Abstract
Both guided bone and guided tissue regeneration are techniques that require the use of barrier membranes. Contamination and infection of the surgical area is one of the most feared complications. Some current lines of research focus on functionalizing these membranes with different antimicrobial agents. The objective of this study was to carry out a review of the use and antibacterial properties of regeneration membranes doped with antimicrobials such as zinc, silver, chlorhexidine, and lauric acid. The protocol was based on PRISMA recommendations, addressing the PICO question: “Do membranes doped with non-antibiotic antimicrobials have antibacterial activity that can reduce or improve infection compared to membranes not impregnated with said antimicrobial?” Methodological quality was evaluated using the RoBDEMAT tool. A total of 329 articles were found, of which 25 met the eligibility criteria and were included in this review. Most studies agree that zinc inhibits bacterial growth as it decreases colony-forming units, depending on the concentration used and the bacterial species studied. Silver compounds also decreased the secretion of proinflammatory cytokines and presented less bacterial adhesion to the membrane. Some concentrations of chlorhexidine that possess antimicrobial activity have shown high toxicity. Finally, lauric acid shows inhibition of bacterial growth measured by the disk diffusion test, the inhibition zone being larger with higher concentrations. Antimicrobial agents such as zinc, silver, chlorhexidine, and lauric acid have effective antibacterial activity and can be used to dope regenerative membranes in order to reduce the risk of bacterial colonization.
1. Introduction
Both guided bone regeneration (GBR) and guided tissue regeneration (GTR) are techniques widely used in dentistry today [1]. The use of these techniques requires the use of membranes with barrier function, allowing the creation of a space, isolating the soft tissues from the bone defect, and thus promoting bone formation [2]. In addition to this barrier function, the ideal membrane should have other characteristics, such as adequate mechanical properties to maintain the regenerative space, tensile strength and pressure resistance, biocompatibility, stability, manageability, so that it can be easily deformed without fracturing and maintain its morphology after implantation, bioactivity and antibacterial properties, among others [3,4,5,6].
In recent years, the use of polymeric membranes in different medical applications has been constantly evolving [7,8]. One of these applications of polymeric membranes in the biomedical field is represented by the developing drug delivery system based on membranes or different separation interest molecules such as antibiotics or proteins [9]. The release of the drug is achieved by the diffusion of the active substance through the polymeric membrane so that the drug release can be controlled and targeted [10].
The most common classification of membranes is according to their ability to degrade, and they can be resorbable or non-resorbable [4]. Non-resorbable membranes are manufactured from synthetic polymers, metals, or composites of these materials [3]. They have high mechanical resistance, so they maintain the surgical space very well; however, they require surgical excision [3]. Resorbable membranes are composed almost exclusively of polymers, natural or synthetic, with the collagen membrane being the most common [4]. It has the advantage of fewer complications and low cost, in addition to the fact that a second surgery is not necessary for its removal. On the other hand, it shows less mechanical resistance and degrades rapidly, compromising the success of the regeneration [11].
The first investigations in dentistry on membranes were carried out by Nyman et al. [12]. These authors used Millipore membranes to maintain space and separate bone defects around a periodontal tooth from adjacent tissue. Subsequently, Dahlin et al. [13] conducted studies on rat jaws where defects were created and covered with Teflon membranes. After 6 weeks, there was complete healing of the defect under these membranes, while defects that were not covered with membranes did not achieve complete healing at 22 weeks. This served as the beginning of the GBR [13].
The first scaffolds used were non-resorbable membranes such as expanded polytetrafluoroethylene (e-PTFE) and high-density polytetrafluoroethylene (d-PTFE), considered the gold standard in the 1990s [14,15]. With the aim of solving the exposure problems and the need for a second surgery presented by the aforementioned membranes, resorbable, natural, and synthetic membranes began to be designed. Within this group were resorbable collagen membranes, non-cross-linked membranes (NCLM), cross-linked membranes (CLM), and synthetic resorbable membranes [2,16]. These scaffolds have variable resorption times, properties, and results depending on their composition.
Currently, the most used membranes are those composed of collagen of different origins since this material is one of the main components of the human organism, being biocompatible and not causing immunogenicity [17]. The most used types of collagen are types I and III derived mainly from bovine or porcine tissue. The porosity of these membranes is variable, allowing the passage of bacteria, cells, and other elements of the organism to a greater or lesser extent. Most of these scaffolds are composed of a homogeneous layer of collagen, although they are also frequently manufactured forming two layers of collagen to prevent the leakage of epithelial cells through it [18].
One of the most feared complications in GBR techniques is bacterial contamination and consequent infection of the surgical site, especially if membrane exposure occurs [19]. The inflammatory response caused by bacterial invasion can inhibit the growth of osteoblasts, thus affecting the regenerative effect and even causing failure of the surgery. Therefore, one of the main current challenges is to try to prevent this contamination [20,21,22]. For this purpose, antibiotic treatment is currently administered systemically; however, the possible risk of toxicity of these drugs is well known, as well as the increase in bacterial resistance and the difficulty in reaching some areas due to insufficient concentration levels [20,23]. Numerous research studies are focusing on functionalizing these membranes by adding antibacterial properties to their occlusive function with the addition of different drugs or substances [3,19]. These substances include antimicrobial agents, which act locally, reducing the side effects that occur when administered systemically and also reducing the appearance of microbial resistance [3,24]. Antibiotic resistance is currently a worldwide health problem, and this is seen as an alternative strategy to combat this situation. Non-antibiotic antimicrobial agents (NMAs) are proving to be a promising therapy to solve this problem [25].
The antimicrobials that have been most studied in recent years and whose results have been most promising have been identified, among which we can highlight zinc (Zn), silver (Ag), chlorhexidine (CHX), and lauric acid (LA). Zn is an essential trace element, present in our body, with a fundamental role in the immune and nervous systems [26]. Studies have shown the antimicrobial properties of zinc oxide (ZnO) since it is capable of inhibiting the formation of bacterial biofilms [27]. It has also been shown to increase cell proliferation and wound healing and to promote osteoblast proliferation by triggering bone neoformation [26]. Ag nanoparticles (AgNPs) are one of the most widely applied antibacterial agents and show broad-spectrum antibacterial activity. In addition to this function, they also have an anti-inflammatory, antifungal, and antiviral effect [28]. Because of these characteristics, they have been widely used in various medical devices [28]. The antiseptic action of CHX is widely known in the field of dentistry. The antibacterial spectrum of this bisbiguanide includes most of the microorganisms present in the oral cavity [29,30]. Finally, LA is a naturally occurring saturated fatty acid. Its high biocompatibility and antibacterial properties have attracted the interest of researchers in studying its use as an antimicrobial agent against various microorganisms, including periodontopathogenic bacteria [31].
There are currently no reviews comparing the antimicrobial effectiveness of membranes impregnated with these compounds. The aim of this work was to perform a review of the use and antibacterial properties of regeneration membranes doped with non-antibiotic antimicrobials such as Zn, Ag, CHX, and LA.
2. Methods
The protocol was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) recommendations. This review addressed the following PICO question: “Do membranes doped with non-antibiotic antimicrobials (NAA) exhibit antibacterial activity that may decrease or improve infection of the membrane versus membranes not impregnated with such antimicrobial?”. P (population): resorbable or non-resorbable for GBR or GTR membranes; I (intervention): doped with NAA; C (comparison): membranes not doped with drugs or doped with other antimicrobial agents; O (results): antimicrobial capacity of the membranes.
Trials performed in vitro that met the following inclusion criteria were included: those that used regenerating membranes, measured antimicrobial activity, and the full text of which was available. We excluded studies that were reviews and case reports, that did not specify the conditions of culture and measurement of activity, and those whose follow-up was less than 24 h.
A literature review was performed using electronic databases such as PubMed, Scopus, and Web of Science (WOS) to perform the literature search. No time or language limits were established. The following combination of terms was used in the electronic search:
(“Guided Tissue Regeneration” OR “Guided Bone Regeneration” OR “Bone Regeneration” OR “Periodontal Regeneration” OR “Bone Tissue Regeneration”) AND (“Barrier Membrane” OR “Membrane” OR “Barrier”) AND (“zinc” OR “ZnO” OR “chlorhexidine” OR “silver” OR “silver nitrate” OR “lauric acid”).
The search for articles and their selection based on eligibility criteria was carried out by two independent investigators (A.A.-J., F.-J.M.M.). Discrepancies between the reviewers were resolved by discussion or, if this was not possible, a third reviewer (C.V.) was consulted. Data were extracted independently in the same manner. The level of concordance between reviewers was expressed with the Kappa index. Search results were cross-checked to eliminate duplicates. All studies that met the eligibility criteria underwent an assessment of methodological quality and risk of bias, performed in the same way by the investigators.
The methodological quality assessment was performed according to the risk of bias tool for pre-clinical dental material research (RoBDEMAT), which assesses the quality of studies of dental laboratory materials. Four main domains were determined: bias related to planning and allocation, sample preparation, outcome assessment, and data processing and reporting of results; and nine items pertaining to different sources of bias within the domains.
3. Results and Discussion
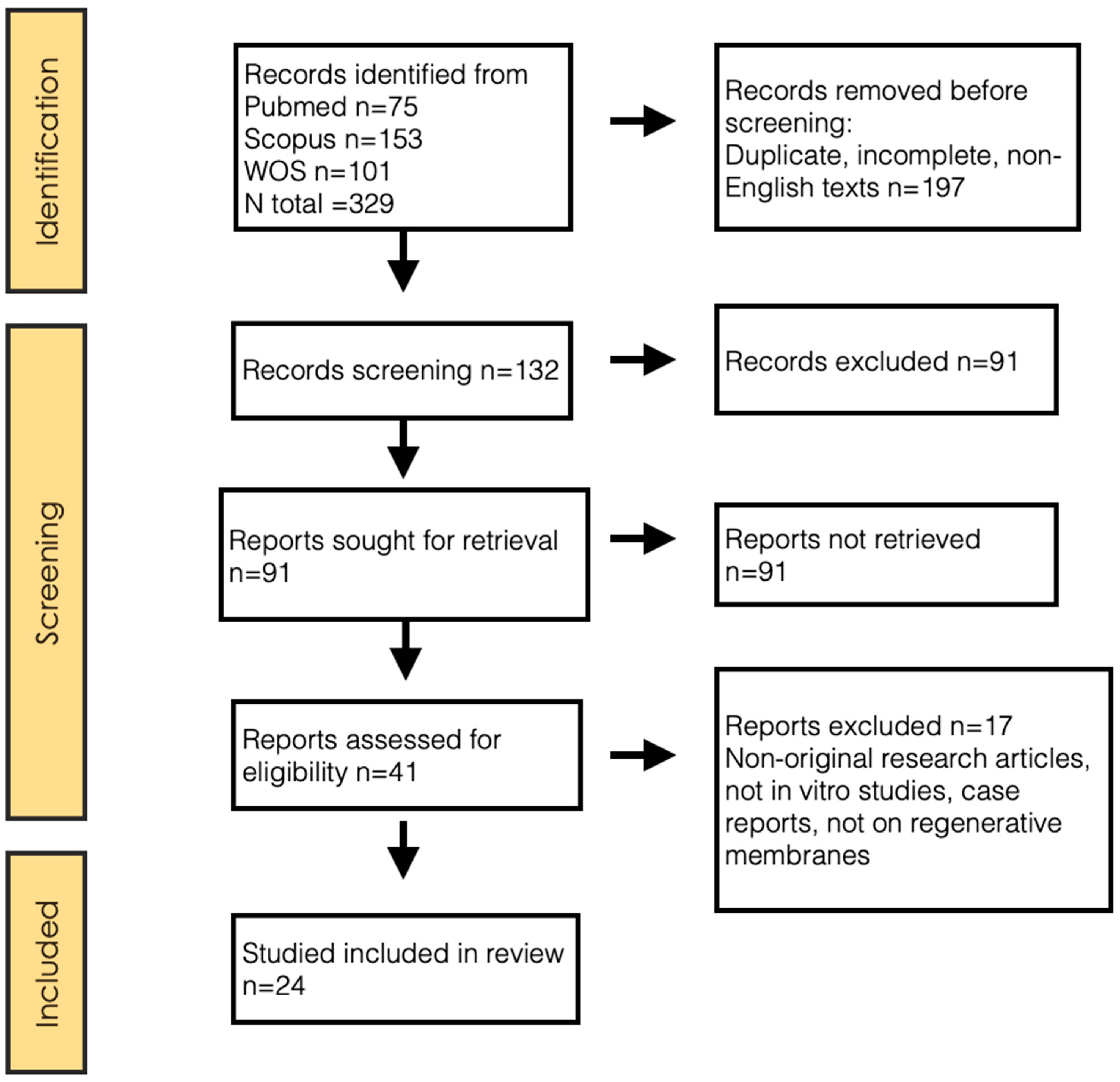
A total of 329 articles were identified through the search described above. After reading the title and/or abstract, discarding duplicates and unavailable full texts, 288 articles were excluded. Of the 41 potentially relevant articles, 17 were discarded after full-text reviews, resulting in a total of 24 articles included in this review (Figure 1). The concordance between reviewers in the inclusion process, both in the title and abstract evaluation and in the full-text evaluation, measured by the Kappa index, was 0.92.

Figure 1.
PRISMA 2020 flow diagram.
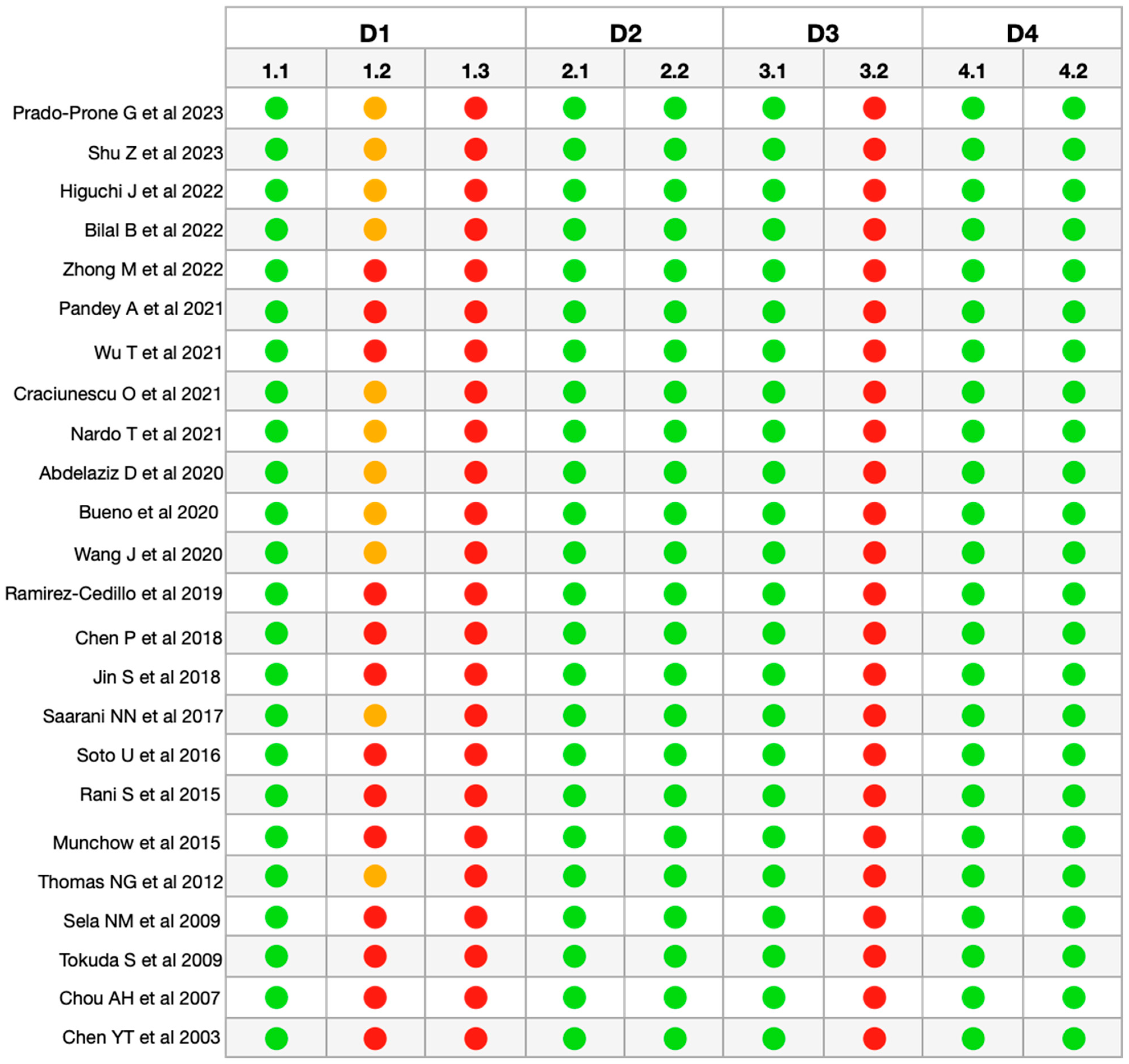
After the final selection of articles, the methodological quality and risk of bias of the articles were evaluated, and a moderate risk value was obtained for all of them (Figure 2). In all the articles analyzed, the information regarding sample randomization (1.2), sample size justification (1.3), and blinding of the test operator (3.2) was insufficiently reported or not reported/not applicable [32].

Figure 2.
RoBDDEMAT analysis for risk of
bias of the included articles [33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56]. D1—Planning
and allocation: 1.1 Control group; 1.2 Randomization of samples; 1.3
Justification of sample size. D2—Sample preparation: 2.1 Standardization of
samples and materials; 2.2 Identical experimental conditions between groups. D3—Evaluation
of results: 3.1 Appropriate and standardized test procedures and results; 3.2
Blinding of the test operator. D4—Data processing and reporting of results: 4.1
Statistical analysis; 4.2 Reporting of study results.  Sufficiently Informed/Adequate.
Sufficiently Informed/Adequate.  Insufficiently
Informed.
Insufficiently
Informed.  Not Informed/Not Applicable.
Not Informed/Not Applicable.
 Sufficiently Informed/Adequate.
Sufficiently Informed/Adequate.  Insufficiently
Informed.
Insufficiently
Informed.  Not Informed/Not Applicable.
Not Informed/Not Applicable.
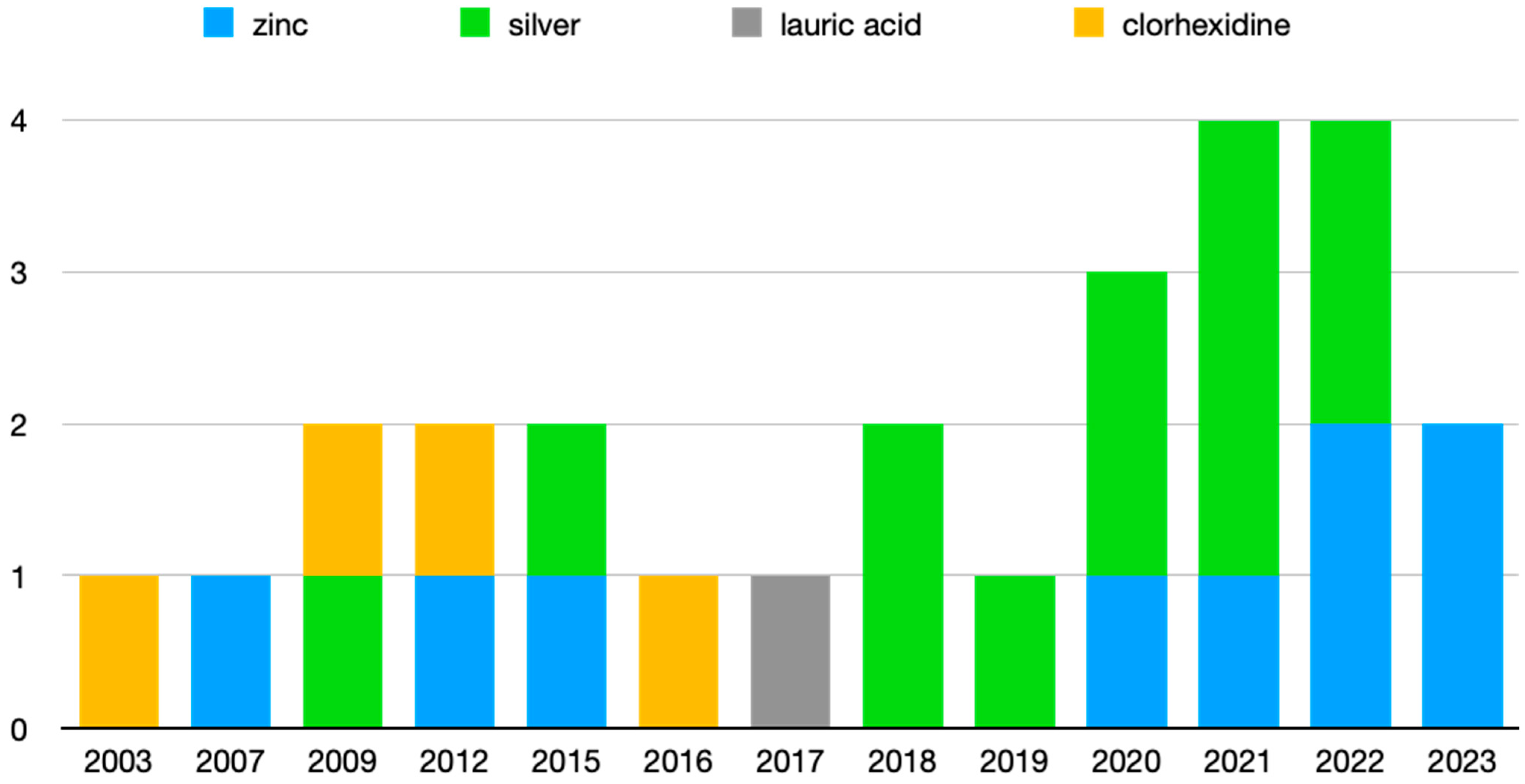
Of the 24 articles reviewed, 8 deal with the antimicrobial activity of Zn, 11 with different Ag compounds, 4 with CHX, and 1 with LA. In addition, one of them analyzes the activity of Zn and Ag together, and another one compares CHX with Ag. Figure 3 shows the articles grouped by antimicrobial agent and year of publication, where it can be seen that the most recent publications are those related to Zn and Ag.

Figure 3.
Articles grouped by publication year.
The results obtained by the different authors are presented in Table 1.

Table 1.
Study selection and data extraction.
3.1. Zinc
In recent works such as those by Prado et al. [33] and Shu et al. [34] published in the same year, inhibition of bacterial growth of between 67 and 87% is shown in membranes impregnated with ZnO, depending on the bacterial species and the concentration of the antimicrobial, in some cases reaching the non-identification of colony-forming units (CFUs) after 48 h of incubation. Along the same lines, Wu et al. [39] demonstrated an inhibition of the CFUs of Porphyromonas gingivalis (PG) and Staphylococcus aureus (SA) on chitin hydrogel membranes, being minimal after 24 h of incubation and showing greater antimicrobial activity on PG. This is corroborated by Chou et al. [55] in previous studies on Actinobacillus actinomycetemcomitans (AA) on collagen membranes. However, Bueno et al. [43] analyzed a greater number of bacterial species, in non-resorbable membranes in this case (PTFE), finding an exponential decrease in CFUs at 12–24 h, but an increase at 48–72 h, a finding that they attribute to a decrease in the release of Zn. Other studies have analyzed this antibacterial action of ZnO by measuring the zone of inhibition around the disc. Bilal et al. [36] concluded that this inhibition is greater the higher the concentration of ZnO to which they subject SA and Escherichia coli (EC), in this case, 1%, 2.5%, and 5% being the concentrations studied. Münchow et al. [51] increased these Zn concentrations, analyzing progressive concentrations of 0, 5, 15, and 30% ZnO, revealing inhibition halos of 6–15 mm after 5 days of incubation, being higher at higher concentrations for Fusobacterium nucleatum (FN), but not for PG. They also added the study of cell viability, establishing 15% ZnO as the highest acceptable concentration from this point of view. Higuchi et al. [35], in 2022, combined the antibacterial activity of ZnO and 1% Ag in the same compound, obtaining as a result inhibition zones between 2 and 8 mm, this effect being more pronounced for SA than for EC.
3.2. Silver
Authors such as Zhong et al. [37] also evaluated the antimicrobial activity of Ag by introducing a 0.5% AgNP layer in a PLGA/gelatin bilayer membrane, observing bacterial inhibition zones against SA and EC of 691.29 ± 30.44 mm2 and 974.23 ± 31.24 mm2, demonstrating an excellent broad-spectrum capacity. In addition, they showed good cell viability and osteoconductive properties at this concentration. The same bacterial species have been used by Nardo et al. [41], who have not only measured this antibacterial activity of AgNPs qualitatively by measuring the inhibition halo in disk diffusion, with results similar to the above, but also added a quantitative study by counting bacterial growth, obtaining a reduction of 92% at 4 h and 38% at 24 h for EC, and of 93% and 49% for SA at 4 h and 24 h of incubation, respectively. This reduction in bacterial inhibition capacity by the gradual release of AgNPs was explained as a function of time in non-resorbable PTFE membranes. Tokuda et al. [54] also analyzed the CFUs of SA after 48 h of incubation in PSCA membranes (polylactic acid/siloxane/calcium carbonate) with 1% Ag particles, observing the elimination of more than 99% of bacteria, and obtaining low cytotoxicity. Wang et al. [44] examined inhibition halos on PLLA membranes after immersion in a 10 mL solution of silver nitrate (AgNO3) for 1, 3, 6, 9, and 24 h, showing inhibition zones between 7 and 9.5 mm, depending on the concentration, after 1 day of incubation. Measurements on subsequent days (3, 7, and 14 days) did not result in larger inhibition zones, but they were maintained in most cases, except for the lowest Ag concentration. Bactericidal efficacy exceeded 95% against SA. In addition to SA, Jin et al. [47] determined an antibacterial activity in PG of 0.2% AgNO3 on calcium phosphate and chitosan membranes by bacterial adhesion to the membrane, which was lower than for the control, and by complete inhibition of bacterial growth after 24 h of incubation in both cases. However, Ramírez-Cedillo et al. [45] only determined a 10% decrease in SA growth due to the low AgNP concentration (<0.5%). Craciunescu et al. [40] and Chen et al. [46] also added to their studies the anti-inflammatory activity of AgNPs by measuring proinflammatory cytokines, such as IL-1β, IL-6, and TNF-α, using the ELISA technique. Thus, they determine decreases in IL-1β and TNF-α secretion of 73% and 62%, respectively, and 40% of IL-6. Abdelazzi et al. [42] measured the inhibition halos of PLA membranes impregnated with 1 and 2% AgNP against Enterococcus faecalis (EF) and EC, with measurements at 8, 16, and 32 days, finding a significant increase in the halo with increasing Ag concentration, being greater against EF and also increasing with time. There was no increase in cytotoxicity. For their part, Pandey et al. [38] obtained an inhibition of bacterial growth, specifically of PG, 3.1 and 6.7 times lower than the control when confronted with 1% silver fluoride and 38% silver diamine fluoride, respectively. However, the cytotoxicity of the latter was not acceptable as cell viability was less than 15%. Four bacterial species were studied by Rani et al. [50], comparing AgNP (0.1 mg/mL) and doxycycline (25%) on collagen membranes, showing a lower bacterial adherence to them for those impregnated with Ag, while the CFUs were lower in the doxycycline group.
3.3. Chlorhexidine
Soto Barreras et al. [49] analyzed the activity of four different concentrations of CHX (0.08, 0.04, 0.02, and 0.01%) against EF, a bacterial species with high resistance to antibiotics, demonstrating high antibacterial activity for all groups compared to the control, finding the highest effectiveness in the highest concentration, where there was no bacterial growth (CFUs). In this sense, Thomas et al. [52] not only analyzed the activity of CHX, but also compared it with the activity of Ag and tetracycline, at concentrations of 0.2%, 0.1%, and 1 mg/mL, respectively, in biodegradable polylactic acid (PLA) membranes. Thus, CHX shows the highest zone of inhibition against methicillin-resistant SA (MRSA), followed by AgNO3 and finally tetracycline. However, they also analyze their cytotoxicity, finding that tetracycline has the best compatibility, and observing a toxic cellular effect for the concentrations of both CHX and Ag used. Chen et al. [56] corroborate the high toxicity of CHX in their study performed on three different types of membranes (expanded polytetrafluoroethylene, glycolide, and collagen), determining that cell viability decreases to approximately 50% with concentrations of 0.0015% CHX. In addition, they found no differences between the different membranes studied in terms of the antimicrobial activity of CHX on AA. Previous studies have also demonstrated that doses of chlorhexidine routinely used in clinical settings produce a decrease in osteoblast proliferation and differentiation, so it should be used with caution in bone regeneration procedures and oral surgery [57].
3.4. Lauric Acid
Lauric acid is a saturated fatty acid that is commonly found in coconut oil and palm kernel oil. It has been studied for its potential antibacterial and antimicrobial properties. The antibacterial effect of lauric acid is primarily attributed to its ability to disrupt the lipid membranes of bacterial cells. This disruption can lead to the breakdown of the bacterial cell membrane and ultimately result in cell death. Lauric acid has shown antibacterial activity against a variety of Gram-positive bacteria, including some strains of Staphylococcus aureus and Streptococcus pneumoniae [31,58]. We have only found one study that measures the antibacterial efficacy of this antimicrobial in regeneration membranes, specifically PLGA and nanoapatite membranes. Saarani et al. [48] have determined that membranes with 2 and 3% LA had long-term antibacterial activity against periodontopathogenic bacteria such as PG and FN, showing zones of inhibition around the disc from 10 to 16 mm for the former, and from 8 to 13 for the latter, in addition to showing an antibacterial efficacy between 65 and 73%, depending on the concentration, through bacterial counts. These results are in agreement with other studies that demonstrate this antibacterial activity and its mechanism of action [31,58].
Drug delivery systems (DDSs) are defined as devices or formulations capable of delivering an active substance to a target tissue to increase the efficacy of the active substance [59]. Polymeric membranes, such as those included in this review, can be used as DDSs. In this way, we can increase the pharmacological activity, thereby reducing side effects, increasing the solubility of the active substance, protecting it from biodegradation, and gradually releasing it.
This review has a number of limitations. Firstly, since these are in vitro studies, the results should be corroborated in subsequent clinical trials on patients. On the other hand, these studies show great variability in the type of membrane, drug concentration, method of execution, and measurement of the results, which makes them difficult to compare with each other. Furthermore, they have been performed on a limited number of bacterial species, and studies covering a greater number of them are needed.
Future research strategies should focus on (i) the standardization of adsorption/release abilities of the different polymeric carriers; (ii) antibacterial activity assays using specific and periodontal clinically relevant biofilm models; and (iii) pre-clinical and randomized clinical trials in order to finally determine the safety and efficacy of these novel and innovative procedures, helping to eliminate the barriers limiting the extension of the experimental results to the clinical situation.
4. Conclusions
Notwithstanding the limitations of this study, we can conclude that non-antibiotic antimicrobial agents such as zinc oxide, chlorhexidine, silver nitrate, or lauric acid possess effective antibacterial activity and can be used to dope regeneration membranes to reduce the risk of bacterial colonization and, consequently, the risk of surgical site infection.
Author Contributions
Conceptualization, A.A.-J., C.V. and F.-J.M.-M.; formal analysis, A.A.-J., C.V. and F.-J.M.-M.; funding acquisition, C.V. and F.-J.M.-M.; investigation, A.A.-J., C.V. and F.-J.M.-M.; methodology, A.A.-J., C.V. and F.-J.M.-M.; supervision, C.V. and F.-J.M.-M.; validation, C.V. and F.-J.M.-M.; visualization, A.A.-J., C.V. and F.-J.M.-M.; writing—original draft, A.A.-J., C.V. and F.-J.M.-M.; writing—review and editing, A.A.-J., C.V. and F.-J.M.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Master of Oral Surgery and Implant Dentistry, University of Granada, Spain.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Glossary
| AA | Actinobacillus actinomycetemcomitans |
| Ag | silver |
| AgNO3 | silver nitrate |
| AgNP | silver nanoparticles |
| AL | lauric acid |
| ANA | non-antibiotic antimicrobial agents |
| CA | cellulose acetate |
| CHX | chlorhexidine |
| COL-CS-FN | collagen chondroitin-4-sulfate fibronectin |
| EC | Escherichia coli |
| EF | Enterococcus faecalis |
| ePTFE | expanded polytetrafluoroethylene |
| FN | Fusobacterium nucleatum |
| IL | interleukin |
| PCL | polycaprolactone |
| PDLLA | poly D-L-lactic acid |
| PG | Porphyromonas gingivalis |
| PLA | polylactic acid |
| PLGA | poly-L-lactic acid co-glycolic acid |
| PLLA | poly L-lactic acid |
| PSCA | polylactic acid/siloxane/calcium carbonate |
| PTFE | polytetrafluoroethylene |
| PVA | polyvinyl alcohol |
| GTR | guided tissue regeneration |
| ROG | guided bone regeneration |
| SA | Staphylococcus aureus |
| TNF | tumoral necrosis factor |
| CFUs | colony forming units |
| Zn | zinc |
| ZnO | zinc oxide |
References
- Retzepi, M.; Donos, N. Guided Bone Regeneration: Biological principle and therapeutic applications. Clin. Oral Implant Res. 2010, 21, 567–576. [Google Scholar] [CrossRef]
- Dimitriou, R.; Mataliotakis, G.I.; Calori, G.M.; Giannoudis, P.V. The role of barrier membranes for guided bone regeneration and restoration of large bone defects: Current experimental and clinical evidence. BMC Med. 2012, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wu, C.; Shi, H.; Luo, X.; Sun, H.; Wang, Q.; Zhang, D. Advances in Barrier Membranes for Guided Bone Regeneration Techniques. Front. Bioeng. Biotechnol. 2022, 10, 921576. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, L.; Zhou, Z.; Lai, H.; Xu, P.; Liao, L.; Wei, J. Biodegradable Polymer Membranes Applied in Guided Bone/Tissue Regeneration: A Review. Polymers 2016, 8, 115. [Google Scholar] [CrossRef]
- Omar, O.; Elgali, I.; Dahlin, C.; Thomsen, P. Barrier membranes: More than the barrier effect? J. Clin. Periodontol. 2019, 46 (Suppl. S21), 103–123. [Google Scholar] [CrossRef] [PubMed]
- Ghavimi, M.A.; Bani Shahabadi, A.; Jarolmasjed, S.; Memar, M.Y.; Maleki Dizaj, S.; Sharifi, S. Nanofibrous asymmetric collagen/curcumin membrane containing aspirin-loaded PLGA nanoparticles for guided bone regeneration. Sci. Rep. 2020, 10, 18200. [Google Scholar] [CrossRef] [PubMed]
- Zagho, M.M.; Hussein, E.A.; Elzatahry, A.A. Recent Overviews in Functional Polymer Composites for Biomedical Applications. Polymers 2018, 10, 739. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, M.; Shi, R.; Zhang, A.; Zhang, J. Advances in Electrostatic Spinning of Polymer Fibers Functionalized with Metal-Based Nanocrystals and Biomedical Applications. Molecules 2022, 27, 5548. [Google Scholar] [CrossRef] [PubMed]
- Radu, E.R.; Semenescu, A.; Voicu, S.I. Recent Advances in Stimuli-Responsive Doxorubicin Delivery Systems for Liver Cancer Therapy. Polymers 2022, 14, 5249. [Google Scholar] [CrossRef]
- Abbasnezhad, N.; Kebdani, M.; Shirinbayan, M.; Champmartin, S.; Tcharkhtchi, A.; Kouidri, S.; Bakir, F. Development of a Model Based on Physical Mechanisms for the Explanation of Drug Release: Application to Diclofenac Release from Polyurethane Films. Polymers 2021, 13, 1230. [Google Scholar] [CrossRef]
- Sasaki, J.-I.; Abe, G.L.; Li, A.; Thongthai, P.; Tsuboi, R.; Kohno, T.; Imazato, S. Barrier membranes for tissue regeneration in dentistry. Biomater. Investig. Dent. 2021, 8, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Nyman, S.; Lindhe, J.; Karring, T.; Rylander, H. New attachment following surgical treatment of human periodontal disease. J. Clin. Periodontol. 1982, 9, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, C.; Linde, A.; Gottlow, J.; Nyman, S. Healing of bone defects by guided tissue regeneration. Plast. Reconstr. Surg. 1988, 81, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Caballé-Serrano, J.; Abdeslam-Mohamed, Y.; Munar-Frau, A.; Fujioka-Kobayashi, M.; Hernández-Alfaro, F.; Miron, R. Adsorption and release kinetics of growth factors on barrier membranes for guided tissue/bone regeneration: A systematic review. Arch. Oral Biol. 2019, 100, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Hämmerle, C.H.F.; Jung, R.E. Bone augmentation by means of barrier membranes. Periodontol. 2000 2003, 33, 36–53. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kerns, D.G. Mechanisms of guided bone regeneration: A review. Open Dent. J. 2014, 8, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Cooperman, L.; Michaeli, D. The immunogenicity of injectable collagen. I. A 1-year prospective study. J. Am. Acad. Dermatol. 1984, 10, 638–646. [Google Scholar] [CrossRef]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef]
- Ul Hassan, S.; Bilal, B.; Nazir, M.S.; Naqvi, S.A.R.; Ali, Z.; Nadeem, S.; Muhammad, N.; Palvasha, B.A.; Mohyuddin, A. Recent progress in materials development and biological properties of GTR membranes for periodontal regeneration. Chem. Biol. Drug Des. 2021, 98, 1007–1024. [Google Scholar] [CrossRef]
- Cao, Y.-B.; Liu, C.; Pan, W.-L.; Tu, Y.; Li, C.-J.; Hua, C.-G. Research progress on the modification of guided bone regeneration membranes. Hua Xi Kou Qiang Yi Xue Za Zhi 2019, 37, 325–329. [Google Scholar] [CrossRef]
- Rudolf, J.-L.; Moser, C.; Sculean, A.; Eick, S. In-vitro antibiofilm activity of chlorhexidine digluconate on polylactide-based and collagen-based membranes. BMC Oral Health 2019, 19, 291. [Google Scholar] [CrossRef]
- Zucchelli, G.; Pollini, F.; Clauser, C.; De Sanctis, M. The effect of chlorhexidine mouthrinses on early bacterial colonization of guided tissue regeneration membranes. An in vivo study. J. Periodontol. 2000, 71, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Prakasam, A.; Elavarasu, S.S.; Natarajan, R.K. Antibiotics in the management of aggressive periodontitis. J. Pharm. Bioallied Sci. 2012, 4, S252–S255. [Google Scholar] [CrossRef] [PubMed]
- Chi, M.; Qi, M.; Wang, P.; Weir, M.D.; Melo, M.A.; Sun, X.; Dong, B.; Li, C.; Wu, J.; Wang, L.; et al. Novel Bioactive and Therapeutic Dental Polymeric Materials to Inhibit Periodontal Pathogens and Biofilms. Int. J. Mol. Sci. 2019, 20, 278. [Google Scholar] [CrossRef] [PubMed]
- Oteo-Iglesias, J. Active surveillance of antimicrobial resistance. Enferm. Infecc. Microbiol. Clin. Engl. Ed. 2019, 37 (Suppl. S1), 26–31. [Google Scholar] [CrossRef] [PubMed]
- Toledano, M.; Vallecillo-Rivas, M.; Osorio, M.T.; Muñoz-Soto, E.; Toledano-Osorio, M.; Vallecillo, C.; Toledano, R.; Lynch, C.D.; Serrera-Figallo, M.-A.; Osorio, R. Zn-Containing Membranes for Guided Bone Regeneration in Dentistry. Polymers 2021, 13, 1797. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhou, G.; Li, Q.; Tang, H.; Wang, S.; Li, P.; Gu, X.; Fan, Y. In vitro degradation, biocompatibility and antibacterial properties of pure zinc: Assessing the potential of Zn as a guided bone regeneration membrane. J. Mater. Chem. B 2021, 9, 5114–5127. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, Z.; Abbott, P.V. The properties and applications of chlorhexidine in endodontics. Int. Endod. J. 2009, 42, 288–302. [Google Scholar] [CrossRef]
- Olvera-Huertas, A.J.; Linares-Recatalá, M.; Herrera-Briones, F.J.; Vallecillo-Capilla, M.F.; Manzano-Moreno, F.J.; Reyes-Botella, C. Microbiological analysis of autologous bone particles obtained by low-speed drilling and treated with different decontamination agents. Int. J. Oral Maxillofac. Surg. 2021, 50, 104–108. [Google Scholar] [CrossRef]
- Dayrit, F.M. The Properties of Lauric Acid and Their Significance in Coconut Oil. J. Am. Oil Chem. Soc. 2015, 92, 1–15. [Google Scholar] [CrossRef]
- Delgado, A.H.; Sauro, S.; Lima, A.F.; Loguercio, A.D.; Della Bona, A.; Mazzoni, A.; Collares, F.M.; Staxrud, F.; Ferracane, J.; Tsoi, J.; et al. RoBDEMAT: A risk of bias tool and guideline to support reporting of pre-clinical dental materials research and assessment of systematic reviews. J. Dent. 2022, 127, 104350. [Google Scholar] [CrossRef] [PubMed]
- Prado-Prone, G.; Silva-Bermudez, P.; Rodil, S.E.; Ganjkhani, Y.; Moradi, A.-R.; Méndez, F.J.; García-Macedo, J.A.; Bazzar, M.; Almaguer-Flores, A. ZnO nanoparticles-modified polycaprolactone-gelatin membranes for guided/bone tissue regeneration, antibacterial and osteogenic differentiation properties. Biomed. Phys. Eng. Express 2023, 9, 035011. [Google Scholar] [CrossRef] [PubMed]
- Shu, Z.; Zhang, C.; Yan, L.; Lei, H.; Peng, C.; Liu, S.; Fan, L.; Chu, Y. Antibacterial and osteoconductive polycaprolactone/polylactic acid/nano-hydroxyapatite/Cu@ZIF-8 GBR membrane with asymmetric porous structure. Int. J. Biol. Macromol. 2023, 224, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, J.; Klimek, K.; Wojnarowicz, J.; Opalińska, A.; Chodara, A.; Szałaj, U.; Dąbrowska, S.; Fudala, D.; Ginalska, G. Electrospun Membrane Surface Modification by Sonocoating with HA and ZnO:Ag Nanoparticles-Characterization and Evaluation of Osteoblasts and Bacterial Cell Behavior In Vitro. Cells 2022, 11, 1582. [Google Scholar] [CrossRef] [PubMed]
- Bilal, B.; Niazi, R.; Nadeem, S.; Farid, M.A.; Nazir, M.S.; Akhter, T.; Javed, M.; Mohyuddin, A.; Rauf, A.; Ali, Z.; et al. Fabrication of Guided Tissue Regeneration Membrane Using Lignin-Mediated ZnO Nanoparticles in Biopolymer Matrix for Antimicrobial Activity. Front. Chem. 2022, 10, 837858. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Lin, J.; Yang, Y.; Liu, M.; Guo, G.; Ji, D.; Zhang, R.; Zhang, J. Bi-layered nanofibrous membrane with osteogenic and antibacterial functions for periodontal tissue regeneration. J. Biomater. Appl. 2022, 36, 1588–1598. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Yang, T.-S.; Yang, T.-I.; Belem, W.F.; Teng, N.-C.; Chen, I.-W.; Huang, C.-S.; Kareiva, A.; Yang, J.-C. An Insight into Nano Silver Fluoride-Coated Silk Fibroin Bioinspired Membrane Properties for Guided Tissue Regeneration. Polymers 2021, 13, 2659. [Google Scholar] [CrossRef]
- Wu, T.; Huang, L.; Sun, J.; Sun, J.; Yan, Q.; Duan, B.; Zhang, L.; Shi, B. Multifunctional chitin-based barrier membrane with antibacterial and osteogenic activities for the treatment of periodontal disease. Carbohydr. Polym. 2021, 269, 118276. [Google Scholar] [CrossRef]
- Craciunescu, O.; Seciu, A.-M.; Zarnescu, O. In vitro and in vivo evaluation of a biomimetic scaffold embedding silver nanoparticles for improved treatment of oral lesions. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 123, 112015. [Google Scholar] [CrossRef]
- Nardo, T.; Chiono, V.; Carmagnola, I.; Fracchia, L.; Ceresa, C.; Tabrizian, M.; Ciardelli, G. Mussel-inspired antimicrobial coating on PTFE barrier membranes for guided tissue regeneration. Biomed. Mater. 2021, 16, 035035. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, D.; Hefnawy, A.; Al-Wakeel, E.; El-Fallal, A.; El-Sherbiny, I.M. New biodegradable nanoparticles-in-nanofibers based membranes for guided periodontal tissue and bone regeneration with enhanced antibacterial activity. J. Adv. Res. 2021, 28, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Bueno, J.; Sánchez, M.C.; Toledano-Osorio, M.; Figuero, E.; Toledano, M.; Medina-Castillo, A.L.; Osorio, R.; Herrera, D.; Sanz, M. Antimicrobial effect of nanostructured membranes for guided tissue regeneration: An in vitro study. Dent. Mater. 2020, 36, 1566–1577. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhan, L.; Zhang, X.; Wu, R.; Liao, L.; Wei, J. Silver Nanoparticles Coated Poly(L-Lactide) Electrospun Membrane for Implant Associated Infections Prevention. Front. Pharmacol. 2020, 11, 431. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Cedillo, E.; Ortega-Lara, W.; Rocha-Pizaña, M.R.; Gutierrez-Uribe, J.A.; Elías-Zúñiga, A.; Rodríguez, C.A. Electrospun Polycaprolactone Fibrous Membranes Containing Ag, TiO2 and Na2Ti6O13 Particles for Potential Use in Bone Regeneration. Membranes 2019, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wu, Z.; Leung, A.; Chen, X.; Landao-Bassonga, E.; Gao, J.; Chen, L.; Zheng, M.; Yao, F.; Yang, H.; et al. Fabrication of a silver nanoparticle-coated collagen membrane with anti-bacterial and anti-inflammatory activities for guided bone regeneration. Biomed. Mater. 2018, 13, 065014. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Li, J.; Wang, J.; Jiang, J.; Zuo, Y.; Li, Y.; Yang, F. Electrospun silver ion-loaded calcium phosphate/chitosan antibacterial composite fibrous membranes for guided bone regeneration. Int. J. Nanomed. 2018, 13, 4591–4605. [Google Scholar] [CrossRef]
- Saarani, N.N.; Jamuna-Thevi, K.; Shahab, N.; Hermawan, H.; Saidin, S. Antibacterial efficacy of triple-layered poly(lactic-co-glycolic acid)/nanoapatite/lauric acid guided bone regeneration membrane on periodontal bacteria. Dent. Mater. J. 2017, 36, 260–265. [Google Scholar] [CrossRef]
- Barreras, U.S.; Méndez, F.T.; Martínez, R.E.M.; Valencia, C.S.; Rodríguez, P.R.M.; Rodríguez, J.P.L. Chitosan nanoparticles enhance the antibacterial activity of chlorhexidine in collagen membranes used for periapical guided tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 1182–1187. [Google Scholar] [CrossRef]
- Rani, S.; Chandra, R.V.; Reddy, A.A.; Reddy, B.H.; Nagarajan, S.; Naveen, A. Evaluation of the Antibacterial Effect of Silver Nanoparticles on Guided Tissue Regeneration Membrane Colonization—An in Vitro Study. J. Int. Acad. Periodontol. 2015, 17, 66–76. [Google Scholar]
- Münchow, E.A.; Albuquerque, M.T.P.; Zero, B.; Kamocki, K.; Piva, E.; Gregory, R.L.; Bottino, M.C. Development and characterization of novel ZnO-loaded electrospun membranes for periodontal regeneration. Dent. Mater. 2015, 31, 1038–1051. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.G.; Sanil, G.P.; Gopimohan, R.; Prabhakaran, J.V.; Thomas, G.; Panda, A.K. Biocompatibility and cytotoxic evaluation of drug-loaded biodegradable guided tissue regeneration membranes. J. Indian Soc. Periodontol. 2012, 16, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Sela, M.N.; Babitski, E.; Steinberg, D.; Kohavi, D.; Rosen, G. Degradation of collagen-guided tissue regeneration membranes by proteolytic enzymes of Porphyromonas gingivalis and its inhibition by antibacterial agents. Clin. Oral Implant Res. 2009, 20, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, S.; Obata, A.; Kasuga, T. Preparation of poly(lactic acid)/siloxane/calcium carbonate composite membranes with antibacterial activity. Acta Biomater. 2009, 5, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Chou, A.H.K.; LeGeros, R.Z.; Chen, Z.; Li, Y. Antibacterial effect of zinc phosphate mineralized guided bone regeneration membranes. Implant Dent. 2007, 16, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-T.; Hung, S.-L.; Lin, L.-W.; Chi, L.-Y.; Ling, L.-J. Attachment of periodontal ligament cells to chlorhexidine-loaded guided tissue regeneration membranes. J. Periodontol. 2003, 74, 1652–1659. [Google Scholar] [CrossRef] [PubMed]
- Olvera-Huertas, A.J.; Costela-Ruiz, V.J.; García-Recio, E.; Melguizo-Rodríguez, L.; Illescas-Montes, R.; Reyes-Botella, C.; Manzano-Moreno, F.J. The Effect of Chlorhexidine, Amoxicillin, and Clindamycin on the Growth and Differentiation of Primary Human Osteoblasts. Int. J. Oral Maxillofac. Implants 2022, 37, 283–288. [Google Scholar] [CrossRef]
- Jin, X.; Zhou, J.; Richey, G.; Wang, M.; Hong, S.M.C.; Hong, S.H. Undecanoic Acid, Lauric Acid, and N-Tridecanoic Acid Inhibit Escherichia coli Persistence and Biofilm Formation. J. Microbiol. Biotechnol. 2021, 31, 130–136. [Google Scholar] [CrossRef]
- Xie, Y.; Chu, Z.; Jin, W. Beyond separation: Membranes towards medicine. J. Membr. Sci. Lett. 2022, 2, 100020. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).