Ammonium Tetrakis(pentafluorophenyl)borate: Preparation and Application in Olefin Coordination Polymerization as the Cocatalyst Compound
Abstract
1. Introduction
2. Research History of Ammonium Tetrakis(pentafluorophenyl)borate
3. Preparation of Ammonium Tetrakis(pentafluorophenyl)borate
3.1. Preparation of Tetrakis(pentafluorophenyl)borate Anions
3.1.1. Pentafluorobenzene Dehydrogenation
3.1.2. Pentafluorohalobenzene Dehalogenation
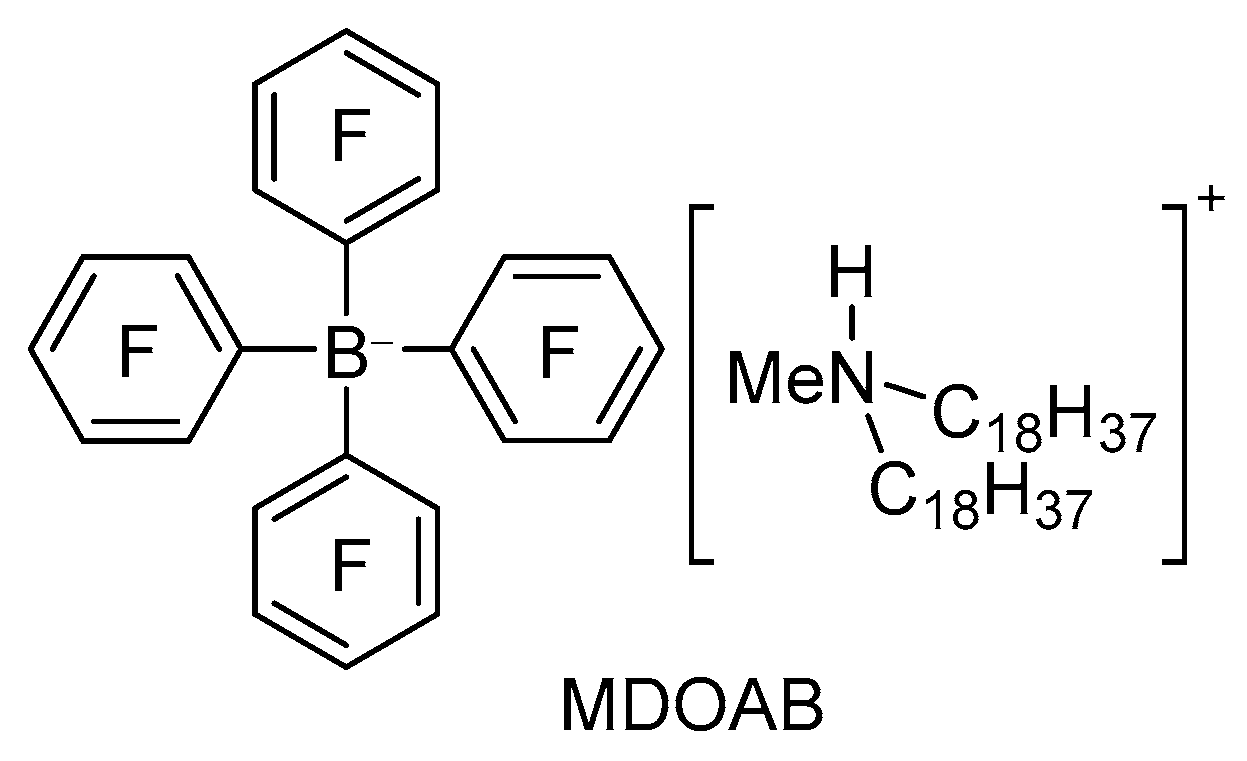
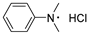
3.2. Preparation for DMAB and MDOAB
4. Research Progress of Ammonium Tetrakis(pentafluorophenyl)borate
4.1. Types of Ammonium Tetrakis(pentafluorophenyl)borate
4.1.1. Dimethylanilinium Tetrakis(pentafluorophenyl)borate
4.1.2. N-Methyl-N,N-dioctadecylammonium Tetrakis(perfluorophenyl)borate
4.2. The Application of Ammonium Tetrakis(pentafluorophenyl)borate in Polymerization
4.2.1. The Ammonium Tetrakis(pentafluorophenyl)borate as a Substitute for MAO
Copolymerization of Ethylene and 1-Octene
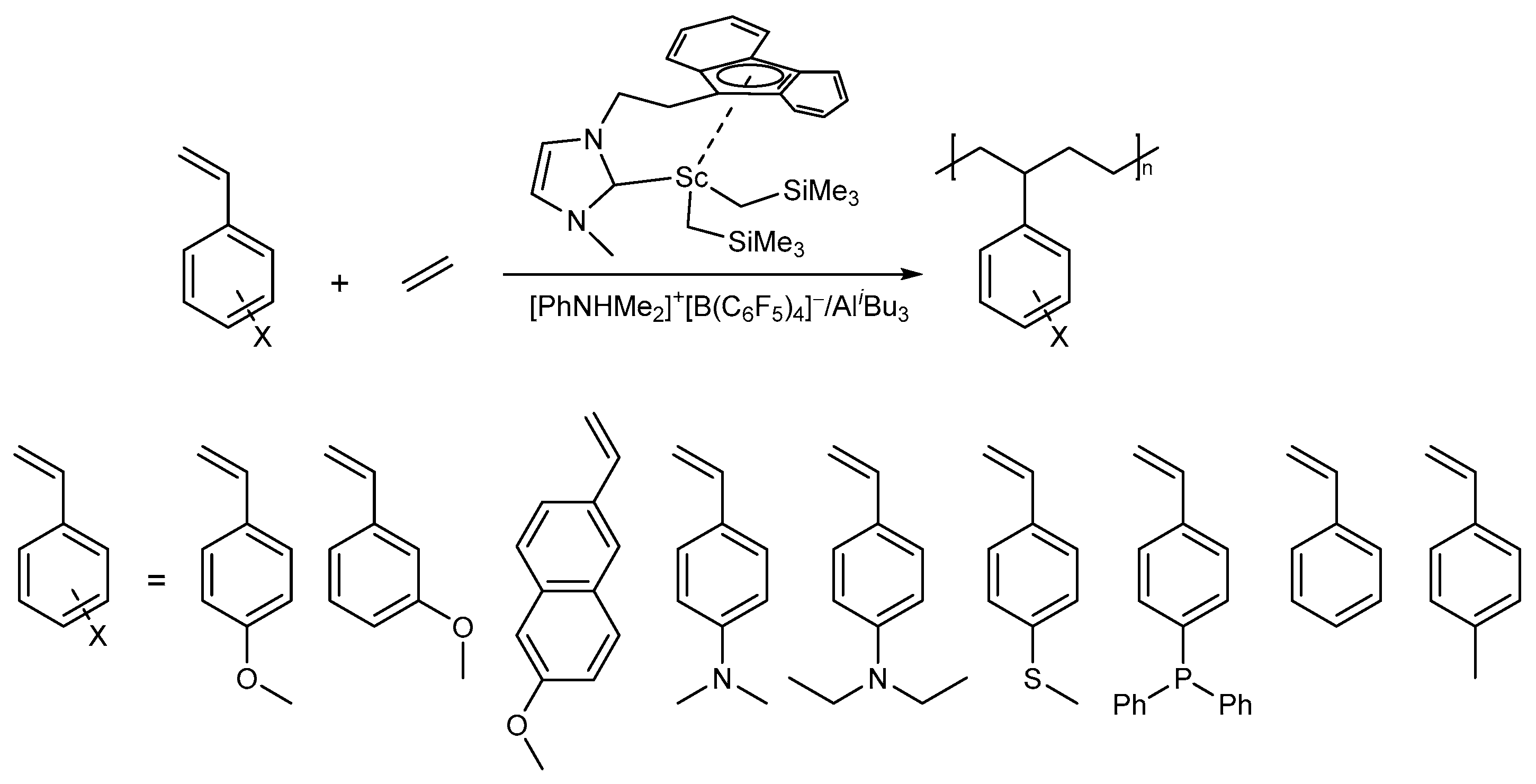
The Polymerization of Ethylene and Styrene Derivatives
The Polymerization of Ethylene and Unsaturated Carboxylate
Copolymerization of Styrene and Butadiene
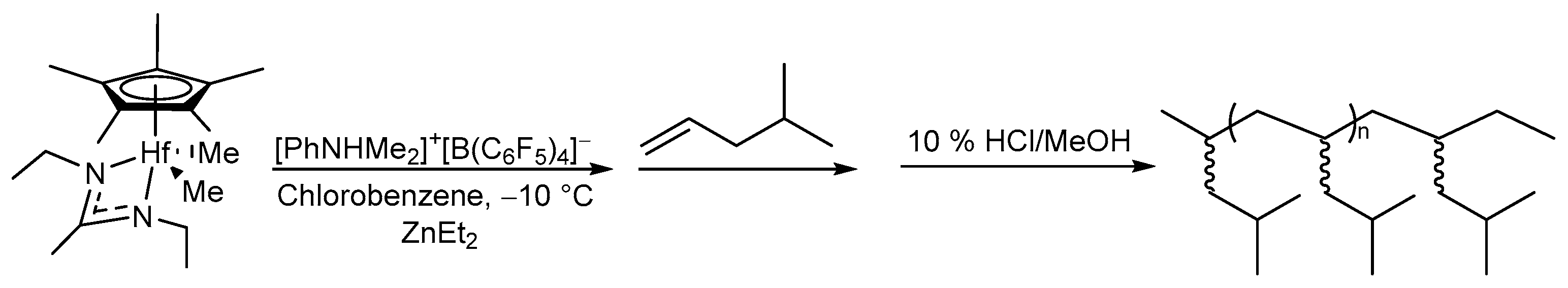
Homopolymerization of 4-methyl-1-pentene
Homopolymerization of Isoprene
4.2.2. Ammonium Tetrakis(pentafluorophenyl)borate as an Excellent Performer Compared with MAO
Coordinative Chain Transfer Polymerization (Hompolymerization of Ethylene)
Oligomerization of 1-Decene
4.2.3. Unique Role of Ammonium Tetrakis(pentafluorophenyl)borate as the Cocatalyst
The Production of Telechelic Polyolefins
The Preparation of Silane-End-Functionalized Polymers
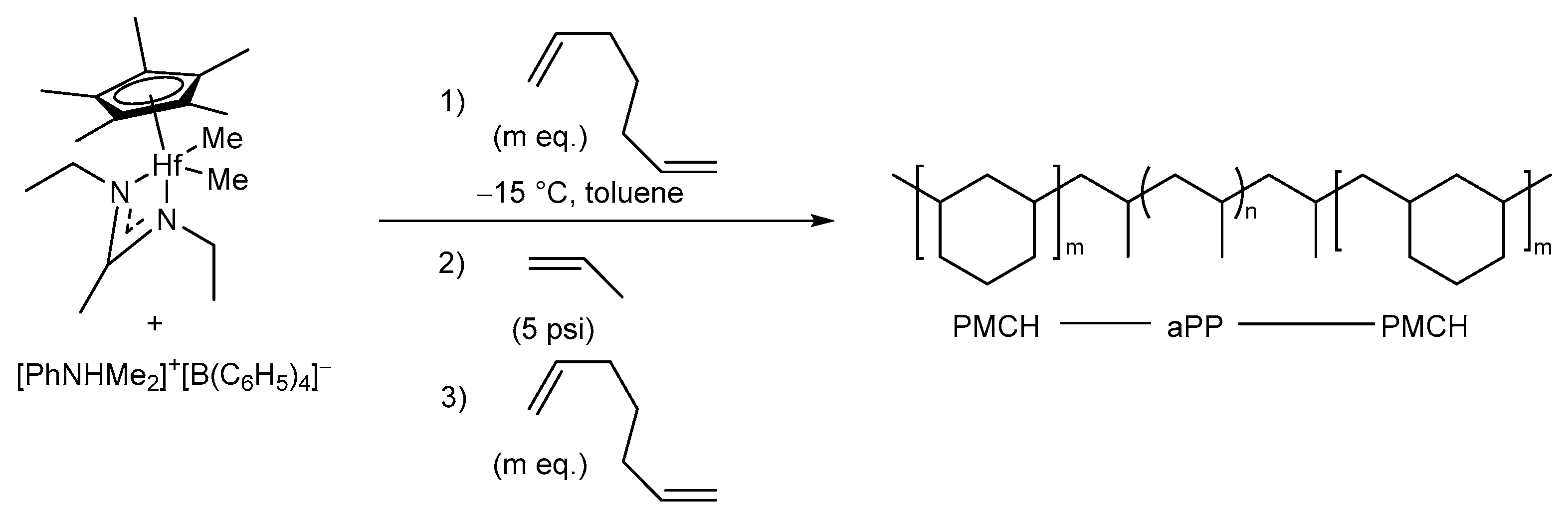
Some Specific Cyclopolymerizations Associated with 1,6-Heptadiene
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kaminsky, W. The discovery of metallocene catalysts and their present state of the art. J. Polym. Sci. Polym. Chem. 2004, 42, 3911–3921. [Google Scholar] [CrossRef]
- Kaminsky, W.; Laban, A. Metallocene catalysis. Appl. Catal. A Gen. 2001, 222, 47–61. [Google Scholar] [CrossRef]
- Janiak, C. Metallocene and related catalysts for olefin, alkyne and silane dimerization and oligomerization. Coord. Chem. Rev. 2006, 250, 66–94. [Google Scholar] [CrossRef]
- Kaminsky, W.; Funck, A.; Hähnsen, H. New application for metallocene catalysts in olefin polymerization. Dalton Trans. 2009, 8803–8810. [Google Scholar] [CrossRef]
- Zhang, H. Headway of Metallocene Catalyst and Metallocene Polymer. Chem. Technol. Econ. 2004, 22, 24–27. [Google Scholar]
- Wu, H.; Zhang, J.; Zhao, C.; Fang, Z.; Liu, W.; Huang, Y. Preparation and Application Progress of Perfluoroaryl Borates. Organo Fluor. Ind. 2018, 3, 38–43. [Google Scholar]
- Kong, Y.; Ma, L.; Huang, Q.; Zhao, Y.; Li, J.; Wang, H.; Deng, K.; Sheng, Y.; Yi, J.; Wang, W.; et al. Advances in living polymerization of polar monomers promoted by metallocene complexes. Chem. Ind. Eng. Prog. 2009, 28, 243–250. [Google Scholar]
- Yu, B.; Wang, L.; Xu, T.; Huo, H.; Wang, Z.; Sun, W. Recent Progresses of the Co-catalyst for Ethylene Selective Oligomerization. Spec. Petrochem. 2017, 34, 78–82. [Google Scholar]
- Sinn, H.; Kaminsky, W.; Vollmer, H.; Woldt, R. “Living Polymers” on Polymerization with Extremely Productive Ziegler Catalysts. Angew. Chem. Int. Ed. Eng. 1980, 19, 390–392. [Google Scholar] [CrossRef]
- Hu, J. Metal Organic Olefin Polymerization Catalyst and Olefin Polymer; Chemical Industry Press: Beijing, China, 2010. [Google Scholar]
- Wu, T.; Qian, Y.; Huang, J. Catalytic trimerization of ethylene by half-sandwich titanium complexes bearing a pendant ethereal group. J. Mol. Catal. A Chem. 2004, 214, 227–229. [Google Scholar] [CrossRef]
- Jiang, T.; Liu, X.; Ning, Y.; Chen, H.; Luo, M.; Wang, L.; Huang, Z. Performance of various aluminoxane activators in ethylene tetramerization based on PNP/Cr(III) catalyst system. Catal. Commun. 2007, 8, 1145–1148. [Google Scholar] [CrossRef]
- Bollmann, A.; Blann, K.; Dixon, J.T.; Hess, F.M.; Killian, E.; Maumela, H.; McGuinness, D.S.; Morgan, D.H.; Neveling, A.; Otto, S.; et al. Ethylene tetramerization: A new route to produce 1-octene in exceptionally high selectivities. J. Am. Chem. Soc. 2004, 126, 14712–14713. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Z.; Liu, B.; Duchateau, R. Selective Ethylene Tri-/Tetramerization by in Situ-Formed Chromium Catalysts Stabilized by N,P-Based Ancillary Ligand Systems. ACS Catal. 2013, 3, 2353–2361. [Google Scholar] [CrossRef]
- Kim, S.; Kim, T.; Chung, J.; Hahn, T.; Chae, S.; Lee, H.; Cheong, M.; Kang, S.O. Bimetallic Ethylene Tetramerization Catalysts Derived from Chiral DPPDME Ligands: Syntheses, Structural Characterizations, and Catalytic Performance of [(DPPDME)CrCl3]2 (DPPDME = S,S- and R,R-chiraphos and meso-achiraphos). Organometallics 2010, 29, 5805–5811. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, H.; Huang, J. Highly selective ethylene trimerization catalyzed by half-sandwich indenyl titanium complexes with pendant arene groups and MAO. J. Mol. Cata. A Chem. 2013, 373, 85–95. [Google Scholar] [CrossRef]
- Briggs, J.R. The selective trimerization of ethylene to hex-1-ene. Chem. Commun. 1989, 674–675. [Google Scholar] [CrossRef]
- Zilbershtein, T.M.; Kardash, V.A.; Suvorova, V.V.; Golovko, A.K. Decene formation in ethylene trimerization reaction catalyzed by Cr–pyrrole system. Appl. Catal. A Gen. 2014, 475, 371–378. [Google Scholar] [CrossRef]
- Luo, H.K.; Li, D.; Li, S. The effect of halide and the coordination geometry of chromium center in homogeneous catalyst system for ethylene trimerization. J. Mol. Catal. A Chem. 2004, 221, 9–17. [Google Scholar] [CrossRef]
- Netalkar, S.P.; Budagumpi, S.; Abdallah, H.H.; Netalkar, P.P.; Revankar, V.K. Sterically modulated binuclear bis-α-diimine Pd(II) complexes: Synthesis, characterization, DFT studies and catalytic behavior towards ethylene oligomerization. J. Mol. Struct. 2014, 1075, 559–565. [Google Scholar] [CrossRef]
- Chen, E.Y.; Marks, T.J. Cocatalysts for Metal-Catalyzed Olefin Polymerization: Activators, Activation Processes, and Structure−Activity Relationships. Chem. Rev. 2000, 100, 1391–1434. [Google Scholar] [CrossRef]
- Ewen, J.A.; Elder, M.J.; Jones, R.L.; Haspeslagh, L.; Atwood, J.L.; Bott, S.G.; Robinson, K. Metallocene/polypropylene structural relationships: Implications on polymerization and stereochemical control mechanisms. Makromol. Chem. Macromol. Symp. 1991, 48–49, 253–295. [Google Scholar] [CrossRef]
- Hlatky, G.G.; Turner, H.W.; Eckman, R.R. Ionic, base-free zirconocene catalysts for ethylene polymerization. J. Am. Chem. Soc. 1989, 111, 2728–2729. [Google Scholar] [CrossRef]
- Yang, X.; Stern, C.; Marks, T.J. Models for organometallic molecule-support complexes. Very large counterion modulation of cationic actinide alkyl reactivity. Organometallics 1991, 10, 840–842. [Google Scholar] [CrossRef]
- Wilmes, G.M.; Polse, J.L.; Waymouth, R.M. Influence of Cocatalyst on the Stereoselectivity of Unbridged 2-Phenylindenyl Metallocene Catalysts. Macromolecules 2002, 35, 6766–6772. [Google Scholar] [CrossRef]
- Wang, Y. Olefin polymerization cocatalysts: Development, applications, and prospects. Chin. Sci. Bull. 2022, 67, 1895–1907. [Google Scholar] [CrossRef]
- Jordan, R.F.; Dasher, W.E.; Echols, S.F. Reactive cationic dicyclopentadienyl zirconium(IV) complexes. J. Am. Chem. Soc. 1986, 108, 1718–1719. [Google Scholar] [CrossRef]
- Jordan, R.F.; Bajgur, C.S.; Willett, R.; Scott, B. Ethylene polymerization by a cationic dicyclopentadienyl zirconium(IV) alkyl complex. J. Am. Chem. Soc. 1986, 108, 7410–7411. [Google Scholar] [CrossRef]
- Massey, A.G.; Park, A.J. Perfluorophenyl derivatives of the elements: I. Tris(pentafluorophenyl)boron. J. Organomet. Chem. 1964, 2, 245–250. [Google Scholar] [CrossRef]
- Massey, A.G.; Park, A.J. Perfluorophenyl derivatives of the elements: VII. further studies on tris(pentafluorophenyl)boron. J. Organomet. Chem. 1966, 5, 218–225. [Google Scholar] [CrossRef]
- Massey, A.G.; Park, A.J. Proceedings of the Chemical Society. July 1963. Proc. Chem. Soc. 1963, 189–228. [Google Scholar] [CrossRef]
- Chen, Y.; Stern, C.L.; Yang, S.; Marks, T.J. Organo-Lewis Acids As Cocatalysts in Cationic Metallocene Polymerization Catalysis. Unusual Characteristics of Sterically Encumbered Tris(perfluorobiphenyl)borane. J. Am. Chem. Soc. 1996, 118, 12451–12452. [Google Scholar] [CrossRef]
- Li, L.; Marks, T.J. New Organo-Lewis Acids. Tris(β-perfluoronaphthyl)borane (PNB) as a Highly Active Cocatalyst for Metallocene-Mediated Ziegler−Natta α-Olefin Polymerization. Organometallics 1998, 17, 3996–4003. [Google Scholar] [CrossRef]
- Williams, V.C.; Piers, W.E.; Clegg, W.; Elsegood, M.R.J.; Collins, S.; Marder, T.B. New Bifunctional Perfluoroaryl Boranes. Synthesis and Reactivity of the ortho-Phenylene-Bridged Diboranes 1,2-[B(C6F5)2]2C6X4 (X = H, F)′. J. Am. Chem. Soc. 1999, 121, 3244–3245. [Google Scholar] [CrossRef]
- Williams, V.C.; Dai, C.; Li, Z.; Collins, S.; Piers, W.E.; Clegg, W.; Elsegood, M.R.J.; Marder, T.B. Activation of [Cp2ZrMe2] with New Perfluoroaryl Diboranes: Solution Chemistry and Ethylene Polymerization Behavior in the Presence of MeAl(BHT)2. Angew. Chem. Int. Ed. 1999, 38, 3695–3698. [Google Scholar] [CrossRef]
- Chien, J.C.W.; Tsai, W.M.; Rausch, M.D. Isospecific polymerization of propylene catalyzed by rac-ethylenebis(indenyl)methylzirconium cation. J. Am. Chem. Soc. 1991, 113, 8570–8571. [Google Scholar] [CrossRef]
- Jia, L.; Yang, X.; Ishihara, A.; Marks, T.J. Protected (Fluoroaryl)borates as Effective Counteranions for Cationic Metallocene Polymerization Catalysts. Organometallics 1995, 14, 3135–3137. [Google Scholar] [CrossRef]
- Ikeda, Y.; Takeo, Y.; Eiichi, K.; Kenji, I. Method of Producing Tetrakis (Pentafluorophenyl) Borate Derivatives Using Pentafluorophenyl Alkali Metal Salt Prepared from Pentafluorobenzene. U.S. Patent 5,493,056, 27 December 1993. [Google Scholar]
- Shimizu, T.; Yoshiko, N.; Kazuya, O. Method for Producing Tetrakis (Fluorophenyl) Borate Derivative. JP 2002265471, 18 September 2002. [Google Scholar]
- Bildinov, I.; Podsevalov, P.; Nazarenko, T.; Deev, L.; Owens, D.W.; Balhoff, J.F. Halogen Exchange Reactions and Uses Thereof. WO/1998/22413, 28 May 1998. [Google Scholar]
- Yan, X.; Zhao, Y.; Chen, J. The Invention Relates to a Synthesis Method of Tetrafluorophenyl Borate. CN 105153209 A, 16 December 2015. [Google Scholar]
- Naganuma, S.; Watanabe, M.; Tomotsu, N. Process for Producing Tetrak-Isflorophenylborate. U.S. Patent 5,600,005, 6 June 1995. [Google Scholar]
- Cao, Y.; Lui, W.; Zhou, H.; Ma, J. The Invention Relates to a Preparation Method of a Fluorinated Aryl Boron Compound. CN 101863913 A, 24 June 2010. [Google Scholar]
- Piotrowski, A.M.; Taylor, D.F. Process for Forming a Bro-Momagnesium Tetrakis(Fluorophenyl) Borate. U.S. Patent 5,473,036, 5 December 1995. [Google Scholar]
- Takeo, Y.; Kenji, I. Production of Tetrakis(Pentaflurophenyl) Borate Derivative by Using Halogenated Alkyl. JP 3743687, 1 May 1996. [Google Scholar]
- Rach, S.F.; Herdtweck, E.; Kühn, F.E. A straightforward synthesis of cationic nitrile ligated transition metal complexes with the [B(C6F5)4]− anion. J. Organomet. Chem. 2011, 696, 1817–1823. [Google Scholar] [CrossRef]
- Fan, A. The Invention Relates to a Synthesis Method of Potassium Salt of Tetrafluorophenylborate. CN 110041354 A, 23 July 2019. [Google Scholar]
- Lee, J.Y.; Strickler, J.R. Preparation of Alkali Metal Tetrafluorophenylborate. U.S. Patent 6,162,950, 19 December 2000. [Google Scholar]
- Mitsui, H.; Ueno, T.; Ikeno, I.; Yamamoto, N. Method for Purifying Tetrakis (Fluoroaryl) Borate/Magnesium Halide, Tetrakis (Fluoroaryl) Borate/Ether Complex and Process for Preparing the Same, and Process for Preparing Tetrakis (Fluoroaryl) Borate Derivative. WO 1998/40389, 10 March 1997. [Google Scholar]
- Takashi, T.; Hang, J. Novel Tetraarylborate Compounds and Preparation Methods. CN 105283459, 24 April 2014. [Google Scholar]
- Fong, P.Y.; Smith, K.R.; Sauer, J.D. Amines for Alcohols. U.S. Patent 4,994,620, 12 February 1991. [Google Scholar]
- Lee, H.-J.; Baek, J.-W.; Seo, Y.-H.; Lee, H.-C.; Jeong, S.-M.; Lee, J.; Lee, C.-G.; Lee, B.-Y. Preparation of High-Purity Ammonium Tetrakis(pentafluorophenyl)borate for the Activation of Olefin Polymerization Catalysts. Molecules 2021, 26, 2827. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, Y.; Dong, W.; Katsumata, T.; Shiotsuki, M.; Masuda, T. Novel Neodymium-Based Ternary Catalyst, Nd(Oi-Pr)3/[HNMe2Ph]+[B(C6F5)4]-/i-Bu3Al, for Isoprene Polymerization. Polym. Bull. 2005, 54, 173–178. [Google Scholar] [CrossRef]
- Heurtefeu, B.; Ibarboure, E.; Cramail, H. ω-Dimethyl ammonium tetrakis-pentafluorophenyl borate polyisoprene as an organic template for alkylated metallocenes toward the synthesis of polyethylene beads. Polym. Chem. 2012, 3, 1133. [Google Scholar] [CrossRef]
- Sian, L.; Macchioni, A.; Zuccaccia, C. Understanding the Role of Metallocenium Ion-Pair Aggregates on the Rate of Olefin Insertion into the Metal–Carbon Bond. ACS Catal. 2020, 10, 1591–1606. [Google Scholar] [CrossRef]
- Parveen, R.; Cundari, T.R.; Younker, J.M.; Rodriguez, G. Computational Assessment of Counterion Effect of Borate Anions on Ethylene Polymerization by Zirconocene and Hafnocene Catalysts. Organometallics 2020, 39, 2068–2079. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, J.; Wang, Y.; Pickens, D.B.; Motta, A.; Wang, Q.J.; Chung, Y.; Lohr, T.L.; Marks, T.J. Highly branched polyethylene oligomers via group IV-catalysed polymerization in very nonpolar media. Nat. Catal. 2019, 2, 236–242. [Google Scholar] [CrossRef]
- Nakashima, T.; Nakayama, Y.; Shiono, T.; Tanaka, R. Neutral, Noncoordinating, and Hydrocarbon-Soluble Protic Cocatalyst for Olefin Polymerization. ACS Catal. 2021, 11, 865–870. [Google Scholar] [CrossRef]
- Zaccaria, F.; Zuccaccia, C.; Cipullo, R.; Budzelaar, P.H.M.; Vittoria, A.; Macchioni, A.; Busico, V.; Ehm, C. Methylaluminoxane’s Molecular Cousin: A Well-defined and “Complete” Al-Activator for Molecular Olefin Polymerization Catalysts. ACS Catal. 2021, 11, 4464–4475. [Google Scholar] [CrossRef]
- Robert, K.R.; VanderLende, D.D. Highly Soluble Olefin Polymerization Catalyst Activator. U.S. Patent 5,919,983, 6 July 1999. [Google Scholar]
- Lee, H.J.; Baek, J.W.; Kim, T.J.; Park, H.S.; Moon, S.H.; Park, K.L.; Bae, S.M.; Park, J.; Lee, B.Y. Synthesis of Long-Chain Branched Polyolefins by Coordinative Chain Transfer Polymerization. Macromolecules 2019, 52, 9311–9320. [Google Scholar] [CrossRef]
- Lee, J.C.; Park, K.L.; Bae, S.M.; Lee, H.J.; Baek, J.W.; Lee, J.; Sa, S.; Shin, E.J.; Lee, K.S.; Lee, B.Y. Styrene Moiety-Carrying Diorganozinc Compound Preparation for Polystyrene-Poly(ethylene-co-1-hexene)-Polystyrene Triblock Copolymer Production. Macromolecules 2020, 53, 7274–7284. [Google Scholar] [CrossRef]
- Lui, W.; Lv, Y.; Zhang, T.; Li, B. Preparation Method of Bimetallic Catalyst, and Process for Preparing High-Molecular Weight Olefin Polymer. CN 116478199 A, 25 July 2023. [Google Scholar]
- Baek, J.W.; Kwon, S.J.; Lee, H.J.; Kim, T.J.; Ryu, J.Y.; Lee, J.; Shin, E.J.; Lee, K.S.; Lee, B.Y. Preparation of Half- and Post-Metallocene Hafnium Complexes with Tetrahydroquinoline and Tetrahydrophenanthroline Frameworks for Olefin Polymerization. Polymers 2019, 11, 1093. [Google Scholar] [CrossRef]
- Metz, M.V.; Schwartz, D.J.; Stern, C.L.; Marks, T.J.; Nickias, P.N. New Perfluoroarylborane Activators for Single-Site Olefin Polymerization. Acidity and Cocatalytic Properties of a “Superacidic” Perfluorodiboraanthracene. Organometallics 2002, 21, 4159–4168. [Google Scholar] [CrossRef]
- Yoon, J.S.; Lee, D.H.; Park, E.S.; Lee, I.M.; Park, D.K.; Jung, S.O. Thermal and mechanical properties of ethylene/α-olefin copolymers produced over (2-MeInd)2ZrCl2/MAO system. Polymer 2000, 41, 4523–4530. [Google Scholar] [CrossRef]
- Li, F.; Liu, W. Progress in the catalyst for ethylene/α-olefin copolymerization at high temperature. Can. J. Chem. Eng. 2023, 101, 4992–5019. [Google Scholar] [CrossRef]
- Lee, C.S.; Park, J.H.; Hwang, E.Y.; Park, G.H.; Go, M.J.; Lee, J.; Lee, B.Y. Preparation of [bis(amido)-phosphine] and [amido-phosphine sulfide or oxide] hafnium and zirconium complexes for olefin polymerization. J. Organomet. Chem. 2014, 772–773, 172–181. [Google Scholar] [CrossRef]
- Hwang, E.Y.; Park, G.H.; Lee, C.S.; Kang, Y.Y.; Lee, J.; Lee, B.Y. Preparation of octahydro- and tetrahydro-[1,10]phenanthroline zirconium and hafnium complexes for olefin polymerization. Dalton Trans. 2015, 44, 3845–3855. [Google Scholar] [CrossRef] [PubMed]
- Gunther, S.O.; Lai, Q.; Senecal, T.; Huacuja, R.; Bremer, S.; Pearson, D.M.; DeMott, J.C.; Bhuvanesh, N.; Ozerov, O.V.; Klosin, J. Highly Efficient Carborane-Based Activators for Molecular Olefin Polymerization Catalysts. ACS Catal. 2021, 11, 3335–3342. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.; Che, C.; Ding, M.; Zhang, P.; Wang, L. Method for Preparing Alpha-Olefin Copolymer. CN 113773430 A, 10 December 2021. [Google Scholar]
- Onwudili, J.A.; Insura, N.; Williams, P.T. Composition of products from the pyrolysis of polyethylene and polystyrene in a closed batch reactor: Effects of temperature and residence time. J. Anal. Appl. Pyrolysis 2009, 86, 293–303. [Google Scholar] [CrossRef]
- Nasir, A.; Kausar, A. A Review on Materials Derived from Polystyrene and Different Types of Nanoparticles. Polym. Plast. Technol. Eng. 2015, 54, 1819–1849. [Google Scholar] [CrossRef]
- Rodrigues, A.; Carpentier, J. Groups 3 and 4 single-site catalysts for styrene–ethylene and styrene–α-olefin copolymerization. Coord. Chem. Rev. 2008, 252, 2137–2154. [Google Scholar] [CrossRef]
- Sernetz, F.G.; Mülhaupt, R.; Waymouth, R.M. Influence of polymerization conditions on the copolymerization of styrene with ethylene using Me2Si(Me4Cp)(N-tert-butyl)TiCl2/methylaluminoxane Ziegler-Natta catalysts. Macromol. Chem. Phys. 1996, 197, 1071–1083. [Google Scholar] [CrossRef]
- Li, S.; Liu, D.; Wang, Z.; Cui, D. Development of Group 3 Catalysts for Alternating Copolymerization of Ethylene and Styrene Derivatives. ACS Catal. 2018, 8, 6086–6093. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, Y.; Lei, R.; Zhang, Y.; Li, S.; Cui, D. Proximity-Driven Synergic Copolymerization of Ethylene and Polar Monomers. Macromolecules 2023, 56, 2476–2483. [Google Scholar] [CrossRef]
- Gao, R.; Gou, Q.; Li, J.; Zhang, X.; Lai, J.; Zhang, L.; Zhou, J.; Lin, J.; Li, X.; Song, J.; et al. Method for Preparing Polymer, and Obtained Polymer. WO 2022/227933, 3 November 2022. [Google Scholar]
- Liu, D.; Hao, H.; Gao, R. Preparation Process of Olefin-Unsaturated Carboxylate Copolymer for Polyolefin Material. CN 115260359 A, 1 November 2022. [Google Scholar]
- Zambelli, A.; Caprio, M.; Grassi, A.; Bowen, D.E. Syndiotactic styrene-butadiene block copolymers synthesized with CpTiX3/MAO (Cp = C5H5, X = Cl, F; Cp = C5Me5, X = Me) and TiXn/MAO (n = 3, X = acac; n = 4, X = O-tert-Bu). Macromol. Chem. Phys. 2000, 201, 393–400. [Google Scholar] [CrossRef]
- Xu, S.; Huo, Y.; Hu, X.; Wang, F.; Pan, L.; Shi, X. Cationic Half-Sandwich Tetrahydrofluorenyl Rare-Earth Metal Complexes as Stable Single-Site Catalysts for (Co)Polymerization of Styrene and Butadiene. Inorg. Chem. 2023, 62, 4980–4989. [Google Scholar] [CrossRef]
- Guo, Y.; Shao, L.; Zhang, R.; Gao, W.; Yu, S.; Du, Y.; Yang, G.; Pan, F.; Li, T.; Jiang, Z. Modified poly (4-methyl-1-pentene) membranes by surface segregation for blood oxygenation. J. Membr. Sci. 2023, 678, 121695. [Google Scholar] [CrossRef]
- Markova, S.Y.; Gries, T.; Teplyakov, V.V. Poly(4-methyl-1-pentene) as a semicrystalline polymeric matrix for gas separating membranes. J. Membr. Sci. 2020, 598, 117754. [Google Scholar] [CrossRef]
- Han, C.; Lyu, D.; Lu, Y.; Men, Y. Crystallinity and temperature dependent mechanical properties of Poly(4-methyl-1-pentene). Polymer 2023, 269, 125734. [Google Scholar] [CrossRef]
- Irwin, L.J.; Miller, S.A. Unprecedented Syndioselectivity and Syndiotactic Polyolefin Melting Temperature: Polypropylene and Poly(4-methyl-1-pentene) from a Highly Active, Sterically Expanded η-Fluorenyl−η1-Amido Zirconium Complex. J. Am. Chem. Soc. 2005, 127, 9972–9973. [Google Scholar] [CrossRef]
- Wentz, C.M.; Fischbach, D.M.; Sita, L.R. Stereomodulation of Poly(4-methyl-1-pentene): Adoption of a Neglected and Misunderstood Commercial Polyolefin. Angew. Chem. Int. Ed. 2022, 61, e202211992. [Google Scholar] [CrossRef]
- Fischbach, D.M.; Wentz, C.M.; Mehdiabadi, S.; Soares, J.; Sita, L.R. Stereoblock vs. Stereoblend: Orchestrating Competing Living Coordination Chain Transfer Polymerizations for the One-Pot Production of New Viscoelastic Grades of Poly(4-methyl-1-pentene). ACS Catal. 2024, 14, 2333–2340. [Google Scholar] [CrossRef]
- Dong, W.; Masuda, T. Novel neodymium (III) isopropoxide-methylaluminoxane catalyst for isoprene polymerization. J. Polym. Sci. A Polym. Chem. 2002, 40, 1838–1844. [Google Scholar] [CrossRef]
- Visseaux, M.; Mainil, M.; Terrier, M.; Mortreux, A.; Roussel, P.; Mathivet, T.; Destarac, M. Cationic borohydrido–neodymium complex: Synthesis, characterization and its application as an efficient pre-catalyst for isoprene polymerisation. Dalton Trans. 2008, 4558–4561. [Google Scholar] [CrossRef]
- Martins, N.; Bonnet, F.; Visseaux, M. Highly efficient cis-1,4 polymerisation of isoprene using simple homoleptic amido rare earth-based catalysts. Polymer 2014, 55, 5013–5016. [Google Scholar] [CrossRef]
- Chu, C.; Wang, X.; Huang, J.; Huang, Z.; Li, Q.; Wang, F. Rare-earth metal complexes supported by indolyl-based NCN-pincer ligand and their efficient catalysis for isoprene polymerization. J. Mol. Struct. 2023, 1285, 135498. [Google Scholar] [CrossRef]
- Moinet, E.C.; Wolf, B.M.; Tardif, O.; Maichle-Mössmer, C.; Anwander, R. Divalent Lanthanide Tetraisobutylaluminates: Reactivity and Living Isoprene Polymerization. Angew. Chem. Int. Ed. 2023, 62, e202219316. [Google Scholar] [CrossRef]
- Goller, A.; Obenauf, J.; Kretschmer, W.P.; Kempe, R. The Highly Controlled and Efficient Polymerization of Ethylene. Angew. Chem. Int. Ed. 2023, 62, e202216464. [Google Scholar] [CrossRef]
- Bochmann, M.; Lancaster, S.J. Monomer-Dimer-Gleichgewichte in homo- und heterodinuclearen kationischen Alkylzirconiumkomplexen: Zur Rolle von Alkylaluminiumverbindungen bei der Stabilisierung katalytisch aktiver Zentren. Angew. Chem. 1994, 106, 1715–1718. [Google Scholar] [CrossRef]
- Nifant Ev, I.; Ivchenko, P. Fair Look at Coordination Oligomerization of Higher α-Olefins. Polymers 2020, 12, 1082. [Google Scholar] [CrossRef]
- Ray, S.; Rao, P.V.C.; Choudary, N.V. Poly-α-olefin-based synthetic lubricants: A short review on various synthetic routes. Lubr. Sci. 2012, 24, 23–44. [Google Scholar] [CrossRef]
- Makaryan, I.A.; Sedov, I.V. Market Potential of Industrial Technologies for Production of Synthetic Bases of Motor Oils. Russ. J. Gen. Chem. 2021, 91, 1243–1259. [Google Scholar] [CrossRef]
- Nifant’Ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Sedov, I.V.; Dorokhov, V.G.; Lyadov, A.S.; Ivchenko, P.V. Structurally uniform 1-hexene, 1-octene, and 1-decene oligomers: Zirconocene/MAO-catalyzed preparation, characterization, and prospects of their use as low-viscosity low-temperature oil base stocks. Appl. Catal. A Gen. 2018, 549, 40–50. [Google Scholar] [CrossRef]
- Nifant’Ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Bagrov, V.V.; Churakov, A.V.; Minyaev, M.E.; Kiselev, A.V.; Salakhov, I.I.; Ivchenko, P.V. A competetive way to low-viscosity PAO base stocks via heterocene-catalyzed oligomerization of dec-1-ene. Mol. Catal. 2022, 529, 112542. [Google Scholar] [CrossRef]
- Burkey, A.A.; Fischbach, D.M.; Wentz, C.M.; Beers, K.L.; Sita, L.R. Highly Versatile Strategy for the Production of Telechelic Polyolefins. ACS Macro Lett. 2022, 11, 402–409. [Google Scholar] [CrossRef]
- Putzien, S.; Nuyken, O.; Kühn, F.E. Functionalized polysilalkylene siloxanes (polycarbosiloxanes) by hydrosilylation—Catalysis and synthesis. Prog. Polym. Sci. 2010, 35, 687–713. [Google Scholar] [CrossRef]
- Chruściel, J.J.; Leśniak, E. Modification of epoxy resins with functional silanes, polysiloxanes, silsesquioxanes, silica and silicates. Prog. Polym. Sci. 2015, 41, 67–121. [Google Scholar] [CrossRef]
- Belay, T.A.; Chen, J.; Xu, H.; Zhang, S.; Chen, S.; Li, X. Functionalization Methodology for Synthesis of Silane-End-Functionalized Linear and Star Poly(aryl isocyanide)s by Combination of Cationic Polymerization and Hydrosilylation Reaction. Macromolecules 2021, 54, 9007–9018. [Google Scholar] [CrossRef]
- Crawford, K.E.; Sita, L.R. Stereoengineering of Poly(1,3-methylenecyclohexane) via Two-State Living Coordination Polymerization of 1,6-Heptadiene. J. Am. Chem. Soc. 2013, 135, 8778–8781. [Google Scholar] [CrossRef]
- Crawford, K.E.; Sita, L.R. De Novo Design of a New Class of “Hard–Soft” Amorphous, Microphase-Separated, Polyolefin Block Copolymer Thermoplastic Elastomers. ACS Macro Lett. 2015, 4, 921–925. [Google Scholar] [CrossRef]
- Burgenson, W.R.; Wentz, C.M.; Sita, L.R. Tailoring Glass Transition Temperature in a Series of Poly(methylene-1,3-cyclopentane-stat-cyclohexane) Statistical Copolymers. ACS Macro Lett. 2023, 12, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Nomura, K. Living Copolymerization of Ethylene with Styrene Catalyzed by (Cyclopentadienyl)(ketimide)titanium(IV) Complex−MAO Catalyst System: Effect of Anionic Ancillary Donor Ligand. Macromolecules 2006, 39, 5266–5274. [Google Scholar] [CrossRef]
- Li, G.; Zuccaccia, C.; Tedesco, C.; D’Auria, I.; Macchioni, A.; Pellecchia, C. NMR Spectroscopy and X-Ray Characterisation of Cationic N-Heteroaryl-Pyridylamido ZrIV Complexes: A Further Level of Complexity for the Elusive Active Species of Pyridylamido Olefin Polymerisation Catalysts. Chem. Eur. J. 2014, 20, 232–244. [Google Scholar] [CrossRef]







| Reactants | Reagents (1) | Intermediate (1) | Reagents (2) | Intermediate (2) | Ref. |
|---|---|---|---|---|---|
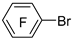
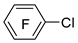 | i-PrMgBr |  | BF3·Et2O | 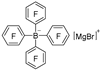 | [40] |
 | n-Butyl lithium | 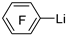 | B(OCH3)3 |  | [41] |
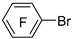 | n-Butyl lithium |  | BCl3 | 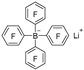 | [42] |
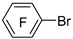 | Mg |  | B(OCH3)3 |  | [43] |
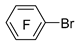 | Mg | 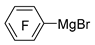 | BF3·Et2O/n-butyl ether | 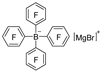 | [44] |
 | Mg | 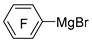 | BF3·Et2O/toluene | 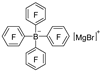 | [45] |
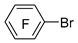 | n-Butyl lithium | 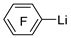 | BCl3 |  | [46] |
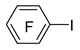 | n-Butyl lithium | 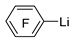 | BBr3 | 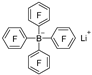 | [46] |
| Intermediate (1) | Reactants | Intermediate (2) | Ref. |
|---|---|---|---|
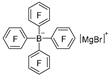 | K2CO3 |  | [47] |
 | KF |  | [48] |
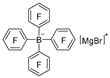 | Na2CO3/H2O | 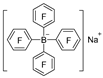 | [49] |
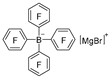 | NaF |  | [41] |
 | NH4Cl/H2O | 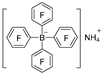 | [50] |
| Intermediate | Reactants | Products | Yield | Ref. |
|---|---|---|---|---|
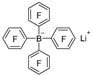 | 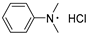 | DMAB | 96.1% | [39] |
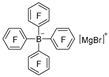 |  | DMAB | 77.7% | [44] |
 |  | DMAB | 87% | [48] |
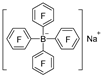 |  | DMAB | 89.9% | [49] |
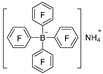 | 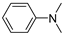 | DMAB | 96.2% | [50] |
| Catalysts | Cocatalysts | Catalytic Activity/kg mol−1 h−1 | Mw/103 | Mw/Mn | Tm/°C | Ref. |
|---|---|---|---|---|---|---|
| Hf-type complex | MMAO | 7800 | 279 | 49.8 | 121 | [68] |
| Hf-type complex | MDOAB | 19,000 | 158 | 2.95 | 118 | [68] |
| Zr-type complex | MDOAB | 28,000 | 200 | 8.4 | 95/120 | [69] |
| Ti-type complex | MDOAB | 966,000 | 21 | 2.9 | — | [70] |
| Bimetallic catalyst | MDOAB | 575,000 | 217 | — | 55.6 | [63] |
| Cr-type complex | MDOAB | 81,000 | 138 | — | — | [71] |
| Catalysts | Cocatalysts | Catalytic Activity/kg mol−1 h−1 | Mn/10−3 | Mw/Mn | Cis-1,4/% | Ref. |
|---|---|---|---|---|---|---|
| Nd-type complex | MAO | — | 417 | 2.17 | 92.0 | [88] |
| Nd-type complex | DMAB | 68 | 55.7 | 1.34 | 92.0 | [89] |
| Nd-type complex | DMAB | 220 | 351 | 1.66 | 79.0 | [90] |
| Nd-type complex | DMAB | — | 201 | 2.27 | 90.4 | [53] |
| Y-type complex | DMAB | — | 127 | — | 86.5 | [91] |
| Yb-type complex | DMAB | — | 54 | — | 54.9 | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Qu, S.; Li, X.; Chen, J.; Wen, Z.; Li, Q.; Wang, W. Ammonium Tetrakis(pentafluorophenyl)borate: Preparation and Application in Olefin Coordination Polymerization as the Cocatalyst Compound. Polymers 2024, 16, 1689. https://doi.org/10.3390/polym16121689
Wei Y, Qu S, Li X, Chen J, Wen Z, Li Q, Wang W. Ammonium Tetrakis(pentafluorophenyl)borate: Preparation and Application in Olefin Coordination Polymerization as the Cocatalyst Compound. Polymers. 2024; 16(12):1689. https://doi.org/10.3390/polym16121689
Chicago/Turabian StyleWei, Yiming, Shuzhang Qu, Xinwei Li, Jian Chen, Zhao Wen, Qian Li, and Wei Wang. 2024. "Ammonium Tetrakis(pentafluorophenyl)borate: Preparation and Application in Olefin Coordination Polymerization as the Cocatalyst Compound" Polymers 16, no. 12: 1689. https://doi.org/10.3390/polym16121689
APA StyleWei, Y., Qu, S., Li, X., Chen, J., Wen, Z., Li, Q., & Wang, W. (2024). Ammonium Tetrakis(pentafluorophenyl)borate: Preparation and Application in Olefin Coordination Polymerization as the Cocatalyst Compound. Polymers, 16(12), 1689. https://doi.org/10.3390/polym16121689







