1291. [Google Scholar] [CrossRef]
- Zhang, H.; Niu, Q.; Wang, N.; Nie, J.; Ma, G. Thermo-sensitive drug controlled release PLA core/PNIPAM shell fibers fabricated using a combination of electrospinning and UV photo-polymerization. Eur. Polym. J. 2015, 71, 440–450. [Google Scholar] [CrossRef]
- Tian, R.; Zhang, P.; Lv, R.; Na, B.; Liu, Q.; Ju, Y. Formation of highly porous structure in the electrospun polylactide fibers by swelling-crystallization in poor solvents. RSC Adv. 2015, 5, 37539–37544. [Google Scholar] [CrossRef]
- Bognitzki, M.; Frese, T.; Steinhart, M.; Greiner, A.; Wendorff, J.H.; Schaper, A.; Hellwig, M. Preparation of fibers with nanoscaled morphologies: Electrospinning of polymer blends. Polym. Eng. Sci. 2001, 41, 982–989. [Google Scholar] [CrossRef]
- Yang, H.; Wang, L.; Xiang, C.; Li, L. Electrospun porous PLLA and poly (LLA-co-CL) fibers by phase separation. New J. Chem. 2018, 42, 5102–5108. [Google Scholar] [CrossRef]
- Zhong, G.; Qiu, M.; Zhang, J.; Jiang, F.; Yue, X.; Huang, C.; Zhao, S.; Zeng, R.; Zhang, C.; Qu, Y. Fabrication and characterization of PVA@PLA electrospinning nanofibers embedded with Bletilla striata polysaccharide and Rosmarinic acid to promote wound healing. Int. J. Biol. Macromol. 2023, 234, 123693. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.H.; Lu, W.P.; Guo, Y.C. Antibacterial Porous Coaxial Drug-Carrying Nanofibers for Sustained Drug-Releasing Applications. Nanomaterials 2021, 11, 1316. [Google Scholar] [CrossRef]
- Tian, L.; Prabhakaran, M.P.; Hu, J.; Chen, M.; Besenbacher, F.; Ramakrishna, S. Coaxial electrospun poly(lactic acid)/silk fibroin nanofibers incorporated with nerve growth factor support the differentiation of neuronal stem cells. RSC Adv. 2015, 5, 49838–49848. [Google Scholar] [CrossRef]
- Zhang, P.; Tian, R.; Lv, R.; Na, B.; Liu, Q. Water-permeable polylactide blend membranes for hydrophilicity-based separation. Chem. Eng. J. 2015, 269, 180–185. [Google Scholar] [CrossRef]
- Lo, J.S.C.; Daoud, W.; Tso, C.Y.; Lee, H.H.; Firdous, I.; Deka, B.J.; Lin, C.S.K. Optimization of polylactic acid-based medical textiles via electrospinning for healthcare apparel and personal protective equipment. Sustain. Chem. Pharm. 2022, 30, 100891. [Google Scholar] [CrossRef]
- Ertek, D.A.; Sanli, N.O.; Menceloglu, Y.Z.; Avaz Seven, S. Environmentally friendly, antibacterial materials from recycled keratin incorporated electrospun PLA films with tunable properties. Eur. Polym. J. 2023, 185, 111804. [Google Scholar] [CrossRef]
- Scaffaro, R.; Lopresti, F.; Botta, L. Preparation, characterization and hydrolytic degradation of PLA/PCL co-mingled nanofibrous mats prepared via dual-jet electrospinning. Eur. Polym. J. 2017, 96, 266–277. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, Q.; Wu, J.; Zhang, Y.; Guo, J.; Yu, J.; Shu, X.; Chen, Q. Flexible, robust and self-peeling PLA/AgNWs nanofiber membranes with photothermally antibacterial properties for wound dressing. Appl. Surf. Sci. 2023, 615, 156284. [Google Scholar] [CrossRef]
- Gulzar, S.; Tagrida, M.; Nilsuwan, K.; Prodpran, T.; Benjakul, S. Electrospinning of gelatin/chitosan nanofibers incorporated with tannic acid and chitooligosaccharides on polylactic acid film: Characteristics and bioactivities. Food Hydrocolloids 2022, 133, 107916. [Google Scholar] [CrossRef]
- Guo, Y.; Ghobeira, R.; Sun, Z.; Shali, P.; Morent, R.; De Geyter, N. Atmospheric pressure plasma jet treatment of PLA/PAni solutions: Enhanced morphology, improved yield of electrospun nanofibers and concomitant doping behaviour. Polymers 2022, 262, 125502. [Google Scholar] [CrossRef]
- Niu, Y.; Stadler, F.J.; Fang, J.; Galluzzi, M. Hyaluronic acid-functionalized poly-lactic acid (PLA) microfibers regulate vascular endothelial cell proliferation and phenotypic shape expression. Colloids Surf. B 2021, 206, 111970. [Google Scholar] [CrossRef]
- Wu, Z.C.; Zhang, Z.J.; Wei, W.; Yin, Y.Q.; Huang, C.X.; Ding, J.; Duan, Q.S. Investigation of a novel poly (lactic acid) porous material toughened by thermoplastic polyurethane. J. Mater. Sci. 2022, 57, 5456–5466. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Gulino, E.F.; Micale, G.D.M. PLA-based functionally graded laminates for tunable controlled release of carvacrol obtained by combining electrospinning with solvent casting. React. Funct. Polym. 2020, 148, 104490. [Google Scholar] [CrossRef]
- Gutiérrez-Sánchez, M.; Escobar-Barrios, V.A.; Pozos-Guillén, A.; Escobar-García, D.M. RGD-functionalization of PLA/starch scaffolds obtained by electrospinning and evaluated in vitro for potential bone regeneration. Mater. Sci. Eng. C 2019, 96, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Min, T.; Zhou, L.; Sun, X.; Du, H.; Bian, X.; Zhu, Z.; Wen, Y. Enzyme-responsive food packaging system based on pectin-coated poly (lactic acid) nanofiber films for controlled release of thymol. Food Res. Int. 2022, 157, 111256. [Google Scholar] [CrossRef]
- François, B.; Pitois, O.; François, J. Polymer films with a self-organized honeycomb morphology. Adv. Mater. 1995, 7, 1041–1044. [Google Scholar] [CrossRef]
- Li, Q.Z.; Zhang, G.Y.; Huang, J.; Zhao, Q.L.; Wei, L.H.; He, Z.H.; Ma, Z. Synthesis and Property of Well-defined Polymethylene/Polylactide Diblock Copolymer. Acta Chim. Sinica 2011, 69, 497–502. [Google Scholar]
- Bertrand, A.; Bousquet, A.; Lartigau-Dagron, C.; Billon, L. Hierarchically porous bio-inspired films prepared by combining “breath figure” templating and selectively degradable block copolymer directed self-assembly. Chem. Commun. 2016, 52, 9562–9565. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, X.-R.; Huang, H.-D.; Xu, L.; Ji, X.; Zhong, G.-J.; Lin, H.; Li, Z.-M. Superhydrophobic, Self-Cleaning, and Robust Properties of Oriented Polylactide Imparted by Surface Structuring. ACS Sustain. Chem. Eng. 2021, 9, 6296–6304. [Google Scholar] [CrossRef]
- Bai, H.; Du, C.; Zhang, A.J.; Li, L. Breath Figure Arrays: Unconventional Fabrications, Functionalizations, and Applications. Angew. Chem. Int. Ed. 2013, 52, 12240–12255. [Google Scholar] [CrossRef] [PubMed]
- Escale, P.; Rubatat, L.; Billon, L.; Save, M. Recent advances in honeycomb-structured porous polymer films prepared via breath figures. Eur. Polym. J. 2012, 48, 1001–1025. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, J.; Sun, W.; Ji, J. Versatile and functional surface patterning of in situ breath figure pore formation via solvent treatment. ACS Appl. Mater. Interfaces 2020, 12, 47048–47058. [Google Scholar] [CrossRef]
- Kong, Q.; Li, Z.; Ding, F.; Ren, X. Hydrophobic N-halamine based POSS block copolymer porous films with antibacterial and resistance of bacterial adsorption performances. Chem. Eng. J. 2021, 410, 128407. [Google Scholar] [CrossRef]
- Preuksarattanawut, C.; Nisaratanaporn, E.; Siralertmukul, K. Highly ordered porous PLA films prepared by breath figure method. J. Met. Mater. Miner. 2019, 29, 106–112. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, X.; Wang, R.; Tang, H.; Wang, L.; Zhang, L.; Qin, S. Construction of porous poly (l-lactic acid) surface via carbon quantum dots-assisted static Breath-Figures method. Colloids Surf. A 2022, 647, 129110. [Google Scholar] [CrossRef]
- Ding, L.Y.; Ju, Y.L.; Sun, W.; Chen, J. Preparation of Functional Patterned Protein Arrays via the Combination of Inverse Emulsion and Breath Figure Method. Chem. J. Chin. Univ. Chin. 2018, 39, 1311–1318. [Google Scholar] [CrossRef]
- Qin, S.; Li, H.; Yuan, W.Z.; Zhang, Y.M. Fabrication of polymeric honeycomb microporous films: Breath figures strategy and stabilization of water droplets by fluorinated diblock copolymer micelles. J. Mater. Sci. 2012, 47, 6862–6871. [Google Scholar] [CrossRef]
- Cheng, K.-Y.; Chang, C.-H.; Yang, Y.-W.; Liao, G.-C.; Liu, C.-T.; Wu, J.-S. Enhancement of cell growth on honeycomb-structured polylactide surface using atmospheric-pressure plasma jet modification. Appl. Surf. Sci. 2017, 394, 534–542. [Google Scholar] [CrossRef]
- Yin, H.; Zhan, F.; Li, Z.; Huang, H.; Marcasuzaa, P.; Luo, X.; Feng, Y.; Billon, L. CO2-Triggered ON/OFF Wettability Switching on Bioinspired Polylactic Acid Porous Films for Controllable Bioadhesion. Biomac. 2021, 22, 1721–1729. [Google Scholar] [CrossRef]
- Yao, B.; Zhu, Q.; Yao, L.; Hao, J. Fabrication of honeycomb-structured poly(ethylene glycol)-block-poly(lactic acid) porous films and biomedical applications for cell growth. Appl. Surf. Sci. 2015, 332, 287–294. [Google Scholar] [CrossRef]
- Ponnusamy, T.; Lawson, L.B.; Freytag, L.C.; Blake, D.A.; Ayyala, R.S.; John, V. In vitro degradation and release characteristics of spin coated thin films of PLGA with a “breath figure” morphology. Biomatter 2012, 2, 77–86. [Google Scholar] [CrossRef]
- Loeb, S.; Sourirajan, S. High-flow semipermeable membranes for separation of water from saline solutions. Adv. Chem. Ser. 1962, 38, 117–132. [Google Scholar] [CrossRef]
- Fijol, N.; Abdelhamid, H.N.; Pillai, B.; Hall, S.A.; Thomas, N.; Mathew, A.P. 3D-printed monolithic biofilters based on a polylactic acid (PLA)—Hydroxyapatite (HAp) composite for heavy metal removal from an aqueous medium. RSC Adv. 2021, 11, 32408–32418. [Google Scholar] [CrossRef]
- Xing, Q.; Dong, X.; Li, R.; Yang, H.; Han, C.C.; Wang, D. Morphology and performance control of PLLA-based porous membranes by phase separation. Polymer 2013, 54, 5965–5973. [Google Scholar] [CrossRef]
- Figoli, A.; Marino, T.; Galiano, F. Polymeric membranes in biorefinery. Membr. Technol. Biorefining 2016, 29–59. [Google Scholar] [CrossRef]
- Lloyd, D.R.; Kinzer, K.E.; Tseng, H. Microporous membrane formation via thermally induced phase separation. I. Solid-liquid phase separation. J. Membr. Sci. 1990, 52, 239–261. [Google Scholar] [CrossRef]
- Lloyd, D.R.; Kim, S.S.; Kinzer, K. Microporous membrane formation via thermally-induced phase separation. II. Liquid—Liquid phase separation. J. Membr. Sci. 1991, 64, 1–11. [Google Scholar] [CrossRef]
- Kim, S.S.; Lloyd, D. Microporous membrane formation via thermally-induced phase separation. III. Effect of thermodynamic interactions on the structure of isotactic polypropylene membranes. J. Membr. Sci. 1991, 64, 13–29. [Google Scholar] [CrossRef]
- Lim, G.B.; Kim, S.S.; Ye, Q.; Wang, Y.F.; Lloyd, D. Microporous membrane formation via thermally-induced phase separation. IV. Effect of isotactic polypropylene crystallization kinetics on membrane structure. J. Membr. Sci. 1991, 64, 31–40. [Google Scholar] [CrossRef]
- Kim, S.S.; Lim, G.B.; Alwattari, A.A.; Wang, Y.F.; Lloyd, D. Microporous membrane formation via thermally-induced phase separation. V. Effect of diluent mobility and crystallization on the structure of isotactic polypropylene membranes. J. Membr. Sci. 1991, 64, 41–53. [Google Scholar] [CrossRef]
- Alwattari, A.A.; Lloyd, D. Microporous membrane formation via thermally-induced phase separation. VI. Effect of diluent morphology and relative crystallization kinetics on polypropylene membrane structure. J. Membr. Sci. 1991, 64, 55–67. [Google Scholar] [CrossRef]
- Lloyd, D.R.; Lim, G. Microporous membrane formation via thermally-induced phase separation. VII. Effect of dilution, cooling rate, and nucleating agent addition on morphology. J. Membr. Sci. 1993, 79, 27–34. [Google Scholar] [CrossRef]
- Chinyerenwa, A.C.; Wang, H.; Zhang, Q.; Zhuang, Y.; Munna, K.H.; Ying, C.; Yang, H.; Xu, W. Structure and thermal properties of porous polylactic acid membranes prepared via phase inversion induced by hot water droplets. Polymer 2018, 141, 62–69. [Google Scholar] [CrossRef]
- Rajabzadeh, S.; Maruyama, T.; Sotani, T.; Matsuyama, H.J.S.; Technology, P. Preparation of PVDF hollow fiber membrane from a ternary polymer/solvent/nonsolvent system via thermally induced phase separation (TIPS) method. Sep. Purif. Technol. 2008, 63, 415–423. [Google Scholar] [CrossRef]
- Cui, Z.; Hassankiadeh, N.T.; Zhuang, Y.; Drioli, E.; Lee, Y.M. Crystalline polymorphism in poly(vinylidenefluoride) membranes. Prog. Polym. Sci. 2015, 51, 94–126. [Google Scholar] [CrossRef]
- Liu, Q.; Tian, S.; Zhao, C.; Chen, X.; Lei, I.; Wang, Z.; Ma, P.X. Porous nanofibrous poly(l-lactic acid) scaffolds supporting cardiovascular progenitor cells for cardiac tissue engineering. Acta Biomater. 2015, 26, 105–114. [Google Scholar] [CrossRef]
- Tanaka, T.; Lloyd, D. Formation of poly (L-lactic acid) microfiltration membranes via thermally induced phase separation. J. Membr. Sci. 2004, 238, 65–73. [Google Scholar] [CrossRef]
- Soltanolkottabi, F. Application of Fourier’s law in thermally induced phase separation (TIPS) process for porous poly(L-lactide) films. Polym. Bull. 2022, 80, 7011–7021. [Google Scholar] [CrossRef]
- Hsu, S.h.; Huang, S.; Wang, Y.C.; Kuo, Y.C. Novel nanostructured biodegradable polymer matrices fabricated by phase separation techniques for tissue regeneration. Acta Biomater. 2013, 9, 6915–6927. [Google Scholar] [CrossRef]
- Önder, Ö.C.; Yilgör, E.; Yilgör, I. Fabrication of rigid poly(lactic acid) foams via thermally induced phase separation. Polymer 2016, 107, 240–248. [Google Scholar] [CrossRef]
- Onder, O.C.; Yilgor, E.; Yilgor, I. Critical parameters controlling the properties of monolithic poly(lactic acid) foams prepared by thermally induced phase separation. J. Polym. Sci. Part B-Polym. Phys. 2019, 57, 98–108. [Google Scholar] [CrossRef]
- Gay, S.; Lefebvre, G.; Bonnin, M.; Nottelet, B.; Boury, F.; Gibaud, A.; Calvignac, B. PLA scaffolds production from Thermally Induced Phase Separation: Effect of process parameters and development of an environmentally improved route assisted by supercritical carbon dioxide. J. Supercrit. Fluids 2018, 136, 123–135. [Google Scholar] [CrossRef]
- Chen, J.-S.; Tu, S.-L.; Tsay, R.-Y. A morphological study of porous polylactide scaffolds prepared by thermally induced phase separation. J. Taiwan Inst. Chem. Eng. 2010, 41, 229–238. [Google Scholar] [CrossRef]
- Kanno, T.; Uyama, H. Unique leafy morphology of poly(lactic acid) monoliths controlled via novel phase separation technology. RSC Adv. 2017, 7, 33726–33732. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Zamparini, F.; Degli Esposti, M.; Chiellini, F.; Aparicio, C.; Fava, F.; Fabbri, P.; Taddei, P.; Prati, C. Polylactic acid-based porous scaffolds doped with calcium silicate and dicalcium phosphate dihydrate designed for biomedical application. Mater. Sci. Eng. 2018, 82, 163–181. [Google Scholar] [CrossRef]
- Farzamfar, S.; Naseri-Nosar, M.; Sahrapeyma, H.; Ehterami, A.; Goodarzi, A.; Rahmati, M.; Lakalayeh, G.A.; Ghorbani, S.; Vaez, A.; Salehi, M. Tetracycline hydrochloride-containing poly (epsilon-caprolactone)/poly lactic acid scaffold for bone tissue engineering application: In vitro and in vivo study. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 472–479. [Google Scholar] [CrossRef]
- Carfì Pavia, F.; Palumbo, F.S.; La Carrubba, V.; Bongiovì, F.; Brucato, V.; Pitarresi, G.; Giammona, G. Modulation of physical and biological properties of a composite PLLA and polyaspartamide derivative obtained via thermally induced phase separation (TIPS) technique. Mater. Sci. Eng. C 2016, 67, 561–569. [Google Scholar] [CrossRef]
- La Carrubba, V.; Pavia, F.C.; Brucato, V.; Piccarolo, S. PLLA/PLA scaffolds prepared via Thermally Induced Phase Separation (TIPS): Tuning of properties and biodegradability. Int. J. Mater. Form. 2008, 1, 619–622. [Google Scholar] [CrossRef]
- Salehi, M.; Bastami, F.; Rad, M.R.; Nokhbatolfoghahaei, H.; Paknejad, Z.; Nazeman, P.; Hassani, A.; Khojasteh, A. Investigation of cell-free poly lactic acid/nanoclay scaffolds prepared via thermally induced phase separation technique containing hydroxyapatite nanocarriers of erythropoietin for bone tissue engineering applications. Polym. Adv. Technol. 2021, 32, 670–680. [Google Scholar] [CrossRef]
- Dong, X.; Lu, D.; Harris, T.A.; Escobar, I. Polymers and solvents used in membrane fabrication: A review focusing on sustainable membrane development. Membranes 2021, 11, 309. [Google Scholar] [CrossRef]
- Guillen, G.R.; Pan, Y.J.; Li, M.H.; Hoek, E.M.V. Preparation and Characterization of Membranes Formed by Nonsolvent Induced Phase Separation: A Review. Ind. Eng. Chem. Res. 2011, 50, 3798–3817. [Google Scholar] [CrossRef]
- Hu, R.; Pi, Y.; Wang, N.; Zhang, Q.; Feng, J.; Xu, W.; Dong, X.; Wang, D.; Yang, H. The formation of the S-shaped edge-on lamellae on the thin porous polylactic acid membrane via phase separation induced by water microdroplets. J. Appl. Polym. Sci. 2016, 133, 43355. [Google Scholar] [CrossRef]
- Moriya, A.; Maruyama, T.; Ohmukai, Y.; Sotani, T.; Matsuyama, H. Preparation of poly (lactic acid) hollow fiber membranes via phase separation methods. J. Membr. Sci. 2009, 342, 307–312. [Google Scholar] [CrossRef]
- Al Tawil, E.; Monnier, A.; Nguyen, Q.T.; Deschrevel, B. Microarchitecture of poly(lactic acid) membranes with an interconnected network of macropores and micropores influences cell behavior. Eur. Polym. J. 2018, 105, 370–388. [Google Scholar] [CrossRef]
- Kang, Y.; Chen, P.; Shi, X.T.; Zhang, G.C.; Wang, C.L. Preparation of open-porous stereocomplex PLA/PBAT scaffolds and correlation between their morphology, mechanical behavior, and cell compatibility. RSC Adv. 2018, 8, 12933–12943. [Google Scholar] [CrossRef]
- Fotsing, E.R.; Rezabeigi, E.; Ross, A.; Wood-Adams, P.M.; Drew, R. Acoustical characteristics of ultralight polylactic acid foams fabricated via solution phase inversion. J. Porous Mater. 2019, 26, 1781–1794. [Google Scholar] [CrossRef]
- Liu, W.; Huang, N.; Yang, J.; Peng, L.; Li, J.; Chen, W. Characterization and application of porous polylactic acid films prepared by nonsolvent-induced phase separation method. Food Chem. 2022, 373, 131525. [Google Scholar] [CrossRef]
- Li, G.; Wang, L.; Lei, X.; Peng, Z.; Wan, T.; Maganti, S.; Huang, M.; Murugadoss, V.; Seok, I.; Jiang, Q.; et al. Flexible, yet robust polyaniline coated foamed polylactic acid composite electrodes for high-performance supercapacitors. Adv. Compos. Hybrid Mater. 2022, 5, 853–863. [Google Scholar] [CrossRef]
- Gao, A.; Zhang, G.; Zhao, S.; Cui, J.; Yan, Y. A solution for trade-off phenomenon based on symmetric-like membrane with nano-scale pore structure. Sep. Purif. Technol. 2019, 227, 115693. [Google Scholar] [CrossRef]
- Ampawan, S.; Phreecha, N.; Chantarak, S.; Chinpa, W. Selective separation of dyes by green composite membrane based on polylactide with carboxylated cellulose microfiber from empty fruit bunch. Int. J. Biol. Macromol. 2023, 225, 1607–1619. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.J.; Liu, F.; Yu, X.M.; Xue, L.X. Poly(Lactic Acid) Hemodialysis Membranes with Poly(Lactic Acid)-block-Poly(2-Hydroxyethyl Methacrylate) Copolymer As Additive: Preparation, Characterization, and Performance. ACS Appl. Mater. Interfaces 2015, 7, 17748–17755. [Google Scholar] [CrossRef]
- Liu, F.; Li, B.B.; Sun, D.; Li, F.G.; Pei, X.Y. The effect of chitosan (CS) coagulation bath on structure and performance of polylactic acid (PLA) microfiltration membrane. Korean J. Chem. Eng. 2022, 39, 1307–1315. [Google Scholar] [CrossRef]
- Shu, Z.; Zhang, C.C.; Yan, L.Z.; Lei, H.Q.; Peng, C.X.; Liu, S.; Fan, L.H.; Chu, Y.Y. Antibacterial and osteoconductive polycaprolactone/polylactic acid/nano-hydroxyapatite/Cu@ZIF-8 GBR membrane with asymmetric porous structure. Int. J. Biol. Macromol. 2023, 224, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Hazra, R.S.; Dutta, D.; Mamnoon, B.; Nair, G.; Knight, A.; Mallik, S.; Ganai, S.; Reindl, K.; Jiang, L.; Quadir, M. Polymeric Composite Matrix with High Biobased Content as Pharmaceutically Relevant Molecular Encapsulation and Release Platform. ACS Appl. Mater. Interfaces 2021, 13, 40229–40248. [Google Scholar] [CrossRef]
- Thiangtham, S.; Runt, J.; Saito, N.; Manuspiya, H. Fabrication of biocomposite membrane with microcrystalline cellulose (MCC) extracted from sugarcane bagasse by phase inversion method. Cellulose 2020, 27, 1367–1384. [Google Scholar] [CrossRef]
- Moriya, A.; Shen, P.; Ohmukai, Y.; Maruyama, T.; Matsuyama, H. Reduction of fouling on poly(lactic acid) hollow fiber membranes by blending with poly(lactic acid)–polyethylene glycol–poly(lactic acid) triblock copolymers. J. Membr. Sci. 2012, 415–416, 712–717. [Google Scholar] [CrossRef]
- Han, W.; Ren, J.; Xuan, H.; Ge, L. Controllable degradation rates, antibacterial, free-standing and highly transparent films based on polylactic acid and chitosan. Colloids Surf. A 2018, 541, 128–136. [Google Scholar] [CrossRef]
- Song, X.; Yin, X.; Cai, Y.; Wei, Q.; Liu, W. Preparation and characterization of PLA porous film by breath figure method. New Chem. Mater. 2018, 46, 59–63. [Google Scholar]
- Hua, M.; Chen, D.; Xu, Z.; Fang, Y.; Song, Y. Fabrication of high-expansion, fully degradable polylactic acid-based foam with exponent oil/water separation. J. Appl. Polym. Sci. 2022, 139, e53234. [Google Scholar] [CrossRef]
- Lopresti, F.; Campora, S.; Tirri, G.; Capuana, E.; Carfì Pavia, F.; Brucato, V.; Ghersi, G.; La Carrubba, V. Core-shell PLA/Kef hybrid scaffolds for skin tissue engineering applications prepared by direct kefiran coating on PLA electrospun fibers optimized via air-plasma treatment. Mater. Sci. Eng. C 2021, 127, 112248. [Google Scholar] [CrossRef]
- Farahani, A.; Zarei-Hanzaki, A.; Abedi, H.R.; Tayebi, L.; Mostafavi, E. Polylactic Acid Piezo-Biopolymers: Chemistry, Structural Evolution, Fabrication Methods, and Tissue Engineering Applications. J. Funct. Biomater. 2021, 12, 71. [Google Scholar] [CrossRef]
- Nie, T.; Xue, L.; Ge, M.; Ma, H.; Zhang, J. Fabrication of poly(L-lactic acid) tissue engineering scaffolds with precisely controlled gradient structure. Mater. Lett. 2016, 176, 25–28. [Google Scholar] [CrossRef]
- Pinto, A.M.; Moreira, S.; Gonçalves, I.C.; Gama, F.M.; Mendes, A.M.; Magalhães, F.D. Biocompatibility of poly (lactic acid) with incorporated graphene-based materials. Colloids Surf. B 2013, 104, 229–238. [Google Scholar] [CrossRef]
- Li, J.; Liu, F.; Yu, X.; Wu, Z.; Wang, Y.; Xiong, Z.; He, J. APTES assisted surface heparinization of polylactide porous membranes for improved hemocompatibility. RSC Adv. 2016, 6, 42684–42692. [Google Scholar] [CrossRef]
- Promnil, S.; Ruksakulpiwat, C.; Numpaisal, P.; Ruksakulpiwat, Y. Electrospun Poly(lactic acid) and Silk Fibroin Based Nanofibrous Scaffold for Meniscus Tissue Engineering. Polymers 2022, 14, 2435. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, H.; Chen, Q.; Gang, H.; Zhou, Y.; Gu, S.; Liu, X.; Xu, W.; Zhang, B.; Yang, H. Polylactic acid scaffold with directional porous structure for large-segment bone repair. Int. J. Biol. Macromol. 2022, 216, 810–819. [Google Scholar] [CrossRef]
- Eftekhari-pournigjeh, F.; Saeed, M.; Rajabi, S.; Tamimi, M.; Pezeshki-Modaress, M. Three-dimensional biomimetic reinforced chitosan/gelatin composite scaffolds containing PLA nano/microfibers for soft tissue engineering application. Int. J. Biol. Macromol. 2023, 225, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Ning, F.; Wang, Y. Additive manufacturing of biodegradable iron-based particle reinforced polylactic acid composite scaffolds for tissue engineering. J. Mater. Process. Technol. 2021, 289, 116952. [Google Scholar] [CrossRef]
- Liu, R.T.; Zhang, S.Y.; Zhao, C.; Yang, D.; Cui, T.T.; Liu, Y.D.; Min, Y.G. Regulated Surface Morphology of Polyaniline/Polylactic Acid Composite Nanofibers via Various Inorganic Acids Doping for Enhancing Biocompatibility in Tissue Engineering. Nanoscale Res. Lett. 2021, 16, 4. [Google Scholar] [CrossRef]
- Oktay, B.; Ahlatcıoğlu Özerol, E.; Sahin, A.; Gunduz, O.; Ustundag, C. Production and characterization of PLA/HA/GO nanocomposite scaffold. Chem. Sel. 2022, 7, e202200697. [Google Scholar] [CrossRef]
- Bogdanova, A.; Pavlova, E.; Polyanskaya, A.; Volkova, M.; Biryukova, E.; Filkov, G.; Trofimenko, A.; Durymanov, M.; Klinov, D.; Bagrov, D. Acceleration of Electrospun PLA Degradation by Addition of Gelatin. Int. J. Mol. Sci. 2023, 24, 3535. [Google Scholar] [CrossRef] [PubMed]
- Kovaleva, P.A.; Pariy, I.O.; Chernozem, R.V.; Zadorozhnyy, M.Y.; Permyakova, E.S.; Kolesnikov, E.A.; Surmeneva, M.A.; Surmenev, R.A.; Senatov, F.S. Shape memory effect in hybrid polylactide-based polymer scaffolds functionalized with reduced graphene oxide for tissue engineering. Eur. Polym. J. 2022, 181, 111694. [Google Scholar] [CrossRef]
- Orafa, Z.; Irani, S.; Zamanian, A.; Bakhshi, H.; Nikukar, H.; Ghalandari, B. Coating of Laponite on PLA Nanofibrous for Bone Tissue Engineering Application. Macromol. Res. 2021, 29, 191–198. [Google Scholar] [CrossRef]
- Shan, X.; Huang, P.; Yang, L.; Feng, R.; Wang, Z. Robust polypropylene/ethylene-propylene-diene terpolymer thermoplastic vulcanizates film for green oil-water separation. J. Polym. Res. 2022, 29, 94. [Google Scholar] [CrossRef]
- Yin, Z.; Pan, Y.; Bao, M.; Li, Y. Superhydrophobic magnetic cotton fabricated under low carbonization temperature for effective oil/water separation. Sep. Purif. Technol. 2021, 266, 118535. [Google Scholar] [CrossRef]
- Zeng, X.; Zhao, L.; Fan, G.; Yan, C. Experimental study on the design of light phase outlets for a novel axial oil-water separator. Chem. Eng. Res. Des. 2021, 165, 308–319. [Google Scholar] [CrossRef]
- Yin, C.; Meng, Z. Evaluation of oil-water sepa tion performance of photocatalytic self-cleaning chitosan/aminated graphene/TiO2 superhydrophobic mesh membrane. Fresenius Environ.Bull. 2022, 31, 238–248. [Google Scholar]
- Mittag, A.; Rahman, M.M.; Hafez, I.; Tajvidi, M. Development of Lignin-Containing Cellulose Nanofibrils Coated Paper-Based Filters for Effective Oil-Water Separation. Membranes 2022, 13, 1. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, L.; Yuan, W. A superhydrophobic poly (lactic acid) electrospun nanofibrous membrane surface-functionalized with TiO2 nanoparticles and methyltrichlorosilane for oil/water separation and dye adsorption. New J. Chem. 2019, 43, 15823–15831. [Google Scholar] [CrossRef]
- Sun, X.; Xue, B.; Tian, Y.; Qin, S.; Xie, L. 3D porous poly (l-lactic acid) materials with controllable multi-scale microstructures and their potential application in oil-water separation. Appl. Surf. Sci. 2018, 462, 633–640. [Google Scholar] [CrossRef]
- Eang, C.; Opaprakasit, P. Electrospun Nanofibers with Superhydrophobicity Derived from Degradable Polylactide for Oil/Water Separation Applications. J. Polym. Environ. 2020, 28, 1484–1491. [Google Scholar] [CrossRef]
- Du, G.; Duan, Y.; Yuan, Q.; Hu, S. Preparation of PLA/rGO nanofiber membrane by electrospinning method and its application in oil-water separation. J. Funct. Mater. 2022, 53, 3162–3166. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, X.; Xue, B.; Zhou, Y.; Xie, L.; Zheng, Q. Carbon quantum dots-driven surface morphology transformation towards superhydrophobic poly(lactic acid) film. Colloids Surf. A 2023, 656, 130547. [Google Scholar] [CrossRef]
- Zeng, Q.; Ma, P.; Su, X.; Lai, D.; Lai, X.; Zeng, X.; Li, H. Facile fabrication of superhydrophobic and magnetic poly (lactic acid) nonwoven fabric for oil–water separation. Ind. Eng. Chem. 2020, 59, 9127–9135. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, H.; Chen, Z.; Chen, N.; Pang, X.; Zhang, L.; Minari, T.; Liu, X.; Liu, H.; Chen, J.; et al. Recyclable oil-absorption foams via secondary phase separation. ACS Sustain. Chem. Eng. 2018, 6, 13834–13843. [Google Scholar] [CrossRef]
- Zhu, C.; Jiang, W.; Hu, J.; Sun, P.; Li, A.; Zhang, Q. Polylactic acid nonwoven fabric surface modified with stereocomplex crystals for recyclable use in oil/water separation. ACS Appl. Polym. Mater. 2020, 2, 2509–2516. [Google Scholar] [CrossRef]
- Wang, X.L.; Pan, Y.M.; Liu, X.H.; Liu, H.; Li, N.; Liu, C.T.; Schubert, D.W.; Shen, C.Y. Facile Fabrication of Superhydrophobic and Eco-Friendly Poly(lactic acid) Foam for Oil-Water Separation via Skin Peeling. ACS Appl. Mater. Interfaces 2019, 11, 14362–14367. [Google Scholar] [CrossRef]
- Mo, J.; Wang, Y.; Wang, J.; Zhao, J.; Ke, Y.; Han, S.; Gan, F.; Wang, L.; Ma, C. Hydrophobic/oleophilic polylactic acid electrospun fibrous membranes with the silicone semi-interpenetrated networks for oil-water separation. J. Mater. Sci. 2022, 57, 16048–16063. [Google Scholar] [CrossRef]
- Liu, W.; Wu, X.; Liu, S.; Cheng, X.; Zhang, C. CNT@LDH functionalized poly(lactic acid) membranes with super oil-water separation and real-time press sensing properties. Polym. Compos. 2022, 43, 6548–6559. [Google Scholar] [CrossRef]
- Nugraha, M.W.; Wirzal, M.D.H.; Ali, F.; Roza, L.; Sambudi, N.S. Electrospun polylactic acid/tungsten oxide/amino-functionalized carbon quantum dots (PLA/WO3/N-CQDs) fibers for oil/water separation and photocatalytic decolorization. J. Environ. Sci. Chem. Eng. 2021, 9, 106033. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Fu, Y. 3D hierarchical biobased gel electrolyte with superior ionic conductivity and flame resistance for suppressing lithium dendrites via alloying and sieving mechanisms. Composites Part B 2022, 230, 109501. [Google Scholar] [CrossRef]
- Khan, N.M.; Kufian, M.Z.; Samsudin, A.S. The Correlation of Free Ions with the Conduction Phase of 1-Ethyl-3-methylimidazolium Chloride in Gel Polymer Electrolyte-Based PMMA/PLA Blend Doped with LiBOB. J. Electron. Mater. 2023, 52, 4247–4260. [Google Scholar] [CrossRef]
- Wan, Y.; Yang, S.; Peng, M.; Gama, M.; Yang, Z.; Deng, X.; Zhou, J.; Ouyang, C.; Luo, H. Controllable synthesis of biomimetic nano/submicro-fibrous tubes for potential small-diameter vascular grafts. J Mater Chem B 2020, 8, 5694–5706. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Hang, C.; Ding, L.; Jia, L.; Tang, L.; Mou, L.; Qi, J.; Dong, R.; Zheng, W.; Zhang, Y.; et al. Electronic Blood Vessel. Matter 2020, 3, 1664–1684. [Google Scholar] [CrossRef]
- Domalik-Pyzik, P.; Morawska-Chochól, A. Preliminary Results on Heparin-Modified Double-Layered PCL and PLA-Based Scaffolds for Tissue Engineering of Small Blood Vessels. J. Funct. Biomater. 2022, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Khalifehzadeh, R.; Ciridon, W.; Ratner, B.D. Surface fluorination of polylactide as a path to improve platelet associated hemocompatibility. Acta Biomater. 2018, 78, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Verduzco, A.A.; Castillo-Ortega, M.M.; Chan-Chan, L.H.; Silva-Campa, E.; Galaz-Méndez, R.; Vera-Graziano, R.; Encinas-Encinas, J.C.; Del Castillo-Castro, T.; Rodríguez-Félix, D.E.; Santacruz-Ortega, H. Electrospun tubes based on PLA, gelatin and genipin in different arrangements for blood vessel tissue engineering. Polym. Bull. 2020, 77, 5985–6003. [Google Scholar] [CrossRef]
- Medina-Gonzalez, Y.; Aimar, P.; Lahitte, J.-F.; Remigy, J. Towards green membranes: Preparation of cellulose acetate ultrafiltration membranes using methyl lactate as a biosolvent. Int. J. Sustain. Eng. 2011, 4, 75–83. [Google Scholar] [CrossRef]
- Ratti, R. Ionic liquids: Synthesis and applications in catalysis. Adv. Chem 2014, 2014, 729842. [Google Scholar] [CrossRef]
- Rogers, R.D.; Seddon, K.R. Ionic liquids—Solvents of the future? Science 2003, 302, 792–793. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).