In Vitro Investigation of the Effects of Various Reducing Agents on Dentin Treated with Hydrogen Peroxide
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation for SBS Testing
2.2. Shear Bond Strength Test
2.3. Specimen Preparation for Microhardness and Roughness Tests
2.4. Microhardness Measurement
2.5. Surface Roughness Measurement
2.6. Smear Layer Removal
2.7. Statistical Analysis
3. Results
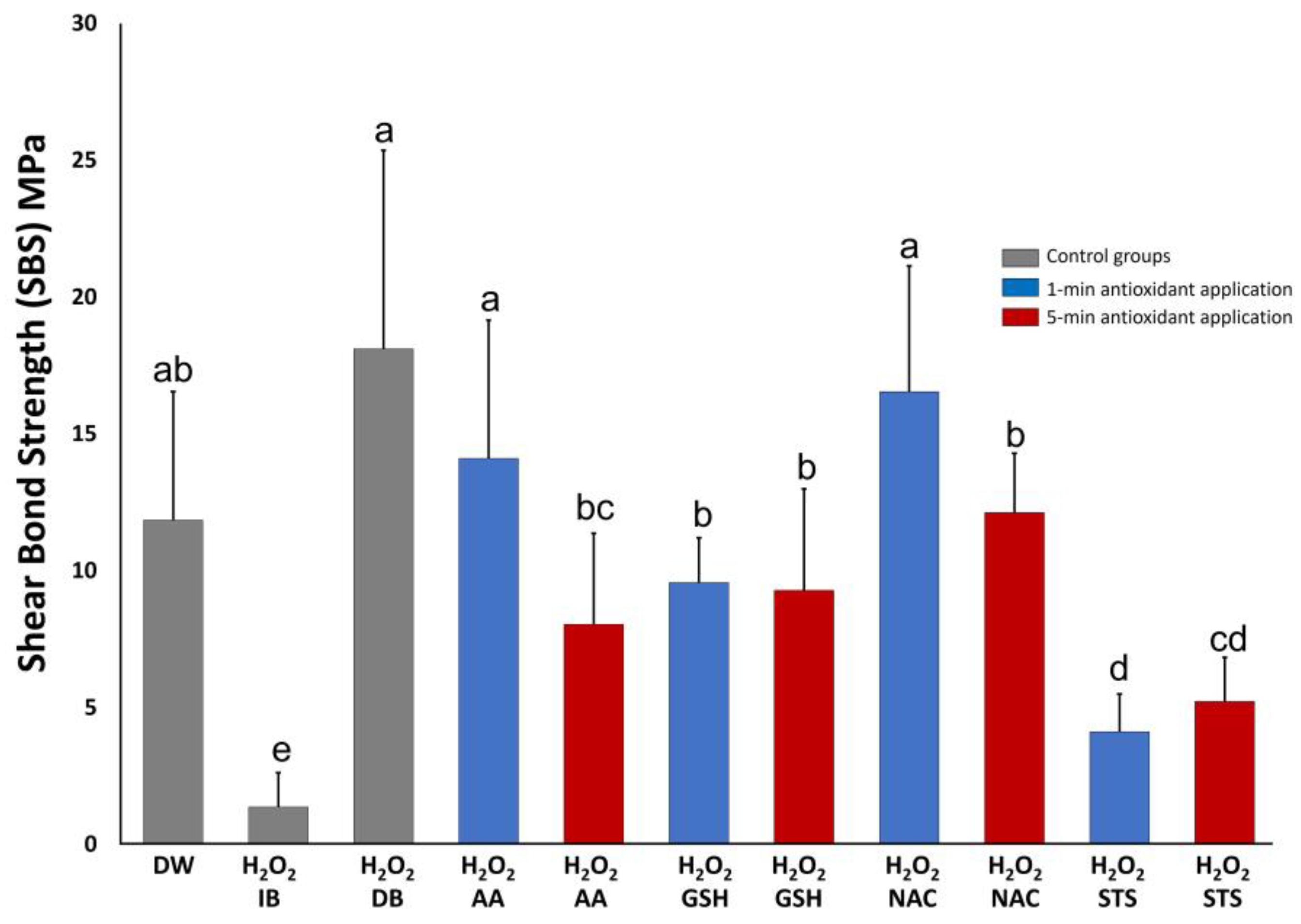
3.1. Shear Bond Strength Test
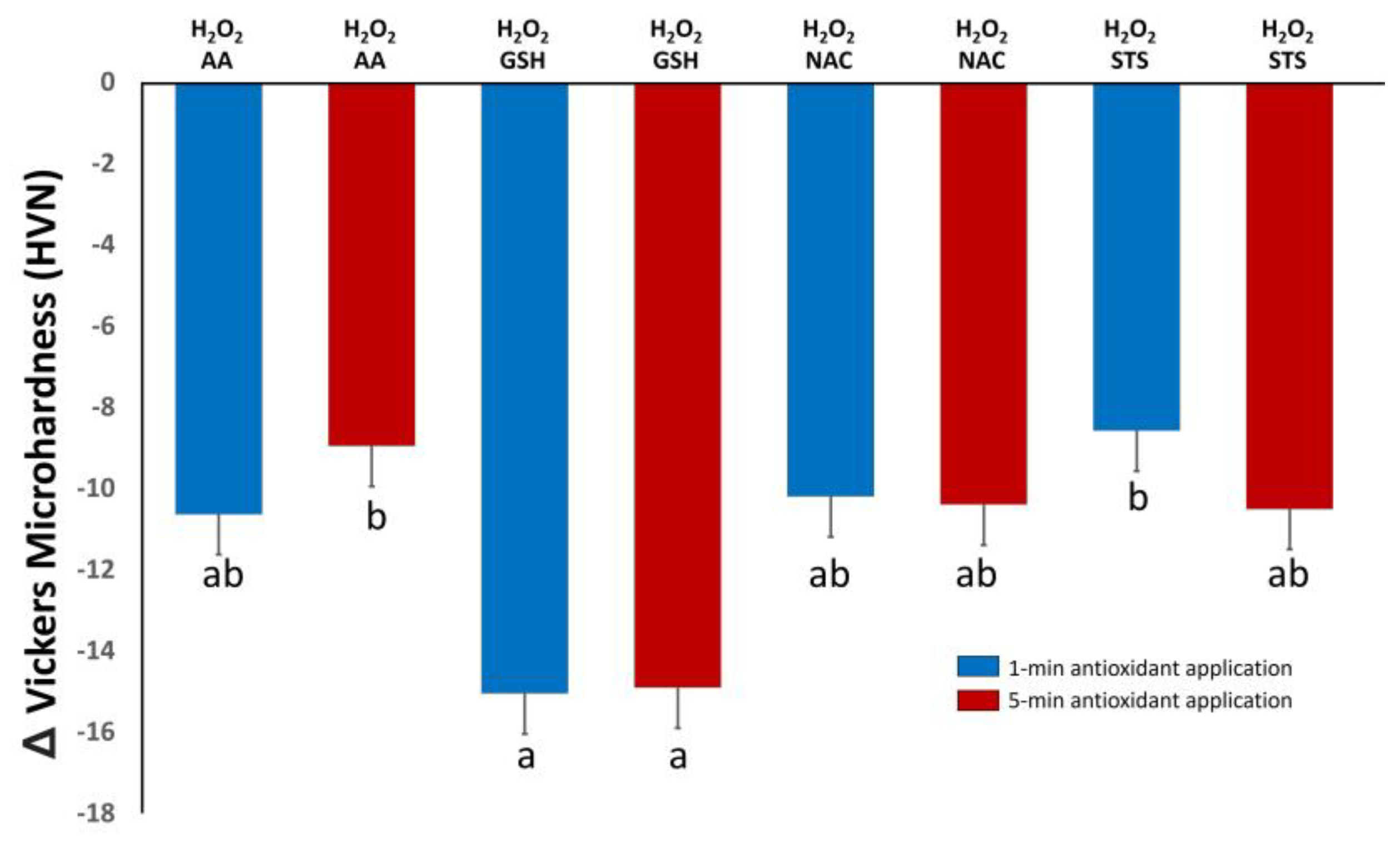
3.2. Microhardness
3.3. Surface Roughness
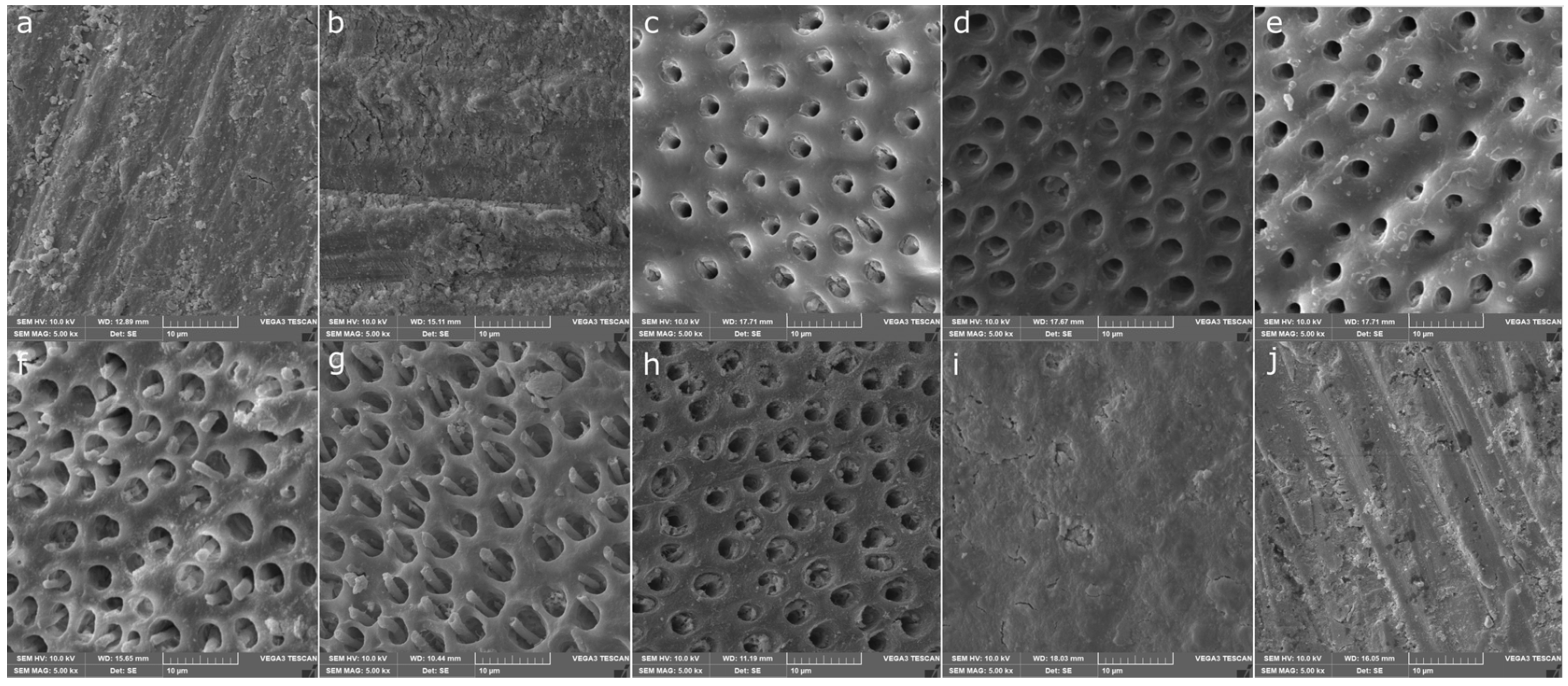
3.4. Smear Layer Removal
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Alqahtani, M.Q. Tooth-bleaching procedures and their controversial effects: A literature review. Saudi Dent. J. 2014, 26, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Féliz-Matos, L.; Hernández, L.M.; Abreu, N. Dental Bleaching Techniques; Hydrogen-carbamide Peroxides and Light Sources for Activation, an Update. Mini Review Article. Open Dent. J. 2015, 8, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Minoux, M.; Serfaty, R. Vital tooth bleaching: Biologic adverse effects-a review. Quintessence Int. 2008, 39, 645–659. [Google Scholar] [PubMed]
- Sun, L.; Liang, S.; Sa, Y.; Wang, Z.; Ma, X.; Jiang, T.; Wang, Y. Surface alteration of human tooth enamel subjected to acidic and neutral 30% hydrogen peroxide. J. Dent. 2011, 39, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Savian, T.G.; Oling, J.; Soares, F.; Rocha, R.O. Vital Bleaching Influences the Bond Strength of Adhesive Systems to Enamel and Dentin: A Systematic Review and Meta-Analysis of In Vitro Studies. Oper Dent. 2021, 46, E80–E97. [Google Scholar] [CrossRef] [PubMed]
- Arslan, S.; Balkaya, H.; Durukan, S.M.; Silici, S. The effect of propolis on the bond strength of composite resin to enamel after intracoronal bleaching with different bleaching agents. Aust. Endod. J. 2023, 49 (Suppl. S1), 366–373. [Google Scholar] [CrossRef] [PubMed]
- Kaya, A.D.; Türkün, M. Reversal of dentin bonding to bleached teeth. Oper. Dent. 2003, 28, 825–829. [Google Scholar] [PubMed]
- Shetti, A.; Keluskar, V.; Aggarwal, A. Antioxidants: Enhancing oral and general health. J. Indian Acad. Oral Med. Radiol. 2009, 21, 1–6. [Google Scholar] [CrossRef]
- Lai, S.C.; Mak, Y.F.; Cheung, G.S.; Osorio, R.; Toledano, M.; Carvalho, R.M.; Tay, F.; Pashley, D. Reversal of compromised bonding to oxidized etched dentin. J. Dent. Res. 2001, 80, 1919–1924. [Google Scholar] [CrossRef]
- Miranda, T.A.; Moura, S.K.; Amorim, V.H.; Terada, R.S.; Pascotto, R.C. Influence of exposure time to saliva and antioxidant treatment on bond strength to enamel after tooth bleaching: An in situ study. J. Appl. Oral Sci. 2013, 21, 567–574. [Google Scholar] [CrossRef]
- Tada, A.; Miura, H. The Relationship between Vitamin C and Periodontal Diseases: A Systematic Review. Int. J. Environ. Res. Public Health 2019, 16, 2472. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cagetti, M.G.; Wolf, T.G.; Tennert, C.; Camoni, N.; Lingström, P.; Campus, G. The Role of Vitamins in Oral Health. A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 938. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nascimento, G.C.R.; Ribeiro, M.E.S.; Guerreiro, M.Y.R.; de Souza Cruz, E.L.; Pinheiro, J.J.V.; Loretto, S.C. Effect of sodium ascorbate on bond strength and metalloproteinases activity in bleached dentin. Clin. Cosmet. Investig. Dent. 2019, 11, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Duane, B.; Stancliffe, R.; Miller, F.A.; Sherman, J.; Pasdeki-Clewer, E. Sustainability in Dentistry: A Multifaceted Approach Needed. J. Dent. Res. 2020, 99, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Marcomini, N.; Albaricci, M.C.D.C.; Costa, J.L.S.G.; Besegato, J.F.; Godoy, E.F.; Dantas, A.A.R.; Kuga, M.C. Effects of alpha-tocopherol antioxidant on fracture strength and adhesion of endodontically treated teeth restored after dental bleaching. Eur. J. Oral Sci. 2024, 132, e12965. [Google Scholar] [CrossRef]
- Haralur, S.B.; Al-Ibrahim, R.M.; Al-Shahrani, F.A.; Al-Qahtani, R.A.; Chaturvedi, S.; Alqahtani, N.M. Efficacy of organic and antioxidant agents to regain bond strength to bleached enamel in different dental adhesive solvents. J. Appl. Biomater. Funct. Mater. 2023, 21, 22808000231198807. [Google Scholar] [CrossRef] [PubMed]
- Almohareb, T.; Al Ahdal, K.; Maawadh, A.M.; Al Deeb, L.; Alshamrani, A.S.; Alrahlah, A. Bleached enamel reversal using grape seed extract, green tea, curcumin-activated photodynamic therapy, and Er: YAG on microleakage and bond integrity of composite material bonded to the enamel surface. Photodiagnosis Photodyn. Ther. 2024, 45, 103943. [Google Scholar] [CrossRef] [PubMed]
- Pimentel Corrêa, A.C.; Cecchin, D.; de Almeida, J.F.; Gomes, B.P.; Zaia, A.A.; Ferraz, C.C. Sodium Thiosulfate for Recovery of Bond Strength to Dentin Treated with Sodium Hypochlorite. J. Endod. 2016, 42, 284–288. [Google Scholar] [CrossRef]
- Yang, Y.; Guan, X. Non-protein thiol imaging and quantification in live cells with a novel benzofurazan sulfide triphenylphosphonium fluorogenic compound. Anal. Bioanal. Chem. 2017, 409, 3417–3427. [Google Scholar] [CrossRef]
- Kerksick, C.; Willoughby, D. The antioxidant role of glutathione and N-acetyl-cysteine supplements and exercise-induced oxidative stress. J. Int. Soc. Sport. Nutr. 2005, 2, 38–44. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sahasrabudhe, S.A.; Terluk, M.R.; Kartha, R.V. N-acetylcysteine Pharmacology and Applications in Rare Diseases-Repurposing an Old Antioxidant. Antioxidants 2023, 12, 1316. [Google Scholar] [CrossRef] [PubMed]
- Nassar, M.; Hiraishi, N.; Islam, M.S.; Tamura, Y.; Otsuki, M.; Kasugai, S.; Ohya, K.; Tagami, J.; Tay, F.R. The effect of glutathione on 2-hydroxyethylmethacrylate cytotoxicity and on resin-dentine bond strength. Int. Endod. J. 2014, 47, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Nassar, M.; Hiraishi, N.; Shimokawa, H.; Tamura, Y.; Otsuki, M.; Kasugai, S.; Ohya, K.; Tagami, J. The inhibition effect of non-protein thiols on dentinal matrix metalloproteinase activity and HEMA cytotoxicity. J. Dent. 2014, 42, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Paranjpe, A.; Sung, E.C.; Cacalano, N.A.; Hume, W.R.; Jewett, A. N-acetyl cysteine protects pulp cells from resin toxins in vivo. J. Dent. Res. 2008, 87, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Liu, H.; Yang, Y.; Yang, Y.; Jiao, Y.; Tay, F.R.; Chen, J. Biological Activities and Potential Oral Applications of N-Acetylcysteine: Progress and Prospects. Oxidative Med. Cell. Longev. 2018, 2018, 2835787. [Google Scholar] [CrossRef] [PubMed]
- Tay, F.R.; Gwinnett, A.J.; Wei, S.H. Ultrastructure of the resin-dentin interface following reversible and irreversible rewetting. Am. J. Dent. 1997, 10, 77–82. [Google Scholar] [PubMed]
- Van Meerbeek, B.; Perdigão, J.; Lambrechts, P.; Vanherle, G. The clinical performance of adhesives. J. Dent. 1998, 26, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.M.; Tjäderhane, L.; Manso, A.P.; Carrilho, M.R.; Carvalho, C.A.R. Dentin as a bonding substrate. Endod. Top. 2009, 21, 62–88. [Google Scholar] [CrossRef]
- Sirisha, K.; Rambabu, T.; Ravishankar, Y.; Ravikumar, P. Validity of bond strength tests: A critical review-Part II. J. Conserv. Dent. 2014, 17, 420–426. [Google Scholar] [CrossRef]
- al-Salehi, S.K.; Burke, F.J. Methods used in dentin bonding tests: An analysis of 50 investigations on bond strength. Quintessence Int. 1997, 28, 717–723. [Google Scholar] [PubMed]
- Ferrari, M.; Goracci, C.; Sadek, F.; Eduardo, P.; Cardoso, C. Microtensile bond strength tests: Scanning electron microscopy evaluation of sample integrity before testing. Eur. J. Oral Sci. 2002, 110, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Sadek, F.T.; Monticelli, F.; Muench, A.; Ferrari, M.; Cardoso, P.E. A novel method to obtain microtensile specimens minimizing cut flaws. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 78, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Titley, K.C.; Torneck, C.D.; Smith, D.C.; Chernecky, R.; Adibfar, A. Scanning electron microscopy observations on the penetration and structure of resin tags in bleached and unbleached bovine enamel. J. Endod. 1991, 17, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Titley, K.C.; Torneck, C.D.; Smith, D.C.; Adibfar, A. Adhesion of composite resin to bleached and unbleached bovine enamel. J. Dent. Res. 1988, 67, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Salz, U.; Bock, T. Testing adhesion of direct restoratives to dental hard tissue—A review. J. Adhes. Dent. 2010, 12, 343–371. [Google Scholar] [CrossRef] [PubMed]
- Perdigão, J.; Lopes, L.; Lambrechts, P.; Leitão, J.; Van Meerbeek, B.; Vanherle, G. Effects of a self-etching primer on enamel shear bond strengths and SEM morphology. Am. J. Dent. 1997, 10, 141–146. [Google Scholar] [PubMed]
- Karadas, M.; Demirbuga, S. Influence of a short-time antioxidant application on the dentin bond strength after intracoronal bleaching. Microsc. Res. Tech. 2019, 82, 1720–1727. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Guerisoli, L.D.; Schiavoni, R.J.; Barroso, J.M.; Guerisoli, D.M.; Pécora, J.D.; Fröner, I.C. Effect of different bleaching systems on the ultrastructure of bovine dentin. Dent. Traumatol. 2009, 25, 176–180. [Google Scholar] [CrossRef]
- Toledano, M.; Yamauti, M.; Osorio, E.; Osorio, R. Bleaching agents increase metalloproteinases-mediated collagen degradation in dentin. J. Endod. 2011, 37, 1668–1672. [Google Scholar] [CrossRef]
- Wang, D.; Kaur, K.; Paranjpe, A.; Lee, E.; Wasilewski, M.; Sung, D.; Han, D.; Sung, E.C.; Jewett, A. N-acetyl cysteine prevents pain and hypersensitivity of bleaching agents without affecting their aesthetic appeal; evidence from in vitro to animal studies and to human clinical trials. Transl. Med. Commun. 2019, 4, 19. [Google Scholar] [CrossRef]
- Ballal, N.V.; Mala, K.; Bhat, K.S. Evaluation of the effect of maleic acid and ethylenediaminetetraacetic acid on the microhardness and surface roughness of human root canal dentin. J. Endod. 2010, 36, 1385–1388. [Google Scholar] [CrossRef] [PubMed]
- Rajakumaran, A.; Ramesh, H.; Ashok, R.; Balaji, L.; Ganesh, A. Smear Layer Removal and Microhardness Alteration Potential of a Naturally Occurring Antioxidant—An In Vitro Study. Cureus 2019, 11, e5241. [Google Scholar] [CrossRef] [PubMed]
| Group | Dentin Surface Treatment |
|---|---|
| Group 1 | 35% H2O2 (pH 4.5) for 15 min and 5% AA (pH = 2.29) for 1 min |
| Group 2 | 35% H2O2 for 15 min and 5% AA for 5 min |
| Group 3 | 35% H2O2 for 15 min and 5% GSH (pH = 2.88) for 1 min |
| Group 4 | 35% H2O2 for 15 min and 5% GSH for 5 min |
| Group 5 | 35% H2O2 for 15 min and 5% NAC (pH = 1.95) for 1 min |
| Group 6 | 35% H2O2 for 15 min and 5% NAC for 5 min |
| Group 7 | 35% H2O2 for 15 min and 5% STS (pH = 7.24) for 1 min |
| Group 8 | 35% H2O2 for 15 min and 5% STS for 5 min |
| Group 9 | (First negative control): DW (pH 7.03) for 15 min |
| Group 10 | (Positive control): 35% H2O2 for 15 min |
| Group 11 | (Second negative control): 35% H2O2 for 15 min (bonding after 2 weeks) |
| Group | Dentin Surface Treatment of the Sectioned Discs |
|---|---|
| Group 1 | Baseline: H2O2 15 min, Treatment: H2O2 15 mins and AA for 1 min |
| Group 2 | Baseline: H2O2 15 min, Treatment: H2O2 15 mins and AA for 5 min |
| Group 3 | Baseline: H2O2,15 min, Treatment: H2O2 15 mins and GSH for 1 min |
| Group 4 | Baseline: H2O2 15 min, Treatment: H2O2 15 mins and GSH for 5 min |
| Group 5 | Baseline: H2O2 15 min, Treatment: H2O2 15 mins and NAC for 1 min |
| Group 6 | Baseline: H2O2 15 min, Treatment: H2O2 15 mins and NAC for 5 min |
| Group 7 | Baseline: H2O2 15 min, Treatment: H2O2 15 mins and STS for 1 min |
| Group 8 | Baseline: H2O2 15 min, Treatment: H2O2 15 mins and STS for 5 min |
| Group | Δ Roughness (Mean ± SD) | |
|---|---|---|
| 1 min Antioxidant Application | 5 min Antioxidant Application | |
| H2O2/AA | 0.00021 ± 0.00023 abc | 0.00042 ± 0.00028 bc |
| H2O2/GSH | −0.00012 ± 0.00039 ab | 0.0011 ± 0.00073 d |
| H2O2/NAC | 0.00006 ± 0.00011 abc | 0.00057 ± 0.00029 cd |
| H2O2/STS | −0.00017 ± 0.00010 a | 0.00016 ± 0.00011 abc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alatta, A.; Nassar, M.; Gorduysus, M.; Alkhatib, W.; Sayed, M. In Vitro Investigation of the Effects of Various Reducing Agents on Dentin Treated with Hydrogen Peroxide. Polymers 2024, 16, 1473. https://doi.org/10.3390/polym16111473
Alatta A, Nassar M, Gorduysus M, Alkhatib W, Sayed M. In Vitro Investigation of the Effects of Various Reducing Agents on Dentin Treated with Hydrogen Peroxide. Polymers. 2024; 16(11):1473. https://doi.org/10.3390/polym16111473
Chicago/Turabian StyleAlatta, Alaa, Mohannad Nassar, Mehmet Gorduysus, Walaa Alkhatib, and Mahmoud Sayed. 2024. "In Vitro Investigation of the Effects of Various Reducing Agents on Dentin Treated with Hydrogen Peroxide" Polymers 16, no. 11: 1473. https://doi.org/10.3390/polym16111473
APA StyleAlatta, A., Nassar, M., Gorduysus, M., Alkhatib, W., & Sayed, M. (2024). In Vitro Investigation of the Effects of Various Reducing Agents on Dentin Treated with Hydrogen Peroxide. Polymers, 16(11), 1473. https://doi.org/10.3390/polym16111473







