A Simple and Efficient Magnesium Hydroxide Modification Strategy for Flame-Retardancy Epoxy Resin
Abstract
1. Introduction
2. Experimental
2.1. Materials
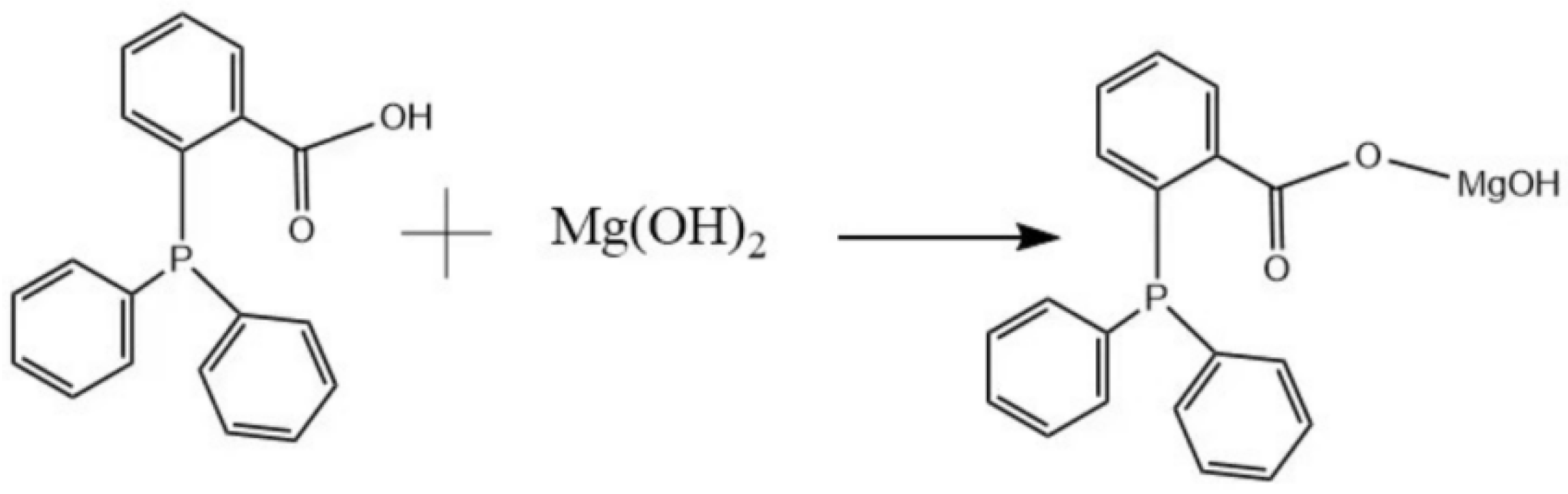
2.2. Preparation of the MH@PPAC
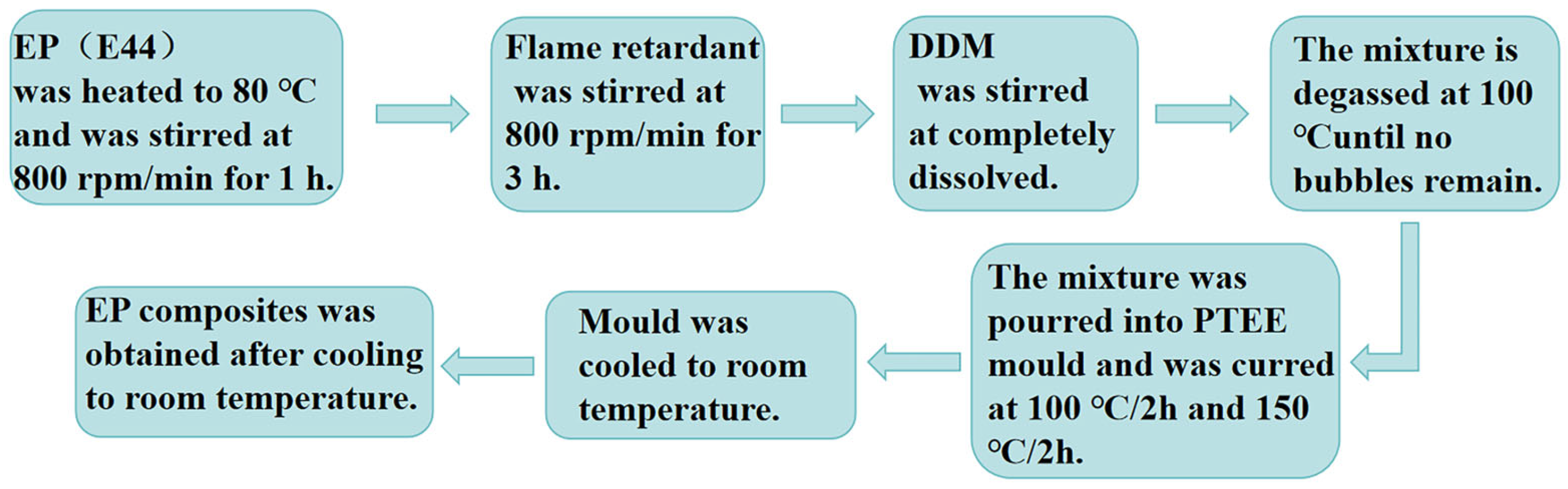
2.3. Preparation for EP Flame-Retardant Composites
2.4. Characterization
3. Results and Discussion
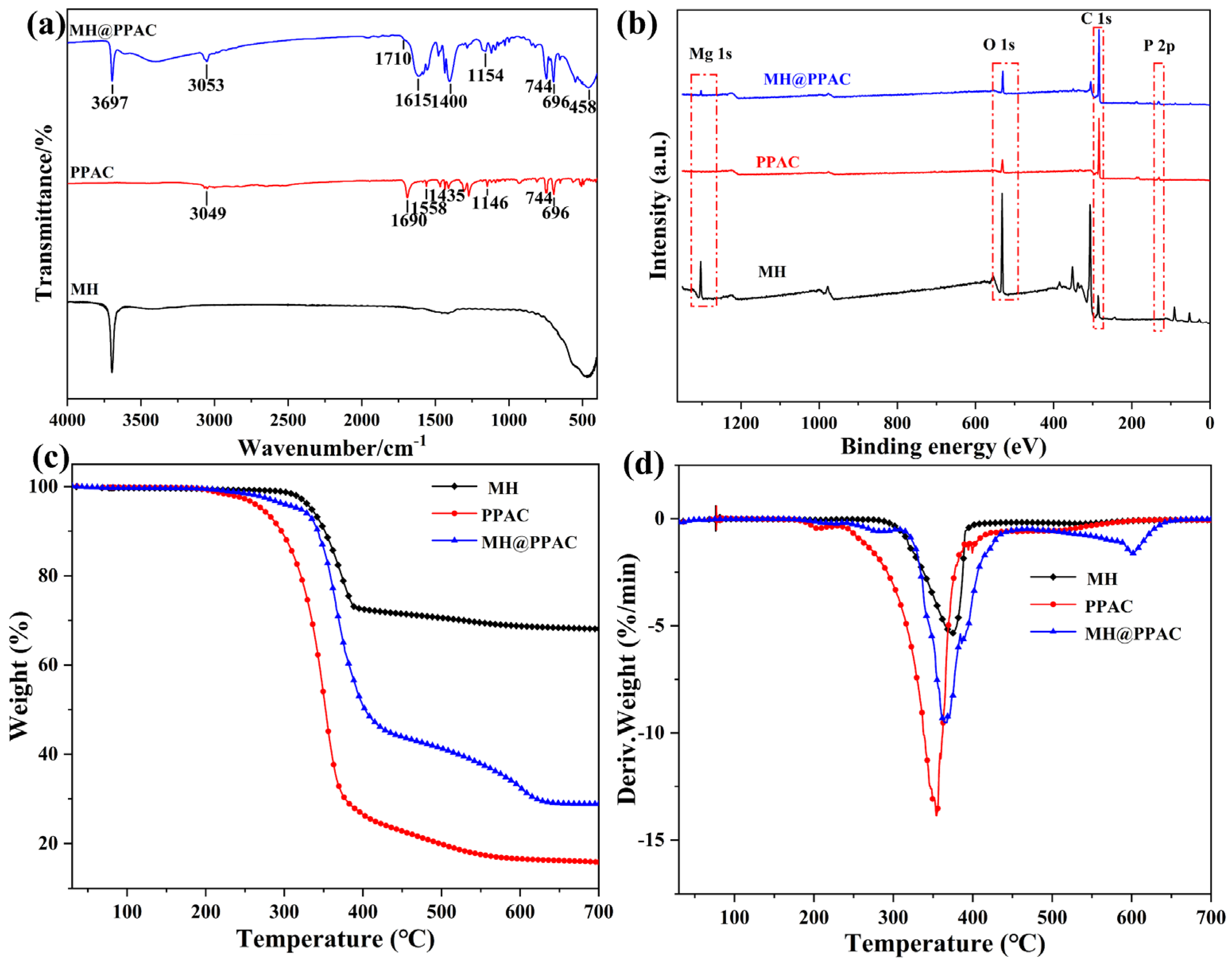
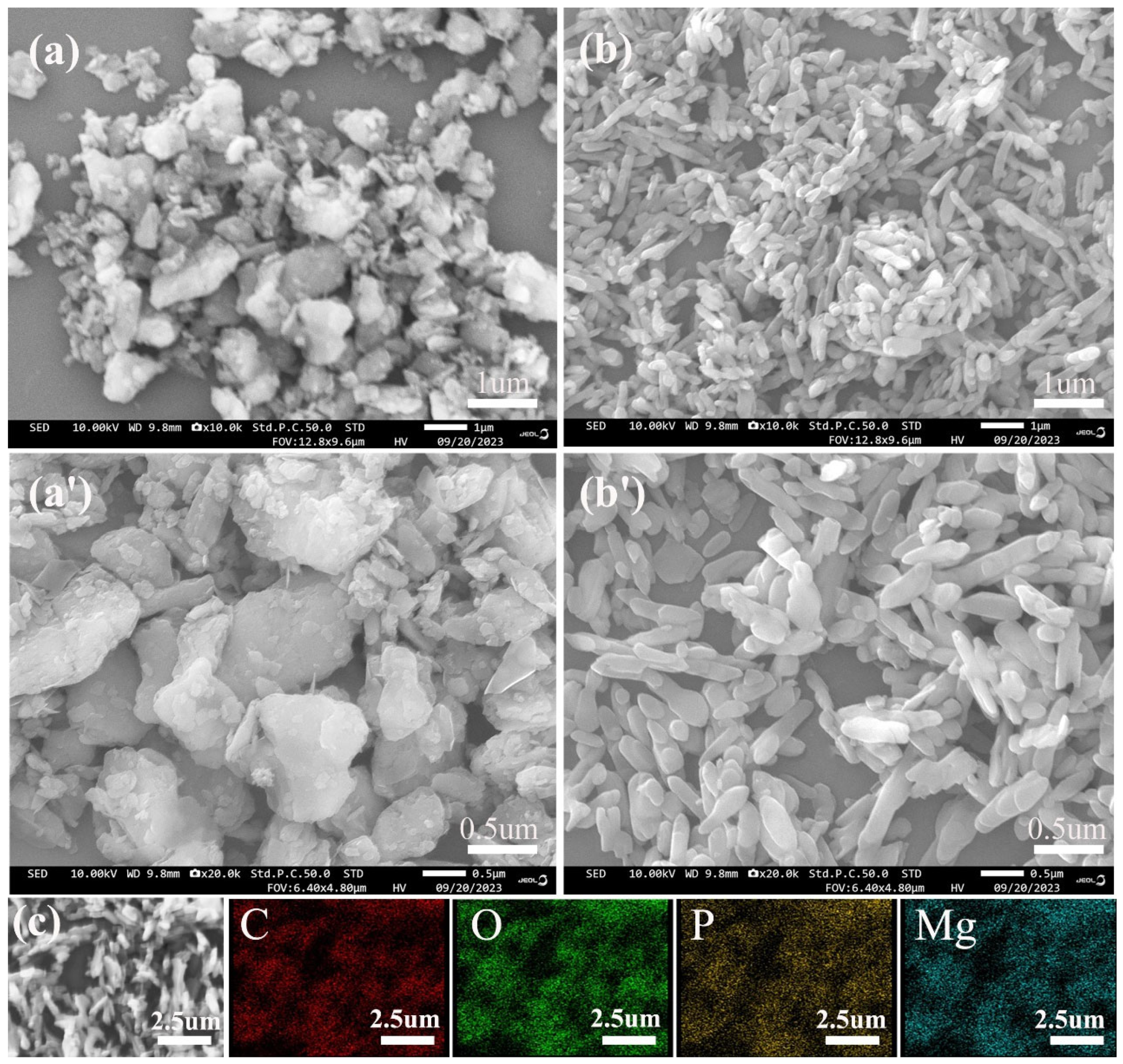
3.1. MH@PPAC Characterization
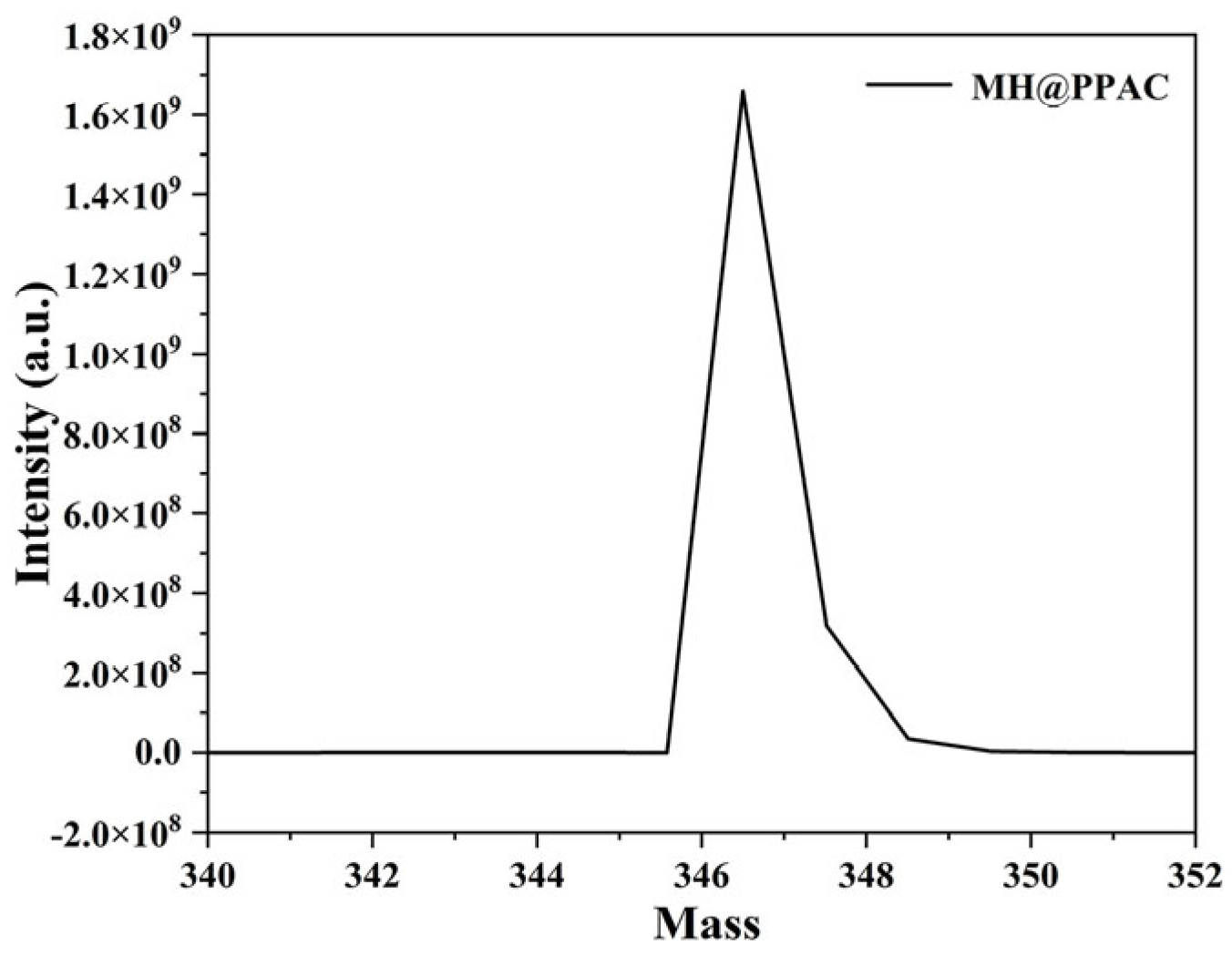
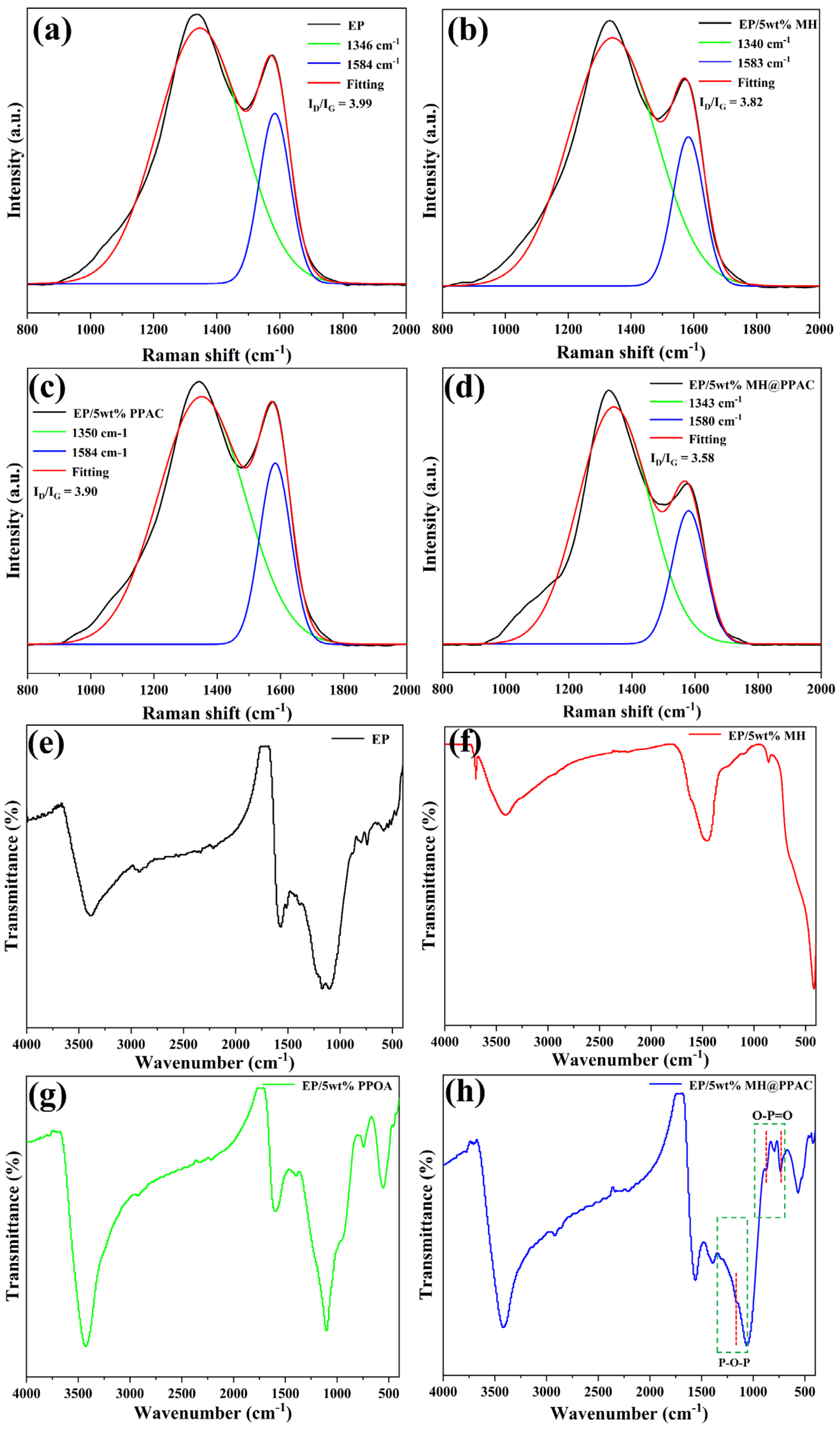
3.1.1. Structure Characterization of MH@PPAC
3.1.2. Morphology Analysis
3.2. Thermal Stability of the EP Blends
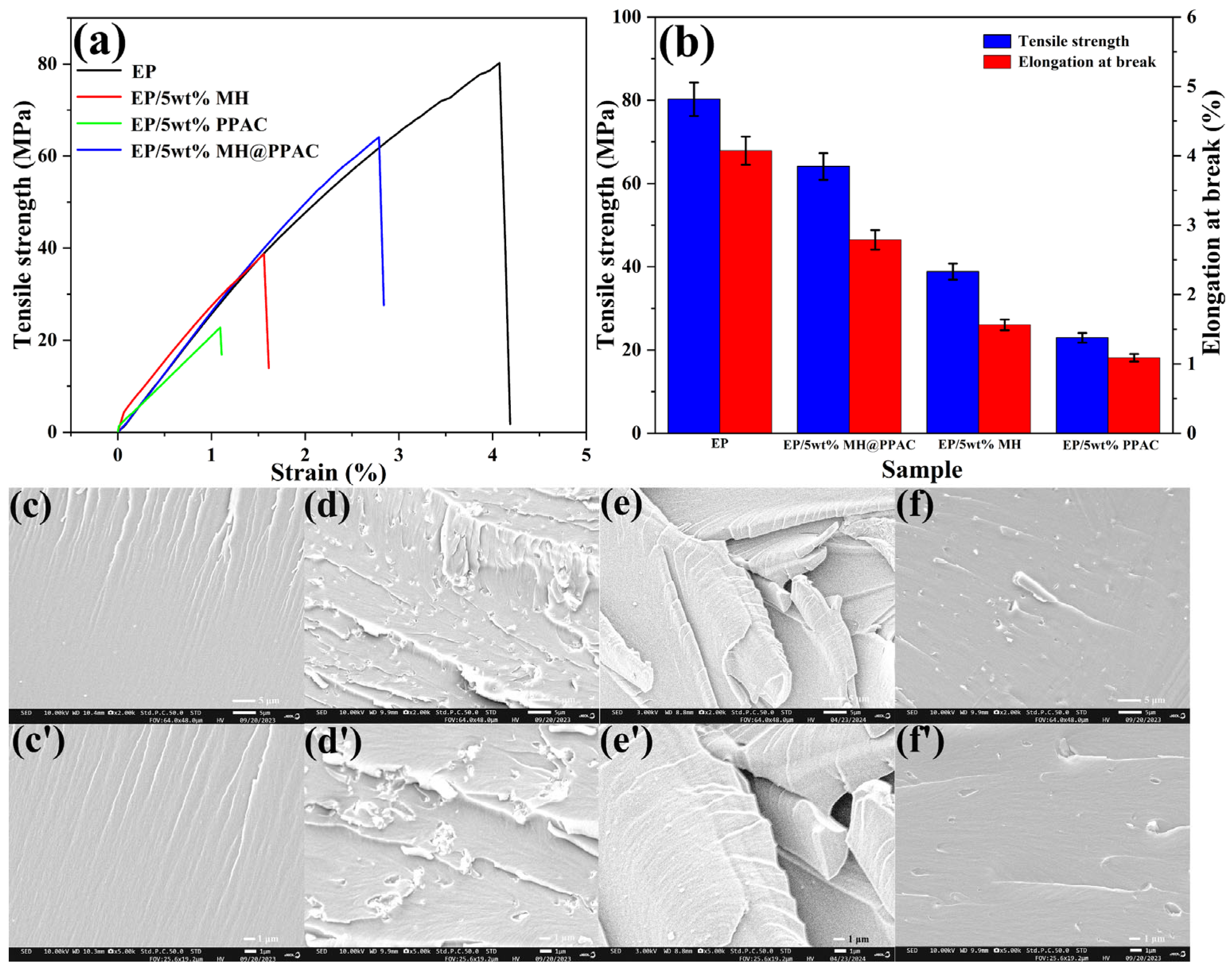
3.3. Mechanical Performance
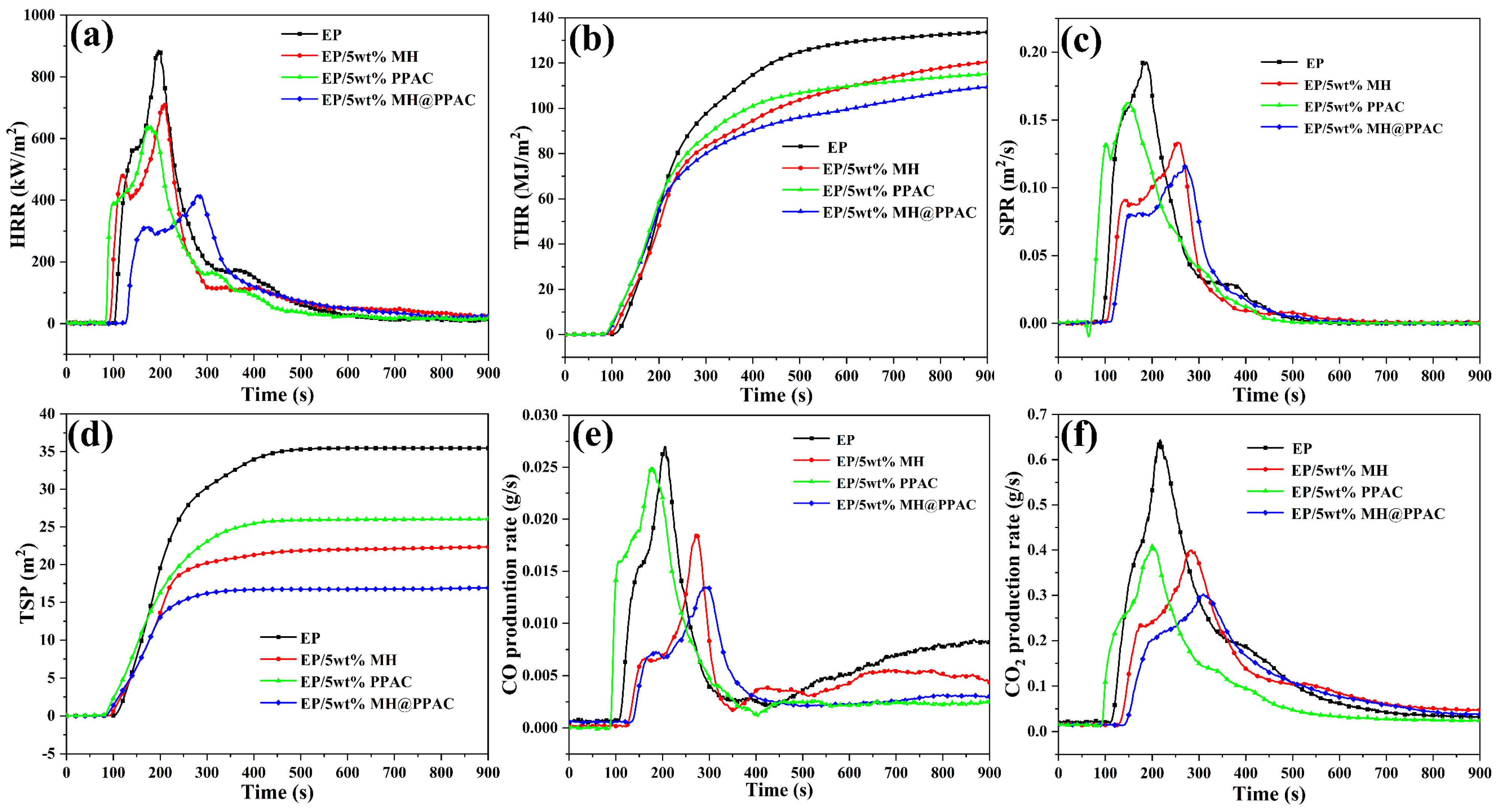
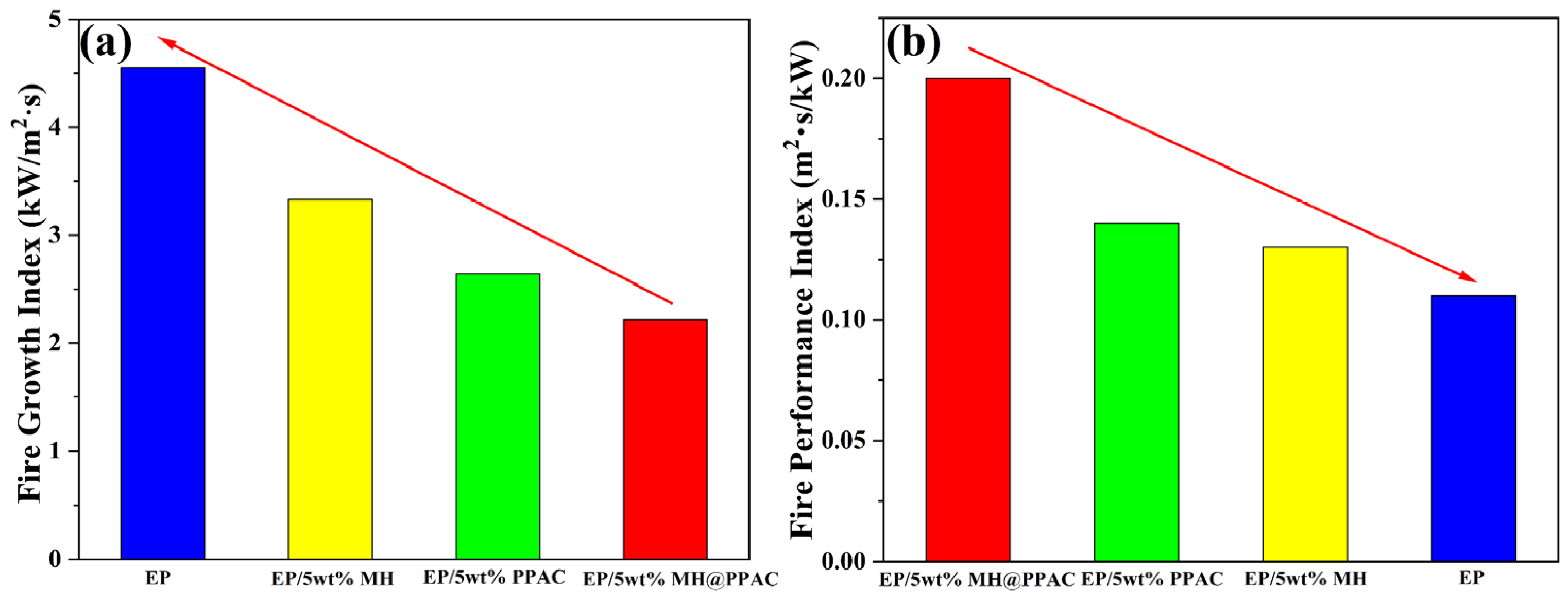
3.4. Flame-Retardancy Behavior for EP-Blend Materials
3.5. Flame-Retardancy Mechanism
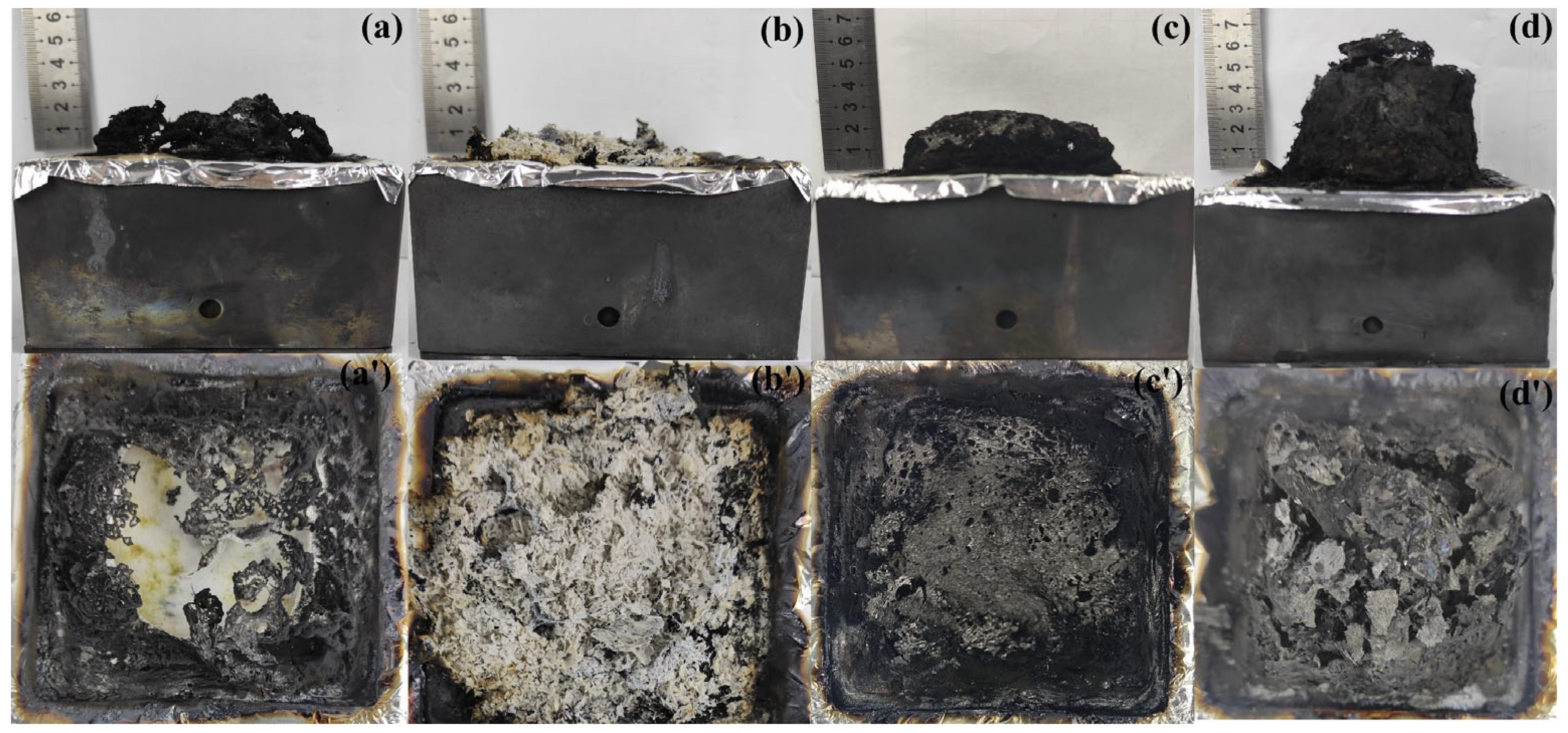
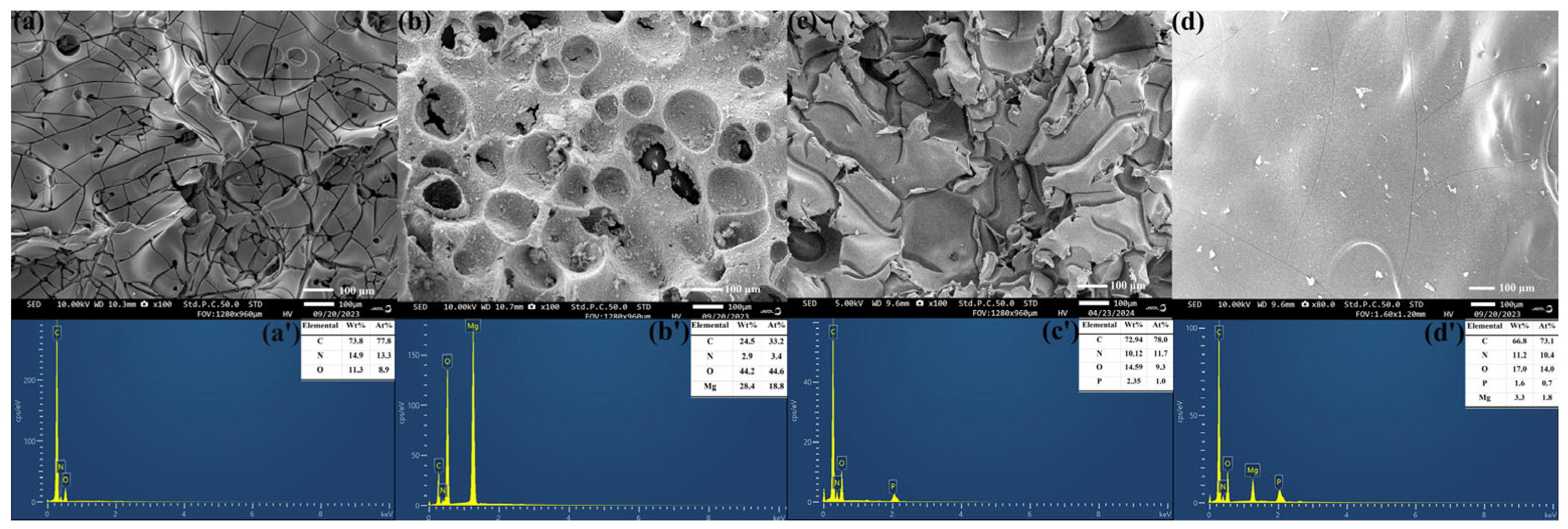
3.5.1. Analysis of the Char Residues
3.5.2. Gaseous Phase Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ren, J.Y.; Wang, Y.X.; Piao, J.X.; Cui, J.H.; Guan, H.C.; Jiao, C.M.; Chen, X.L. Facile construction of phosphorus-free and green organic-inorganic hybrid flame-retardant system: For improving fire safety of EP. Prog. Org. Coat. 2023, 179, 107489. [Google Scholar] [CrossRef]
- Zhou, H.B.; Wang, Z.R.; Wang, J.L.; Yu, S. Ternary MXenes-based nanostructure enabled fire-safe and mechanic-robust EP composites with markedly impeded toxicants releases. Compos. Part A 2022, 162, 107137. [Google Scholar] [CrossRef]
- Xiao, Y.L.; Jiang, G.Y.; Ma, C.; Zhou, X.; Wang, C.Y.; Xu, Z.M.; Mu, X.W.; Song, L.; Hu, Y. Construction of multifunctional linear polyphosphazene and molybdenum diselenide hybrids for efficient fire retardant and toughening epoxy resins. Chem. Eng. J. 2021, 426, 131839. [Google Scholar] [CrossRef]
- Qian, Y.X.; Luo, Y.B.; Li, Y.; Xiong, T.S.; Wang, L.Y.; Zhang, W.G.; Gang, S.F.; Li, X.; Jiang, Q.H.; Yang, J.Y. Enhanced electromagnetic wave absorption, thermal conductivity and flame retardancy of BCN@LDH/EP for advanced electronic packing materials. Chem. Eng. J. 2023, 467, 143433. [Google Scholar] [CrossRef]
- Qian, Y.X.; Luo, Y.N.; Haruna, A.Y.; Xiao, B.; Li, W.; Li, Y.; Xiong, T.S.; Jiang, Q.H.; Yang, J.Y. Multifunctional Epoxy-Based Electronic Packaging Material MDCF@LDH/EP for Electromagnetic Wave Absorption, Thermal Management, and Flame Retardancy. Small 2022, 220, 4303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.C.; Wang, K.J.; Sun, Y.S.; Xu, M.S.; Cheng, Z.Y. Novel Biphasically and Reversibly Transparent Phase Change Material to Solve the Thermal Issues in Transparent Electronics. ACS Appl. Mater. Interfaces 2022, 14, 31245–31256. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Qin, J.B.; Guo, B.R.; Fan, X.; Wang, F.P.; Zhang, Y.; Xiao, R.L.; Huang, F.; Shi, X.T.; Zhang, G.C. High-performance electromagnetic interference shielding epoxy/Ag nanowire/thermal annealed graphene aerogel composite with bicontinuous three-dimensional conductive skeleton. Compos. Part A 2021, 151, 106648. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Han, S.H.; Hu, X.P.; Li, W.X.; Na, B.; Xie, C.Q.; Wang, X.L. Benefiting from the multiple effects of ferrocene and cyclotriphosphazene bi-based hierarchical layered nanosheets towards improving fire safety and mechanical properties of epoxy resin. Compos. Part B 2023, 264, 110914. [Google Scholar] [CrossRef]
- Farzanehfar, N.; Taheri, A.; Rafiemanzela, F.; Jazani, O.M. High-performance epoxy nanocomposite adhesives with enhanced mechanical, thermal and adhesion properties based on new nanoscale ionic materials. Chem. Eng. J. 2023, 471, 144428. [Google Scholar] [CrossRef]
- Kong, Q.H.; Sun, Y.L.; Zhang, C.J.; Guan, H.M.; Zhang, J.H.; Wang, D.Y.; Zhang, F. Ultrathin iron phenyl phosphonate nanosheets with appropriate thermal stability for improving fire safety in epoxy. Compos. Sci. Technol. 2019, 182, 107748. [Google Scholar] [CrossRef]
- Xu, Y.J.; Chen, L.; Rao, W.H.; Qi, M.; Guo, D.M.; Liao, W.; Wang, Y.Z. Latent curing epoxy system with excellent thermal stability, flame retardance and dielectric property. Chem. Eng. J. 2018, 347, 223–232. [Google Scholar] [CrossRef]
- Ye, X.M.; Han, Z.Q.; Tian, P.P.; Liu, T.; Meng, X.N.; Yuan, C.K.; Wang, W.S.; Li, Y.C. Copper-containing tetra-phenyl polyhedral oligomeric silsesquioxane endows epoxy resin with reinforcements in the thermal stability, flame retardancy and smoke suppression. Compos. Commun. 2023, 37, 101459. [Google Scholar] [CrossRef]
- Awais, M.; Chen, X.R.; Hong, Z.L.; Wang, Q.L.; Shi, Y.W.; Meng, F.B.; Dai, C.; Paramane, A. Synergistic effects of Micro-hBN and core-shell Nano-TiO2@SiO2 on thermal and electrical properties of epoxy at high frequencies and temperatures. Compos. Sci. Technol. 2022, 227, 109576. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, C.; Wen, Y.F.; Xue, Z.G.; Zhou, X.P.; Shi, D.; Hu, G.H.; Xie, X.L. Novel micro-nano epoxy composites for electronic packaging application: Balance of thermal conductivity and processability. Compos. Sci. Technol. 2021, 209, 108760. [Google Scholar] [CrossRef]
- Bi, X.; Di, H.; Liu, J.; Meng, Y.F.; Song, Y.Y.; Meng, W.H.; Qu, H.Q.; Fang, L.D.; Song, P.A.; Xu, J.Z. A core–shell-structured APP@COFs hybrid for enhanced flame retardancy and mechanical property of epoxy resin (EP). Adv. Compos. 2022, 5, 1743–1755. [Google Scholar] [CrossRef]
- Li, M.G.; Li, S.Q.; Liu, J.; Wen, X.; Tang, T. Striking effect of epoxy resin on improving mechanical properties of poly (butylene terephthalate)/recycled carbon fibre composites. Compos. Sci. Technol. 2016, 125, 9–16. [Google Scholar] [CrossRef]
- Liu, N.; Wang, H.; Xu, B.S.; Qu, L.J.; Fang, D.N. Cross-linkable phosphorus/nitrogen-containing aromatic ethylenediamine endowing epoxy resin with excellent flame retardancy and mechanical properties. Compos. Part A 2022, 162, 107145. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Lv, L.H.; Cui, Y.Z.; Wei, C.Y.; Pang, G.B. Preparation of Mg(OH)2 hybrid pigment by direct precipitation and graft onto cellulose fiber via surface-initiated atom transfer radical polymerization. Appl. Surf. Sci. 2016, 363, 189–196. [Google Scholar] [CrossRef]
- Chen, H.; Wang, T.; Wen, Y.L.; Wen, X.; Gao, D.D.; Yu, R.H.; Chen, X.C.; Mijowska, E.; Tang, T. Expanded graphite assistant construction of gradient-structured char layer in PBS/Mg(OH)2 composites for improving flame retardancy, thermal stability and mechanical properties. Compos. Part B 2019, 177, 107402. [Google Scholar] [CrossRef]
- Yang, J.; Liang, J.Z.; Tang, C.Y. Studies on melt flow properties during capillary extrusion of PP/Al(OH)3/Mg(OH)2 flame retardant composites. Polym. Test. 2009, 28, 907–911. [Google Scholar] [CrossRef]
- Li, P.; Li, L.; Ji, L.; Dang, L.; Lan, S.; Zhu, D. Functionalized magnesium hydroxide with zinc borate and 3-aminopropyltriethoxysilane for enhanced flame retardant and smoke suppressant properties of epoxy resins. J. Appl. Polym. Sci. 2023, 140, 53941. [Google Scholar] [CrossRef]
- Piperopoulos, E.; Scionti, G.; Atria, M.; Calabrese, L.; Proverbio, E. Flame-Retardant Performance Evaluation of Functional Coatings Filled with Mg(OH)2 and Al(OH)3. Polymers 2022, 14, 372. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.Y.; Fan, H.; Zeng, W.; Yang, Z.W.; Wang, Y.; Lei, Z.Q. Novel nanocomposites based on epoxy resin and modified magnesium hydroxide: Focus on flame retardancy and mechanical properties. Polym. Adv. Technol. 2019, 14, 1. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, W.S.; Wang, M.X.; Liu, P.; Pan, Y.H.; Liu, D.F. Synergistic effect of an aromatic boronic acid derivative and magnesium hydroxide on the flame retardancy of epoxy resin. Polym. Degrad. Stab. 2016, 130, 257. [Google Scholar] [CrossRef]
- Huo, S.Q.; Song, P.A.; Yua, B.; Ran, S.Y.; Chevali, V.S.; Liu, L.; Fang, Z.P.; Wang, H. Phosphorus-containing flame retardant epoxy thermosets: Recent advances and future perspectives. Prog. Polym. Sci. 2021, 114, 101366. [Google Scholar] [CrossRef]
- Zhi, M.Y.; Yang, X.; Fan, R.; Yue, S.; Zheng, L.L.; Liu, Q.Y.; He, Y.H. A comprehensive review of reactive flame-retardant epoxy resin: Fundamentals, recent developments, and perspectives. Polym. Degrad. Stab. 2022, 201, 109976. [Google Scholar] [CrossRef]
- Guo, W.W.; Zhao, Y.Y.; Wang, X.; Cai, W.; Wang, J.L.; Song, L.; Hu, Y. Multifunctional epoxy composites with highly flame retardant and effective electromagnetic interference shielding performances. Compos. Part B 2020, 192, 107990. [Google Scholar] [CrossRef]
- Jiao, D.L.; Zhao, H.J.; Sima, H.F.; Cheng, C.Z.; Zhang, C.L. Engineering flame retardant epoxy resins with strengthened mechanical property by using reactive catechol functionalized DOPO compounds. Chem. Eng. J. 2024, 485, 149910. [Google Scholar] [CrossRef]
- Giebultowicz, J.; Ruzycka, M.; Wroczynski, P.; Purser, D.A.; Stec, A.A. Analysis of fire deaths in Poland and influence of smoke toxicity. Forensic Sci. Int. 2017, 277, 77. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, M.H.; Ma, Z.W.; Xu, X.D.; Seraji, S.M.; Yu, B.; Sun, Z.Q.; Wang, H.; Song, P.A. A reactive copper-organophosphate-MXene heterostructure enabled antibacterial, self-extinguishing and mechanically robust polymer nanocomposites. Chem. Eng. J. 2022, 430, 132712. [Google Scholar] [CrossRef]
- Chen, X.L.; Wang, W.D.; Jiao, C.M. A recycled environmental friendly flame retardant by modifying para-aramid fiber with phosphorus acid for thermoplastic polyurethane elastomer. J. Hazard. Mater. 2017, 331, 257. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.H.; Chen, S.S.; Zeng, J.M.; Yang, L.; Liu, P. Synergistic flame retardant effect of an intumescent flame retardant containing boron and magnesium hydroxide. ACS Omega 2019, 4, 3314–3321. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.M.; Ren, M.S.; Chu, Y.J.; Sun, J.L.; Xie, W. An interlayer flame retardant method to effectively fire retardant and reinforce CF/EP composites by nanofiber film intercalation. Chem. Eng. J. 2023, 467, 143545. [Google Scholar] [CrossRef]
- Zhang, W.J.; Zhou, M.T.; Kan, Y.C.; Chen, J.; Hu, Y.; Xing, W.Y. Synthesis and flame retardant efficiency study of two phosphorus-nitrogen type flame retardants containing triazole units. Polym. Degrad. Stabil. 2023, 208, 110236. [Google Scholar] [CrossRef]
- Sun, J.H.; Wang, B.T.; Guo, Z.H.; Fang, Z.P.; Li, J. Flame retardant epoxy resin toughened and strengthened by a reactive compatibilizer. Polymer 2022, 248, 124798. [Google Scholar] [CrossRef]
- Jiang, J.W.; Huo, S.Q.; Zheng, Y.; Yang, C.Y.; Yan, H.Q.; Ran, S.Y.; Fang, Z.P. A novel synergistic flame retardant of hexaphenoxycyclotriphosphazene for epoxy resin. Polymers 2021, 13, 3648. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Cai, H.P.; Gao, L.H.; Mao, Y.W.; Huang, W.Y. The synthesis of biphenyl ether/vanillin-based flame retardants for enhancing both the flame retardant properties and mechanical performance of epoxy resin. J. Appl. Polym. Sci. 2024, 141, 55281. [Google Scholar] [CrossRef]
- Ou, M.Y.; Lian, R.C.; Cui, J.H.; Guan, H.C.; Liu, L.; Jiao, C.M.; Chen, X.L. Co-curing preparation of flame retardant and smoke-suppressive epoxy resin with a novel phosphorus-containing ionic liquid. Chemosphere 2023, 311, 137061. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.X.; Zhu, X.J.; Dai, P.; Lu, X.Y.; Guo, H.Q.; Que, H.; Wang, D.D.; He, T.; Xu, C.Z.; Robin, H.M.; et al. Preparation of a novel lignin-based flame retardant for epoxy resin. Mater. Chem. Phys. 2021, 259, 124101. [Google Scholar] [CrossRef]
- Wang, X.Y.; Liu, Q.; Wang, J.M.; Liu, J.; Long, S.J.; Wang, D. Synthesis of multifunctional flame retardant with toughening and transparency and its application in epoxy resin. React. Funct. Polym. 2022, 176, 105289. [Google Scholar] [CrossRef]
- Chen, C.; Wang, X.; Luo, T.; Zhen, H.; Yang, X.; Yang, L.; Yan, Z. Synthesis of solid reactive organophosphorus-nitrogen flame retardant and its application in epoxy resin. J. Appl. Polym. Sci. 2023, 140, 54282. [Google Scholar] [CrossRef]
- Zhao, P.F.; Zeng, W.; Yang, Z.W.; Yang, Y.X.; Li, J.; Shi, J.P.; Wen, N.; Li, H.T.; Guan, J.; Lei, Z.Q.; et al. Preparation of a novel functionalized magnesium-based curing agent as an intrinsic flame retardant for epoxy resin. Chemosphere 2021, 273, 129658. [Google Scholar] [CrossRef]
- Wang, X.; Kalali, E.N.; Wan, J.T.; Wang, D.Y. Carbon-family materials for flame retardant polymeric materials. Prog. Polym. Sci. 2017, 69, 22. [Google Scholar] [CrossRef]
- Bi, Q.Q.; Yao, D.W.; Yin, G.Z.; You, J.Q.; Liu, X.Q.; Wang, N.; Wang, D.Y. Surface engineering of magnesium hydroxide via bioinspired iron-loaded polydopamine as green and efficient strategy to epoxy composites with improved flame retardancy and reduced smoke release. React. Funct. Polym. 2020, 155, 104690. [Google Scholar] [CrossRef]
- Guo, F.; Zhang, Y.Z.; Cai, L.; Li, L.J. Functionalized graphene with Platelet-like magnesium hydroxide for enhancing fire safety, smoke suppression and toxicity reduction of Epoxy resin. Appl. Surf. Sci. 2022, 578, 152052. [Google Scholar] [CrossRef]
- Zhang, G.; Dong, Y.L.; Yao, M.; Cui, Y.M.; Meng, W.H.; Wang, S.S.; Qu, H.Q.; Xu, J.Z. Preparation of a MOF flame retardant containing phosphazene ring and its effect on the flame retardant of epoxy resin. React. Funct. Polym. 2023, 191, 105670. [Google Scholar] [CrossRef]
- Qi, Y.Z.; Hou, Z.M.; Xu, S.J.; Xu, Q.; Huan, X.Y.; Bao, D.M.; Zhang, D.H.; Zhou, G.Y.; Zhang, Y.P.; Tan, F. The flame retardant properties and mechanism of the composites based on DOPS and triazine-trione groups in epoxy resin. Polym. Degrad. Stab. 2023, 216, 110482. [Google Scholar] [CrossRef]
- Lian, R.; Guan, H.C.; Zhang, Y.Q.; Ou, M.Y.; Jiang, Y.P.; Liu, L.; Jiao, C.M.; Chen, X.L. A green organic-inorganic PAbz@ZIF hybrid towards efficient flame-retardant and smoke-suppressive epoxy coatings with enhanced mechanical properties. Polym. Degrad. Stab. 2023, 217, 110534. [Google Scholar] [CrossRef]
- Huo, S.Q.; Sai, T.; Ran, S.Y.; Guo, Z.H.; Fang, Z.P.; Song, P.A.; Wang, H. A hyperbranched P/N/B-containing oligomer as multifunctional flame retardant for epoxy resins. Compos. Part B 2022, 234, 109701. [Google Scholar] [CrossRef]
- Yang, Z.W.; Xiao, G.Q.; Chen, C.L.; Chen, C.Y.; Zhong, F.; Cao, M.; Wang, M.T.; Zou, R.; Li, R.L.; Li, Y.Y. Bio-inspired adenosine triphosphate-modified h-BN-based coral-like Cu/Al-LDH nanosheets as a functional green flame retardant to improve the fire safety of epoxy resins via catalyzing intumescent char formation. Prog. Org. Coat. 2023, 182, 107648. [Google Scholar] [CrossRef]
- Lu, J.Y.; Jia, P.F.; Liao, C.; Xu, Z.M.; Chu, F.K.; Zhou, M.T.; Yu, B.; Wang, B.B.; Song, L. Leaf vein-inspired engineering of MXene@SrSn(OH)6 nanorods towards super-tough elastomer nanocomposites with outstanding fire safety. Compos. Part B 2022, 228, 109425. [Google Scholar] [CrossRef]
- Howell, B.A. Thermal Degradation of organophosphorus flame retardants. Polymers 2022, 14, 4929. [Google Scholar] [CrossRef] [PubMed]
- Howel, B.A. Toxicity of organophosphorus flame retardants. J. Fire Sci. 2023, 41, 102–104. [Google Scholar] [CrossRef]


| Samples | EP (wt.%) | DDM (wt%) | MH (wt%) | PPAC (wt%) | MH@PPAC (wt%) |
|---|---|---|---|---|---|
| EP | 82.0 | 18.0 | 0.0 | 0.0 | 0.0 |
| EP/5 wt% MH | 77.4 | 17.6 | 5.0 | 0.0 | 0.0 |
| EP/5 wt% PPAC | 77.4 | 17.6 | 0 | 5.0 | 0 |
| EP/5 wt% MH@PPAC | 77.4 | 17.6 | 0.0 | 0.0 | 5.0 |
| Samples | T-5% a (°C) | Tmax b (°C) | Char c (%) |
|---|---|---|---|
| MH | 334 | 383 | 68.12 |
| PPAC | 273 | 367 | 16.08 |
| MH@PPAC | 316 | 356 | 28.98 |
| Samples | T-5% a (°C) | T-50% b (°C) | Tmax c (°C) | Char d (%) |
|---|---|---|---|---|
| EP | 367 | 401 | 384 | 14.97 |
| EP/5 wt% MH | 346 | 370 | 368 | 18.50 |
| EP/5 wt% PPAC | 355 | 395 | 375 | 15.51 |
| EP/5 wt% MH@PPAC | 333 | 369 | 355 | 18.90 |
| Samples | EP | EP/5 wt% MH | EP/5 wt% PPAC | EP/5 wt% MH@PPAC |
|---|---|---|---|---|
| LOI (%) | 24.5 ± 0.20 | 26.9 ± 0.30 | 26.3 ± 0.21 | 38.9 ± 0.26 |
| TTI (s) | 102 ± 3 | 92 ± 2 | 83 ± 3 | 85 ± 2 |
| t PHRR (s) | 198 ± 3 | 210 ± 2 | 241 ± 5 | 283 ± 2 |
| PHRR (kW/m2·S) | 883 ± 15 | 710 ± 21 | 637 ± 16 | 415 ± 19 |
| FGI (kW/(m2·S)) | 4.55 ± 0.31 | 3.33 ± 0.23 | 2.64 ± 0.32 | 2.22 ± 0.16 |
| FPI (S·m2/kW) | 0.11 ± 0.06 | 0.13 ± 0.09 | 0.14 ± 0.10 | 0.20 ± 0.12 |
| PSPR (m2/s) | 0.20 ± 0.10 | 0.13 ± 0.08 | 0.16 ± 0.03 | 0.11 ± 0.07 |
| TSP (m2) | 35.4 ± 5.61 | 22.4 ± 3.91 | 26.10 ± 2.36 | 16.8 ± 2.30 |
| PCOP (g/s) | 0.027 ± 0.005 | 0.018 ± 0.003 | 0.025 ± 0.009 | 0.013 ± 0.003 |
| PCO2P (g/s) | 0.64 ± 0.12 | 0.40 ± 0.09 | 0.41 ± 0.008 | 0.30 ± 0.12 |
| UL-94 | NR | NR | NR | V-0 |
| Reference | LOI (%) | PHRR (kW/m2·S) | THR (MJ/m2) | TSP (m2) | UL-94 |
|---|---|---|---|---|---|
| [32] | 31.5 | 535 | 67 | 38.9 | V-1 |
| [33] | 33.0 | 471.3 | 69.5 | 34.4 | V-1 |
| [34] | 30.0 | 1059.1 | 66.8 | 8.20 | V-1 |
| [35] | 34.2 | 557 | 90.1 | 32.02 | V-0 |
| [36] | 35.2 | 658 | 51.3 | 26.96 | V-0 |
| [37] | 36.7 | 507.2 | 25.9 | - | V-0 |
| [38] | 30.3 | 696.12 | 79.09 | 26.14 | V-0 |
| [39] | 36.1 | 385.4 | 53.1 | 9.9 | V-0 |
| [40] | 35.2 | 625.5 | 62.9 | - | V-0 |
| [41] | 27.9 | 450.65 | 124.98 | 21.87 | V-0 |
| This work | 38.9 | 415 | 109.40 | 16.8 | V-0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dun, L.; Ouyang, Z.; Sun, Q.; Yue, X.; Wu, G.; Li, B.; Kang, W.; Wang, Y. A Simple and Efficient Magnesium Hydroxide Modification Strategy for Flame-Retardancy Epoxy Resin. Polymers 2024, 16, 1471. https://doi.org/10.3390/polym16111471
Dun L, Ouyang Z, Sun Q, Yue X, Wu G, Li B, Kang W, Wang Y. A Simple and Efficient Magnesium Hydroxide Modification Strategy for Flame-Retardancy Epoxy Resin. Polymers. 2024; 16(11):1471. https://doi.org/10.3390/polym16111471
Chicago/Turabian StyleDun, Linan, Zeen Ouyang, Qihao Sun, Xiaoju Yue, Guodong Wu, Bohan Li, Weidong Kang, and Yuanhao Wang. 2024. "A Simple and Efficient Magnesium Hydroxide Modification Strategy for Flame-Retardancy Epoxy Resin" Polymers 16, no. 11: 1471. https://doi.org/10.3390/polym16111471
APA StyleDun, L., Ouyang, Z., Sun, Q., Yue, X., Wu, G., Li, B., Kang, W., & Wang, Y. (2024). A Simple and Efficient Magnesium Hydroxide Modification Strategy for Flame-Retardancy Epoxy Resin. Polymers, 16(11), 1471. https://doi.org/10.3390/polym16111471





