Synthetic Degradable Polyvinyl Alcohol Polymer and Its Blends with Starch and Cellulose—A Comprehensive Overview
Abstract
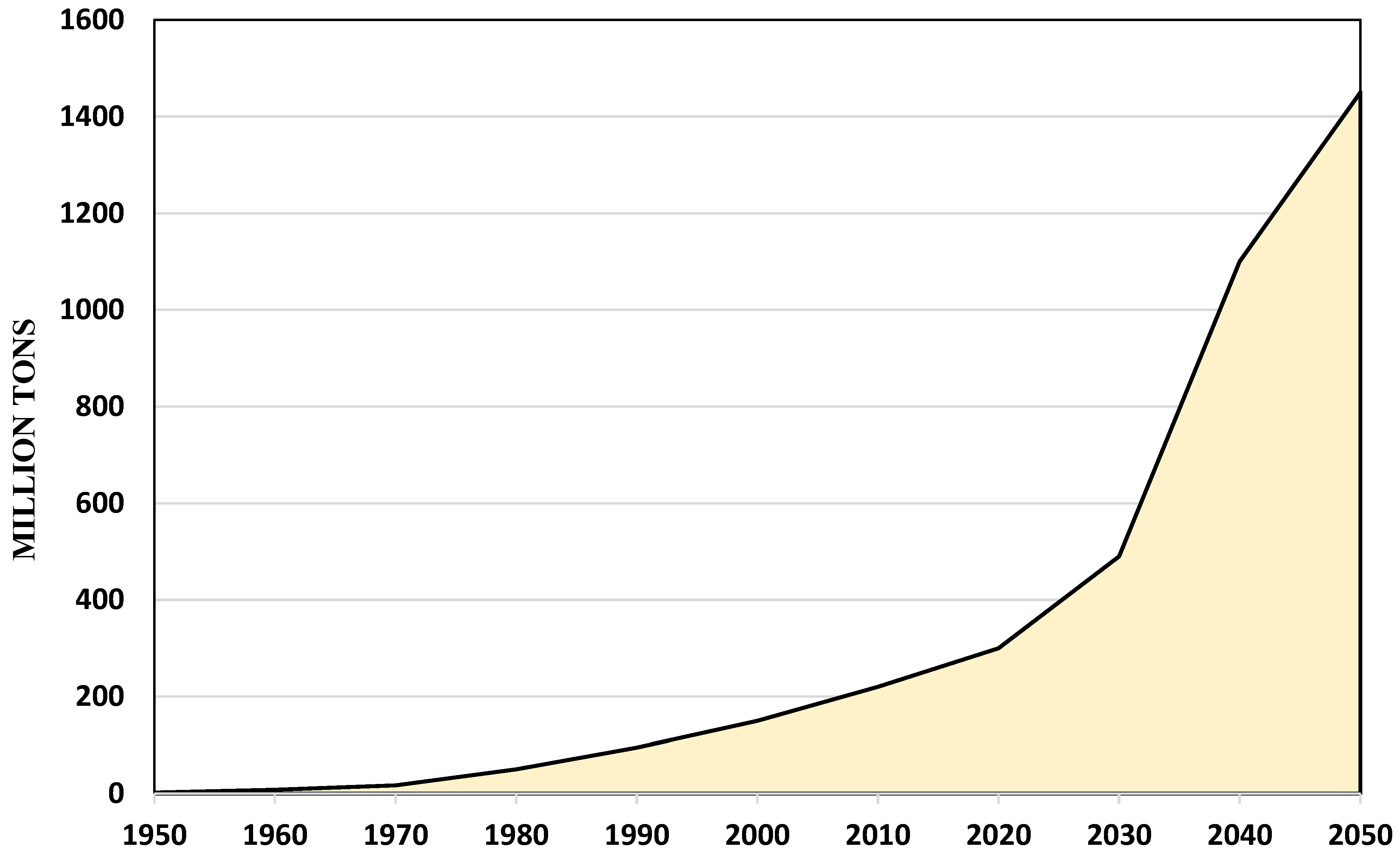
1. Introduction
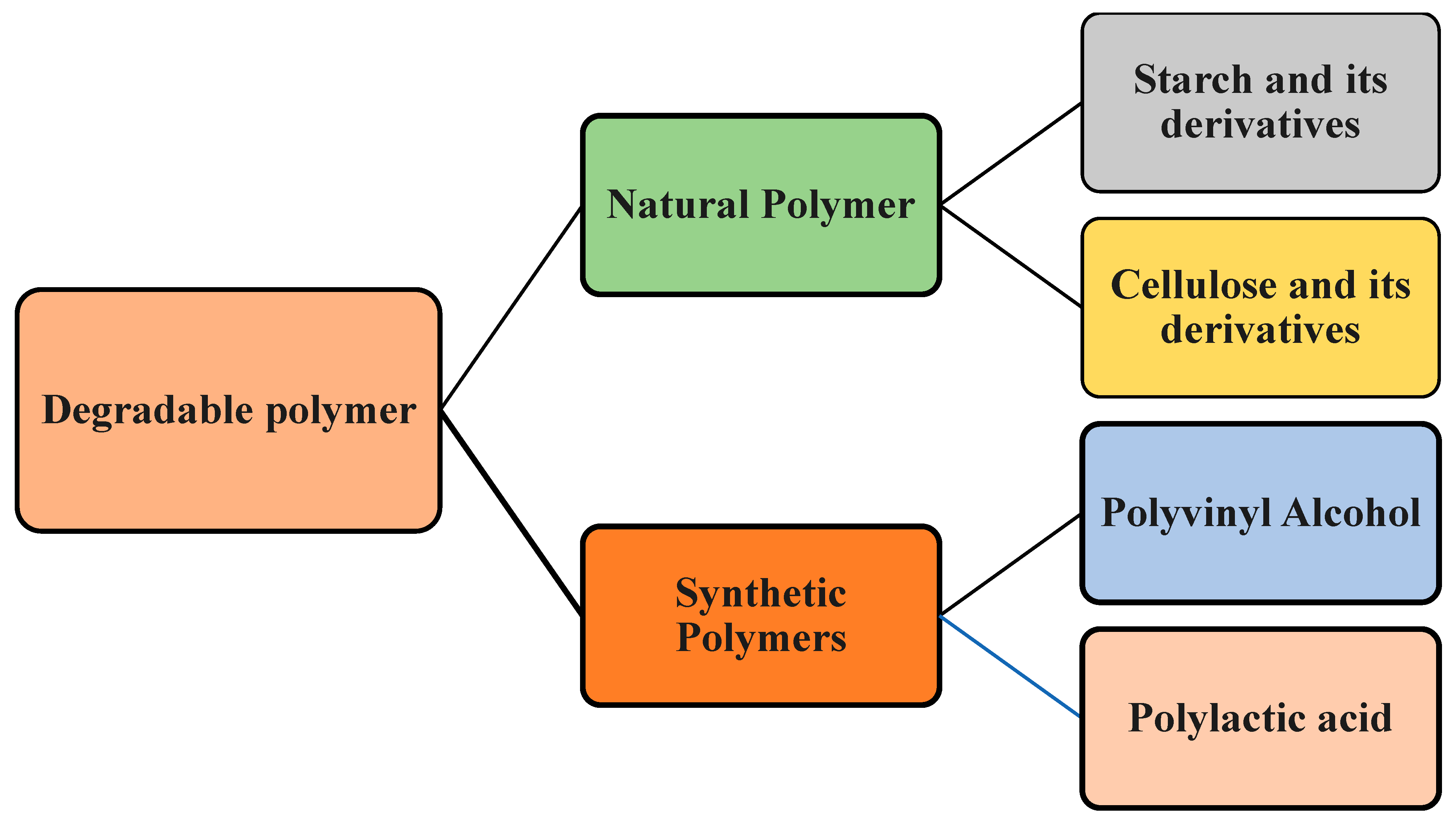
2. Biodegradable Polymers and Biopolymers
3. Polylactic Acid
4. Polyvinyl Alcohol
5. Starch
6. Cellulose
7. Hydroxypropyl Methylcellulose
8. Blending of PVA with Natural Polymers
8.1. Polyvinyl Alcohol and Starch
8.2. Polyvinyl Alcohol and Cellulose Derivatives
8.2.1. Polyvinyl Alcohol and Carboxy Methyl Cellulose
8.2.2. Polyvinyl Alcohol and Hydroxypropyl Methylcellulose
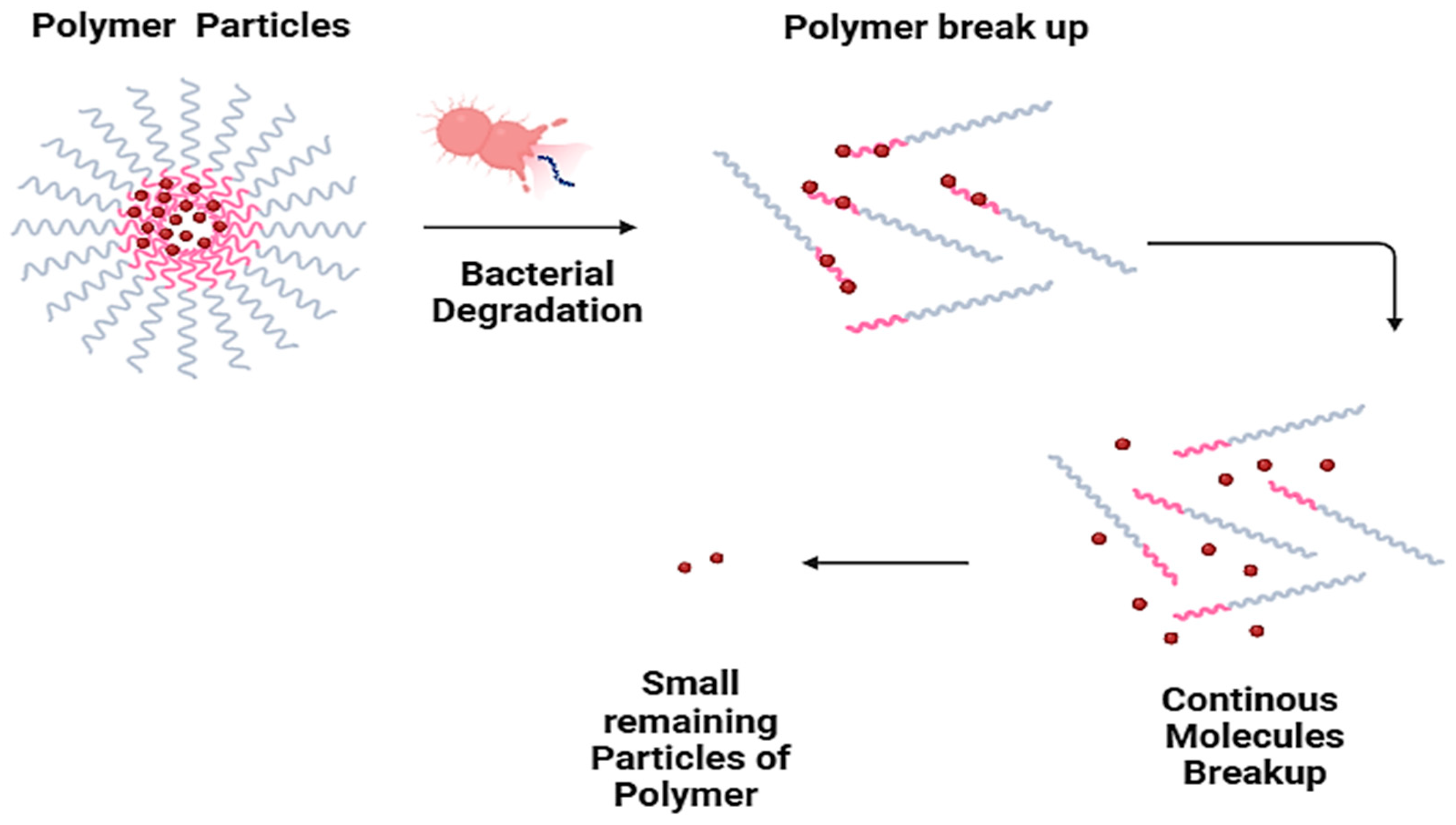
8.3. Biodegradation of Polyvinyl Alcohol
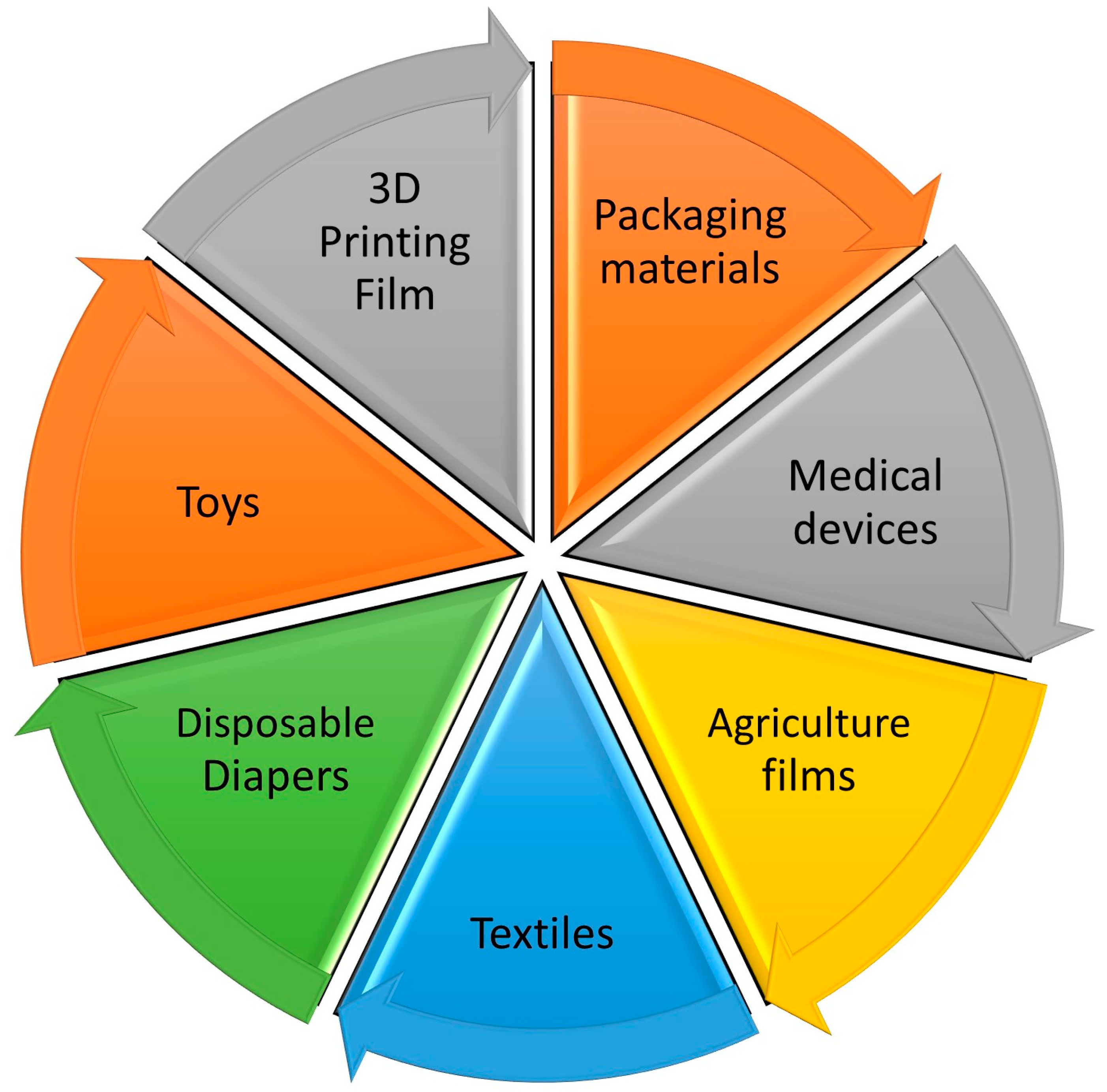
9. Applications
10. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kasznik, D.; Łapniewska, Z. The end of plastic? The EU’s directive on single-use plastics and its implementation in Poland. Environ. Sci. Policy 2023, 145, 151–163. [Google Scholar] [CrossRef]
- Borrelle, S.B.; Ringma, J.; Law, K.L.; Monnahan, C.C.; Lebreton, L.; McGivern, A.; Murphy, E.; Jambeck, J.; Leonard, G.H.; Hilleary, M.A.; et al. Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science 2020, 369, 1515–1518. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.W.Y.; Shiran, Y.; Bailey, R.M.; Cok, E.; Stuchtey, M.R.; Koskella, J.; Velis, C.A.; Godfrey, L.; Boucher, J.; Murphy, M.B.; et al. Evaluating scenarios toward zero plastic pollution. Science 2020, 369, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Simantiris, N. Single-Use Plastic or Paper Products? A Dilemma That Requires Societal Change. Clean. Waste Syst. 2024, 7, 100128. [Google Scholar] [CrossRef]
- Kitz, R.; Walker, T.; Charlebois, S.; Music, J. Food packaging during the COVID-19 pandemic: Consumer perceptions. Int. J. Consum. Stud. 2021, 46, 434–448. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Verma, A.; Shome, A.; Sinha, R.; Sinha, S.; Jha, P.K.; Kumar, R.; Kumar, P.; Shubham; Das, S.; et al. Impacts of Plastic Pollution on Ecosystem Services, Sustainable Development Goals, and Need to Focus on Circular Economy and Policy Interventions. Sustainability 2021, 13, 9963. [Google Scholar] [CrossRef]
- Dimassi, S.N.; Hahladakis, J.N.; Yahia, M.N.D.; Ahmad, M.I.; Sayadi, S.; Al-Ghouti, M.A. Degradation-Fragmentation of Marine Plastic Waste and Their Environmental Implications: A Critical Review. Arab. J. Chem. 2022, 15, 104262. [Google Scholar] [CrossRef]
- Brooks, A.L.; Wang, S.; Jambeck, J.R. The Chinese Import Ban and Its Impact on Global Plastic Waste Trade. Sci. Adv. 2018, 4, eaat0131. [Google Scholar] [CrossRef] [PubMed]
- Le Breton, M.; Baillet, L.; Larose, E.; Rey, E.; Benech, P.; Jongmans, D.; Guyoton, F.; Jaboyedoff, M. Passive radio-frequency identification ranging, a dense and weather-robust technique for landslide displacement monitoring. Eng. Geol. 2019, 250, 1–10. [Google Scholar] [CrossRef]
- Villarrubia-Gómez, P.; Cornell, S.E.; Fabres, J. Marine plastic pollution as a planetary boundary threat—The drifting piece in the sustainability puzzle. Mar. Policy 2018, 96, 213–220. [Google Scholar] [CrossRef]
- Babaremu, K.O.; Okoya, S.A.; Hughes, E.; Tijani, B.; Teidi, D.; Akpan, A.; Igwe, J.; Karera, S.; Oyinlola, M.; Akinlabi, E.T. Sustainable Plastic Waste Management in a Circular Economy. Heliyon 2022, 8, e09984. [Google Scholar] [CrossRef]
- Kurtela, A.; Antolović, N. The problem of plastic waste and microplastics in the seas and oceans: Impact on marine organisms. Croat. J. Fish. 2019, 77, 51–56. [Google Scholar] [CrossRef]
- Schmaltz, E.; Melvin, E.C.; Diana, Z.; Gunady, E.F.; Rittschof, D.; Somarelli, J.A.; Virdin, J.; Dunphy-Daly, M.M. Plastic pollution solutions: Emerging technologies to prevent and collect marine plastic pollution. Environ. Int. 2020, 144, 106067. [Google Scholar] [CrossRef] [PubMed]
- Kehinde, O.; Ramonu, O.J.; Babaremu, K.O.; Justin, L.D. Plastic wastes: Environmental hazard and instrument for wealth creation in Nigeria. Heliyon 2020, 6, e05131. [Google Scholar] [CrossRef] [PubMed]
- Gallo, F.; Fossi, C.; Weber, R.; Santillo, D.; Sousa, J.; Ingram, I.; Nadal, A.; Romano, D. Marine litter plastics and microplastics and their toxic chemicals components: The need for urgent preventive measures. In Analysis of Nanoplastics and Microplastics in Food; CRC Press: Boca Raton, FL, USA, 2020; pp. 159–179. [Google Scholar]
- Ghosh, S.; Sinha, J.K.; Ghosh, S.; Vashisth, K.; Han, S.; Bhaskar, R. Microplastics as an Emerging Threat to the Global Environment and Human Health. Sustainability 2023, 15, 10821. [Google Scholar] [CrossRef]
- Carbery, M.; O’Connor, W.; Palanisami, T. Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environ. Int. 2018, 115, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Cordier, M.; Uehara, T. How much innovation is needed to protect the ocean from plastic contamination? Sci. Total Environ. 2019, 670, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Barboza, L.G.A.; Vethaak, A.D.; Lavorante, B.R.B.O.; Lundebye, A.-K.; Guilhermino, L. Marine microplastic debris: An emerging issue for food security, food safety and human health. Mar. Pollut. Bull. 2018, 133, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Benson, N.U.; Bassey, D.E.; Palanisami, T. COVID pollution: Impact of COVID-19 pandemic on global plastic waste footprint. Heliyon 2021, 7, e06343. [Google Scholar] [CrossRef]
- Pawar, P.R.; Shirgaonkar, S.S.; Patil, R.B. Plastic marine debris: Sources, distribution and impacts on coastal and ocean biodiversity. PENCIL Publ. Biol. Sci. 2016, 3, 40–54. [Google Scholar]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Huysman, S.; De Schaepmeester, J.; Ragaert, K.; Dewulf, J.; De Meester, S. Performance indicators for a circular economy: A case study on post-industrial plastic waste. Resour. Conserv. Recycl. 2017, 120, 46–54. [Google Scholar] [CrossRef]
- Ángeles-Hurtado, L.A.; Rodríguez-Reséndiz, J.; Salazar-Colores, S.; Torres-Salinas, H.; Sevilla-Camacho, P.Y. Viable disposal of post-consumer polymers in Mexico: A review. Front. Environ. Sci. 2021, 9, 749775. [Google Scholar] [CrossRef]
- Kibria, M.G.; Masuk, N.I.; Safayet, R.; Nguyen, H.Q.; Mourshed, M. Plastic Waste: Challenges and Opportunities to Mitigate Pollution and Effective Management. Int. J. Environ. Res. 2023, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- Babaremu, K.; Adediji, A.; Olumba, N.; Okoya, S.; Akinlabi, E.; Oyinlola, M. Technological Advances in Mechanical Recycling Innovations and Corresponding Impacts on the Circular Economy of Plastics. Environments 2024, 11, 38. [Google Scholar] [CrossRef]
- Santos, G.; Esmizadeh, E.; Riahinezhad, M. Recycling Construction, Renovation, and Demolition Plastic Waste: Review of the Status Quo, Challenges and Opportunities. J. Polym. Environ. 2024, 32, 479–509. [Google Scholar] [CrossRef]
- Yang, C.; Wu, H.; Cai, M.; Zhou, Y.; Guo, C.; Han, Y.; Zhang, L. Valorization of Biomass-Derived Polymers to Functional Biochar Materials for Supercapacitor Applications via Pyrolysis: Advances and Perspectives. Polymers 2023, 15, 2741. [Google Scholar] [CrossRef] [PubMed]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Vinoda, K.S.; Papireddy, M.; Gowda, A.N.S. Toxic Pollutants from Plastic Waste—A Review. Procedia Environ. Sci. 2016, 35, 701–708. [Google Scholar] [CrossRef]
- Jeswani, H.; Krüger, C.; Russ, M.; Horlacher, M.; Antony, F.; Hann, S.; Azapagic, A. Life cycle environmental impacts of chemical recycling via pyrolysis of mixed plastic waste in comparison with mechanical recycling and energy recovery. Sci. Total Environ. 2021, 769, 144483. [Google Scholar] [CrossRef]
- EN 13432:2000; Packaging. Requirements for Packaging Recoverable through Composting and Biodegradation. Test Scheme and Evaluation Criteria for the Final Acceptance of Packaging. British Standards Institution: London, UK, 2000.
- Silva, R.R.A.; Marques, C.S.; Arruda, T.R.; Teixeira, S.C.; de Oliveira, T.V. Biodegradation of Polymers: Stages, Measurement, Standards and Prospects. Macromol 2023, 3, 371–399. [Google Scholar] [CrossRef]
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent Advances in Biodegradable Polymers for Sustainable Applications. npj Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Silva, A.C.Q.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Natural Polymers-Based Materials: A Contribution to a Greener Future. Molecules 2021, 27, 94. [Google Scholar] [CrossRef] [PubMed]
- Rosenboom, J.-G.; Langer, R.; Traverso, G. Bioplastics for a Circular Economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef]
- Joseph, T.M.; Unni, A.B.; Joshy, K.S.; Kar Mahapatra, D.; Haponiuk, J.; Thomas, S. Emerging Bio-Based Polymers from Lab to Market: Current Strategies, Market Dynamics and Research Trends. C 2023, 9, 30. [Google Scholar] [CrossRef]
- Syed, M.H.; Qutaba, S.; Zahari, M.A.; Abdullah, N.; Shoaib, M.; Bashir, H.F.; Ali, M.Q.; Ali, R.; Iqbal, A.; Ali, W. Eco-friendly antimicrobial finishing of cotton fabrics using bioactive agents from novel Melia azedarachayan berries extract and their performance after subsequent washings. Egypt. J. Chem. 2023, 66, 255–268. [Google Scholar]
- Mukherjee, C.; Varghese, D.; Krishna, J.S.; Boominathan, T.; Rakeshkumar, R.; Dineshkumar, S.; Rao, C.V.S.B.; Sivaramakrishna, A. Recent Advances in Biodegradable Polymers–Properties, Applications and Future Prospects. Eur. Polym. J. 2023, 192, 112068. [Google Scholar] [CrossRef]
- Bishai, M.; De, S.; Adhikari, B.; Banerjee, R. A comprehensive study on enhanced characteristics of modified polylactic acid based versatile biopolymer. Eur. Polym. J. 2014, 54, 52–61. [Google Scholar] [CrossRef]
- Vatanpour, V.; Dehqan, A.; Paziresh, S.; Zinadini, S.; Zinatizadeh, A.A.; Koyuncu, I. Polylactic acid in the fabrication of separation membranes: A review. Sep. Purif. Technol. 2022, 296, 121433. [Google Scholar] [CrossRef]
- Castro-Aguirre, E.; Iniguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly (lactic acid)—Mass production, processing, industrial applications, and end of life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef]
- Taib, N.-A.A.B.; Rahman, M.R.; Huda, D.; Kuok, K.K.; Hamdan, S.; Bin Bakri, M.K.; Bin Julaihi, M.R.M.; Khan, A. A Review on Poly Lactic Acid (PLA) as a Biodegradable Polymer. Polym. Bull. 2023, 80, 1179–1213. [Google Scholar] [CrossRef]
- Sangeetha, V.H.; Deka, H.; Varghese, T.O.; Nayak, S.K. State of the art and future prospectives of poly(lactic acid) based blends and composites. Polym. Compos. 2016, 39, 81–101. [Google Scholar] [CrossRef]
- Baran, E.H.; Erbil, H.Y. Surface Modification of 3D Printed PLA Objects by Fused Deposition Modeling: A Review. Colloids Interfaces 2019, 3, 43. [Google Scholar] [CrossRef]
- Casalini, T.; Rossi, F.; Castrovinci, A.; Perale, G. A perspective on polylactic acid-based polymers use for nanoparticles synthesis and applications. Front. Bioeng. Biotechnol. 2019, 7, 259. [Google Scholar] [CrossRef]
- Shah, M.; Shah, S.N. A novel method to evaluate performance of chemical particulates for fluid diversion during hydraulic fracturing treatment. J. Nat. Gas. Sci. Eng. 2021, 95, 104178. [Google Scholar] [CrossRef]
- Chen, X.; Lu, Y. A perspective review on degradable polylactic acid diverters for well stimulations. Fuel 2023, 348, 128557. [Google Scholar] [CrossRef]
- Pyda, M.; Czerniecka-Kubicka, A. Thermal Properties and Thermodynamics of Poly (L-Lactic Acid). In Synthesis, Structure and Properties of Poly (Lactic Acid); Springer: Cham, Switzerland, 2018; pp. 153–193. [Google Scholar]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in Modern Medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Li, H.; Wu, C.; Yu, X.; Zhang, W. Recent Advances of PVA-Based Hydrogels in Cartilage Repair Application. J. Mater. Res. Technol. 2023, 24, 2279–2298. [Google Scholar] [CrossRef]
- Thong, C.C.; Teo, D.C.L.; Ng, C.K. Application of Polyvinyl Alcohol (PVA) in Cement-Based Composite Materials: A Review of Its Engineering Properties and Microstructure Behavior. Constr. Build. Mater. 2016, 107, 172–180. [Google Scholar] [CrossRef]
- Couți, N.; Porfire, A.; Iovanov, R.; Crișan, A.G.; Iurian, S.; Casian, T.; Tomuță, I. Polyvinyl Alcohol, a Versatile Excipient for Pharmaceutical 3D Printing. Polymers 2024, 16, 517. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, E.A.; Chen, X.; Eldin, M.S.M.; Kenawy, E.-R.S. Crosslinked poly (vinyl alcohol) hydrogels for wound dressing applications: A review of remarkably blended polymers. Arab. J. Chem. 2015, 8, 1–14. [Google Scholar] [CrossRef]
- Abdullah, Z.W.; Dong, Y.; Davies, I.J.; Barbhuiya, S. PVA, PVA blends, and their nanocomposites for biodegradable packaging application. Polym. Plast. Technol. Eng. 2017, 56, 1307–1344. [Google Scholar] [CrossRef]
- Sarwar, M.S.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Hussain, A. Preparation and characterization of PVA/nanocellulose/Ag nanocomposite films for antimicrobial food packaging. Carbohydr. Polym. 2018, 184, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Compart, J.; Singh, A.; Fettke, J.; Apriyanto, A. Customizing Starch Properties: A Review of Starch Modifications and Their Applications. Polymers 2023, 15, 3491. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.; Dharmalingam, K.; Nishizu, T.; Katsuno, N.; Anandalakshmi, R. Effect of Amylose–Amylopectin Ratios on Physical, Mechanical, and Thermal Properties of Starch-based Bionanocomposite Films Incorporated with CMC and Nanoclay. Starch-Stärke 2020, 72, 1900121. [Google Scholar] [CrossRef]
- Guarás, M.P.; Ludueña, L.N.; Alvarez, V.A. Recent Advances in Thermoplastic Starch Biodegradable Nanocomposites. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Springer: Cham, Switzerland, 2020; pp. 1–24. [Google Scholar]
- Katayama, M.; Mitsuno, D.; Ueda, K. Clinical Application to Improve the “Depth Perception Problem” by Combining Augmented Reality and a 3D Printing Model. Plast. Reconstr. Surg. Glob. Open 2023, 11, e5071. [Google Scholar] [CrossRef]
- Kumari, S.V.G.; Pakshirajan, K.; Pugazhenthi, G. Recent Advances and Future Prospects of Cellulose, Starch, Chitosan, Polylactic Acid and Polyhydroxyalkanoates for Sustainable Food Packaging Applications. Int. J. Biol. Macromol. 2022, 221, 163–182. [Google Scholar] [CrossRef]
- Dong, J.; Zeng, J.; Li, P.; Li, J.; Wang, B.; Xu, J.; Gao, W.; Chen, K. Mechanically Strong Nanopapers Based on Lignin Containing Cellulose Micro-and Nano-Hybrid Fibrils: Lignin Content-Fibrils Morphology-Strengthening Mechanism. Carbohydr. Polym. 2023, 311, 120753. [Google Scholar] [CrossRef]
- Jebel, F.S.; Almasi, H. Morphological, physical, antimicrobial and release properties of ZnO nanoparticles-loaded bacterial cellulose films. Carbohydr. Polym. 2016, 149, 8–19. [Google Scholar] [CrossRef]
- Salari, M.; Khiabani, M.S.; Mokarram, R.R.; Ghanbarzadeh, B.; Kafil, H.S. Development and evaluation of chitosan based active nanocomposite films containing bacterial cellulose nanocrystals and silver nanoparticles. Food Hydrocoll. 2018, 84, 414–423. [Google Scholar] [CrossRef]
- Syafri, E.; Kasim, A.; Asben, A.; Senthamaraikannan, P.; Sanjay, M.R. Studies on Ramie cellulose microfibrils reinforced cassava starch composite: Influence of microfibrils loading. J. Nat. Fibers 2018, 17, 122–131. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, W.; Kong, F.; Lin, M.; Mustapha, A. Cellulose nanofibril/silver nanoparticle composite as an active food packaging system and its toxicity to human colon cells. Int. J. Biol. Macromol. 2019, 129, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and Its Derivatives: Towards Biomedical Applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Silva, P.M.; Prieto, C.; Lagarón, J.M.; Pastrana, L.M.; Coimbra, M.A.; Vicente, A.A.; Cerqueira, M.A. Food-Grade Hydroxypropyl Methylcellulose-Based Formulations for Electrohydrodynamic Processing: Part I–Role of Solution Parameters on Fibre and Particle Production. Food Hydrocoll. 2021, 118, 106761. [Google Scholar] [CrossRef]
- Drago, E.; Campardelli, R.; Lagazzo, A.; Firpo, G.; Perego, P. Improvement of Natural Polymeric Films Properties by Blend Formulation for Sustainable Active Food Packaging. Polymers 2023, 15, 2231. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zha, D.; Zhang, X.; Xu, J.; Guo, B.; Huang, Y. Ordered long polyvinyl alcohol fiber-reinforced thermoplastic starch composite having comparable mechanical properties with polyethylene and polypropylene. Carbohydr. Polym. 2020, 250, 116913. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Zha, D.; Li, B.; Yin, P.; Li, P. Polyvinyl Alcohol Microspheres Reinforced Thermoplastic Starch Composites. Materials 2018, 11, 640. [Google Scholar] [CrossRef] [PubMed]
- Vineeth, S.K.; Gadhave, R.V. Corn Starch Blended Polyvinyl Alcohol Adhesive Chemically Modified by Crosslinking and Its Applicability as Polyvinyl Acetate Wood Adhesive. Polym. Bull. 2024, 81, 811–825. [Google Scholar] [CrossRef]
- Kochkina, N.E.; Lukin, N.D. Structure and properties of biodegradable maize starch/chitosan composite films as affected by PVA additions. Int. J. Biol. Macromol. 2020, 157, 377–384. [Google Scholar] [CrossRef]
- Gómez-Aldapa, C.A.; Velazquez, G.; Gutierrez, M.C.; Rangel-Vargas, E.; Castro-Rosas, J.; Aguirre-Loredo, R.Y. Effect of polyvinyl alcohol on the physicochemical properties of biodegradable starch films. Mater. Chem. Phys. 2019, 239, 122027. [Google Scholar] [CrossRef]
- Phattarateera, S.; Sangthongdee, M.; Subsomboon, T.; Threepopnatkul, P. Assessing Antibacterial Properties of Polyvinyl Alcohol/Pregelatinized Starch Films for Outbreak Prevention. Ind. Crops Prod. 2024, 211, 118214. [Google Scholar] [CrossRef]
- Zhang, K.; Huang, T.-S.; Yan, H.; Hu, X.; Ren, T. Novel pH-sensitive films based on starch/polyvinyl alcohol and food anthocyanins as a visual indicator of shrimp deterioration. Int. J. Biol. Macromol. 2020, 145, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Lin, X.; Wu, H.; Huang, Z.; Gan, T.; Hu, H.; Qin, Y.; Zhang, Y. Fabrication of Biodegradable and Cold-Water-Soluble Starch/Polyvinyl Alcohol Films as Inner Packaging Materials of Pesticides: Enhanced Emulsification, Dispersibility, and Efficacy. Carbohydr. Polym. 2024, 328, 121713. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Huang, Y.; Xiao, L.; Lin, D.; Yang, Y.; Wang, H.; Yang, Y.; Wu, D.; Chen, H.; Zhang, Q.; et al. Physical properties and structural characterization of starch/polyvinyl alcohol/graphene oxide composite films. Int. J. Biol. Macromol. 2018, 123, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Usman, A.; Hussain, Z.; Riaz, A.; Khan, A.N. Enhanced mechanical, thermal and antimicrobial properties of poly (vinyl alcohol)/graphene oxide/starch/silver nanocomposites films. Carbohydr. Polym. 2016, 153, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Tao, H.; Tan, C.; Xie, J.; Yuan, F.; Guo, L.; Cui, B.; Zou, F.; Gao, W.; Liu, P.; et al. Intermolecular Interactions between Starch and Polyvinyl Alcohol for Improving Mechanical Properties of Starch-Based Straws. Int. J. Biol. Macromol. 2023, 239, 124211. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Hu, Y.; Su, C.; Liang, W.; Liu, X.; Zhao, W.; Sun, Z.; Zhang, X.; Lu, Y.; Shen, H.; et al. Structural, Physicochemical and Biodegradable Properties of Composite Plastics Prepared with Polyvinyl Alcohol (PVA), OSA Potato Starch and Gliadin. J. Food Eng. 2023, 339, 111278. [Google Scholar] [CrossRef]
- Varghese, S.A.; Pulikkalparambil, H.; Rangappa, S.M.; Parameswaranpillai, J.; Siengchin, S. Antimicrobial Active Packaging Based on PVA/Starch Films Incorporating Basil Leaf Extracts. Mater. Today Proc. 2023, 72, 3056–3062. [Google Scholar] [CrossRef]
- Liu, D.; Dang, S.; Zhang, L.; Munsop, K.; Li, X. Corn starch/polyvinyl alcohol based films incorporated with curcumin-loaded Pickering emulsion for application in intelligent packaging. Int. J. Biol. Macromol. 2021, 188, 974–982. [Google Scholar] [CrossRef]
- Terzioğlu, P.; Parin, F.N. Polyvinyl alcohol-corn starch-lemon peel biocomposite films as potential food packaging. Celal Bayar Univ. J. Sci. 2020, 16, 373–378. [Google Scholar]
- Kumar, P.; Tanwar, R.; Gupta, V.; Upadhyay, A.; Kumar, A.; Gaikwad, K.K. Pineapple peel extract incorporated poly (vinyl alcohol)-corn starch film for active food packaging: Preparation, characterization and antioxidant activity. Int. J. Biol. Macromol. 2021, 187, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Xu, F.; Yuan, L.; Hu, H.; Yao, X.; Liu, J. Comparison of the physical and functional properties of starch/polyvinyl alcohol films containing anthocyanins and/or betacyanins. Int. J. Biol. Macromol. 2020, 163, 898–909. [Google Scholar] [CrossRef]
- Yurong, G.; Dapeng, L. Preparation and characterization of corn starch/PVA/glycerol composite films incorporated with ε-polylysine as a novel antimicrobial packaging material. e-Polymers 2020, 20, 154–161. [Google Scholar] [CrossRef]
- Ali, M.A.S.S.; Jimat, D.N.; Nawawi, W.M.F.W.; Sulaiman, S. Antibacterial, Mechanical and Thermal Properties of PVA/Starch Composite Film Reinforced with Cellulose Nanofiber of Sugarcane Bagasse. Arab. J. Sci. Eng. 2022, 47, 5747–5754. [Google Scholar] [CrossRef]
- Kan, J.; Liu, J.; Xu, F.; Yun, D.; Yong, H.; Liu, J. Development of pork and shrimp freshness monitoring labels based on starch/polyvinyl alcohol matrices and anthocyanins from 14 plants: A comparative study. Food Hydrocoll. 2022, 124, 107293. [Google Scholar] [CrossRef]
- Mathew, S.; Jayakumar, A.; Kumar, V.P.; Mathew, J.; Radhakrishnan, E.K. One-step synthesis of eco-friendly boiled rice starch blended polyvinyl alcohol bionanocomposite films decorated with in situ generated silver nanoparticles for food packaging purpose. Int. J. Biol. Macromol. 2019, 139, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Phattarateera, S.; Xin, L.; Amphong, C.; Limsamran, V.; Threepopnatkul, P. Comparative studies of starch blends on the properties of PVA films. Carbohydr. Polym. Technol. Appl. 2023, 6, 100340. [Google Scholar] [CrossRef]
- Patil, S.; Bharimalla, A.K.; Mahapatra, A.; Dhakane-Lad, J.; Arputharaj, A.; Kumar, M.; Raja, A.; Kambli, N. Effect of polymer blending on mechanical and barrier properties of starch-polyvinyl alcohol based biodegradable composite films. Food Biosci. 2021, 44, 101352. [Google Scholar] [CrossRef]
- Hu, D.; Qiang, T.; Wang, L. Quaternized chitosan/polyvinyl alcohol/sodium carboxymethylcellulose blend film for potential wound dressing application. Wound Med. 2017, 16, 15–21. [Google Scholar] [CrossRef]
- Saadiah, M.A.; Zhang, D.; Nagao, Y.; Muzakir, S.K.; Samsudin, A.S. Reducing crystallinity on thin film based CMC/PVA hybrid polymer for application as a host in polymer electrolytes. J. Non-Cryst. Solids 2019, 511, 201–211. [Google Scholar] [CrossRef]
- Youssef, A.M.; Assem, F.M.; El-Sayed, H.S.; El-Sayed, S.M.; Elaaser, M.; El-Salam, M.H.A. Synthesis and evaluation of eco-friendly carboxymethyl cellulose/polyvinyl alcohol/CuO bionanocomposites and their use in coating processed cheese. RSC Adv. 2020, 10, 37857–37870. [Google Scholar] [CrossRef] [PubMed]
- Muppalla, S.R.; Kanatt, S.R.; Chawla, S.P.; Sharma, A. Carboxymethyl cellulose–polyvinyl alcohol films with clove oil for active packaging of ground chicken meat. Food Packag. Shelf Life 2014, 2, 51–58. [Google Scholar] [CrossRef]
- Fasihi, H.; Fazilati, M.; Hashemi, M.; Noshirvani, N. Novel carboxymethyl cellulose-polyvinyl alcohol blend films stabilized by Pickering emulsion incorporation method. Carbohydr. Polym. 2017, 167, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Fasihi, H.; Noshirvani, N.; Hashemi, M.; Fazilati, M.; Salavati, H.; Coma, V. Antioxidant and antimicrobial properties of carbohydrate-based films enriched with cinnamon essential oil by Pickering emulsion method. Food Packag. Shelf Life 2019, 19, 147–154. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M.; Velazquez, G. Cellulose-glycerol-polyvinyl alcohol composite films for food packaging: Evaluation of water adsorption, mechanical properties, light-barrier properties and transparency. Carbohydr. Polym. 2018, 195, 432–443. [Google Scholar] [CrossRef]
- Huang, J.; Liu, J.; Chen, M.; Yao, Q.; Hu, Y. Immobilization of roselle anthocyanins into polyvinyl alcohol/hydroxypropyl methylcellulose film matrix: Study on the interaction behavior and mechanism for better shrimp freshness monitoring. Int. J. Biol. Macromol. 2021, 184, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, M.; Zhou, Y.; Li, Y.; Hu, Y. Functional characteristics improvement by structural modification of hydroxypropyl methylcellulose modified polyvinyl alcohol films incorporating roselle anthocyanins for shrimp freshness monitoring. Int. J. Biol. Macromol. 2020, 162, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Laoasoke, W.; Choipang, C.; Chaiarwut, S.; Suwantong, O.; Chuysinuan, P.; Techasakul, S.; Sangsanoh, P.; Supaphol, P. Preparation and Characterization of Hydroxypropyl Methylcellulose/Polyvinyl Alcohol Mucoadhesive Buccal Film of Cannabidiol. J. Polym. Environ. 2024, 1–10. [Google Scholar] [CrossRef]
- Chen, J.; Su, X.; Zheng, M.; Lin, J.; Tan, K.B. Enhanced properties of chitosan/hydroxypropyl methylcellulose/polyvinyl alcohol green bacteriostatic film composited with bamboo fiber and silane-modified bamboo fiber. Polym. Compos. 2022, 43, 2440–2449. [Google Scholar] [CrossRef]
- Kida, D.; Gładysz, O.; Szulc, M.; Zborowski, J.; Junka, A.; Janeczek, M.; Lipińska, A.; Skalec, A.; Karolewicz, B. Development and Evaluation of a Polyvinylalcohol-Cellulose Derivative-Based Film with Povidone-Iodine Predicted for Wound Treatment. Polymers 2020, 12, 1271. [Google Scholar] [CrossRef] [PubMed]
- Olechno, K.; Basa, A.; Winnicka, K. “Success Depends on Your Backbone”—About the Use of Polymers as Essential Materials Forming Orodispersible Films. Materials 2021, 14, 4872. [Google Scholar] [CrossRef] [PubMed]
- Ayyubi, S.N.; Purbasari, A. The effect of composition on mechanical properties of biodegradable plastic based on chitosan/cassava starch/PVA/crude glycerol: Optimization of the composition using Box Behnken Design. Mater. Today Proc. 2022, 63, S78–S83. [Google Scholar] [CrossRef]
- Chokboribal, J.; Nantachai, T.; Jongnimitphaiboon, K.; Chumprasert, S.; Suchaiya, V. Tapioca starch/PVA plastic films with water hyacinth powder: Enhanced stability in direct contact with moisture. Mater. Today Proc. 2022, 65, 2380–2388. [Google Scholar] [CrossRef]
- Negim, E.S.M.; Rakhmetullayeva, R.K.; Yeligbayeva, G.Z.; Urkimbaeva, P.I.; Primzharova, S.T.; Kaldybekov, D.B.; Khatib, J.M.; Mun, G.A.; Craig, W. Improving biodegradability of polyvinyl alcohol/starch blend films for packaging applications. Int. J. Basic. Appl. Sci. 2014, 3, 263. [Google Scholar] [CrossRef]
- Mittal, A.; Garg, S.; Bajpai, S. Fabrication and characteristics of poly (vinyl alcohol)-starch-cellulosic material based biodegradable composite film for packaging application. Mater. Today Proc. 2019, 21, 1577–1582. [Google Scholar] [CrossRef]
- Chai, W.-L.; Chow, J.-D.; Chen, C.-C.; Chuang, F.-S.; Lu, W.-C. Evaluation of the biodegradability of polyvinyl alcohol/starch blends: A methodological comparison of environmentally friendly materials. J. Polym. Environ. 2009, 17, 71–82. [Google Scholar] [CrossRef]
- Abdullah, Z.W.; Dong, Y. Biodegradable and water resistant poly (vinyl) alcohol (PVA)/starch (ST)/glycerol (GL)/halloysite nanotube (HNT) nanocomposite films for sustainable food packaging. Front. Mater. 2019, 6, 58. [Google Scholar] [CrossRef]
- Priya, B.; Gupta, V.K.; Pathania, D.; Singha, A.S. Synthesis, characterization and antibacterial activity of biodegradable starch/PVA composite films reinforced with cellulosic fibre. Carbohydr. Polym. 2014, 109, 171–179. [Google Scholar] [CrossRef]
- Ghatge, S.; Yang, Y.; Ahn, J.-H.; Hur, H.-G. Biodegradation of Polyethylene: A Brief Review. Appl. Biol. Chem. 2020, 63, 27. [Google Scholar] [CrossRef]
- Oliveira, J.; Belchior, A.; da Silva, V.D.; Rotter, A.; Petrovski, Ž.; Almeida, P.L.; Lourenço, N.D.; Gaudêncio, S.P. Marine Environmental Plastic Pollution: Mitigation by Microorganism Degradation and Recycling Valorization. Front. Mar. Sci. 2020, 7, 567126. [Google Scholar] [CrossRef]
| Process | Definition | Advantages/Disadvantages |
|---|---|---|
| Traditional recycling |
|
|
| Traditional mechanical recycling |
|
|
| Chemical recycling |
|
|
| Gasification (chemical recycling) |
|
|
| Pyrolysis (chemical recycling) |
|
|
| Landfilling |
| |
| Burning plastic |
|
|
| Type | Biodegradable Polymers | Biopolymers |
|---|---|---|
| Definition |
|
|
| Examples |
|
|
| Advantages | Have the least detrimental impact on the environment in terms of pollution | Have the least detrimental impact on the environment in terms of pollution |
| Quantity produced | The overall quantity of bio-based polymers produced in 2020 was 4.2 million tons, or 1% of the total amount of fossil-fuel-based polymers produced. The CAGR is now, with 8%, much higher than the growth of polymers (3–4%) for the first time in a long time, and this trend is anticipated to last through 2025 [38] | |
| Polymer Blend Type | Effect | Ref. |
|---|---|---|
| PVA/corn starch mixes designed for wood adhesives that are used with polyvinyl acetate (PVAc)-white glue. Glyoxal, boric acid, citric acid, and glutaraldehyde were used in low concentrations (0.1 wt%) |
| [73] |
| Low-weight chitosan, polyvinyl alcohol, and maize starch; the PVA concentration ranged from 0 to 40 wt.%, while the St/Chit weight ratio was set at 70/30 |
| [74] |
| Polyvinyl alcohol (PVOH)/starch ratios of 0–60% |
| [75] |
| Polyvinyl alcohol (PVOH) mixed with pregelatinized starch (PSt) by incorporating antibacterial agents, such as dodecyl dipropylene triamine (TRIAMEEN) and 2-hydroxypropyl-3-piperazinylquinolinecarboxylic acid methacrylate (HPQM) |
| [76] |
| Polyvinyl alcohol (PVA) and maize starch with the addition of purple sweet potato extracts (PSPE) and red cabbage extracts (RCE) |
| [77] |
| St/PVA films produced using the blowing extrusion technique with a St/PVA ratio of 4:6 |
| [78] |
| Starch, polyvinyl alcohol, and graphene oxide. |
| [79] |
| Nanocomposite films of polyvinyl alcohol, graphene oxide, starch, and silver (PVA/GO/Starch/Ag) |
| [80] |
| Starch/polyvinyl alcohol (PVA) degradable straws using a twin-screw extrusion technique, with varying PVA concentrations |
| [81] |
| By using 88% hydrolyzed (PVA/cassava starches (NCS, HCS, and PCS), a mixture of the mixes was plasticized with glycerol or a glycerol–sorbitol mixture by solution-casting |
| [82] |
| PVA/starch films with water extracts from basil leaves were added as antibacterial agents following the addition of basil leaf extracts |
| [83] |
| Corn starch (CS) and polyvinyl alcohol (PVA) matrix for a Pickering emulsion loaded with curcumin |
| [84] |
| Lemon peel/polyvinyl alcohol/starch matrix |
| [85] |
| Polyvinyl alcohol (PVOH) and corn starch (ST) with a pineapple peel extract (PPE), with PPE concentrations of 5%, 10%, 15%, and 20% |
| [86] |
| Anthocyanins or betacyanins or anthocyanin/betacyanin mixtures (in various weight ratios of 3:1, 1:1, and 1:3) in starch/polyvinyl alcohol (PVA) films |
| [87] |
| Corn starch, polyvinyl alcohol (PVA), and glycerol that also contained polylysine |
| [88] |
| PVA/starch nanocomposite film reinforced with sugarcane bagasse cellulose nanofiber (CNF), with a varying ratio of 1–6 wt% of the cellulose nanofiber suspension applied to the PVA/starch film |
| [89] |
| Intelligent packaging labels in which anthocyanin-rich extract was immobilized in starch/polyvinyl alcohol matrices |
| [90] |
| Polyvinyl alcohol/boiled rice starch blend film in the presence of solar irradiation and the addition of silver nanoparticles (PVA/BRS/sAgNPs) |
| [91] |
| Hydrolyzed starch (HST)/pregelatinized starch (PST) at different concentrations with 20% glycerol |
| [92] |
| Starch/polyvinyl alcohol (PVA) straws with varying PVA contents |
| [81] |
| Starch/PVA (10, 30, 50, 70, and 90% of the starch weight) composite films |
| [93] |
| Polymer Blend | Effect | Ref. |
|---|---|---|
| Polyvinyl alcohol (PVA), sodium carboxymethylcellulose (CMC), and N-(2-hydroxyl) propyl-3-trimethylammonium chitosan chloride (HTCC) |
| [94] |
| Carboxymethyl cellulose and polyvinyl alcohol (CMC/PVA)-based hybrid polymer (HPe) system with different ratios of composition |
| [95] |
| CMC/PVA/CuO bio-nanocomposites for covering processed cheese |
| [96] |
| Polyvinyl alcohol (PVOH), clove oil, and carboxymethyl cellulose (CMC) |
| [97] |
| Carboxymethyl cellulose (CMC)/polyvinyl alcohol (PVA) film emulsified with oleic acid (OL) and mixed with rosemary essential oil (REO) at various concentrations of REO (0.5, 1.5, and 3%) |
| [98] |
| Polyvinyl alcohol (PVA) and carboxymethyl cellulose (CMC) |
| [99] |
| Cellulose, glycerol, and polyvinyl alcohol |
| [100] |
| Polymer Blend | Effect | Ref. |
|---|---|---|
| Polyvinyl alcohol (PVA)/hydroxypropyl methylcellulose (HPMC) film matrix to immobilize roselle anthocyanin extract (RAE) |
| [101] |
| Polyvinyl alcohol (PVA) modified with hydroxypropyl methylcellulose (HPMC) |
| [102] |
| Hydroxypropylmethylcellulose (HPMC) to prepare thin films containing up to 20% cannabidiol (CBD). Soft and flexible polyvinyl alcohol (PVA) was used as the supporting layer |
| [103] |
| Chitosan/hydroxypropyl methylcellulose/polyvinyl alcohol mix film (CHP) with modified bamboo fiber treated with coupling agent |
| [104] |
| Povidone-iodine (PVP-I)-integrated polyvinyl alcohol-hydroxypropyl methylcellulose (PVA/HPMC_B)-based film |
| [105] |
| HPMC and PVA ODF (orally dissolving film) (HPMC: PVA) with ratios of F1 (3:0), F2 (2:1), F3 (1.5:1.5), F4 (1:2), and F5 (0:3) |
| [106] |
| Polyvinyl alcohol/hydroxypropyl methylcellulose/chitosan blend film (CHP) using bamboo fiber |
| [104] |
| Polymer Blend | Effect | Ref. |
|---|---|---|
| Chitosan/cassava starch/PVA |
| [107] |
| Different amounts of cellulosic fibers in the form of powder (water hyacinth powder, “WHp”) were added to the topical starch (TS)/PVA blend |
| [108] |
| Octenyl succinic anhydride (OSA) esterified potato starch, gliadin, and polyvinyl alcohol (PVA) |
| [82] |
| PVA (10, 30, 50, 70, and 90% of the starch weight per gram) |
| [93] |
| Polyvinyl alcohol (PVA) starch (S) in the presence of glacial acetic acid as a crosslinking agent |
| [109] |
| Cellulosic material barley husk (BH) and PVA (polyvinyl alcohol)/starch and starch-based composite sheets for packaging applications |
| [110] |
| Modified maize starch combined with PVA in various ratios |
| [111] |
| Neat polyvinyl alcohol (PVA)/starch (ST)/glycerol (GL)/halloysite nanotube (HNT) nanocomposite films with different HNT contents |
| [112] |
| PVA/starch and citric acid as plasticizing agent (buried for 120 days in a pot of farm soil) |
| [113] |
| Maize starch/chitosan composite film |
| [74] |
| Type of Biodegrading Enzyme/Bacteria | Polymer Type | Biodegradation Mechanism | Mode of Action and Mechanism |
|---|---|---|---|
| Amylase | Starch | Hydrolysis | Breaks down the α-1,4-glycosidic bonds in starch, producing glucose |
| Cellulases | Cellulose | Hydrolysis | Breaks down the β-1,4-glycosidic bonds in cellulose, producing glucose |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elgharbawy, A.S.; El Demerdash, A.-G.M.; Sadik, W.A.; Kasaby, M.A.; Lotfy, A.H.; Osman, A.I. Synthetic Degradable Polyvinyl Alcohol Polymer and Its Blends with Starch and Cellulose—A Comprehensive Overview. Polymers 2024, 16, 1356. https://doi.org/10.3390/polym16101356
Elgharbawy AS, El Demerdash A-GM, Sadik WA, Kasaby MA, Lotfy AH, Osman AI. Synthetic Degradable Polyvinyl Alcohol Polymer and Its Blends with Starch and Cellulose—A Comprehensive Overview. Polymers. 2024; 16(10):1356. https://doi.org/10.3390/polym16101356
Chicago/Turabian StyleElgharbawy, Abdallah S., Abdel-Ghaffar M. El Demerdash, Wagih A. Sadik, Mosaad A. Kasaby, Ahmed H. Lotfy, and Ahmed I. Osman. 2024. "Synthetic Degradable Polyvinyl Alcohol Polymer and Its Blends with Starch and Cellulose—A Comprehensive Overview" Polymers 16, no. 10: 1356. https://doi.org/10.3390/polym16101356
APA StyleElgharbawy, A. S., El Demerdash, A.-G. M., Sadik, W. A., Kasaby, M. A., Lotfy, A. H., & Osman, A. I. (2024). Synthetic Degradable Polyvinyl Alcohol Polymer and Its Blends with Starch and Cellulose—A Comprehensive Overview. Polymers, 16(10), 1356. https://doi.org/10.3390/polym16101356








