Development and Characterization of the Shale Stratum Well Wall Stabilized with Nanosomal Sealing Agent
Abstract
1. Introduction
2. Experimental Section
2.1. Experimental Materials
2.2. Sample Preparation
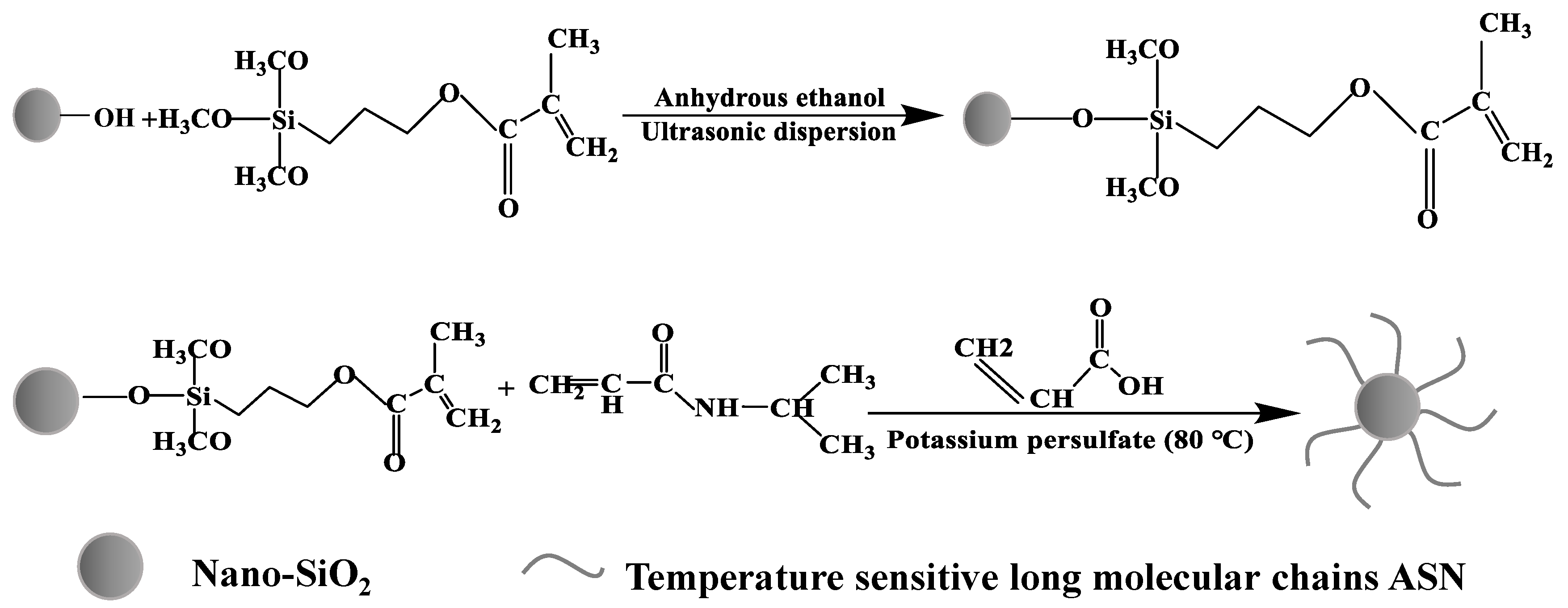
2.2.1. Surface Modification of Nano SiO2
2.2.2. Synthesis of ASN Nanosealing Agent
2.3. Temperature-Responsive Nanosealing Agent Characterization
2.4. Comprehensive Performance Evaluation
2.4.1. Core Sealing Performance
2.4.2. Temperature Response Performance
2.4.3. Wetting Performance
2.4.4. Toxicity Evaluation
3. Results and Discussion
3.1. Characterization of ASN
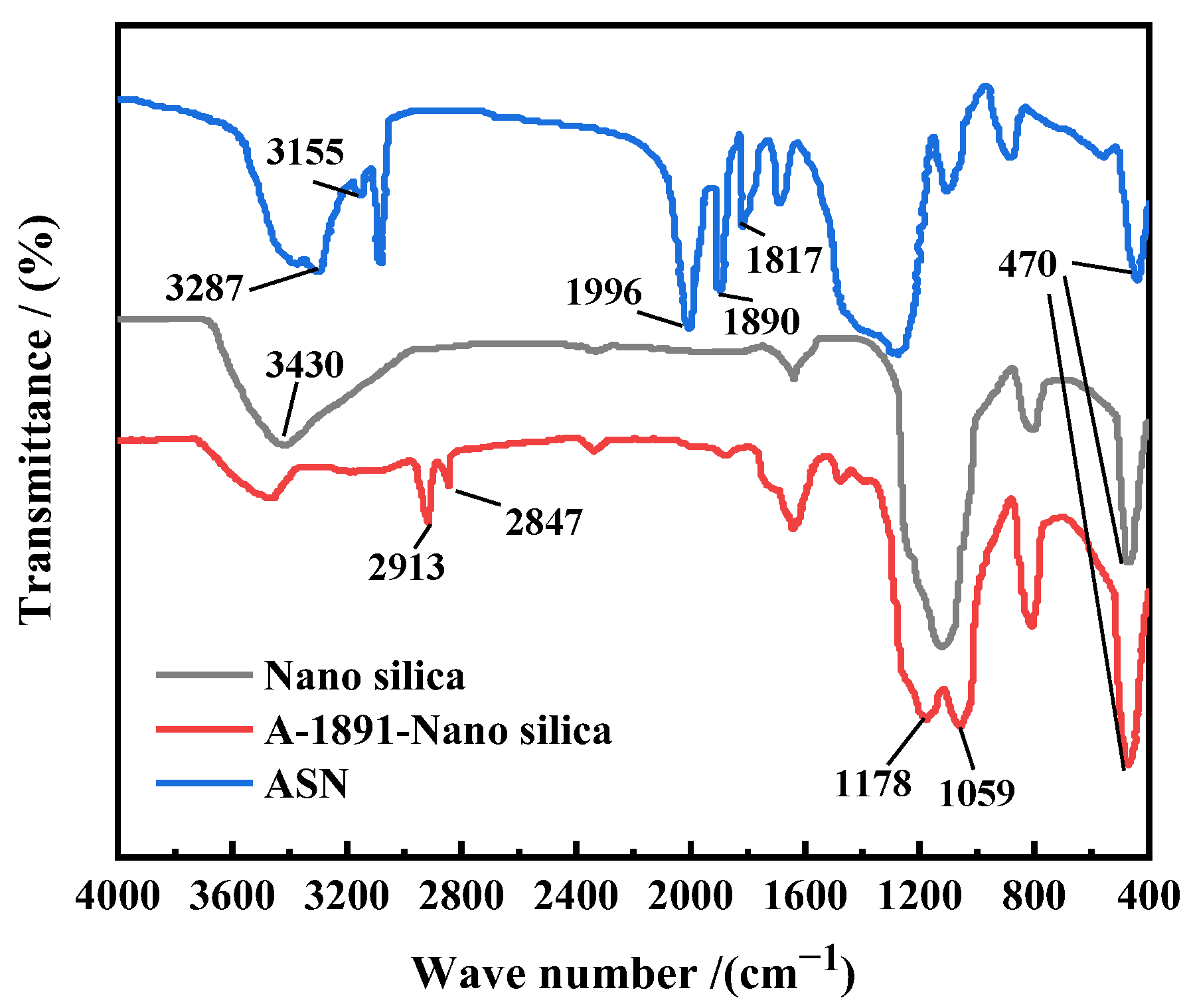
3.1.1. Characterization of FT-IR
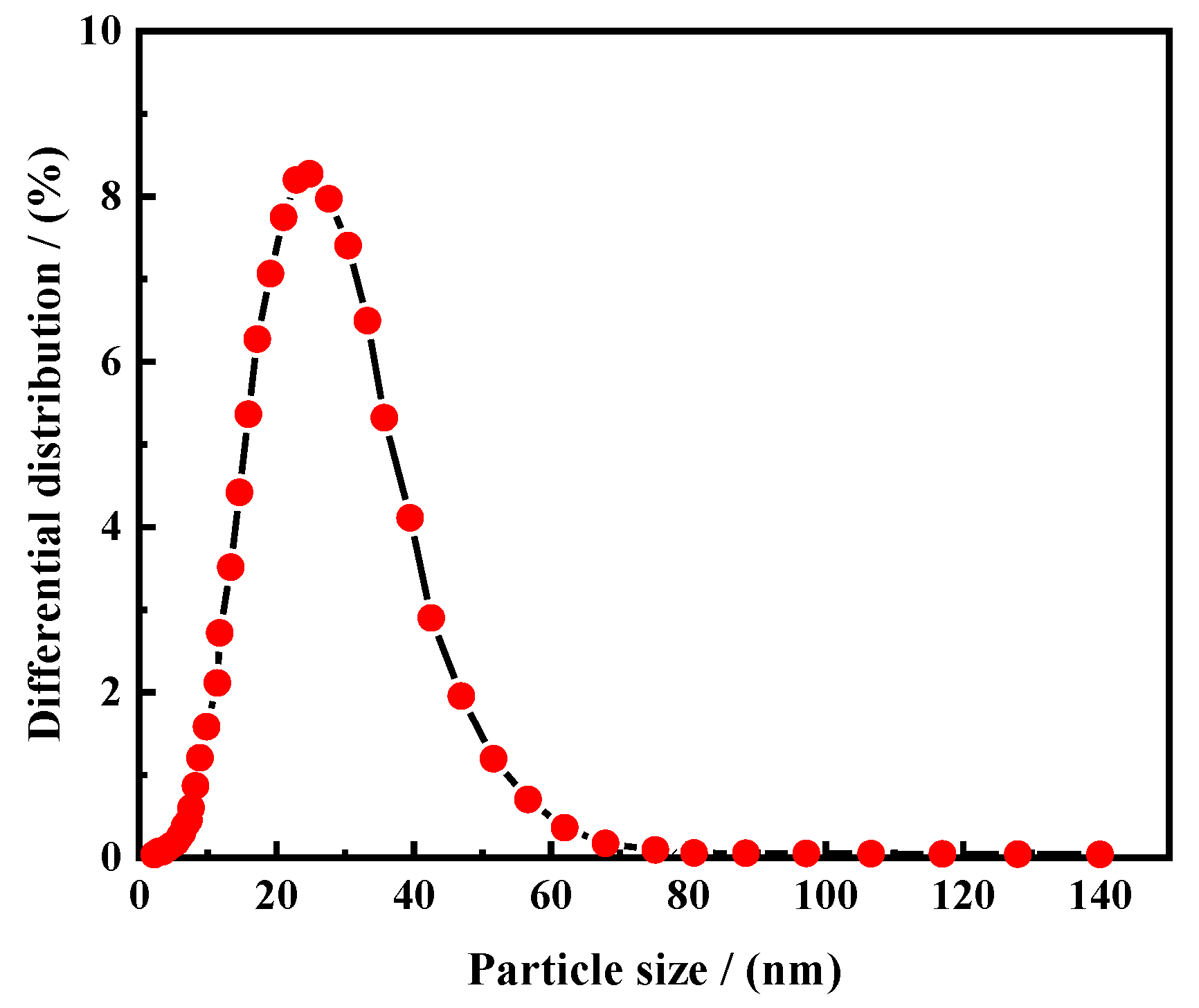
3.1.2. Particle Size Characterization
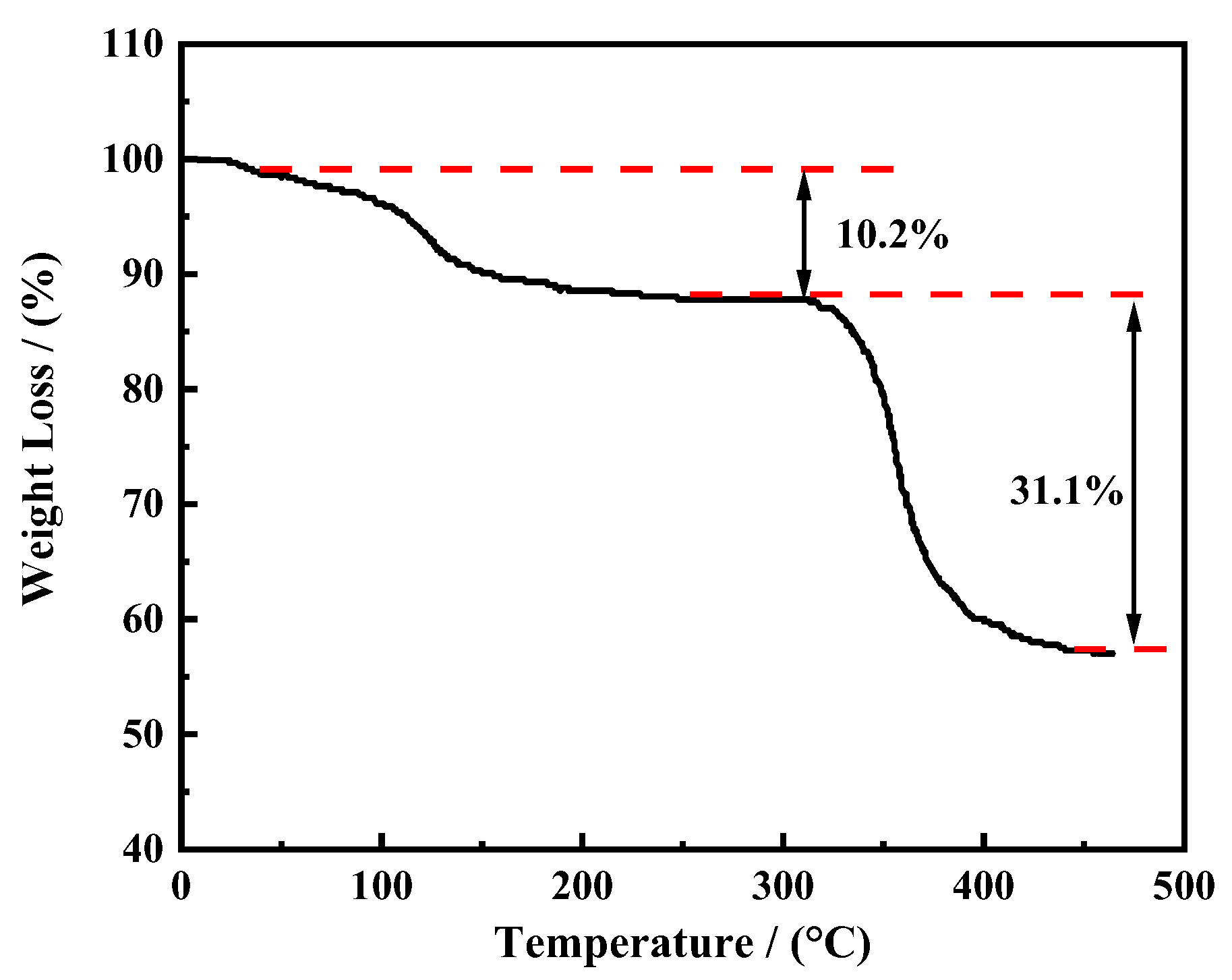
3.1.3. Thermogravimetric Analysis (TG)
3.1.4. ASN Biotoxicity Evaluation
3.2. Performance Testing
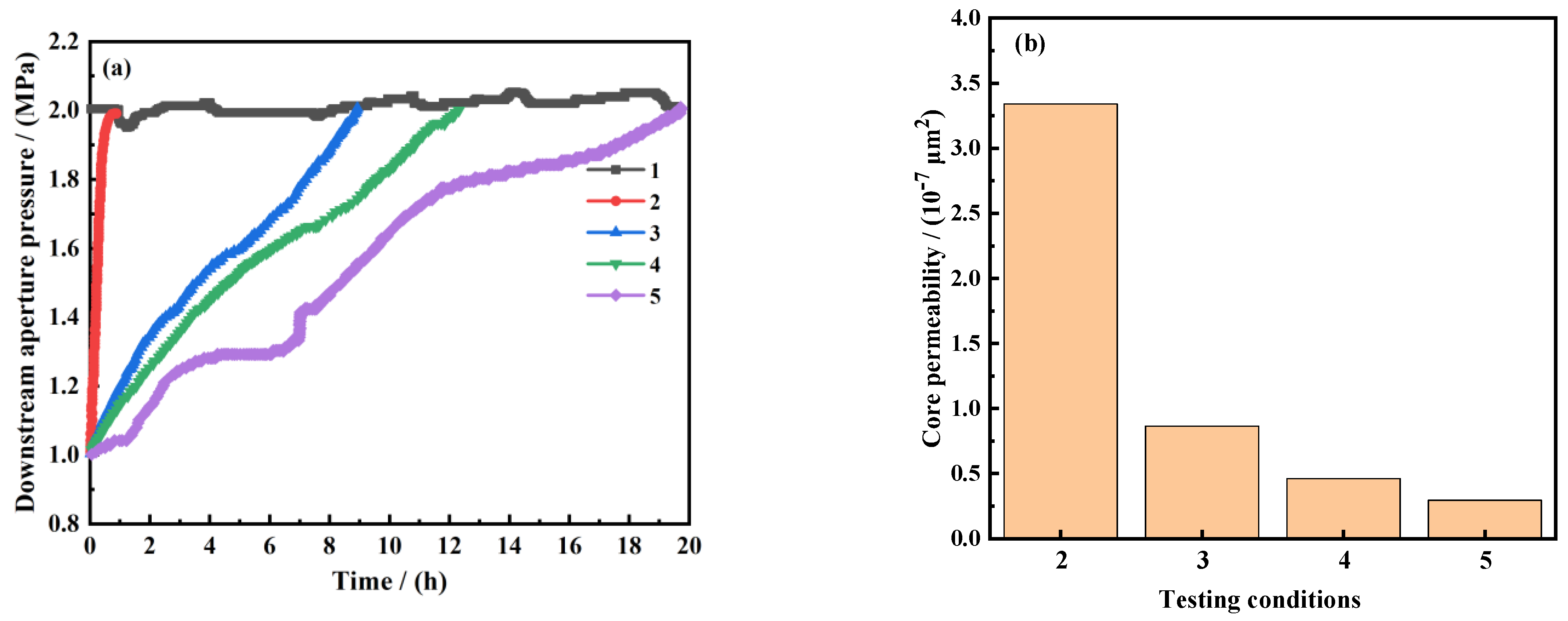
3.2.1. Sealing Performance Evaluation
- (1)
- Pressure transmission experiment
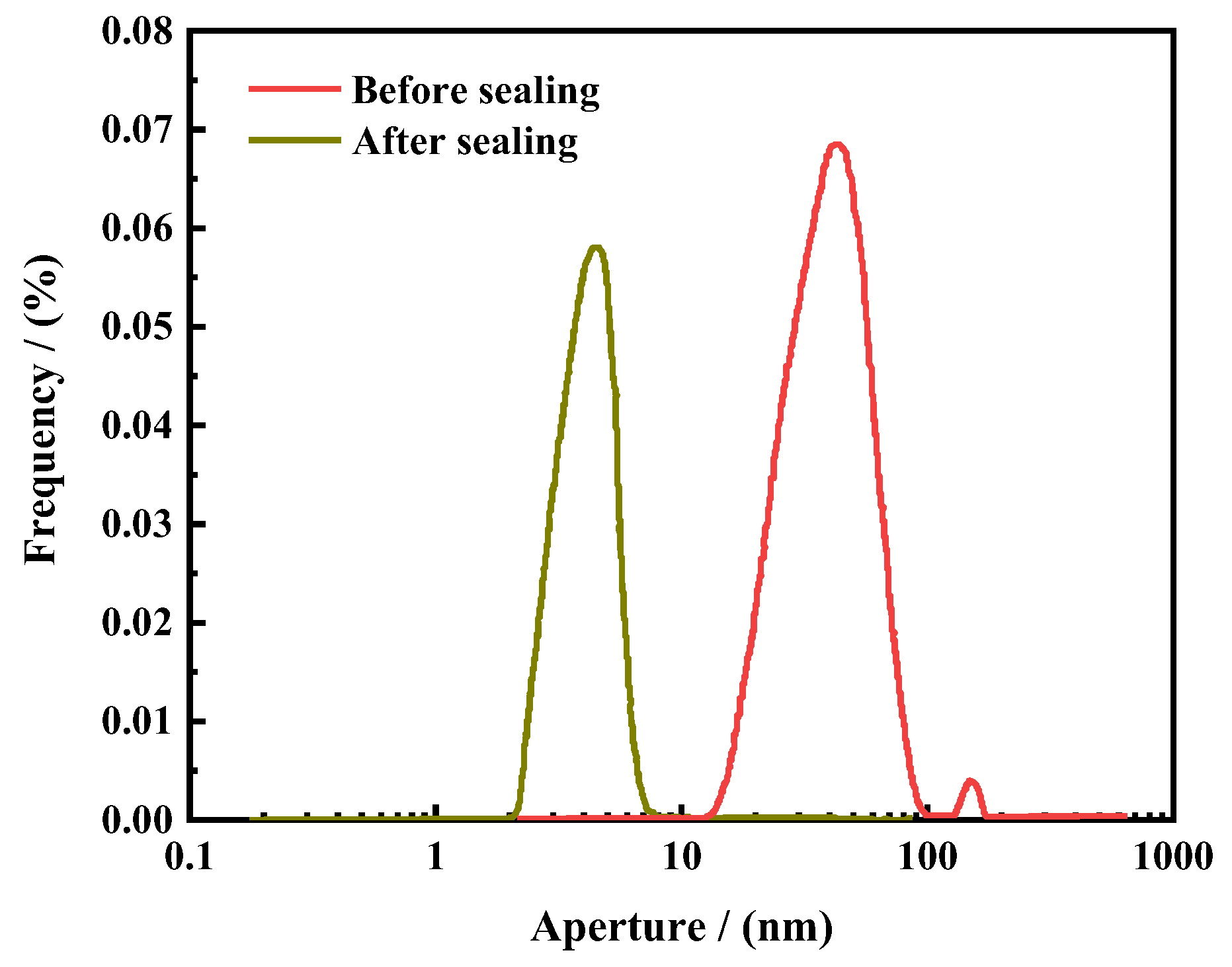
- (2)
- Nuclear magnetic resonance core pore size analysis
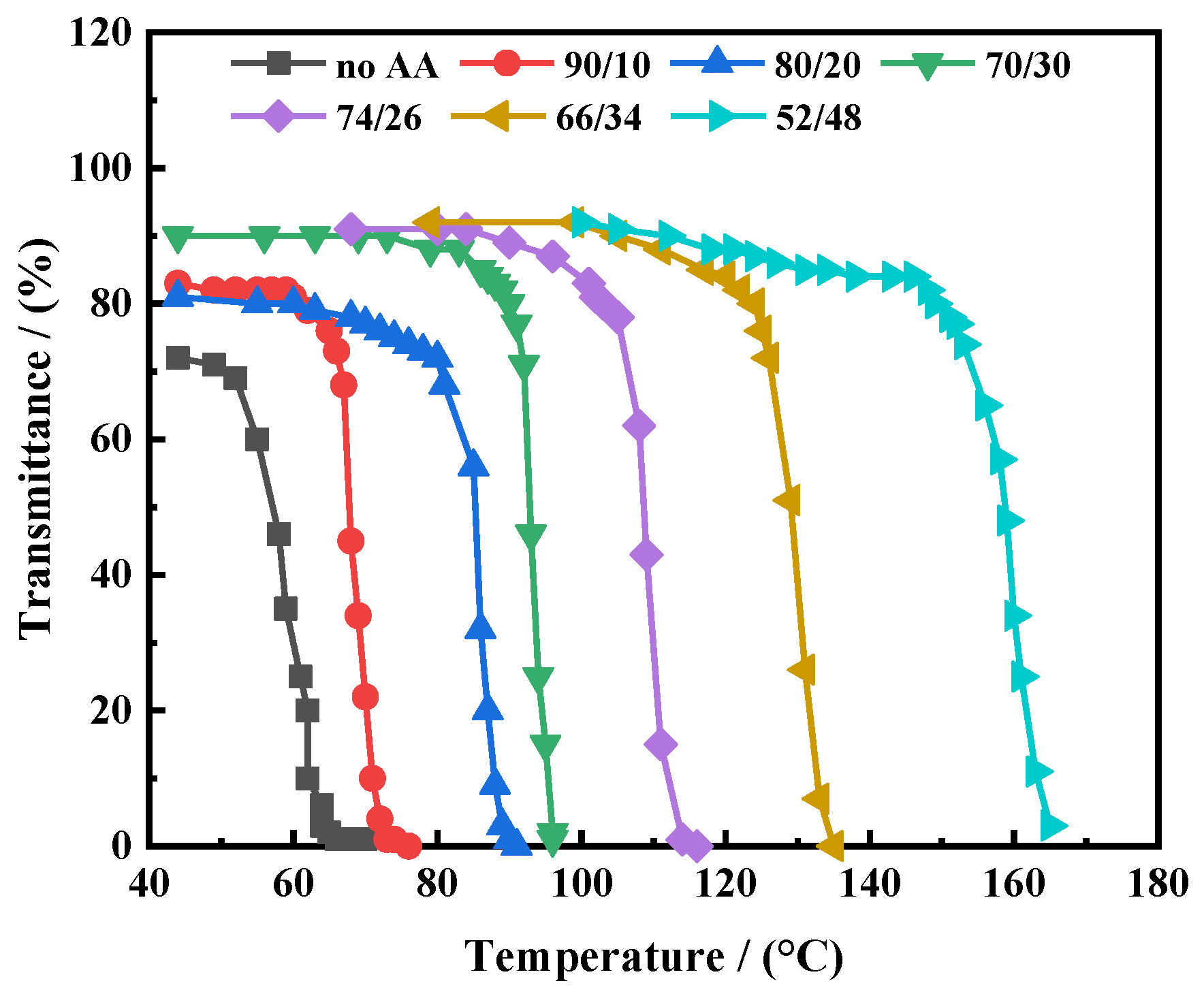
3.2.2. Temperature-Sensitive Characteristics
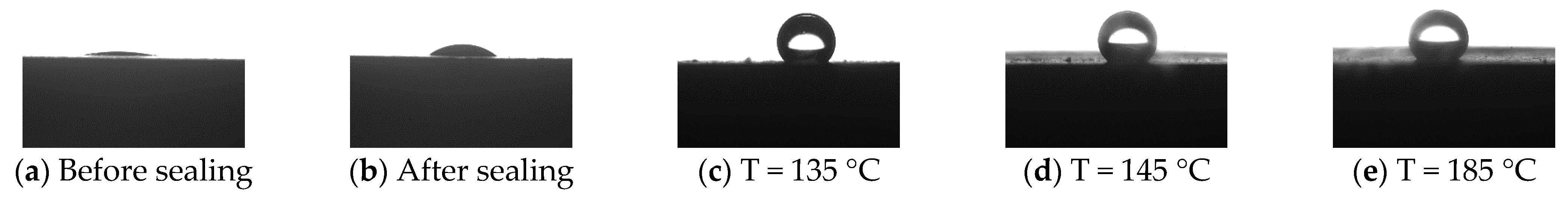
3.2.3. Wettability Testing
3.3. Comprehensive Analysis of the Mechanism of Action
4. Conclusions
- The SiO2 nanoparticles were surface-treated with the silane-coupling agent A-1891 to obtain the temperature-responsive nanoparticle ASN, which possesses good thermal stability and strong temperature resistance. ASN is also an environmentally friendly treatment agent.
- ASN can effectively minimize the pore dimensions of shale and make it denser through entry into the nanoscale micropores and microcracks of shale. When the external temperature surpasses the LCST of the nanosealing agent ASN, its surface undergoes a transition from hydrophilic to hydrophobic and forms a hydrophobic layer. This, in turn, enhances the wellbore stability of the rock strata.
- Consequently, ASN, under optimal temperature and pressure conditions, is capable of concurrently performing physical sealing and chemical inhibition, resulting in the formation of a continuous and dense pressure-resistant sealing layer around the wellbore.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Yang, H.; Wei, Y.; Feng, Y.; Yang, H.; Yan, W.; Fu, L. Wellbore instability in naturally fractured formations: Experimental study and 3D numerical investigation. Gas Sci. Eng. 2024, 124, 205265. [Google Scholar] [CrossRef]
- Wei, Y.; Feng, Y.; Tan, Z.; Yang, T.; Yan, S.; Li, X.; Deng, J. Simultaneously improving ROP and maintaining wellbore stability in shale gas well: A case study of Luzhou shale gas reservoirs. Rock Mech. Bull. 2024; 3, 100124. [Google Scholar]
- Li, M.; Xu, J.; Pei, D.; Su, K.; Wang, L. Evaluation of Aminated Nano-Silica as a Novel Shale Stabilizer to Improve Wellbore Stability. Materials 2024, 17, 1776. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, X.; Liu, X.; Zhai, Y.; Fang, F.; Guo, W.; Qian, C.; Han, L.; Cui, Y.; Jia, Y. Review of the productivity evaluation methods for shale gas wells. J. Pet. Explor. Prod. Technol. 2024, 14, 25–39. [Google Scholar] [CrossRef]
- Wang, C.; Lin, Y. The characteristic analysis of micro-nano pore structure in shale gas formation and its sealing evaluation. Sci. Technol. Eng. 2017, 17, 32–38. [Google Scholar]
- Mahetaji, M.; Brahma, J. Prediction of minimum mud weight for prevention of breakout using new 3D failure criterion to maintain wellbore stability. Rock Mech. Rock Eng. 2024, 57, 2231–2252. [Google Scholar] [CrossRef]
- He, Z.; Yang, Y.; Qi, J.; Lin, X.; Wang, N.; Wang, L.; Dai, H.; Lu, H. Hyperbranched polymer nanocomposite as a potential shale stabilizer in water-based drilling fluids for improving wellbore stability. J. Mol. Liq. 2024, 395, 123903. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, Y.; Zhu, K.; Ding, Y.; Liang, L. The analyses on wellbore stability in hard brittle shale in Dongying formation of Jidong oilfield. Sci. Technol. Eng. 2018, 18, 109–115. [Google Scholar]
- Wen, H.; Chen, M.; Jin, Y.; Wang, K.; Xia, Y.; Dong, J.; Niu, C. A chemo-mechanical coupling model of deviated borehole stability in hard brittle shale. Pet. Explor. Dev. 2014, 41, 748–754. [Google Scholar] [CrossRef]
- Liu, W.; Li, A.; Zhu, X. The mechanism of wellbore instability in high-temperature fractured granite formation. Energy 2024, 299, 131425. [Google Scholar] [CrossRef]
- Ning, W.; Ju, W.; Guo, W. The present-day in-situ stress field and its effect on shale gas development in Zigong area of southern Sichuan Basin. Unconv. Resour. 2024, 4, 100078. [Google Scholar] [CrossRef]
- Lei, M.; Li, N. The damage mechanism of oil-based drilling fluid for tight sandstone gas reservoir and its optimization. J. Pet. Sci. Eng. 2017, 158, 616–625. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, G.; Wu, H.; Yang, Y.; Lu, C.; Cheng, Z. Study and application of a graphene lugging agent for water based drilling fluids. Drill. Fluid Complet. Fluid 2023, 40, 462–466. [Google Scholar]
- Teng, C.; Zhen, J.; Luo, H.; Jiang, S. A strongly adsorptive hydrophobically modified nano SiO2 plugging agent. Drill. Fluid Complet. Fluid 2022, 39, 307–312. [Google Scholar]
- Wang, W.; Zhao, C.; Luo, J.; Li, C.; Liu, G.; Geng, T. Development and Application of the High Temperature Plugging Agent PF-MOSHIELD for Oil Base Drilling Fluids. Drill. Fluid Complet. Fluid 2019, 36, 153–159. [Google Scholar]
- Xu, L.; Wang, D.; Liu, L.; Wang, C.; Zhu, H.; Tang, X. Review of Shale Oil and Gas Refracturing: Techniques and Field Applications. Processes 2024, 12, 965. [Google Scholar] [CrossRef]
- Gai, B.; Duan, H.; Jiang, Y. Development of environmentally friendly hydrophilic polyurethane anti-seepage sealing agent. New Chem. Mater. 2024, 6, 1–10. [Google Scholar]
- Shu, R.; Long, J.; Zhang, Q.; Wang, T.; Ma, L.; Yuan, Z.; Wu, Q. The preparation and application of core-shell structure materials. Adv. New Renew. Energy 2014, 2, 423–429. [Google Scholar]
- Xu, L.; Deng, M.; Guo, Y. Research on application of nano-plugging agent in drilling fluid. Appl. Chem. Ind. 2016, 45, 742–746. [Google Scholar]
- Wu, Y.; Zhu, Z.; Yue, Q.; Jiang, W.; Lin, Y.; Zheng, Z.; Ding, X. Research progress of nanoparticles modified water-based drilling fluid. Polym. Bull. 2014, 1, 1–6. [Google Scholar] [CrossRef]
- Li, L.; Sun, J.; Liu, Y. Recent progress of application of nanomaterials in drilling/completion fluids and reservoir protection. Oilfield Chem. 2013, 30, 139–144. [Google Scholar]
- Zhang, T.; Wu, Y.; Zheng, Z.; Ding, X.; Peng, Y. Preparation of dual responsive gold nanoparticles using click chemistry and living radical polymerization. Chem. J. Chin. Univ. 2010, 31, 2303–2307. [Google Scholar]
- Choi, S.; Choi, R.; Han, S.; Park, J.T. Synthesis and characterization of Pt9Co nanocubes with high activity for oxygen reduction. Chem. Commun. 2010, 46, 4950–4952. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fan, Y.; Wang, X. Study on migration law of multiscale temporary plugging agent in rough fractures of shale oil reservoirs. Front. Phys. 2023, 11, 1228006. [Google Scholar] [CrossRef]
- Liu, W.; Xu, Q.; Lin, C.; Yuan, Z.; Xu, X. Experimental study of a novel oil-based crosslinking-curing lost circulation material for shale gas well. Pet. Sci. Technol. 2023, 41, 1492–1509. [Google Scholar] [CrossRef]
- An, Q.; Guo, K. The surface modification and its research progress in composite materials of nano SiO2. Nanotechnology 2007, 5, 9–15. [Google Scholar]
- Dong, H.; Li, Z.; Xu, D.; Yan, L.; Wang, L.; Ye, Y. Study on the Dispersion Stability and Sealing Performance of Nanoscale Plugging Materials for Shale Formations. SPE J. 2024, 1–12. [Google Scholar] [CrossRef]
- Pan, Y.; Cui, X.; Wang, H.; Lou, X.; Yang, S.; Oluwabusuyi, F.F. Research Progress of Intelligent Polymer Plugging Materials. Molecules 2023, 28, 2975. [Google Scholar] [CrossRef]
- Hayeemasae, N.; Ajaman, S.; Soontaranon, S.; Mohamad Rasidi, M.S.; Masa, A. Optimising silane coupling agent content in phenolic-resin-cured sepiolite-filled natural rubber composites. J. Rubber Res. 2023, 26, 303–312. [Google Scholar] [CrossRef]
- Guo, Z.; Shangguan, H.; Liu, Q.; Li, Y.; Liu, X.; Sun, A.; Li, Y.; Wei, L. Synthesis of hydroxyl silane coupling agent and its application in preparation of silane-modified polyurethane. Polym. Eng. Sci. 2023, 63, 2325–2335. [Google Scholar] [CrossRef]
- Chumnanwat, S.; Ota, S.; Nishizawa, J.; Sonthichai, C.; Takiguchi, N.; Kodama, A.; Kumita, M. Formation of adsorbent thin layer on aluminum sheet by using a silane coupling agent for vapor adsorption refrigeration system. Int. J. Refrig. 2023, 146, 40–46. [Google Scholar] [CrossRef]
- Liu, J. Study on Wellbore Strengthening Drilling Fluid Technology. Ph.D. Thesis, China University of Petroleum (East China), Qingdao, China, 2016. [Google Scholar]
- Zheng, G.; Ling, B.; Zheng, D.; Lian, H.Q.; Zhu, X.Z. Application of NMR technology in coal pore size analysis. J. North China Inst. Sci. Technol. 2014, 11, 1–7. [Google Scholar]
- Li, Z. Quantitative characterization of pore structure continuity based on NMR and Hg dataa case study of the middle third member of Shahejie Formation in Bonan Oilfield. J. Shengli Coll. China Univ. Pet. 2016, 30, 11–14. [Google Scholar]
- Wang, Z.; Huang, C.; Wang, T. Measurement of pore size distribution of cement-based materials by nuclear magnetic resonance freeze hole method. J. Build. Mater. 2012, 15, 6–10. [Google Scholar]
- Liu, K.; Wang, R.; Rong, K.; Yin, Z.; Lu, T.; Yu, Y.; Li, Y.; Yang, Z.; Yang, J.; Zhao, Z. Synthesis and Plugging Performance of Nano-Micron Polymeric Gel Microsphere Plugging Agents for Oil-Based Drilling Fluids. Gels 2023, 9, 290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q. Development and Application of a Novel Nano-Micron Plugging Agent with Core-Shell Structure for Oil-Based Drilling Fluids. Chem. Technol. Fuels Oils 2023, 59, 891–900. [Google Scholar] [CrossRef]
- Wang, L.; Yang, J.; Peng, J.; Han, H.; Wang, Y.; Lv, Z. Temporary Plugging Agent Evaluation Technology and Its Applications in Shale Reservoirs in the Sichuan Basin. Processes 2023, 11, 2799. [Google Scholar] [CrossRef]
- Tchameni, A.P.; Djouonkep, L.D.W.; Nagre, R.D.; Wang, X. Thermo-responsive polymer-based Janus biogenic-nanosilica composite, part B: Experimental study as a multi-functional synergistic shale stabilizer for water-based drilling fluids. J. Mol. Liq. 2024, 395, 123921. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.; Cai, J.; Zhang, S. Application of Micro-Seismic Monitoring in Post-Fracturing Evaluation of Shale Gas: A Case Study of Well X from Puguang Area, China. Processes 2023, 11, 1863. [Google Scholar] [CrossRef]
- GB/T 15441-1995; Water Quality-Determination of the Acute Toxicity-Luminescent Bacteria Test. Standardization Administration of China: Beijing, China, 1995.
- Q/SY 111-2007; Grading and Determination of the Biotoxicity of Chemicals and Drilling Fluids—Luminescent Bacteria Test. National Energy Administration: Beijing, China, 2007.
- Zhang, C.; Sun, J.; Huang, X.; Zhang, Y.; Zong, J.; Lv, K.; Dai, J. Use of modified polystyrene micro-nano spheres to improve the inhibition and plugging performance of water-based drilling fluids. Colloids Surf. A Physicochem. Eng. Asp. 2023, 668, 131409. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, C.; Li, G.; Wang, R.; Liu, D.; Luo, P. Based on self-made shale formation simulated artificial cores to evaluate water-based drilling fluids plugging effect technology. Pet. Res. 2023, 8, 11–17. [Google Scholar] [CrossRef]


| Mass Concentration/(106 mg∙L−1) | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|
| Result 1/% | 96.84 | 79.46 | 61.69 | 50.55 | 28.12 | 24.72 | 17.30 |
| Result 2/% | 90.35 | 79.18 | 59.95 | 41.41 | 31.61 | 25.23 | 12.99 |
| Result 3/% | 92.25 | 82.20 | 63.21 | 48.26 | 31.33 | 30.22 | 17.88 |
| Average value/% | 93.15 | 80.28 | 61.62 | 46.74 | 30.35 | 26.72 | 16.06 |
| Test Conditions | Contact Angle (°) |
|---|---|
| Before sealing | 10 |
| After sealing | 37 |
| T = 135 °C | 136 |
| T = 145 °C | 142 |
| T = 185 °C | 139 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Gao, S.; Tang, Z.; Wu, H.; Zhang, Y. Development and Characterization of the Shale Stratum Well Wall Stabilized with Nanosomal Sealing Agent. Polymers 2024, 16, 1614. https://doi.org/10.3390/polym16121614
Li D, Gao S, Tang Z, Wu H, Zhang Y. Development and Characterization of the Shale Stratum Well Wall Stabilized with Nanosomal Sealing Agent. Polymers. 2024; 16(12):1614. https://doi.org/10.3390/polym16121614
Chicago/Turabian StyleLi, Daqi, Shuyang Gao, Zhichuan Tang, Huimei Wu, and Yayun Zhang. 2024. "Development and Characterization of the Shale Stratum Well Wall Stabilized with Nanosomal Sealing Agent" Polymers 16, no. 12: 1614. https://doi.org/10.3390/polym16121614
APA StyleLi, D., Gao, S., Tang, Z., Wu, H., & Zhang, Y. (2024). Development and Characterization of the Shale Stratum Well Wall Stabilized with Nanosomal Sealing Agent. Polymers, 16(12), 1614. https://doi.org/10.3390/polym16121614






