Biopolymer-Based Hydrogel Incorporated with Naproxen Sodium and Lidocaine Hydrochloride for Controlled Drug Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Hydrogel Preparation
2.2. Viscosity Study
2.3. Release Study
2.4. Difference Factor and Similarity Factor
2.5. Kinetic Study
2.6. Statistical Analysis
2.7. FTIR Measurements
2.8. DSC Measurements
3. Results and Discussion
3.1. Viscosity Study
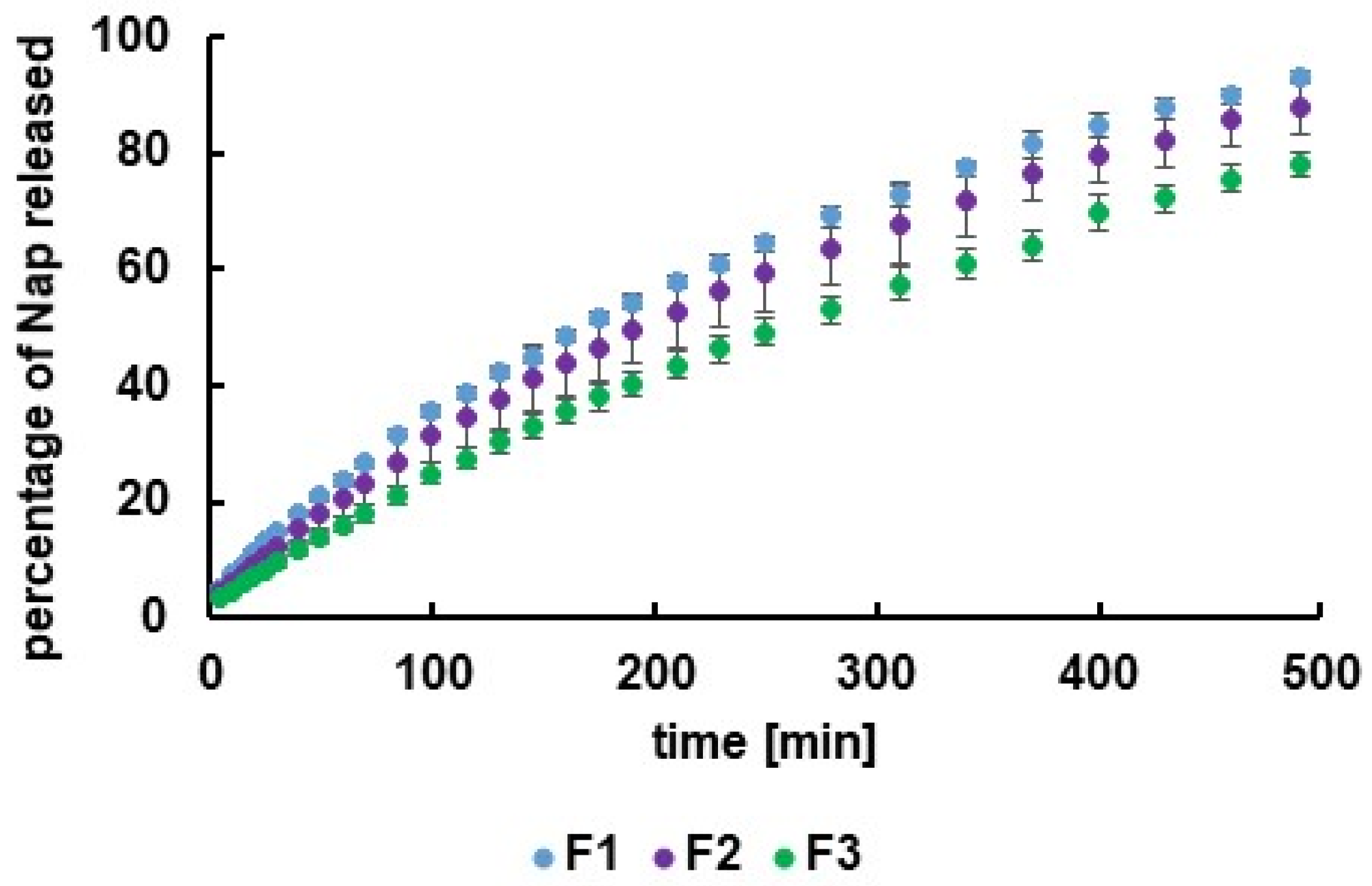
3.2. Release Study
3.3. Release Curves Assesment
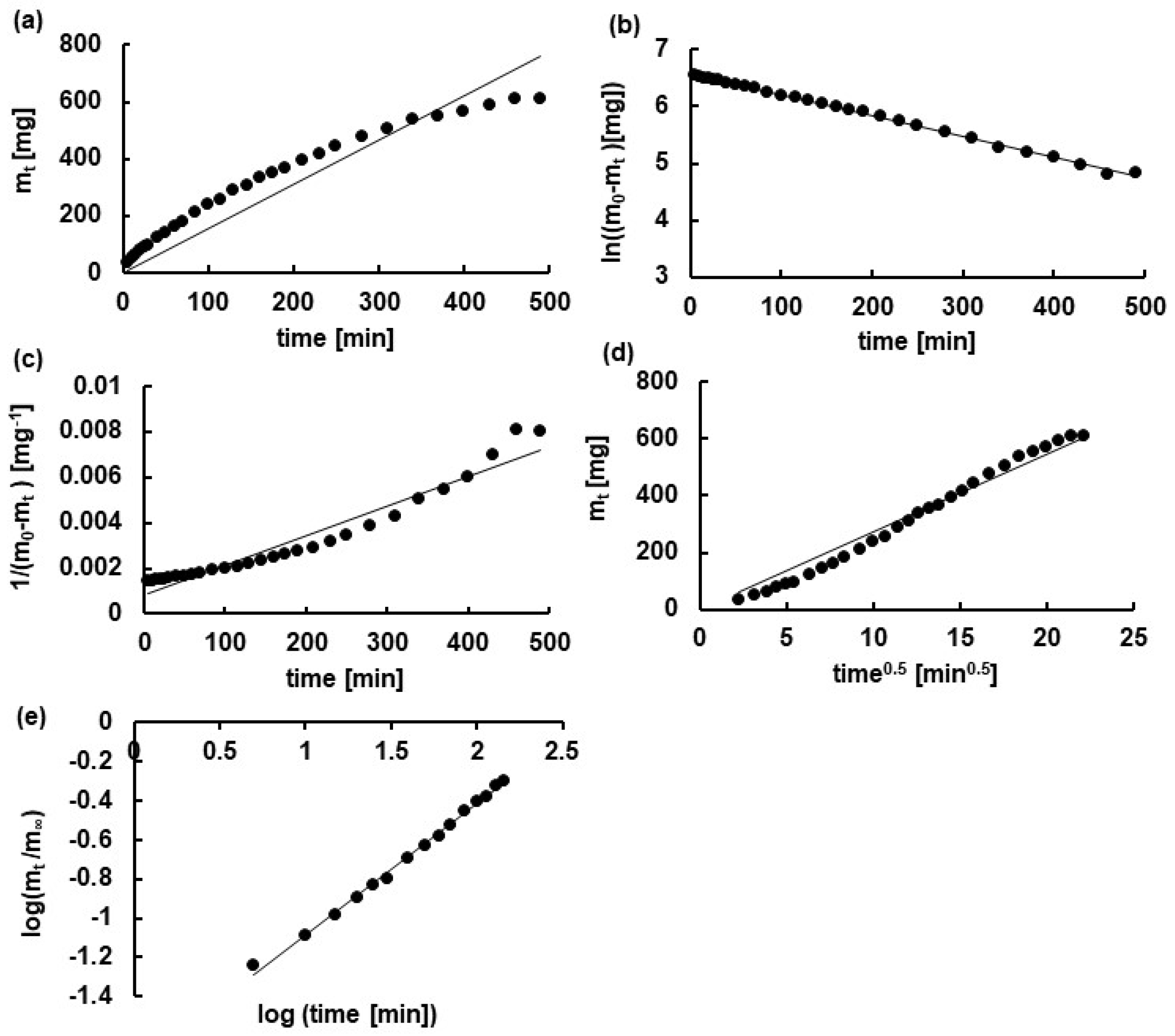
3.4. Kinetic Analysis
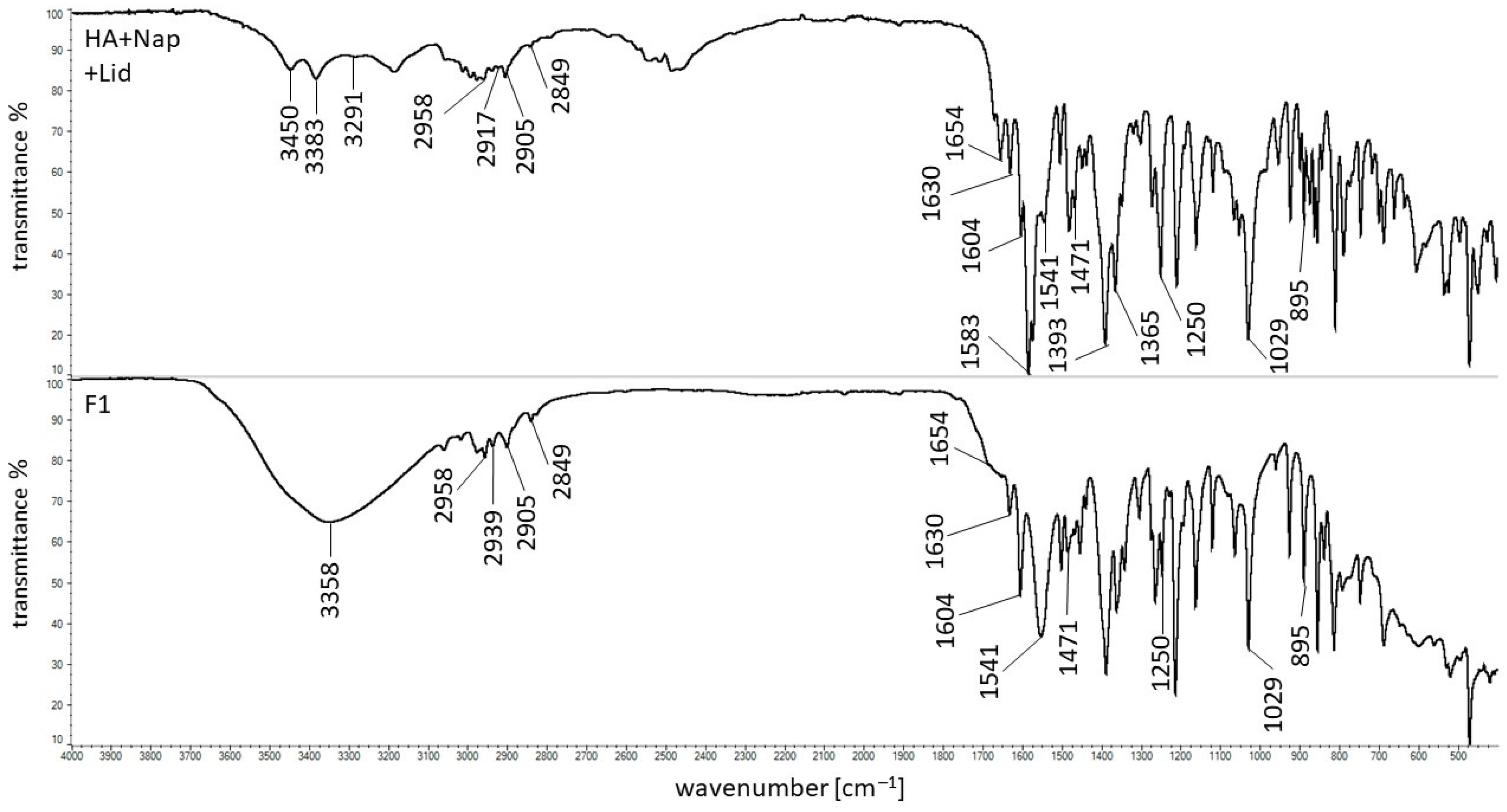
3.5. FTIR Study
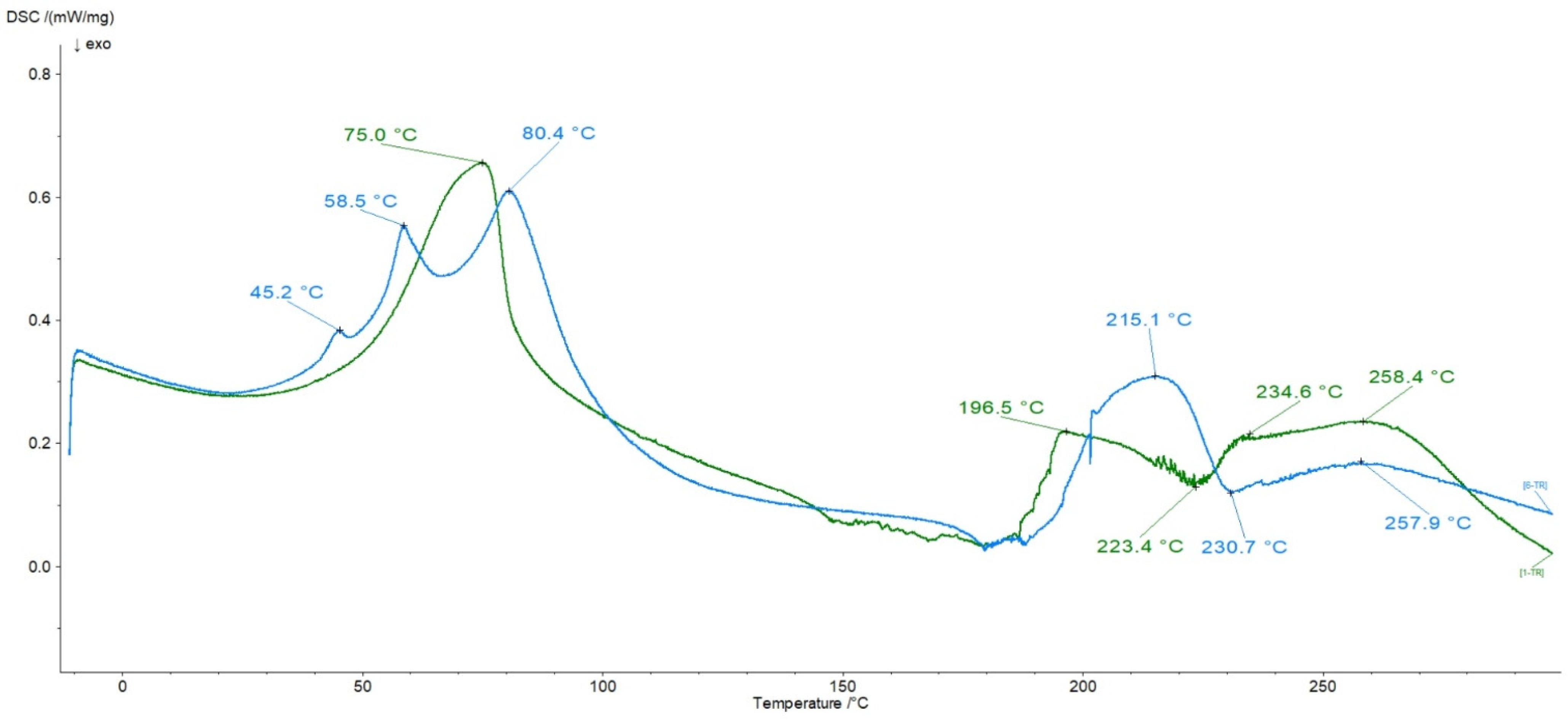
3.6. DSC Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pluta, J.; Karolewicz, B. Hydrożele: Właściwości i zastosowanie w technologii postaci leku. I. Charakterystyka hydrożeli. Polim. Med. 2004, 34, 63–81. [Google Scholar]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-Based Hydrogels Applied in Drug Delivery: An Overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef]
- Mahmood, A.; Patel, D.; Hickson, B.; Desrochers, J.; Hu, X. Recent Progress in Biopolymer-Based Hydrogel Materials for Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 1415. [Google Scholar] [CrossRef]
- Kogan, G.; Šoltés, L.; Stern, R.; Gemeiner, P. Hyaluronic acid: A natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol. Lett. 2007, 29, 17–25. [Google Scholar] [CrossRef]
- Kobayashi, T.; Chanmee, T.; Itano, N. Hyaluronan: Metabolism and function. Biomolecules 2020, 10, 1525. [Google Scholar] [CrossRef]
- Salwowska, N.M.; Bebenek, K.A.; Żądło, D.A.; Wcisło-Dziadecka, D.L. Physiochemical properties and application of hyaluronic acid: A systematic review. J. Cosmet. Dermatol. 2016, 15, 520–526. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef]
- Kumar, R.; Lee, Y.K.; Jho, Y.S. Martini coarse-grained model of hyaluronic acid for the structural change of its gel in the presence of monovalent and divalent salts. Int. J. Mol. Sci. 2020, 21, 4602. [Google Scholar] [CrossRef]
- Tezel, A.; Fredrickson, G.H. The science of hyaluronic acid dermal fillers. J. Cosmet. Laser Ther. 2008, 10, 35–42. [Google Scholar] [CrossRef]
- Gall, Y. Hyaluronic acid: Structure, metabolism and implication in cicatrisation. Ann. Dermatol. Venereol. 2010, 137, S30–S39. [Google Scholar] [CrossRef]
- Magaña-Vergara, N.E.; De La Cruz-Cruz, P.; Peraza-Campos, A.L.; Martínez-Martínez, F.J.; González-González, J.S. Mechanochemical synthesis and crystal structure of the lidocaine-phloroglucinol hydrate 1:1:1 complex. Crystals 2018, 8, 130. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, E.; Li, L.; Bai, L.; Zhang, W. Facile design of lidocaine-loaded polymeric hydrogel to persuade effects of local anesthesia drug delivery system: Complete in vitro and in vivo toxicity analyses. Drug Deliv. 2021, 28, 1080–1092. [Google Scholar] [CrossRef]
- Barletta, M.; Reed, R. Local Anesthetics: Pharmacology and Special Preparations. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 1109–1125. [Google Scholar] [CrossRef] [PubMed]
- Hermanns, H.; Hollmann, M.W.; Stevens, M.F.; Lirk, P.; Brandenburger, T.; Piegeler, T.; Werdehausen, R. Molecular mechanisms of action of systemic lidocaine in acute and chronic pain: A narrative review. Br. J. Anaesth. 2019, 123, 335–349. [Google Scholar] [CrossRef]
- Abu-Huwaij, R.; Assaf, S.; Salem, M.; Sallam, A. Mucoadhesive dosage form of lidocaine hydrochloride: I. Mucoadhesive and physicochemical characterization. Drug Dev. Ind. Pharm. 2007, 33, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Jamrógiewicz, M.; Milewska, K.; Mikolaszek, B. Spectroscopic evaluation on pseudopolymorphs of sodium naproxen. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 261, 120018. [Google Scholar] [CrossRef] [PubMed]
- Leung, G.J.; Rainsford, K.D.; Kean, W.F. Osteoarthritis of the hand I: Aetiology and pathogenesis, risk factors, investigation and diagnosis. J. Pharm. Pharmacol. 2014, 66, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Angiolillo, D.J.; Weisman, S.M. Clinical Pharmacology and Cardiovascular Safety of Naproxen. Am. J. Cardiovasc. Drugs 2017, 17, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Davies, N.M.; Anderson, K.E. Clinical pharmacokinetics of naproxen. Clin. Pharmacokinet. 1997, 32, 268–293. [Google Scholar] [CrossRef]
- Zid, L.; Zeleňák, V.; Almáši, M.; Zeleňáková, A.; Szücsová, J.; Bednarčík, J.; Šuleková, M.; Hudák, A.; VáhovskáLucia, L. Mesoporous Silica as a Drug Delivery System for Naproxen:Influence of Surface Functionalization. Molecules 2020, 25, 4722. [Google Scholar] [CrossRef]
- Halamová, D.; Badaničová, M.; Zeleňák, V.; Gondová, T.; Vainio, U. Naproxen drug delivery using periodic mesoporous silica SBA-15. Appl. Surf. Sci. 2010, 256, 6489–6494. [Google Scholar] [CrossRef]
- Farsi, F.; Dogru, B.; Kumar, U.D.; Almareddy, S.R.; Kadriye, A.; Evin, H. Gel Formulation Comprising Analgesic and Anesthetic Agents. EP3097907A1, 15 August 2018. [Google Scholar]
- Wójcik-Pastuszka, D.; Stawicka, K.; Dryś, A.; Musiał, W. Influence of HA on Release Process of Anionic and Cationic API Incorporated into Hydrophilic Gel. Int. J. Mol. Sci. 2023, 24, 5606. [Google Scholar] [CrossRef]
- Council of Europe, European Directorate for the Quality of Medicines & Health Care (EDQM). European Pharmacopeia 10.8; Council of Europe: Strasbourg, France, 2022. [Google Scholar]
- U.S. Food & Drug Administration. Approval Package for: APPLICATION NUMBER: ANDA 040837; U.S. Food & Drug Administration: Rockville, MD, USA, 2011; pp. 1–160. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/anda/2011/040837Orig1s000.pdf (accessed on 28 March 2024).
- Available online: https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=317a8784-5fdd-429f-9b74-14d89f372a32&type=display (accessed on 28 April 2024).
- Package Leaflet: Information for the User, Momendol 10% w/w Gel, Naproxen. Available online: https://www.hpra.ie/img/uploaded/swedocuments/2145676.PA0959_002_002.72697853-cf95-4aa2-a0e5-9a31ca013ef3.000001Product%20Leaflet%20Approved.150323.pdf (accessed on 1 March 2024).
- Leaflet Naproxen HASCO 100 mg/g (10%) Gel. Available online: https://www.hasco-lek.pl/wp-content/uploads/2023/02/Ulotka-naproxen-hasco-100-mg-10-zel.pdf (accessed on 1 March 2024).
- Council of Europe, European Directorate for the Quality of Medicines & Health Care (EDQM). European Pharmacopoeia 10.5, Dissolution Test for Patches; Council of Europe: Strasbourg, France, 2021; pp. 5675–5677. [Google Scholar]
- US Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research. Guidance for Industry, Dissolution Testing of Immediate Release Solid Oral Dosage Forms; Part 4; Food and Drug Administration: Rockville, MD, USA, 1997; pp. 15–22.
- Costa, P.; Sousa Lobo, J.M. Evaluation of mathematical models describing drug release from estradiol transdermal systems. Drug Dev. Ind. Pharm. 2003, 29, 89–97. [Google Scholar] [CrossRef]
- Gouda, R.; Baishya, H.; Qing, Z. Application of Mathematical Models in Drug Release Kinetics of Carbidopa and Levodopa ER Tablets. J. Dev. Drugs 2017, 6, 171. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Higuchi equation: Derivation, applications, use and misuse. Int. J. Pharm. 2011, 418, 6–12. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2001, 48, 139–157. [Google Scholar] [CrossRef]
- Wójcik-Pastuszka, D.; Krzak, J.; Prządka, P.; Twarda, M.; Osiński, B.; Musiał, W. Release of bupivacaine from artificial ligament implants modified with the silica coating. Ceram. Int. 2023, 49, 2852–2859. [Google Scholar] [CrossRef]
- Wójcik-Pastuszka, D.; Skrzypczyk, A.; Musiał, W. The interactions and release kinetics of sodium hyaluronate implemented in nonionic and anionic polymeric hydrogels, studied by immunoenzymatic ELISA test. Pharmaceutics 2022, 14, 58. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm.-Drug Res. 2010, 67, 217–223. [Google Scholar]
- Costa, P.; Lobo, J.M.S. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Bhatt, D.C.; Dhake, A.S.; Khar, R.K.; Mishra, D.N. Development and in vitro evaluation of transdermal matrix films of metoprolol tartrate. Yakugaku Zasshi 2008, 128, 1325–1331. [Google Scholar] [CrossRef]
- Paarakh, M.P.; Jose, P.A.N.I.; Setty, C.M.; Christoperr, P.G. V Release Kinetics—Concepts and Applications. Int. J. Pharm. Res. Technol. 2018, 8, 12–20. [Google Scholar] [CrossRef]
- Alam, K.; Iqbal, M.; Hasan, A.; Al-Maskari, N. Rheological characterization of biological hydrogels in aqueous state. J. Appl. Biotechnol. Rep. 2020, 7, 172–176. [Google Scholar] [CrossRef]
- Kar, F.; Arslan, N. Effect of temperature and concentration on viscosity of orange peel pectin solutions and intrinsic viscosity-molecular weight relationship. Carbohydr. Polym. 1999, 40, 277–284. [Google Scholar] [CrossRef]
- Das, S.; Subuddhi, U. Cyclodextrin mediated controlled release of naproxen from pH-sensitive chitosan/poly(vinyl alcohol) hydrogels for colon targeted delivery. Ind. Eng. Chem. Res. 2013, 52, 14192–14200. [Google Scholar] [CrossRef]
- Gadekar, V. Formulation and Evaluation of Naproxen Proniosomal Gel for the Treatment of Inflammatory and Degenerative Disorders of the Musculoskeletal System. J. Drug Deliv. Ther. 2013, 3, 36–41. [Google Scholar] [CrossRef]
- Hima, B.E.; Radha, G.V. Design and in Vivo Evaluation of Naproxen-Loaded Transferosomal Gel for Transdermal Delivery. Int. J. Appl. Pharm. 2024, 16, 272–284. [Google Scholar] [CrossRef]
- Noreen, S.; Pervaiz, F.; Ashames, A.; Buabeid, M.; Fahelelbom, K.; Shoukat, H.; Maqbool, I.; Murtaza, G. Optimization of novel naproxen-loaded chitosan/carrageenan nanocarrier-based gel for topical delivery: Ex vivo, histopathological, and in vivo evaluation. Pharmaceuticals 2021, 14, 557. [Google Scholar] [CrossRef]
- Lee, P.I. Kinetics of drug release from hydrogel matrices. J. Control. Release 1985, 2, 277–288. [Google Scholar] [CrossRef]
- Mrestani, Y.; Hammitzch, M.; Neubert, R.H.H. Investigation of the interaction between lidocaine and the components of hyaluronic acid using frontal analysis continuous capillary electrophoresis. Chromatographia 2009, 69, 1321–1324. [Google Scholar] [CrossRef]
- Larrañeta, E.; Henry, M.; Irwin, N.J.; Trotter, J.; Perminova, A.A.; Donnelly, R.F. Synthesis and characterization of hyaluronic acid hydrogels crosslinked using a solvent-free process for potential biomedical applications. Carbohydr. Polym. 2018, 181, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
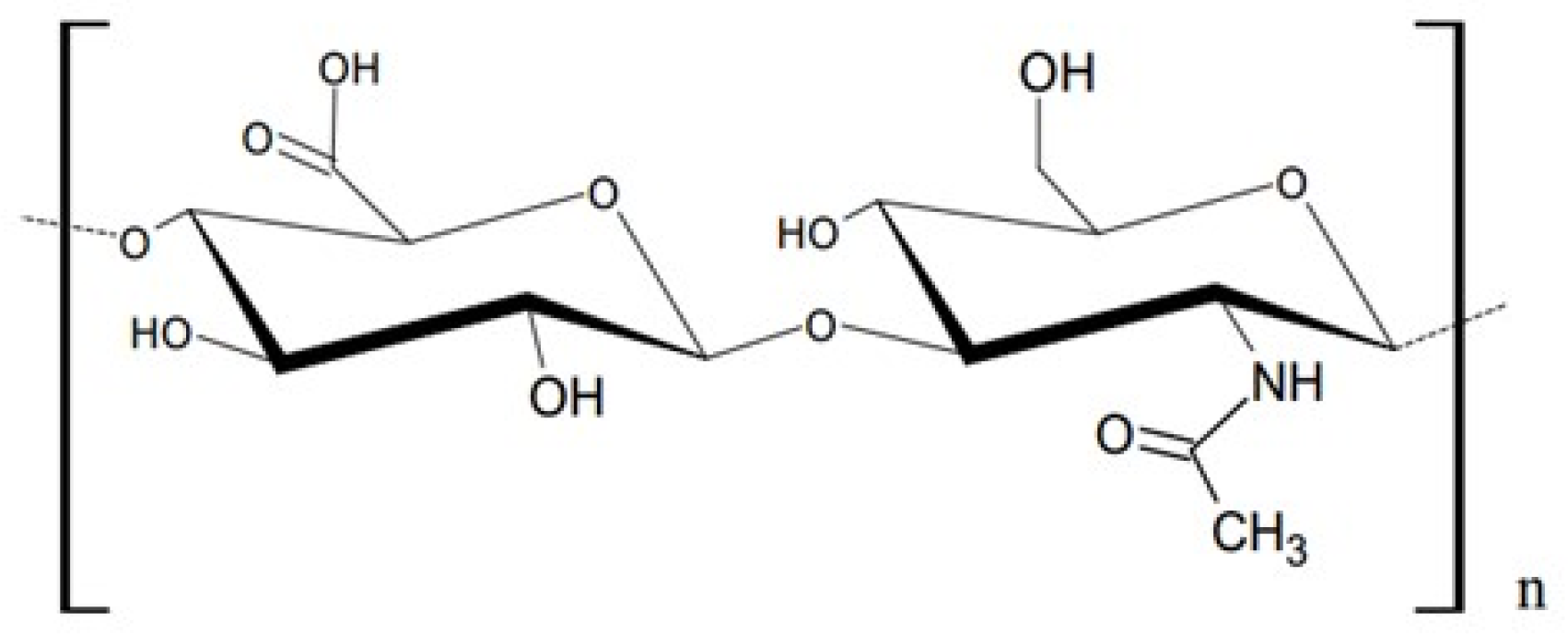
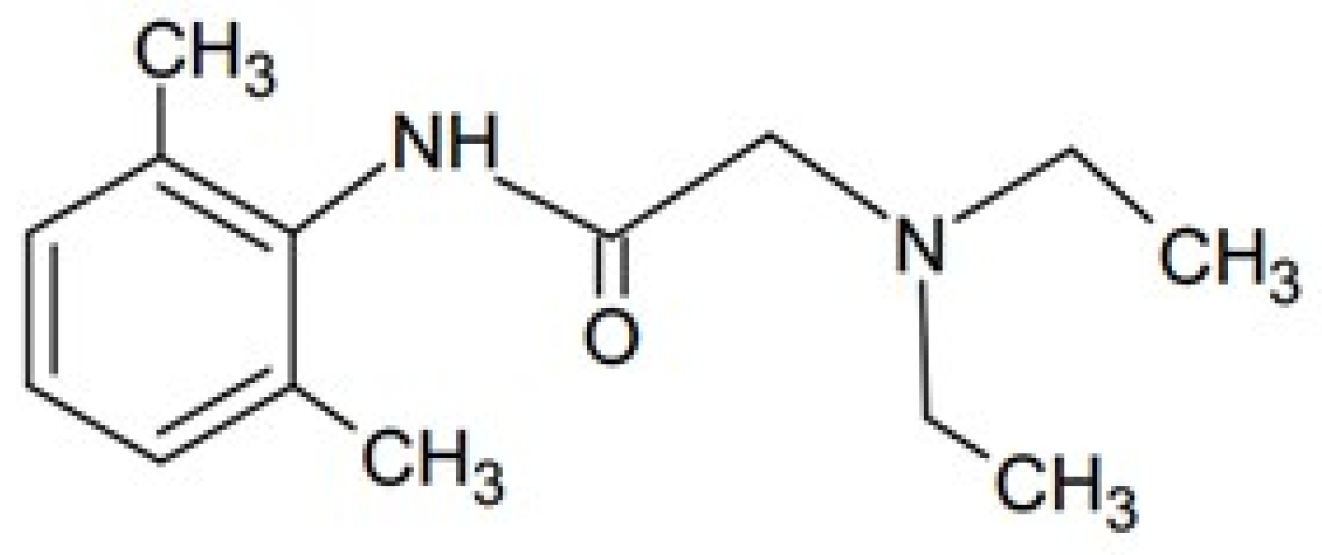
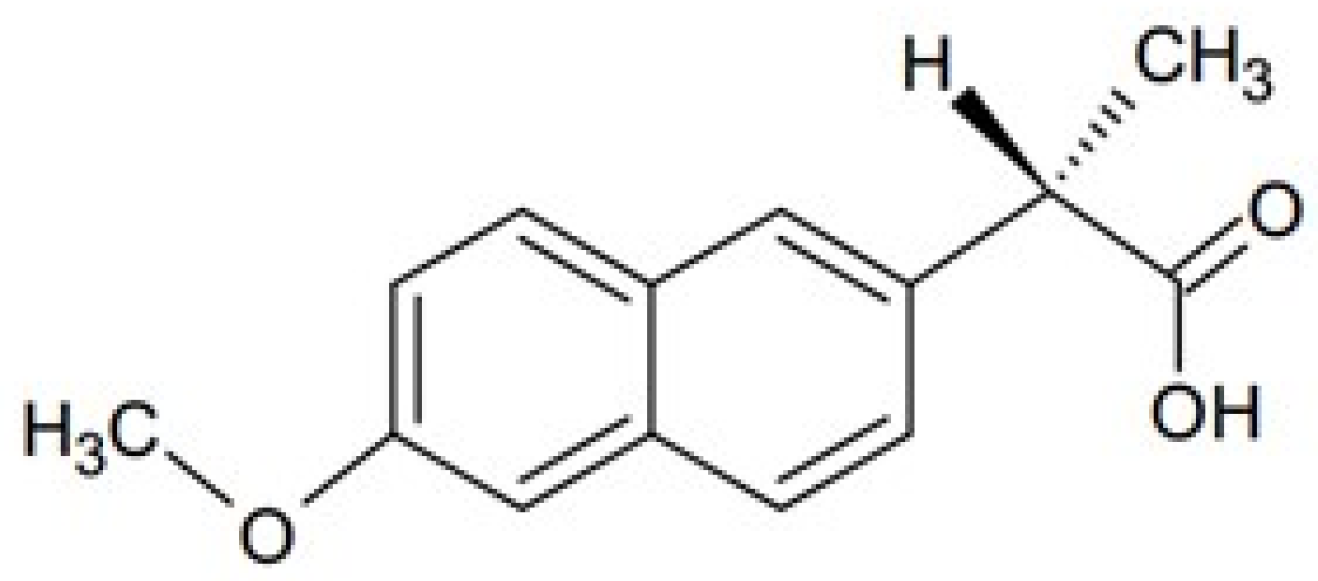
| Formulation | F1 | F2 | F3 |
|---|---|---|---|
| HA [g] | 3.0 | 4.0 | 5.0 |
| Water [g] | 173.0 | 172.0 | 171.0 |
| L [g] | 4.0 | 4.0 | 4.0 |
| N [g] | 20.0 | 20.0 | 20.0 |
| Formulation | F1 | F2 | F3 |
|---|---|---|---|
| Viscosity [cP] | 1905.2 ± 58.6 | 4308.0 ± 95.8 | 8946.0 ± 417.4 |
| Reference formulation * | F1r | F2r | F3r |
| Viscosity [cP] | 2850.1 ± 42.8 | 5888.0 ± 143.1 | 13,860.9 ± 638.5 |
| f1 | f2 | |||
|---|---|---|---|---|
| Formulation | F2 | F3 | F2 | F3 |
| F1 | 8.49 | 31.15 | 68.54 | 46.19 |
| F2 | ― | 20.01 | ― | 55.30 |
| Kinetic Model | Kinetic Parameters | F1 | F2 | F3 |
|---|---|---|---|---|
| Z-O | k0 [mg × min−1] | 1.5 ± 0.1 | 1.4 ± 0.1 | 1.4 ± 0.1 |
| t0.5 [min] | 219.5 ± 15.5 | 237.1 ± 14.2 | 277.0 ± 12.9 | |
| R2 | 0.87 ± 0.01 | 0.87 ± 0.02 | 0.95 ± 0.01 | |
| F-O | k1 ×103 [min−1] | 4.8 ± 0.3 | 4.1 ± 0.2 | 2.9 ± 0.1 |
| t0.5 [min] | 144.1 ± 8.2 | 172.5 ± 7.5 | 235.9 ± 8.2 | |
| R2 | 0.98 ± 0.01 | 0.99 ± 0.01 | 0.99 ± 0.01 | |
| S-O | k2 × 105 [mg−1 × min−1] | 2.7 ± 0.6 | 2.0 ± 0.4 | 0.8 ± 0.09 |
| t0.5 [min] | 55.2 ± 11.5 | 87.2 ± 13.7 | 161.1 ± 18.6 | |
| R2 | 0.77 ± 0.04 | 0.84 ± 0.05 | 0.92 ± 0.01 | |
| H | kH [mg × min−1/2] | 31.5 ± 0.7 | 24.9 ± 1.1 | 24.9 ± 1.4 |
| t0.5 [min] | 114.2 ± 5.2 | 186.4 ± 16.6 | 257.6 ± 28.2 | |
| R2 | 0.97 ± 0.01 | 0.95 ± 0.01 | 0.94 ± 0.01 | |
| K-P | kK-P × 102 [min−n] | 1.7 ± 0.1 | 1.5 ± 0.1 | 1.0 ± 0.1 |
| t0.5 [min] | 160.0 ± 13.8 | 164.8 ± 19.5 | 189.5 ± 20.1 | |
| R2 | 0.99 ± 0.01 | 0.99 ± 0.01 | 0.99 ± 0.01 | |
| n | 0.67 ± 0.01 | 0.69 ± 0.02 | 0.74 ± 0.02 | |
| best fit | K-P, | K-P, F-O | K-P, F-O | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójcik-Pastuszka, D.; Stawicka, K.; Musiał, W. Biopolymer-Based Hydrogel Incorporated with Naproxen Sodium and Lidocaine Hydrochloride for Controlled Drug Delivery. Polymers 2024, 16, 1353. https://doi.org/10.3390/polym16101353
Wójcik-Pastuszka D, Stawicka K, Musiał W. Biopolymer-Based Hydrogel Incorporated with Naproxen Sodium and Lidocaine Hydrochloride for Controlled Drug Delivery. Polymers. 2024; 16(10):1353. https://doi.org/10.3390/polym16101353
Chicago/Turabian StyleWójcik-Pastuszka, Dorota, Karolina Stawicka, and Witold Musiał. 2024. "Biopolymer-Based Hydrogel Incorporated with Naproxen Sodium and Lidocaine Hydrochloride for Controlled Drug Delivery" Polymers 16, no. 10: 1353. https://doi.org/10.3390/polym16101353
APA StyleWójcik-Pastuszka, D., Stawicka, K., & Musiał, W. (2024). Biopolymer-Based Hydrogel Incorporated with Naproxen Sodium and Lidocaine Hydrochloride for Controlled Drug Delivery. Polymers, 16(10), 1353. https://doi.org/10.3390/polym16101353






