Facile Fabrication of Highly Efficient Chitosan-Based Multifunctional Coating for Cotton Fabrics with Excellent Flame-Retardant and Antibacterial Properties
Abstract
1. Introduction
2. Experiment Section
2.1. Material
2.2. Preparation of ATMP-CS Coating
2.3. Preparation of Flame-Retardant Cotton Fabric
2.4. Characterization
3. Results and Discussion
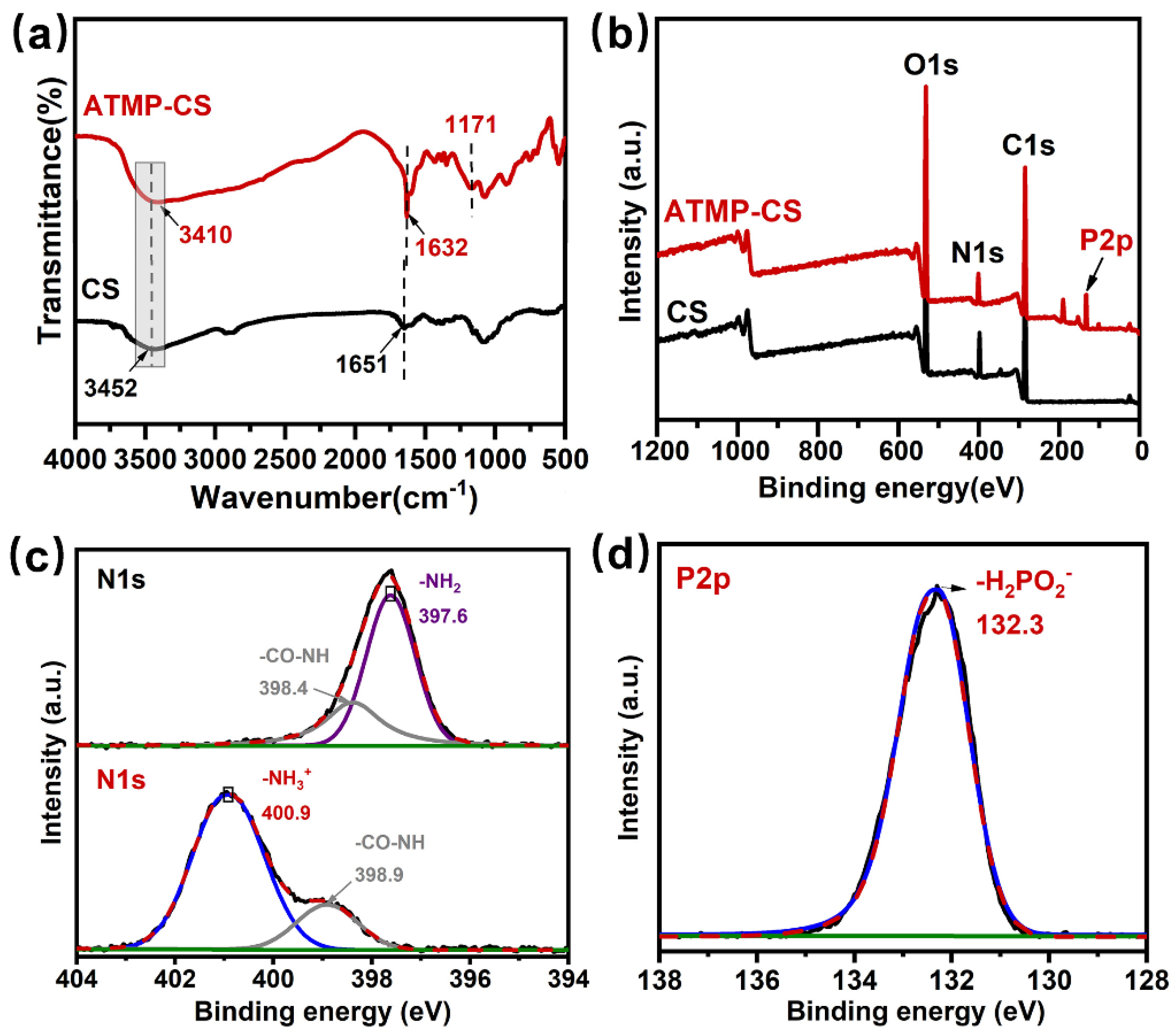
3.1. Characterization of ATMP-CS Coating
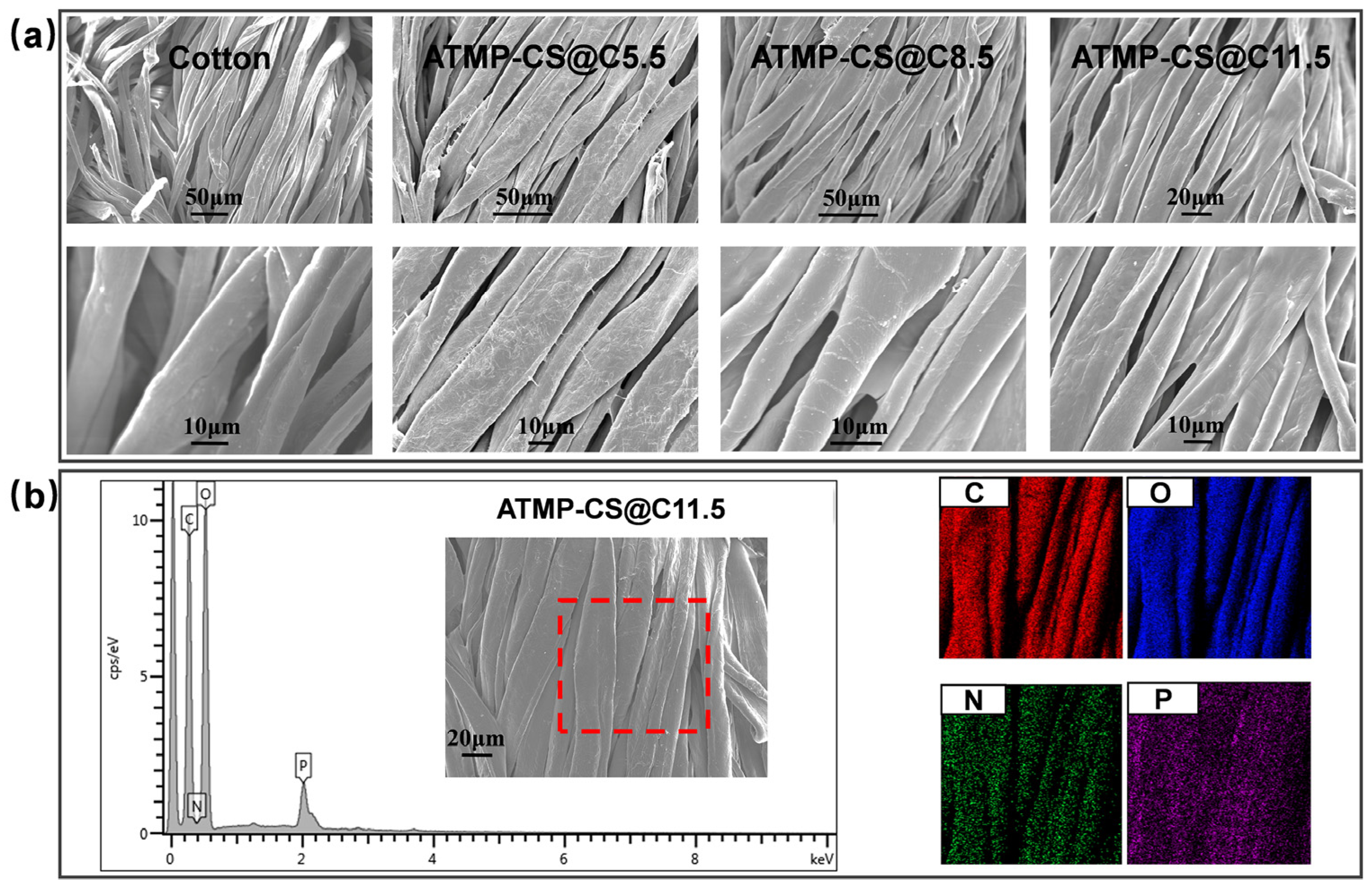
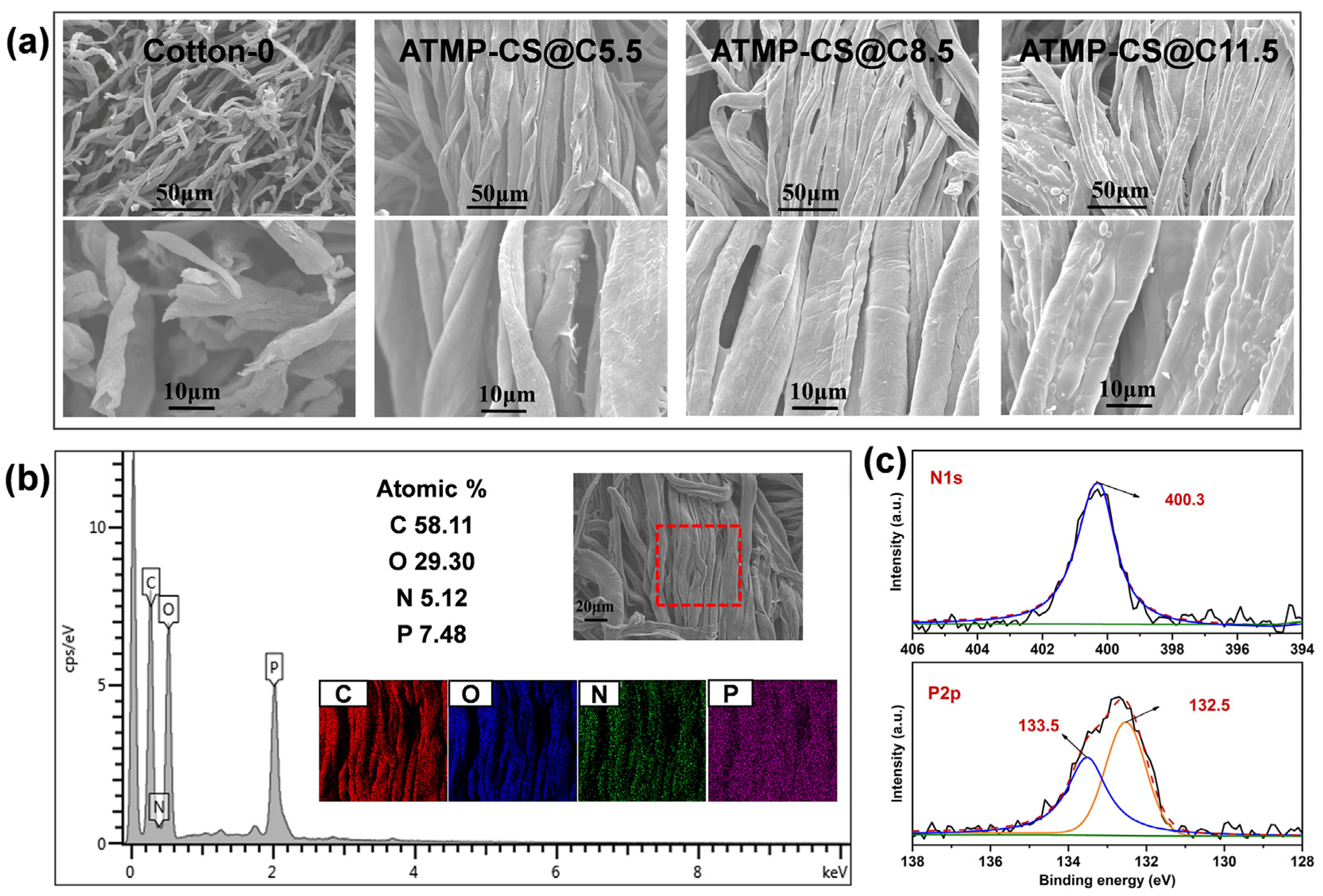
3.2. Morphology and Element Analysis
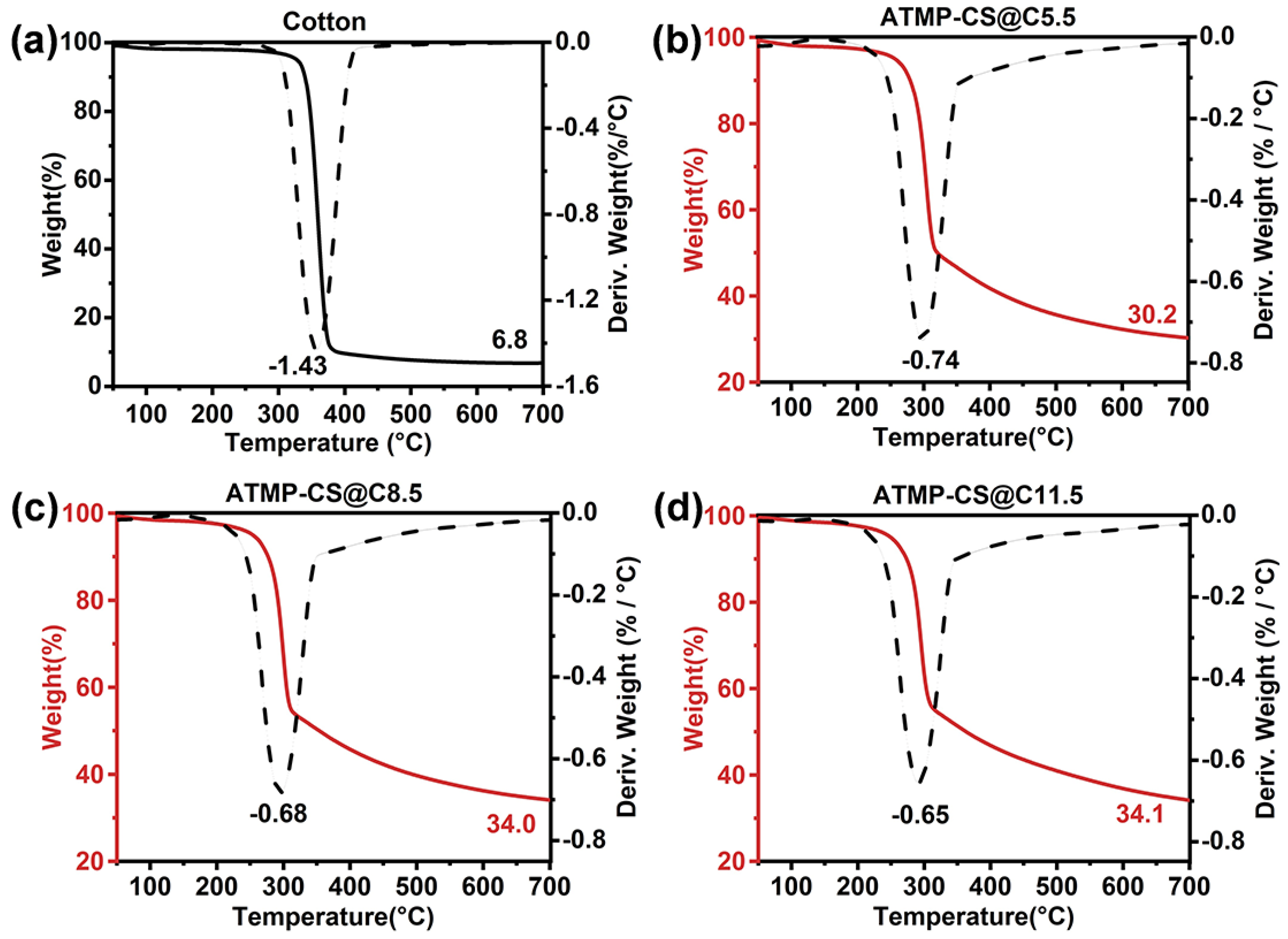
3.3. Thermal Stability
3.4. Flame Retardant Performance
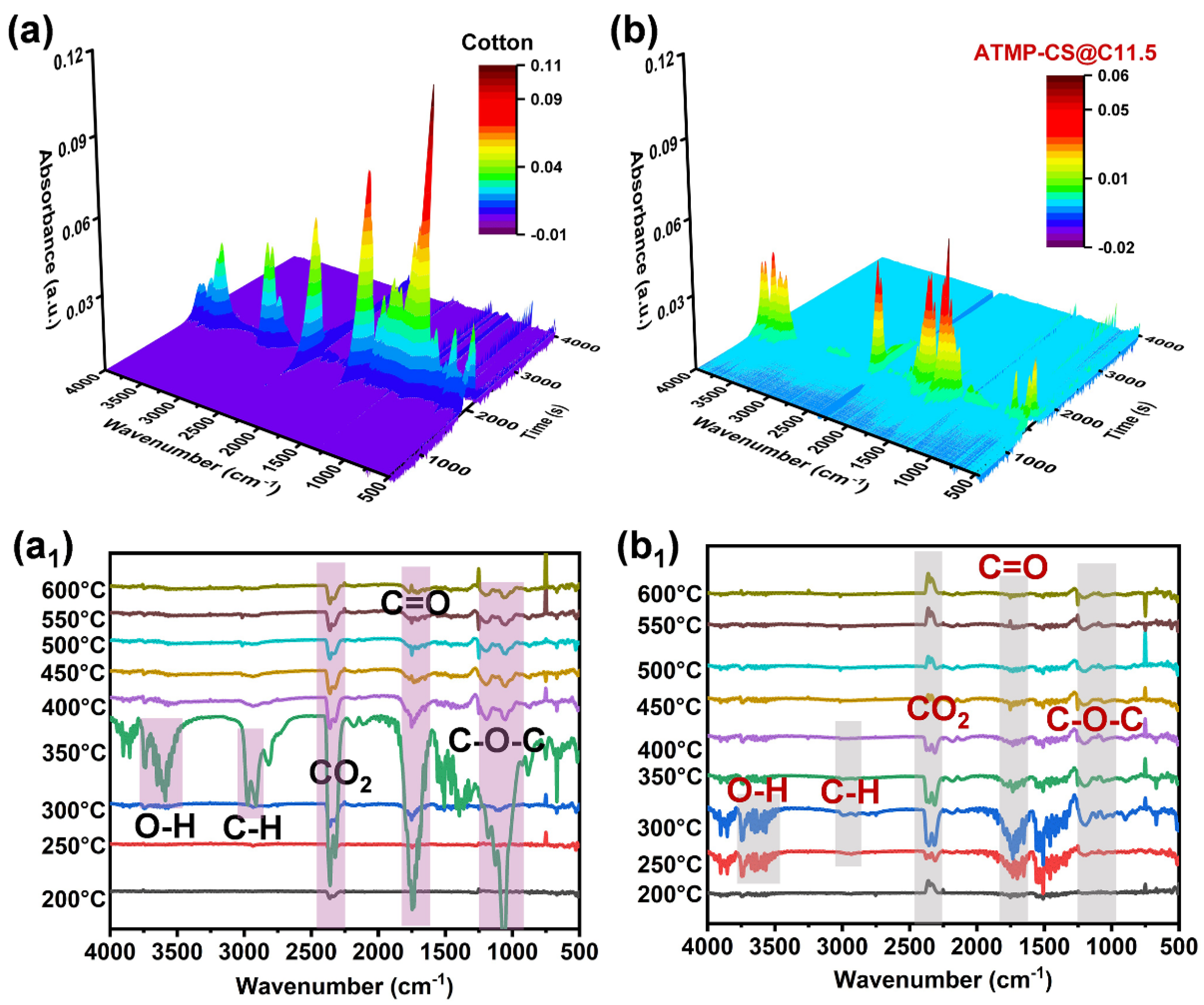
3.5. Flame-Retardant Mechanism Analysis
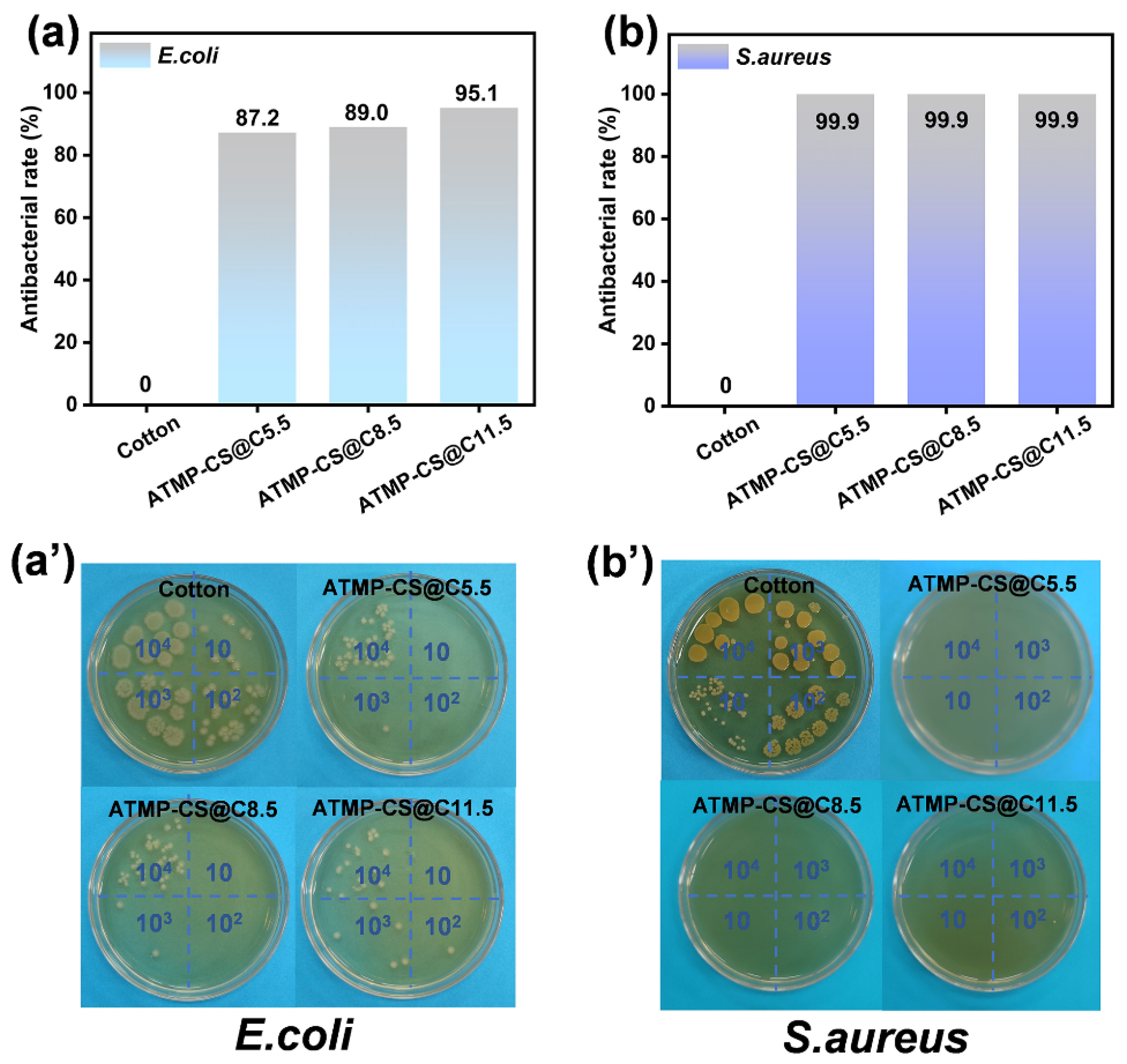
3.6. Antibacterial Performance
3.7. Whiteness, Air Permeability, and Mechanical Property
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Li, G.-W.; Huang, J.-W.; Zhou, J.; Zhang, Y.-C.; Zhang, C.-C.; Rao, Z.-G.; Fei, L.-F. A flame-retardant wood-based composite with magnesium-aluminium layered double hydroxides for efficient daytime radiative cooling. J. Mater. Chem. A 2024, 12, 1609–1616. [Google Scholar] [CrossRef]
- Chen, T.; Peng, C.-H.; Lin, Z.-Y.; Chen, G.-R.; Luo, W.-A.; Yuan, C.-H.; Liu, C.; Xu, Y.-T.; Dai, L.-Z. Hierarchical structure coating modified cotton fabric with superhydrophobic and flame-retardant performances. Prog. Org. Coat. 2024, 186, 108038. [Google Scholar] [CrossRef]
- Rahman, M.-M.; Koh, J.; Hong, K.-H. Sustainable Chitosan Biomordant Dyeing and Functionalization of Cotton Fabrics Using Pomegranate Rind and Onion Peel Extracts. J. Nat. Fibers. 2024, 21, 2290856. [Google Scholar] [CrossRef]
- Lokhande, K.-D.; Bhakare, M.-A.; Bondarde, M.-P.; Dhumal, P.-S.; Some, S. Bio-derived efficient flame-retardants for cotton fabric. Cellulose 2022, 29, 3583–3593. [Google Scholar] [CrossRef]
- Tang, Q.; Lu, Y.; Diao, S.; Yang, Y.; Liang, M.; Zhou, H.; Zhang, G. Formaldehyde-free and durable phosphorus-containing cotton flame retardant with -N=P-(N)3- and reactive ammonium phosphoric acid groups. Int. J. Biol. Macromol. 2024, 260, 129293. [Google Scholar] [CrossRef]
- Wu, X.; Gou, T.; Zhao, Q.; Chen, L.; Wang, P. High-efficiency durable flame retardant with ammonium phosphate ester and phosphine oxide groups for cotton cellulose biomacromolecule. Int. J. Biol. Macromol. 2022, 194, 945–953. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Y.; Ren, Y.; Liu, X. A sustainable strategy for preparation of flame-retardant cotton fabric by phosphorylation of recycled cotton. Text. Res. J. 2022, 92, 3766–3781. [Google Scholar] [CrossRef]
- Liu, L.; Pan, Y.; Wang, Z.; Hou, Y.; Gui, Z.; Hu, Y. Layer-by-Layer Assembly of Hypophosphorous Acid-Modified Chitosan Based Coating for Flame-Retardant Polyester–Cotton Blends. Ind. Eng. Chem. Res. 2017, 56, 9429–9436. [Google Scholar] [CrossRef]
- Bentis, A.; Boukhriss, A.; Boyer, D.; Gmouh, S. Development of flame retardant cotton fabric based on ionic liquids via sol-gel technique. Mat. Sci. Eng. 2017, 254, 122001. [Google Scholar] [CrossRef]
- Liu, K.; Lu, Y.; Cheng, Y.; Li, J.; Zhang, G.; Zhang, F. Flame retardancy and mechanism of polymer flame retardant containing P–N bonds for cotton fabrics modified by chemical surface grafting. Cellulose 2024, 31, 3243–3258. [Google Scholar] [CrossRef]
- Li, X.-L.; Shi, X.-H.; Chen, M.-J.; Liu, Q.-Y.; Li, Y.-M.; Li, Z.; Huang, Y.-H.; Wang, D.-Y. Biomass-based coating from chitosan for cotton fabric with excellent flame retardancy and improved durability. Cellulose 2022, 29, 5289–5303. [Google Scholar] [CrossRef]
- Qin, R.; Du, L.; Li, H.; Shao, Z.-B.; Jiang, Z.; Zhu, P. Reasonable multi-functionality of cotton fabric with superior flame retardancy and antibacterial ability using ammonium diphosphate as cross-linker. Chem. Eng. J. 2024, 485, 149848. [Google Scholar] [CrossRef]
- Wang, T.-C.; He, X.-H.; Hu, W.; Zhu, L.; Shao, Z.-B. Facile construction of bio-based high fire-safety cellulose fabrics with well wearing performance. Int. J. Biol. Macromol. 2023, 253, 127349. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liang, J.; Bao, L.; Gu, X.; Zha, S.; Chen, X. Competitive and cooperative sorption between triclosan and methyl triclosan on microplastics and soil. Environ. Res. 2022, 212, 113548. [Google Scholar] [CrossRef]
- De Boer, J.; Wester, P.-G.; Klamer, H.-J.-C.; Lewis, W.-E.; Boon, J.-P. Do flame retardants threaten ocean life? Nature 1998, 394, 28–29. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.-W.; Guan, J.-P.; Tang, R.-C.; Liu, K.-Q. Phytic acid as a bio-based phosphorus flame retardant for poly(lactic acid) nonwoven fabric. J. Clean. Prod. 2016, 124, 114–119. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S.; Chen, H.; Chen, C.; Luan, J.; Dong, C.; Lu, Z. Preparation and thermostability of a Si/P/N synergistic flame retardant containing triazine ring structure for cotton fabrics. Int. J. Biol. Macromol. 2024, 260, 129497. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.; Wang, S.; Wang, W.; Sun, J.; Yuan, H.; Zhang, S. Chitosan/sodium polyborate based micro-nano coating with high flame retardancy and superhydrophobicity for cotton fabric. Int. J. Biol. Macromol. 2022, 205, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-T.; Ning, K.; Zhao, B. Two birds, one stone: Enhancement of flame retardancy and antibacterial property of viscose fabric using an aminoazole-based cyclotriphosphazene. Int. J. Biol. Macromol. 2023, 253, 126875. [Google Scholar] [CrossRef]
- Aenishänslin, R.; Guth, C.; Hofmann, P.; Maeder, A.; Nachbur, H. A New Chemical Approach to Durable Flame-Retardant Cotton Fabrics. Text. Res. J. 1969, 39, 375–381. [Google Scholar] [CrossRef]
- Wu, W.; Yang, C.-Q. Comparison of different reactive organophosphorus flame retardant agents for cotton: Part I. The bonding of the flame retardant agents to cotton. Polym. Degrad. Stabil. 2006, 91, 2541–2548. [Google Scholar] [CrossRef]
- Feng, J.; Sun, Y.; Song, P.; Lei, W.; Wu, Q.; Liu, L.; Yu, Y.; Wang, H. Fire-Resistant, Strong, and Green Polymer Nanocomposites Based on Poly(lactic acid) and Core–Shell Nanofibrous Flame Retardants. ACS Sustain. Chem. Eng. 2017, 5, 7894–7904. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhang, Y.; Du, X.; Song, P.; Fang, Z. Green and Scalable Fabrication of Core–Shell Biobased Flame Retardants for Reducing Flammability of Polylactic Acid. ACS Sustain. Chem. Eng. 2019, 7, 8954–8963. [Google Scholar] [CrossRef]
- Song, W.-M.; Zhang, L.-Y.; Li, P.; Ni, Y.-P.; Liu, Y. The fabrication of flame-retardant viscose fabrics with phytic acid-based flame retardants: Balancing efficient flame retardancy and tensile strength. Int. J. Biol. Macromol. 2024, 260, 129596. [Google Scholar] [CrossRef]
- Rathod, N.-B.; Bangar, S.-P.; Šimat, V.; Ozogul, F. Chitosan and gelatine biopolymer-based active/biodegradable packaging for the preservation of fish and fishery products. Int. J. Food. Sci. Technol. 2023, 58, 854–861. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, P.; Liu, Y.; Zhu, P. Flame retardant cotton fabrics with anti-UV properties based on tea polyphenol-melamine-phenylphosphonic acid. J. Colloid Interface Sci. 2023, 629, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, C.; Xu, Y.-J.; Jiang, Z.-M.; Liu, Y.; Zhu, P. Novel and eco-friendly flame-retardant cotton fabrics with lignosulfonate and chitosan through LbL: Flame retardancy, smoke suppression and flame-retardant mechanism. Polym. Degrad. Stabil. 2020, 181, 109302. [Google Scholar] [CrossRef]
- Nam, S.; Condon, B.-D.; Xia, Z.; Nagarajan, R.; Hinchliffe, D.-J.; Madison, C.-A. Intumescent flame-retardant cotton produced by tannic acid and sodium hydroxide. J. Anal. Appl. Pyrol. 2017, 126, 239–246. [Google Scholar] [CrossRef]
- Zhang, A.-N.; Zhao, H.-B.; Cheng, J.-B.; Li, M.-E.; Li, S.-L.; Cao, M.; Wang, Y.-Z. Construction of durable eco-friendly biomass-based flame-retardant coating for cotton fabrics. Chem. Eng. J. 2021, 410, 128361. [Google Scholar] [CrossRef]
- Zheng, X.-T.; Dong, Y.-Q.; Liu, X.-D.; Xu, Y.-L.; Jian, R.-K. Fully bio-based flame-retardant cotton fabrics via layer-by-layer self assembly of laccase and phytic acid. J. Clean. Prod. 2022, 350, 131525. [Google Scholar] [CrossRef]
- Makhlouf, G.; Abdelkhalik, A.; Ameen, H. Preparation of highly efficient chitosan-based flame retardant coatings with good antibacterial properties for cotton fabrics. Prog. Org. Coat. 2022, 163, 106627. [Google Scholar] [CrossRef]
- Wang, T.; Xu, J.; Zhan, Y.-J.; He, L.; Deng, J.; Fu, Z.-C.; Zhao, H.-B.; Chen, M.-J. Eco-Friendly and Facile Integrated Intumescent Polyelectrolyte Complex Coating with Universal Flame Retardancy and Smoke Suppression for Cotton and its Blending Fabrics. ACS Sustain. Chem. Eng. 2023, 11, 4838–4849. [Google Scholar] [CrossRef]
- Kumar Kundu, C.; Wang, W.; Zhou, S.; Wang, X.; Sheng, H.; Pan, Y.; Song, L.; Hu, Y. A green approach to constructing multilayered nanocoating for flame retardant treatment of polyamide 66 fabric from chitosan and sodium alginate. Carbohyd. Polym. 2017, 166, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, B.; Liu, Y.-Y.; Xu, Y.-J.; Jiang, Z.-M.; Dong, C.-H.; Zhang, L.; Liu, Y.; Zhu, P. Fully bio-based coating from chitosan and phytate for fire-safety and antibacterial cotton fabrics. Carbohyd. Polym. 2020, 237, 116173. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Luo, C.-Y.; Zhu, P.; Liu, Y.; Xu, Y.-J. Facile construction of H3PO3-modified chitosan/montmorillonite coatings for highly efficient flame retardation of polyester–cotton fabrics. Prog. Org. Coat. 2023, 184, 107864. [Google Scholar] [CrossRef]
- Amaral, I.-F.; Granja, P.-L.; Barbosa, M.-A. Chemical modification of chitosan by phosphorylation: An XPS, FT-IR and SEM study. J. Biomat. Sci.-Polym. E 2005, 16, 1575–1593. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dong, C.; Zhang, Z.; Sun, H.; Kong, D.; Lu, Z. Durable flame retardant cotton fabrics modified with a novel silicon–phosphorus–nitrogen synergistic flame retardant. Cellulose 2020, 27, 9027–9043. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Cheng, C.; Lyu, S.; Zhu, Z. Preparation of phosphorus-doped chitosan derivative and its applications in polylactic acid: Crystallization, flame retardancy, anti-dripping and mechanical properties. Int. J. Biol. Macromol. 2024, 265, 130648. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Liu, R.; Chi, W.; An, X.; Zhu, Q.; Xu, S.; Wang, L. A chitosan derivative/phytic acid polyelectrolyte complex endowing polyvinyl alcohol film with high barrier, flame-retardant, and antibacterial effects. Int. J. Biol. Macromol. 2024, 259, 129240. [Google Scholar] [CrossRef]
- Zhang, L.-Y.; Song, W.-M.; Li, P.; Liu, Y. A P/N flame retardant for polyester-cotton fabrics: Flame retardancy, mechanical properties and antibacterial property. Int. J. Biol. Macromol. 2024, 261, 129767. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Q. Adsorption of phosphorylated chitosan on mineral surfaces. Colloids. Surf. A Physicochem. Eng. Asp. 2013, 436, 656–663. [Google Scholar] [CrossRef]
- Li, P.; Liu, H.; Xu, Y.-J.; Wang, D.-Y.; Liu, Y.; Zhu, P. Flame-retardant and antibacterial flexible polyurethane foams with high resilience based on a P/N/Si-containing system. J. Mater. Sci. Technol. 2024, 182, 141–151. [Google Scholar] [CrossRef]
- Song, W.-M.; Zhang, L.-Y.; Li, P.; Liu, Y. High-Efficient Fame-Retardant Finishing of Cotton Fabrics Based on Phytic Acid. Int. J. Mol. Sci. 2023, 24, 24021093. [Google Scholar]
- Zhang, L.-P.; Zhao, Z.-G.; Huang, Y.-Y.; Zhu, C.-J.; Cao, X.; Ni, Y.-P. Robust, Flame-Retardant, and Anti-Corrosive Waterborne Polyurethane Enabled by a PN Synergistic Flame-Retardant Containing Benzimidazole and Phosphinate Groups. Polymers 2023, 15, 2400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-Y.; Song, W.-M.; Li, P.; Wang, J.-S.; Liu, Y.; Zhu, P. Green flame-retardant coatings based on iron alginate for polyester fabrics: Thermal stability, flame retardancy and mechanical properties. Polym. Degrad. Stabil. 2022, 206, 110207. [Google Scholar] [CrossRef]
- Wang, T.-C.; Jia, M.-H.; Xu, N.-T.; Hu, W.; Jiang, Z.; Zhao, B.; Ni, Y.-P.; Shao, Z.-B. Facile fabrication of adenosine triphosphate/chitosan/polyethyleneimine coating for high flame-retardant lyocell fabrics with outstanding antibacteria. Int. J. Biol. Macromol. 2024, 260, 129599. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Fu, R.; Xiao, M.; Zheng, Q.; Wu, L.; Fang, K.; Ren, Y. Synergetic construction of color and multifunction for sustainable lyocell fabric by microbial nano pigment. Chem. Eng. J. 2024, 481, 148453. [Google Scholar] [CrossRef]



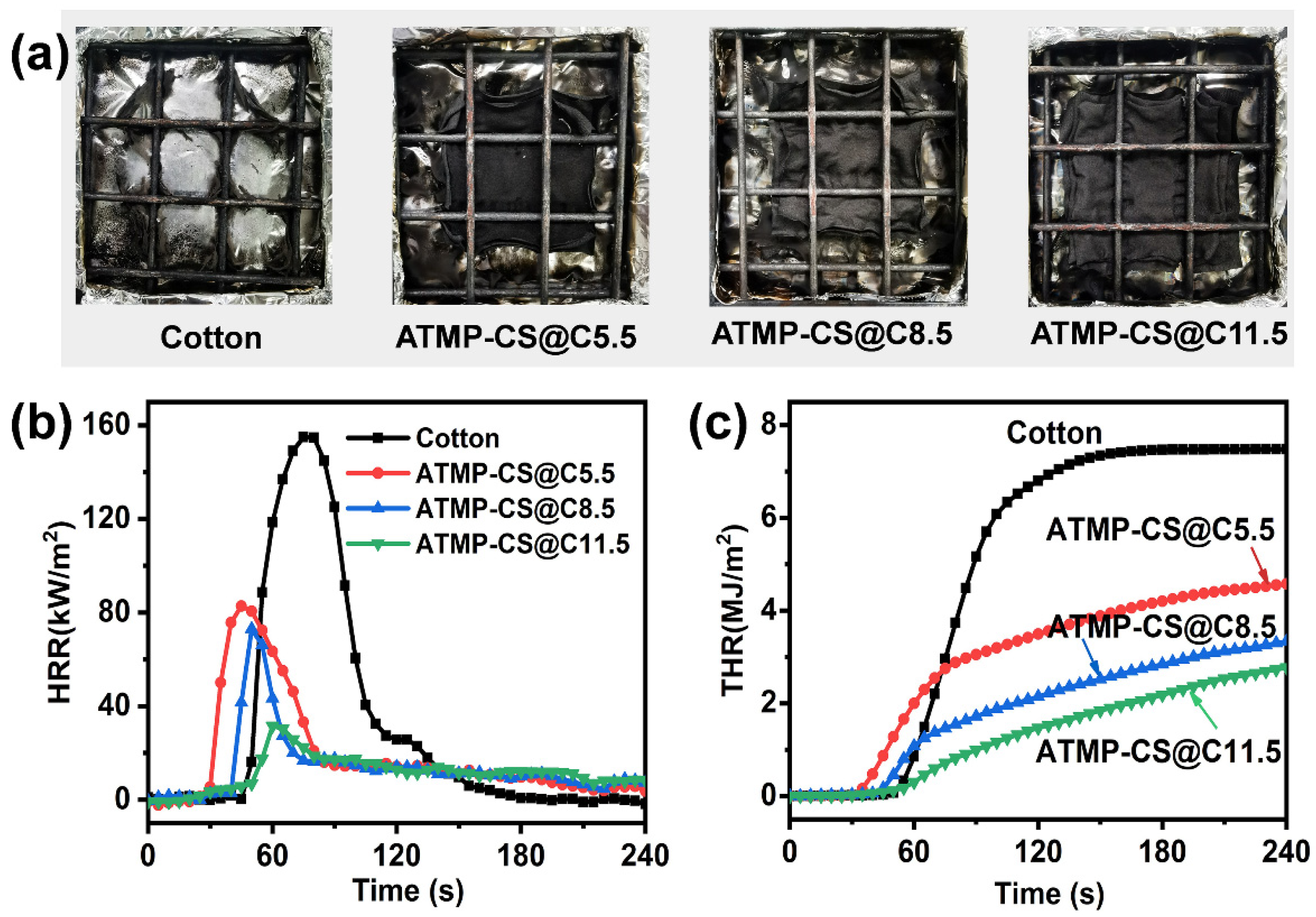

| Samples | Add-on (%) | After-Flame Time (s) | After-Glow Time (s) | Damaged Length (cm) | LOI (%) |
|---|---|---|---|---|---|
| Cotton | 0 | 20.0 | 24.0 | 30.0 | 18.0 |
| ATMP-CS@C5.5 | 5.5 | 10.0 | 0 | 30.0 | 23.5 |
| ATMP-CS@C8.5 | 8.5 | 8.0 | 0 | 30.0 | 25.9 |
| ATMP-CS@C11.5 | 11.5 | 0 | 0 | 6.3 | 29.7 |
| Samples | TTI (s) | pHRR (kW/m2) | THR (MJ/m2) | TSR (m2) | Av-HRR (kW/m2) | FIGRA (kW/(m2.s)) | Residues (%) |
|---|---|---|---|---|---|---|---|
| Cotton | 42.5 ± 2.1 | 146.6 ± 11.8 | 7.4 ± 0.5 | 0.04 ± 0.01 | 29.1 ± 0.4 | 2.0 ± 0.2 | 1.7 ± 0.2 |
| ATMP-CS@C5.5 | 24.0 ± 2.8 | 87.1 ± 6.1 | 4.9 ± 0.2 | 0.02 ± 0.001 | 17.9 ± 0.6 | 1.8 ± 0.008 | 16.2 ± 1.2 |
| ATMP-CS@C8.5 | 37.5 ± 0.7 | 74.4 ± 2.0 | 3.9 ± 0.3 | 0.09 ± 0.02 | 14.8 ± 1.1 | 1.4 ± 0.06 | 28.7 ± 0.9 |
| ATMP-CS@C11.5 | 47.0 ± 1.5 | 19.3 ± 4.5 | 2.8 ± 0.3 | 0.02 ± 0.002 | 10.6 ± 0.6 | 0.2 ± 0.08 | 77.2 ± 2.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-Y.; Zhang, L.-P.; Cao, X.; Tian, X.-Y.; Ni, Y.-P. Facile Fabrication of Highly Efficient Chitosan-Based Multifunctional Coating for Cotton Fabrics with Excellent Flame-Retardant and Antibacterial Properties. Polymers 2024, 16, 1409. https://doi.org/10.3390/polym16101409
Huang Y-Y, Zhang L-P, Cao X, Tian X-Y, Ni Y-P. Facile Fabrication of Highly Efficient Chitosan-Based Multifunctional Coating for Cotton Fabrics with Excellent Flame-Retardant and Antibacterial Properties. Polymers. 2024; 16(10):1409. https://doi.org/10.3390/polym16101409
Chicago/Turabian StyleHuang, Yuan-Yuan, Li-Ping Zhang, Xing Cao, Xin-Yu Tian, and Yan-Peng Ni. 2024. "Facile Fabrication of Highly Efficient Chitosan-Based Multifunctional Coating for Cotton Fabrics with Excellent Flame-Retardant and Antibacterial Properties" Polymers 16, no. 10: 1409. https://doi.org/10.3390/polym16101409
APA StyleHuang, Y.-Y., Zhang, L.-P., Cao, X., Tian, X.-Y., & Ni, Y.-P. (2024). Facile Fabrication of Highly Efficient Chitosan-Based Multifunctional Coating for Cotton Fabrics with Excellent Flame-Retardant and Antibacterial Properties. Polymers, 16(10), 1409. https://doi.org/10.3390/polym16101409





