Development and Evaluation of an Innovative Approach Using Niosomes Based Polymeric Microneedles to Deliver Dual Antioxidant Drugs
Abstract
1. Introduction
2. Materials and Methods
2.1. Methods
Preparation of Niosomes
2.2. Characterization of Niosomal Nanoparticles
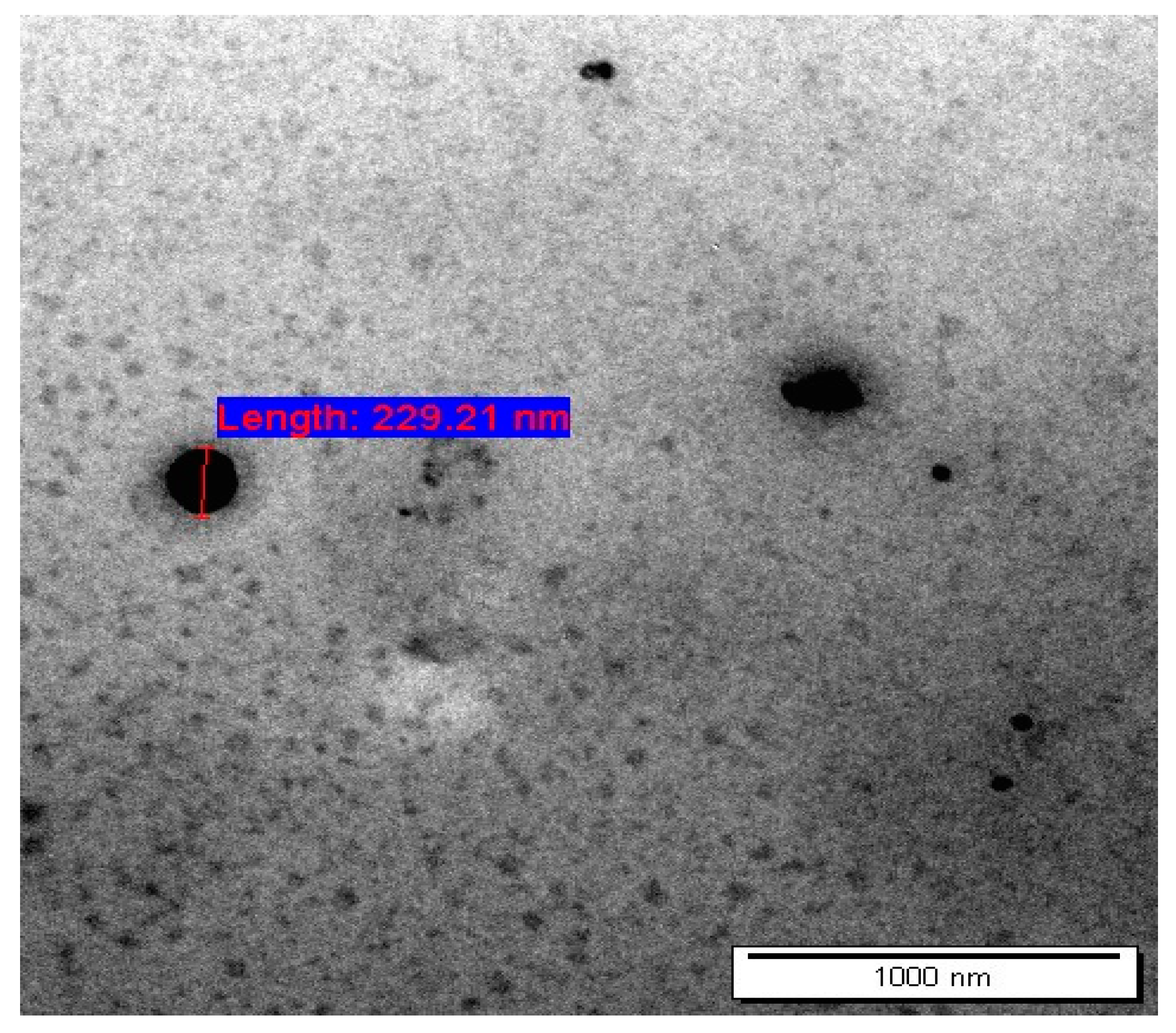
2.2.1. Transmission Electron Microscope (TEM)
2.2.2. Particle Size (PS) and Zeta Potential (ZP)
2.2.3. Drug Entrapment Efficiency
2.3. Attenuated Total Reflectance—Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.4. Stability Studies
2.5. In Vitro Drug Release Study
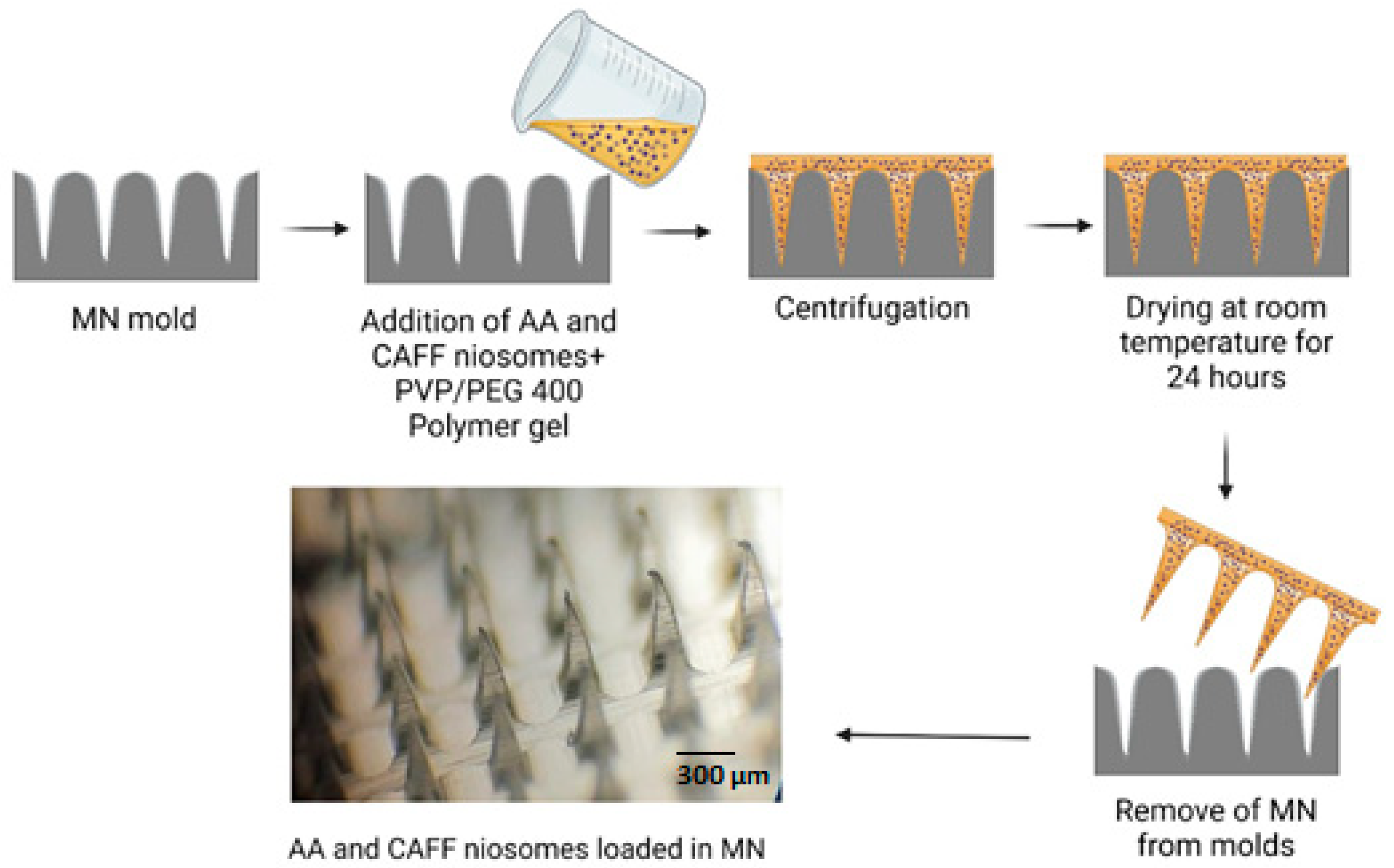
2.6. Fabrication of Dissolving MN
2.7. Characterization of Dissolving MNs
2.7.1. MNs Dissolution Rate
2.7.2. Evaluation of Skin Insertion Ability of DMN
2.7.3. Determination of AA and CAFF Recovery from DMN
2.7.4. Mechanical Characterization
2.8. Short Term Stability Study
2.9. Drug Permeation Study
2.10. In Vitro Skin Deposition Analysis
2.11. Kinetic of Drug Release
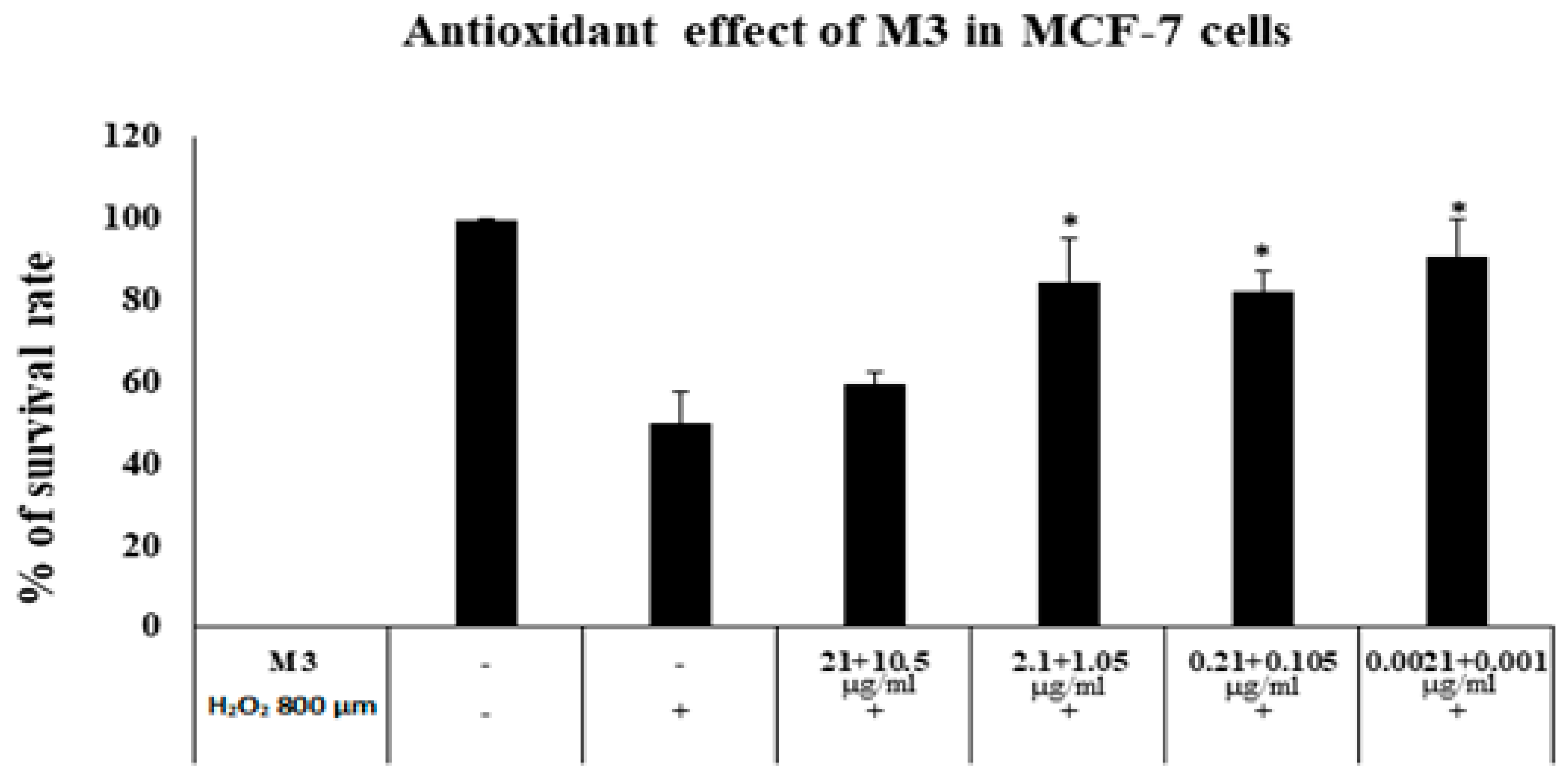
2.12. Antioxidant Activity against H2O2 Induced Cytotoxicity
2.13. Cytotoxicity Assay
2.14. Analysis of the AA and CAFF
2.15. Statistical Analysis
3. Results and Discussion
3.1. Characterization of AA and CAFF Loaded Niosomes
3.2. Attenuated Total Reflectance—Fourier Transform Infrared (ATR—FTIR) Analysis
3.3. Stability Studies
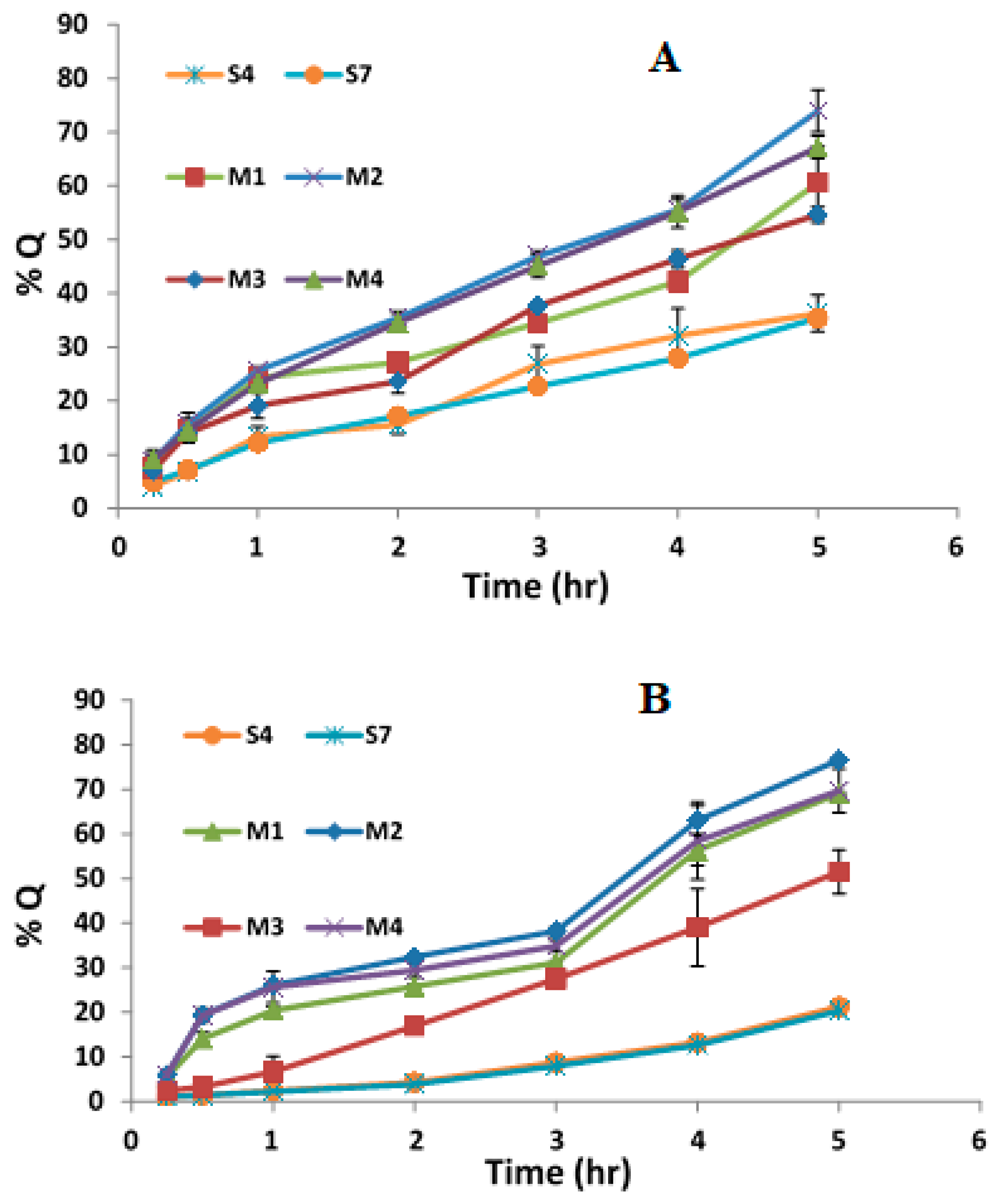
3.4. In Vitro Release Study
3.5. Fabrication of DMN
3.6. Mechanical Characterization of DMN
3.7. DMNs Dissolution in Skin
3.8. Microneedles Insertion Studies
3.9. Drug Content and Content Uniformity
3.10. Stability Study
3.11. Ex Vivo Permeation Study
3.12. Skin Deposition
3.13. Drug Release Kinetics
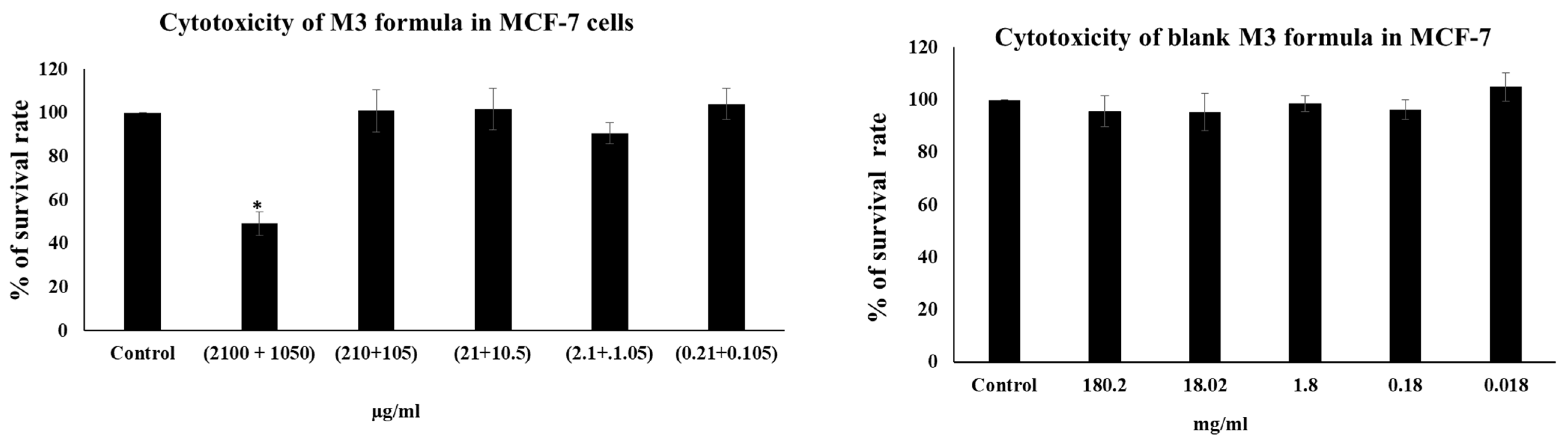
3.14. MTT Cytotoxicity Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alkilani, A.Z.; McCrudden, M.T.; Donnelly, R.F. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the stratum corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Alkilani, A.Z.; Nasereddin, J.; Hamed, R.; Nimrawi, S.; Hussein, G.; Abo-Zour, H.; Donnelly, R.F. Beneath the Skin: A Review of Current Trends and Future Prospects of Transdermal Drug Delivery Systems. Pharmaceutics 2022, 14, 1152. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.Y.; Kwon, M.; Choi, H.E.; Kim, K.S. Recent advances in transdermal drug delivery systems: A review. Biomater. Res. 2021, 25, 24. [Google Scholar] [CrossRef] [PubMed]
- Edis, Z.; Wang, J.; Waqas, M.K.; Ijaz, M.; Ijaz, M. Nanocarriers-Mediated Drug Delivery Systems for Anticancer Agents: An Overview and Perspectives. Int. J. Nanomed. 2021, 16, 1313–1330. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Durga, B.; Veera, L. Recent advances of non-ionic surfactant-based nano-vesicles (niosomes and proniosomes): A brief review of these in enhancing transdermal delivery of drug. Futur. J. Pharm. Sci. 2020, 6, 100. [Google Scholar]
- Ita, K. Transdermal Delivery of Drugs with Microneedles—Potential and Challenges. Pharmaceutics 2015, 7, 90–105. [Google Scholar] [CrossRef]
- Alkilani, A.Z.; Abu-Zour, H.; Alshishani, A.; Abu-Huwaij, R.; Basheer, H.A.; Abo-Zour, H. Formulation and Evaluation of Niosomal Alendronate Sodium Encapsulated in Polymeric Microneedles: In Vitro Studies, Stability Study and Cytotoxicity Study. Nanomaterials 2022, 12, 3570. [Google Scholar] [CrossRef]
- Alkilani, A.Z.; Hamed, R.; Abdo, H.; Swellmeen, L.; Basheer, H.A.; Wahdan, W.; Abu Kwiak, A.D. Formulation and Evaluation of Azithromycin-Loaded Niosomal Gel: Optimization, In Vitro Studies, Rheological Characterization, and Cytotoxicity Study. ACS Omega 2022, 7, 39782–39793. [Google Scholar] [CrossRef]
- Bnyan, R.; Khan, I.; Ehtezazi, T.; Saleem, I.; Gordon, S.; Neill, F.O.; Roberts, M. Surfactant Effects on Lipid-Based Vesicles Properties. J. Pharm. Sci. 2018, 107, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- ZZhou, X.; Hao, Y.; Yuan, L.; Pradhan, S.; Shrestha, K.; Pradhan, O.; Liu, H.; Li, W. Nano-formulations for transdermal drug delivery: A review. Chin. Chem. Lett. 2018, 29, 1713–1724. [Google Scholar] [CrossRef]
- Kaul, S.; Gulati, N.; Verma, D.; Mukherjee, S.; Nagaich, U. Role of Nanotechnology in Cosmeceuticals: A Review of Recent Advances. J. Pharm. 2018, 2018, 3420204. [Google Scholar] [CrossRef] [PubMed]
- Dugam, S.; Tade, R.; Dhole, R.; Nangare, S. Emerging era of microneedle array for pharmaceutical and biomedical applications: Recent advances and toxicological perspectives. Futur. J. Pharm. Sci. 2021, 7, 19. [Google Scholar] [CrossRef]
- Nagarkar, R.; Singh, M.; Nguyen, H.X.; Jonnalagadda, S. A review of recent advances in microneedle technology for transdermal drug delivery. J. Drug Deliv. Sci. Technol. 2020, 59, 101923. [Google Scholar] [CrossRef]
- Larrañeta, E.; Lutton, R.E.M.; Woolfson, A.D.; Donnelly, R.F. Microneedle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commercial development. Mater. Sci. Eng. R Rep. 2016, 104, 1–32. [Google Scholar] [CrossRef]
- McAlister, E.; Kirkby, M.; Domínguez-Robles, J.; Paredes, A.J.; Anjani, Q.K.; Moffatt, K.; Vora, L.K.; Hutton, A.R.; McKenna, P.E.; Larrañeta, E.; et al. The role of microneedle arrays in drug delivery and patient monitoring to prevent diabetes induced fibrosis. Adv. Drug Deliv. Rev. 2021, 175, 113825. [Google Scholar] [CrossRef] [PubMed]
- Franco, P.; De Marco, I. The Use of Poly(N-vinyl pyrrolidone) in the Delivery of Drugs: A Review. Polymers 2020, 12, 1114. [Google Scholar] [CrossRef]
- D’souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in Drug Delivery: Pros and Cons as Well as Potential Alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef]
- Yang, S.-J.; Jeong, J.-O.; Lim, Y.-M.; Park, J.-S. Synthesis and characterization of PVP microneedle patch using metal bioelectrodes for novel drug delivery system. Mater. Des. 2021, 201, 109485. [Google Scholar] [CrossRef]
- Homayouni, A.; Sadeghi, F.; Nokhodchi, A.; Varshosaz, J.; Garekani, H.A. Preparation and characterization of celecoxib solid dispersions; comparison of poloxamer-188 and PVP-K30 as carriers. Iran. J. Basic Med. Sci. 2014, 17, 322–331. [Google Scholar] [PubMed]
- Cordani, M.; Donadelli, M.; Strippoli, R.; Bazhin, A.V.; Sánchez-Álvarez, M. Interplay between ROS and Autophagy in Cancer and Aging: From Molecular Mechanisms to Novel Therapeutic Approaches. Oxidative Med. Cell. Longev. 2019, 2019, 8794612. [Google Scholar] [CrossRef]
- Kruk, J.; Duchnik, E. Oxidative Stress and Skin Diseases: Possible Role of Physical Activity. Asian Pac. J. Cancer Prev. 2014, 15, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Pandel, R.; Poljšak, B.; Godic, A.; Dahmane, R. Skin Photoaging and the Role of Antioxidants in Its Prevention. ISRN Dermatol. 2013, 2013, 930164. [Google Scholar] [CrossRef]
- Burke, K.E. Mechanisms of aging and development—A new understanding of environmental damage to the skin and prevention with topical antioxidants. Mech. Ageing Dev. 2018, 172, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Rhie, G.-E.; Shin, M.H.; Seo, J.Y.; Choi, W.W.; Cho, K.H.; Kim, K.H.; Park, K.C.; Eun, H.C.; Chung, J.H. Aging- and Photoaging-Dependent Changes of Enzymic and Nonenzymic Antioxidants in the Epidermis and Dermis of Human Skin In Vivo. J. Investig. Dermatol. 2001, 117, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- McArdle, F.; Rhodes, L.; Parslew, R.; Jack, C.; Friedmann, P.; Jackson, M. UVR-induced oxidative stress in human skin in vivo: Effects of oral vitamin C supplementation. Free Radic. Biol. Med. 2002, 33, 1355–1362. [Google Scholar] [CrossRef]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The Roles of Vitamin C in Skin Health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef]
- Rodrigues, F.; Alves, A.C.; Nunes, C.; Sarmento, B.; Amaral, M.H.; Reis, S.; Oliveira, M.B.P. Permeation of topically applied caffeine from a food by—Product in cosmetic formulations: Is nanoscale in vitro approach an option? Int. J. Pharm. 2016, 513, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.; Herman, A.P. Caffeine’s Mechanisms of Action and Its Cosmetic Use. Ski. Pharmacol. Physiol. 2013, 26, 8–14. [Google Scholar] [CrossRef]
- Dewi, S.R.; Cahyono, A.; Anggana Rafika, P.; Mawardi, P. The Comparison of the Effects of Caffeine Topical 0.25% and 0.5% as Anti-wrinkle Therapy. In Proceedings of the 23rd Regional Conference of Dermatology; SciTePress: Setúbal, Portugal, 2021; pp. 113–117. [Google Scholar] [CrossRef]
- Dargah, M.S.; Hadjizadeh, A. Improvement of ascorbic acid delivery into human skin via hyaluronic acid-coated niosomes. J. Microencapsul. 2022, 39, 552–562. [Google Scholar] [CrossRef]
- Khazaeli, P.; Pardakhty, A.; Shoorabi, H. Caffeine-Loaded Niosomes: Characterization and in Vitro Release Studies. Drug Deliv. 2007, 14, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Dangol, M.; Kim, S.; Li, C.G.; Lahiji, S.F.; Jang, M.; Ma, Y.; Huh, I.; Jung, H. Anti-obesity effect of a novel caffeine-loaded dissolving microneedle patch in high-fat diet-induced obese C57BL/6J mice. J. Control. Release 2017, 265, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Sawutdeechaikul, P.; Kanokrungsee, S.; Sahaspot, T.; Thadvibun, K.; Banlunara, W.; Limcharoen, B.; Sansureerungsikul, T.; Rutwaree, T.; Oungeun, M.; Wanichwecharungruang, S. Detachable dissolvable microneedles: Intra-epidermal and intradermal diffusion, effect on skin surface, and application in hyperpigmentation treatment. Sci. Rep. 2021, 11, 24114. [Google Scholar] [CrossRef]
- Alhawarat, F.M.; Hammad, H.M.; Hijjawi, M.S.; Sharab, A.S.; Abuarqoub, D.A.; Al Shhab, M.A.; Zihlif, M.A. The effect of cycling hypoxia on MCF-7 cancer stem cells and the impact of their microenvironment on angiogenesis using human umbilical vein endothelial cells (HUVECs) as a model. PeerJ 2019, 7, e5990. [Google Scholar] [CrossRef] [PubMed]
- Nasereddin, J.; Shakib, M. Ira: A free and open-source Fourier transform infrared (FTIR) data analysis widget for pharmaceutical applications. Anal. Lett. 2023, 1–12. [Google Scholar] [CrossRef]
- Abdelghany, S.; Tekko, I.A.; Vora, L.; Larrañeta, E.; Permana, A.D.; Donnelly, R.F. Nanosuspension-Based Dissolving Microneedle Arrays for Intradermal Delivery of Curcumin. Pharmaceutics 2019, 11, 308. [Google Scholar] [CrossRef] [PubMed]
- Larrañeta, E.; Moore, J.; Vicente-Pérez, E.M.; González-Vázquez, P.; Lutton, R.; Woolfson, A.D.; Donnelly, R.F. A proposed model membrane and test method for microneedle insertion studies. Int. J. Pharm. 2014, 472, 65–73. [Google Scholar] [CrossRef]
- Vora, L.; Vavia, P.R.; Larrañeta, E.; Bell, S.E.; Donnelly, R.F. Novel nanosuspension-based dissolving microneedle arrays for transdermal delivery of a hydrophobic drug. J. Interdiscip. Nanomed. 2018, 3, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Lin, H.; Wang, Z.; Yang, X.; Zhang, M.; Liu, X.; Wang, B.; Wu, Z.; Chen, D. Preparation and characterization of dissolving hyaluronic acid composite microneedles loaded micelles for delivery of curcumin. Drug Deliv. Transl. Res. 2020, 10, 1520–1530. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Kao, W.J. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin. Drug Deliv. 2010, 7, 429–444. [Google Scholar] [CrossRef]
- Alkilani, A.Z.; Musleh, B.; Hamed, R.; Swellmeen, L.; Basheer, H.A. Preparation and Characterization of Patch Loaded with Clarithromycin Nanovesicles for Transdermal Drug Delivery. J. Funct. Biomater. 2023, 14, 57. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, P.; Tripathi, P.; Gupta, R.; Pandey, S. Niosomes: A review on niosomal research in the last decade. J. Drug Deliv. Sci. Technol. 2020, 56, 101581. [Google Scholar] [CrossRef]
- Muzzalupo, R.; Tavano, L. Niosomal drug delivery for transdermal targeting: Recent advances. Res. Rep. Transdermal Drug Deliv. 2015, 4, 23–33. [Google Scholar] [CrossRef]
- Mansouri, M.; Khayam, N.; Jamshidifar, E.; Pourseif, T.; Kianian, S.; Mirzaie, A.; Akbarzadeh, I.; Ren, Q. Streptomycin Sulfate–Loaded Niosomes Enables Increased Antimicrobial and Anti-Biofilm Activities. Front. Bioeng. Biotechnol. 2021, 9, 745099. [Google Scholar] [CrossRef]
- Miatmoko, A.; Safitri, S.; Aquila, F.; Cahyani, D.; Hariawan, B.; Hendrianto, E.; Hendradi, E.; Sari, R. Characterization and distribution of niosomes containing ursolic acid coated with chitosan layer. Res. Pharm. Sci. 2021, 16, 660. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Goodyear, B.; Haq, A.; Puri, V.; Michniak-Kohn, B. Evaluations of Quality by Design (QbD) Elements Impact for Developing Niosomes as a Promising Topical Drug Delivery Platform. Pharmaceutics 2020, 12, 246. [Google Scholar] [CrossRef] [PubMed]
- Nowroozi, F.; Almasi, A.; Javidi, J.; Haeri, A.; Dadashzadeh, S. Effect of Surfactant Type, Cholesterol Content and Various Downsizing Methods on the Particle Size of Niosomes. Iran. J. Pharm. Res. IJPR 2018, 17 (Suppl. 2), 1–11. [Google Scholar]
- Sezgin-Bayindir, Z.; Antep, M.N.; Yuksel, N. Development and Characterization of Mixed Niosomes for Oral Delivery Using Candesartan Cilexetil as a Model Poorly Water-Soluble Drug. AAPS PharmSciTech 2015, 16, 108–117. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Jing, Q.; Hu, H.; He, Z.; Wu, T.; Guo, T.; Feng, N. Sodium dodecyl sulfate improved stability and transdermal delivery of salidroside-encapsulated niosomes via effects on zeta potential. Int. J. Pharm. 2020, 580, 119183. [Google Scholar] [CrossRef] [PubMed]
- Radhi, A.A. Benazepril hydrochloride loaded niosomal formulation for oral delivery: Formulation and characterization. Int. J. Appl. Pharm. 2018, 10, 66–70. [Google Scholar] [CrossRef]
- Aboubakr, E.M.; Mohammed, H.A.; Hassan, A.S.; Mohamed, H.B.; El Dosoky, M.I.; Ahmad, A.M. Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione. Nanotechnol. Rev. 2022, 11, 117–137. [Google Scholar] [CrossRef]
- Xiang, H.; Xu, S.; Li, J.; Pan, S.; Miao, X. Particle Size Effect of Curcumin Nanocrystals on Transdermal and Transfollicular Penetration by Hyaluronic Acid-Dissolving Microneedle Delivery. Pharmaceuticals 2022, 15, 206. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Martini, N.; Wu, Z.; Chen, S.; Falconer, J.R.; Locke, M.; Zhang, Z.; Wen, J. Niosomal Nanocarriers for Enhanced Dermal Delivery of Epigallocatechin Gallate for Protection against Oxidative Stress of the Skin. Pharmaceutics 2022, 14, 726. [Google Scholar] [CrossRef]
- Elhabak, M.; Ibrahim, S.; Abouelatta, S.M. Topical delivery of l-ascorbic acid spanlastics for stability enhancement and treatment of UVB induced damaged skin. Drug. Deliv. 2021, 28, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Butt, S.; Hasan, S.M.F.; Hassan, M.M.; Alkharfy, K.M.; Neau, S.H. Directly compressed rosuvastatin calcium tablets that offer hydrotropic and micellar solubilization for improved dissolution rate and extent of drug release. Saudi Pharm. J. 2019, 27, 619–628. [Google Scholar] [CrossRef]
- Kura, A.U.; Hussein-Al-Ali, S.H.; Hussein, M.Z.; Fakurazi, S. Preparation of Tween 80-Zn/Al-Levodopa-Layered Double Hydroxides Nanocomposite for Drug Delivery System. Sci. World J. 2014, 2014, 104246. [Google Scholar] [CrossRef]
- Anbarasan, B.; Rekha, S.; Elango, K.; Shriya, B.; Ramaprabhu, S. Optimization of the formulation and in-vitro evaluation of capecitabine niosomes for the treatment of colon cancer. Int. J. Pharm. Sci. Res. 2013, 4, 1504. [Google Scholar]
- Farmoudeh, A.; Akbari, J.; Saeedi, M.; Ghasemi, M.; Asemi, N.; Nokhodchi, A. Methylene blue-loaded niosome: Preparation, physicochemical characterization, and in vivo wound healing assessment. Drug Deliv. Transl. Res. 2020, 10, 1428–1441. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.H.; Bashir, S.; Figueiredo, P.; Santos, H.A.; Khan, M.I.; Peltonen, L. Process optimization of ecological probe sonication technique for production of rifampicin loaded niosomes. J. Drug Deliv. Sci. Technol. 2019, 50, 27–33. [Google Scholar] [CrossRef]
- Pardakhty, A.; Varshosaz, J.; Rouholamini, A. In vitro study of polyoxyethylene alkyl ether niosomes for delivery of insulin. Int. J. Pharm. 2007, 328, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Varshosaz, J.; Taymouri, S.; Pardakhty, A.; Asadi-Shekaari, M.; Babaee, A. Niosomes of Ascorbic Acid and α-Tocopherol in the Cerebral Ischemia-Reperfusion Model in Male Rats. BioMed Res. Int. 2014, 2014, 816103. [Google Scholar] [CrossRef] [PubMed]
- Kurakula, M.; Rao, G.K. Pharmaceutical assessment of polyvinylpyrrolidone (PVP): As excipient from conventional to controlled delivery systems with a spotlight on COVID-19 inhibition. J. Drug Deliv. Sci. Technol. 2020, 60, 102046. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-C.; Wu, Y.-C.; Tsai, S.-W.; Chen, C.-H.; Wu, M.-H. Fabrication of two-layer dissolving polyvinylpyrrolidone microneedles with different molecular weights for in vivo insulin transdermal delivery. RSC Adv. 2017, 7, 5067–5075. [Google Scholar] [CrossRef]
- Sun, W.; Araci, Z.; Inayathullah, M.; Manickam, S.; Zhang, X.; Bruce, M.A.; Marinkovich, M.P.; Lane, A.T.; Milla, C.; Rajadas, J.; et al. Polyvinylpyrrolidone microneedles enable delivery of intact proteins for diagnostic and therapeutic applications. Acta Biomater. 2013, 9, 7767–7774. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, D.; Xuan, X.; He, H. Advances of Microneedles in Biomedical Applications. Molecules 2021, 26, 5912. [Google Scholar] [CrossRef] [PubMed]
- Tekko, I.; Permana, A.D.; Vora, L.; Hatahet, T.; McCarthy, H.; Donnelly, R.F. Localised and sustained intradermal delivery of methotrexate using nanocrystal-loaded microneedle arrays: Potential for enhanced treatment of psoriasis. Eur. J. Pharm. Sci. 2020, 152, 105469. [Google Scholar] [CrossRef]
- Permana, A.D.; Paredes, A.J.; Volpe-Zanutto, F.; Anjani, Q.K.; Utomo, E.; Donnelly, R.F. Dissolving microneedle-mediated dermal delivery of itraconazole nanocrystals for improved treatment of cutaneous candidiasis. Eur. J. Pharm. Biopharm. 2020, 154, 50–61. [Google Scholar] [CrossRef]
- Rojekar, S.; Vora, L.K.; Tekko, I.A.; Volpe-Zanutto, F.; McCarthy, H.O.; Vavia, P.R.; Donnelly, R.F. Etravirine-loaded dissolving microneedle arrays for long-acting delivery. Eur. J. Pharm. Biopharm. 2021, 165, 41–51. [Google Scholar] [CrossRef]
- Shim, W.S.; Hwang, Y.M.; Park, S.G.; Lee, C.K.; Kang, N.G. Role of Polyvinylpyrrolidone in Dissolving Microneedle for Efficient Transdermal Drug Delivery: In vitro and Clinical Studies. Bull. Korean Chem. Soc. 2018, 39, 789–793. [Google Scholar] [CrossRef]
- Hanif, M.; Ameer, N.; Ahmad, Q.-U.; Aziz, M.; Mahmood, K.; Ramzan, N.; Rahman, H.M.A. Improved solubility and corneal permeation of PEGylated curcumin complex used for the treatment of ophthalmic bacterial infections. PLoS ONE 2022, 17, e0258355. [Google Scholar] [CrossRef]
- Ma, B.-L.; Yang, Y.; Dai, Y.; Li, Q.; Lin, G.; Ma, Y.-M. Polyethylene glycol 400 (PEG400) affects the systemic exposure of oral drugs based on multiple mechanisms: Taking berberine as an example. RSC Adv. 2017, 7, 2435–2442. [Google Scholar] [CrossRef]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, Z.; Zheng, X.; Li, L.; Qi, J.; Wu, W.; Lu, Y. Design and Evaluation of Dissolving Microneedles for Enhanced Dermal Delivery of Propranolol Hydrochloride. Pharmaceutics 2021, 13, 579. [Google Scholar] [CrossRef] [PubMed]
- Permana, A.D.; McCrudden, M.T.C.; Donnelly, R.F. Enhanced Intradermal Delivery of Nanosuspensions of Antifilariasis Drugs Using Dissolving Microneedles: A Proof of Concept Study. Pharmaceutics 2019, 11, 346. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, J.; Shayan, F.L.; Kim, S.; Huh, I.; Ma, Y.; Yang, H.; Kang, G.; Jung, H. Physicochemical study of ascorbic acid 2-glucoside loaded hyaluronic acid dissolving microneedles irradiated by electron beam and gamma ray. Carbohydr. Polym. 2018, 180, 297–303. [Google Scholar] [CrossRef]
- Chandran, R.; Mohd Tohit, E.R.; Stanslas, J.; Salim, N.; Tuan Mahmood, T.M. Investigation and Optimization of Hydrogel Microneedles for Transdermal Delivery of Caffeine. Tissue Eng. Part C Methods 2022, 28, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Lisik, A.; Musiał, W. Conductomeric Evaluation of the Release Kinetics of Active Substances from Pharmaceutical Preparations Containing Iron Ions. Materials 2019, 12, 730. [Google Scholar] [CrossRef]
- Shaghlil, L.; Alshishani, A.; Abu Sa’Aleek, A.; Abdelkader, H.; Al-Ebini, Y. Formulation and evaluation of nasal insert for nose-to-brain drug delivery of rivastigmine tartrate. J. Drug Deliv. Sci. Technol. 2022, 76, 103736. [Google Scholar] [CrossRef]
- Hamed, R.; Aburezeq, A.; Tarawneh, O. Development of hydrogels, oleogels, and bigels as local drug delivery systems for periodontitis. Drug Dev. Ind. Pharm. 2018, 44, 1488–1497. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Rogoff, H.A. Implications of reactive oxygen species on cancer formation and its treatment. Semin. Oncol. 2021, 48, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Caritá, A.C.; Fonseca-Santos, B.; Shultz, J.D.; Michniak-Kohn, B.; Chorilli, M.; Leonardi, G.R. Vitamin C: One compound, several uses. Advances for delivery, efficiency and stability. Nanomedicine 2020, 24, 102117. [Google Scholar] [PubMed]
- Ősz, B.-E.; Jîtcă, G.; Ștefănescu, R.-E.; Pușcaș, A.; Tero-Vescan, A.; Vari, C.-E. Caffeine and Its Antioxidant Properties—It Is All about Dose and Source. Int. J. Mol. Sci. 2022, 23, 13074. [Google Scholar] [CrossRef]
- Böttger, F.; Vallés-Martí, A.; Cahn, L.; Jimenez, C.R. High-dose intravenous vitamin C, a promising multi-targeting agent in the treatment of cancer. J. Exp. Clin. Cancer Res. 2021, 40, 343. [Google Scholar] [CrossRef]
- Lee, S.J.; Jeong, J.-H.; Lee, I.H.; Lee, J.; Jung, J.H.; Park, H.Y.; Lee, D.H.; Chae, Y.S. Effect of High-dose Vitamin C Combined With Anti-cancer Treatment on Breast Cancer Cells. Anticancer Res. 2019, 39, 751–758. [Google Scholar] [CrossRef]
- Kazaks, A.; Collier, M.; Conley, M. Cytotoxicity of Caffeine on MCF-7 Cells Measured by XTT Cell Proliferation Assay (P06-038-19). Curr. Dev. Nutr. 2019, 3 (Suppl. 1), 3013217. [Google Scholar] [CrossRef]
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B.T. Two Faces of Vitamin C—Antioxidative and Pro-Oxidative Agent. Nutrients 2020, 12, 1501. [Google Scholar] [CrossRef] [PubMed]
- Gaascht, F.; Dicato, M.; Diederich, M. Coffee provides a natural multitarget pharmacopeia against the hallmarks of cancer. Genes Nutr. 2015, 10, 51. [Google Scholar] [CrossRef]
- Ngo, B.; Van Riper, J.M.; Cantley, L.C.; Yun, J. Targeting cancer vulnerabilities with high-dose vitamin C. Nat. Rev. Cancer 2019, 19, 271–282. [Google Scholar] [CrossRef] [PubMed]




| Code | Span 60 | Tween 60 | Tween 80 | Chol | DCP | CAFF | AA | HLB Value |
|---|---|---|---|---|---|---|---|---|
| (mg) | (mg) | (mg) | (mg) | (mg) | (mg) | (mg) | ||
| S1 | 150 | - | - | 150 | 2 | 50 | 100 | 4.7 |
| S2 | 300 | - | - | 150 | 2 | 50 | 100 | 4.7 |
| S3 | 150 | - | - | 75 | 2 | 50 | 100 | 4.7 |
| S4 | 125 | 25 | - | 150 | 2 | 50 | 100 | 6.4 |
| S5 | 250 | 50 | - | 150 | 2 | 50 | 100 | 6.4 |
| S6 | 125 | 25 | - | 75 | 2 | 50 | 100 | 6.4 |
| S7 | 120 | - | 30 | 150 | 2 | 50 | 100 | 6.8 |
| S8 | 240 | - | 60 | 150 | 2 | 50 | 100 | 6.8 |
| S9 | 120 | - | 30 | 75 | 2 | 50 | 100 | 6.8 |
| Code | PVP | PEG 400 | AA and CAFF Niosomes |
|---|---|---|---|
| M1 | 95 | 5 | 0.3 mL of S4 niosomes |
| M2 | 95 | 5 | 0.3 mL of S7 niosomes |
| M3 | 93 | 7 | 0.3 mL of S4 niosomes |
| M4 | 93 | 7 | 0.3 mL of S7 niosomes |
| Code | PS (nm) | PDI | ZP (mV) | EE (%) | |
|---|---|---|---|---|---|
| CAFF | AA | ||||
| S1 | 150.70 ± 10.10 | 0.005 ± 0.00 | −27.53 ± 0.54 | 35.83 ± 1.20 | 39.68 ± 1.30 |
| S2 | 170.00 ± 3.70 | 0.031 ± 0.00 | −25.40 ± 0.52 | 15.16 ± 0.99 | 10.88 ± 1.51 |
| S3 | 130.60 ± 7.50 | 0.255 ± 0.03 | −30.30 ± 0.80 | 16.37 ± 0.11 | 13.78 ± 1.82 |
| S4 | 411.20 ± 4.51 | 0.027 ± 0.01 | −41.13 ± 7.41 | 59.71 ± 0.09 | 61.98 ± 0.19 |
| S5 | 302.90 ± 1.50 | 0.228 ± 0.00 | −50.20 ± 3.74 | 42.76 ± 0.25 | 35.12 ± 1.72 |
| S6 | 391.50 ± 1.10 | 0.213 ± 0.02 | −48.30 ± 2.10 | 45.87 ± 0.51 | 36.42 ± 1.26 |
| S7 | 369.50 ± 3.21 | 0.204 ± 0.03 | −40.20 ± 5.61 | 67.07 ± 0.30 | 70.89 ± 0.42 |
| S8 | 280.10 ± 0.60 | 0.256 ± 0.00 | −40.70 ± 0.77 | 37.97 ± 0.69 | 42.52 ± 0.49 |
| S9 | 386.50 ± 2.34 | 0.005 ± 0.00 | −41.76 ± 0.64 | 49.90 ± 0.15 | 43.13 ± 0.25 |
| Code | PS (nm) | PDI | ZP (mv) | %EE | ||
|---|---|---|---|---|---|---|
| AA | CAFF | |||||
| S4 | Freshly prepared | 411.20 ± 4.51 | 0.02 ± 0.01 | −41.13 ± 7.41 | 59.71 ± 0.09 | 61.98 ± 0.19 |
| One month | 415.50 ± 6.50 | 0.25 ± 0.01 | −36.5 ± 7.66 | 61.32 ± 2.02 | 58.99 ± 2.04 | |
| Two months | 410.30 ± 7.50 | 0.221 ± 0.02 | −39.3 ± 6.30 | 59.46 ± 0.44 | 56.09 ± 0.34 | |
| S7 | Freshly prepared | 369.50 ± 3.21 | 0.20 ± 0.03 | −40.20 ± 5.61 | 67.07 ± 0.30 | 70.89 ± 0.42 |
| One month | 374.30 ± 5.90 | 0.11 ± 0.01 | −33.8 ± 0.53 | 69.77 ± 0.02 | 67.80 ± 0.01 | |
| Two months | 363.00 ± 4.50 | 0.14 ± 0.00 | −36.7 ± 0.66 | 69.76 ± 0.40 | 66.96 ± 0.14 | |
| Formulation | Forces Applied per Array | |||
|---|---|---|---|---|
| Control | 200 gm | 500 gm | 1000 gm | |
| M1 | 595 ± 10 | 580 ± 17 | 575 ± 30 | 565 ± 17 |
| M2 | 600 ± 00 | 590 ± 00 | 570 ± 17 | 560 ± 30 |
| M3 | 593 ± 05 | 585 ± 17 | 580 ± 17 | 570 ± 17 |
| M4 | 600 ± 00 | 590 ± 00 | 570 ± 34 | 560 ± 30 |
| Formulation | % Recovery | |
|---|---|---|
| AA | CAFF | |
| M1 | 99.33 ± 1.11 | 98.55 ± 0.17 |
| M2 | 99.66 ± 0.47 | 99.19 ± 0.15 |
| M3 | 98.72 ± 0.79 | 98.03 ± 0.40 |
| M4 | 99.67 ± 0.41 | 99.25 ± 0.50 |
| Formulations | %Moisture Content | %Recovery | ||||
|---|---|---|---|---|---|---|
| One Month | Two Months b | One Month | Two Months b | |||
| AA | CAFF | AA | CAFF | |||
| M1 | 4.35 ± 0.12 | 5.17 ± 0.16 | 98.25 ± 1.05 | 98.25 ± 0.29 | 98.12 ± 0.45 | 97.87 ± 0.88 |
| M2 | 4.76 ± 0.13 | 5.98 ± 0.15 | 98.71 ± 0.25 | 98.97 ± 0.94 | 98.64 ± 0.66 | 98.55 ± 1.40 |
| M3 | 3.56 ± 0.11 | 3.71 ± 0.05 | 98.21 ± 0.25 | 97.89 ± 0.67 | 98.17 ± 0.75 | 97.52 ± 0.85 |
| M4 | 2.22 ± 0.10 | 2.54 ± 0.09 | 99.14 ± 0.23 | 99.05 ± 1.20 | 99.25 ± 0.58 | 98.76 ± 1.30 |
| Code | Q% | Q/A (µg/cm2) | Jss (µg/cm2/h) | P × 10−2 (cm/h) | |
|---|---|---|---|---|---|
| M1 | AA | 60.55 ± 6.82 | 616.8 ± 69.5 | 110.1 ± 4.2 | 6.1 ± 0.3 |
| CAFF | 69.08 ± 0.33 | 351.8 ± 85.2 | 78.9 ± 3.6 | 8.8 ± 0.2 | |
| M2 | AA | 73.95 ± 3.93 | 753.3 ± 40.1 | 126.2 ± 6.5 | 7.0 ± 0.2 |
| CAFF | 76.58 ± 0.39 | 390.0 ± 2.0 | 80.1 ± 4.3 | 8.9 ± 0.02 | |
| M3 | AA b | 54.59 ± 1.43 | 649.8 ± 17.7 | 100.8 ± 6.3 | 4.8 ± 0.04 |
| CAFF b | 51.51 ± 7.90 | 306.1 ± 47.1 | 71.3 ± 3.6 | 6.8 ± 0.02 | |
| M4 | AA | 67.19 ± 2.08 | 798.5 ± 19.2 | 128.4 ± 8.4 | 6.1 ± 0.07 |
| CAFF | 69.70 ± 4.92 | 414.2 ± 29.2 | 85.7 ± 5.3 | 8.2 ± 0.02 |
| % Deposited in Skin | ||
|---|---|---|
| CAFF | AA | |
| M1 | 36.62 ± 3.26 | 41.08 ± 4.26 |
| M2 | 31.81 ± 3.26 | 30.40 ± 3.22 |
| M3 | 49.03 ± 6.95 b | 43.70 ± 2.83 b |
| M4 | 30.64 ± 3.78 | 36.99 ± 3.69 |
| API | First Order Model | Korsmeyer-Peppas Model | |||
|---|---|---|---|---|---|
| R2 | K | R2 | K | n | |
| AA | 0.9723 | 0.003 | 0.9548 | 0.231 | 0.962 |
| CAFF | 0.9659 | 0.002 | 0.9851 | 0.172 | 0.994 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaid Alkilani, A.; Abo-Zour, H.; Basheer, H.A.; Abu-Zour, H.; Donnelly, R.F. Development and Evaluation of an Innovative Approach Using Niosomes Based Polymeric Microneedles to Deliver Dual Antioxidant Drugs. Polymers 2023, 15, 1962. https://doi.org/10.3390/polym15081962
Zaid Alkilani A, Abo-Zour H, Basheer HA, Abu-Zour H, Donnelly RF. Development and Evaluation of an Innovative Approach Using Niosomes Based Polymeric Microneedles to Deliver Dual Antioxidant Drugs. Polymers. 2023; 15(8):1962. https://doi.org/10.3390/polym15081962
Chicago/Turabian StyleZaid Alkilani, Ahlam, Hadeel Abo-Zour, Haneen A. Basheer, Hana Abu-Zour, and Ryan F. Donnelly. 2023. "Development and Evaluation of an Innovative Approach Using Niosomes Based Polymeric Microneedles to Deliver Dual Antioxidant Drugs" Polymers 15, no. 8: 1962. https://doi.org/10.3390/polym15081962
APA StyleZaid Alkilani, A., Abo-Zour, H., Basheer, H. A., Abu-Zour, H., & Donnelly, R. F. (2023). Development and Evaluation of an Innovative Approach Using Niosomes Based Polymeric Microneedles to Deliver Dual Antioxidant Drugs. Polymers, 15(8), 1962. https://doi.org/10.3390/polym15081962







