Self-Organization of Polyurethane Ionomers Based on Organophosphorus-Branched Polyols
Abstract
1. Introduction
2. Materials and Methods
2.1. Solvents and Reagents
2.2. Synthetic Procedures
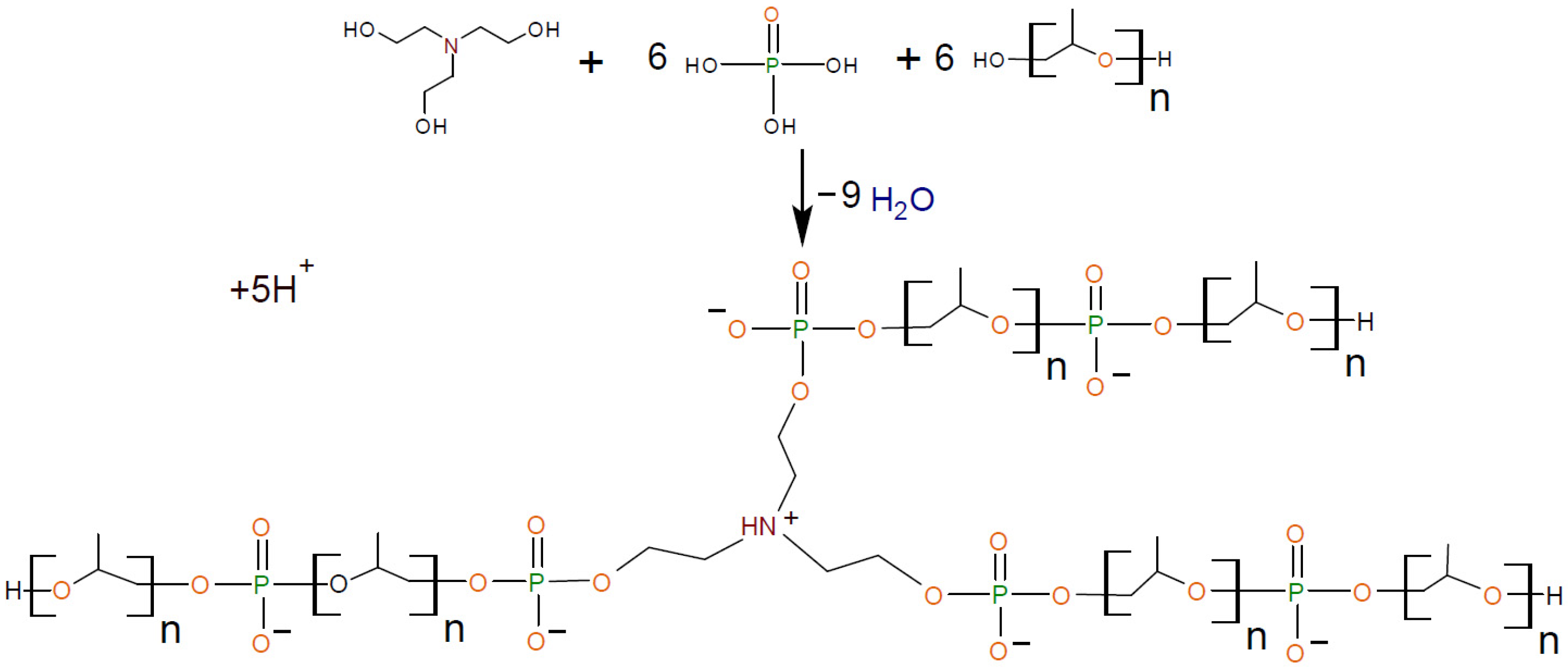
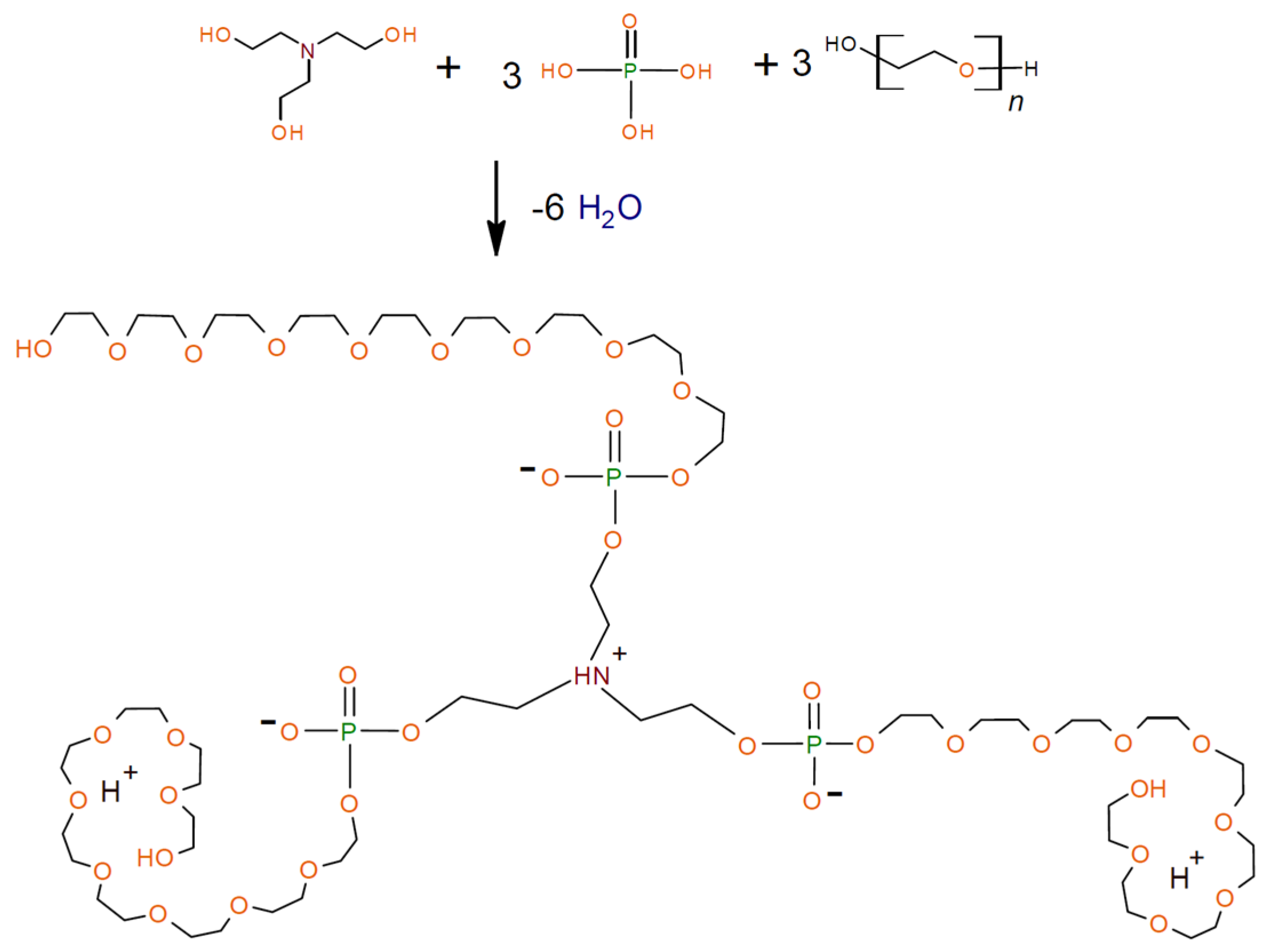
2.2.1. General Procedure for Synthesis of Aminoethers of Ortho-Phosphoric Acid (AEPA-PEG)
2.2.2. General Procedure for Synthesis of Polyurethanes Based on Aminoethers of Ortho-Phosphoric (AEPA-(2–9)-PEG-PU)
2.3. Measurements
2.3.1. Determination of the Content of Ortho-Phosphoric Acid by Titration
2.3.2. NMR Spectroscopy
2.3.3. Light-Scattering of Solution
3. Results
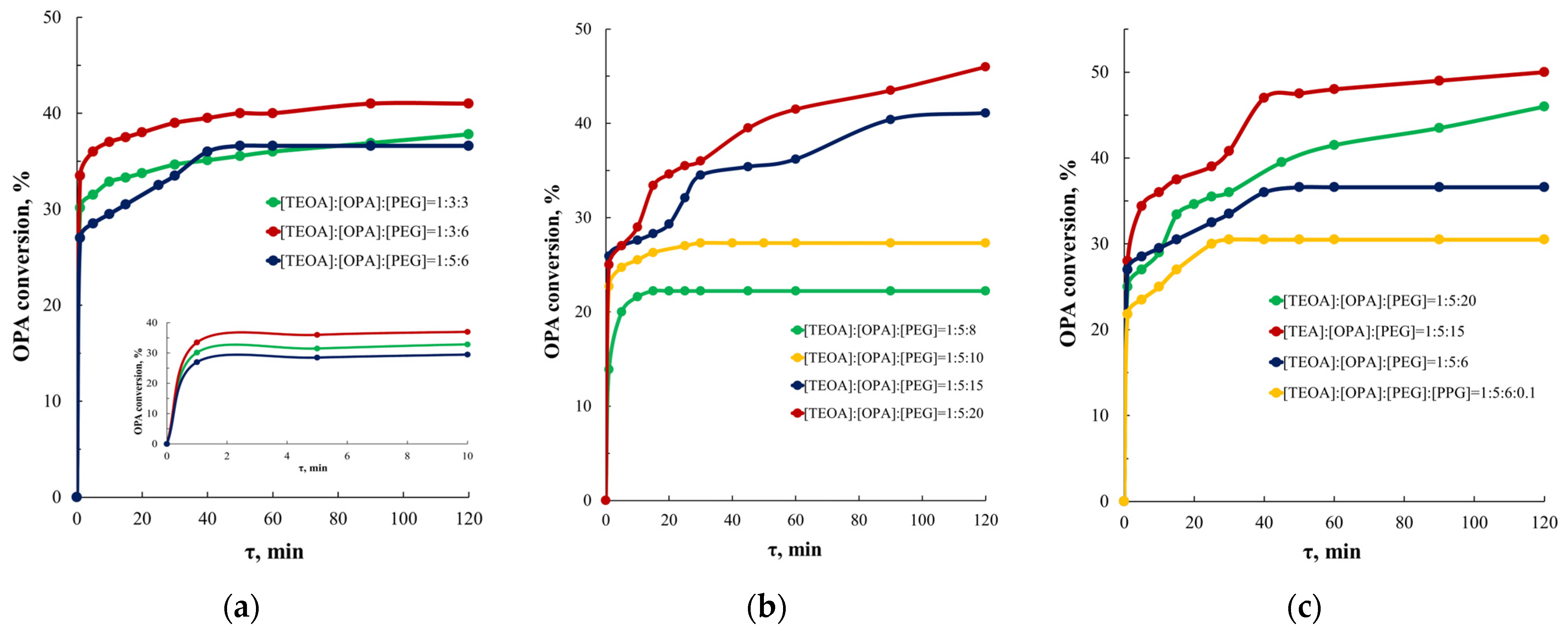
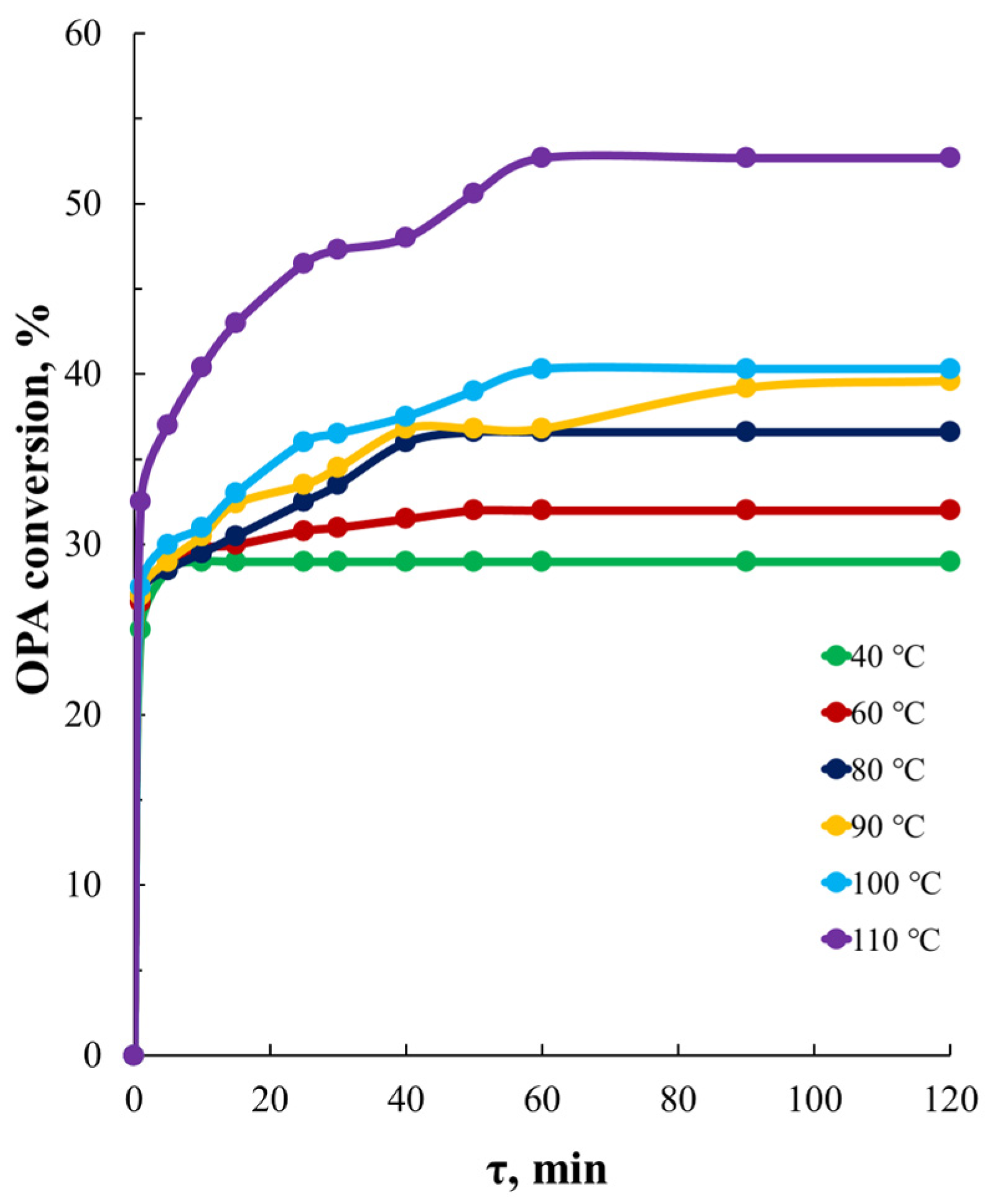
3.1. Study of the OPA Etherification
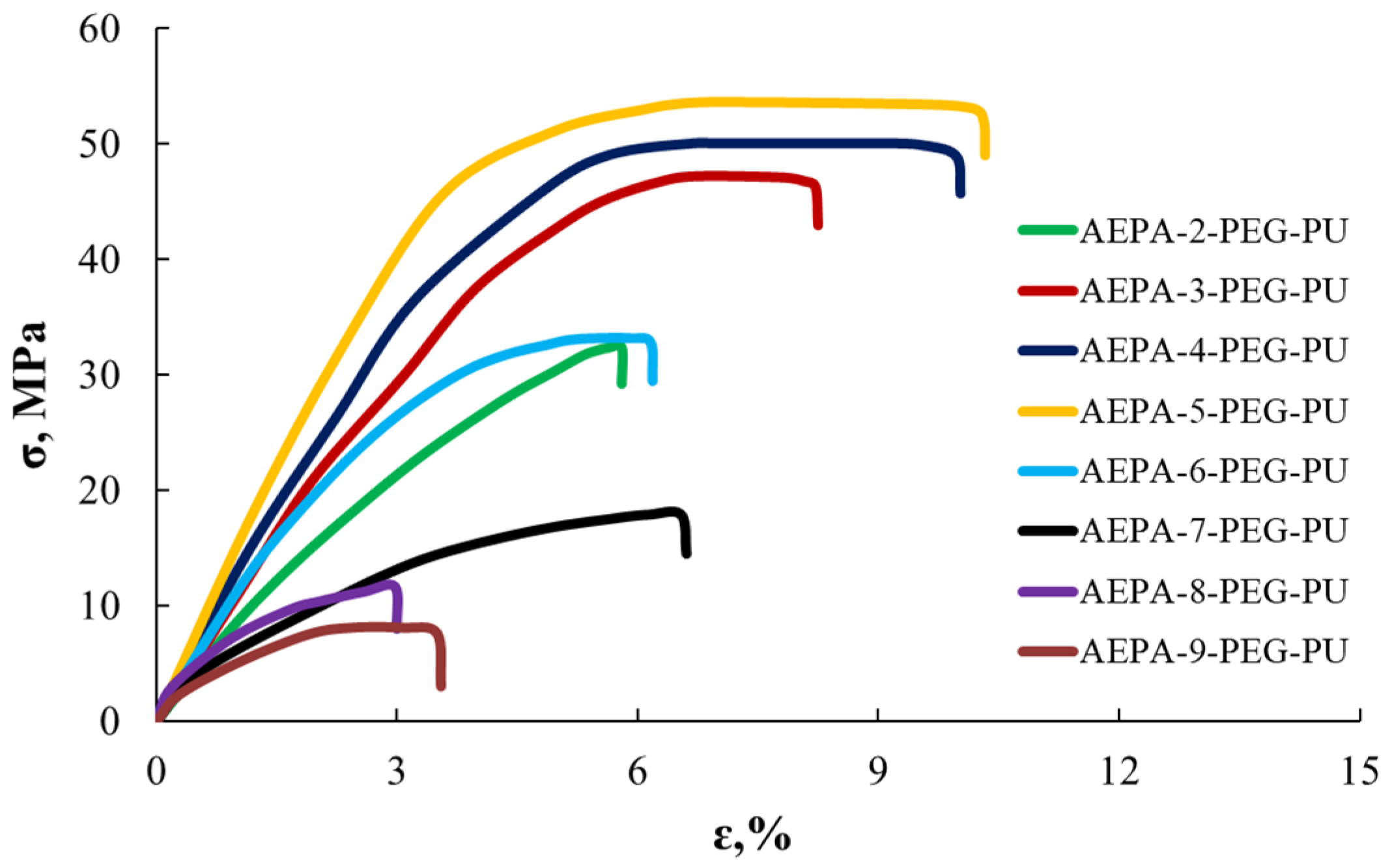
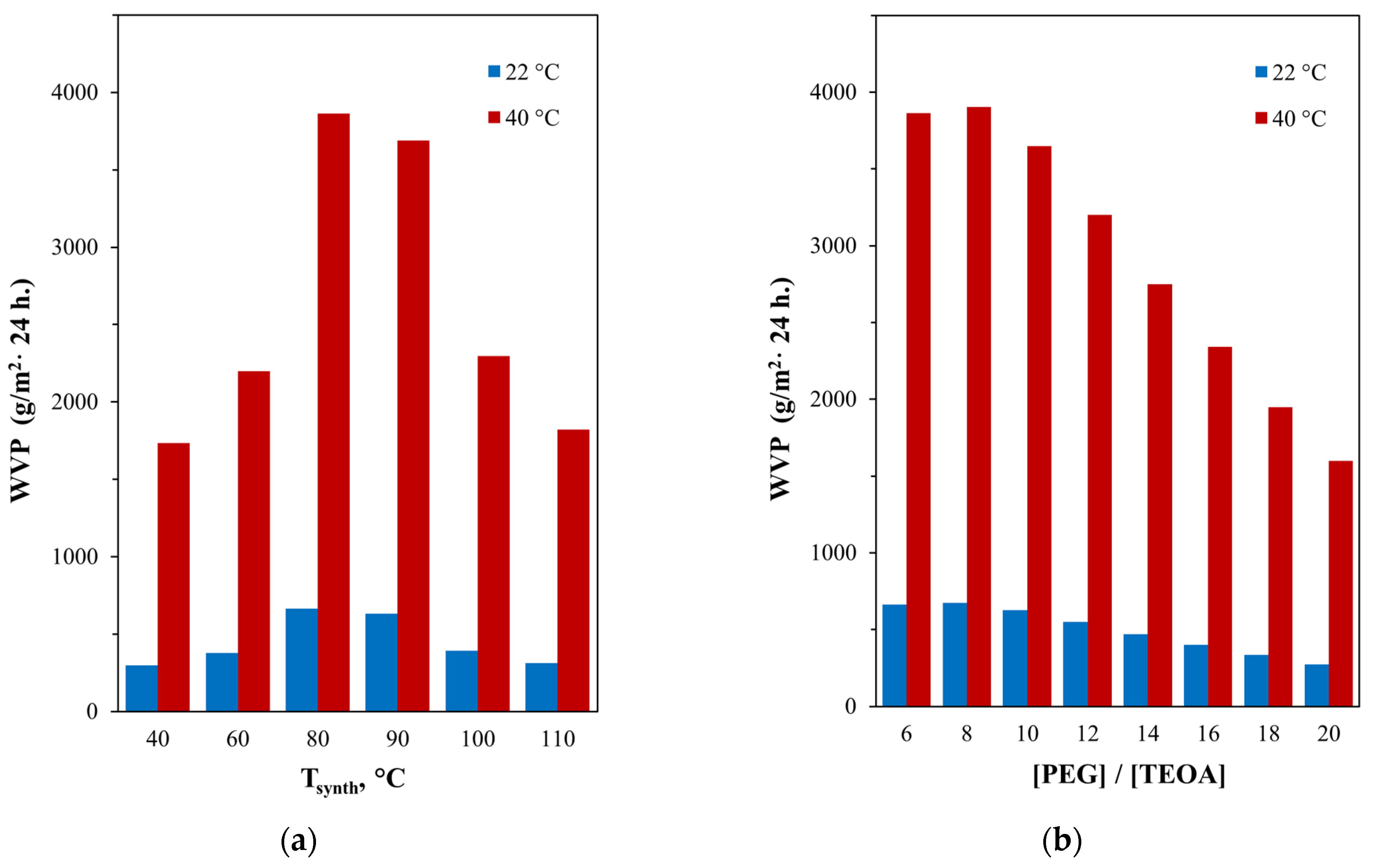
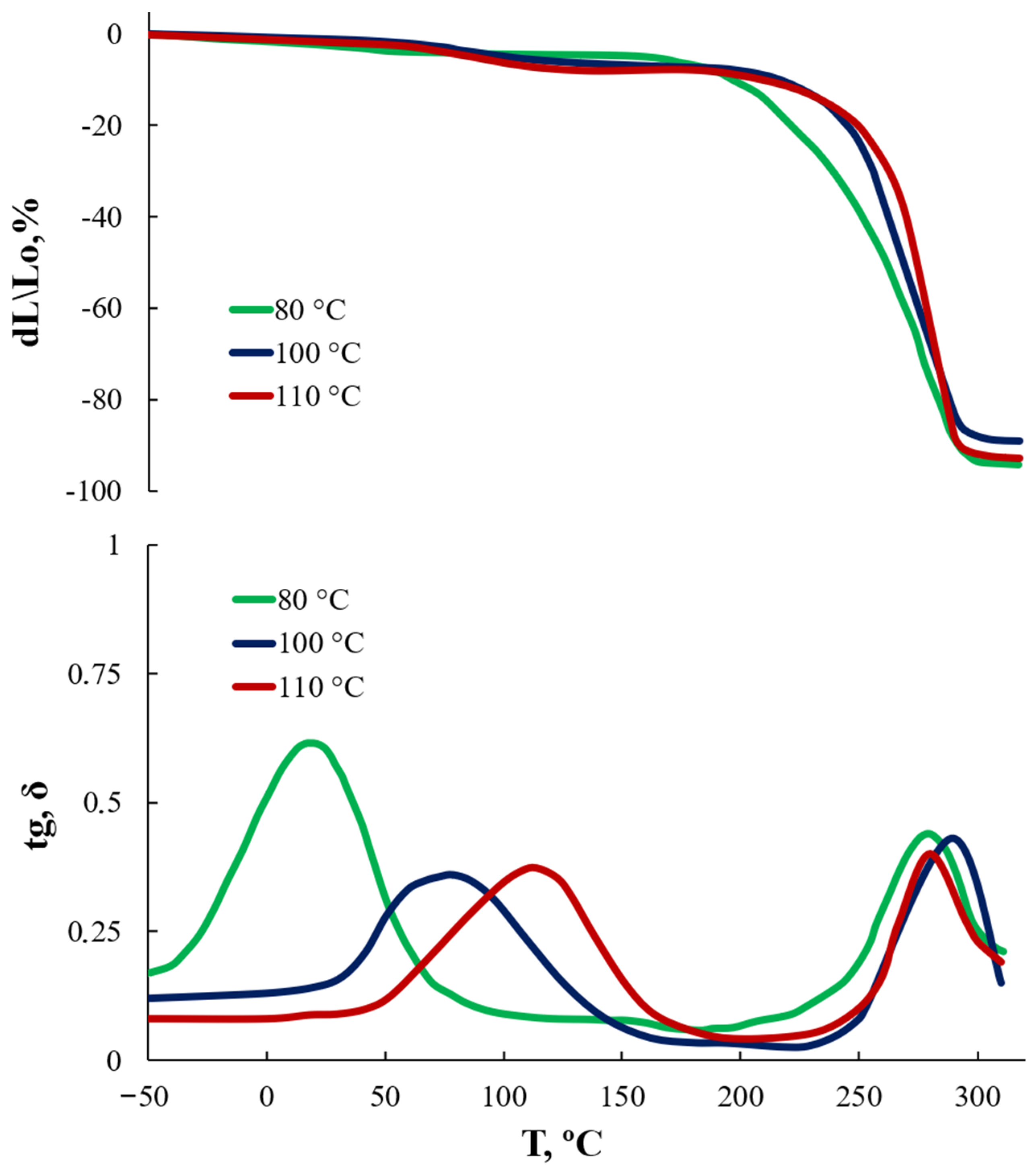
3.2. Effect of the AEPA-5-PEG Synthesis Temperature on the Some AEPA-5-PEG-PU Properties
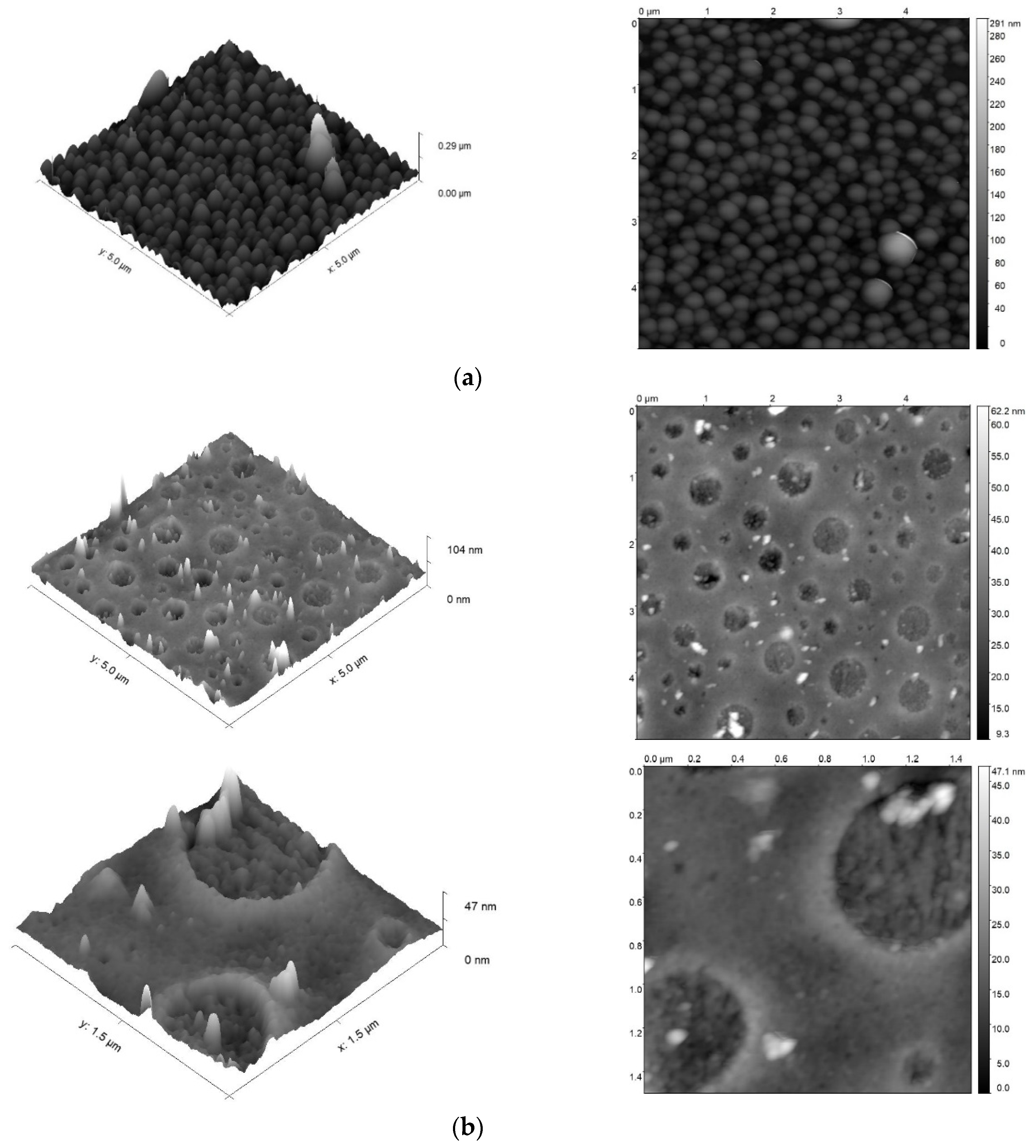
3.3. Comparison of Surface Morphology of AEPA-6-PPG-PU and AEPA-5-PEG-PU
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Das, A.; Mahanwar, P. A brief discussion on advances in polyurethane applications. Adv. Ind. Eng. Polym. Res. 2020, 3, 93–101. [Google Scholar] [CrossRef]
- Ahmadi, Y.; Kim, K.-H.; Tabatabaei, M. Recent advances in polyurethanes as efficient media for thermal energy. Energy Storage Mater. 2020, 30, 74–86. [Google Scholar] [CrossRef]
- Khatoon, H.; Iqbal, S.; Irfan, M.; Darda, A.; Rawat, N.K. A review on the production, properties and applications of non-isocyanate polyurethane: A greener perspective. Prog. Org. Coat. 2021, 154, 106124. [Google Scholar] [CrossRef]
- Gama, N.; Ferreira, A.; Barros-Timmons, A. Polyurethane Foams: Past, Present, and Future. Materials 2018, 11, 1841. [Google Scholar] [CrossRef]
- Tian, S. Recent Advances in Functional Polyurethane and Its Application in Leather Manufacture: A Review. Polymers 2020, 12, 1996. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Maharjan, A.; Kim, B.S. Shape Memory Polyurethane and its Composites for Various Applications. Appl. Sci. 2019, 9, 4694. [Google Scholar] [CrossRef]
- Somarathna, H.M.C.C.; Raman, S.N.; Mohotti, D.; Mutalib, A.A.; Badri, K.H. The use of polyurethane for structural and infrastructural engineering applications: A state-of-the-art review. Constr. Build. Mater. 2018, 190, 995–1014. [Google Scholar] [CrossRef]
- Kausar, A. Polyurethane Composite Foams in High-Performance Applications: A Review. Polym.-Plast. Technol. Eng. 2017, 57, 346–369. [Google Scholar] [CrossRef]
- Cheng, B.-X.; Gao, W.-C.; Ren, X.-M.; Ouyang, X.-Y.; Zhao, Y.; Zhao, H.; Wu, W.; Huang, C.-X.; Liu, Y.; Liu, X.-Y.; et al. A review of microphase separation of polyurethane: Characterization and applications. Polym. Test. 2022, 107, 107489. [Google Scholar] [CrossRef]
- Joseph, J.; Patel, R.M.; Wenham, A.; Smith, J.R. Biomedical applications of polyurethane materials and coatings. Trans. IMF 2018, 96, 121–129. [Google Scholar] [CrossRef]
- Xuerui, J.; Jixin, D.; Xufan, G.; Mingzhu, D.; Rui, B.; Yunzi, L. Current advances in polyurethane biodegradation. Polym. Int. 2021, 71, 1384–1392. [Google Scholar]
- Murat, A.; Selin, K.; Aysegul, A.E.; Bulent, E. Polyurethane foam materials and their industrial applications. Polym. Int. 2022, 71, 1157–1163. [Google Scholar]
- Shubham, M.D.; Rushikesh, Y.S.; Aarti, P.M. Thermoplastic polyurethane for three-dimensional printing applications: A review. Polym. Adv. Tech. 2023, 34, 2061–2082. [Google Scholar]
- Wang, C.; Mu, C.; Lin, W.; Xiao, H. Functional-modified polyurethanes for rendering surfaces antimicrobial: An overview. Adv. Colloid Interface Sci. 2020, 283, 102235. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.-H.; Deng, C.; Chen, H.; Wei, Y.-X.; Wang, Y.-Z. A novel Schiff-base polyphosphate ester: Highly-efficient flame retardant for polyurethane elastomer. Polym. Degr. Stab. 2017, 144, 82. [Google Scholar] [CrossRef]
- Yin, X.; Li, L.; Pang, H.; Luo, Y.; Zhang, B. Halogen-free instinct flame-retardant waterborne polyurethanes: Composition, performance, and application. RSC Adv. 2022, 12, 14509–14520. [Google Scholar] [CrossRef] [PubMed]
- Wlodarczyk, D.; Urban, M.; Strankowski, M. Chemical modifications of graphene and their influence on properties of polyurethane composites: A review. Phys. Scr. 2016, 91, 104003. [Google Scholar] [CrossRef]
- Namita, K.; Girish, M.J.; Mhaske, S.T. Structure-property relationship of silane-modified polyurethane: A review. Prog. Org. Coat. 2023, 176, 107377. [Google Scholar]
- Naureen, B.; Haseeb, A.S.M.A.; Basirun, W.J.; Muhamad, F. Recent advances in tissue engineering scaffolds based on polyurethane and modified polyurethane. Mater. Sci. Eng. C 2021, 118, 111228. [Google Scholar] [CrossRef]
- Adipurnama, I.; Yang, M.-C.; Ciach, T.; Butruk-Raszeja, B. Surface modification and endothelialization of polyurethane for vascular tissue engineering applications: A review. Biomater. Sci. 2017, 5, 22–37. [Google Scholar] [CrossRef]
- Santamaria-Echart, A.; Fernandes, I.; Barreiro, F.; Corcuera, M.A.; Eceiza, A. Advances in Waterborne Polyurethane and Polyurethane-Urea Dispersions and Their Eco-friendly Derivatives: A Review. Polymers 2021, 13, 409. [Google Scholar] [CrossRef] [PubMed]
- Zemla, M.; Prociak, A.; Michalowski, S. Bio-Based Rigid Polyurethane Foams Modified with Phosphorus Flame Retardants. Polymers 2022, 14, 102. [Google Scholar] [CrossRef] [PubMed]
- Jang, T.; Kim, H.J.; Jang, J.B.; Kim, T.H.; Lee, W.; Seo, B.; Ko, W.B.; Lim, C.-S. Synthesis of Waterborne Polyurethane Using Phosphorus-Modified Rigid Polyol and its Physical Properties. Polymers 2021, 13, 432. [Google Scholar] [CrossRef] [PubMed]
- Sui, Z.; Li, Y.; Guo, Z.; Zhang, Q.; Xu, Y.; Zhao, X. Preparation and properties of polysiloxane modified fluorine-containing waterborne polyurethane emulsion. Prog. Org. Coat. 2022, 166, 106783. [Google Scholar] [CrossRef]
- Li, D.; Yu, L.; Lu, Z.; Kang, H.; Li, L.; Zhao, S.; Shi, N.; You, S. Synthesis, Structure, Properties, and Applications of Fluorinated Polyurethane. Polymers 2024, 16, 959. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Gao, W.-C.; Li, Q.; Khan, M.R.; Hu, G.-H.; Liu, Y.; Wu, W.; Huang, C.-X.; Li, R.K.Y. Recent advances in superhydrophobic polyurethane: Preparations and applications. Adv. Colloid Interface Sci. 2022, 303, 102644. [Google Scholar] [CrossRef]
- Wang, X.; Cui, Y.; Wang, Y.; Ban, T.; Zhang, Y.; Zhang, J.; Zhu, X. Preparation and characteristics of crosslinked fluorinated acrylate modified waterborne polyurethane for metal protection coating. Prog. Org. Coat. 2021, 158, 106371. [Google Scholar] [CrossRef]
- Krol, P.; Krol, B. Structures, properties and applications of the polyurethane ionomers. J. Mater. Sci. 2020, 55, 73–87. [Google Scholar] [CrossRef]
- Krol, P. Synthesis methods, chemical structures and phase structures of linear polyurethanes. Properties and applications of linear polyurethanes in polyurethane elastomers, copolymers and ionomers. Prog. Mater. Sci. 2007, 52, 915–1015. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications—A review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Jaudouin, O.; Robin, J.J.; Lopez-Cuesta, J.M.; Perrin, D.; Imbert, C. Ionomer-based polyurethanes: A comparative study of properties and applications. Polym. Int. 2012, 61, 495–510. [Google Scholar] [CrossRef]
- Fragiadakis, D.; Dou, S.; Colby, R.H.; Runt, J. Molecular mobility, ion mobility, and mobile ion concentration in poly(ethylene oxide)-based polyurethane ionomers. Macromolecules 2008, 41, 5723–5728. [Google Scholar] [CrossRef]
- Buruiana, T.; Airinei, A.; Buruiana, E.C.; Robila, G. Polyurethane cationomers containing anthryl and nitroaromatic chromophores. Eur. Polym. J. 1997, 33, 877–880. [Google Scholar] [CrossRef]
- Charnetskaya, A.G.; Polizos, G.; Shtompel, V.I.; Privalko, E.G.; Kercha, Y.Y.; Pissis, P. Phase morphology and molecular dynamics of a polyurethane ionomer reinforced with a liquid crystalline caller. Eur. Polym. J. 2003, 39, 2167–2174. [Google Scholar] [CrossRef]
- Zhu, Y.; Hu, J.L.; Choi, K.F.; Meng, Q.H.; Chen, S.J.; Yeung, K.W. Shape memory effect and reversible phase crystallization process in SMPU ionomer. Polym. Adv. Technol. 2008, 19, 328–333. [Google Scholar] [CrossRef]
- Zhu, Y.; Hu, J.L.; Lu, J.; Yeung, L.Y.; Yeung, K.W. Shape memory ber spun with segmented polyurethane ionomer. Polym. Adv. Technol. 2008, 19, 1745–1753. [Google Scholar] [CrossRef]
- Raasch, J.; Ivey, M.; Aldrich, D.; Nobes, D.S.; Ayranci, C. Characterization of polyurethane shape memory polymer processedby material extrusion additive manufacturing. Addit. Manuf. 2015, 8, 132–141. [Google Scholar]
- Wang, C.C.; Zhao, Y.; Purnawali, H.; Huang, W.M.; Sun, L. Chemically induced morphing in polyurethane shape memory polymer micro bers/springs. React. Funct. Polym. 2012, 72, 757–764. [Google Scholar] [CrossRef]
- Casado, U.M.; Quintanilla, R.M.; Aranguren, M.I.; Marcovich, N.E. Composite lms based on shape memory polyurethanes and nanostructured, polyaniline or cellulose–polyaniline particles. Synth. Met. 2012, 162, 1654–1664. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Z.X.; Huang, W.B.; Li, J.L.; Yang, J.H.; Wang, Y.; Zhou, Z.W.; Zhang, J.H. Carbon nanotube network structure induced strain sensitivity and shape memory behavior changes of thermoplastic polyurethane. Mater. Des. 2015, 69, 105–113. [Google Scholar] [CrossRef]
- Gu, S.; Yan, B.; Liu, L.; Ren, J. Carbon nanotube–polyurethane shape memory nanocomposites with low trigger temperature. Eur. Polym. J. 2013, 49, 3867–3877. [Google Scholar] [CrossRef]
- Peponi, L.; Navarro-Baena, I.; Sonseca, A.; Gimenez, E.; Marcos-Fernandez, A.; Kenny, J.M. Synthesis and characterization of PCL–PLLA polyurethane with shape memory behavior. Eur. Polym. J. 2013, 49, 893–903. [Google Scholar] [CrossRef]
- Takahashi, T.; Hayashi, N.; Hayashi, S. Structure and properties of shape-memory polyurethane block copolymers. J. Appl. Polym. Sci. 1996, 60, 1061–1069. [Google Scholar] [CrossRef]
- Takahara, A.; Hergenrother, R.W.; Coury, A.J.; Cooper, S.L. Effect of soft segment chemistry on the biostability of segmented polyurethanes. II. In vitro hydrolytic degradation and lipod sorption. J. Biomed. Mater. Res. 1992, 26, 801–818. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Li, Y.J.; Nakaya, T. Synthesis and properties of polyurethanes containing phosphatidylcholine analogues in the polymer backbone. Macromol. Rapid Commun. 1995, 16, 25–30. [Google Scholar] [CrossRef]
- Li, Y.J.; Nakamura, N.; Wang, Y.F.; Kodama, M.; Nakaya, T. Synthesis and Hemocompatibilities of New Segmented Polyurethanes and Poly(urethane urea)s with Poly(butadiene) and Phosphatidylcholine Analogues in the Main Chains and Long-Chain Alkyl Groups in the Side Chains. Chem. Mater. 1997, 9, 1570–1577. [Google Scholar] [CrossRef]
- Zhang, L.; Brostowitz, N.R.; Cavicchi, K.A. Perspective: Ionomer Research and Applications. Macromol. React. Eng. 2014, 8, 81–99. [Google Scholar] [CrossRef]
- Narayan, R.; Chattopadhyay, D.K.; Sreedhar, B.; Raju, K.V.S.N.; Mallikarjuna, N.N.; Aminabhavi, T.M. Synthesis and characterization of crosslinked polyurethane dispersions based on hydroxylated polyesters. J. Appl. Polym. Sci. 2006, 99, 368–380. [Google Scholar] [CrossRef]
- Nakayama, Y.; Inaba, Y.; Toda, Y.; Tanaka, R.; Cai, Z.; Shiono, T.; Shirahama, H.; Tsutsumi, C. Synthesis and properties of cationic ionomers from poly(ester-urethane)s based on polylactide. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 4423–4428. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Z.; Zhang, V.; Liang, Y.; Liu, Y. Polyurethanes Modified by Ionic Liquids and Their Applications. Int. J. Mol. Sci. 2023, 24, 11627. [Google Scholar] [CrossRef]
- Eisenberg, A. Clustering of Ions in Organic Polymers. A Theoretical Approach. Macromolecules 1970, 3, 147–154. [Google Scholar] [CrossRef]
- Tadano, K.; Hirasawa, E.; Yamamoto, H.; Yano, S. Order-disorder transition of ionic clusters in ionomers. Macromolecules 1989, 22, 226–233. [Google Scholar] [CrossRef]
- Eisenberg, A.; Hird, B.; Moore, R.B. A new multiplet-cluster model for the morphology of random ionomers. Macromolecules 1990, 23, 4098–4107. [Google Scholar] [CrossRef]
- Nyrkova, I.A.; Khokhlov, A.R.; Doi, M. Microdomains in block copolymers and multiplets in ionomers: Parallels in behavior. Macromolecules 1993, 26, 3601–3610. [Google Scholar] [CrossRef]
- Semenov, A.N.; Nyrkova, I.A.; Khokhlov, A.R. Polymers with Strongly Interacting Groups: Theory for Nonspherical Multiplets. Macromolecules 1995, 28, 7491–7500. [Google Scholar] [CrossRef]
- Davletbaeva, I.M.; Sazonov, O.O.; Fazlyev, A.R.; Zakirov, I.N.; Davletbaev, R.S.; Efimov, S.V.; Klochkov, V.V. Thermal behavior of polyurethane ionomers based on amino ethers of ortho-phosphoric acid. Polym. Sci. Ser. A 2020, 62, 337–349. [Google Scholar] [CrossRef]
- Davletbaeva, I.M.; Sazonov, O.O.; Fazlyev, A.R.; Davletbaev, R.S.; Efimov, S.V.; Klochkov, V.V. Polyurethane ionomers based on amino ethers of orto-phosphoric acid. RSC Adv. 2019, 9, 18599–18608. [Google Scholar] [CrossRef]
- Davletbaeva, I.M.; Sazonov, O.O.; Zakirov, I.N.; Davletbaev, R.S.; Efimov, S.V.; Klochkov, V.V. Catalytic Etherification of ortho-Phosphoric Acid for the Synthesis of Polyurethane Ionomer Films. Polymers 2022, 14, 3295. [Google Scholar] [CrossRef]
- Davletbaeva, I.M.; Sazonov, O.O.; Zakirov, I.N.; Gumerov, A.M.; Klinov, A.V.; Fazlyev, A.R.; Malygin, A.V. Organophosphorus polyurethane ionomers as water vapor permeable and pervaporation membranes. Polymers 2021, 14, 1442. [Google Scholar] [CrossRef]
- Vögtle, F.; Weber, E. Host Guest Complex Chemistry. In Macrocycles, Synthesis, Structures, Applications; Springer: Berlin/Heidelberg, Germany; New York, NY, USA; Tokyo, Japan, 1985; p. 421. [Google Scholar]
- Tai, N.L.; Adhikari, R.; Shanks, R.; Halley, P.; Adhikari, B. Flexible starch-polyurethane films: Effect mixed macrodiol polyurethane ionomers on physicochemical characteristics and hydrophobicity. Carbohydr. Polym. 2018, 197, 321–335. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davletbaeva, I.M.; Sazonov, O.O.; Zakirov, I.N.; Arkhipov, A.V.; Davletbaev, R.S. Self-Organization of Polyurethane Ionomers Based on Organophosphorus-Branched Polyols. Polymers 2024, 16, 1773. https://doi.org/10.3390/polym16131773
Davletbaeva IM, Sazonov OO, Zakirov IN, Arkhipov AV, Davletbaev RS. Self-Organization of Polyurethane Ionomers Based on Organophosphorus-Branched Polyols. Polymers. 2024; 16(13):1773. https://doi.org/10.3390/polym16131773
Chicago/Turabian StyleDavletbaeva, Ilsiya M., Oleg O. Sazonov, Ilyas N. Zakirov, Alexander V. Arkhipov, and Ruslan S. Davletbaev. 2024. "Self-Organization of Polyurethane Ionomers Based on Organophosphorus-Branched Polyols" Polymers 16, no. 13: 1773. https://doi.org/10.3390/polym16131773
APA StyleDavletbaeva, I. M., Sazonov, O. O., Zakirov, I. N., Arkhipov, A. V., & Davletbaev, R. S. (2024). Self-Organization of Polyurethane Ionomers Based on Organophosphorus-Branched Polyols. Polymers, 16(13), 1773. https://doi.org/10.3390/polym16131773








