Melting Behaviors of Bio-Based Poly(propylene 2,5-furan dicarboxylate)-b-poly(ethylene glycol) Co Polymers Related to Their Crystal Morphology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
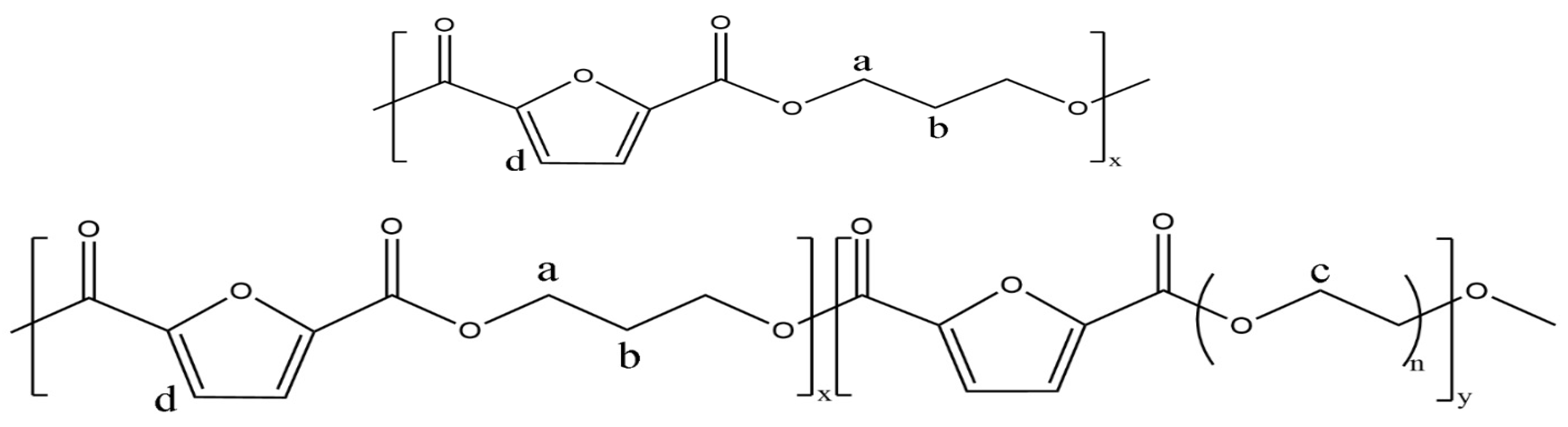
2.2. Synthesis of PPFEG Copolymers and Corresponding PPF Homopolymer
2.3. Thermal Characterization
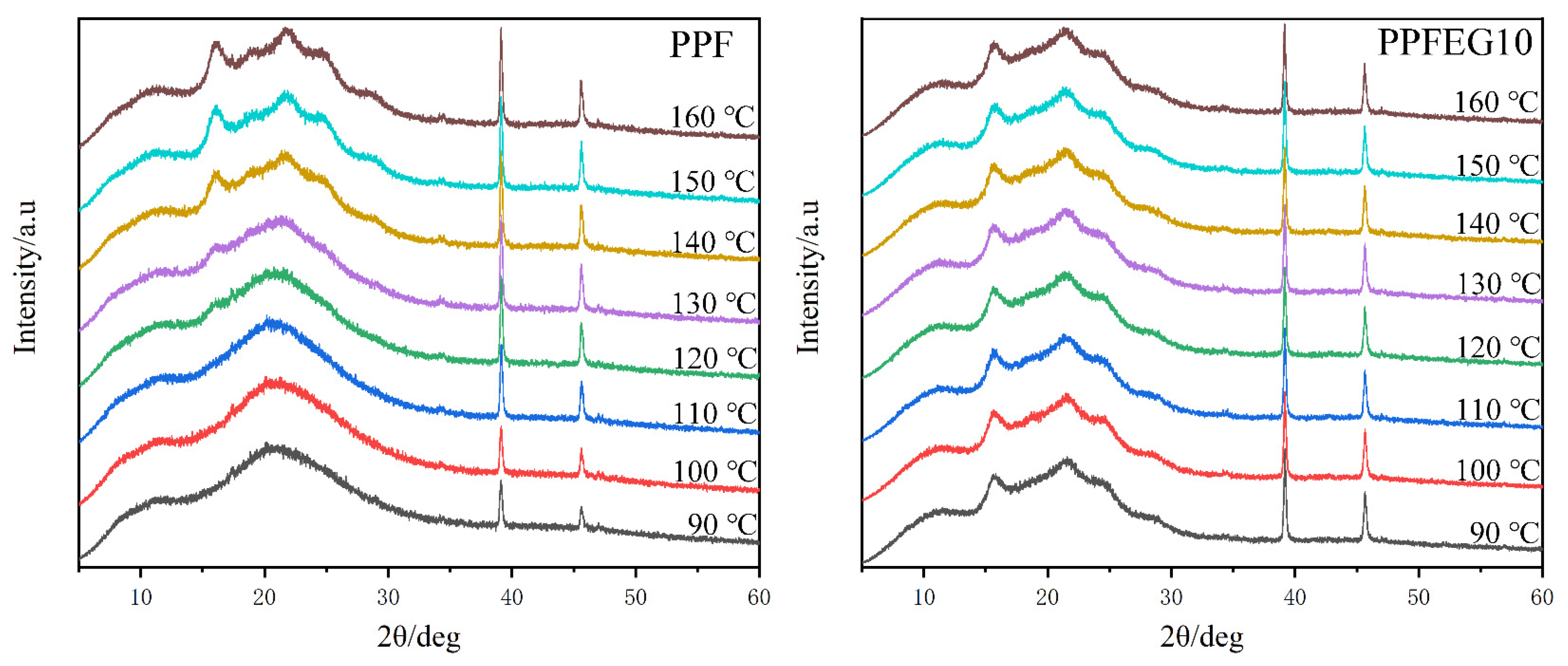
2.4. Wideangle X-ray Scattering Characterization
2.5. Morphological Characterization
3. Results and Discussion
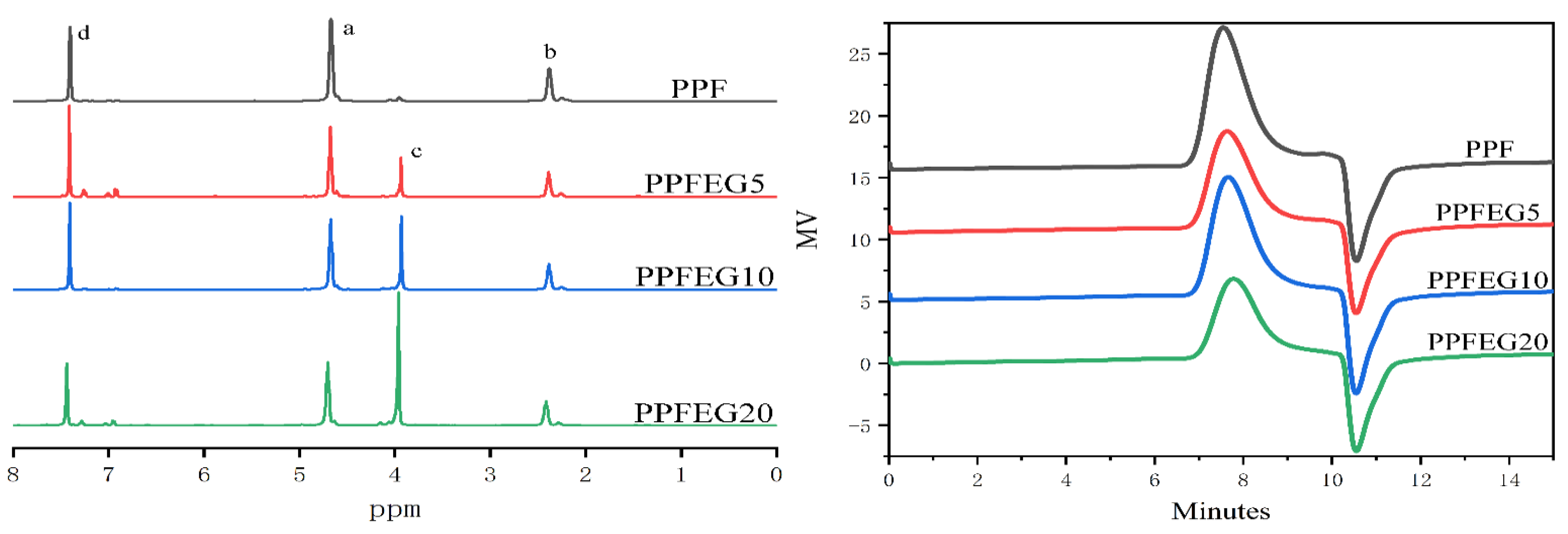
3.1. Composition of PPF and PPFEG Copolymers
3.2. Thermal Behavior of PPFEG Copolymers
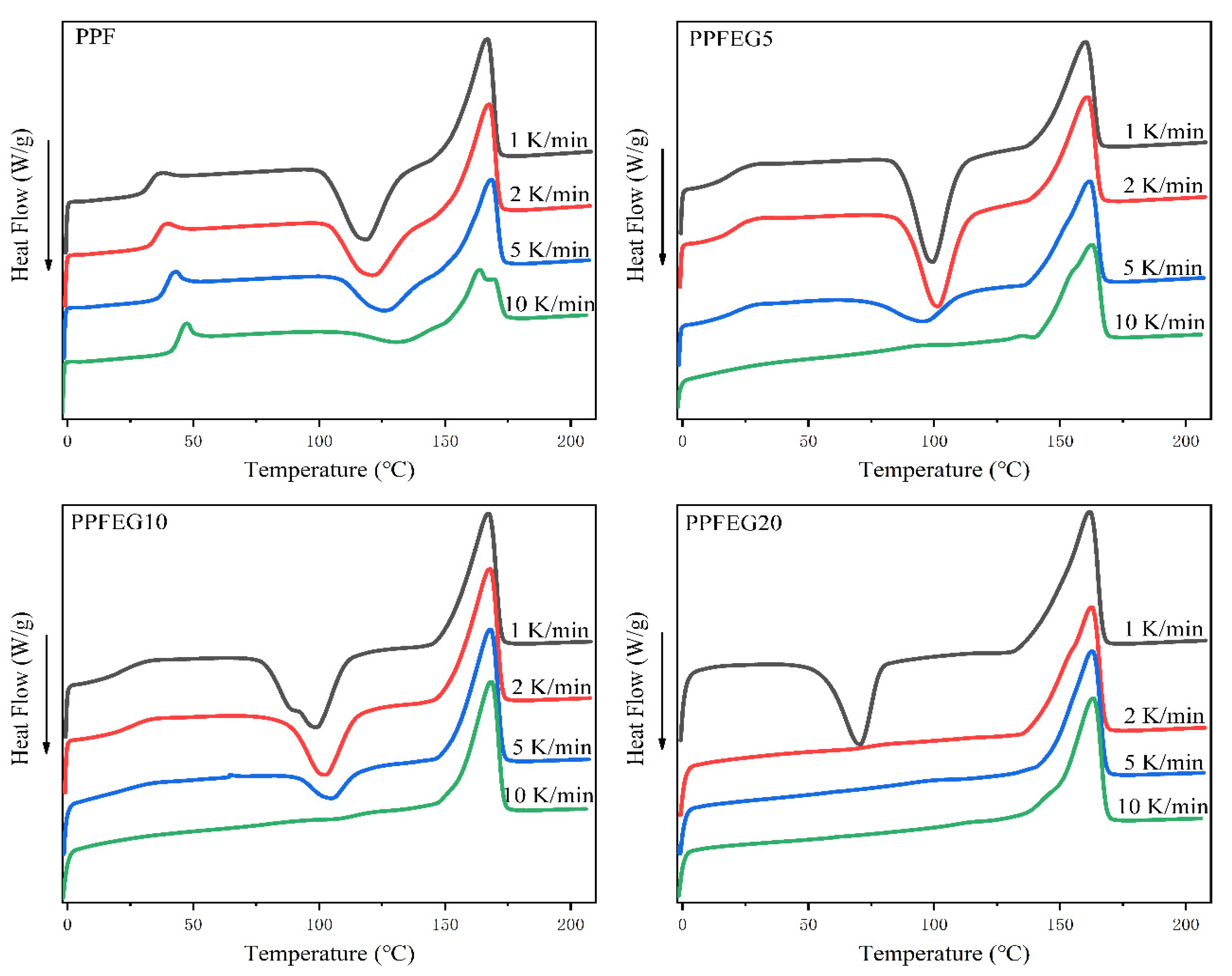
3.2.1. DSC Characterization of PPF and PPFEG Copolymers
3.2.2. Isothermal Crystallization of PPFEG Copolymers
3.2.3. Non–Isothermal Crystallization of PPFEG Copolymers
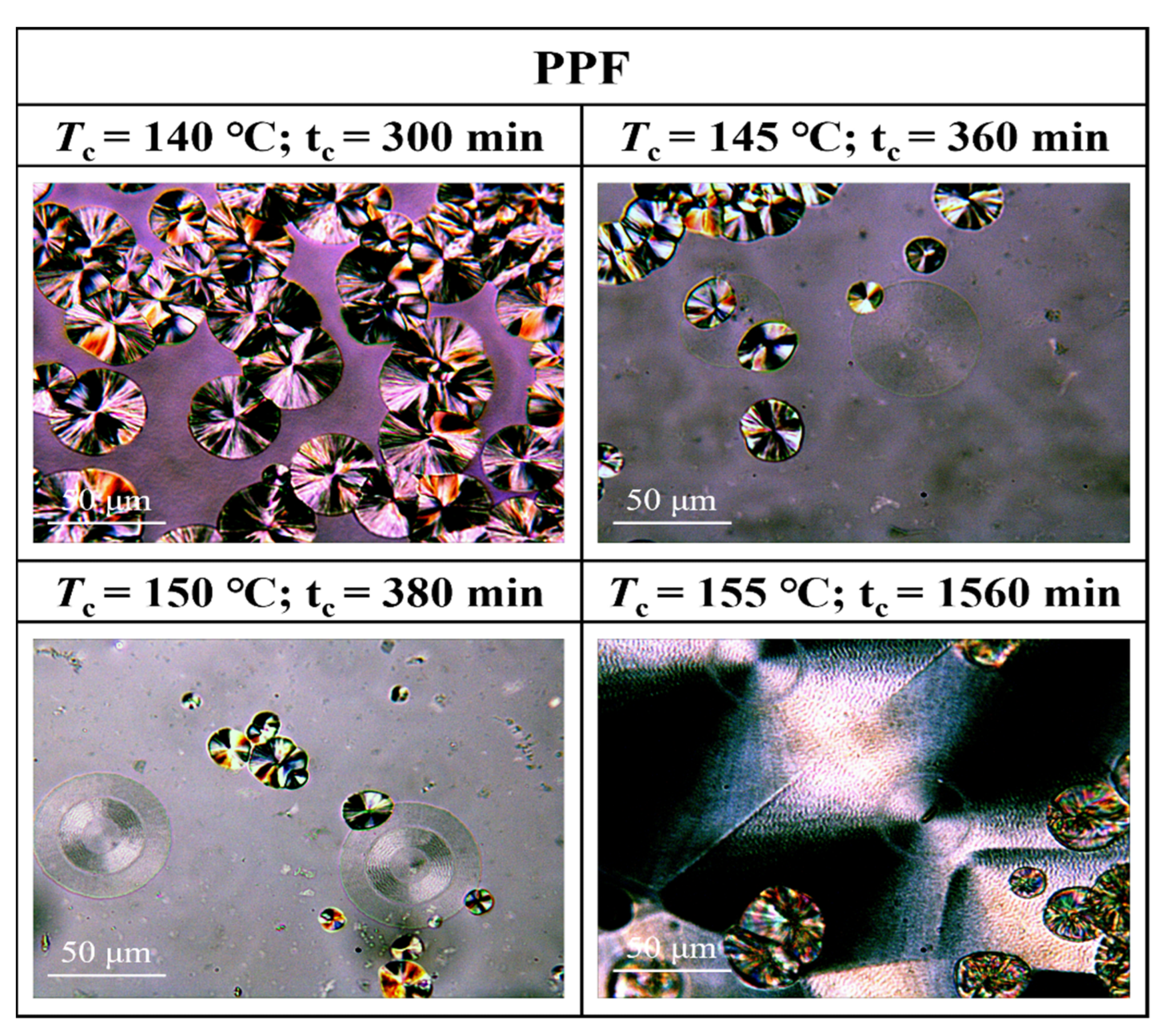
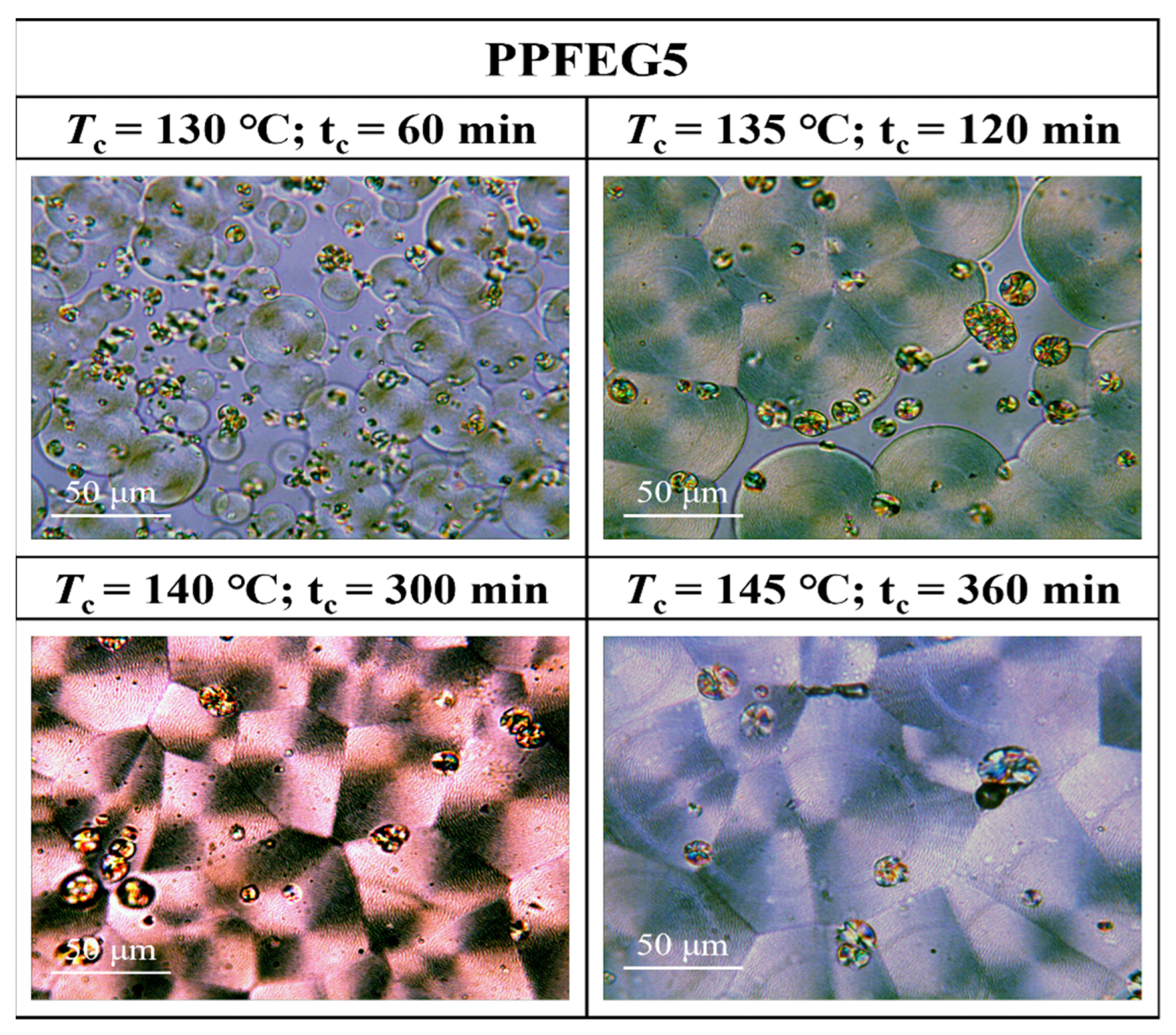
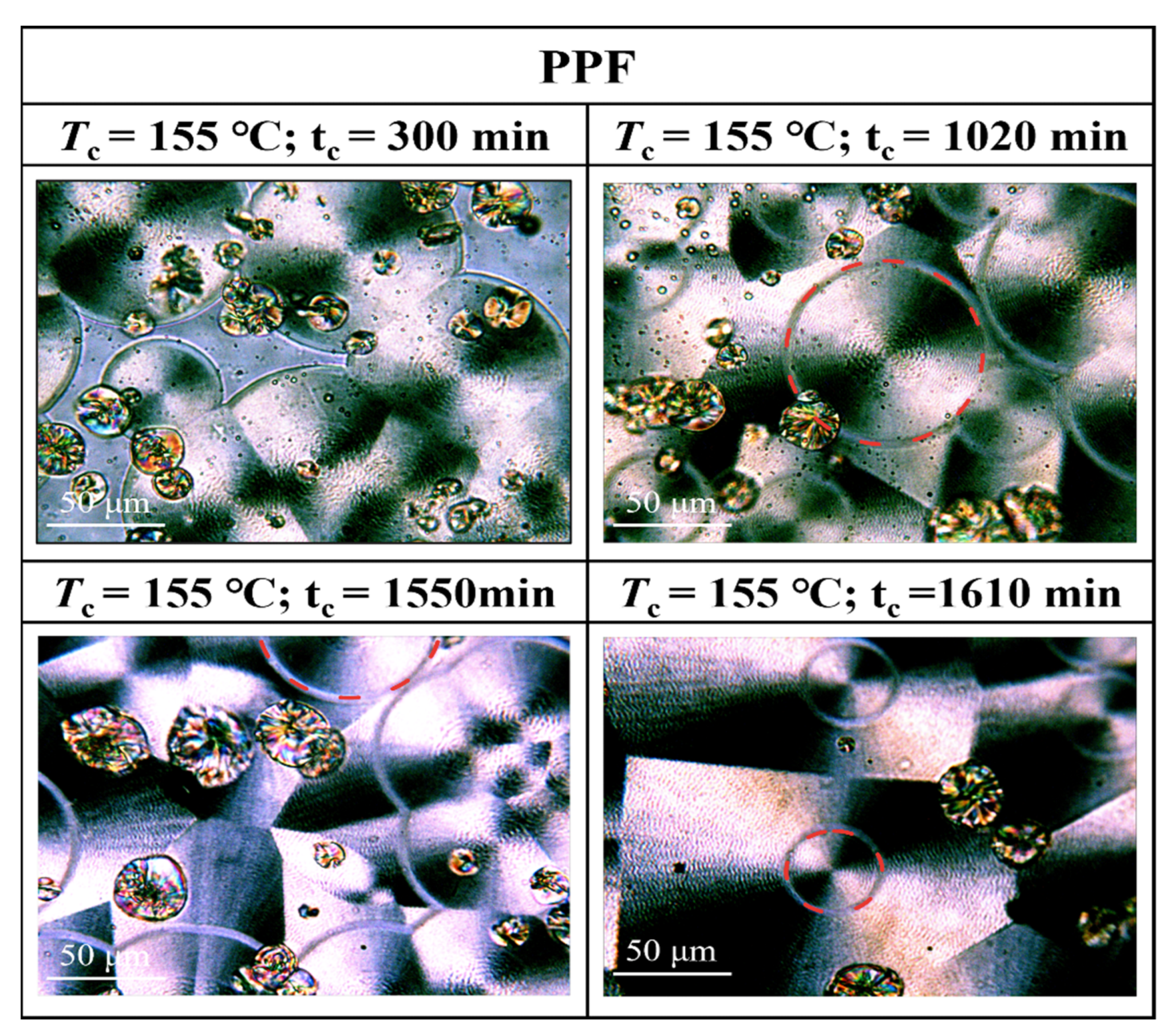
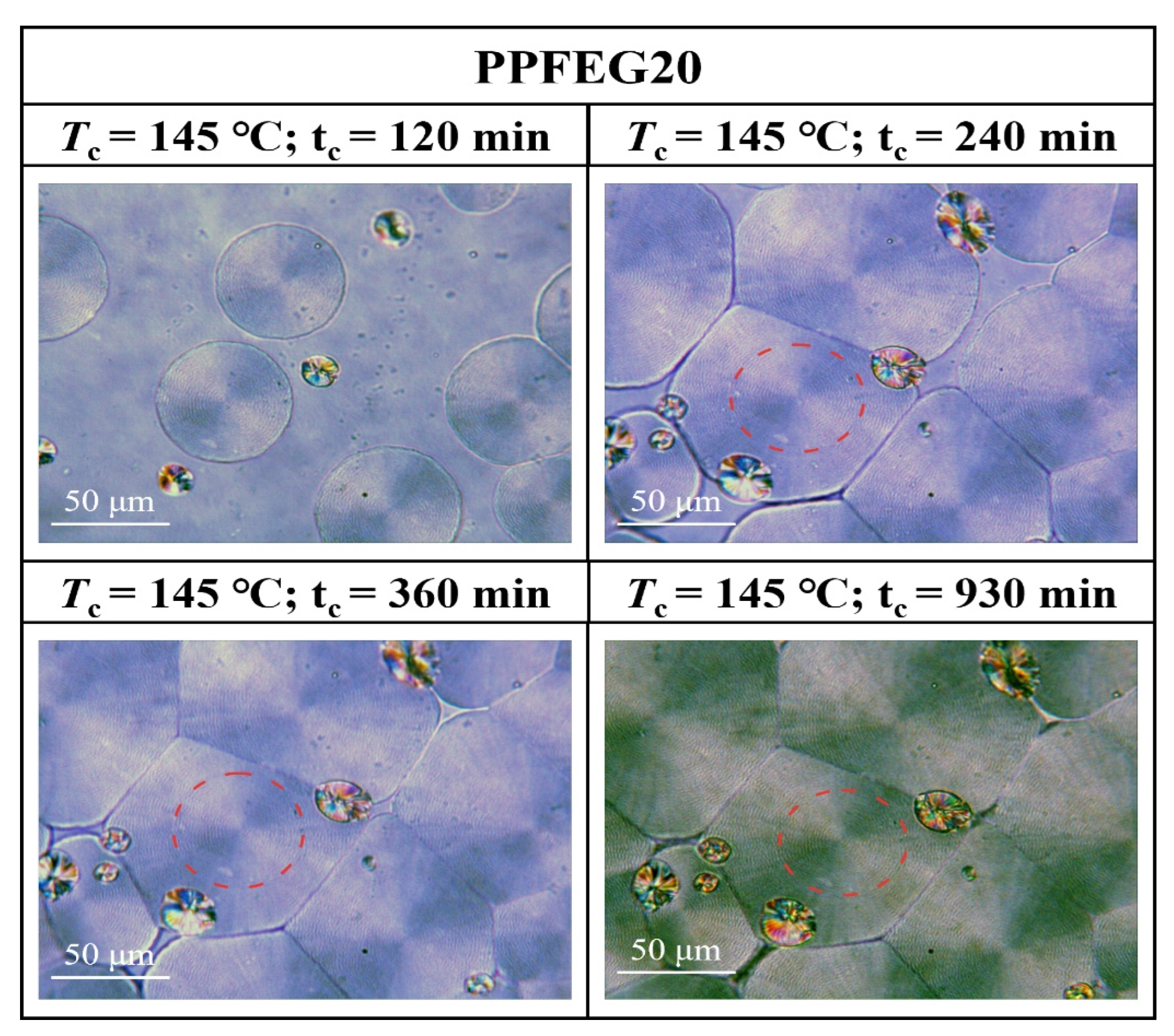
3.3. Crystalline Morphology of PPFEG Copolymers
3.4. WAXD Characteristics of PPFEG Copolymers
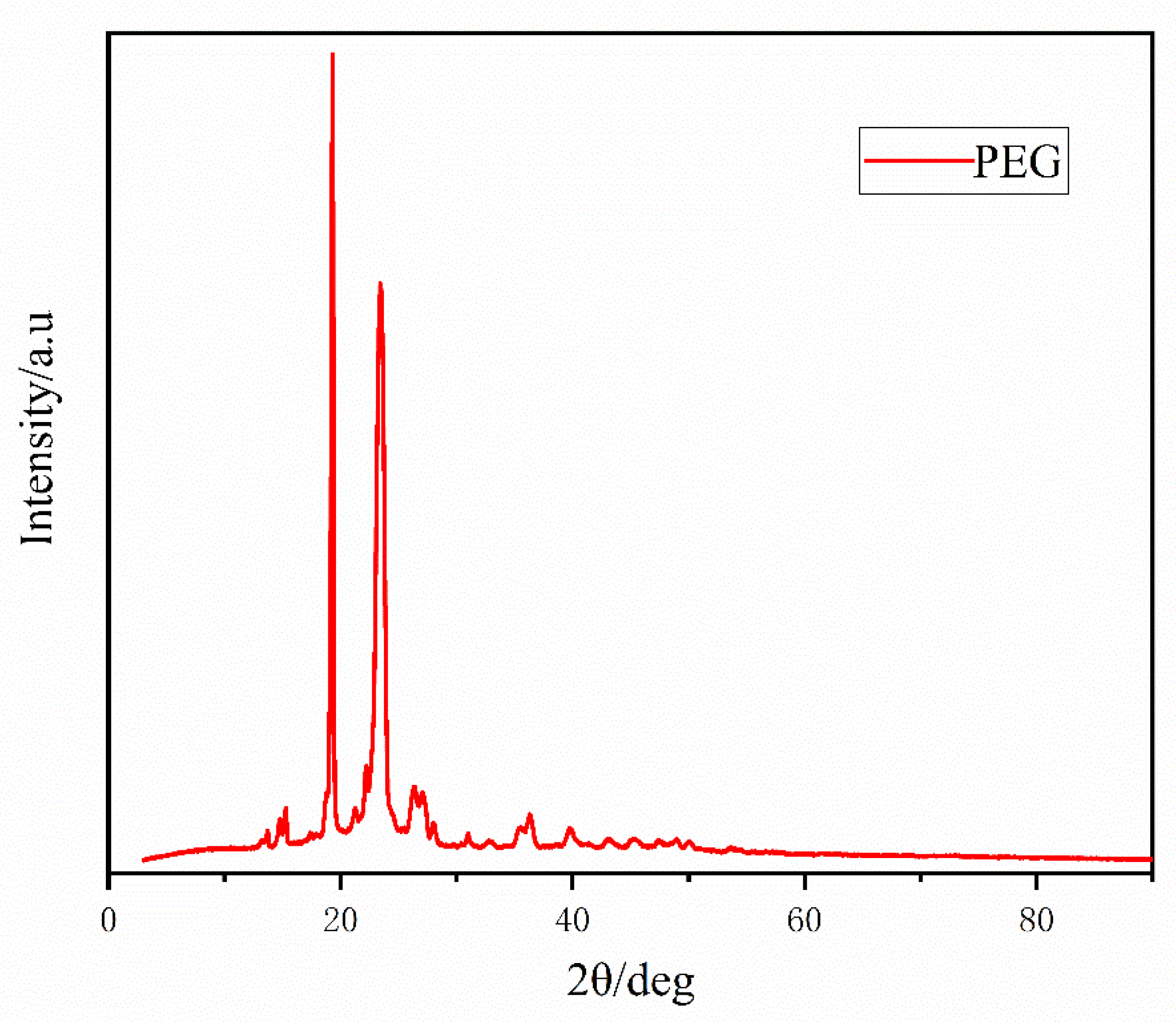
3.4.1. WAXD Patterns of PEG
3.4.2. WAXS Patterns upon Heating as a Function of the Isothermal Crystallization Temperature
3.4.3. WAXS Patterns upon Heating as a Function of the Non–Isothermal Crystallization Temperature
4. Conclusions
- (1)
- The structure of PEG consists of repeated glycol units embedded in PPF as a soft segment material, and the addition of PEG does not change the crystal structure of PPF. However, the addition of PEG affects the crystal structure of the copolymer to a certain extent and enhances its crystallization ability, and a PEG will inhibit the crystallization of the copolymer to a certain extent.
- (2)
- The isothermal crystallization and melting behavior of the PPFEG polyester shows that when the Tc temperature continues to increase, the polyester crystal structure gradually becomes perfect. The PPF copolymer enhanced the crystallization ability of the molecular chains, and the crystallization rate was accelerated. The multiple melt crystallization behavior of the copolymers gradually becomes increasingly pronounced, as seen by their enhanced crystallization ability at this crystallization temperature compared to pure PPF.
- (3)
- The addition of PEG decreased the crystallization temperature of PPF but increased the crystalline perfection of the polyester. The crystallization rate of the bio–based PPFEG polyesters tended to increase and then decrease with increasing amounts of flexible chain segment PEG and Tc.
- (4)
- From the crystalline morphology, the Tm of PPFEG decreased significantly with increasing proportion of PEG chain segments, which caused the crystalline body to change gradually to α-spherical crystals. With increasing crystallization time, ring–band spherical crystals appear slowly, and spherical crystals will also increase slowly.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Richter, S.M. New polymer syntheses. CIX. Biodegadable, alternating copolyesters of terephthalic acid, aliphatic dicarboxyclic acids, and alkane diols. J. Polym. Sci. A Polym. Chem. 2001, 39, 3371–3382. [Google Scholar]
- Samperi, F.; Puglisi, C.; Alicata, R.; Montaudo, G. Thermal degradation of poly(butylene terephthalate) at the processing temperature. Polym. Degrad. Stab. 2004, 83, 11–17. [Google Scholar] [CrossRef]
- Konstantopoulou, M.; Terzopoulou, Z.; Nerantzaki, M.; Tsagkalias, J.; Achilias, D.S.; Bikiaris, D.N.; Exarhopoulos, S.; Papageorgiou, D.G.; Papageorgiou, G.Z. Poly(ethylene furanoate-co-ethylene terephthalate) biobased copolymers: Synthesis, thermal properties and cocrystallization behavior. Eur. Polym. J. 2017, 89, 349–366. [Google Scholar] [CrossRef]
- Araujo, C.F.; Nolasco, M.M.; Ribeiro-Claro, P.J.A.; Rudić, S.; Silvestre, A.J.D.; Vaz, P.D.; Sousa, A.F. Inside PEF: Chain Conformation and Dynamics in Crystalline and Amorphous Domains. Macromolecules 2018, 51, 3515–3526. [Google Scholar] [CrossRef]
- van Berkel, J.G.; Guigo, N.; Visser, H.A.; Sbirrazzuoli, N. Chain Structure and Molecular Weight Dependent Mechanics of Poly(ethylene 2,5-furandicarboxylate) Compared to Poly(ethylene terephthalate). Macromolecules 2018, 51, 8539–8549. [Google Scholar] [CrossRef]
- Banella, M.B.; Bonucci, J.; Vannini, M.; Marchese, P.; Lorenzetti, C.; Celli, A. Insights into the Synthesis of Poly(ethylene 2,5-Furandicarboxylate) from 2,5-Furandicarboxylic Acid: Steps toward Environmental and Food Safety Excellence in Packaging Applications. Ind. Eng. Chem. Res. 2019, 58, 8955–8962. [Google Scholar] [CrossRef]
- Jan-Georg, R.; Kay, H.D.; Peter, F.; Giuseppe, S.; Massimo, M. Bottle-grade polyethylene furanoate from ring-opening polymerisation of cyclic oligomers. Nat. Commun. 2018, 9, 2701. [Google Scholar]
- Papamokos, G.; Dimitriadis, T.; Bikiaris, D.N.; Papageorgiou, G.Z.; Floudas, G. Chain Conformation, Molecular Dynamics, and Thermal Properties of Poly(n-methylene 2,5-furanoates) as a Function of Methylene Unit Sequence Length. Macromolecules 2019, 52, 6533–6546. [Google Scholar] [CrossRef]
- Wang, J.; Sun, L.; Shen, Z.; Zhu, J.; Song, X.; Liu, X. Effects of Various 1,3-Propanediols on the Properties of Poly(propylene furandicarboxylate). ACS Sustai. Chem. Eng. 2019, 7, 3282–3291. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Ward, I.M.; Wilding, M.A.; Brody, H.J. The mechanical properties and structure of poly(m-methylene terephthalate) fibers. J. Polym. Sci. Polym. Phys. 2010, 14, 263–274. [Google Scholar] [CrossRef]
- DeJong, E.; Dam, R.; Sipos, L.; Ouden, D.D.; Gruter, G.J. Furandicarboxylic acid (FDCA) a versatile building block for a very interesting class of polyesters. In Biobased Monomers, Polymers, and Materials; American Chemical Society: Washington, DC, USA, 2012; pp. 1–13. [Google Scholar] [CrossRef]
- Fan-Chiang, C.C.; Chiu, W.Y.; Hsieh, K.H.; Chen, L.W. Crystallization of polypropylene II. Non-isothermal kinetics. Mater. Chem. Phys. 1993, 34, 52–57. [Google Scholar] [CrossRef]
- Rastogi, S.; Terry, A.E.; Vinken, E. Dissolution of Hydrogen-Bonded Polymers in Water: A Study of Nylon-4,6. Macromolecules 2004, 37, 8825–8828. [Google Scholar] [CrossRef]
- Qu, D.; Gao, H.; Wang, Q.; Bai, Y.; Li, N. Non-isothermal crystallization kinetics of bio-based poly(butylene-co-isosorbide succinate) (PBIS). J. Therm. Anal. Calorim. 2019, 139, 1931–1939. [Google Scholar] [CrossRef]
- Zhang, F.; Jiang, Z.G.; Qiu, Z.B. Biobased Poly(ethylene succinate)-b-poly(triethylene terephthalate) multiblock copolyesters with high melting temperature and improved crystallization rate and mechanical property. Polymer 2022, 254, 125061. [Google Scholar] [CrossRef]
- Righetti, M.C.; Marchese, P.; Vannini, M.; Celli, A.; Lorenzetti, C.; Cavallo, D.; Ocando, C.; Muller, A.J.; Androsch, R. Polymorphism and Multiple Melting Behavior of Bio-Based Poly(propylene 2,5-furandicarboxylate). Biomacromolecules 2020, 21, 2622–2634. [Google Scholar] [CrossRef]
- Wei, X.-W.; Zhao, X.-X.; Li, Y.; Meng, X.-Y.; Zhou, Q.; Ye, H.-M. Distinctive Polymorphism-like Isodimorphism in Poly(propylene succinate-ran-propylene fumarate). Chin. J. Poly. Sci. 2022, 40, 602–610. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, K.; Jiang, Z.; Qiu, Z. Biobased Poly(1,3-propylene 2,5-furandicarboxylate)-Carbon Nanotubes Nanocomposites with Enhanced Thermal, Mechanical Properties and Crystallization Behavior. J. Polym. Environ. 2021, 30, 555–561. [Google Scholar] [CrossRef]
- Pan, S.; Jiang, Z.; Qiu, Z. Influence of low contents of cellulose nanocrystals on the crystallization behavior of biobased Poly(propylene 2,5-furandicarboxylate). Giant 2023, 17, 100212. [Google Scholar] [CrossRef]
- Righetti, M.C.; Marchese, P.; Vannini, M.; Celli, A.; Tricoli, F.; Lorenzetti, C. Temperature-induced polymorphism in bio-based poly(propylene 2,5-furandicarboxylate). Thermochim. Acta 2019, 677, 186–193. [Google Scholar] [CrossRef]
- Chen, M.; Jiang, Z.; Qiu, Z. In Situ Synthesis, Crystallization Behavior, and Mechanical Property of Biobased Poly(hexamethylene 2,5-furandicarboxylate)/Multiwalled Carbon Nanotube Nanocomposites. Ind. Eng. Chem. Res. 2022, 61, 9745–9754. [Google Scholar] [CrossRef]
- Wang, G.; Dong, Y. Bio-based poly(propylene 2,5-furandicarboxylate-co-propylene 2,5-thiophenedicarboxylate): Synthesis and characterization. Polym. Bull. 2023, 95, 1–19. [Google Scholar] [CrossRef]







| Mn | Mw | Mp | Mz | Mz/Mw | |
|---|---|---|---|---|---|
| PPF | 18,394 | 47,865 | 46,141 | 85,151 | 1.778986 |
| PPFEG5 | 14,731 | 41,383 | 37,767 | 76,948 | 1.859430 |
| PPFEG10 | 12,570 | 37,588 | 35,644 | 69,488 | 1.848691 |
| PPFEG20 | 12,176 | 31,785 | 27,065 | 59,545 | 1.873384 |
| Polymer | Tm (°C) | △Hm (J/g) | Xc (%) |
|---|---|---|---|
| PPF | 169.5 | 25.956 | 18.28 |
| PPFEG5 | 163.9 | 30.180 | 21.25 |
| PPFEG10 | 163.2 | 37.599 | 26.48 |
| PPFEG20 | 163.1 | 45.128 | 31.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, O.; Li, P.; Yang, C.; Jiang, H.; Qin, L.; Liu, W.; Li, X.; Chen, Z. Melting Behaviors of Bio-Based Poly(propylene 2,5-furan dicarboxylate)-b-poly(ethylene glycol) Co Polymers Related to Their Crystal Morphology. Polymers 2024, 16, 97. https://doi.org/10.3390/polym16010097
Shi O, Li P, Yang C, Jiang H, Qin L, Liu W, Li X, Chen Z. Melting Behaviors of Bio-Based Poly(propylene 2,5-furan dicarboxylate)-b-poly(ethylene glycol) Co Polymers Related to Their Crystal Morphology. Polymers. 2024; 16(1):97. https://doi.org/10.3390/polym16010097
Chicago/Turabian StyleShi, Ouyang, Peng Li, Chao Yang, Haitian Jiang, Liyue Qin, Wentao Liu, Xiaolin Li, and Zhenming Chen. 2024. "Melting Behaviors of Bio-Based Poly(propylene 2,5-furan dicarboxylate)-b-poly(ethylene glycol) Co Polymers Related to Their Crystal Morphology" Polymers 16, no. 1: 97. https://doi.org/10.3390/polym16010097
APA StyleShi, O., Li, P., Yang, C., Jiang, H., Qin, L., Liu, W., Li, X., & Chen, Z. (2024). Melting Behaviors of Bio-Based Poly(propylene 2,5-furan dicarboxylate)-b-poly(ethylene glycol) Co Polymers Related to Their Crystal Morphology. Polymers, 16(1), 97. https://doi.org/10.3390/polym16010097






