Evaluation of PLA-Based Composite Films Filled with Cu2(OH)3NO3 Nanoparticles as an Active Material for the Food Industry: Biocidal Properties and Environmental Sustainability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. CuHS Synthesis
2.2.2. Preparation of PLA/CuHS Composite Films
2.2.3. PLA/CuHS Composite Films Characterization
2.2.4. Antibacterial Activity of the Composite Films against Food Microbiota
2.2.5. Migration Assay and Swelling
2.2.6. Cytotoxicity Assay
2.2.7. Disintegration Test under Aerobic Composting Conditions
2.3. Statistical Analysis
3. Results and Discussion
3.1. PLA/CuHS Composite Films Characterization
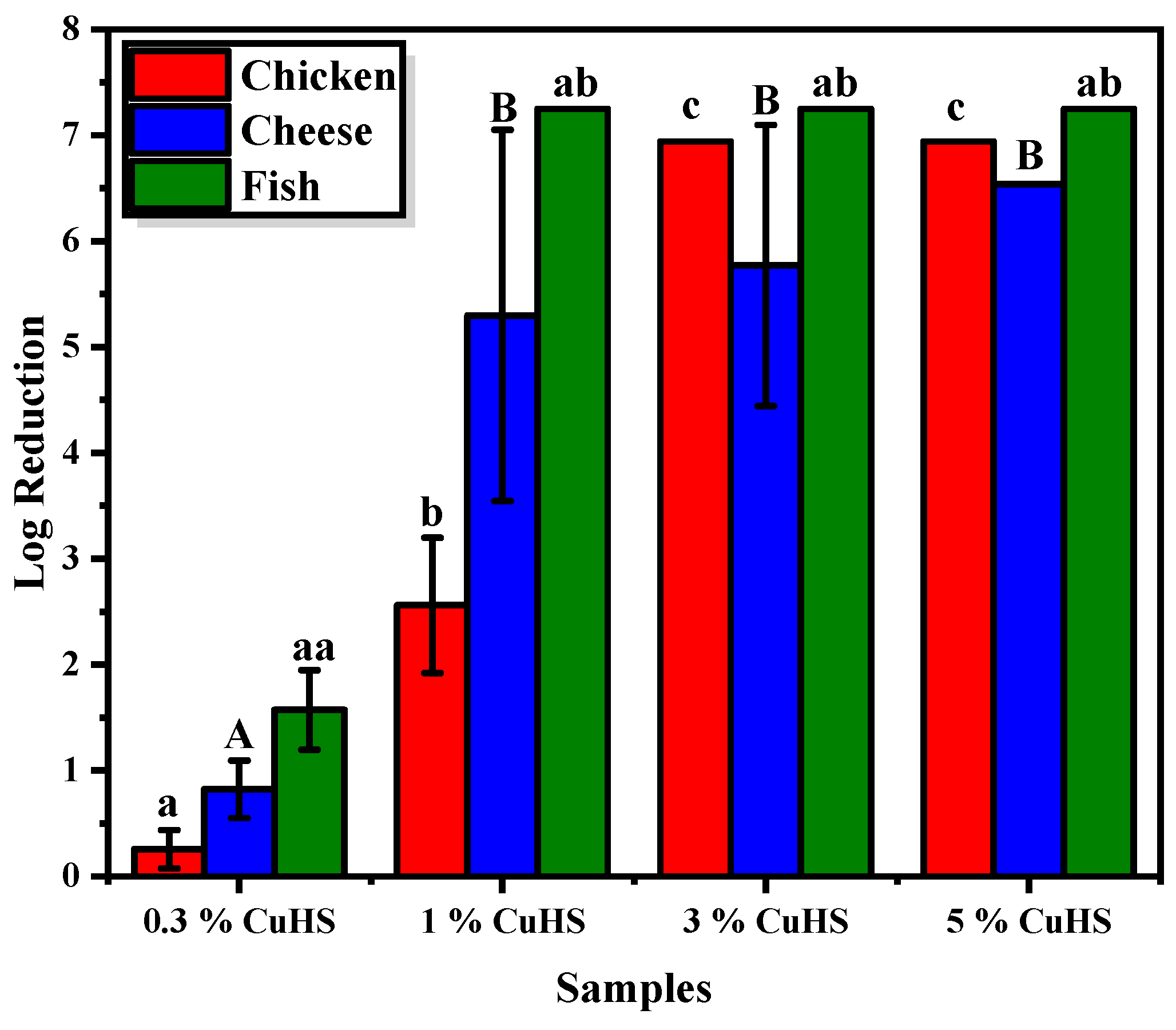
3.2. Antibacterial Capacity of the Composite Films against Food Microbiota
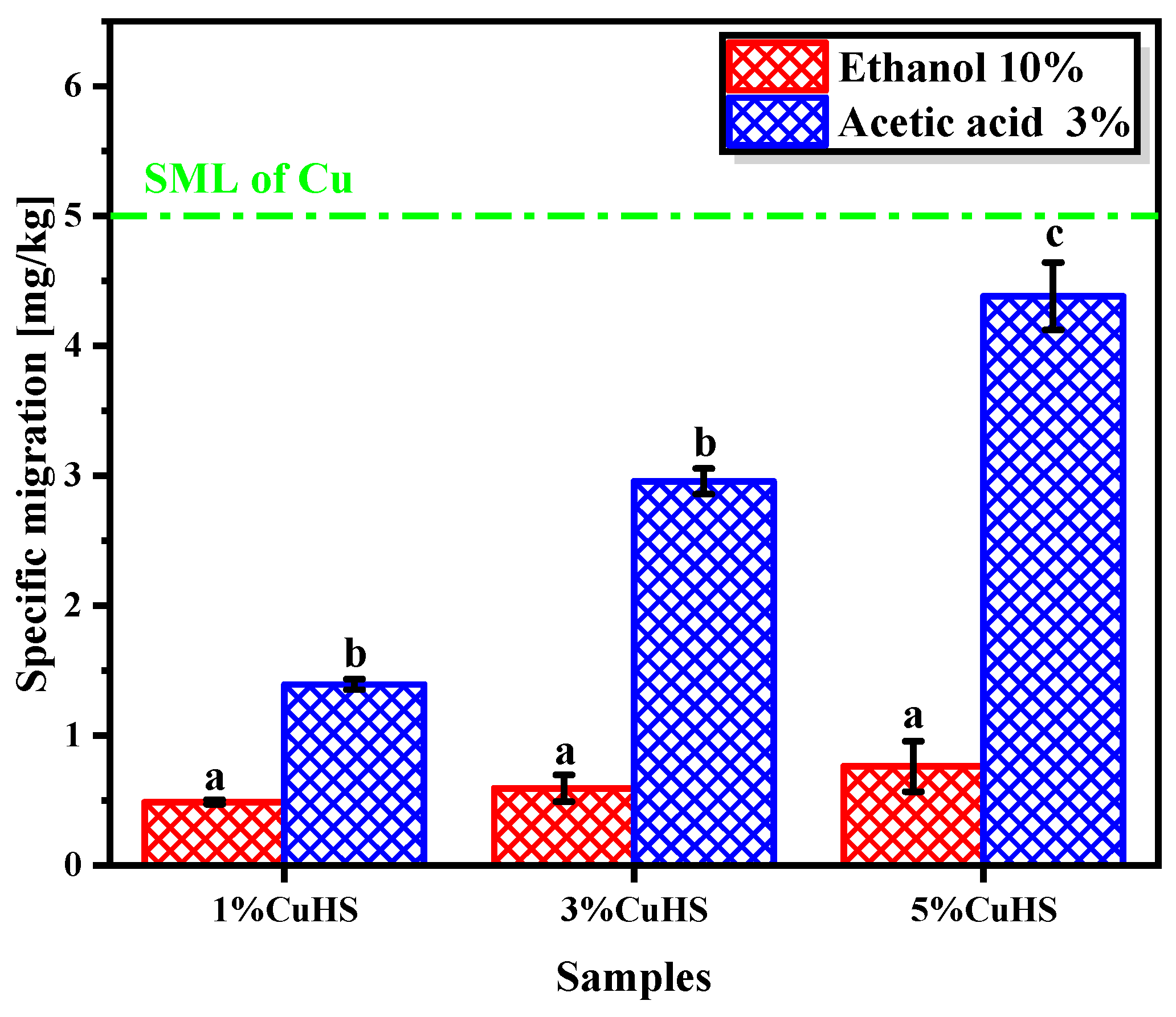
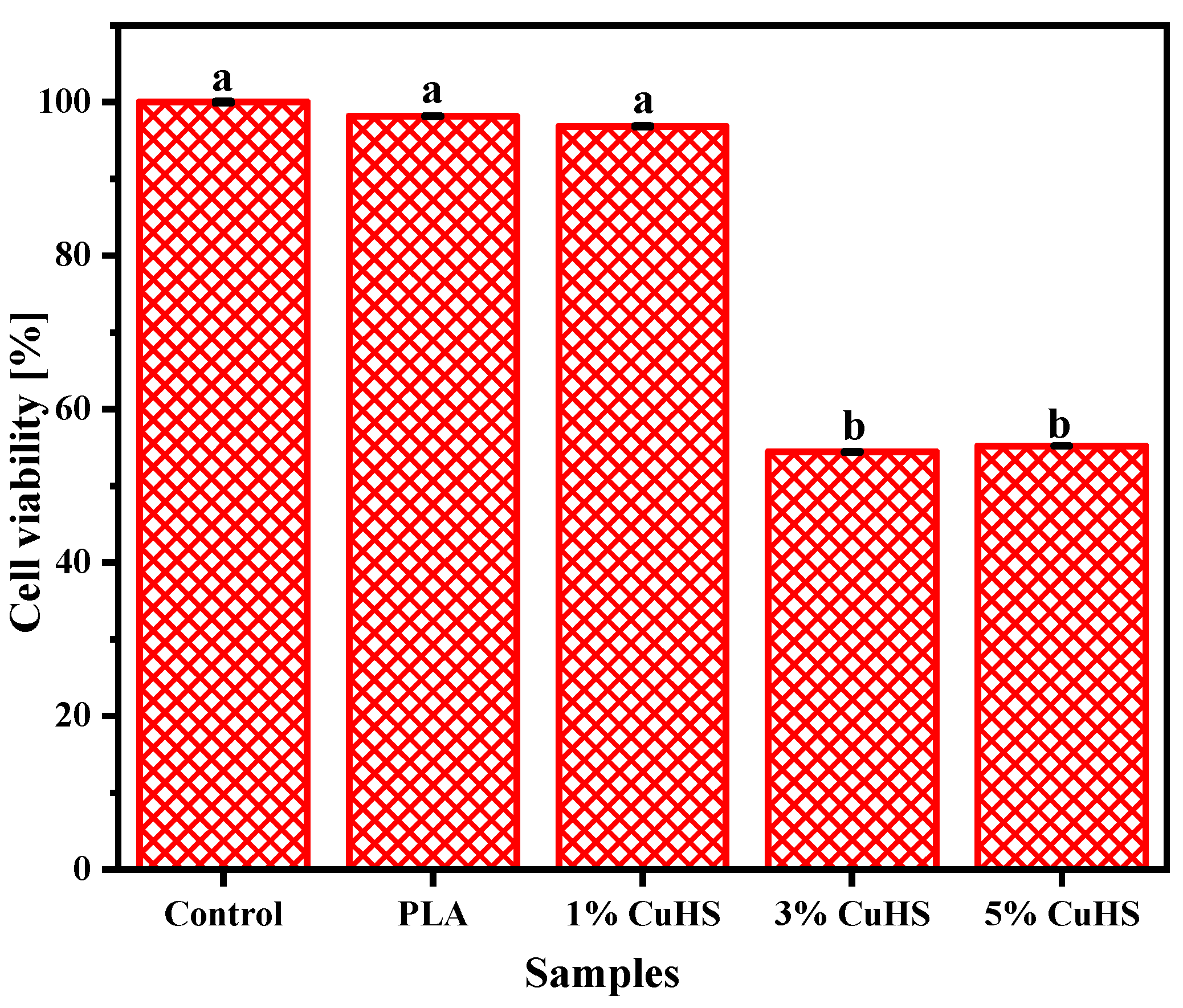
3.3. Cu (II) Migration, Swelling and Cytotoxicity of the Composite Films
3.4. Biodegradation Assessment under Composting Conditions
3.4.1. Disintegration Process
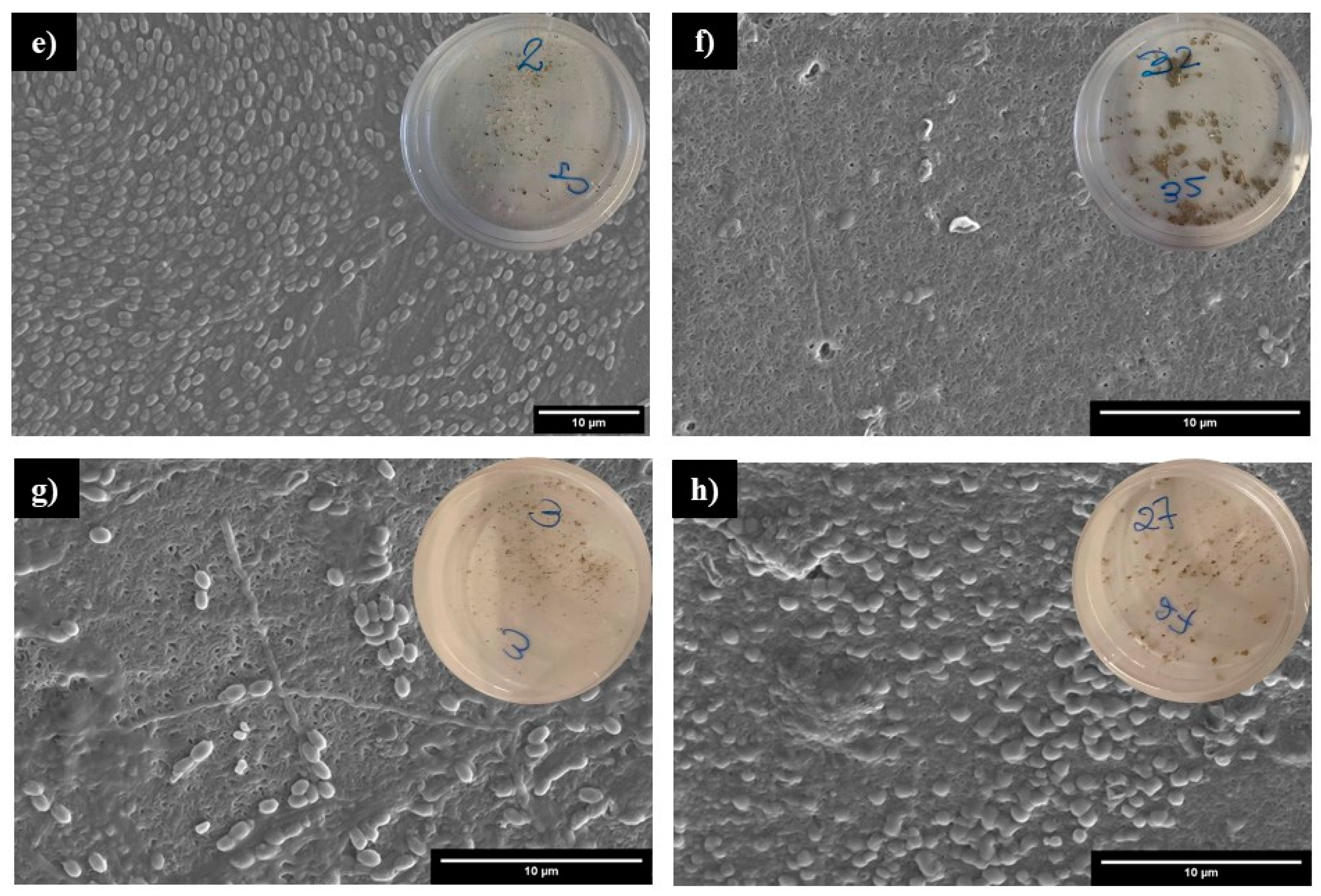
3.4.2. Characterization of Plastic Residue
3.4.3. Compost Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M.D. Biodegradable Polymers for Food Packaging: A Review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Luo, Y.; Lin, Z.; Guo, G. Biodegradation Assessment of Poly (Lactic Acid) Filled with Functionalized Titania Nanoparticles (PLA/TiO2) under Compost Conditions. Nanoscale Res. Lett. 2019, 14, 56. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of Food and Agriculture 2019. Moving Forward on Food Loss and Waste Reduction; FAO: Rome, Italy, 2019. [Google Scholar]
- United Nations Environment Programme. Food Waste Index Report 2021; United Nations Environment Programme: Nairobi, Kenya, 2021. [Google Scholar]
- Santos, X.; Rodríguez, J.; Guillén, F.; Pozuelo, J.; Molina-Guijarro, J.M.; Videira-Quintela, D.; Martín, O. Capability of Copper Hydroxy Nitrate (Cu2(OH)3NO3) as an Additive to Develop Antibacterial Polymer Contact Surfaces: Potential for Food Packaging Applications. Polymers 2023, 15, 1661. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, S.; Ahari, H.; Sahraeyan, R. The Effect of Silver Nanocomposite Packaging Based on Melt Mixing and Sol–Gel Methods on Shelf Life Extension of Fresh Chicken Stored at 4 °C. J. Food Saf. 2019, 39, e12625. [Google Scholar] [CrossRef]
- Moreira, M.d.R.; Pereda, M.; Marcovich, N.E.; Roura, S.I. Antimicrobial Effectiveness of Bioactive Packaging Materials from Edible Chitosan and Casein Polymers: Assessment on Carrot, Cheese, and Salami. J. Food Sci. 2011, 76, M54–M63. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Xue, L.; Duraiarasan, S.; Haiying, C. Preparation of ε-Polylysine/Chitosan Nanofibers for Food Packaging against Salmonella on Chicken. Food Packag. Shelf Life 2018, 17, 134–141. [Google Scholar] [CrossRef]
- Beigmohammadi, F.; Peighambardoust, S.H.; Hesari, J.; Azadmard-Damirchi, S.; Peighambardoust, S.J.; Khosrowshahi, N.K. Antibacterial Properties of LDPE Nanocomposite Films in Packaging of UF Cheese. LWT 2016, 65, 106–111. [Google Scholar] [CrossRef]
- Widsten, P.; Mesic, B.B.; Cruz, C.D.; Fletcher, G.C.; Chycka, M.A. Inhibition of Foodborne Bacteria by Antibacterial Coatings Printed onto Food Packaging Films. J. Food Sci. Technol. 2017, 54, 2379–2386. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Rhim, J.W. Preparation and Characterization of Agar/Lignin/Silver Nanoparticles Composite Films with Ultraviolet Light Barrier and Antibacterial Properties. Food Hydrocoll. 2017, 71, 76–84. [Google Scholar] [CrossRef]
- Longano, D.; Ditaranto, N.; Cioffi, N.; Di Niso, F.; Sibillano, T.; Ancona, A.; Conte, A.; Del Nobile, M.A.; Sabbatini, L.; Torsi, L. Analytical Characterization of Laser-Generated Copper Nanoparticles for Antibacterial Composite Food Packaging. Anal. Bioanal. Chem. 2012, 403, 1179–1186. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Sarbon, N.M. A Comparative Study: Physical, Mechanical and Antibacterial Properties of Bio-Composite Gelatin Films as Influenced by Chitosan and Zinc Oxide Nanoparticles Incorporation. Food Biosci. 2021, 43, 101250. [Google Scholar] [CrossRef]
- Jing, F.; Suo, H.; Cui, S.; Tang, X.; Zhang, M.; Shen, X.; Lin, B.; Jiang, G.; Wu, X. Facile Synthesis of TiO2/Ag Composite Aerogel with Excellent Antibacterial Properties. J. Solgel. Sci. Technol. 2018, 86, 590–598. [Google Scholar] [CrossRef]
- Gordon, T.; Perlstein, B.; Houbara, O.; Felner, I.; Banin, E.; Margel, S. Synthesis and Characterization of Zinc/Iron Oxide Composite Nanoparticles and Their Antibacterial Properties. Colloids Surf. A Physicochem. Eng. Asp. 2011, 374, 1–8. [Google Scholar] [CrossRef]
- Salmas, C.E.; Giannakas, A.E.; Baikousi, M.; Kollia, E.; Tsigkou, V.; Proestos, C. Effect of Copper and Titanium-Exchanged Montmorillonite Nanostructures on the Packaging Performance of Chitosan/Poly-Vinyl-Alcohol-Based Active Packaging Nanocomposite Films. Foods 2021, 10, 3038. [Google Scholar] [CrossRef] [PubMed]
- Shabestari, M.E.; Martín, O.; Díaz-García, D.; Gómez-Ruiz, S.; Gonzalez, V.J.; Baselga, J. Facile and Rapid Decoration of Graphene Oxide with Copper Double Salt, Oxides and Metallic Copper as Catalysts in Oxidation and Coupling Reactions. Carbon N. Y. 2020, 161, 7–16. [Google Scholar] [CrossRef]
- Santos, X.; Álvarez, M.; Videira-Quintela, D.; Mediero, A.; Rodríguez, J.; Guillén, F.; Pozuelo, J.; Martín, O. Antibacterial Capability of MXene (Ti3C2Tx) to Produce PLA Active Contact Surfaces for Food Packaging Applications. Membranes 2022, 12, 1146. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.R.; Ponce, A.G.; Del Valle, C.E.; Roura, S.I. Effects of clove and tea tree oils on Escherichia coli O157:H7 in blanched spinach and minced cooked beef. JFP 2007, 75, 488–496. [Google Scholar] [CrossRef]
- Videira-Quintela, D.; Guillén, F.; Martin, O.; Montalvo, G. Antibacterial LDPE Films for Food Packaging Application Filled with Metal-Fumed Silica Dual-Side Fillers. Food Packag. Shelf Life 2022, 31, 100772. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food. Off. J. Eur. Union 2011, 12, 1–89. [Google Scholar]
- UNE-EN ISO 20200:2004; Plastics. Determination of the degree of disintegration of plastic materials under simulated composting conditions in a laboratory-scale test (ISO 20200:2004). Asociación Española de Normalización: Madrid, Spain, 2004.
- Gong, X.; Pan, L.; Tang, C.Y.; Chen, L.; Hao, Z.; Law, W.C.; Wang, X.; Tsui, C.P.; Wu, C. Preparation, Optical and Thermal Properties of CdSe-ZnS/Poly(Lactic Acid) (PLA) Nanocomposites. Compos. B Eng. 2014, 66, 494–499. [Google Scholar] [CrossRef]
- Ikram, H.; Al Rashid, A.; Koç, M. Synthesis and Characterization of Hematite (α-Fe2O3) Reinforced Polylactic Acid (PLA) Nanocomposites for Biomedical Applications. Compos. Part C Open Access 2022, 9, 100331. [Google Scholar] [CrossRef]
- Jonoobi, M.; Harun, J.; Mathew, A.P.; Oksman, K. Mechanical Properties of Cellulose Nanofiber (CNF) Reinforced Polylactic Acid (PLA) Prepared by Twin Screw Extrusion. Compos. Sci. Technol. 2010, 70, 1742–1747. [Google Scholar] [CrossRef]
- Petersson, L.; Oksman, K. Biopolymer Based Nanocomposites: Comparing Layered Silicates and Microcrystalline Cellulose as Nanoreinforcement. Compos. Sci. Technol. 2006, 66, 2187–2196. [Google Scholar] [CrossRef]
- Shirai, M.A.; Grossmann, M.V.E.; Mali, S.; Yamashita, F.; Garcia, P.S.; Müller, C.M.O. Development of Biodegradable Flexible Films of Starch and Poly(Lactic Acid) Plasticized with Adipate or Citrate Esters. Carbohydr. Polym. 2013, 92, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and Mechanical Properties of PLA, and Their Functions in Widespread Applications—A Comprehensive Review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Guinault, A.; Sollogoub, C.; Domenek, S.; Grandmontagne, A.; Ducruet, V. Influence of Crystallinity on Gas Barrier and Mechanical Properties of Pla Food Packaging Films. Int. J. Mater. Form. 2010, 3, 603–606. [Google Scholar] [CrossRef]
- Siracusa, V.; Blanco, I.; Romani, S.; Tylewicz, U.; Rocculi, P.; Rosa, M.D. Poly(Lactic Acid)-Modified Films for Food Packaging Application: Physical, Mechanical, and Barrier Behavior. J. Appl. Polym. Sci. 2012, 125, E390–E401. [Google Scholar] [CrossRef]
- Andrade, M.S.; Ishikawa, O.H.; Costa, R.S.; Seixas, M.V.S.; Rodrigues, R.C.L.B.; Moura, E.A.B. Development of sustainable food packaging material based on biodegradable polymer reinforced with cellulose nanocrystals. Food Packag. Shelf Life 2022, 31, 100807. [Google Scholar] [CrossRef]
- Valerini, D.; Tammaro, L.; Villani, F.; Rizzo, A.; Paolella, G.; Vigliotta, G.; Caputo, I. Antibacterial Al-doped ZnO coatings on PLA films. J. Mater. Sci. 2020, 55, 4830–4847. [Google Scholar] [CrossRef]
- Silva, F.; Domingues, F.C.; Nerín, C. Control Microbial Growth on Fresh Chicken Meat Using Pinosylvin Inclusion Complexes Based Packaging Absorbent Pads. LWT 2018, 89, 148–154. [Google Scholar] [CrossRef]
- Mild, R.M.; Joens, L.A.; Friedman, M.; Olsen, C.W.; Mchugh, T.H.; Law, B.; Ravishankar, S. Antimicrobial Edible Apple Films Inactivate Antibiotic Resistant and Susceptible Campylobacter Jejuni Strains on Chicken Breast. J. Food Sci. 2011, 76, M163–M168. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Chen, Y.J.; Tseng, H.J.; Hsiao, H.I.; Chai, H.J.; Shang, K.C.; Pan, C.L.; Tsai, G.J. Applications of Nisin and Edta in Food Packaging for Improving Fabricated Chitosan-Polylactate Plastic Film Performance and Fish Fillet Preservation. Membranes 2021, 11, 852. [Google Scholar] [CrossRef] [PubMed]
- Govaris, A.; Botsoglou, E.; Sergelidis, D.; Chatzopoulou, P.S. Antibacterial Activity of Oregano and Thyme Essential Oils against Listeria Monocytogenes and Escherichia Coli O157:H7 in Feta Cheese Packaged under Modified Atmosphere. LWT 2011, 44, 1240–1244. [Google Scholar] [CrossRef]
- Requena, R.; Vargas, M.; Chiralt, A. Eugenol and Carvacrol Migration from PHBV Films and Antibacterial Action in Different Food Matrices. Food Chem. 2019, 277, 38–45. [Google Scholar] [CrossRef]
- Collins, C.H.; Lyne, P.M.; Grange, J.M.; Falkinham, J.O. Microbiological Methods Collins and Lyne’s, 8th ed.; Arnold: London, UK, 2004. [Google Scholar]
- Lejon, D.P.H.; Pascault, N.; Ranjard, L. Differential Copper Impact on Density, Diversity and Resistance of Adapted Culturable Bacterial Populations According to Soil Organic Status. Eur. J. Soil. Biol. 2010, 46, 168–174. [Google Scholar] [CrossRef]
- Vasiliev, G.; Kubo, A.L.; Vija, H.; Kahru, A.; Bondar, D.; Karpichev, Y.; Bondarenko, O. Synergistic Antibacterial Effect of Copper and Silver Nanoparticles and Their Mechanism of Action. Sci. Rep. 2023, 13, 9202. [Google Scholar] [CrossRef] [PubMed]
- Videira-Quintela, D.; Guillén, F.; Montalvo, G.; Martin, O. Silver, Copper, and Copper Hydroxy Salt Decorated Fumed Silica Hybrid Composites as Antibacterial Agents. Colloids Surf. B Biointerfaces 2020, 195, 111216. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Scattareggia Marchese, A.; Destro, E.; Boselli, C.; Barbero, F.; Malandrino, M.; Cardeti, G.; Fenoglio, I.; Lanni, L. Inhibitory Effect against Listeria Monocytogenes of Carbon Nanoparticles Loaded with Copper as Precursors of Food Active Packaging. Foods 2022, 11, 2941. [Google Scholar] [CrossRef]
- Jastrzębska, A.M.; Szuplewska, A.; Wojciechowski, T.; Chudy, M.; Ziemkowska, W.; Chlubny, L.; Rozmysłowska, A.; Olszyna, A. In Vitro Studies on Cytotoxicity of Delaminated Ti3C2 MXene. J. Hazard. Mater. 2017, 339, 1–8. [Google Scholar] [CrossRef]
- Luzi, F.; Fortunati, E.; Puglia, D.; Petrucci, R.; Kenny, J.M.; Torre, L. Study of Disintegrability in Compost and Enzymatic Degradation of PLA and PLA Nanocomposites Reinforced with Cellulose Nanocrystals Extracted from Posidonia Oceanica. Polym. Degrad. Stab. 2015, 121, 105–115. [Google Scholar] [CrossRef]
- Zaaba, N.F.; Jaafar, M. A Review on Degradation Mechanisms of Polylactic Acid: Hydrolytic, Photodegradative, Microbial, and Enzymatic Degradation. Polym. Eng. Sci. 2020, 60, 2061–2075. [Google Scholar] [CrossRef]
- Avella, A.; Ruda, M.; Gioia, C.; Sessini, V.; Roulin, T.; Carrick, C.; Verendel, J.; Lo Re, G. Lignin Valorization in Thermoplastic Biomaterials: From Reactive Melt Processing to Recyclable and Biodegradable Packaging. Chem. Eng. J. 2023, 463, 142245. [Google Scholar] [CrossRef]
- Hernández-García, E.; Vargas, M.; González-Martínez, C.; Chiralt, A. Biodegradable Antimicrobial Films for Food Packaging: Effect of Antimicrobials on Degradation. Foods 2021, 10, 14. [Google Scholar] [CrossRef]
- Gorrasi, G.; Pantani, R. Effect of PLA Grades and Morphologies on Hydrolytic Degradation at Composting Temperature: Assessment of Structural Modification and Kinetic Parameters. Polym. Degrad. Stab. 2013, 98, 1006–1014. [Google Scholar] [CrossRef]
- Paul, M.A.; Delcourt, C.; Alexandre, M.; Degée, P.; Monteverde, F.; Dubois, P. Polylactide/Montmorillonite Nanocomposites: Study of the Hydrolytic Degradation. Polym. Degrad. Stab. 2005, 87, 535–542. [Google Scholar] [CrossRef]
- Akratos, C.S.; Tekerlekopoulou, A.G.; Vasiliadou, I.A.; Vayenas, D.V. Cocomposting of Olive Mill Waste for the Production of Soil Amendments. In Olive Mill Waste: Recent Advances for Sustainable Management; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 161–182. [Google Scholar] [CrossRef]



| Samples | PLA | PLA/CuHS 0.3% (w/w) | PLA/CuHS 1% (w/w) | PLA/CuHS 3% (w/w) | PLA/CuHS 5% (w/w) | |
|---|---|---|---|---|---|---|
| Parameters | ||||||
| Tg [°C] | 57 | 57 | 59 | 60 | 58 | |
| Tconset [°C] | 106 | 106 | 110 | 108 | 110 | |
| Tcon peak [°C] | 118 | 120 | 126 | 126 | 127 | |
| Tmonset [°C] | 142 | 143 | 147 | 146 | 146 | |
| Tmon peak [°C] | 148 | 149 | 151 | 152 | 152 | |
| ΔHc [J/g] | −16 | −17 | −7 | −4 | −4 | |
| ΔHm [J/g] | 16 | 17 | 6 | 3 | 4 | |
| Tdonset [°C] | 355 | 349 | 297 | 295 | 295 | |
| Tdon peak [°C] | 373 | 379 | 374 | 377 | 377 | |
| Ref. | This study | [5] | This study | |||
| Samples | σmax [MPa] | εmax [%] | E [GPa] |
|---|---|---|---|
| PLA | 40 ± 10 a | 15 ± 6 A | 2.5 ± 0.4 aa |
| PLA/CuHS 1% (w/w) | 70 ± 7 b | 12 ± 5 A | 3.5 ± 0.5 ab |
| PLA/CuHS 3% (w/w) | 65 ± 8 b | 16 ± 6 A | 3.4 ± 0.4 ab |
| PLA/CuHS 5% (w/w) | 65 ± 6 b | 15 ± 3 A | 3.5 ± 0.4 ab |
| Parameters | Tg [°C] | Tmonset [°C] | Tmon peak [°C] | ΔHm [J/g] | Tdon peak [°C] | |
|---|---|---|---|---|---|---|
| Samples | ||||||
| PLA d0 | 67 | 143 | 148 | 2 | 373 | |
| PLA d2 | 58 | 146 | 153 | 22 | 362 | |
| PLA d4 | 66 | 135 | 154 | 58 | 371 | |
| PLA d7 | - | 138 | 154 | 64 | 369 | |
| PLA/CuHS d0 | 67 | 145 | 152 | 3 | 374 | |
| PLA/CuHS d2 | 57 | 147 | 152 | 8 | 356 | |
| PLA/CuHS d4 | 67 | 137 | 154 | 53 | 367 | |
| PLA/CuHS d7 | 60 | 136 | 154 | 66 | 267.5 | |
| Samples | σmax [MPa] | εmax [%] | E [GPa] |
|---|---|---|---|
| PLA_d2 | 81 ± 13 a | 4 ± 1 A | 4.2 ± 0.5 aa |
| PLA_d4 | 13 ± 5 b | 1.0 ± 0.3 A | 1.4 ± 0.2 ab |
| PLA/CuHS_d2 | 27 ± 8 b | 7 ± 2 B | 1.3 ± 0.3 ab |
| PLA/CuHS_d4 | 11 ± 3 b | 1.7 ± 0.2 A | 1.0 ± 0.1 ab |
| Reactor | Dry massinitial [%] | Dry massfinal [%] | R [%] | C/Ninitial | C/Nfinal |
|---|---|---|---|---|---|
| Control | 72 ± 3 a | 66 ± 3 b | 38 ± 2 A | 31 ± 2 aa | 36 ± 3 aa |
| PLA | 72 ± 3 a | 68 ± 3 b | 34 ± 3 A | 31 ± 2 aa | 30 ± 9 aa |
| PLA/CuHS | 72 ± 3 a | 67 ± 4 b | 35 ± 3 A | 31 ± 2 aa | 30 ± 5 aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, X.; Domínguez, G.; Rodríguez, J.; Pozuelo, J.; Hernández, M.; Martín, O.; Fajardo, C. Evaluation of PLA-Based Composite Films Filled with Cu2(OH)3NO3 Nanoparticles as an Active Material for the Food Industry: Biocidal Properties and Environmental Sustainability. Polymers 2024, 16, 1772. https://doi.org/10.3390/polym16131772
Santos X, Domínguez G, Rodríguez J, Pozuelo J, Hernández M, Martín O, Fajardo C. Evaluation of PLA-Based Composite Films Filled with Cu2(OH)3NO3 Nanoparticles as an Active Material for the Food Industry: Biocidal Properties and Environmental Sustainability. Polymers. 2024; 16(13):1772. https://doi.org/10.3390/polym16131772
Chicago/Turabian StyleSantos, Xiomara, Gabriela Domínguez, Juana Rodríguez, Javier Pozuelo, Manuel Hernández, Olga Martín, and Carmen Fajardo. 2024. "Evaluation of PLA-Based Composite Films Filled with Cu2(OH)3NO3 Nanoparticles as an Active Material for the Food Industry: Biocidal Properties and Environmental Sustainability" Polymers 16, no. 13: 1772. https://doi.org/10.3390/polym16131772
APA StyleSantos, X., Domínguez, G., Rodríguez, J., Pozuelo, J., Hernández, M., Martín, O., & Fajardo, C. (2024). Evaluation of PLA-Based Composite Films Filled with Cu2(OH)3NO3 Nanoparticles as an Active Material for the Food Industry: Biocidal Properties and Environmental Sustainability. Polymers, 16(13), 1772. https://doi.org/10.3390/polym16131772







