Self-Healing of Recombinant Spider Silk Gel and Coating
Abstract
1. Introduction
2. Materials and Methods
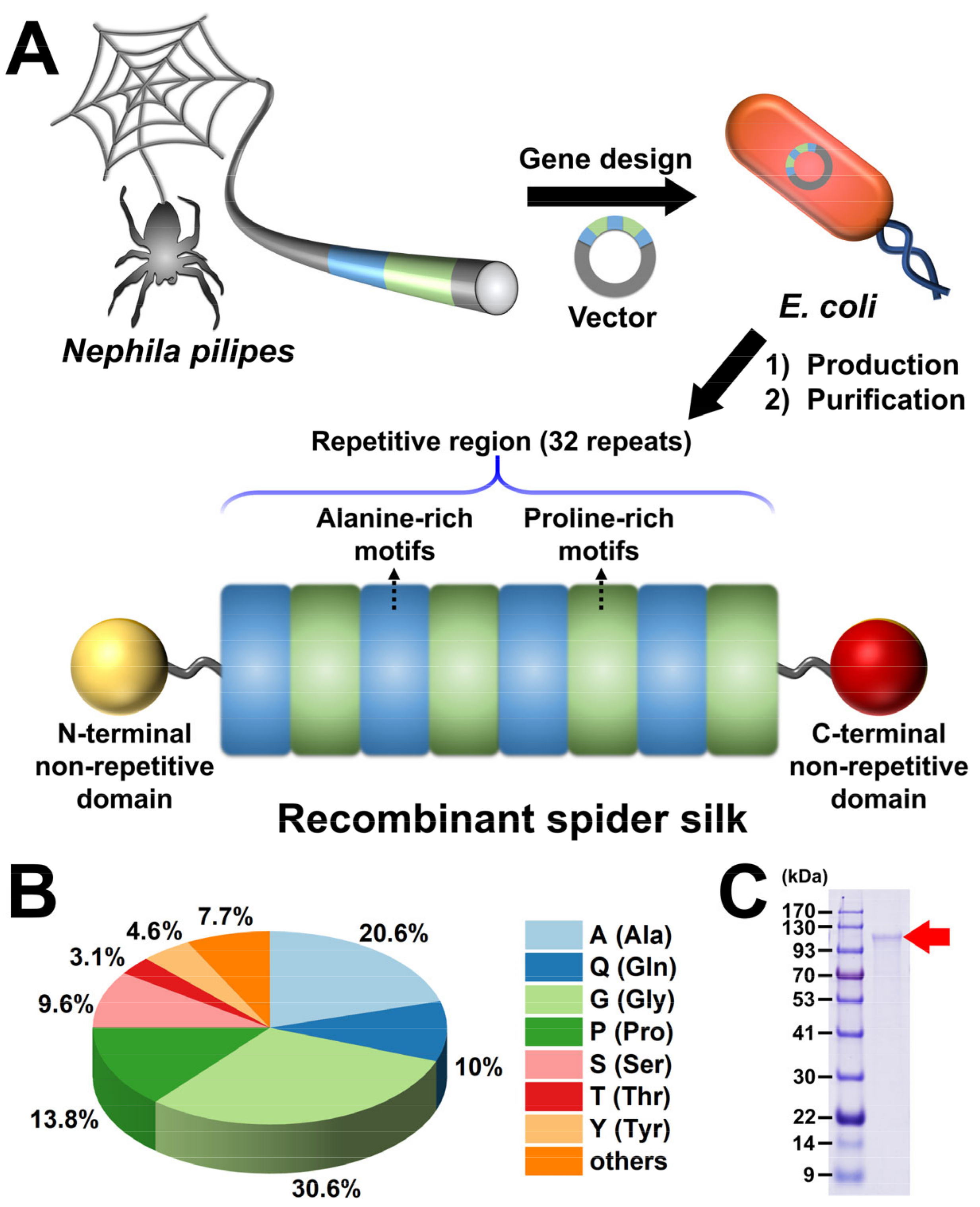
2.1. Production of Recombinant Spider Silk: Gene Construction and Protein Expression
2.2. Purification of Recombinant Spider Silk Protein
2.3. Protein Analysis of Recombinant Spider Silk
2.4. Fabrication of Recombinant Spider Silk Hydrogel and Coated Surface
2.5. FT-IR Analysis of Recombinant Spider Silk Hydrogel
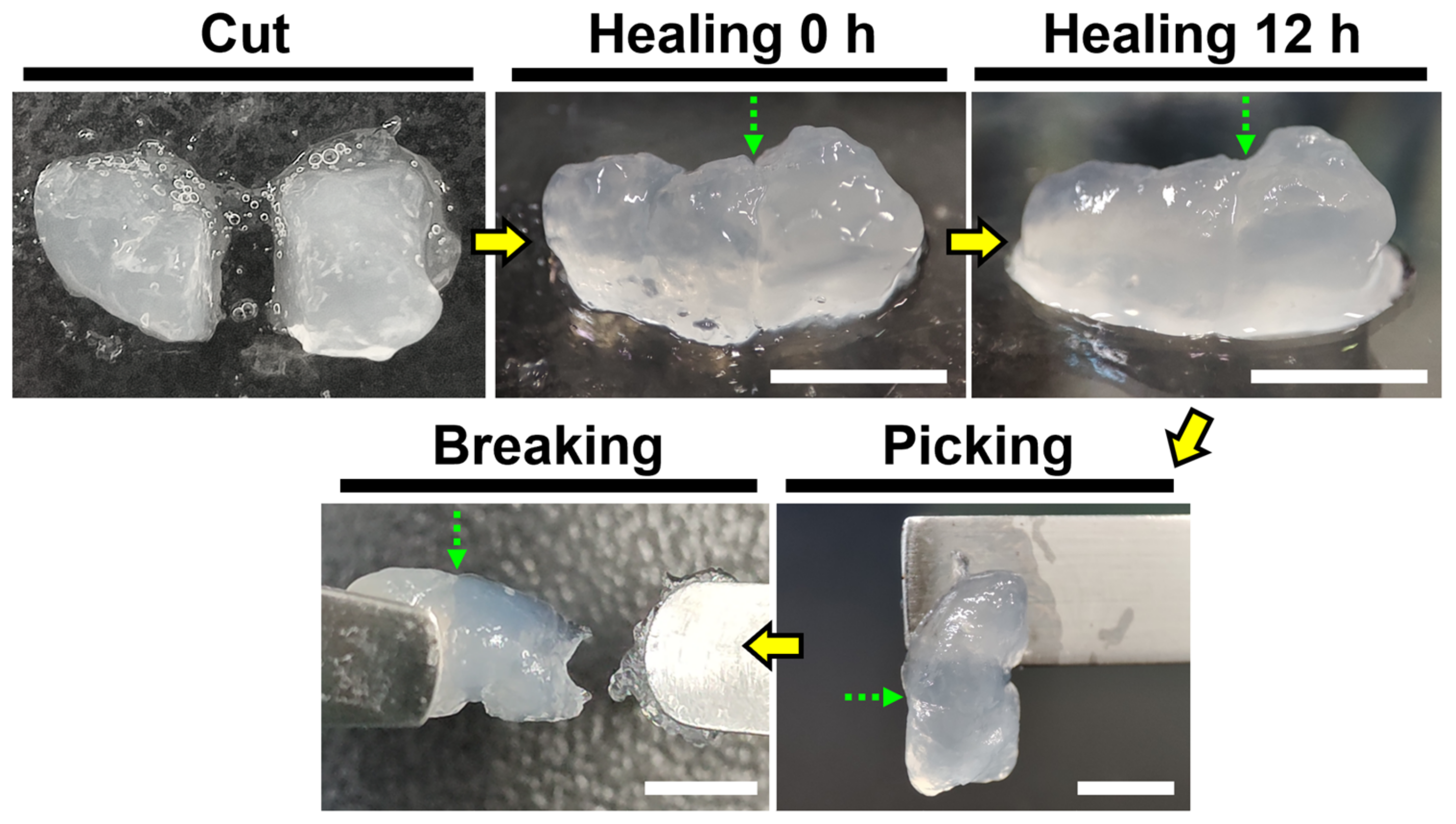
2.6. Self-Healing Properties of Recombinant Spider Silk Hydrogel and Coated Films
2.7. Rheological Properties of Recombinant Spider Silk Hydrogel
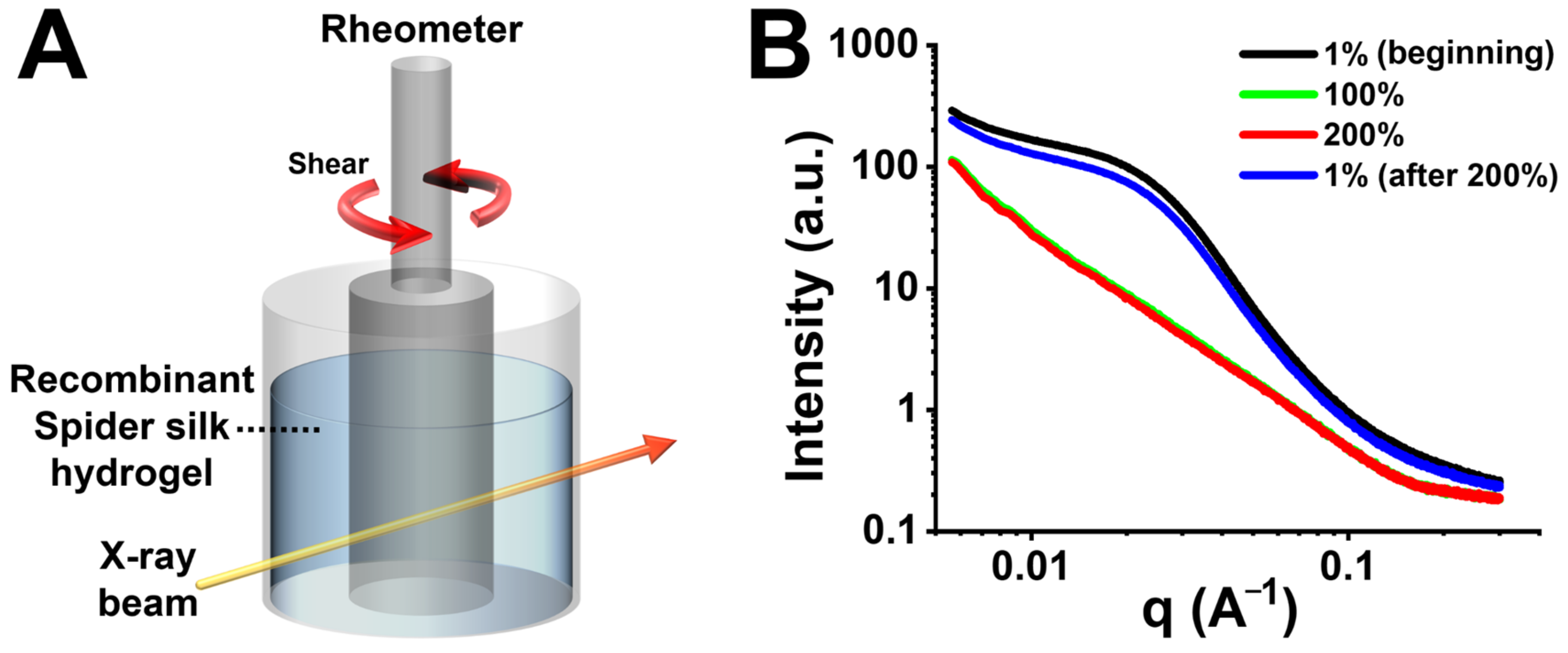
2.8. In Situ SAXS Analysis of Recombinant Spider Silk Hydrogel
2.9. Cell Culture on Recombinant Spider Silk Substrate
2.10. Conductivity of the Recombinant Spider Silk Substrate
3. Results
3.1. Fabrication of Recombinant Spider Silk
3.2. Fabrication of Recombinant Spider Silk Hydrogel
3.3. FTIR Analysis of Recombinant Spider Silk Hydrogel
3.4. Self-Healing Property of Recombinant Spider Silk Hydrogel
3.5. Rheological Properties of Self-Healing Recombinant Spider Silk Hydrogel
3.6. In Situ SAXS Analysis of Self-Healing Recombinant Spider Silk Hydrogel
3.7. Self-Healing Mechanism of Recombinant Spider Silk Hydrogel
3.8. Fabrication and Characterization of Recombinant Spider Silk-Coated Substrate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Omenetto, F.G.; Kaplan, D.L. New Opportunities for an Ancient Material. Science 2010, 329, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Bell, F.I.; McEwen, I.J.; Viney, C. Supercontraction stress in wet spider dragline. Nature 2002, 416, 37. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Kim, T.Y.; Lee, S.Y. Recent advances in production of recombinant spider silk proteins. Curr. Opin. Biotechnol. 2012, 23, 957–964. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Huang, J.; Khan, A.Q.; An, B.; Zhou, X.; Liu, Z.; Zhu, M. Spider Silk-Inspired Artificial Fibers. Adv. Sci. 2022, 9, 2103965. [Google Scholar] [CrossRef]
- Bittencourt, D.M.d.C.; Oliveira, P.; Michalczechen-Lacerda, V.A.; Rosinha, G.M.S.; Jones, J.; Rech Filho, E.L. Bioengineering of spider silks for the production of biomedical materials. Front. Bioeng. Biotechnol. 2022, 10, 958486. [Google Scholar] [CrossRef]
- Debabov, V.G.; Bogush, V.G. Recombinant Spidroins as the Basis for New Materials. ACS Biomater. Sci. Eng. 2020, 6, 3745–3761. [Google Scholar] [CrossRef]
- Liu, T.; Liang, A.; Liang, Z.; Li, G.; Wang, F. Construction of a synthetic Araneus ventricosus dragline silk gene multimer and its expression in Escherichia coli. 3 Biotech 2018, 8, 252. [Google Scholar] [CrossRef]
- Edlund, A.M.; Jones, J.; Lewis, R.; Quinn, J.C. Economic feasibility and environmental impact of synthetic spider silk production from Escherichia coli. New Biotechnol. 2018, 42, 12–18. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Humenik, M.; Smith, A.M.; Scheibel, T. Recombinant Spider Silks—Biopolymers with Potential for Future Applications. Polymers 2011, 3, 640–661. [Google Scholar] [CrossRef]
- Rammensee, S.; Huemmerich, D.; Hermanson, K.D.; Scheibel, T.; Bausch, A.R. Rheological characterization of hydrogels formed by recombinantly produced spider silk. Appl. Phys. A 2006, 82, 261–264. [Google Scholar] [CrossRef]
- Schacht, K.; Scheibel, T. Controlled Hydrogel Formation of a Recombinant Spider Silk Protein. Biomacromolecules 2011, 12, 2488–2495. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, V.J.; Trossmann, V.T.; Jacobi, S.; Döbl, A.; Scheibel, T. Recombinant Spider Silk Gels Derived from Aqueous–Organic Solvents as Depots for Drugs. Angew. Chem. Int. Ed. 2021, 60, 11847–11851. [Google Scholar] [CrossRef]
- Slotta, U.K.; Rammensee, S.; Gorb, S.; Scheibel, T. An Engineered Spider Silk Protein Forms Microspheres. Angew. Chem. Int. Ed. 2008, 47, 4592–4594. [Google Scholar] [CrossRef]
- Kim, U.-J.; Park, J.; Li, C.; Jin, H.-J.; Valluzzi, R.; Kaplan, D.L. Structure and Properties of Silk Hydrogels. Biomacromolecules 2004, 5, 786–792. [Google Scholar] [CrossRef]
- Matsumoto, A.; Chen, J.; Collette, A.L.; Kim, U.-J.; Altman, G.H.; Cebe, P.; Kaplan, D.L. Mechanisms of Silk Fibroin Sol−Gel Transitions. J. Phys. Chem. B 2006, 110, 21630–21638. [Google Scholar] [CrossRef]
- Wang, X.; Kluge, J.A.; Leisk, G.G.; Kaplan, D.L. Sonication-induced gelation of silk fibroin for cell encapsulation. Biomaterials 2008, 29, 1054–1064. [Google Scholar] [CrossRef]
- Hsu, S.-h.; Hung, K.-C.; Lin, Y.-Y.; Su, C.-H.; Yeh, H.-Y.; Jeng, U.S.; Lu, C.-Y.; Dai, S.A.; Fu, W.-E.; Lin, J.-C. Water-based synthesis and processing of novel biodegradable elastomers for medical applications. J. Mater. Chem. B 2014, 2, 5083–5092. [Google Scholar] [CrossRef]
- Hu, X.; Liao, M.; Gong, H.; Zhang, L.; Cox, H.; Waigh, T.A.; Lu, J.R. Recent advances in short peptide self-assembly: From rational design to novel applications. Curr. Opin. Colloid Interface Sci. 2020, 45, 1–13. [Google Scholar] [CrossRef]
- Diesendruck, C.E.; Sottos, N.R.; Moore, J.S.; White, S.R. Biomimetic Self-Healing. Angew. Chem. Int. Ed. 2015, 54, 10428–10447. [Google Scholar] [CrossRef]
- Becker, N.; Oroudjev, E.; Mutz, S.; Cleveland, J.P.; Hansma, P.K.; Hayashi, C.Y.; Makarov, D.E.; Hansma, H.G. Molecular nanosprings in spider capture-silk threads. Nat. Mater. 2003, 2, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.; Bruchmann, B.; Lehn, J.-M. DYNAMERS: Dynamic polymers as self-healing materials. Chem. Soc. Rev. 2015, 44, 3786–3807. [Google Scholar] [CrossRef] [PubMed]
- Ke, R.; Lin, Z.; Zhang, H.; Zhou, S. Research Progress in Intrinsic Self-healing Polyurethane Materials Based on Dynamic Reversible Non-Covalent Bonds. J. Phys. Conf. Ser. 2022, 2324, 012007. [Google Scholar] [CrossRef]
- Xie, Z.; Hu, B.-L.; Li, R.-W.; Zhang, Q. Hydrogen Bonding in Self-Healing Elastomers. ACS Omega 2021, 6, 9319–9333. [Google Scholar] [CrossRef] [PubMed]
- Koga, T.; Morishita, T.; Harumoto, Y.; Nishimura, S.-n.; Higashi, N. Spider silk-inspired peptide multiblock hybrid copolymers for self-healable thin film materials. Mater. Adv. 2021, 2, 7851–7860. [Google Scholar] [CrossRef]
- Larrañeta, E.; Henry, M.; Irwin, N.J.; Trotter, J.; Perminova, A.A.; Donnelly, R.F. Synthesis and characterization of hyaluronic acid hydrogels crosslinked using a solvent-free process for potential biomedical applications. Carbohydr. Polym. 2018, 181, 1194–1205. [Google Scholar] [CrossRef]
- Xu, J.; Fu, C.-Y.; Tsai, Y.-L.; Wong, C.-W.; Hsu, S.-h. Thermoresponsive and Conductive Chitosan-Polyurethane Biocompatible Thin Films with Potential Coating Application. Polymers 2021, 13, 326. [Google Scholar] [CrossRef]
- Boulet-Audet, M.; Vollrath, F.; Holland, C. Identification and classification of silks using infrared spectroscopy. J. Exp. Biol. 2015, 218, 3138–3149. [Google Scholar] [CrossRef]
- Tso, I.M.; Wu, H.-C.; Hwang, I.-R. Giant wood spider Nephila pilipes alters silk protein in response to prey variation. J. Exp. Biol. 2005, 208, 1053–1061. [Google Scholar] [CrossRef]
- Jin, H.-J.; Kaplan, D.L. Mechanism of silk processing in insects and spiders. Nature 2003, 424, 1057–1061. [Google Scholar] [CrossRef]
- Martel, A.; Burghammer, M.; Davies, R.J.; Di Cola, E.; Vendrely, C.; Riekel, C. Silk Fiber Assembly Studied by Synchrotron Radiation SAXS/WAXS and Raman Spectroscopy. J. Am. Chem. Soc. 2008, 130, 17070–17074. [Google Scholar] [CrossRef]
- Thomas, S.; Shanks, R.; Joy, J. Micro- and Nanostructured Polymer Systems: From Synthesis to Applications; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Saric, M.; Eisoldt, L.; Döring, V.; Scheibel, T. Interplay of Different Major Ampullate Spidroins during Assembly and Implications for Fiber Mechanics. Adv. Mater. 2021, 33, 2006499. [Google Scholar] [CrossRef]
- Tucker, C.L.; Jones, J.A.; Bringhurst, H.N.; Copeland, C.G.; Addison, J.B.; Weber, W.S.; Mou, Q.; Yarger, J.L.; Lewis, R.V. Mechanical and Physical Properties of Recombinant Spider Silk Films Using Organic and Aqueous Solvents. Biomacromolecules 2014, 15, 3158–3170. [Google Scholar] [CrossRef]
- Cao, H.; Parveen, S.; Ding, D.; Xu, H.; Tan, T.; Liu, L. Metabolic engineering for recombinant major ampullate spidroin 2 (MaSp2) synthesis in Escherichia coli. Sci. Rep. 2017, 7, 11365. [Google Scholar] [CrossRef]
- Hayashi, C.Y.; Lewis, R.V. Evidence from flagelliform silk cDNA for the structural basis of elasticity and modular nature of spider silks. J. Mol. Biol. 1998, 275, 773–784. [Google Scholar] [CrossRef]
- Suzuki, Y.; Higashi, T.; Yamamoto, T.; Okamura, H.; Sato, T.K.; Asakura, T. Presence of β-Turn Structure in Recombinant Spider Silk Dissolved in Formic Acid Revealed with NMR. Molecules 2022, 27, 511. [Google Scholar] [CrossRef]
- Ramezaniaghdam, M.; Nahdi, N.D.; Reski, R. Recombinant spider silk: Promises and bottlenecks. Front. Bioeng. Biotechnol. 2022, 10, 835637. [Google Scholar] [CrossRef]
- Rosano, G.L.; Ceccarelli, E.A. Recombinant protein expression in Escherichia coli: Advances and challenges. Front. Microbiol. 2014, 5, 172. [Google Scholar] [CrossRef]
- Hayashi, C.Y.; Shipley, N.H.; Lewis, R.V. Hypotheses that correlate the sequence, structure, and mechanical properties of spider silk proteins. Int. J. Biol. Macromol. 1999, 24, 271–275. [Google Scholar] [CrossRef]
- Rising, A.; Nimmervoll, H.; Grip, S.; Fernandez-Arias, A.; Storckenfeldt, E.; Knight, D.P.; Vollrath, F.; Engström, W. Spider silk proteins–mechanical property and gene sequence. Zool. Sci. 2005, 22, 273–281. [Google Scholar] [CrossRef]
- Hagn, F.; Thamm, C.; Scheibel, T.; Kessler, H. pH-Dependent Dimerization and Salt-Dependent Stabilization of the N-terminal Domain of Spider Dragline Silk—Implications for Fiber Formation. Angew. Chem. Int. Ed. 2011, 50, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Eisoldt, L.; Smith, A.; Scheibel, T. Decoding the secrets of spider silk. Mater. Today 2011, 14, 80–86. [Google Scholar] [CrossRef]
- Hagn, F.; Eisoldt, L.; Hardy, J.G.; Vendrely, C.; Coles, M.; Scheibel, T.; Kessler, H. A conserved spider silk domain acts as a molecular switch that controls fibre assembly. Nature 2010, 465, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.; Wu, J.; Zhao, X. Hydrogel interfaces for merging humans and machines. Nat. Rev. Mater. 2022, 7, 935–952. [Google Scholar] [CrossRef]
- Song, G.; Zhao, Z.; Peng, X.; He, C.; Weiss, R.A.; Wang, H. Rheological Behavior of Tough PVP-in Situ-PAAm Hydrogels Physically Cross-Linked by Cooperative Hydrogen Bonding. Macromolecules 2016, 49, 8265–8273. [Google Scholar] [CrossRef]
- Fujikura, K.; Maeda, H.; Obata, A.; Inukai, K.; Kato, K.; Kasuga, T. Preparation and rheological characterization of imogolite hydrogels. J. Nanomater. 2014, 2014, 727254. [Google Scholar] [CrossRef]
- Shao, C.; Chang, H.; Wang, M.; Xu, F.; Yang, J. High-Strength, Tough, and Self-Healing Nanocomposite Physical Hydrogels Based on the Synergistic Effects of Dynamic Hydrogen Bond and Dual Coordination Bonds. ACS Appl. Mater. Interfaces 2017, 9, 28305–28318. [Google Scholar] [CrossRef]
- Maitra, J.; Shukla, V.K. Cross-linking in hydrogels-a review. Am. J. Polym. Sci 2014, 4, 25–31. [Google Scholar]
- Tang, S.; Liu, Z.; Xiang, X. Graphene oxide composite hydrogels for wearable devices. Carbon Lett. 2022, 32, 1395–1410. [Google Scholar] [CrossRef]
- Hu, X.; Vatankhah-Varnoosfaderani, M.; Zhou, J.; Li, Q.; Sheiko, S.S. Weak Hydrogen Bonding Enables Hard, Strong, Tough, and Elastic Hydrogels. Adv. Mater. 2015, 27, 6899–6905. [Google Scholar] [CrossRef]
- Quan, L.; Xin, Y.; Wu, X.; Ao, Q. Mechanism of Self-Healing Hydrogels and Application in Tissue Engineering. Polymers 2022, 14, 2184. [Google Scholar] [CrossRef]
- Luo, J.; Wang, T.; Sim, C.; Li, Y. Mini-Review of Self-Healing Mechanism and Formulation Optimization of Polyurea Coating. Polymers 2022, 14, 2808. [Google Scholar] [CrossRef]
- Goyal, M.; Agarwal, S.N.; Bhatnagar, N. A review on self-healing polymers for applications in spacecraft and construction of roads. J. Appl. Polym. Sci. 2022, 139, e52816. [Google Scholar] [CrossRef]
- Liu, Y.; Hsu, S.-h. Synthesis and biomedical applications of self-healing hydrogels. Front. Chem. 2018, 6, 449. [Google Scholar] [CrossRef]
- Chan, N.J.-A.; Gu, D.; Tan, S.; Fu, Q.; Pattison, T.G.; O’Connor, A.J.; Qiao, G.G. Spider-silk inspired polymeric networks by harnessing the mechanical potential of β-sheets through network guided assembly. Nat. Commun. 2020, 11, 1630. [Google Scholar] [CrossRef]
- Du, N.; Yang, Z.; Liu, X.Y.; Li, Y.; Xu, H.Y. Structural Origin of the Strain-Hardening of Spider Silk. Adv. Funct. Mater. 2011, 21, 772–778. [Google Scholar] [CrossRef]
- Keten, S.; Xu, Z.; Ihle, B.; Buehler, M.J. Nanoconfinement controls stiffness, strength and mechanical toughness of β-sheet crystals in silk. Nat. Mater. 2010, 9, 359–367. [Google Scholar] [CrossRef]
- Ling, S.; Kaplan, D.L.; Buehler, M.J. Nanofibrils in nature and materials engineering. Nat. Rev. Mater. 2018, 3, 18016. [Google Scholar] [CrossRef]
- Ding, D.; Guerette, P.A.; Fu, J.; Zhang, L.; Irvine, S.A.; Miserez, A. From Soft Self-Healing Gels to Stiff Films in Suckerin-Based Materials through Modulation of Crosslink Density and β-Sheet Content. Adv. Mater. 2015, 27, 3953–3961. [Google Scholar] [CrossRef]
- Pena-Francesch, A.; Jung, H.; Demirel, M.C.; Sitti, M. Biosynthetic self-healing materials for soft machines. Nat. Mater. 2020, 19, 1230–1235. [Google Scholar] [CrossRef]
- Gaddes, D.; Jung, H.; Pena-Francesch, A.; Dion, G.; Tadigadapa, S.; Dressick, W.J.; Demirel, M.C. Self-Healing Textile: Enzyme Encapsulated Layer-by-Layer Structural Proteins. ACS Appl. Mater. Interfaces 2016, 8, 20371–20378. [Google Scholar] [CrossRef] [PubMed]
- Némethy, G.; Steinberg, I.Z.; Scheraga, H.A. Influence of water structure and of hydrophobic interactions on the strength of side-chain hydrogen bonds in proteins. Biopolymers 1963, 1, 43–69. [Google Scholar] [CrossRef]
- Cohen, N.; Levin, M.; Eisenbach, C.D. On the Origin of Supercontraction in Spider Silk. Biomacromolecules 2021, 22, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shao, Z.; Vollrath, F. Relationships between supercontraction and mechanical properties of spider silk. Nat. Mater. 2005, 4, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Steven, E.; Park, J.G.; Paravastu, A.; Lopes, E.B.; Brooks, J.S.; Englander, O.; Siegrist, T.; Kaner, P.; Alamo, R.G. Physical characterization of functionalized spider silk: Electronic and sensing properties. Sci. Technol. Adv. Mater. 2011, 12, 055002. [Google Scholar] [CrossRef]
- Yang, Z.; Liivak, O.; Seidel, A.; LaVerde, G.; Zax, D.B.; Jelinski, L.W. Supercontraction and Backbone Dynamics in Spider Silk: 13C and 2H NMR Studies. J. Am. Chem. Soc. 2000, 122, 9019–9025. [Google Scholar] [CrossRef]
- Holland, G.P.; Lewis, R.V.; Yarger, J.L. WISE NMR Characterization of Nanoscale Heterogeneity and Mobility in Supercontracted Nephila clavipes Spider Dragline Silk. J. Am. Chem. Soc. 2004, 126, 5867–5872. [Google Scholar] [CrossRef]
- Agnarsson, I.; Boutry, C.; Wong, S.-C.; Baji, A.; Dhinojwala, A.; Sensenig, A.T.; Blackledge, T.A. Supercontraction forces in spider dragline silk depend on hydration rate. Zoology 2009, 112, 325–331. [Google Scholar] [CrossRef]
- Lefèvre, T.; Auger, M. Spider silk as a blueprint for greener materials: A review. Int. Mater. Rev. 2016, 61, 127–153. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.-D.; Chuang, W.-T.; Ho, J.-C.; Wu, H.-C.; Hsu, S.-h. Self-Healing of Recombinant Spider Silk Gel and Coating. Polymers 2023, 15, 1855. https://doi.org/10.3390/polym15081855
Wu S-D, Chuang W-T, Ho J-C, Wu H-C, Hsu S-h. Self-Healing of Recombinant Spider Silk Gel and Coating. Polymers. 2023; 15(8):1855. https://doi.org/10.3390/polym15081855
Chicago/Turabian StyleWu, Shin-Da, Wei-Tsung Chuang, Jo-Chen Ho, Hsuan-Chen Wu, and Shan-hui Hsu. 2023. "Self-Healing of Recombinant Spider Silk Gel and Coating" Polymers 15, no. 8: 1855. https://doi.org/10.3390/polym15081855
APA StyleWu, S.-D., Chuang, W.-T., Ho, J.-C., Wu, H.-C., & Hsu, S.-h. (2023). Self-Healing of Recombinant Spider Silk Gel and Coating. Polymers, 15(8), 1855. https://doi.org/10.3390/polym15081855








