Optical Limiting Properties of DNA Biopolymer Doped with Natural Dyes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Characterization
2.1.1. UV-VIS-NIR Spectroscopy
2.1.2. Photostability of Prepared Solutions
2.1.3. Thermal Degradation
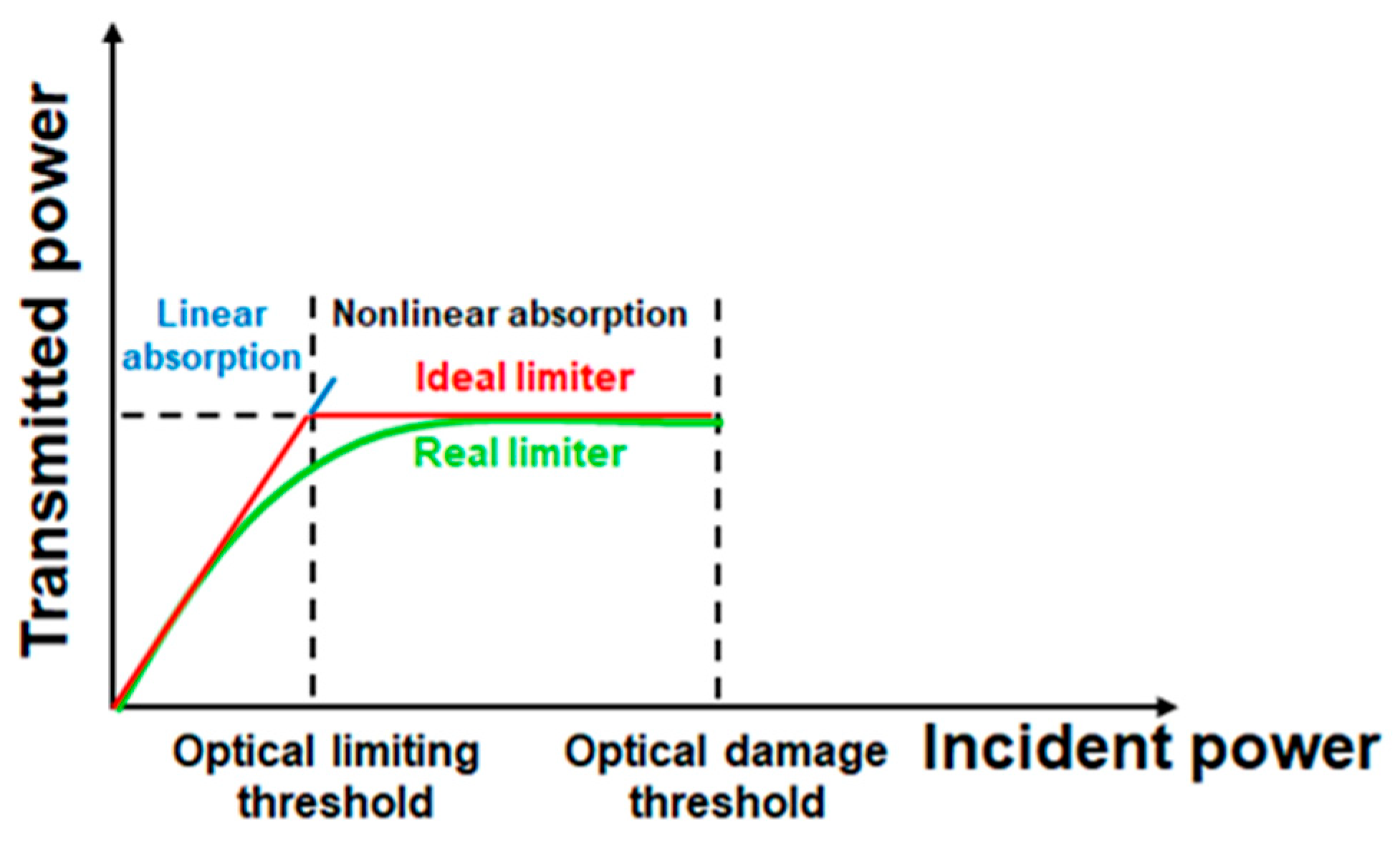
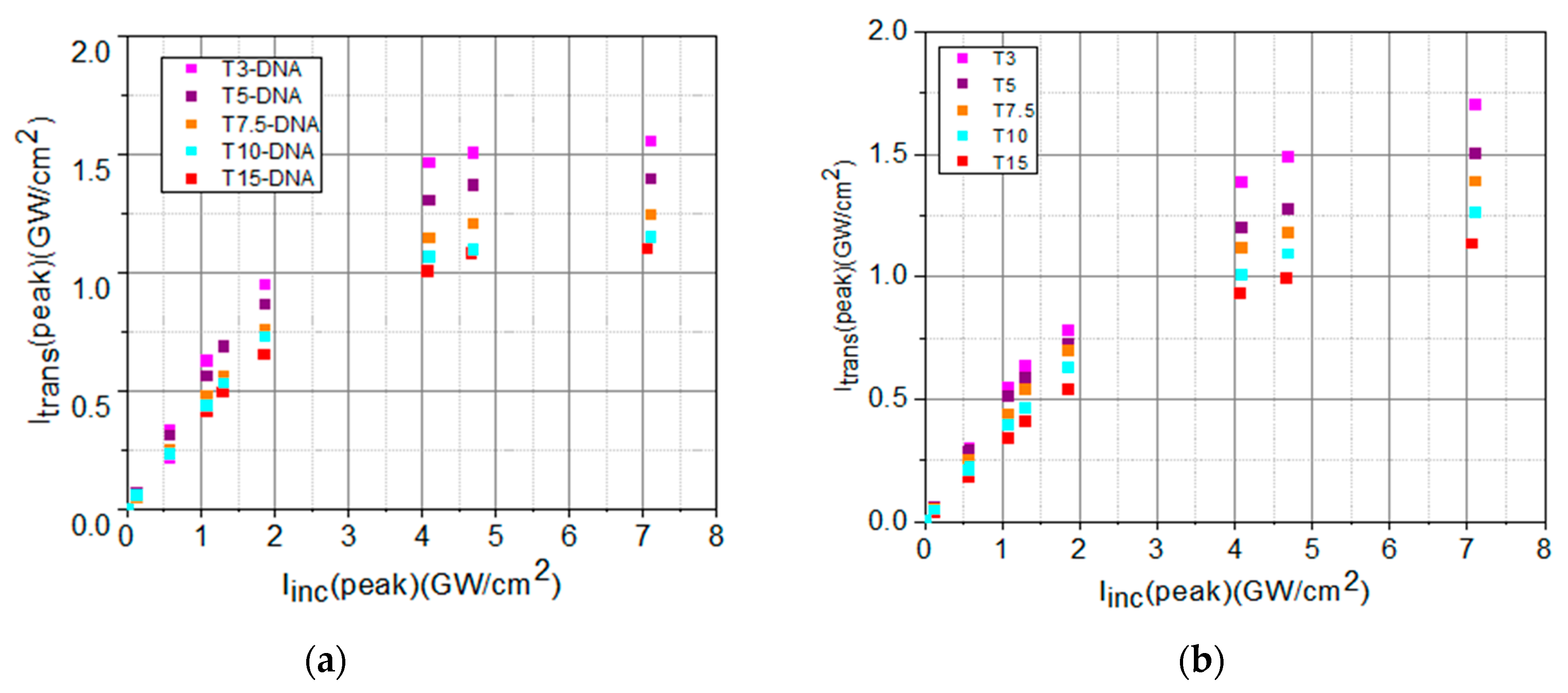
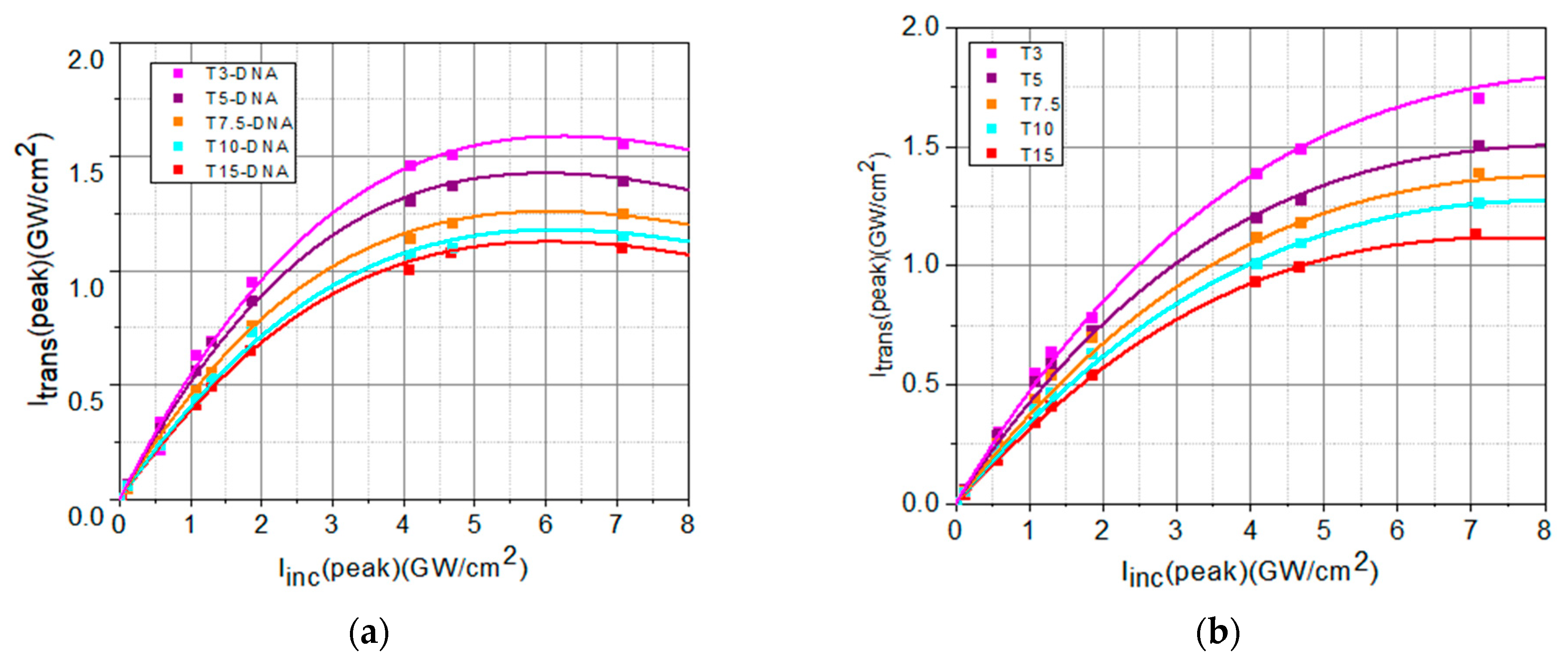
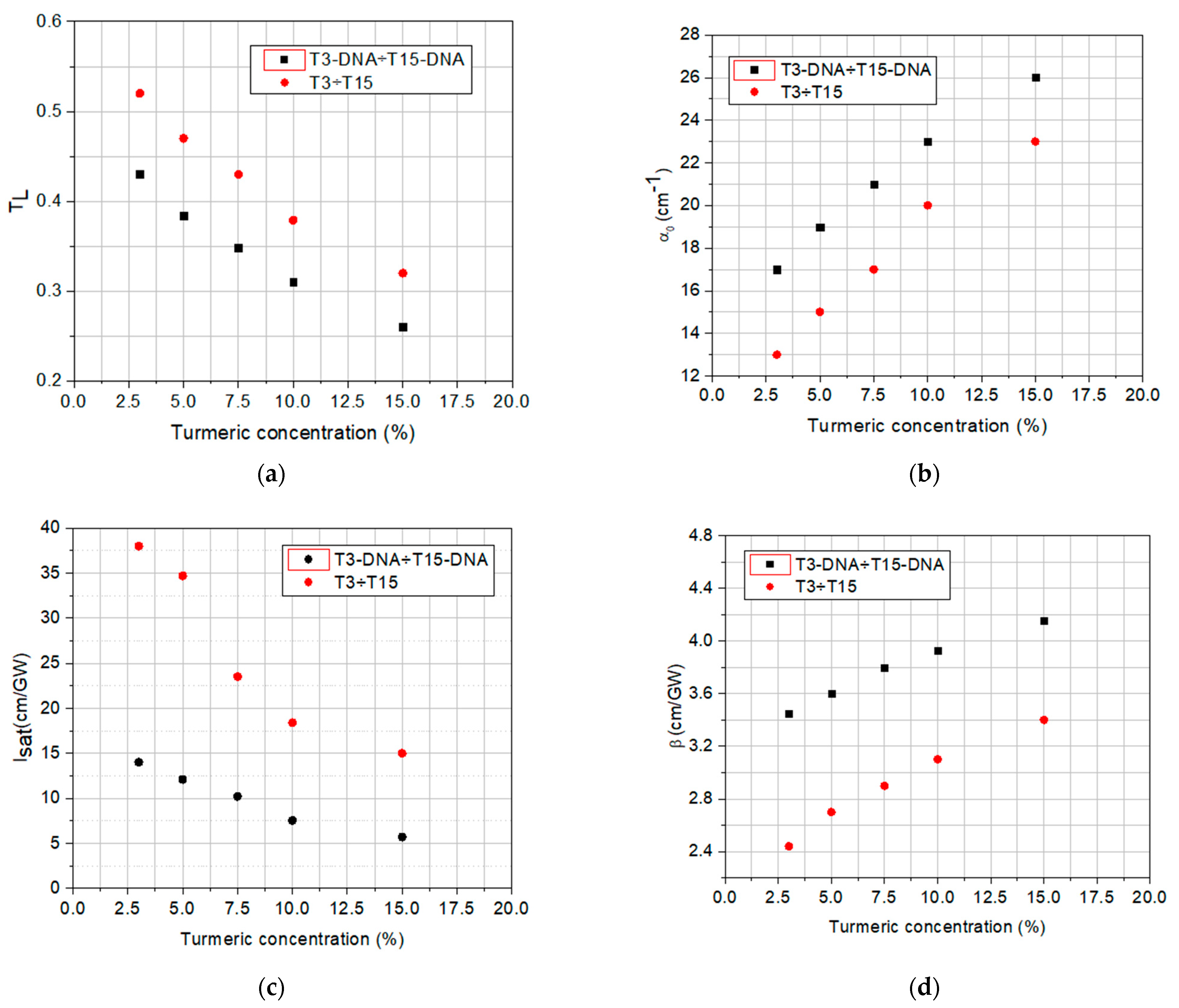
3. Optical Limiting Capability: Measurements and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Das, B.C.; Reji, N.; Philip, R. Optical limiting behavior of the natural dye extract from Indigofera Tinctoria leaves. Opt. Mater. 2021, 114, 110925. [Google Scholar]
- Marbello, O.; Valbuena, S.D.; Racedo, F.J.N. Optical limiting phenomenon study in oils of vegetable origin. J. Appl. Res. Technol. 2020, 18, 333–340. [Google Scholar]
- Haripadmam, P.C.; Beryl, C.; Philip, R. Optical Limiting Properties of the Natural Dye Extract from Alternanthera brasiliana Leaves. J. Electron. Mater. 2022, 51, 3364–3371. [Google Scholar] [CrossRef]
- Petris, A.; Gheorghe, P.; Rau, I. DNA—CTMA matrix influence on Rhodamine 610 light emission in thin films. Polymers 2023, 15, 3105. [Google Scholar] [CrossRef]
- Petris, A.; Gheorghe, P.; Rau, I. Influence of continuous wave laser light at 532 nm on transmittance and on photoluminescence of DNA-CTMA-RhB solutions. Heliyon 2023, 9, e20410. [Google Scholar] [CrossRef]
- Bazaru Rujoiu, T.; Petris, A.; Vlad, V.I.; Rau, I.; Manea, A.M.; Kajzar, F. Lasing in DNA–CTMA doped with Rhodamine 610 in butanol. Phys. Chem. Chem. Phys. 2015, 17, 13104–13111. [Google Scholar] [CrossRef]
- Xia, T.; Hagan, D.J.; Dogariu, A.; Said, A.A.; Van Stryland, E.W. Optimization of optical limiting devices based on excited-state absorption. Appl. Opt. 1997, 36, 4110. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, B.; Chen, Y. Recent Progress in Two-Dimensional Nanomaterials for Laser Protection. Chemistry 2019, 1, 17–43. [Google Scholar] [CrossRef]
- Barbinta-Patrascu, M.-E.; Iordache, S.M. DNA—The fascinating biomacromolecule in optoelectronics and photonics applications. J. Optoelectron. Adv. Mater. 2022, 24, 563. [Google Scholar]
- Lundén, H.; Glimsdal, E.; Lindgren, M.; Lopesa, C. How to assess good candidate molecules for self-activated optical power limiting. Opt. Eng. 2018, 57, 030802. [Google Scholar] [CrossRef]
- Dini, D.; Calvete, M.J.F.; Hanack, M. Nonlinear Optical Materials for the Smart Filtering of Optical Radiation. Chem. Rev. 2016, 116, 13043–13233. [Google Scholar] [CrossRef]
- Anton, A.M.; Rau, I.; Kajzar, F.; Simion, A.M.; Pirvu, C.; Radu, N.; Simion, C. Natural materials with enhanced optical damage threshold. Opt. Mater. 2018, 86, 1–6. [Google Scholar] [CrossRef]
- Liang, L.; Fu, Y.; Wang, D.; Wei, Y.; Kobayashi, N.; Minari, T. DNA as Functional Material in Organic-Based Electronics. Appl. Sci. 2018, 8, 90. [Google Scholar] [CrossRef]
- Aithal, S.; Aithal, P.S.; Bhat, G.K. Characteristics of ideal optical limiter and realization scenarios using nonlinear organic materials. Int. J. Adv. Trends Eng. Technol. 2016, 1, 2456. [Google Scholar]
- Sun, Y.P.; Riggs, J.E. Organic and inorganic optical limiting materials. From fullerenes to nanoparticles. Int. Rev. Phys. Chem. 1999, 18, 43–90. [Google Scholar] [CrossRef]
- Wang, J.; Werner, J.B. Inorganic and hybrid nanostructures for optical limiting. J. Opt. Pure Appl. Opt. 2009, 11, 024001. [Google Scholar] [CrossRef]
- Parola, S.; Julián-López, B.; Carlos, L.D.; Sanchez, C. Optical Properties of Hybrid Organic–Inorganic Materials and their Applications—Part II: Nonlinear Optics and Plasmonics. In Handbook of Solid State Chemistry; Part 4 Nano and Hybrid Materials; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Jancy, J.; Vinoy, T.; Sujesh, B.; Ramakrishnan, J.; Sebastian, M.; Ibrahimkutty, R.; Abdulhassan, M. Open-aperture Z-scan and optical limiting of plasmonic silver-polymer system. J. Optoelectron. Adv. Mater. 2022, 24, 250. [Google Scholar]
- Chen, Y.; Bai, T.; Dong, N.; Fan, F.; Zhang, S.; Zhuang, X.; Sun, J.; Zhang, B.; Zhang, X.; Wang, J.; et al. Graphene and its derivatives for laser protection. Prog. Mater. Sci. 2016, 84, 118–157. [Google Scholar] [CrossRef]
- Wang, A.J.; Yu, W.; Fang, Y.; Song, Y.L.; Jia, D.; Long, L.L.; Cifuentes, M.P.; Humphrey, M.G.; Zhang, C. Facile Hydrothermal Synthesis and Optical Limiting Properties of TiO2-Reduced Graphene Oxide Nanocomposites. Carbon 2015, 89, 130–141. [Google Scholar] [CrossRef]
- Zhao, M.; Peng, R.; Zheng, Q.; Wang, Q.; Chang, M.J.; Liu, Y.; Song, Y.L.; Zhang, H.L. Broadband optical limiting response of a graphene–PbS nanohybrid. Nanoscale 2015, 7, 9268–9274. [Google Scholar] [CrossRef]
- Rau, I.; Grote, J.G.; Kajzar, F.; Pawlicka, A. DNA–novel nanomaterial for applications in photonics and in electronics. Comptes Rendus Phys. 2012, 13, 853–864. [Google Scholar] [CrossRef]
- Petris, A.; Vasiliu, I.C.; Gheorghe, P.; Iordache, A.M.; Ionel, L.; Rusen, L.; Iordache, S.; Elisa, M.; Trusca, R.; Ulieru, D.; et al. Graphene Oxide-Based Silico-Phosphate Composite Films for Optical Limiting of Ultrashort Near-Infrared Laser Pulses. Nanomaterials 2020, 10, 1638. [Google Scholar] [CrossRef]
- Graphene Report 2020, Description. Available online: https://www.researchandmarkets.com/reports/4901148/the-graphene-report-2020 (accessed on 1 June 2020).
- Liu, Z.; Wang, Y.; Zhang, X.; Xu, Y.; Chen, Y.; Tian, J. Nonlinear optical properties of graphene oxide in nanosecond and picosecond regimes. Appl. Phys. Lett. 2009, 94, 021902. [Google Scholar] [CrossRef]
- Liaros, N.; Aloukos, P.; Kolokithas-Ntoukas, A.; Bakandritsos, A.; Szabo, T.; Zboril, R.; Couris, S. Nonlinear Optical Properties and Broadband Optical Power Limiting Action of Graphene Oxide Colloids. J. Phys. Chem. C 2013, 117, 6842–6850. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Z.; Zhang, X.; Wang, Y.; Tian, J.; Huang, Y.; Ma, Y.; Zhang, X.; Chen, Y. A Graphene Hybrid Material Covalently Functionalized with Porphyrin: Synthesis and Optical Limiting Property. Adv. Mater. 2009, 21, 1275–1279. [Google Scholar] [CrossRef]
- Liaros, N.; Orfanos, I.; Papadakis, I.; Couris, S. Nonlinear optical response of some Graphene oxide and Graphene fluoride derivatives. Optofluid. Microfluid. Nanofluid. 2016, 3, 53–58. [Google Scholar] [CrossRef]
- Stathis, A.; Stavrou, M.; Papadakis, I.; Obratzov, I.; Couris, S. Enhancing and Tuning the Nonlinear Optical Response and Wavelength-Agile Strong Optical Limiting Action of N-octylamine Modified Fluorographenes. Nanomaterials 2020, 10, 2319. [Google Scholar] [CrossRef]
- Jiang, X.F.; Polavarapu, L.; Neo, S.T.; Venkatesan, T.; Xu, Q. Graphene Oxides as Tunable Broadband Nonlinear Optical Materials for Femtosecond Laser Pulses. J. Phys. Chem. Lett. 2012, 3, 785–790. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhu, L.; Zhao, F. Nonlinear Optical and Optical Limiting Properties of Graphene Oxide Dispersion in Femtosecond Regime. Proc. SPIE 2014, 9283, 92830V-1. [Google Scholar]
- Ren, J.; Zheng, X.; Tian, Z.; Li, D.; Wang, P.; Jia, B. Giant third-order nonlinearity from low-loss electrochemical graphene oxide film with a high power stability. Appl. Phys. Lett. 2016, 109, 221105. [Google Scholar] [CrossRef]
- Oluwafemi, O.S.; Sreekanth, P.; Philip, R.; Thomas, S.; Kalarikkal, N. Improved nonlinear optical and optical limiting properties in non-covalent functionalized reduced graphene oxide/silver nanoparticle (NF-RGO/Ag-NPs) hybrid. Opt. Mater. 2016, 58, 476–483. [Google Scholar]
- Sooraj, B.N.S.; Pradeep, T. Chapter 4—Optical properties of metal clusters. In Atomically Precise Metal Nanoclusters; Elsevier: Amsterdam, The Netherlands, 2023; pp. 83–101. [Google Scholar]
- Xing, F.; Wang, Y.; Wang, J.; Zhou, S.; Zhao, J.; Xie, Z. Highly dispersed antimonene oxide quantum dots and their hybrid gel glasses for broadband nonlinear optical limiting. J. Mater. Chem. C 2021, 9, 10084–10088. [Google Scholar] [CrossRef]
- Sun, X.; Hu, X.; Sun, J.; Xie, Z.; Zhou, S. Strong optical limiting properties of Ormosil gel glasses doped with silver nano-particles. New J. Chem. 2019, 43, 6274–6278. [Google Scholar] [CrossRef]
- Zidan, M.D.; Ajji, Z. Optical limiting behavior of disperse red 1 dye doped polymer. Opt. Laser Technol. 2011, 43, 934–937. [Google Scholar] [CrossRef]
- Aithal, S.; Aithal, P.S.; Bhat, G.K. CW Optical Limiting Study in Disperse Yellow Dye-Doped PMMA-MA Polymer Films. IRA-Int. J. Appl. Sci. 2016, 5, 129–146. [Google Scholar] [CrossRef]
- Rau, I.; Kajzar, F. Biopolymers for application in photonics. Sci. J. Volgograd State Univ. 2014, 4, 29–41. [Google Scholar]
- Moldoveanu, M.; Meghea, A.; Popescu, R.; Grote, J.; Kajzar, F.; Rau, I. On the stability and degradation of DNA based thin films. Mol. Cryst. Liq. Cryst. 2010, 523, 182–190. [Google Scholar] [CrossRef]
- Steckl, A.J. DNA—A new material for photonics? Nat. Photonics 2007, 1, 3–5. [Google Scholar] [CrossRef]
- Petris, A.; Gheorghe, P.; Vlad, V.I.; Rau, I.; Kajzar, F. Interferometric method for the study of spatial phase modulation induced by light in dye-doped DNA complexes. Rom. Rep. Phys. 2015, 67, 1373–1382. [Google Scholar]
- Gheorghe, P.; Petris, A.; Vlad, V.I.; Rau, I.; Kajzar, F.; Manea, A.M. Temporal evolution of the laser recording of gratings in DNA-CTMA:Rh610 films. Rom. Rep. Phys. 2015, 67, 1412–1420. [Google Scholar]
- Khazaeinezhad, R.; Kassani, S.H.; Paulson, B.; Jeong, H.; Gwak, J.; Rotermund, F.; Yeom, D.; Oh, K. Ultrafast nonlinear optical properties of thin-solid DNA film and their application as a saturable absorber in femtosecond mode-locked fiber laser. Sci. Rep. 2017, 7, 41480. [Google Scholar] [CrossRef]
- Grote, J. Biotechnology in biopolymers: Developments, applications & challenging areas. SPIE Newsroom 2008, 15. [Google Scholar]
- Petris, A.; Gheorghe, P.S.; Rau, I.; Manea-Saghin, A.M.; Kajzar, F. All-optical spatial phase modulation in films of dye-doped DNA biopolymer. Eur. Polym. J. 2019, 110, 130–137. [Google Scholar] [CrossRef]
- Dancus, I.; Vlad, V.I.; Petris, A.; Bazaru Rujoiu, T.; Rau, I.; Kajzar, F.; Meghea, A.; Tane, A. Z-Scan and I-Scan methods for characterization of DNA Optical Nonlinearities. Rom. Rep. Phys. 2013, 65, 966. [Google Scholar]
- Gonzalez, A.M.; Vilhena, J.G.; Perez, R.; Herrero, F.M. A molecular view of DNA flexibility. Q. Rev. Biophys. 2021, 54, e8. [Google Scholar]
- Gonzalez, A.M.; Vilhena, J.G.; Herrero, F.M.; Perez, R. DNA Crookedness Regulates DNA Mechanical Properties at Short Length Scales. Phys. Rev. Lett. 2019, 122, 048102. [Google Scholar] [CrossRef]
- Gonzalez, A.M.; Vilhena, J.G.; Herrero, F.M.; Perez, R. Sequence-dependent mechanical properties of double-stranded RNA. Nanoscale 2019, 11, 21471–21478. [Google Scholar] [CrossRef]
- Moldoveanu, M.; Popescu, R.; Pîrvu, C.; Grote, J.G.; Kajzar, F.; Rau, I. Biopolymer thin films for optoelectronics applications. Mol. Cryst. Liq. Cryst. 2010, 522, 530–539. [Google Scholar]
- Rau, I.; Tane, A.; Zgarian, R.; Meghea, A.; Grote, J.G.; Kajzar, F. Stability of Selected Chromophores in Biopolymer Matrix. Mol. Cryst. Liq. Cryst. 2012, 554, 43–55. [Google Scholar] [CrossRef]
- Grote, J.G. Materials Science of DNA—Conclusions and Perspectives. In Materials Science of DNA; Jin, J.-I., Grote, J., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 311–317. [Google Scholar]
- Mindroiu, M.; Manea, A.-M.; Rau, I.; Grote, J.G.; Oliveira, H.; Pawlicka, A.; Kajzar, F. DNA- and DNA-CTMA: Novel bio-nanomaterials for application in photonics and in electronics. Proc. SPIE 2013, 8882, 888202. [Google Scholar]
- Anton, A.-M.; Rau, I.; Kajzar, F.; Simion, A.-M.; Simion, C. Third order nonlinear optical properties of DNA-based biopolymers thin films doped with selected natural chromophores. Opt. Mater. 2019, 88, 181. [Google Scholar] [CrossRef]
- Nizioł, J.; Makyła-Juzak, K.; Marzec, M.M.; Ekiert, R.; Marzec, M.; Gondek, E. Thermal stability of the solid DNA as a novel optical material. Opt. Mater. 2017, 66, 344–350. [Google Scholar] [CrossRef]
- Nizioł, J.; Fiedor, J.; Pagacz, J.; Hebda, E.; Marzec, M.; Gondek, E.; Kityk, I.V. DNA-hexadecyltrimethyl ammonium chloride complex with enhanced thermostability as promising electronic and optoelectronic material. J. Mater. Sci Mater. Electron. 2017, 28, 259–268. [Google Scholar] [CrossRef][Green Version]
- Manea-Saghin, A.M.; Paduret, C.C.; Kajzar, F. Spectroscopy and non-linear optical properties of DNA—Bilberry complex. Opt. Mater. 2020, 100, 109669. [Google Scholar] [CrossRef]
- Manea-Saghin, A.M.; Paduret, C.C.; Kajzar, F. Optical properties of DNA doped with blackcurrant and bilberry extracts. Opt. Mater. 2020, 101, 109721. [Google Scholar] [CrossRef]
- Grote, J.G.; Diggs, D.E.; Nelson, R.L.; Zetts, J.S.; Hopkins, F.K.; Ogata, N.; Hagen, J.A.; Heckman, E.; Yaney, P.P.; Stone, M.O.; et al. DNA photonics [deoxyribonucleic acid]. Mol. Cryst. Liq. 2005, 426, 3–17. [Google Scholar] [CrossRef]
- Singh, T.B.; Sariciftci, N.S.; Grote, J.G. Bio-Organic Optoelectronic Devices Using DNA. In Organic Electronics, Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Wang, L.; Yoshida, I.; Ogata, N. Self-Assembled Supramolecular Films Derived from Marine Deoxyribonucleic Acid (DNA)−Cationic Surfactant Complexes: Large-Scale Preparation and Optical and Thermal Properties. Chem. Mater. 2001, 13, 1273–1281. [Google Scholar] [CrossRef]
- Prenting, M.M.; Shilikin, M.; Dreier, T.; Schultz, C.; Endres, T. Characterization of tracers for two-color laser-induced fluorescence thermometry of liquid-phase temperature in ethanol, 2–ethylhexanoic-acid/ethanol mixtures, 1-butanol, and o-xylene. Appl. Opt. 2021, 60, C98–C113. [Google Scholar] [CrossRef]
- You, H.; Spaeth, H.; Linhard, V.N.L.; Steckl, A.J. Role of Surfactants in the Interaction of Dye Molecules in Natural DNA Polymers. Langmuir 2009, 25, 11698–11702. [Google Scholar] [CrossRef]
- Patra, D.; Barakat, C. Synchronous fluorescence spectroscopic study of solvatochromic curcumin dye. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 1034–1041. [Google Scholar] [CrossRef]
- Shanti, R.; Hadi, A.N.; Salim, Y.S.; Chee, S.Y.; Ramesh, S.; Ramesha, K. Degradation of ultra-high molecular weight poly(methyl methacrylate-co-butyl acrylate-co-acrylic acid) under ultra violet irradiation. RSC Adv. 2017, 7, 112–120. [Google Scholar] [CrossRef]
- Anton, A.M.; Rau, I.; Kajzar, F.; Simion, A.M.; Simion, C. Stability studies of some DNA based materials doped with natural extracts. U.P.B. Sci. Bull. B 2019, 81, 121–129. [Google Scholar]
- Taheri, A.; Liu, H.; Jassemnejad, B.; Appling, D.; Powell, R.C.; Song, J.J. Intensity scan and two photon absorption and nonlinear refraction of C60 in toluene. Appl. Phys. Lett. 1996, 68, 1317–1319. [Google Scholar] [CrossRef]
- SheikBahae, M.; Said, A.A.; Wei, T.; Hagan, D.J.; Van Stryland, E.W. Sensitive measurement of optical nonlinearities using a single beam. IEEE J. Quantum Electron. 1990, 26, 760–769. [Google Scholar] [CrossRef]

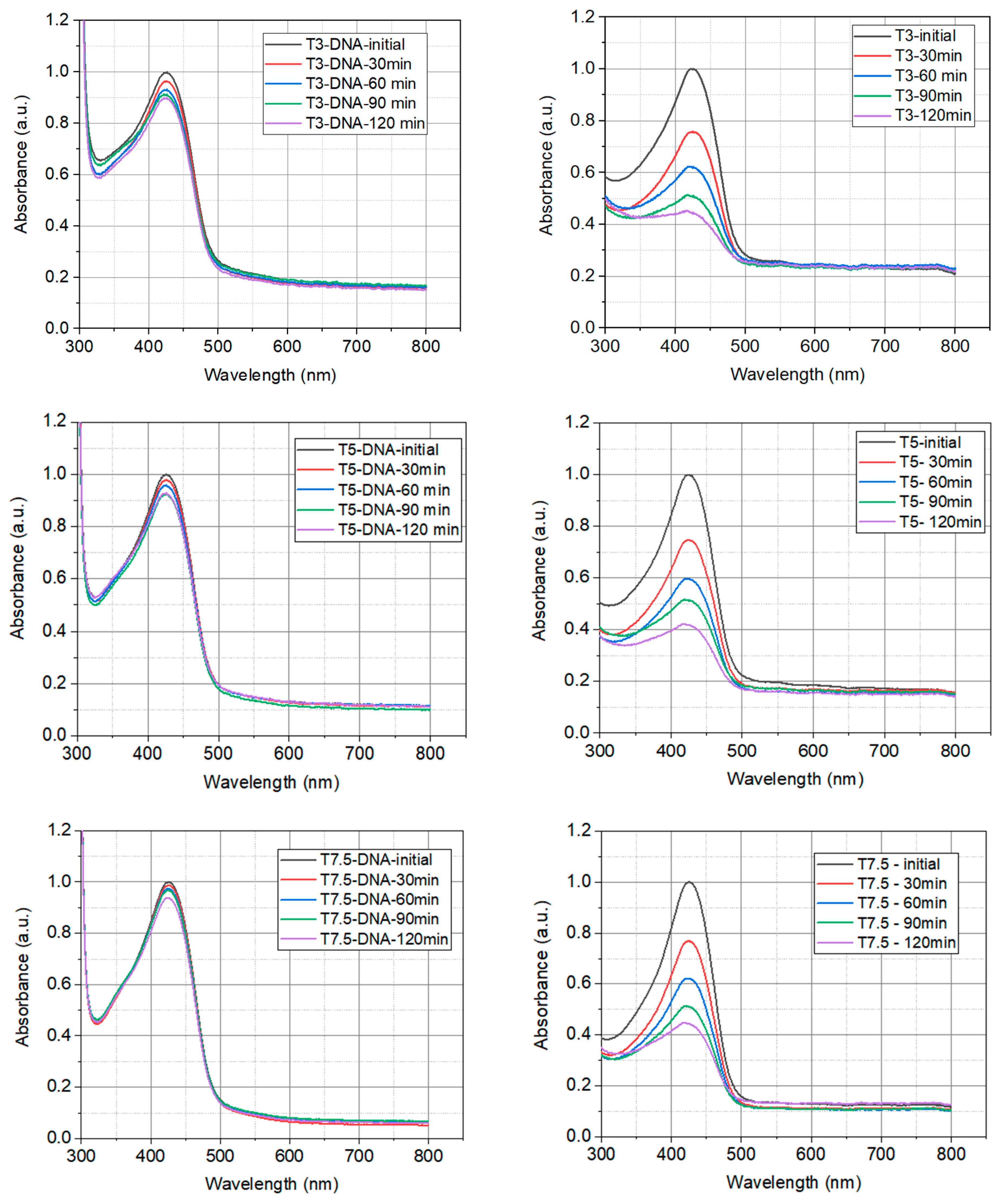
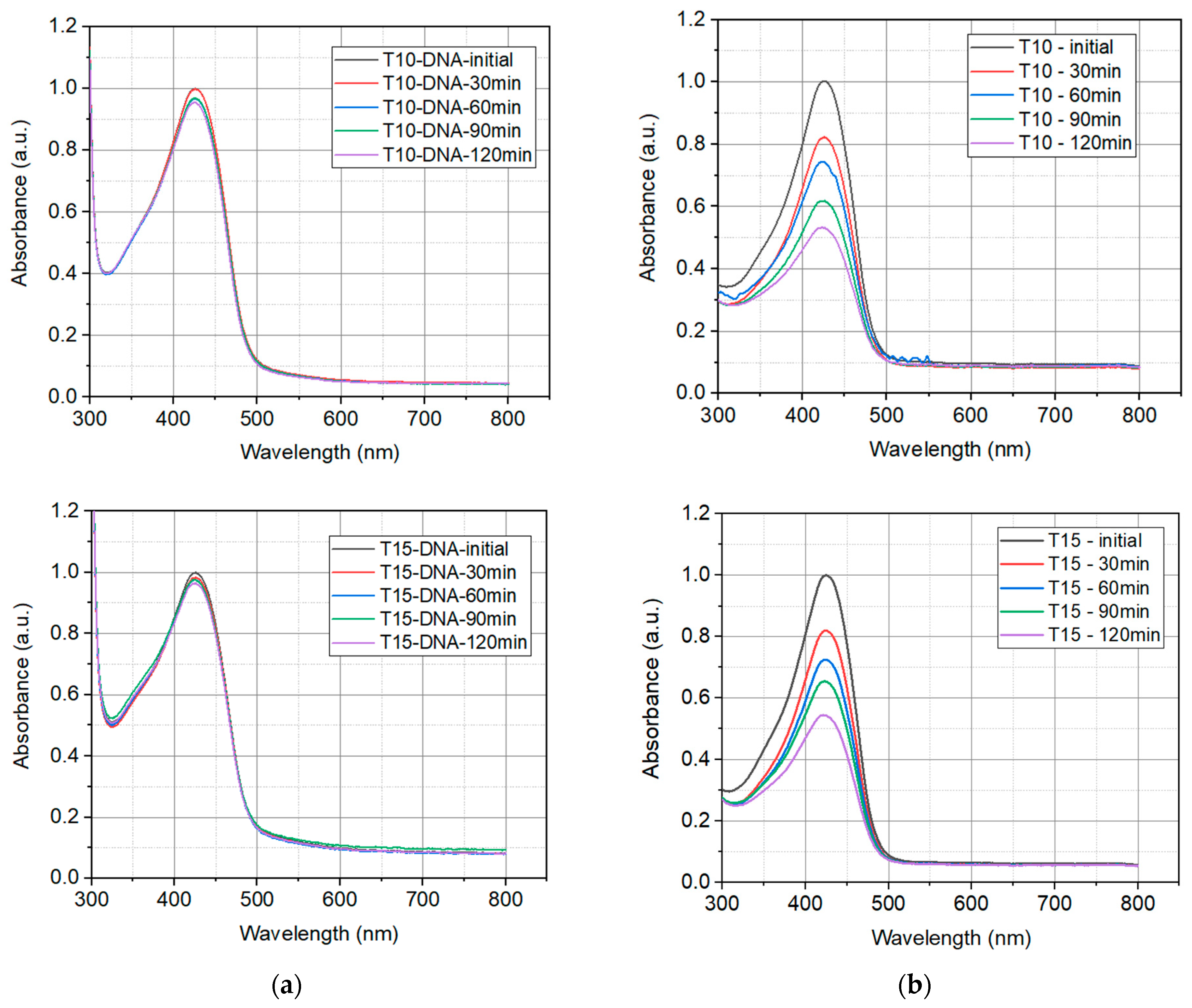


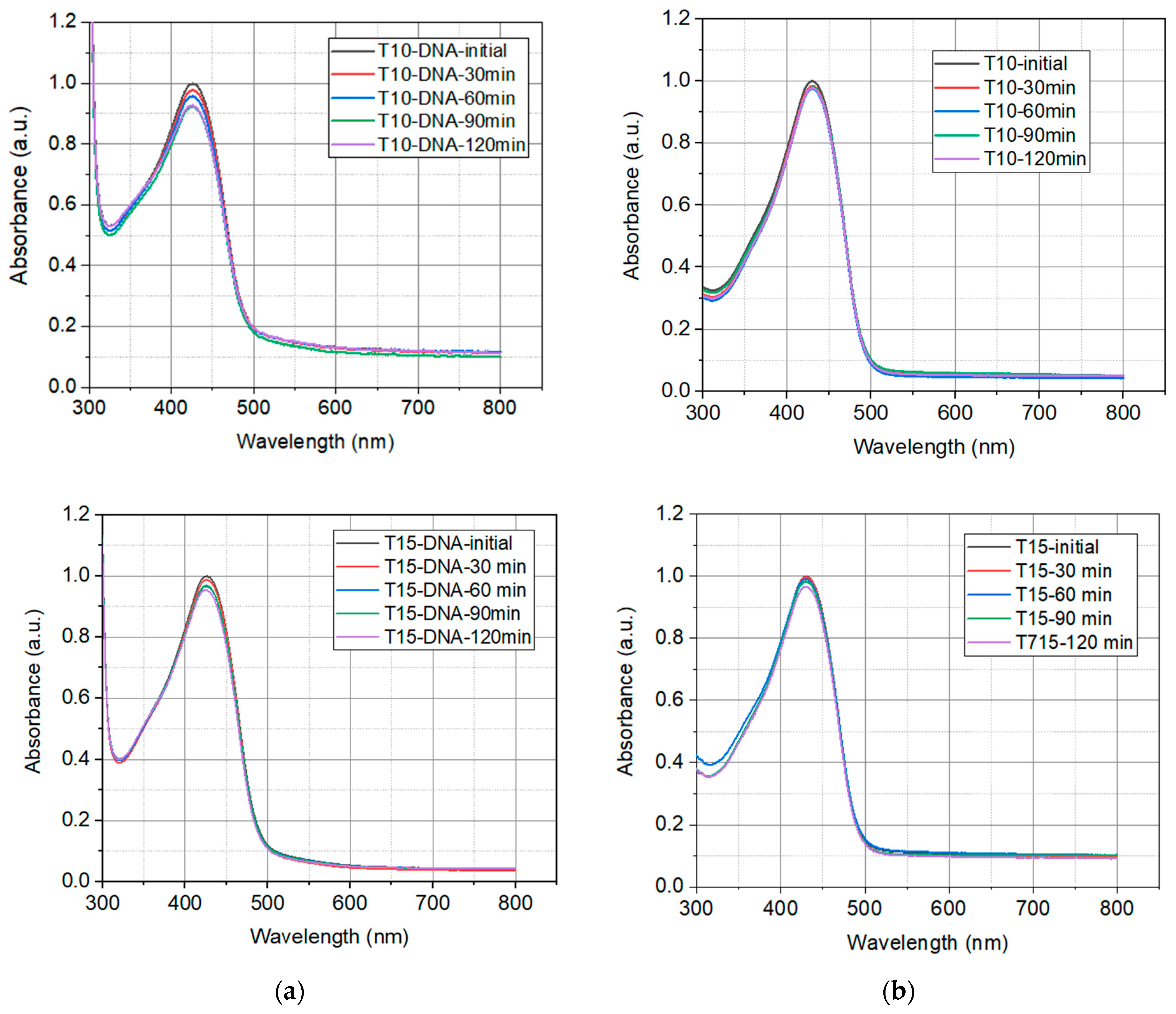



| Sample | Turmeric Concentration in Solution g/L |
|---|---|
| T3-DNA/T3 | 3 |
| T5-DNA/T5 | 5 |
| T7.5-DNA/T7.5 | 7.5 |
| T10-DNA/T10 | 10 |
| T15-DNA/T15 | 15 |
| Film | T3-DNA/T3 | T5-DNA/T5 | T7.5-DNA/T7.5 | T10-DNA/T10 | T15-DNA/T15 | |
|---|---|---|---|---|---|---|
| Fitting Parameter | ||||||
| y0Pi | 0.8666/ 0.33452 | 0.87049/ 0.34271 | 0.87542/ 0.33081 | 0.90768/ 0.39744 | 0.94231/ 0.51004 | |
| APi | 0.13429/ 0.66048 | 0.13029/ 0.65415 | 0.12578/ 0.66785 | 0.09363/ 0.59953 | 0.05685/ 0.48695 | |
| kPi (min−1) | 0.01259/ 0.01569 | 0.00969/ 0.01453 | 0.00721/ 0.01405 | 0.00698/ 0.01246 | 0.00645/ 0.01117 | |
| Film | T3-DNA/T3 | T5-DNA/T5 | T7.5-DNA/T7.5 | T10-DNA/T10 | T15-DNA/T15 | |
|---|---|---|---|---|---|---|
| Fitting Parameter | ||||||
| y0Ti | 0.8839/ 0.87714 | 0.9000/ 0.90468 | 0.9158/ 0.91292 | 0.92052/ 0.9204 | 0.92547/ 0.9310 | |
| ATi | 0.11726/ 0.12183 | 0.10091/ 0.0953 | 0.08525/ 0.08672 | 0.08021/ 0.7964 | 0.07502/ 0.07001 | |
| kTi (min−1) | 0.0141/ 0.01512 | 0.01159/ 0.01449 | 0.01151/ 0.01299 | 0.00882/ 0.01111 | 0.00703/ 0.00943 | |
| Sample | Linear Transmittance | α0 (cm−1) | Isat (GW/cm2) | β (cm/GW) |
|---|---|---|---|---|
| T3-DNA/T3 | 0.43/0.52 | 17/13 | 14/38 | 3.5/2.4 |
| T5-DNA/T5 | 0.38/0.47 | 19/15 | 12/35 | 3.6/2.7 |
| T7.5-DNA/T7.5 | 0.36/0.43 | 21/17 | 11/21 | 3.8/2.9 |
| T10-DNA/T10 | 0.31/0.37 | 23/20 | 7/18 | 3.9/3.0 |
| T15-DNA/T15 | 0.27/0.32 | 26/23 | 6/16 | 4.0/3.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gheorghe, P.; Petris, A.; Anton, A.M. Optical Limiting Properties of DNA Biopolymer Doped with Natural Dyes. Polymers 2024, 16, 96. https://doi.org/10.3390/polym16010096
Gheorghe P, Petris A, Anton AM. Optical Limiting Properties of DNA Biopolymer Doped with Natural Dyes. Polymers. 2024; 16(1):96. https://doi.org/10.3390/polym16010096
Chicago/Turabian StyleGheorghe, Petronela, Adrian Petris, and Adina Mirela Anton. 2024. "Optical Limiting Properties of DNA Biopolymer Doped with Natural Dyes" Polymers 16, no. 1: 96. https://doi.org/10.3390/polym16010096
APA StyleGheorghe, P., Petris, A., & Anton, A. M. (2024). Optical Limiting Properties of DNA Biopolymer Doped with Natural Dyes. Polymers, 16(1), 96. https://doi.org/10.3390/polym16010096





