TM-Free and TM-Catalyzed Mechanosynthesis of Functional Polymers
Abstract
Highlights
- The most representative examples for the TM-free and TM-catalyzed mechano-synthesis of functional polymers are reported;
- The most common applications for the various types of functional polymers are precented;
- The advantage of solvent-free mechanosynthesis over conventional solvent-based synthesis are highlighted;
- In many cases the better performance of the mechanchemically-prepared polymers over those obtained by using conventional methods are demonstrated.
Abstract
1. Introduction
2. Results and Discussion
2.1. Mechanosynthesis of Conductive Polymers
2.2. Mechanosynthesis of Polystyrenes and Poly(2-vinylnaphthalene)
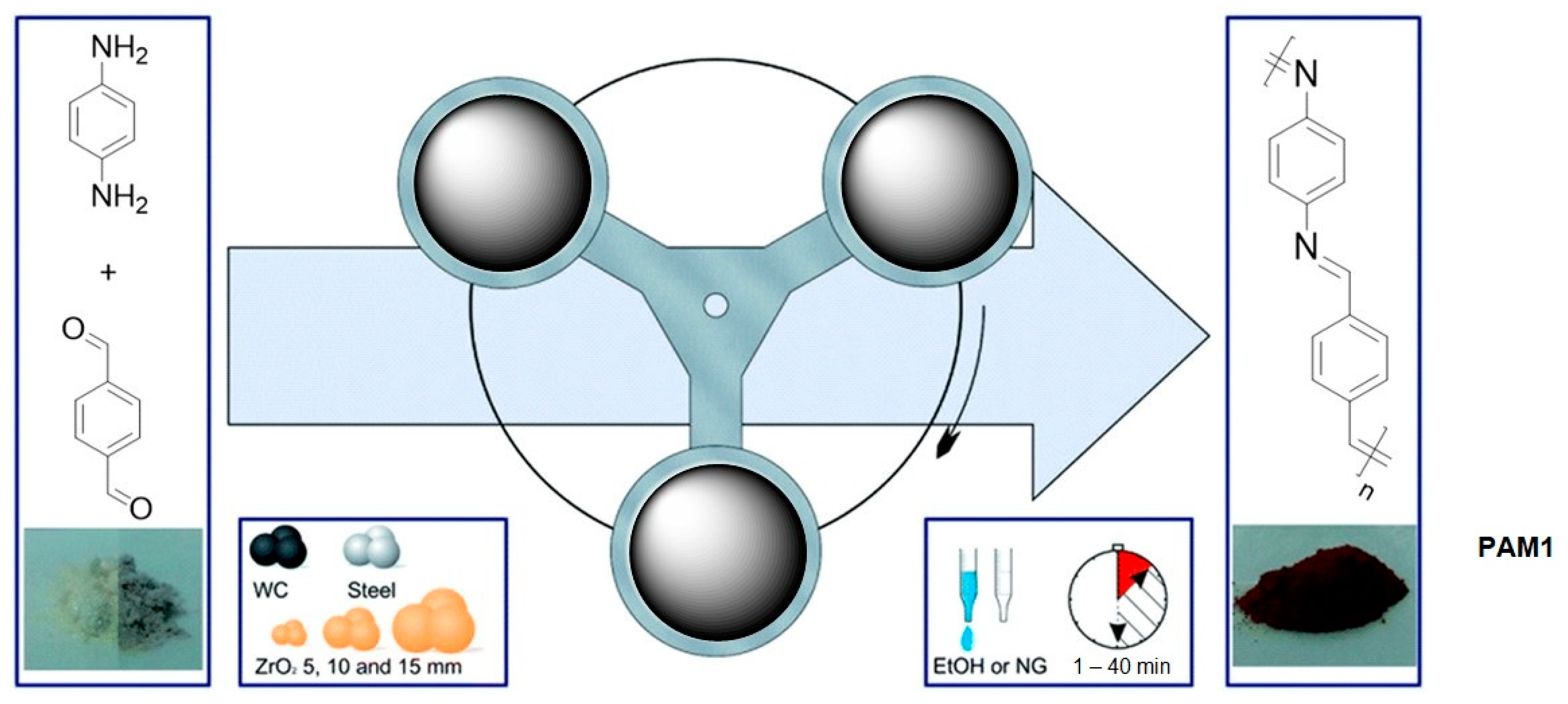
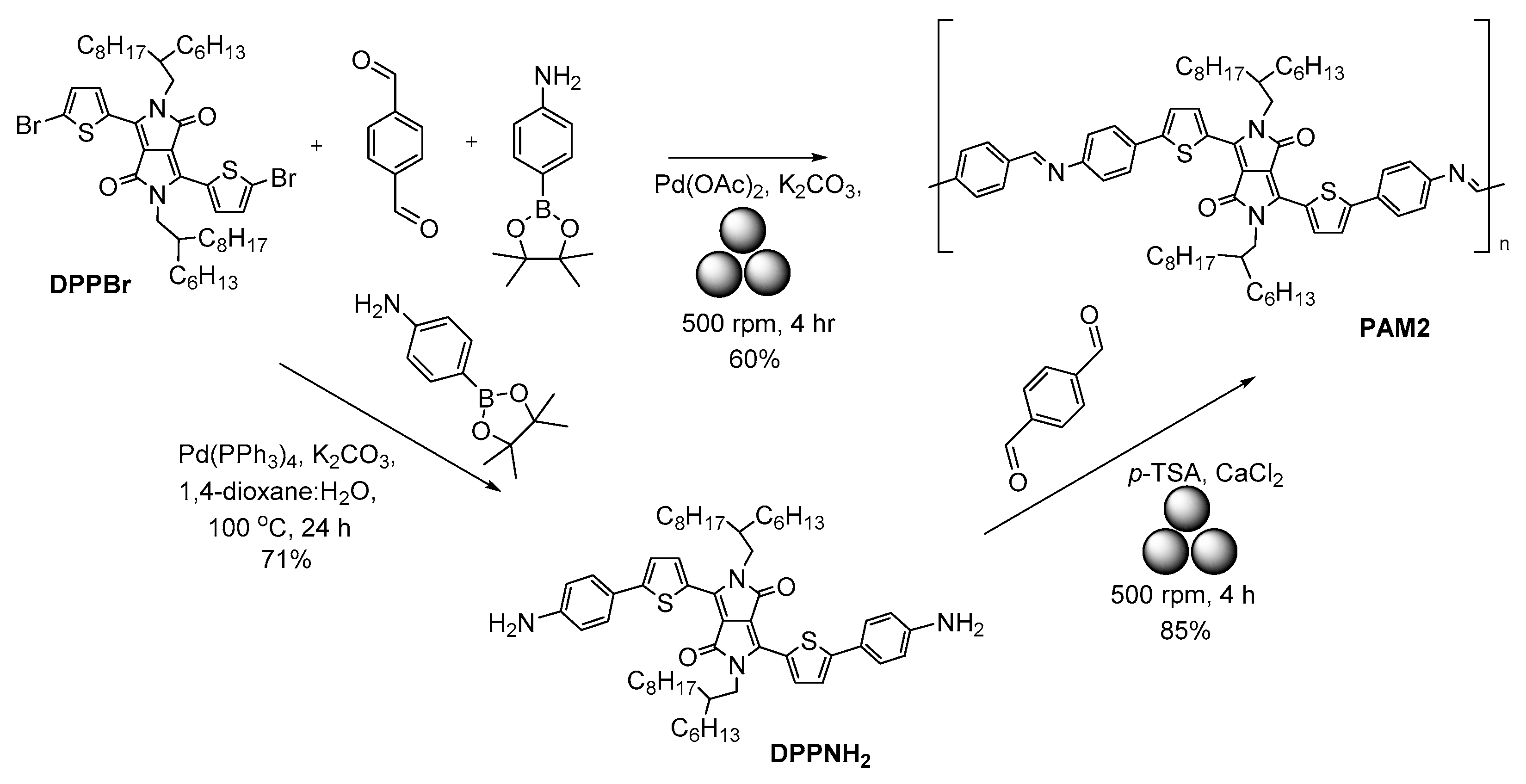
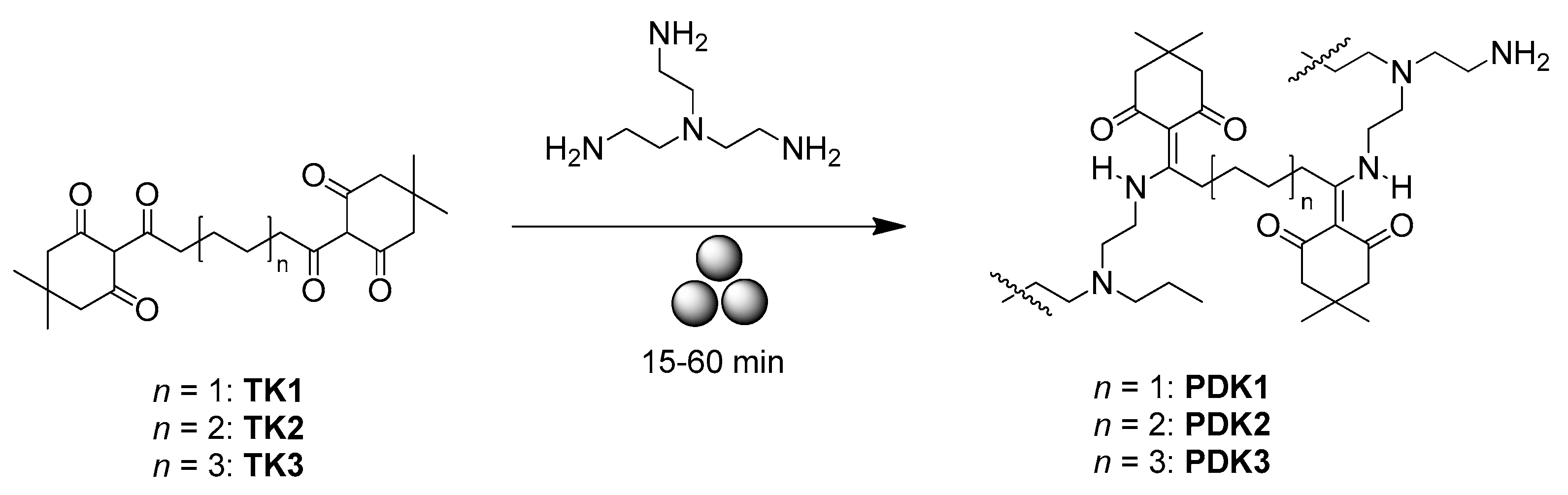
2.3. Mechanosynthesis of Polyazomethines
2.4. Mechanosynthesis of Biopolymers and (Bio)Degradable Polymers
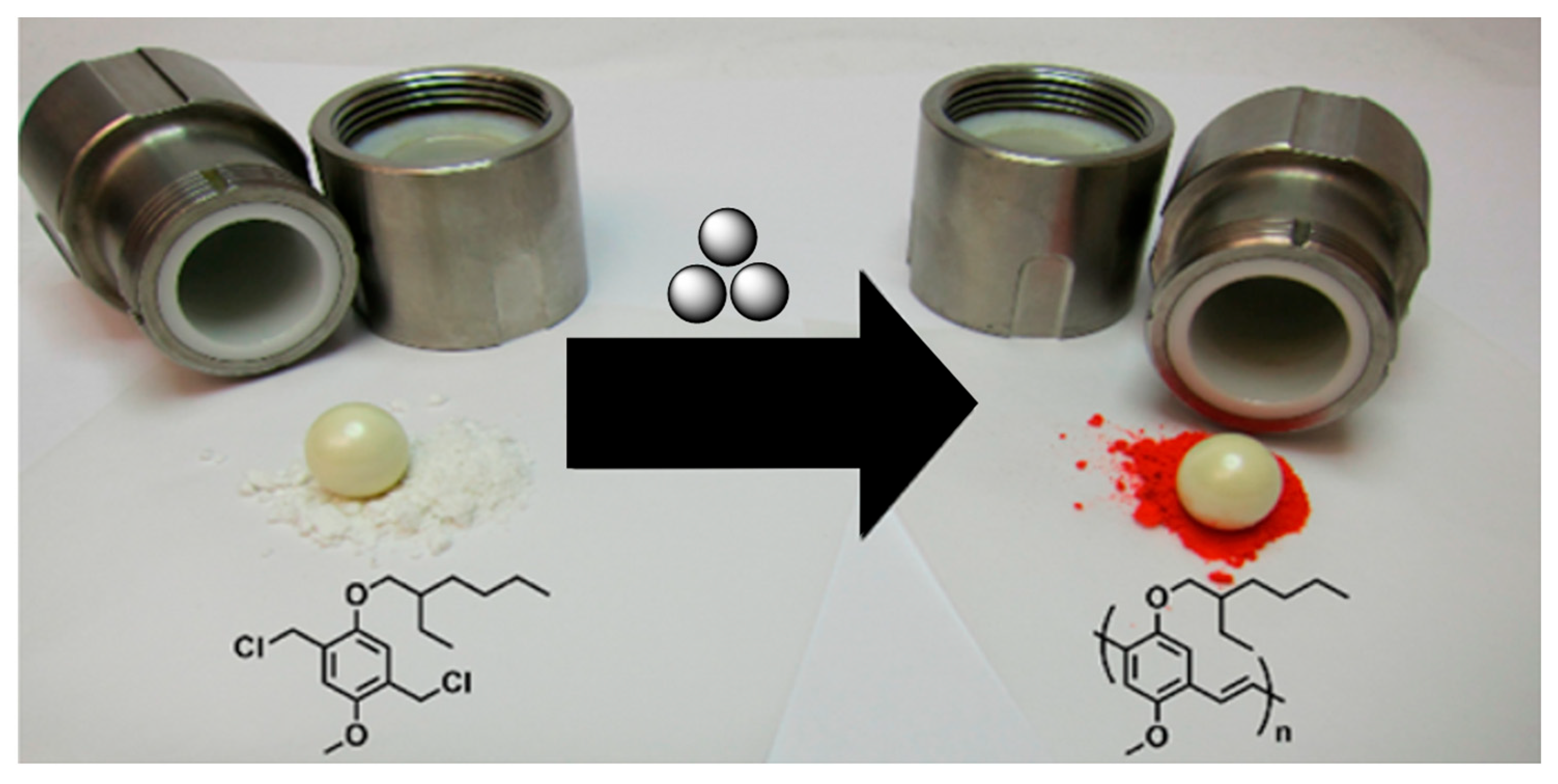
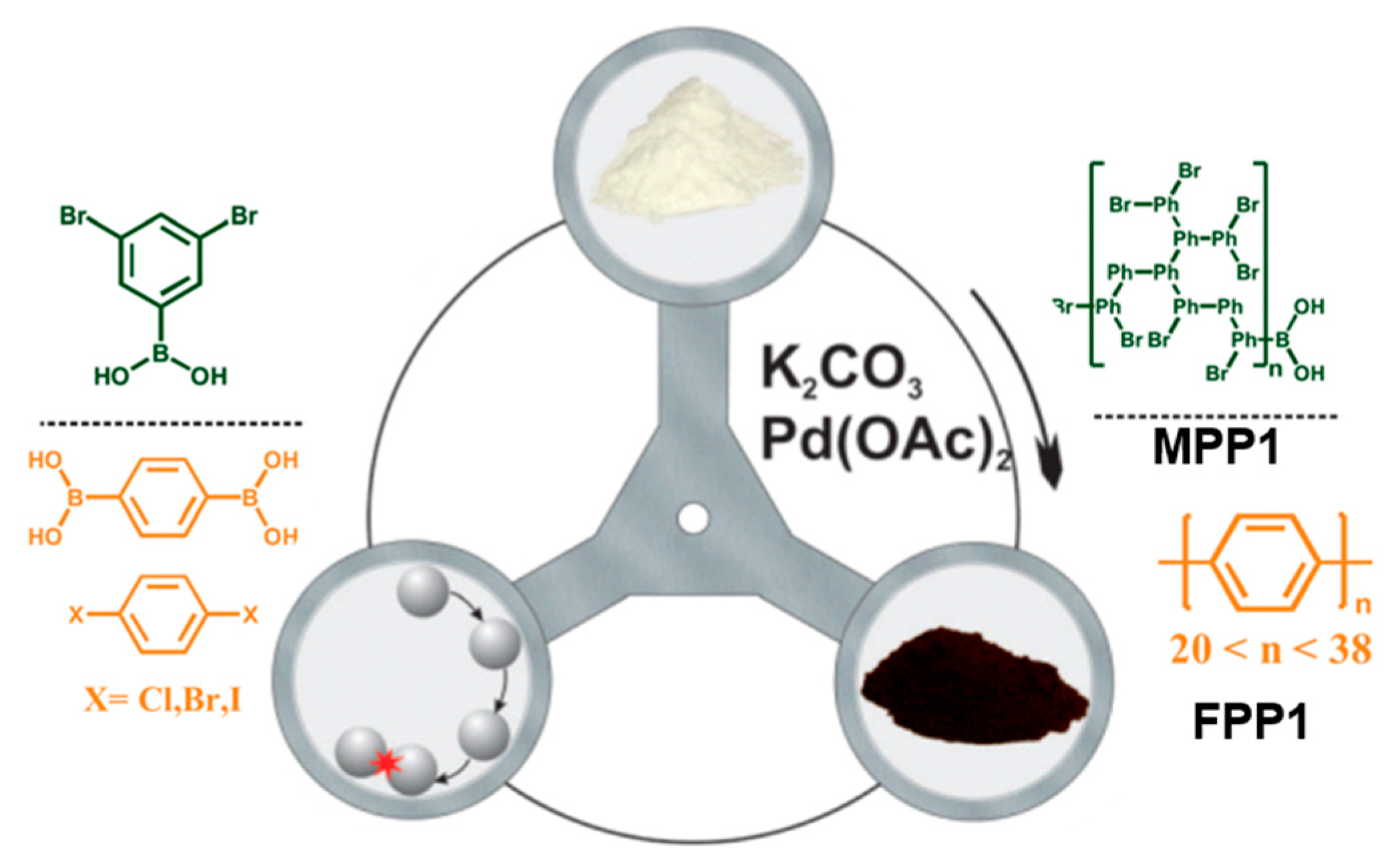
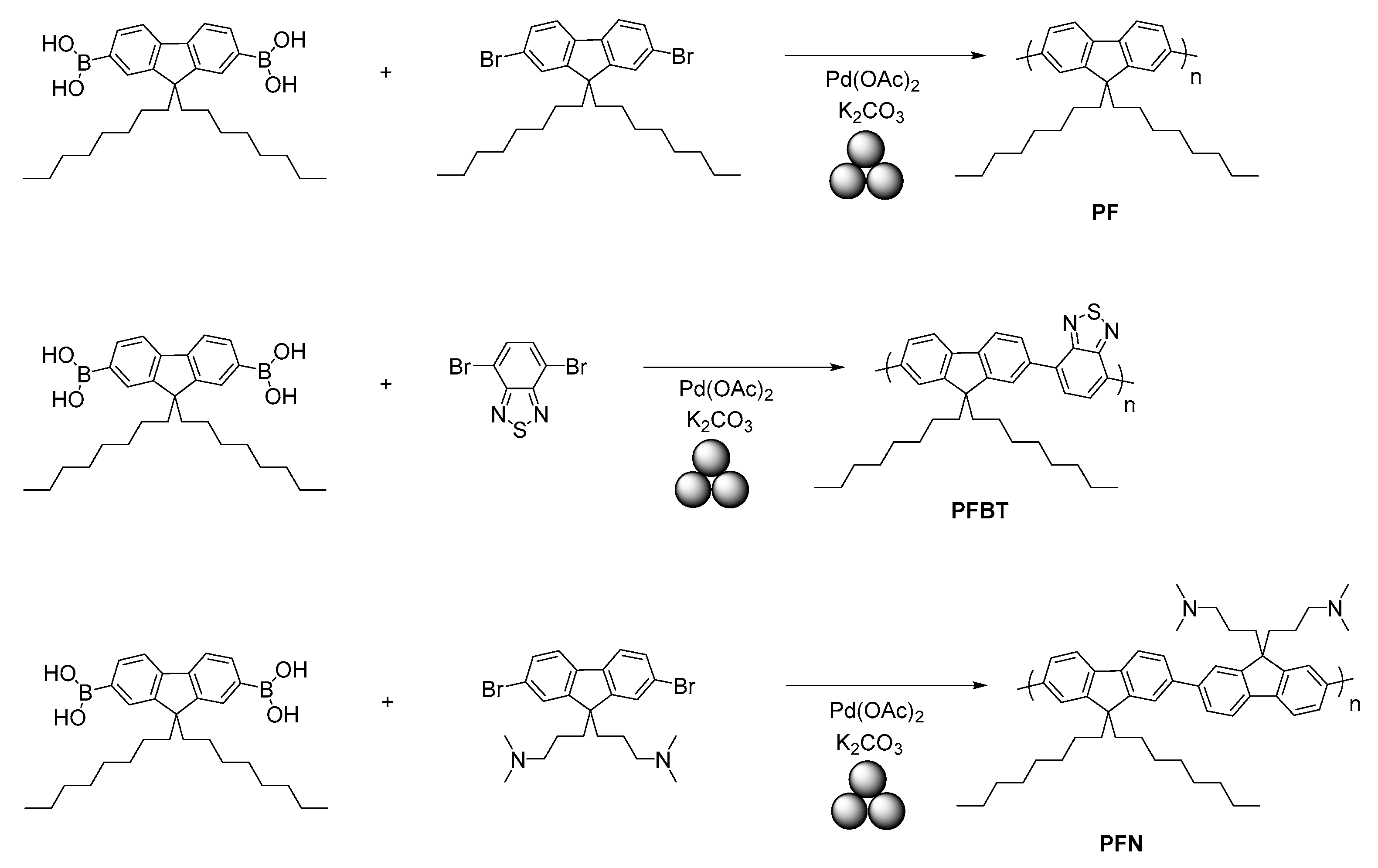
2.5. Mechanosynthesis of Polyphenylenes
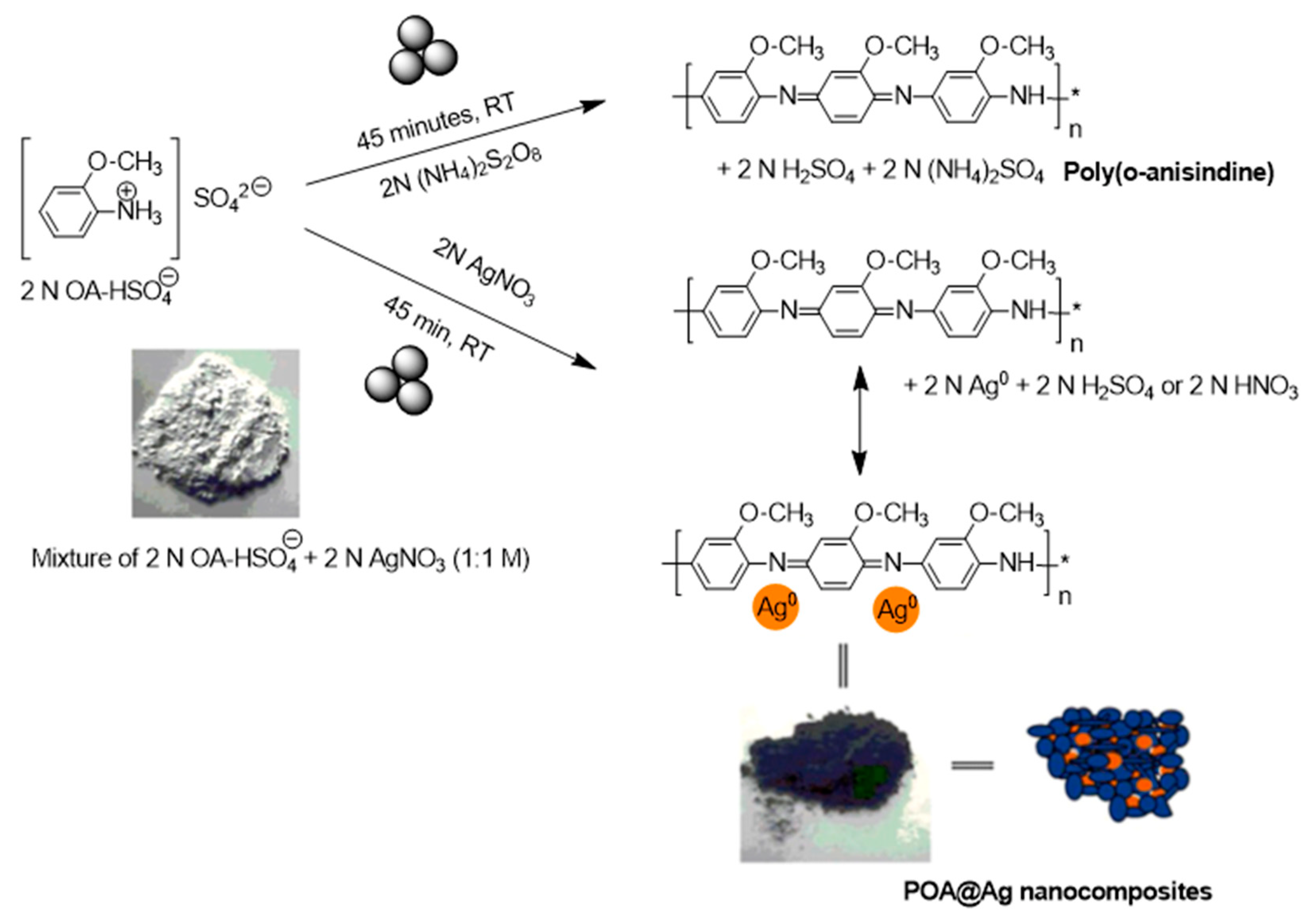
2.6. Mechanosynthesis of Polyanylines and Polyamines
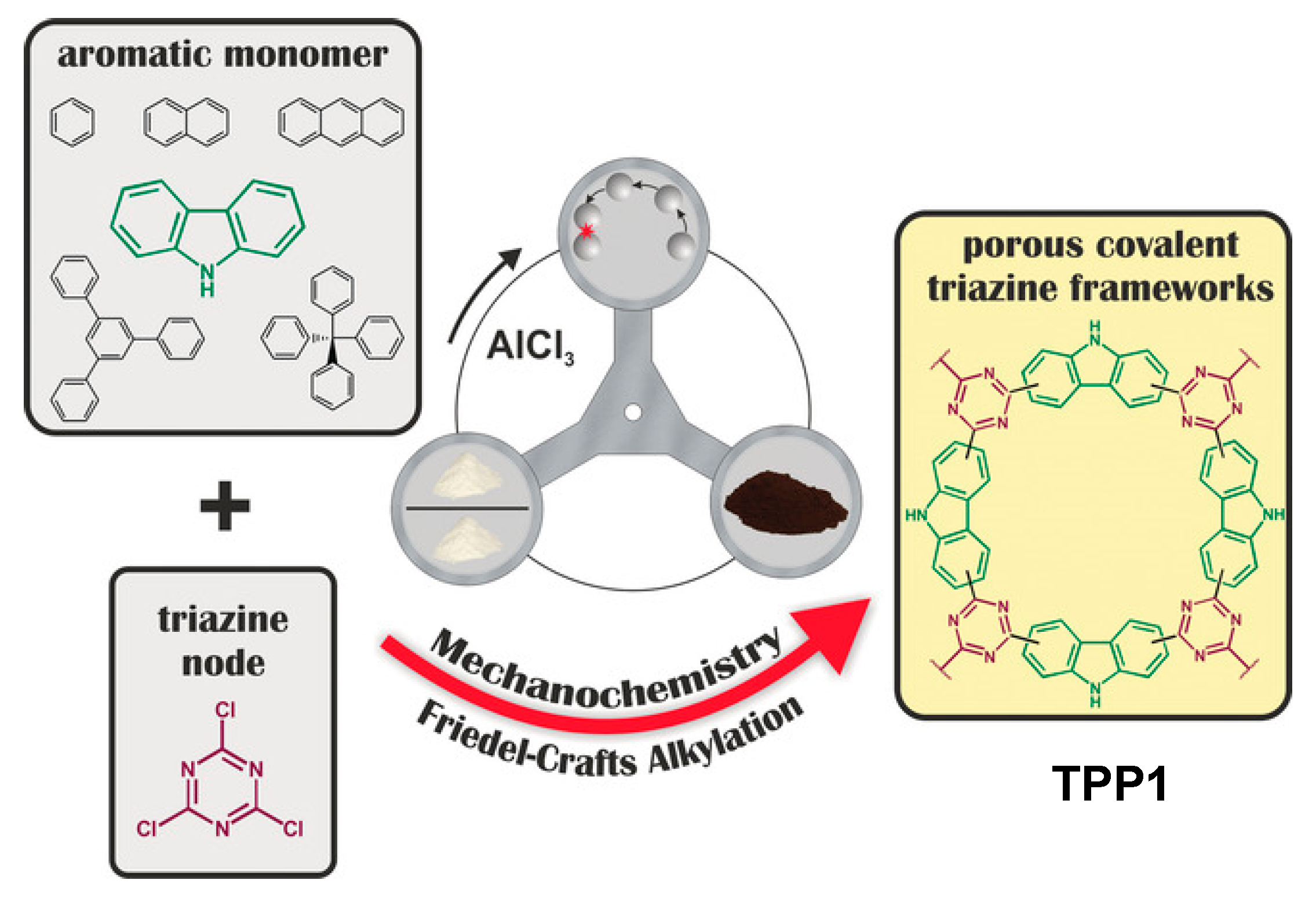
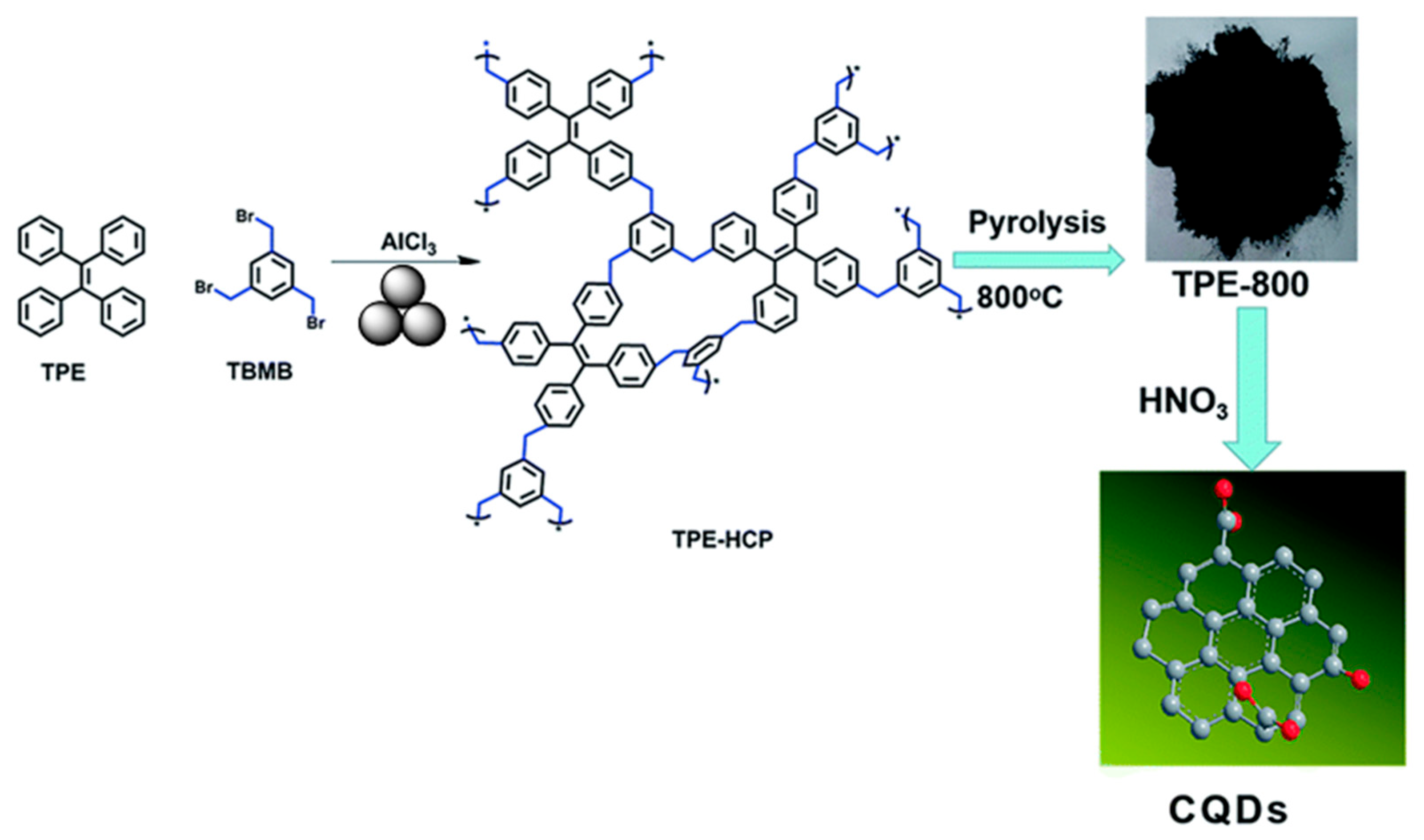
2.7. Mechanosynthesis of Organic Porous Polymers
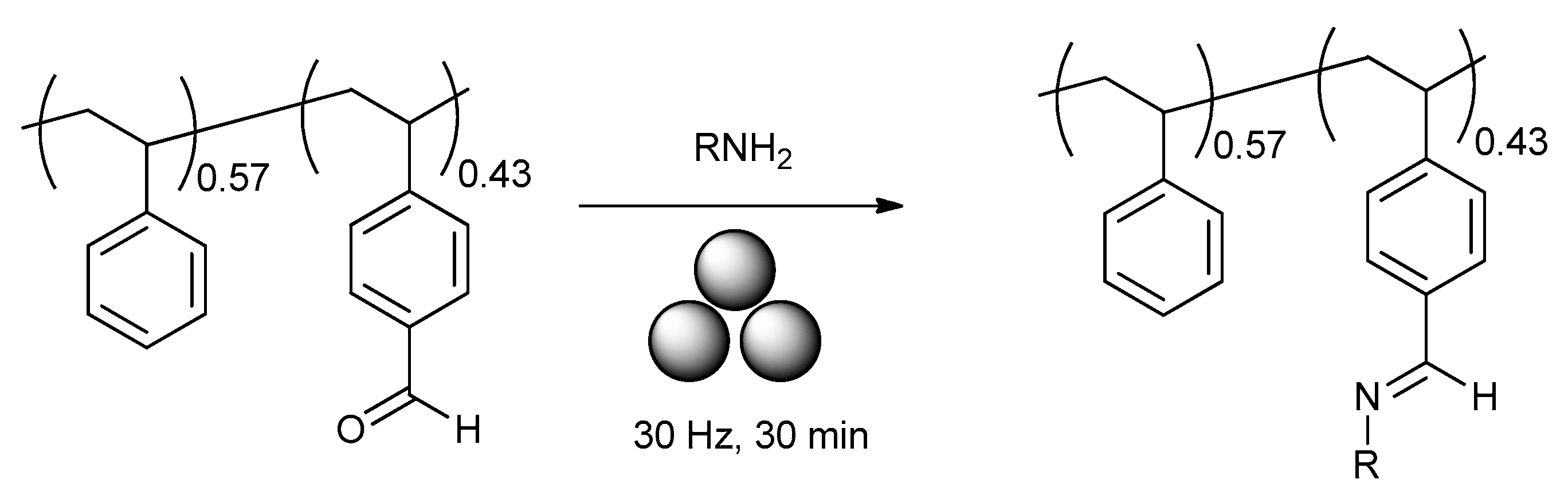
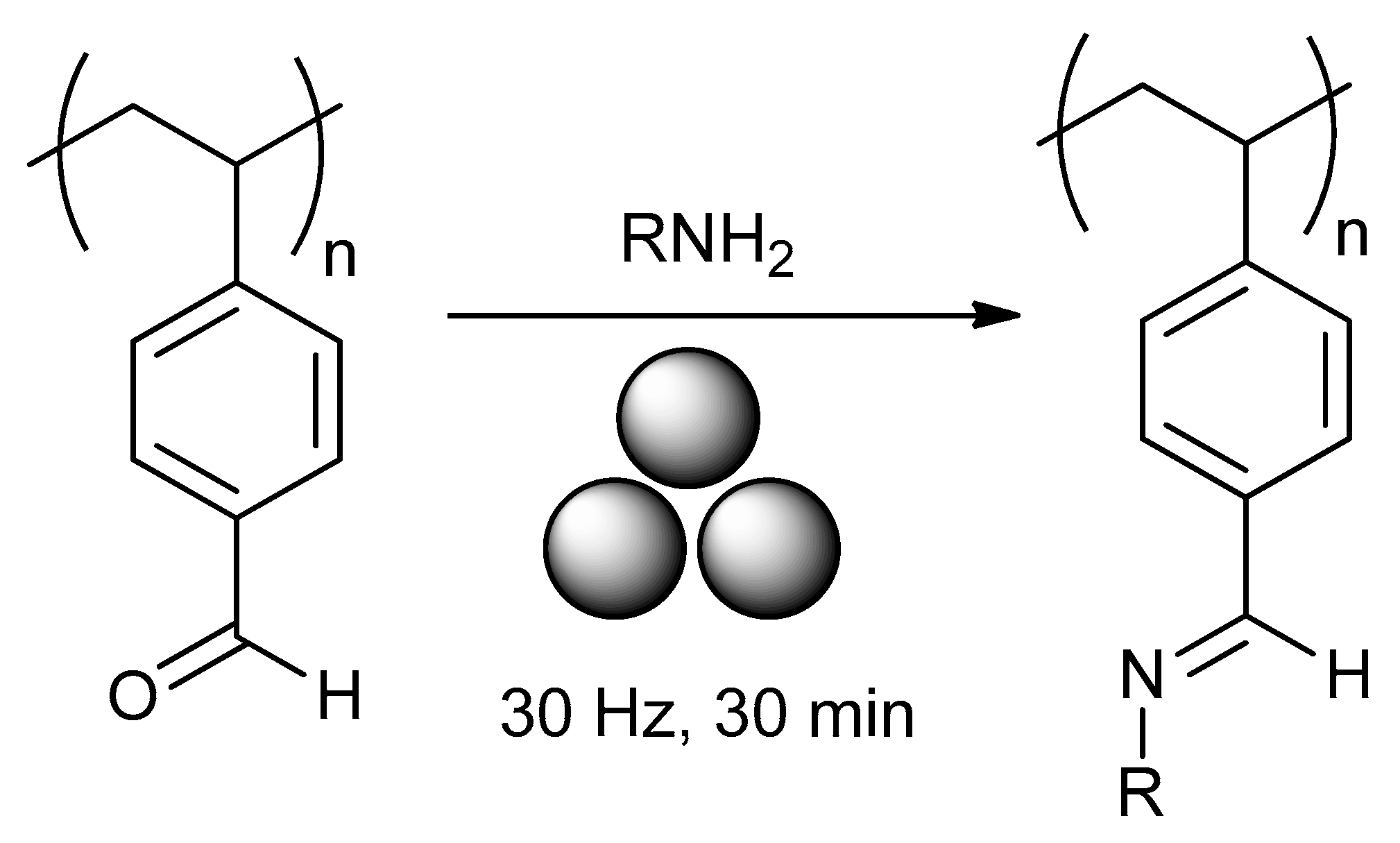
2.8. Mechanochemical Post-Modification of Polymers
3. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cintas, P.; Tabasso, S.; Veselov, V.V.; Cravotto, G. Alternative reaction conditions: Enabling technologies in solvent-free protocols. Curr. Opin. Green Sustain. Chem. 2020, 21, 44–49. [Google Scholar] [CrossRef]
- Mottillo, C.; Friščić, T. Advances in Solid-State Transformations of Coordination Bonds: From the Ball Mill to the Aging Chamber. Molecules 2017, 22, 144. [Google Scholar] [CrossRef] [PubMed]
- Krusenbaum, A.; Grätz, S.; Tigineh, G.T.; Borchardt, L.; Kim, J.G. The mechanochemical synthesis of polymers. Chem. Soc. Rev. 2022, 51, 2873–2905. [Google Scholar] [CrossRef] [PubMed]
- Cuccu, F.; De Luca, L.; Delogu, F.; Colacino, E.; Solin, N.; Mocci, R.; Porcheddu, A. Mechanochemistry: New Tools to Navigate the Uncharted Territory of “Impossible” Reactions. ChemSusChem 2022, 15, e202200362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Y.; Zhang, F.-S.; Yao, T.Q. An environmentally friendly ball milling process for recovery of valuable metals from e-waste scraps. Waste Manag. 2017, 68, 490–497. [Google Scholar] [CrossRef]
- Capuano, R.; Bonadies, I.; Castaldo, R.; Cocca, M.; Gentile, G.; Protopapa, A.; Avolio, R.; Errico, M.E. Valorization and Mechanical Recycling of Heterogeneous Post-Consumer Polymer Waste through a Mechano-Chemical Process. Polymers 2021, 13, 2783. [Google Scholar] [CrossRef]
- Bento, O.; Luttringer, F.; El Dine, T.M.; Pétry, N.; Bantreil, X.; Lamaty, F. Sustainable Mechanosynthesis of Biologically Active Molecules. Eur. J. Org. Chem. 2022, 2022, e202101516. [Google Scholar] [CrossRef]
- Ying, P.; Yu, J.; Su, W. Liquid-assisted grinding mechanochemistry in the synthesis of pharmaceuticals. Adv. Synth. Catal. 2021, 363, 1246–1271. [Google Scholar] [CrossRef]
- Pedrazzo, A.R.; Cecone, C.; Trotta, F.; Zanetti, M. Mechanosynthesis of β-cyclodextrin polymers based on natural deep eutectic solvents. ACS Sustain. Chem. Eng. 2021, 9, 14881–14889. [Google Scholar] [CrossRef]
- Xuan, M.; Schumacher, C.; Bolm, C.; Göstl, R.; Herrmann, A. The mechanochemical synthesis and activation of carbon-rich π-conjugated materials. Adv. Sci. 2022, 9, 2105497. [Google Scholar] [CrossRef]
- Friščić, T.; Mottillo, C.; Titi, H.M. Mechanochemistry for Synthesis. Angew. Chem. Int. Ed. 2020, 59, 1018–1029. [Google Scholar] [CrossRef]
- Chiang, C.K.; Druy, M.A.; Gau, S.C.; Heeger, A.J.; Louis, E.J.; MacDiarmid, A.G.; Park, Y.W.; Shirakawa, H. Synthesis of Highly Conducting Films of Derivatives of Polyacetylene, (CH)x. J. Am. Chem. Soc. 1978, 100, 1013–1015. [Google Scholar] [CrossRef]
- Basescu, N.; Liu, Z.-X.; Moses, D.; Heeger, A.J.; Naarmann, H.; Theophilou, N. High electrical conductivity in doped polyacetylene. Nature 1987, 327, 403–405. [Google Scholar] [CrossRef]
- Foyle, L.D.P.; Hicks, G.E.J.; Pollit, A.A.; Seferos, D.S. Polyacetylene Revisited: A Computational Study of the Molecular Engineering of N-type Polyacetylene. J. Phys. Chem. Lett. 2021, 12, 7745–7751. [Google Scholar] [CrossRef]
- Hernangómez-Pérez, D.; Gunasekaran, S.; Venkataraman, L.; Evers, F. Solitonics with polyacetylenes. Nano Lett. 2020, 20, 2615–2619. [Google Scholar] [CrossRef]
- Bustamantea, C.M.; Scherlis, D.A. Doping and coupling strength in molecular conductors: Polyacetylene as a case study. Phys. Chem. Chem. Phys. 2021, 23, 26974–26980. [Google Scholar] [CrossRef]
- Banerjee, J.; Dutta, K. A short overview on the synthesis, properties and major applications of poly(p-phenylene vinylene). Chem. Pap. 2021, 75, 5139–5151. [Google Scholar] [CrossRef]
- Burroughes, J.H.; Bradley, D.D.C.; Brown, A.R.; Marks, R.N.; Mackay, K.; Friend, R.H.; Burns, P.L.; Holmes, A.B. Light-emitting diodes based on conjugated polymers. Nature 1990, 347, 539–541. [Google Scholar] [CrossRef]
- Ravnsbæk, J.B.; Swager, T.M. Mechanochemical Synthesis of Poly(Phenylene Vinylenes). ACS Macro. Lett. 2014, 3, 305–309. [Google Scholar] [CrossRef]
- Wang, L.-X.; Li, X.-G.; Yang, Y.-L. Preparation, properties and applications of polypyrroles. React. Funct. Polym. 2001, 47, 125–139. [Google Scholar] [CrossRef]
- Grgur, B.N.; Gvozdenovi, M.M.; Stevanovi, J.; Jugović, B.Z.; Marinović, V.M. Polypyrrole as possible electrode materials for the aqueous-based rechargeable zinc batteries. Electrochim. Acta 2008, 53, 4627–4632. [Google Scholar] [CrossRef]
- Wang, G.; Qu, Q.; Wang, B.; Shi, Y.; Tian, S.; Wu, Y. An Aqueous Electrochemical Energy Storage System Based on Doping and Intercalation: Ppy//LiMn2O4. ChemPhysChem 2008, 9, 2299–2301. [Google Scholar] [CrossRef] [PubMed]
- Carquignya, S.; Sanchez, J.-B.; Berger, F.; Lakard, B.; Lallemand, F. Ammonia gas sensor based on electrosynthesized polypyrrole films. Talanta 2009, 78, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lau, K.T.; Shepherd, R.; Wu, Y.; Wallace, G.; Diamond, D. Performance characteristics of a polypyrrole modified polydimethylsiloxane (PDMS) membrane based microfluidic pump. Sens. Actuators A 2008, 148, 239–244. [Google Scholar] [CrossRef]
- Cui, S.; Mao, J.; Rouabhia, M.; Elkoun, S.; Zhang, Z. A biocompatible polypyrrole membrane for biomedical applications. RSC Adv. 2021, 11, 16996–17006. [Google Scholar] [CrossRef]
- Sun, J.; Wang, G.; Zhang, H.; Zhang, B.; Hu, C. Facile fabrication of a conductive polypyrrole membrane for anti-fouling enhancement by electrical repulsion and in situ oxidation. Chemosphere 2021, 270, 129416. [Google Scholar] [CrossRef]
- Pang, A.L.; Arsad, A.; Ahmadipour, M. Synthesis and factor affecting on the conductivity of polypyrrole: A short review. Polym. Adv. Technol. 2021, 32, 1428–1454. [Google Scholar] [CrossRef]
- Carrasco, P.M.; Grande, H.J.; Cortazar, M.; Alberdi, J.M.; Areizaga, J.; Pomposo, J.A. Structure–conductivity relationships in chemical polypyrroles of low, medium and high conductivity. Synth. Met. 2006, 156, 420–425. [Google Scholar] [CrossRef]
- Blinova, N.V.; Stejskal, J.; Trchová, M.; Prokeš, J.; Omastová, M. Polyaniline and polypyrrole: A comparative study of the preparation. Eur. Polym. J. 2007, 43, 2331–2341. [Google Scholar] [CrossRef]
- Posudievsky, O.Y.; Goncharuk, O.A.; Pokhodenko, V.D. Mechanochemical preparation of conducting polymers and oligomers. Synth. Met. 2010, 160, 47–51. [Google Scholar] [CrossRef]
- Posudievsky, O.Y.; Kozarenkoa, O.A. Effect of monomer/oxidant mole ratio on polymerization mechanism, conductivity and spectral characteristics of mechanochemically prepared polypyrrole. Polym. Chem. 2011, 2, 216–220. [Google Scholar] [CrossRef]
- Ueno, N.; Sugita, K.; Seki, K.; Inokuchi, H. Electron Affinities of polystyrene and poly(2-vinylpyridine) by low-energy electron inelastic scattering. Jpn. J. Appl. Phys. 1985, 24, 1156. [Google Scholar] [CrossRef]
- Semerak, S.N.; Frank, C.W. Energy migration in the aromatic vinyl polymers. 5. Poly(2-vinyl naphthalene) and polystyrene. Can. J. Chem. 1985, 63, 1328–1332. [Google Scholar] [CrossRef]
- Frank, C.W.; Harrah, L.A. Excimer formation in vinyl polymers. II. Rigid solutions of poly(2-vinylnaphthalene) and polystyrene. J. Chem. Phys. 1974, 61, 1526. [Google Scholar] [CrossRef]
- Masayuki, A.; Takeshi, T.; Hiroaki, B.; Masahiro, I.; Koichiro, H. Intramolecular Dimer and Excimer Phosphorescence of Poly(2-vinylnaphthalene) and Copolymers of 2-Vinylnaphthalene and Phenyl Vinyl Ketone. Bull. Chem. Soc. Jpn. 1978, 51, 3643–3644. [Google Scholar]
- Nakamura, H.; Kitamura, H.; Shinji, O.; Saito, K.; Shirakawa, Y.; Takahashi, S. Development of polystyrene-based scintillation materials and its mechanisms. Appl. Phys. Lett. 2012, 101, 261110. [Google Scholar] [CrossRef]
- Sen, I.; Penumadu, D.; Williamson, M.; Miller, L.F.; Green, A.D.; Mabe, A.N. Thermal neutron scintillator detectors based on poly (2-vinylnaphthalene) composite films. IEEE Trans. Nucl. Sci. 2011, 58, 1386–1393. [Google Scholar] [CrossRef]
- Berns, S.; Boyarintsev, A.; Hugon, S.; Kose, U.; Sgalaberna, D.; De Roeck, A.; Lebedynskiy, A.; Sibilievad, T.; Zhmurin, P. A novel polystyrene-based scintillator production process involving additive manufacturing. JINST 2020, 15, P10019. [Google Scholar] [CrossRef]
- Kapłon, Ł.; Moskal, G. Blue-emitting polystyrene scintillators for plastic scintillation dosimetry. Bio-Algorithms Med-Syst. 2021, 17, 191–197. [Google Scholar] [CrossRef]
- Cho, H.Y.; Bielawski, C.W. Atom transfer radical polymerization in the solid-state. Angew. Chem. 2020, 132, 14033–14039. [Google Scholar] [CrossRef]
- Hasegawa, M.; Kimata, M.; Takahashi, I. Mechanochemical polymerization of styrene initiated by the grinding of layered clay minerals. Adv. Powder Technol. 2007, 18, 541–554. [Google Scholar] [CrossRef]
- Yoo, K.; Lee, G.S.; Lee, H.W.; Kim, B.-S.; Kim, J.G. Mechanochemical solid-state vinyl polymerization with anionic initiator. Faraday Discuss. 2023, 241, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.C.; Kim, J.S.; Kim, K.S.; Park, H.K.; Baek, S.; Ree, M. Synthesis and characterization of new, soluble polyazomethines bearing fluorene and carbazole units in the backbone and solubility-improving moieties in the side group. J. Polym. Sci. A 2004, 42, 825–834. [Google Scholar] [CrossRef]
- Iwan, A.; Boharewicz, B.; Parafiniuk, K.; Tazbir, I.; Gorecki, L.; Sikora, A.; Filapek, M.; Schab-Balcerzak, E. New air-stable aromatic polyazomethines with triphenylamine or phenylenevinylene moieties towards photovoltaic application. Synth. Met. 2014, 195, 341–349. [Google Scholar] [CrossRef]
- Wojtkiewicz, J.; Iwan, A.; Pilch, M.; Boharewicz, B.; Wójcik, K.; Tazbir, I.; Kaminska, M. Towards designing polymers for photovoltaic applications: A DFT and experimental study of polyazomethines with various chemical structures. Spectrochim. Acta A 2017, 181, 208–217. [Google Scholar] [CrossRef]
- Bolduc, A.; Al Ouahabi, A.; Mallet, C.; Skene, W.G. Insight into the isoelectronic character of azomethines and vinylenes using representative models: A spectroscopic and electrochemical Study. J. Org. Chem. 2013, 78, 9258–9269. [Google Scholar] [CrossRef]
- Şenol, D.; Kolcu, F.; Kaya, İ. Synthesis, characterization, electrical conductivity and fluorescence properties of polyimine bearing phenylacetylene units. J. Fluoresc. 2016, 26, 1579–1590. [Google Scholar] [CrossRef]
- Yen, H.J.; Liou, G.S. Recent advances in triphenylamine-based electrochromic derivatives and polymers. Polym. Chem. 2018, 9, 3001–3018. [Google Scholar] [CrossRef]
- Kaya, I.; Kilavuz, E.; Temizkan, K. Synthesis, characterization and quantum yields of multichromic poly(azomethine)s containing carbazole unit. Arab. J. Chem. 2020, 13, 1335. [Google Scholar] [CrossRef]
- Lei, T.; Guan, M.; Liu, J.; Lin, H.-C.; Pfattner, R.; Shaw, L.; McGuire, A.F.; Huang, T.-C.; Shao, L.; Cheng, K.-T.; et al. Biocompatible and totally disintegrable semiconducting polymer for ultrathin and ultralight weight transient electronics. Proc. Natl. Acad. Sci. USA 2017, 114, 5107–5112. [Google Scholar] [CrossRef]
- Grätz, S.; Borchardt, L. Mechanochemical polymerization—Controlling a polycondensation reaction between a diamine and a dialdehyde in a ball mill. RSC Adv. 2016, 6, 64799. [Google Scholar] [CrossRef]
- Al-Ithawi, W.K.A.; Khasanov, A.F.; Valieva, M.I.; Baklykov, A.V.; Chistiakov, K.A.; Ladin, E.D.; Kovalev, I.S.; Nikonov, I.L.; Kim, G.A.; Platonov, V.A.; et al. (Mechano)synthesis of azomethine- and terpyridine-linked diketopyrrolopyrrole-based polymers. Chim. Techno. Acta. 2023, 10, 202310204. [Google Scholar] [CrossRef]
- Bao, W.W.; Li, R.; Dai, Z.C.; Tang, J.; Shi, X.; Geng, J.T.; Deng, Z.F.; Hua, J. Diketopyrrolopyrrole (DPP)-Based Materials and Its Applications: A Review. Front. Chem. 2020, 8, 679. [Google Scholar] [CrossRef]
- Al-Ithawi, W.K.A.; Platonov, V.A.; Kovalev, I.S.; Kopchuk, D.S.; Nikonov, I.L.; Zyryanov, G.V.; Ranu, B.C. Mechanosynthesis of aza-linked dibenzo[a,c]phenazine-containing polymers. Butl. Commun. B 2023, 5, 8. [Google Scholar]
- Helms, B.A.; Russell, T.P. Polymer chemistries enabling cradle-to-cradle life cycles for plastics. Chem 2016, 1, 813–819. [Google Scholar] [CrossRef]
- Rahimi, A.R.; García, J.M. Chemical recycling of waste plastics for new materials production. Nat. Rev. Chem. 2017, 1, 0046. [Google Scholar] [CrossRef]
- García, J.M.; Robertson, M.L. The future of plastics recycling. Science 2017, 358, 870–872. [Google Scholar] [CrossRef]
- Hong, M.; Chen, E.Y.-X. Chemically recyclable polymers: A circular economy approach to sustainability. Green Chem. 2017, 19, 3692–3706. [Google Scholar] [CrossRef]
- MacArthur, E. Beyond plastic waste. Science 2017, 358, 843. [Google Scholar] [CrossRef]
- Schneiderman, D.K.; Hillmyer, M.A. 50th Anniversary perspective: There is a great future in sustainable polymers. Macromolecules 2017, 50, 3733–3749. [Google Scholar] [CrossRef]
- La Manria, F.P. Closed loop recycling: A case study of films for greenhouse. Polym. Degrad. Stab. 2010, 95, 285–288. [Google Scholar] [CrossRef]
- Min, H. Analysis for strategy of closed-loop supply chain with dual-recycling channel. Int. J. Prod. Econ. 2013, 144, 510–520. [Google Scholar]
- Bruno, L. Emergy assessment of benefits of closed-loop recycling accounting for material losses. Ecol. Model. 2015, 315, 77–87. [Google Scholar]
- Denissen, W.; Rivero, G.; Nicolaÿ, R.; Leibler, L.; Winne, J.M.; Du Prez, F.E. Vinylogous urethane vitrimers. Adv. Funct. Mater. 2015, 25, 2451–2457. [Google Scholar] [CrossRef]
- Fortman, D.J.; Brutman, J.P.; Cramer, C.J.; Hillmyer, M.A.; Dichtel, W.R. Mechanically activated, catalyst-free polyhydroxyurethane vitrimers. J. Am. Chem. Soc. 2015, 137, 14019–14022. [Google Scholar] [CrossRef]
- Obadia, M.M.; Mudraboyina, B.P.; Serghei, A.; Montarnal, D.; Drockenmuller, E. Reprocessing and recycling of highly cross-linked ion-conduction networks through trans-alkylation exchanges of C–N bonds. J. Am. Chem. Soc. 2015, 137, 6078–6083. [Google Scholar] [CrossRef]
- Taynton, P.; Ni, H.; Zhu, C.; Yu, K.; Loob, S.; Jin, Y.; Qi, H.J.; Zhang, W. Repairable woven carbon-fiber composites with full recyclability enabled by malleable polyimine networks. Adv. Mater. 2016, 28, 2904–2909. [Google Scholar] [CrossRef]
- Yu, K.; Shi, Q.; Dunn, M.L.; Wang, T.; Qi, H.J. Carbon fiber reinforced thermoset composite with near 100% recyclability. Adv. Funct. Mater. 2016, 26, 6098–6106. [Google Scholar] [CrossRef]
- Röttger, M.; Domenech, T.; Van Der Weegen, R.; Breuillac, A.; Nicolaÿ, R.; Leibler, L. High-performance vitrimers from commodity thermoplastics through dioxaborolane metathesis. Science 2017, 356, 62–65. [Google Scholar] [CrossRef]
- Snyder, R.L.; Fortman, D.J.; De Hoe, G.X.; Hillmyer, M.A.; Dichtel, W.R. Reprocessable acid-degradable polycarbonate vitrimers. Macromolecules 2018, 51, 389–397. [Google Scholar] [CrossRef]
- Christensen, P.R.; Scheuermann, A.M.; Loeffler, K.E.; Helms, B.A. Closed-loop recycling of plastics enabled by dynamic covalent diketoenamine bonds. Nat. Chem. 2019, 11, 442–448. [Google Scholar] [CrossRef]
- Nagarajan, V.; Mohanty, A.K.; Misra, M. Perspective on polylactic acid (pla) based sustainable materials for durable applications: Focus on toughness and heat resistance. ACS Sustain. Chem. Eng. 2016, 4, 2899–2916. [Google Scholar] [CrossRef]
- Lee, G.S.; Moon, B.R.; Jeong, H.; Shin, J.; Kim, J.G. Mechanochemical synthesis of poly(lactic acid) block copolymers: Overcoming the miscibility of the macroinitiator, monomer and catalyst under solvent-free conditions. Polym. Chem. 2019, 10, 539–545. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass, Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; Pacific Northwest National Laboratory, US Department of Energy: Richland, WA, USA, 2004. [Google Scholar]
- Marshall, A.; Jiang, B.; Gauvin, R.M.; Thomas, C.M. 2,5-Furandicarboxylic Acid: An intriguing precursor for monomer and polymer synthesis. Molecules 2022, 27, 4071. [Google Scholar] [CrossRef]
- Biddy, M.J.; Davis, R.; Humbird, D.; Tao, L.; Dowe, N.; Guarnieri, M.T.; Linger, J.G.; Karp, E.M.; Salvachúa, D.; Vardon, D.R.; et al. The techno-economic basis for coproduct manufacturing to enable hydrocarbon fuel production from lignocellulosic biomass. ACS Sustain. Chem. Eng. 2016, 4, 3196–3211. [Google Scholar] [CrossRef]
- Oh, C.; Choi, E.H.; Choi, E.J.; Premkumar, T.; Song, C. Facile solid-state mechanochemical synthesis of eco-friendly thermoplastic polyurethanes and copolymers using a biomass-derived furan diol. ACS Sustain. Chem. Eng. 2020, 8, 4400–4406. [Google Scholar] [CrossRef]
- Schmaltz, B.; Weil, T.; Müllen, K. Polyphenylene-based materials: Control of the electronic function by molecular and supramolecular complexity. Adv. Mat. 2009, 21, 1067–1078. [Google Scholar] [CrossRef]
- Baumgarten, M.; Müllen, K. Chapter 6, Functional Polyphenylenes for Supramolecular Ordering and Application in Organic Electronics. In Functional Supramolecular Architectures; Samorì, P., Cacialli, F., Eds.; Wiley: Hoboken, NJ, USA, 2011; ISBN 9783527689897. [Google Scholar] [CrossRef]
- Li, C.; Liu, M.; Pschirer, N.G.; Baumgarten, M.; Müllen, K. Polyphenylene-based materials for organic photovoltaics. Chem. Rev. 2010, 110, 6817–6855. [Google Scholar] [CrossRef]
- Chang, Y.; Cao, H.; Feng, Q.; Wei, Y.; Bian, L.; Ling, H.; Lin, D.; Xie, L.; Huang, W. Organic semiconductors based on complex diarylfluorenes via Friedel-Crafts protocols of fluorenols. Chin. Sci. Bull. 2021, 66, 4268–4283. [Google Scholar] [CrossRef]
- Lipton-Duffin, J.A.; Ivasenko, O.; Perepichka, D.F.; Rosei, F. Synthesis of polyphenylene molecular wires by surface-confined polymerization. Small 2009, 5, 592–597. [Google Scholar] [CrossRef]
- Abe, M.; Yamamoto, T. Modification of soluble polyphenylene by Friedel–Crafts reactions: Introduction of benzyl and toluoyl groups to the polyphenylene and effects of the introduced group on optical properties of the polymer. Synth. Met. 2006, 156, 1118–1122. [Google Scholar] [CrossRef]
- Vogt, C.G.; Grätz, S.; Lukin, S.; Halasz, I.; Etter, M.; Evans, J.D.; Borchardt, L. Direct Mechanocatalysis: Palladium as milling media and catalyst in the mechanochemical Suzuki polymerization. Angew. Chem. Int. Ed. 2019, 58, 18942–18947. [Google Scholar] [CrossRef] [PubMed]
- Grätz, S.; Wolfrum, B.; Borchardt, L. Mechanochemical Suzuki polycondensation—From linear to hyperbranched polyphenylenes. Green Chem. 2017, 19, 2973–2979. [Google Scholar] [CrossRef]
- Skotheim, T.A.; Elsenbaumer, R.L.; Reynolds, J.R. (Eds.) Handbook of Conducting Polymer Second Edition Revices and Expanded; Marcel Dekker Inc.: New York, NY, USA, 1998. [Google Scholar]
- Pal, B.; Yen, W.; Yang, J.; Su, W. Substituent effect on the optoelectronic properties of alternating fluorene-thiophene copolymers. Macromoloecules 2007, 40, 8189–8194. [Google Scholar] [CrossRef]
- Inagi, S.; Hayashi, S.; Hosaka, K.; Fuchigami, T. Facile functionalization of a thiophenefluorene alternating copolymer via electrochemical polymer reaction. Macromolecules 2009, 42, 3881–3883. [Google Scholar] [CrossRef]
- Ego, C.; Marsitzky, D.; Becker, S.; Zhang, J.; Grimsdale, A.C.; Müllen, K.; MacKenzie, J.D.; Silva, C.; Friend, R.H. Attaching perylene dyes to polyfluorene: Three simple, efficient methods for facile color tuning of light-emitting polymers. J. Am. Chem. Soc. 2003, 125, 437–443. [Google Scholar] [CrossRef]
- Coffin, R.C.; Peet, J.; Rogers, J.; Bazan, G.C. Streamlined microwave-assisted preparation of narrow-bandgap conjugated polymers for high-performance bulk heterojunction solar cells. Nat. Chem. 2009, 1, 657–661. [Google Scholar] [CrossRef]
- Zhang, Y.; Tajima, K.; Hirota, K.; Hashimoto, K. Synthesis of all-conjugated diblock copolymers by quasi-living polymerization and observation of their microphase separation. J. Am. Chem. Soc. 2008, 130, 7812–7813. [Google Scholar] [CrossRef]
- Rothe, C.; Galbrecht, F.; Scherf, U.; Monkman, A. The β-phase of poly(9,9-dioctylfluorene) as a potential system for electrically pumped organic lasing. Adv. Mater. 2006, 18, 2137–2140. [Google Scholar] [CrossRef]
- Grell, M.; Bradley, D.D.C.; Ungar, G.; Hill, J.; Whitehead, K.S. Interplay of physical structure and photophysics for a liquid crystalline polyfluorene. Macromolecules 1999, 32, 5810–5817. [Google Scholar] [CrossRef]
- Hayashi, S.; Inagi, S.; Fuchigami, T. Macrostructural order and optical properties of polyfluorene-based polymer films. Polym. J. 2010, 42, 772–775. [Google Scholar] [CrossRef]
- Neher, D. Polyfluorene homopolymers: Conjugated liquid-crystalline polymers for bright blue emission and polarized electroluminescence. Macromol. Rapid Commun. 2001, 22, 1365–1385. [Google Scholar] [CrossRef]
- Scherf, U.; List, E.J.W. Semiconducting polyfluorenes-towards reliable structure–property relationships. Adv. Mater. 2002, 14, 477–487. [Google Scholar] [CrossRef]
- Knaapila, M.; Monkman, A.P. Methods for controlling structure and photophysical properties in polyfluorene solutions and gels. Adv. Mater. 2013, 25, 1090–1108. [Google Scholar] [CrossRef]
- Nirmani, L.P.T.; Pary, F.F.; Nelson, T.L. Mechanochemical Suzuki polymerization for the synthesis of polyfluorenes. Green Chem. Lett. Rev. 2022, 15, 863–868. [Google Scholar] [CrossRef]
- Bhadra, J.; Alkareem, A.; Al-Thani, N. A review of advances in the preparation and application of polyaniline based thermoset blends and composites. J. Polym. Res. 2020, 27, 122. [Google Scholar] [CrossRef]
- Gómez, I.J.; Sulleiro, M.V.; Mantione, D.; Alegret, N. Carbon nanomaterials embedded in conductive polymers: A state of the art. Polymers 2021, 13, 745. [Google Scholar] [CrossRef]
- Beygisangchin, M.; Rashid, S.A.; Shafie, S.; Sadrolhosseini, A.R.; Lim, H.N. Preparations, properties, and applications of polyaniline and polyaniline thin films—A review. Polymers 2021, 13, 2003. [Google Scholar] [CrossRef]
- Jaymand, M. Recent progress in chemical modification of polyaniline. Prog. Polym. Sci. 2013, 38, 1287–1306. [Google Scholar] [CrossRef]
- Liao, G. Green preparation of sulfonated polystyrene/polyaniline/silver composites with enhanced anticorrosive properties. Int. J. Chem. 2018, 10, 81. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, W.; Wang, C.; Wen, T.C.; Wei, Y. One-dimensional conducting polymer nanocomposites: Synthesis, properties and applications. Prog. Polym. Sci. 2011, 36, 671–712. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Zhang, X.X.; Tian, D.; Xiong, R.; Lu, C.H. Solvent free synthesis of polyaniline with improved molecular weight through solid state mechanochemical milling at ambient temperature. Mater. Res. Innov. 2013, 17, 84–91. [Google Scholar] [CrossRef]
- Šeděnková, I.; Konyushenko, E.N.; Stejskal, J.; Trchová, M.; Prokeš, J. Solid-state oxidation of anilinium chloride with various oxidants. Synth. Met. 2011, 161, 1353–1360. [Google Scholar] [CrossRef]
- Posudievsky, O.Y.; Goncharuk, O.A.; Barillé, R.; Pokhodenko, V.D. Structure-property relationship in mechanochemically prepared polyaniline. Synth. Met. 2010, 160, 462–467. [Google Scholar] [CrossRef]
- Huang, J.; Moore, J.A.; Acquaye, J.H.; Kaner, R.B. Mechanochemical route to the conducting polymer polyaniline. Macromolecules 2005, 38, 317–321. [Google Scholar] [CrossRef]
- Bhandari, S.; Khastgir, D. Template-free solid state synthesis of ultra-long hairy polyaniline nanowire supercapacitor. Mater. Lett. 2014, 135, 202–205. [Google Scholar] [CrossRef]
- Pandian, P.; Kalimuthu, R.; Arumugam, S.; Kannaiyan, P. Solid phase mechanochemical synthesis of poly(o-anisidine) protected silver nanoparticles for electrochemical dopamine sensor. Mater. Today Commun. 2021, 26, 102191. [Google Scholar] [CrossRef]
- Barbero, C.A.; Acevedo, D.F. Mechanochemical synthesis of polyanilines and their nanocomposites: A critical review. Polymers 2023, 15, 133. [Google Scholar] [CrossRef]
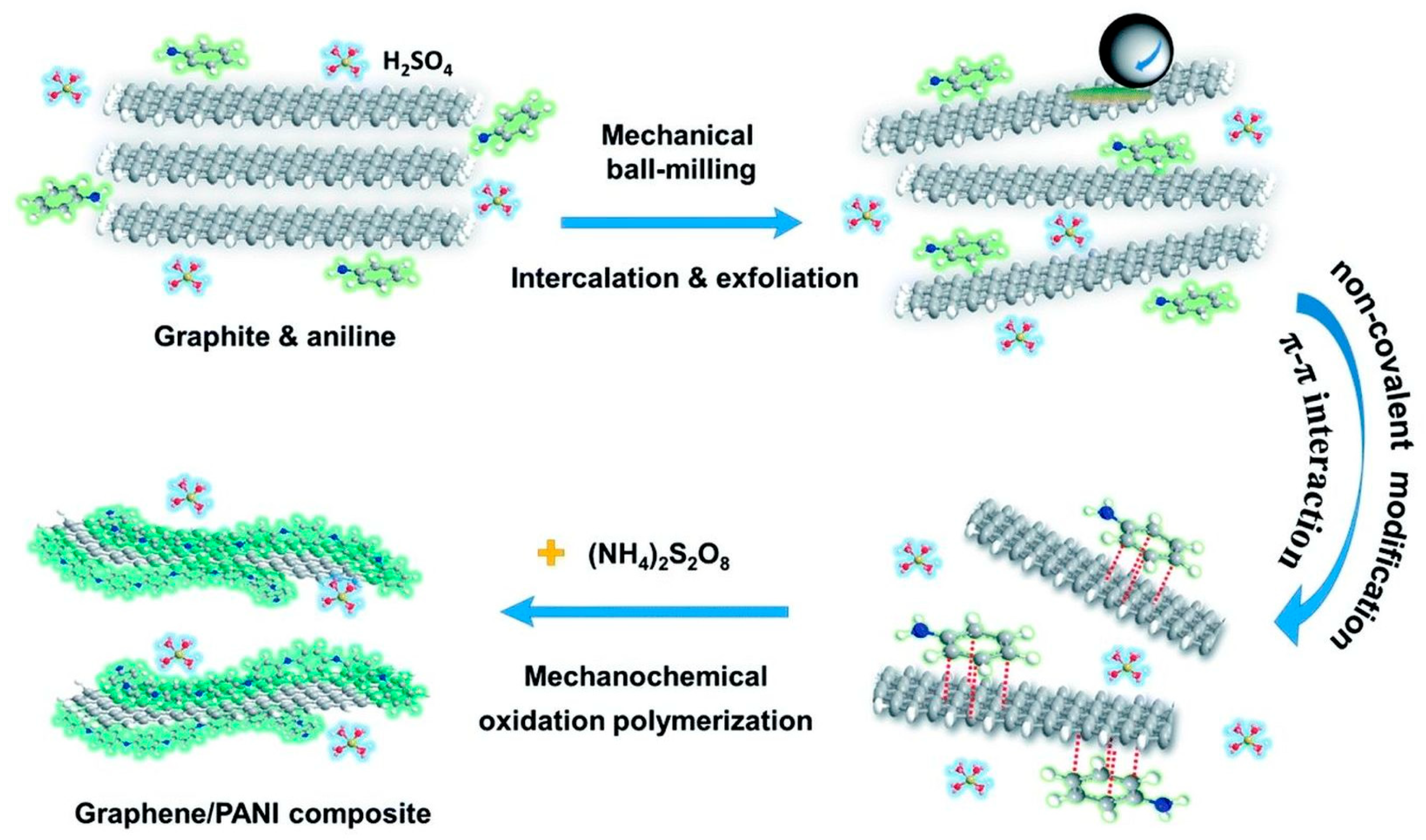
- Jiang, Y.; Ji, J.; Huang, L.; He, C.; Zhang, J.; Wang, X.; Yang, Y. One-pot mechanochemical exfoliation of graphite and in situ polymerization of aniline for the production of graphene/polyaniline composites for high-performance supercapacitors. RSC Adv. 2020, 10, 44688. [Google Scholar] [CrossRef]
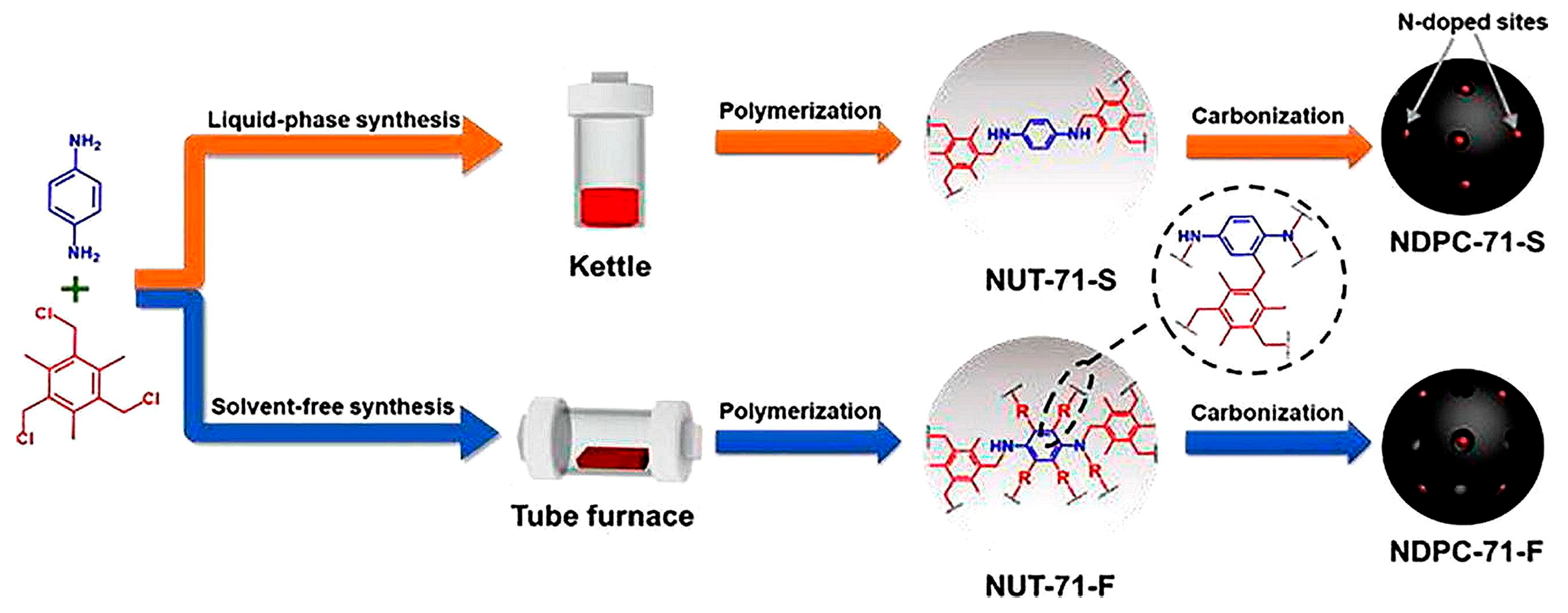
- Lou, Y.-C.; Qi, S.-C.; Xue, D.-M.; Gu, C.; Zhou, R.; Liu, X.-Q.; Sun, L.-B. Solvent-free synthesis of n-containing polymers with high cross-linking degree to generate n-doped porous carbons for high-efficiency CO2 capture. Chem. Eng. J. 2020, 399, 125845. [Google Scholar] [CrossRef]
- Das, S.; Heasman, P.; Ben, T.; Qiu, S. Porous organic materials: Strategic design and structure–function correlation. Chem. Rev. 2016, 117, 1515–1563. [Google Scholar] [CrossRef]
- Xu, Y.; Jin, S.; Xu, H.; Nagai, A.; Jiang, D. Conjugated microporous polymers: Design, synthesis and application. Chem. Soc. Rev. 2013, 42, 8012–8031. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, X.; Zheng, B.; Huang, L.; Hang, C.; Jiao, Y.; Cao, X.; Zeng, W.; Yun, R. Highly selective carbon dioxide capture and cooperative catalysis of a water-stable acylamide-functionalized metal–organic framework. Eur. J. Inorg. Chem. 2018, 2018, 1309–1314. [Google Scholar] [CrossRef]
- Yoon, M.; Srirambalaji, R.; Kim, K. Homochiral metal–organic frameworks for asymmetric heterogeneous catalysis. Chem. Rev. 2011, 112, 1196–1231. [Google Scholar] [CrossRef]
- Xu, S.; Song, K.; Li, T.; Tan, B. Palladium catalyst coordinated in knitting N-heterocyclic carbene porous polymers for efficient Suzuki–Miyaura coupling reactions. J. Mater. Chem. A 2015, 3, 1272–1278. [Google Scholar] [CrossRef]
- Pan, L.; Xu, M.-Y.; Feng, L.-J.; Chen, Q.; He, Y.-J.; Han, B.-H. Conjugated microporous polycarbazole containing tris (2-phenylpyridine) iridium (III) complexes: Phosphorescence, porosity, and heterogeneous organic photocatalysis. Polym. Chem. 2016, 7, 2299–2307. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, S.; Yan, J.; Li, L.; Wu, G.; Shi, W.; Yang, G.; Guan, N.; Cheng, P. Polyoxometalate-based metal–organic frameworks as visible-light-induced photocatalysts. Inorg. Chem. 2018, 57, 5030–5037. [Google Scholar] [CrossRef]
- Zheng, B.; Liu, H.; Wang, Z.; Yu, X.; Yi, P.; Bai, J. Porous NbO-type metal–organic framework with inserted acylamide groups exhibiting highly selective CO2 capture. CrystEngComm 2013, 15, 3517–3520. [Google Scholar] [CrossRef]
- Lu, Z.; Du, L.; Zheng, B.; Bai, J.; Zhang, M.; Yun, R. A highly porous agw-type metal–organic framework and its CO2 and H2 adsorption capacity. CrystEngComm 2013, 15, 9348–9351. [Google Scholar] [CrossRef]
- Du, N.; Park, H.B.; Robertson, G.P.; Dal-Cin, M.M.; Visser, T.; Scoles, L.; Guiver, M.D. Polymer nanosieve membranes for CO2-capture applications. Nat. Mater. 2011, 10, 372–375. [Google Scholar] [CrossRef]
- Li, G.; Liu, Q.; Xia, B.; Huang, J.; Li, S.; Guan, Y.; Zhou, H.; Liao, B.; Zhou, Z.; Liu, B. Synthesis of stable metal-containing porous organic polymers for gas storage. Eur. Polym. J. 2017, 91, 242–247. [Google Scholar] [CrossRef]
- Zheng, B.; Huang, L.; Cao, X.; Shen, S.; Cao, H.; Hang, C.; Zeng, W.; Wang, Z. A highly porous acylamide decorated MOF-505 analogue exhibiting high and selective CO2 gas uptake capability. CrystEngComm 2018, 20, 1874–1881. [Google Scholar] [CrossRef]
- Zheng, B.; Luo, X.; Wang, Z.; Zhang, S.; Yun, R.; Huang, L.; Zeng, W.; Liu, W. An unprecedented water stable acylamide-functionalized metal–organic framework for highly efficient CH4/CO2 gas storage/separation and acid–base cooperative catalytic activity. Inorg. Chem. Front. 2018, 5, 2355–2363. [Google Scholar] [CrossRef]
- Sun, H.; Ren, D.; Kong, R.; Wang, D.; Jiang, H.; Tan, J.; Wu, D.; Chen, S.; Shen, B. Tuning 1-hexene/n-hexane adsorption on MOF-74 via constructing Co-Mg bimetallic frameworks. Microporous Mesoporous Mater. 2019, 284, 151–160. [Google Scholar] [CrossRef]
- Novoa-Cid, M.; Melillo, A.; Ferrer, B.; Alvaro, M.; Baldovi, H.G. Photocatalytic water splitting promoted by 2d and 3d porphyrin covalent organic polymers synthesized by Suzuki-Miyaura carbon-carbon coupling. Nanomaterials 2022, 12, 3197. [Google Scholar] [CrossRef]
- Martín, C.F.; Stöckel, E.; Clowes, R.; Adams, D.J.; Cooper, A.I.; Pis, J.J.; Rubiera, F.; Pevida, C. Hypercrosslinked organic polymer networks as potential adsorbents for pre-combustion CO2 capture. J. Mater. Chem. 2011, 21, 5475–5483. [Google Scholar] [CrossRef]
- Kuhn, P.; Antonietti, M.; Thomas, A. Porous, covalent triazine-based frameworks prepared by ionothermal synthesis. Angew. Chem. Int. Ed. 2008, 47, 3450–3453. [Google Scholar] [CrossRef]
- Pandey, P.; Katsoulidis, A.P.; Eryazici, I.; Wu, Y.; Kanatzidis, M.G.; Nguyen, S.T. Imine-linked microporous polymer organic frameworks. Chem. Mater. 2010, 22, 4974–4979. [Google Scholar] [CrossRef]
- Ritter, N.; Senkovska, I.; Kaskel, S.; Weber, J. Towards chiral microporous soluble polymers—Binaphthalene-based polyimides. Macromol. Rapid Commun. 2011, 32, 438–443. [Google Scholar] [CrossRef]
- Tan, L.; Tan, B. Hypercrosslinked porous polymer materials: Design, synthesis, and applications. Chem. Soc. Rev. 2017, 46, 3322–3356. [Google Scholar] [CrossRef]
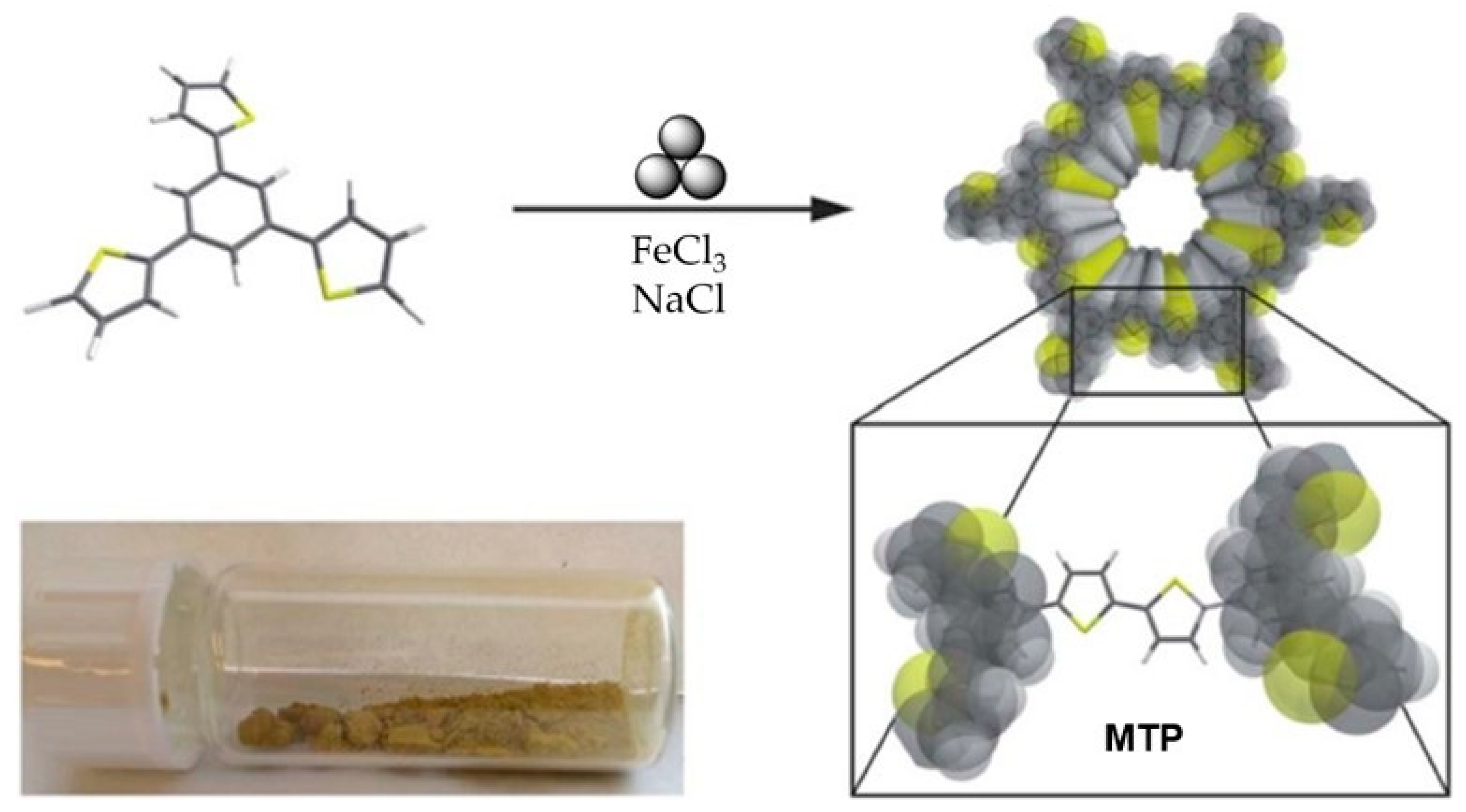
- Grätz, S.; Zink, S.; Kraffczyk, H.; Rose, M.; Borchardt, L. Mechanochemical synthesis of hyper-crosslinked polymers: Influences on their pore structure and adsorption behaviour for organic vapors. Beilstein J. Org. Chem. 2019, 15, 1154–1161. [Google Scholar] [CrossRef]
- Giri, A.; Biswas, S.; Hussain, M.W.; Dutta, T.K.; Patra, A. Nanostructured Hypercrosslinked Porous Organic Polymers: Morphological Evolution and Rapid Separation of Polar Organic Micropollutants. ACS Appl. Mater. Interfaces. 2022, 14, 7369–7381. [Google Scholar] [CrossRef]
- Grätz, S.; Oltermann, M.; Troschke, E.; Paasch, S.; Krause, S.; Brunner, E.; Borchardt, L. Solvent-free synthesis of a porous thiophene polymer by mechanochemical oxidative polymerization. J. Mater. Chem. A 2018, 6, 21901–21905. [Google Scholar] [CrossRef]
- Konnert, L.; Gauliard, A.; Lamaty, F.; Martinez, J.; Colacino, E. Solventless synthesis of N-protected amino acids in a ball mill. ACS Sustain. Chem. Eng. 2013, 1, 1186–1191. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, D.-P.; Luo, M.; Feng, L.-J.; Zhao, Y.-C.; Han, B.-H. Nitrogen-containing microporous conjugated polymers via carbazole-based oxidative coupling polymerization: Preparation, porosity, and gas uptake. Small 2014, 10, 308–315. [Google Scholar] [CrossRef]
- Zhu, X.; Tian, C.; Jin, T.; Browning, K.L.; Sacci, R.L.; Veith, G.M.; Dai, S. Solid-state synthesis of conjugated nanoporous polycarbazoles. ACS Macro Lett. 2017, 6, 1056–1059. [Google Scholar] [CrossRef]
- Krusenbaum, A.; Geisler, J.; Kraus, F.J.L.; Grätz, S.; Höfler, M.V.; Gutmann, T.; Borchardt, L. The mechanochemical Friedel-Crafts polymerization as a solvent-free cross-linking approach toward microporous polymers. J. Polym. Sci. 2022, 60, 62–71. [Google Scholar] [CrossRef]
- McKeown, N.B.; Budd, P.M. Polymers of intrinsic microporosity (PIMs): Organic materials for membrane separations, heterogeneous catalysis and hydrogen storage. Chem. Soc. Rev. 2006, 35, 675–683. [Google Scholar] [CrossRef]
- McKeown, N.B.; Budd, P.M.; Msayib, K.J.; Ghanem, B.S.; Kingston, H.J.; Tattershall, C.E.; Makhseed, S.; Reynolds, K.J.; Fritsch, D. Polymers of Intrinsic Microporosity (PIMs): Bridging the Void between Microporous and Polymeric Materials. Chem. Eur. J. 2005, 11, 2610–2620. [Google Scholar] [CrossRef]
- Zhu, X.; Hua, Y.; Tian, C.; Abney, C.W.; Zhang, P.; Jin, T.; Liu, G.; Browning, K.L.; Sacci, R.L.; Veith, G.M.; et al. Accelerating membrane-based CO2 separation by soluble nanoporous polymer networks produced by mechanochemical oxidative coupling. Angew. Chem. Int. Ed. 2018, 57, 2816–2821. [Google Scholar] [CrossRef]
- Ma, H.; Ren, H.; Meng, S.; Sun, F.; Zhu, G. Novel porphyrinic porous organic frameworks for high performance separation of small hydrocarbons. Sci. Rep. 2013, 3, 2611. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, P.; Forget, A.; Su, D.; Thomas, A.; Antonietti, M. From microporous regular frameworks to mesoporous materials with ultrahigh surface area: Dynamic reorganization of porous polymer networks. J. Am. Chem. Soc. 2008, 130, 13333–13337. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, P.; Thomas, A.; Antonietti, M. Toward tailorable porous organic polymer networks: A high-temperature dynamic polymerization scheme based on aromatic nitriles. Macromolecules 2009, 42, 319–326. [Google Scholar] [CrossRef]
- Liao, L.; Li, M.; Yin, Y.; Chen, J.; Zhong, Q.; Du, R.; Liu, S.; He, Y.; Fu, W.; Zeng, F. Advances in the Synthesis of Covalent Triazine Frameworks. ACS Omega 2023, 8, 4527–4542. [Google Scholar] [CrossRef]
- Ren, S.; Bojdys, M.J.; Dawson, R.; Laybourn, A.; Khimyak, Y.Z.; Adams, D.J.; Cooper, A.I. Porous, fluorescent, covalent triazine-based frameworks via room-temperature and microwave-assisted synthesis. Adv. Mater. 2012, 24, 2357–2361. [Google Scholar] [CrossRef]
- Kuecken, S.; Schmidt, J.; Zhi, L.; Thomas, A. Conversion of amorphous polymer networks to covalent organic frameworks under ionothermal conditions: A facile synthesis route for covalent triazine frameworks. J. Mater. Chem. A. 2015, 3, 24422–24427. [Google Scholar] [CrossRef]
- Hao, L.; Ning, J.; Luo, B.; Wang, B.; Zhang, Y.; Tang, Z.; Yang, J.; Thomas, A.; Zhi, L. Structural evolution of 2D microporous covalent triazine-based framework toward the study of high-performance supercapacitors. J. Am. Chem. Soc. 2015, 137, 219–225. [Google Scholar] [CrossRef]
- Liao, H.; Ding, H.; Li, B.; Ai, X.; Wang, C. Covalent-organic frameworks: Potential host materials for sulfur impregnation in lithium–sulfur batteries. J. Mater. Chem. A 2014, 2, 8854–8858. [Google Scholar] [CrossRef]
- Talapaneni, S.N.; Hwang, T.H.; Je, S.H.; Buyukcakir, O.; Choi, J.W.; Coskun, A. Elemental-Sulfur-Mediated Facile Synthesis of a Covalent Triazine Framework for High-Performance Lithium–Sulfur Batteries. Angew. Chem. Int. Ed. 2016, 55, 3106–3111. [Google Scholar] [CrossRef]
- Borchardt, L.; Oschatz, M.; Kaskel, S. Carbon Materials for Lithium Sulfur Batteries—Ten Critical Questions. Chem. Eur. J. 2016, 22, 7324–7351. [Google Scholar] [CrossRef]
- Dey, S.; Bhunia, A.; Esquivel, D.; Janiak, C. Covalent triazine-based frameworks (CTFs) from triptycene and fluorene motifs for CO2 adsorption. J. Mater. Chem. A 2016, 4, 6259–6263. [Google Scholar] [CrossRef]
- Hug, S.; Stegbauer, L.; Oh, H.; Hirscher, M.; Lotsch, B.V. Nitrogen-rich covalent triazine frameworks as high-performance platforms for selective carbon capture and storage. Chem. Mater. 2015, 27, 8001–8010. [Google Scholar] [CrossRef]
- Zhao, Y.; Yao, K.X.; Teng, B.; Zhang, T.; Han, Y. A perfluorinated covalent triazine-based framework for highly selective and water–tolerant CO2 capture. Energy Environ. Sci. 2013, 6, 3684–3692. [Google Scholar] [CrossRef]
- Troschke, E.; Grätz, S.; Lübken, T.; Borchardt, L. Mechanochemical Friedel–Crafts alkylation—A sustainable pathway towards porous organic polymers. Angew. Chem. 2017, 129, 6963–6967. [Google Scholar] [CrossRef]
- Yuan, R.; Yan, Z.; Shaga, A.; He, H. Solvent-free mechanochemical synthesis of a carbazole-based porous organic polymer with high CO2 capture and separation. J. Solid State Chem. 2020, 287, 121327. [Google Scholar] [CrossRef]
- Wang, S.; Dai, T.; Lu, Y.; Chen, Q.; Feng, L.; Sui, Z. Fullerene-bearing porous polymer via ball-milling approach and its palladium composite for catalytic deallylation. Microporous Mesoporous Mater. 2020, 302, 110187. [Google Scholar] [CrossRef]
- Giacalone, F.; Martín, N. Fullerene polymers: Synthesis and properties. Chem. Rev. 2006, 106, 5136–5190. [Google Scholar] [CrossRef]
- Balch, A.L.; Winkler, K. Two-component polymeric materials of fullerenes and the transition metal complexes: A bridge between metal–organic frameworks and conducting polymers. Chem. Rev. 2016, 116, 3812–3882. [Google Scholar] [CrossRef]
- Wang, C.; Guo, Z.-X.; Fu, S.; Wu, W.; Zhu, D. Polymers containing fullerene or carbon nanotube structures. Prog. Polym. Sci. 2004, 29, 1079–1141. [Google Scholar] [CrossRef]
- Kang, H.; Cho, C.-H.; Cho, H.-H.; Kang, T.E.; Kim, H.J.; Kim, K.-H.; Yoon, S.C.; Kim, B.J. Controlling number of indene solubilizing groups in multiadduct fullerenes for tuning optoelectronic properties and open-circuit voltage in organic solar cells. ACS Appl. Mater. Interfaces 2012, 4, 110–116. [Google Scholar] [CrossRef]
- Pan, Q.; Xu, Z.; Deng, S.; Zhang, F.; Li, H.; Cheng, Y.; Wei, L.; Wang, J.; Zhou, B. A Mechanochemically synthesized porous organic polymer derived CQD/chitosan–graphene composite film electrode for electrochemiluminescence determination of dopamine. RSC Adv. 2019, 9, 39332–39337. [Google Scholar] [CrossRef] [PubMed]
- Ohura, T.; Tsutaki, Y.; Sakaguchi, M. Novel synthesis of cellulose-based diblock copolymer of poly(hydroxyethyl methacrylate) by mechanochemical reaction. Sci. World J. 2014, 2014, 127506. [Google Scholar] [CrossRef] [PubMed]
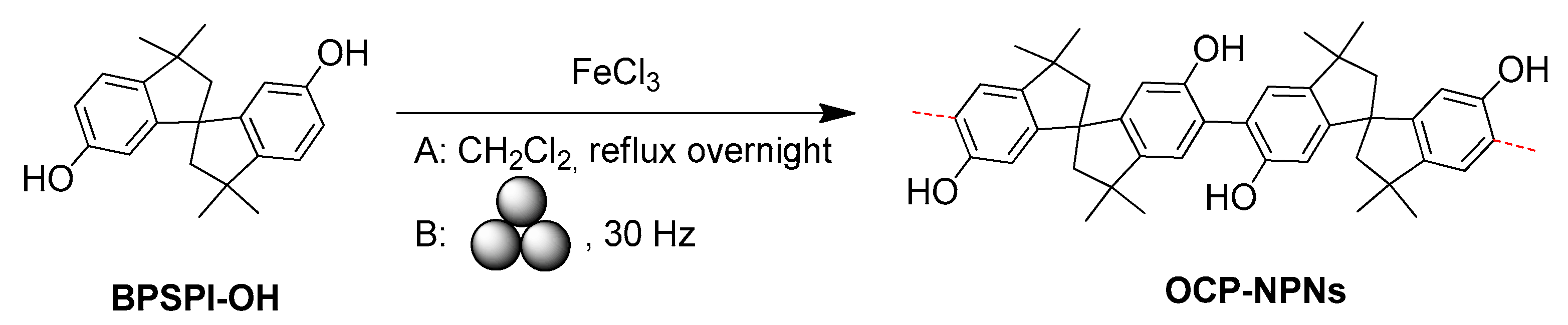
- Ohn, N.; Kim, J.G. Mechanochemical post-polymerization modification: Solvent-free solid-state synthesis of functional polymers. ACS Macro Lett. 2018, 7, 561–565. [Google Scholar] [CrossRef] [PubMed]
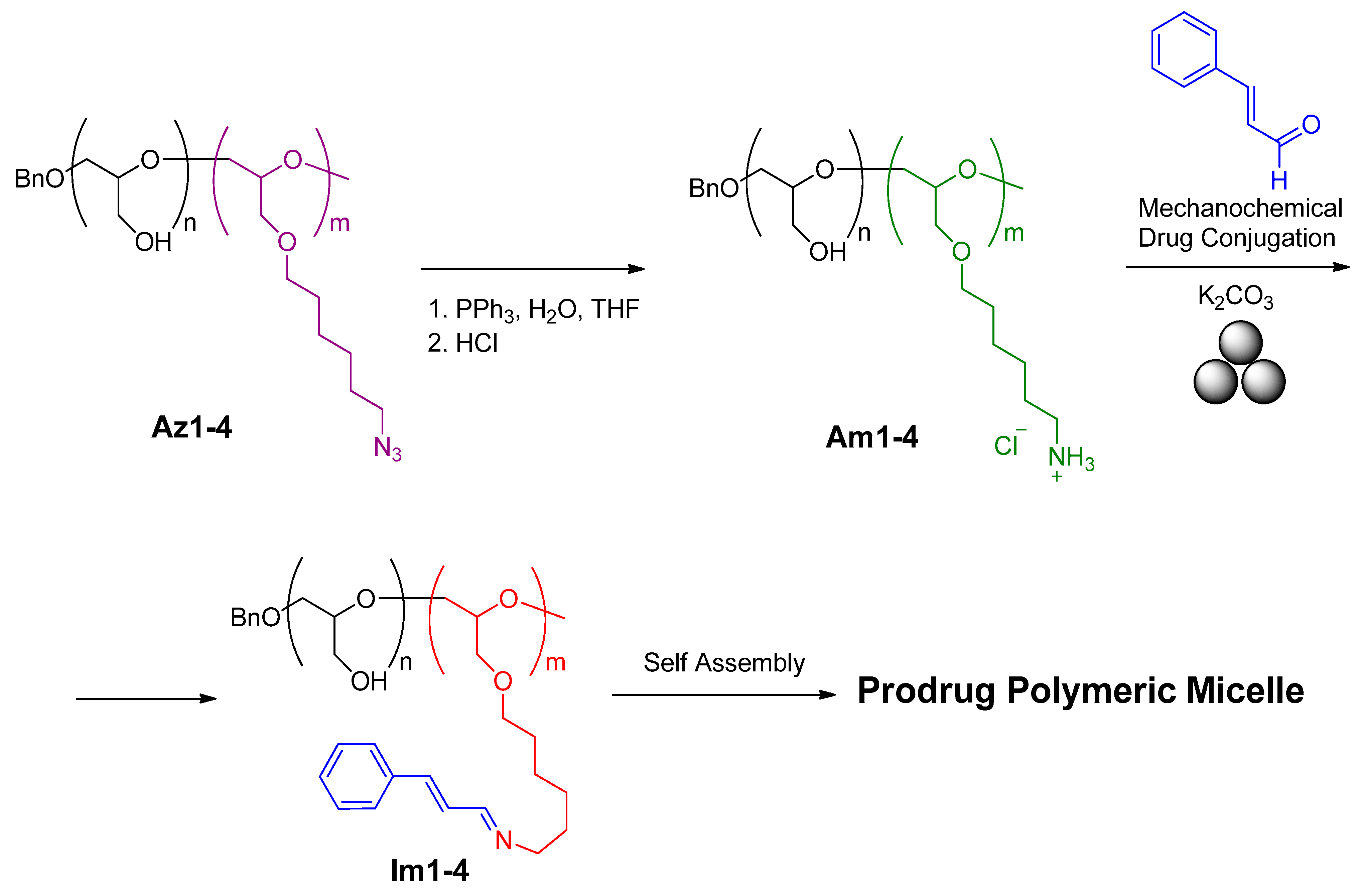
- Han, S.; Lee, J.; Jung, E.; Park, S.; Sagawa, A.; Shibasaki, Y.; Kim, B.-S. Mechanochemical drug conjugation via ph-responsive imine linkage for polyether prodrug micelles. ACS Appl. Bio Mater. 2021, 4, 2465–2474. [Google Scholar] [CrossRef] [PubMed]
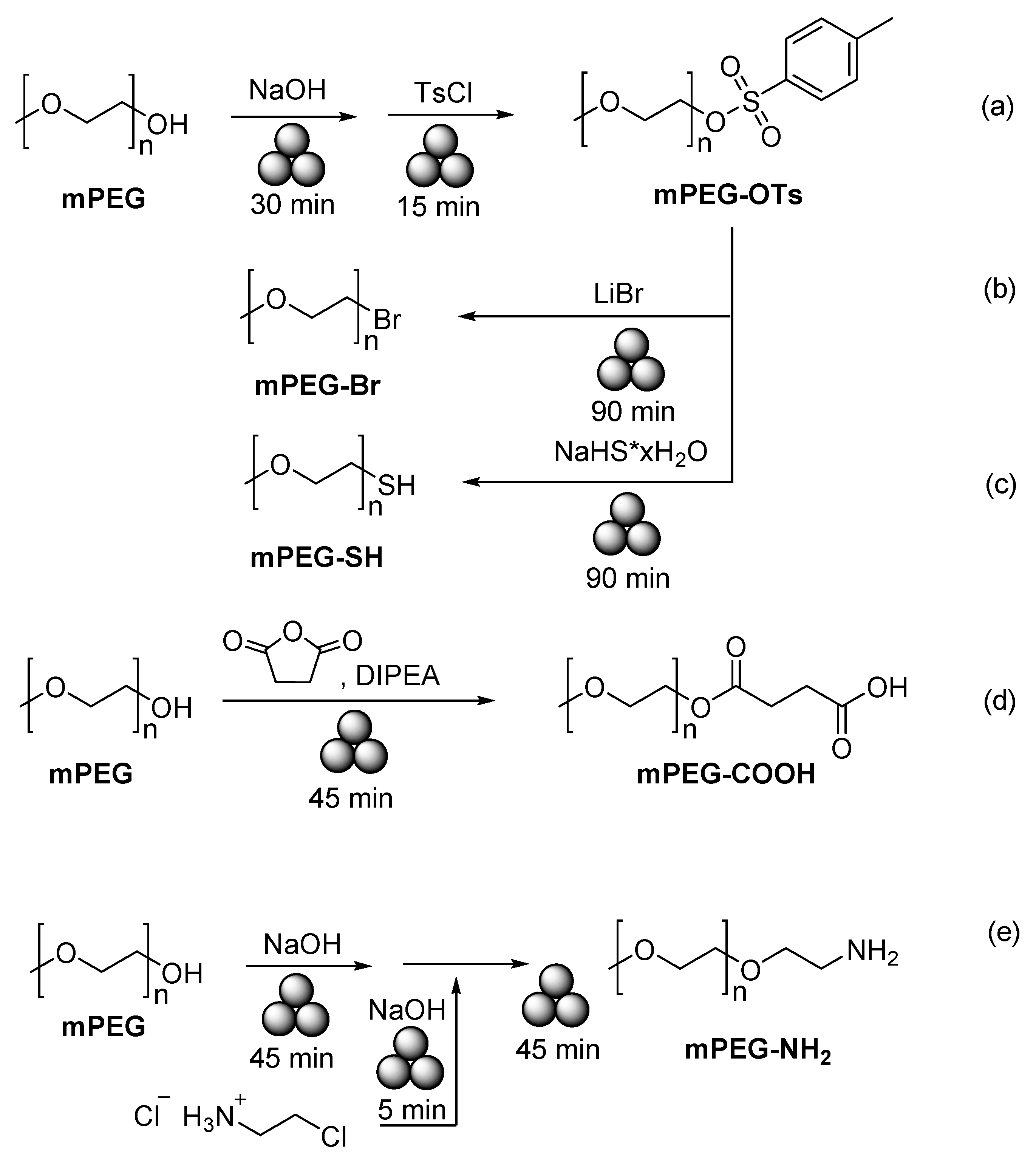
- Malca, M.Y.; Ferko, P.-O.; Friščić, T.; Moores, A. Solid-state mechanochemical ω functionalization of poly(ethylene glycol). Beilstein J. Org. Chem. 2017, 13, 1963–1968. [Google Scholar] [CrossRef]
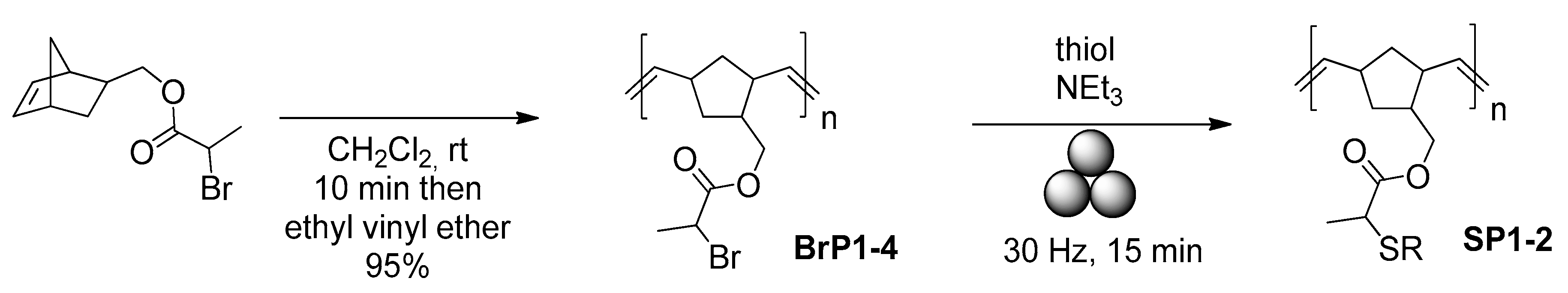
- Ashlin, M.; Hobbs, C.E. Post-polymerization thiol substitutions facilitated by mechanochemistry. Macromol. Chem. Phys. 2019, 220, 1900350. [Google Scholar] [CrossRef]
- Lee, G.S.; Lee, H.W.; Lee, H.S.; Do, T.; Do, J.-L.; Lim, J.; Peterson, G.I.; Friščić, T.; Kim, J.G. Mechanochemical ring-opening metathesis polymerization: Development, scope, and mechano-exclusive polymer synthesis. Chem. Sci. 2022, 13, 11496–11505. [Google Scholar] [CrossRef]
- Do, J.-L.; Mottillo, C.; Tan, D.; Štrukil, V.; Friščić, T. Mechanochemical ruthenium-catalyzed olefin metathesis. J. Am. Chem. Soc. 2015, 137, 2476–2479. [Google Scholar] [CrossRef]
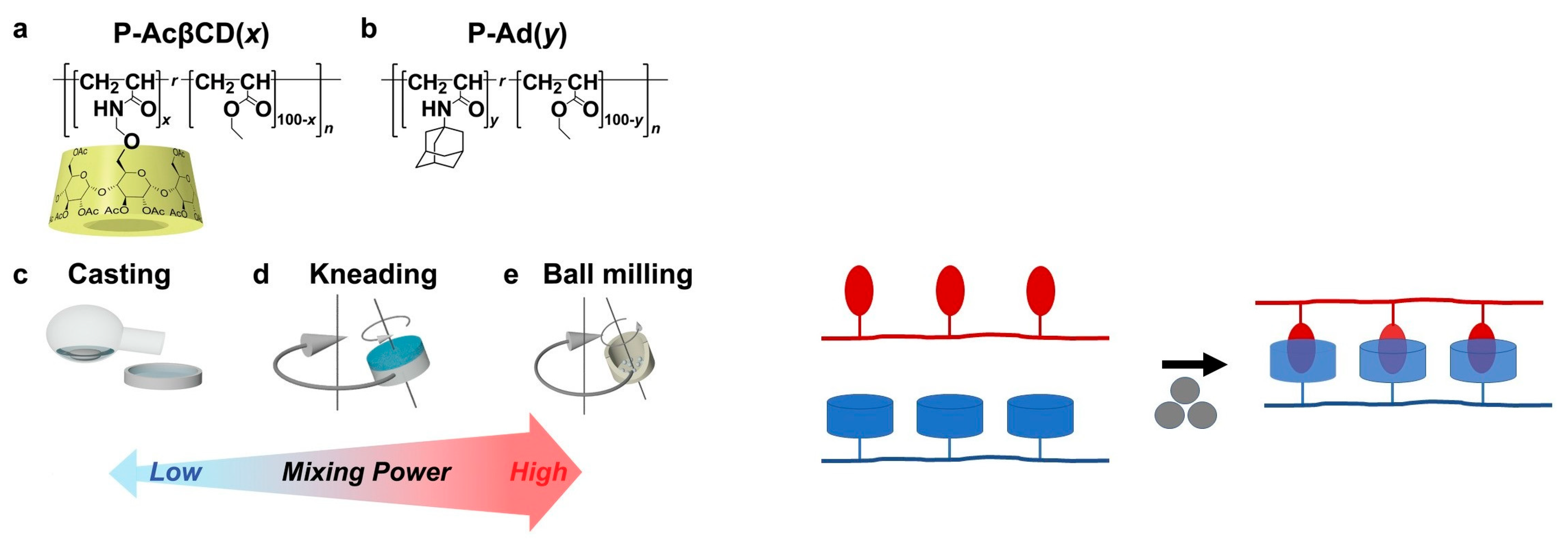
- Park, J.; Murayama, S.; Osaki, M.; Yamaguchi, H.; Harada, A.; Matsuba, G.; Takashima, Y. Extremely rapid self-healable and recyclable supramolecular materials through planetary ball milling and host–guest interactions. Adv. Mat. 2020, 32, 2002008. [Google Scholar] [CrossRef]
- Kubota, K.; Toyoshima, N.; Miura, D.; Jiang, J.; Maeda, S.; Jin, M.; Ito, H. Introduction of a luminophore into generic polymers via mechanoradical coupling with a prefluorescent reagent. Angew. Chem. Int. Ed. 2021, 60, 16003–16008. [Google Scholar] [CrossRef]
- Chung, W.J.; Griebel, J.J.; Kim, E.T.; Yoon, H.; Simmonds, A.G.; Ji, H.J.; Dirlam, P.T.; Glass, R.S.; Wie, J.J.; Nguyen, N.A.; et al. The use of elemental sulfur as an alternative feedstock for polymeric materials. Nat. Chem. 2013, 5, 518–524. [Google Scholar] [CrossRef]
- Yan, P.; Zhao, W.; McBride, F.; Cai, D.; Dale, J.; Hanna, V.; Hasell, T. Mechanochemical synthesis of inverse vulcanized polymers. Nat. Commun. 2022, 13, 4824. [Google Scholar] [CrossRef]
- Cavalieri, F.; Padella, F. Development of composite materials by mechanochemical treatment of post-consumer plastic waste. Waste Manag. 2002, 22, 913–916. [Google Scholar] [CrossRef]
- Baheti, V.; Abbasi, R.; Militky, J. Ball milling of jute fiber wastes to prepare nanocellulose. World J. Eng. 2012, 9, 45–50. [Google Scholar] [CrossRef]
- Razzak, S.A.-A.; Lofgren, E.A.; Jabarin, S.A. End-group determination in poly(ethylene terephthalate) by infrared spectroscopy. Polym. Int. 2002, 51, 174–182. [Google Scholar]
- Takacs, L.; McHenry, J.S. Temperature of the milling balls in shaker and planetary mills. J. Mater Sci. 2006, 41, 5246–5249. [Google Scholar] [CrossRef]
- Kubota, K.; Ito, H. Mechanochemical Cross-Coupling Reactions. Trends Chem. 2020, 2, 1066–1081. [Google Scholar] [CrossRef]
- Post, W.; Susa, A.; Blaauw, R.; Molenveld, K.; Knoop, R.J.I. A review on the potential and limitations of recyclable thermosets for structural applications. Polym. Rev. 2020, 60, 359–388. [Google Scholar] [CrossRef]
- Worch, J.C.; Dove, A.P. 100th anniversary of macromolecular science viewpoint: Toward catalytic chemical recycling of waste (and future) plastics. ACS Macro Lett. 2020, 9, 1494–1506. [Google Scholar] [CrossRef]
- Qin, B.; Liu, S.; Huang, Z.; Zeng, L.; Xu, J.-F.; Zhang, X. Closed-loop chemical recycling of cross-linked polymeric materials based on reversible amidation chemistry. Nat. Commun. 2022, 13, 7595. [Google Scholar] [CrossRef]
- Hussain, Z.; Khan, K.M.; Hussain, K. Microwave–metal interaction pyrolysis of polystyrene. J. Anal. Appl. Pyrolysis 2010, 89, 39–43. [Google Scholar] [CrossRef]
- Bartoli, M.; Rosi, L.; Frediani, M.; Undri, A.; Frediani, P. Depolymerization of polystyrene at reduced pressure through a microwave assisted pyrolysis. J. Anal. Appl. Pyrolysis 2015, 113, 281–287. [Google Scholar] [CrossRef]
- Onwudili, J.A.; Insura, N.; Williams, P.T. Composition of products from the pyrolysis of polyethylene and polystyrene in a closed batch reactor: Effects of temperature and residence time. J. Anal. Appl. Pyrolysis 2009, 86, 293–303. [Google Scholar] [CrossRef]
- Undri, A.; Frediani, M.; Rosi, L.; Frediani, P. Reverse polymerization of waste polystyrene through microwave assisted pyrolysis. J. Anal. Appl. Pyrolysis 2014, 105, 35–42. [Google Scholar] [CrossRef]
- Faravelli, T.; Pinciroli, M.; Pisano, F.; Bozzano, G.; Dente, M.; Ranzi, E. Thermal degradation of polystyrene. J. Anal. Appl. Pyrolysis 2001, 60, 103–121. [Google Scholar] [CrossRef]
- Štrukil, V. Highly efficient solid-state hydrolysis of waste polyethylene terephthalate by mechanochemical milling and vapor-assisted aging. ChemSusChem 2021, 14, 330–338. [Google Scholar] [CrossRef]
- Balema, V.P.; Hlova, I.Z.; Carnahan, S.L.; Seyedi, M.; Dolotko, O.; Rossini, A.J.; Luzinov, I. Depolymerization of polystyrene under ambient conditions. New J. Chem. 2021, 45, 2935–2938. [Google Scholar] [CrossRef]
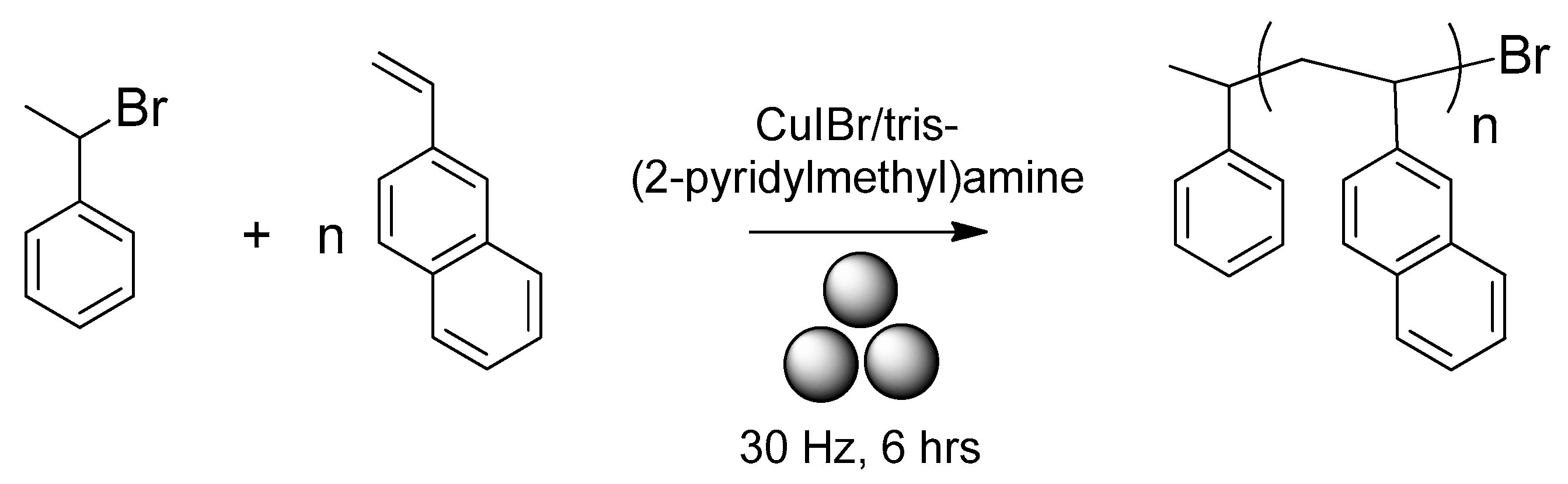









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ithawi, W.K.A.; Khasanov, A.F.; Kovalev, I.S.; Nikonov, I.L.; Platonov, V.A.; Kopchuk, D.S.; Santra, S.; Zyryanov, G.V.; Ranu, B.C. TM-Free and TM-Catalyzed Mechanosynthesis of Functional Polymers. Polymers 2023, 15, 1853. https://doi.org/10.3390/polym15081853
Al-Ithawi WKA, Khasanov AF, Kovalev IS, Nikonov IL, Platonov VA, Kopchuk DS, Santra S, Zyryanov GV, Ranu BC. TM-Free and TM-Catalyzed Mechanosynthesis of Functional Polymers. Polymers. 2023; 15(8):1853. https://doi.org/10.3390/polym15081853
Chicago/Turabian StyleAl-Ithawi, Wahab K. A., Albert F. Khasanov, Igor S. Kovalev, Igor L. Nikonov, Vadim A. Platonov, Dmitry S. Kopchuk, Sougata Santra, Grigory V. Zyryanov, and Brindaban C. Ranu. 2023. "TM-Free and TM-Catalyzed Mechanosynthesis of Functional Polymers" Polymers 15, no. 8: 1853. https://doi.org/10.3390/polym15081853
APA StyleAl-Ithawi, W. K. A., Khasanov, A. F., Kovalev, I. S., Nikonov, I. L., Platonov, V. A., Kopchuk, D. S., Santra, S., Zyryanov, G. V., & Ranu, B. C. (2023). TM-Free and TM-Catalyzed Mechanosynthesis of Functional Polymers. Polymers, 15(8), 1853. https://doi.org/10.3390/polym15081853










