Recent Advances in Nanoparticle Development for Drug Delivery: A Comprehensive Review of Polycaprolactone-Based Multi-Arm Architectures
Abstract
1. Introduction
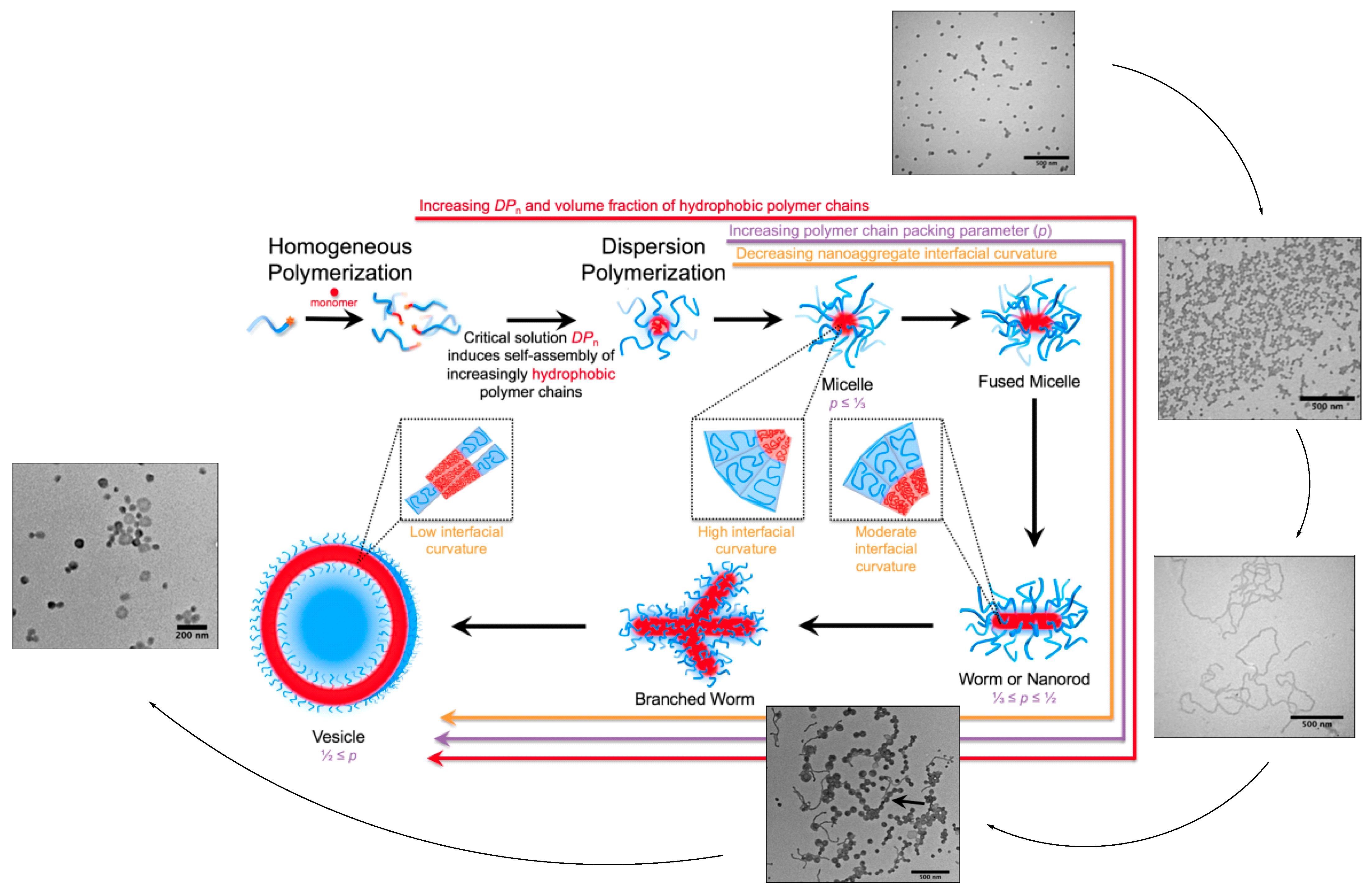
2. Block Copolymer Self-Assembly: Strategies for Controlling Nanostructure Formation
3. The Advantages of Polycaprolactone for Advanced Polymer Architecture Formation
4. Application of PCL-Based Materials in Nanoformulations
4.1. Electrospun Mats, Films and Scaffolds
4.2. Hydrogels
4.3. Nanoparticles
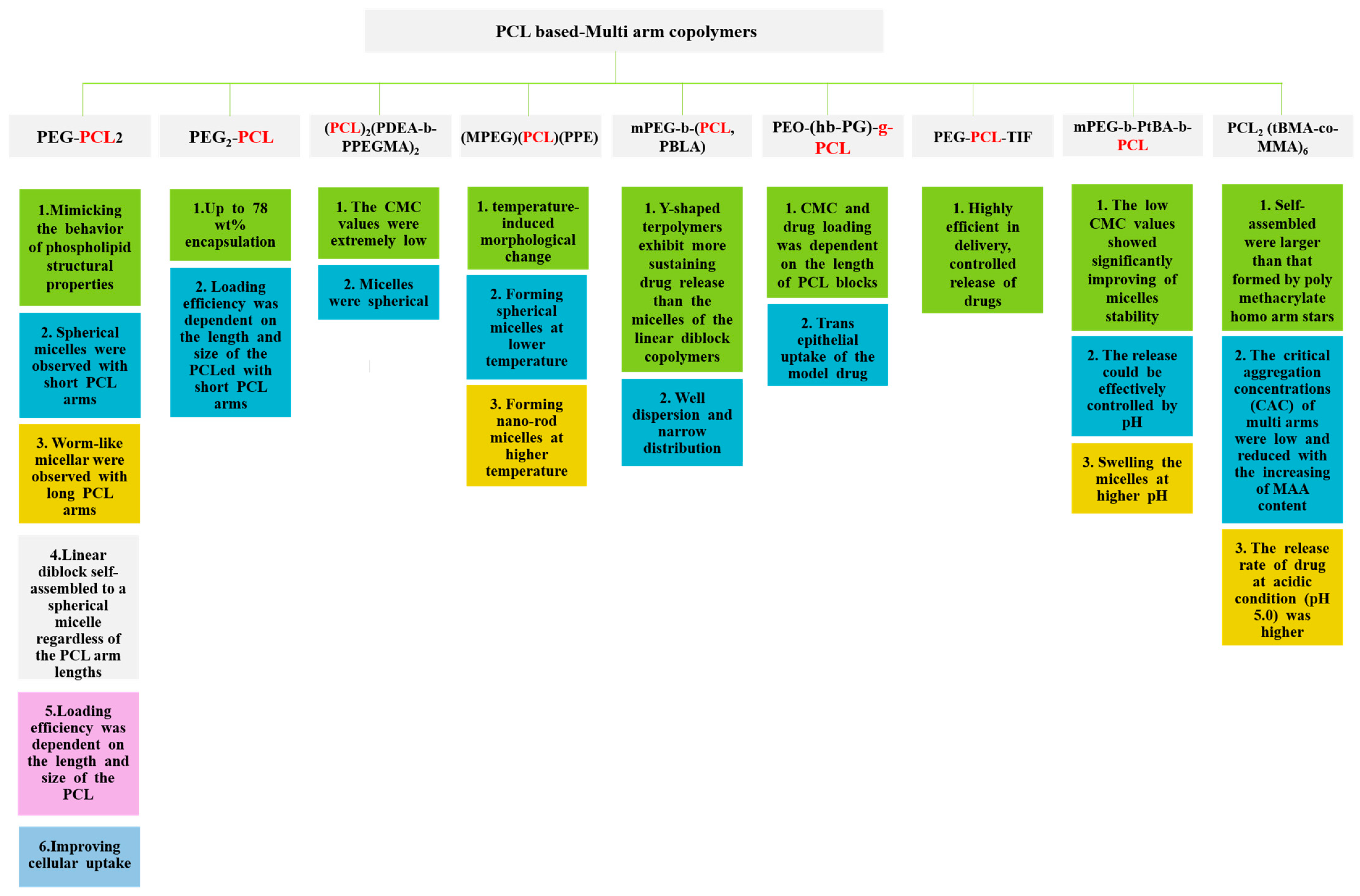
5. Exploring the Potential of Multi-Arm Polymers as Nanocarriers
6. Impact of Multi-Arm Topology on PCL-Based Polymer Properties for Drug Delivery
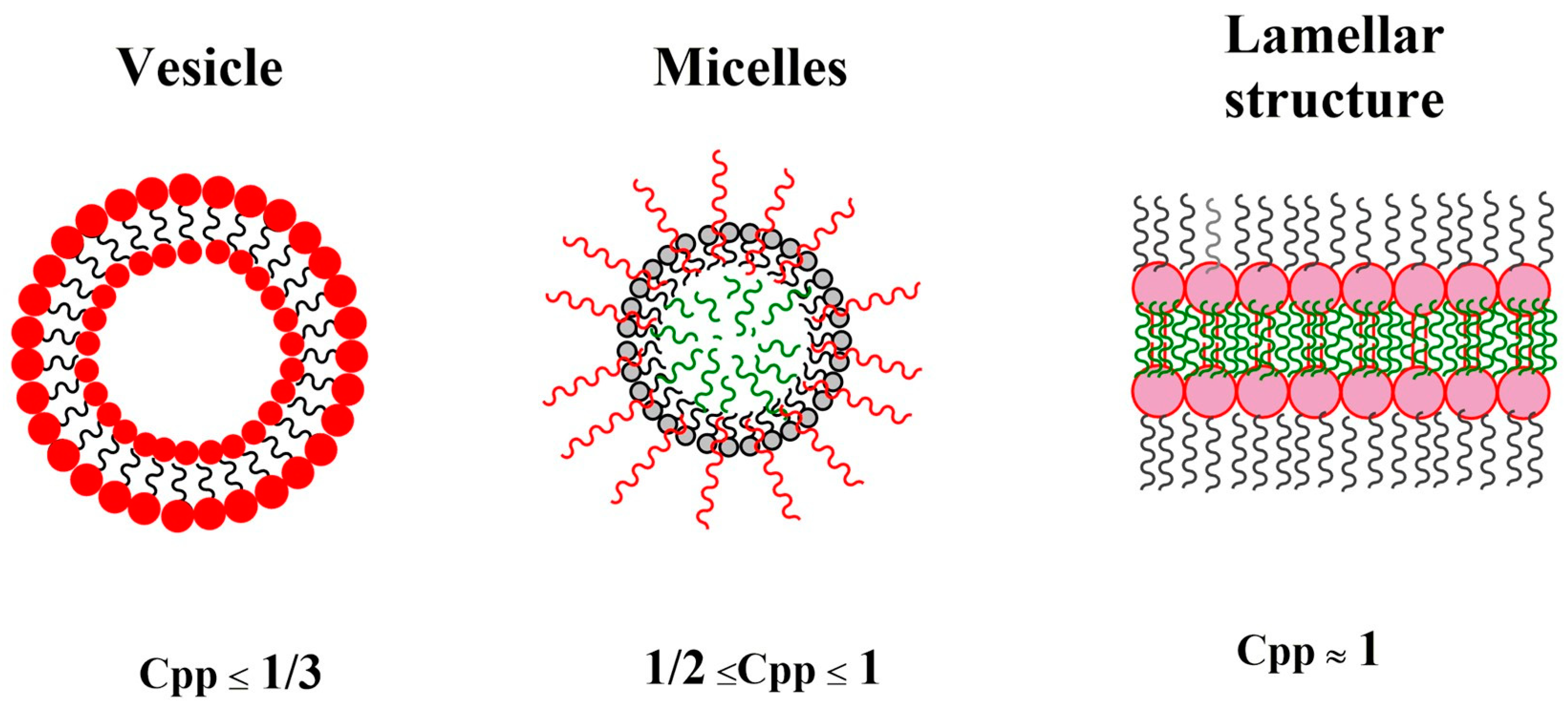
7. The Key Parameters of Self-Assembly in Multi-Arm Polymers
7.1. Solvophobic/Solvophilic Balance
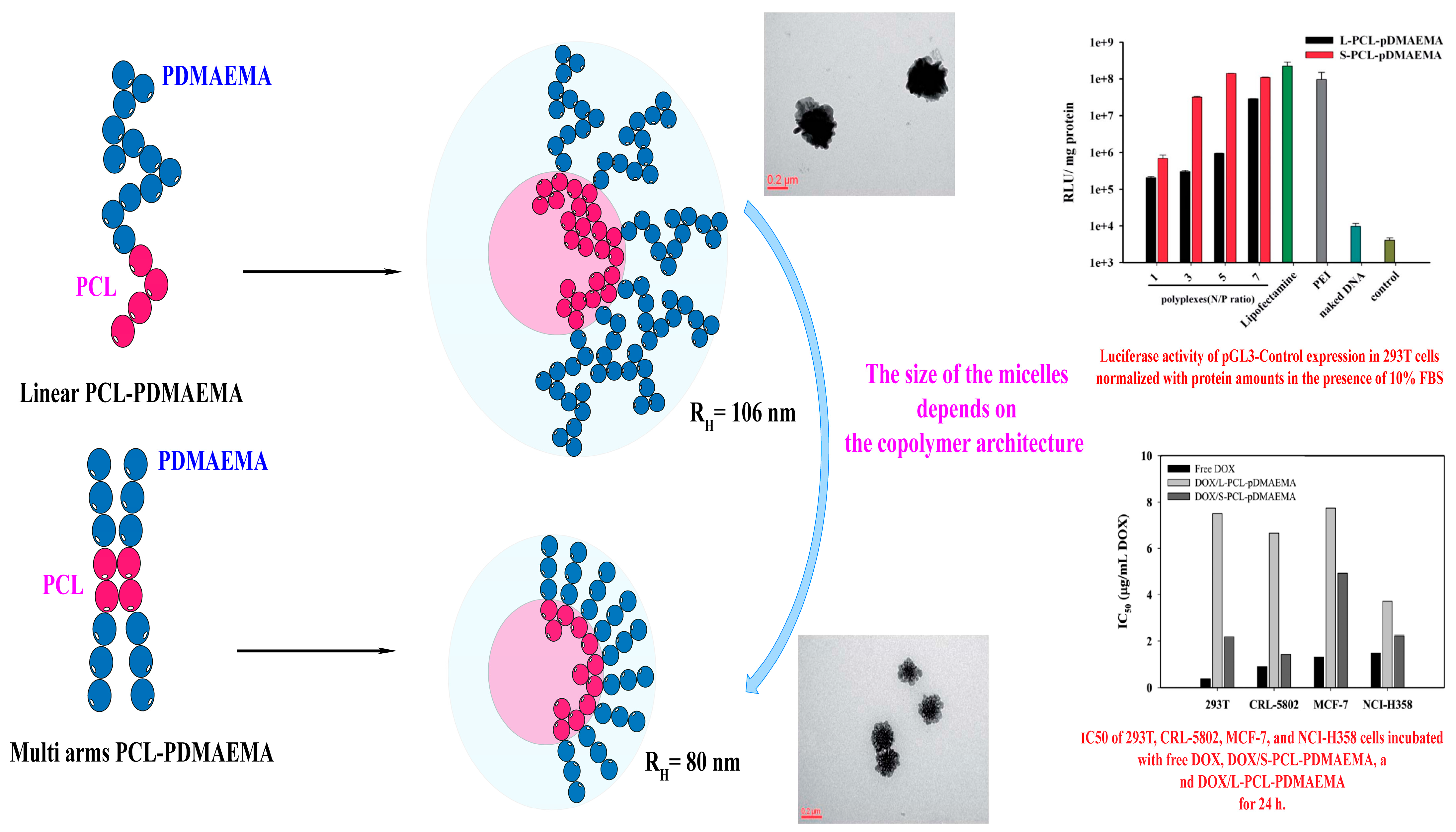
7.2. Linear versus Multi-Arm Copolymers
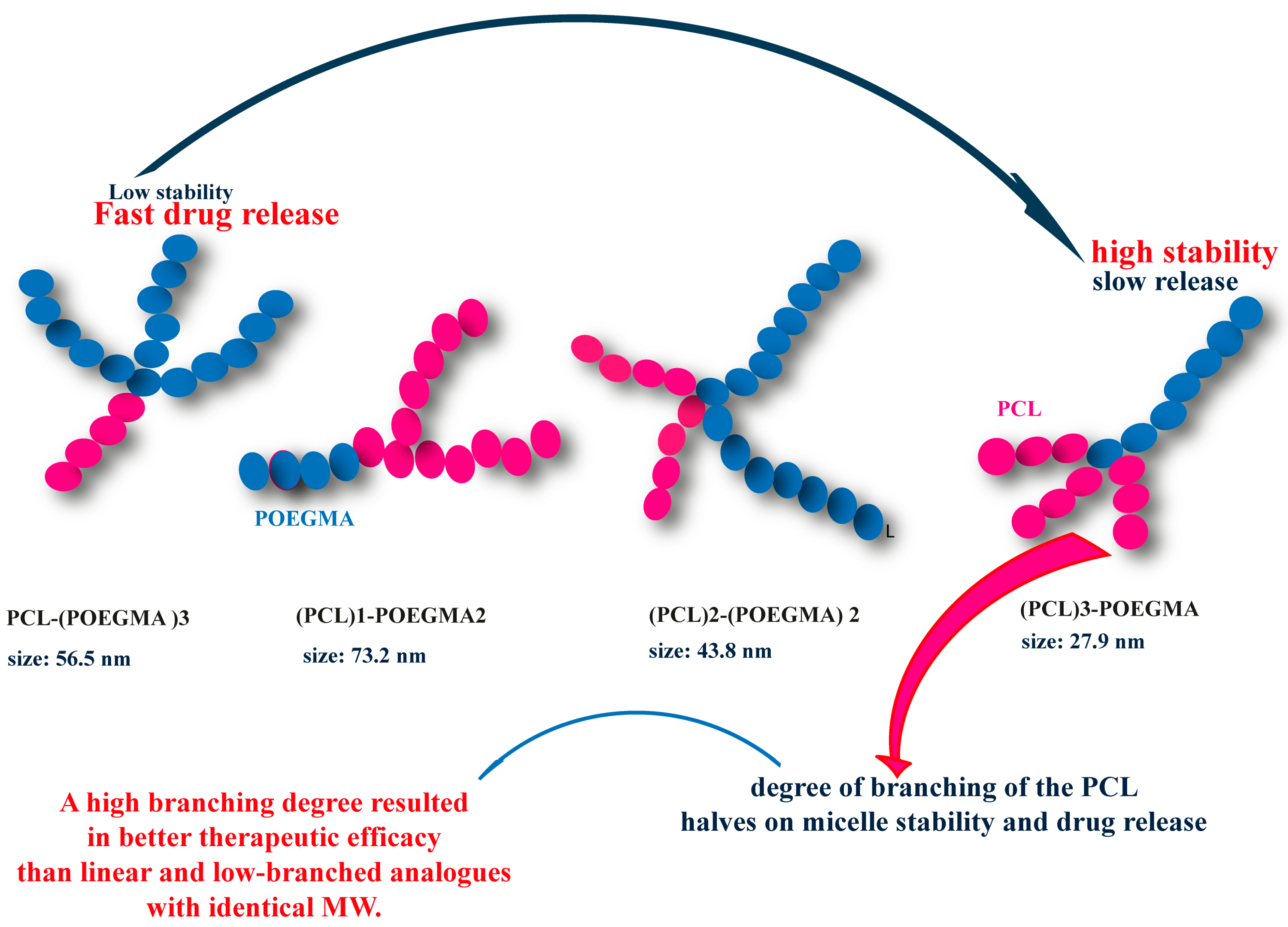
7.3. Arm Number and Branching Degree
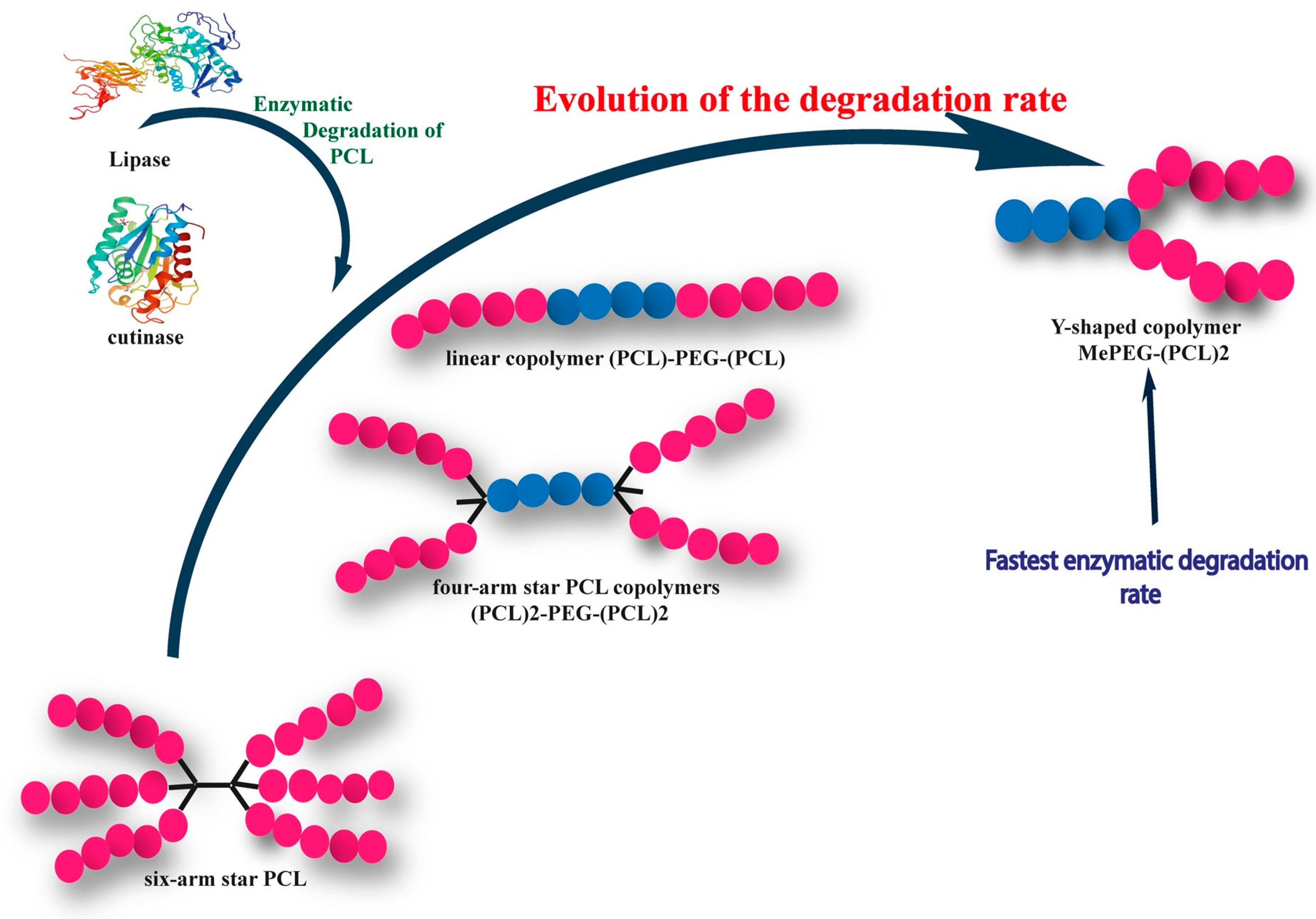
8. Degradation of PCL-Based Multiarms
9. Limitations and Future Perspectives
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, G.; Cheng, X.; Wang, Y.; Zhang, W. Supramolecular chiral polymeric aggregates: Construction and applications. Aggregate 2022, 4, e262. [Google Scholar] [CrossRef]
- Kulshrestha, A.; Gehlot, P.; Kumar, A. Paramagnetic surface active ionic liquids: Synthesis, properties, and applications. Mater. Today Chem. 2021, 21, 100522. [Google Scholar] [CrossRef]
- Pei, Y.; Lowe, A.B.; Roth, P.J. Stimulus-Responsive Nanoparticles and Associated (Reversible) Polymorphism via Polymerization Induced Self-assembly (PISA). Macromol. Rapid Commun. 2017, 38, 1600528. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, H.; Shi, J. In vivo bio-safety evaluations and diagnostic/therapeutic applications of chemically designed mesoporous silica nanoparticles. Adv. Mater. 2013, 25, 3144–3176. [Google Scholar] [CrossRef]
- Mulikova, T.; Abduraimova, A.; Molkenova, A.; Em, S.; Duisenbayeva, B.; Han, D.-W.; Atabaev, T.S. Mesoporous silica decorated with gold nanoparticles as a promising nanoprobe for effective CT X-ray attenuation and potential drug delivery. Nano-Struct. Nano-Objects 2021, 26, 100712. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric nanoparticles for drug delivery: Recent developments and future prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- Chen, M.; Jafvert, C.T.; Wu, Y.; Cao, X.; Hankins, N.P. Inorganic anion removal using micellar enhanced ultrafiltration (MEUF), modeling anion distribution and suggested improvements of MEUF: A review. Chem. Eng. J. 2020, 398, 125413. [Google Scholar] [CrossRef]
- Hassan, S.; Prakash, G.; Ozturk, A.B.; Saghazadeh, S.; Sohail, M.F.; Seo, J.; Dokmeci, M.R.; Zhang, Y.S.; Khademhosseini, A. Evolution and clinical translation of drug delivery nanomaterials. Nano Today 2017, 15, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Chen, X.; Zhang, X. Recent advances in stimuli-responsive polymeric micelles via click chemistry. Polym. Chem. 2019, 10, 34–44. [Google Scholar] [CrossRef]
- Hanafy, N.A.; El-Kemary, M.; Leporatti, S. Micelles structure development as a strategy to improve smart cancer therapy. Cancers 2018, 10, 238. [Google Scholar] [CrossRef]
- Kenchegowda, M.; Rahamathulla, M.; Hani, U.; Begum, M.Y.; Guruswamy, S.; Osmani, R.A.M.; Gowrav, M.P.; Alshehri, S.; Ghoneim, M.M.; Alshlowi, A. Smart nanocarriers as an emerging platform for cancer therapy: A review. Molecules 2021, 27, 146. [Google Scholar] [CrossRef]
- Khan, M.I.; Hossain, M.I.; Hossain, M.K.; Rubel, M.; Hossain, K.; Mahfuz, A.; Anik, M.I. Recent progress in nanostructured smart drug delivery systems for cancer therapy: A review. ACS Appl. Bio Mater. 2022, 5, 971–1012. [Google Scholar] [CrossRef] [PubMed]
- Ouarga, A.; Lebaz, N.; Tarhini, M.; Noukrati, H.; Barroug, A.; Elaissari, A. Towards smart self-healing coatings: Advances in micro/nano-encapsulation processes as carriers for anti-corrosion coatings development. J. Mol. Liq. 2022, 118862. [Google Scholar] [CrossRef]
- Meng, Q.; Zhong, S.; Xu, L.; Wang, J.; Zhang, Z.; Gao, Y.; Cui, X. Review on design strategies and considerations of polysaccharide-based smart drug delivery systems for cancer therapy. Carbohydr. Polym. 2022, 279, 119013. [Google Scholar] [CrossRef] [PubMed]
- Doerk, G.S.; Yager, K.G. Beyond native block copolymer morphologies. Mol. Syst. Des. Eng. 2017, 2, 518–538. [Google Scholar] [CrossRef]
- Yang, C.; Liu, S.Q.; Venkataraman, S.; Gao, S.J.; Ke, X.; Chia, X.T.; Hedrick, J.L.; Yang, Y.Y. Structure-directing star-shaped block copolymers: Supramolecular vesicles for the delivery of anticancer drugs. J. Control. Release 2015, 208, 93–105. [Google Scholar] [CrossRef]
- Sharma, A.; Pande, P. Recent advancement in comb-like polymers: A review. J. Polym. Mater. 2019, 36, 175–194. [Google Scholar] [CrossRef]
- Hofman, A.H.; ten Brinke, G.; Loos, K. Hierarchical structure formation in supramolecular comb-shaped block copolymers. Polymer 2016, 107, 343–356. [Google Scholar] [CrossRef]
- Liberman-Martin, A.L.; Chu, C.K.; Grubbs, R.H. Application of bottlebrush block copolymers as photonic crystals. Macromol. Rapid Commun. 2017, 38, 1700058. [Google Scholar] [CrossRef]
- Feng, C.; Huang, X. Polymer brushes: Efficient synthesis and applications. Acc. Chem. Res. 2018, 51, 2314–2323. [Google Scholar] [CrossRef]
- Ray, S.; Li, Z.; Hsu, C.H.; Hwang, L.P.; Lin, Y.C.; Chou, P.T.; Lin, Y.-Y. Dendrimer-and copolymer-based nanoparticles for magnetic resonance cancer theranostics. Theranostics 2018, 8, 6322. [Google Scholar] [CrossRef]
- Hsu, H.J.; Bugno, J.; Lee, S.R.; Hong, S. Dendrimer-based nanocarriers: A versatile platform for drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1409. [Google Scholar] [CrossRef] [PubMed]
- Moini, N.; Jahandideh, A.; Shahkarami, F.; Kabiri, K.; Piri, F. Linear and star-shaped π-conjugated oligoanilines: A review on molecular design in syntheses and properties. Polym. Chem. 2022, 13, 2714–2756. [Google Scholar] [CrossRef]
- Klajnert, B.; Bryszewska, M. Dendrimers: Properties and applications. Acta Biochim. Pol. 2001, 48, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Sahranavard, M.; Shahriari, M.; Abnous, K.; Hadizadeh, F.; Taghdisi, S.M.; Zolfaghari, R.; Ramezani, M.; Alibolandi, M. Design and synthesis of targeted star-shaped micelle for guided delivery of camptothecin: In vitro and in vivo evaluation. Mater. Sci. Eng. C 2021, 131, 112529. [Google Scholar] [CrossRef]
- Wang, T.W.; Golder, M.R. Advancing macromolecular hoop construction: Recent developments in synthetic cyclic polymer chemistry. Polym. Chem. 2021, 12, 958–969. [Google Scholar] [CrossRef]
- Zhao, X.; Li, J.; Liu, J.; Zhou, W.; Peng, S. Recent progress of preparation of branched poly (lactic acid) and its application in the modification of polylactic acid materials. Int. J. Biol. Macromol. 2021, 193, 874–892. [Google Scholar] [CrossRef]
- Takata, T.; Aoki, D. Topology-transformable polymers: Linear-branched polymer structural transformation via the mechanical linking of polymer chains. Polym. J. 2018, 50, 127–147. [Google Scholar] [CrossRef]
- Zhao, X.; Si, J.; Huang, D.; Li, K.; Xin, Y.; Sui, M. Application of star poly (ethylene glycol) derivatives in drug delivery and controlled release. J. Control. Release 2020, 323, 565–577. [Google Scholar] [CrossRef]
- Liu, Z.; Ye, L.; Xi, J.; Wang, J.; Feng, Z.G. Cyclodextrin polymers: Structure, synthesis, and use as drug carriers. Prog. Polym. Sci. 2021, 118, 101408. [Google Scholar] [CrossRef]
- Shahrokhinia, A.; Biswas, P.; Reuther, J.F. Orthogonal synthesis and modification of polymer materials. J. Polym. Sci. 2021, 59, 1748–1786. [Google Scholar] [CrossRef]
- Kakkar, A.; Traverso, G.; Farokhzad, O.C.; Weissleder, R.; Langer, R. Evolution of macromolecular complexity in drug delivery systems. Nat. Rev. Chem. 2017, 1, 0063. [Google Scholar] [CrossRef]
- Yan, J.; Bockstaller, M.R.; Matyjaszewski, K. Brush-modified materials: Control of molecular architecture, assembly behavior, properties and applications. Prog. Polym. Sci. 2020, 100, 101180. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Yusoh, K. A review on the recent research of polycaprolactone (PCL). Adv. Mater. Res. 2016, 1134, 249–255. [Google Scholar]
- Archer, E.; Torretti, M.; Madbouly, S. Biodegradable polycaprolactone (PCL) based polymer and composites. Phys. Sci. Rev. 2021. [Google Scholar] [CrossRef]
- Abdal-hay, A.; Sheikh, F.A.; Gómez-Cerezo, N.; Alneairi, A.; Luqman, M.; Pant, H.R.; Ivanovski, S. A review of protein adsorption and bioactivity characteristics of poly ε-caprolactone scaffolds in regenerative medicine. Eur. Polym. J. 2022, 162, 110892. [Google Scholar] [CrossRef]
- Borandeh, S.; van Bochove, B.; Teotia, A.; Seppälä, J. Polymeric drug delivery systems by additive manufacturing. Adv. Drug Deliv. Rev. 2021, 173, 349–373. [Google Scholar] [CrossRef] [PubMed]
- Duchiron, S.W.; Pollet, E.; Givry, S.; Avérous, L. Enzymatic synthesis of poly (ε-caprolactone-co-ε-thiocaprolactone). Eur. Polym. J. 2017, 87, 147–158. [Google Scholar] [CrossRef]
- Coudane, J.; Nottelet, B.; Mouton, J.; Garric, X.; Van Den Berghe, H. Poly (ε-caprolactone)-Based Graft Copolymers: Synthesis Methods and Applications in the Biomedical Field: A Review. Molecules 2022, 27, 7339. [Google Scholar] [CrossRef]
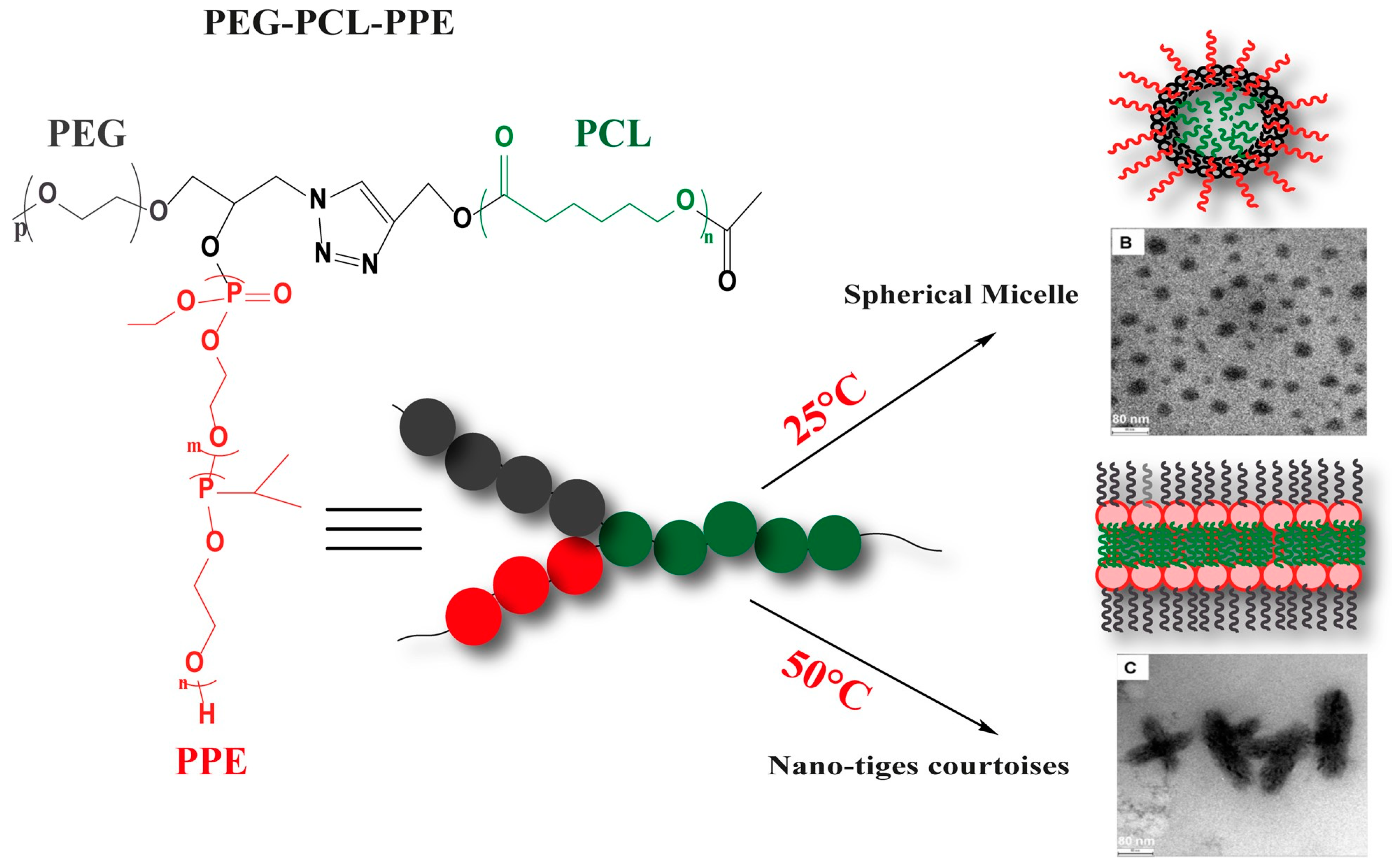
- Grossen, P.; Witzigmann, D.; Sieber, S.; Huwyler, J. PEG-PCL-based nanomedicines: A biodegradable drug delivery system and its application. J. Control. Release 2017, 260, 46–60. [Google Scholar] [CrossRef]
- Behl, A.; Parmar, V.S.; Malhotra, S.; Chhillar, A.K. Biodegradable diblock copolymeric PEG-PCL nanoparticles: Synthesis, characterization and applications as anticancer drug delivery agents. Polymer 2020, 207, 122901. [Google Scholar] [CrossRef]
- Baghbanbashi, M.; Kakkar, A. Polymersomes: Soft Nanoparticles from Miktoarm Stars for Applications in Drug Delivery. Mol. Pharm. 2022, 19, 1687–1703. [Google Scholar] [CrossRef] [PubMed]
- Lotocki, V.; Kakkar, A. Miktoarm star polymers: Branched architectures in drug delivery. Pharmaceutics 2020, 12, 827. [Google Scholar] [CrossRef] [PubMed]
- Villemin, E.; Ong, Y.C.; Thomas, C.M.; Gasser, G. Polymer encapsulation of ruthenium complexes for biological and medicinal applications. Nat. Rev. Chem. 2019, 3, 261–282. [Google Scholar] [CrossRef]
- Probst, C.E.; Zrazhevskiy, P.; Bagalkot, V.; Gao, X. Quantum dots as a platform for nanoparticle drug delivery vehicle design. Adv. Drug Deliv. Rev. 2013, 65, 703–718. [Google Scholar] [CrossRef]
- Ramasamy, T.; Ruttala, H.B.; Gupta, B.; Poudel, B.K.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Smart chemistry-based nanosized drug delivery systems for systemic applications: A comprehensive review. J. Control. Release 2017, 258, 226–253. [Google Scholar] [CrossRef]
- Karayianni, M.; Pispas, S. Block copolymer solution self-assembly: Recent advances, emerging trends, and applications. J. Polym. Sci. 2021, 59, 1874–1898. [Google Scholar] [CrossRef]
- Rodichkin, I.D.; Gumerov, R.A.; Potemkin, I.I. Self-assembly of miktoarm palm tree-like star copolymers in a selective solvent. J. Colloid Interface Sci. 2022, 606, 1966–1973. [Google Scholar] [CrossRef]
- Kindt, J.T.; Szostak, J.W.; Wang, A. Bulk self-assembly of giant, unilamellar vesicles. ACS Nano 2020, 14, 14627–14634. [Google Scholar] [CrossRef]
- Go, Y.K.; Kambar, N.; Leal, C. Hybrid unilamellar vesicles of phospholipids and block copolymers with crystalline domains. Polymers 2020, 12, 1232. [Google Scholar] [CrossRef]
- Didiot, C.; Pons, S.; Kierren, B.; Fagot-Revurat, Y.; Malterre, D. Nanopatterning the electronic properties of gold surfaces with self-organized superlattices of metallic nanostructures. Nat. Nanotechnol. 2007, 2, 617–621. [Google Scholar] [CrossRef]
- Joachim, C.; Gimzewski, J.K.; Aviram, A. Electronics using hybrid-molecular and mono-molecular devices. Nature 2000, 408, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Mateescu, M.A.; Ispas-Szabo, P.; Assaad, E. Controlled Drug Delivery: The Role of Self-Assembling Multi-Task Excipients; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Qi, M.; Zhou, Y. Multimicelle aggregate mechanism for spherical multimolecular micelles: From theories, characteristics and properties to applications. Mater. Chem. Front. 2019, 3, 1994–2009. [Google Scholar] [CrossRef]
- Jahan, R.; Bodratti, A.M.; Tsianou, M.; Alexandridis, P. Biosurfactants, natural alternatives to synthetic surfactants: Physicochemical properties and applications. Adv. Colloid Interface Sci. 2020, 275, 102061. [Google Scholar] [CrossRef]
- Mandal, T. Scission energy and topology of micelles controlled by the molecular structure of additives. Soft Matter 2022, 18, 1678–1687. [Google Scholar] [CrossRef]
- Gröschel, T.I.; Wong, C.K.; Haataja, J.S.; Dias, M.A.; Gröschel, A.H. Direct observation of topological defects in striped block copolymer discs and polymersomes. ACS Nano 2020, 14, 4829–4838. [Google Scholar] [CrossRef]
- Zhang, L.; Eisenberg, A. Multiple morphologies of “crew-cut” aggregates of polystyrene-b-poly (acrylic acid) block copolymers. Science 1995, 268, 1728–1731. [Google Scholar] [CrossRef]
- Luo, L.; Eisenberg, A. Thermodynamic stabilization mechanism of block copolymer vesicles. J. Am. Chem. Soc. 2001, 123, 1012–1013. [Google Scholar] [CrossRef] [PubMed]
- Terreau, O.; Bartels, C.; Eisenberg, A. Effect of poly (acrylic acid) block length distribution on polystyrene-b-poly (acrylic acid) block copolymer aggregates in solution. 2. A partial phase diagram. Langmuir 2004, 20, 637–645. [Google Scholar] [CrossRef]
- Discher, B.M.; Won, Y.-Y.; Ege, D.S.; Lee, J.C.; Bates, F.S.; Discher, D.E.; Hammer, D.A. Polymersomes: Tough vesicles made from diblock copolymers. Science 1999, 284, 1143–1146. [Google Scholar] [CrossRef]
- Ali, I.; Althakfi, S.H.; Suhail, M.; Locatelli, M.; Hsieh, M.-F.; Alsehli, M.; Hameed, A.M. Advances in Polymeric Colloids for Cancer Treatment. Polymers 2022, 14, 5445. [Google Scholar] [CrossRef] [PubMed]
- Araste, F.; Aliabadi, A.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Self-assembled polymeric vesicles: Focus on polymersomes in cancer treatment. J. Control. Release 2021, 330, 502–528. [Google Scholar] [CrossRef] [PubMed]
- Bangham, A. Physical structure and behavior of lipids and lipid enzymes. Adv. Lipid Res. 1963, 1, 65–104. [Google Scholar]
- Ventola, C.L. Progress in nanomedicine: Approved and investigational nanodrugs. Pharm. Ther. 2017, 42, 742. [Google Scholar]
- Danafar, H.; Rostamizadeh, K.; Davaran, S.; Hamidi, M. PLA-PEG-PLA copolymer-based polymersomes as nanocarriers for delivery of hydrophilic and hydrophobic drugs: Preparation and evaluation with atorvastatin and lisinopril. Drug Dev. Ind. Pharm. 2014, 40, 1411–1420. [Google Scholar] [CrossRef]
- Fetsch, C.; Gaitzsch, J.; Messager, L.; Battaglia, G.; Luxenhofer, R. Self-Assembly of amphiphilic block copolypeptoids–Micelles, worms and polymersomes. Sci. Rep. 2016, 6, 33491. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A.; Caccamo, M.T. Smart nanoparticles for drug delivery application: Development of versatile nanocarrier platforms in biotechnology and nanomedicine. J. Nanomater. 2019, 2019. [Google Scholar] [CrossRef]
- Yorulmaz Avsar, S.; Kyropoulou, M.; Di Leone, S.; Schoenenberger, C.-A.; Meier, W.P.; Palivan, C.G. Biomolecules turn self-assembling amphiphilic block co-polymer platforms into biomimetic interfaces. Front. Chem. 2019, 6, 645. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Feijen, J. Polymersomes for drug delivery: Design, formation and characterization. J. Control. Release 2012, 161, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 1–9. [Google Scholar] [CrossRef]
- Bermudez, H.; Brannan, A.K.; Hammer, D.A.; Bates, F.S.; Discher, D.E. Molecular weight dependence of polymersome membrane structure, elasticity, and stability. Macromolecules 2002, 35, 8203–8208. [Google Scholar] [CrossRef]
- Bermudez, H.; Hammer, D.; Discher, D. Effect of bilayer thickness on membrane bending rigidity. Langmuir 2004, 20, 540–543. [Google Scholar] [CrossRef] [PubMed]
- Blanazs, A.; Armes, S.P.; Ryan, A.J. Self-assembled block copolymer aggregates: From micelles to vesicles and their biological applications. Macromol. Rapid Commun. 2009, 30, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; McCabe, J.; Hill, K.; Beales, P.A. Biodegradable hybrid block copolymer–lipid vesicles as potential drug delivery systems. J. Colloid Interface Sci. 2020, 562, 418–428. [Google Scholar] [CrossRef]
- Patel, K.D.; Silva, L.B.; Park, Y.; Shakouri, T.; Keskin-Erdogan, Z.; Sawadkar, P.; Cho, K.J.; Knowles, J.C.; Chau, D.Y.; Kim, H.-W. Recent advances in drug delivery systems for glaucoma treatment. Mater. Today Nano 2022, 18, 100178. [Google Scholar] [CrossRef]
- Pang, Z.; Lu, W.; Gao, H.; Hu, K.; Chen, J.; Zhang, C.; Gao, X.; Jiang, X.; Zhu, C. Preparation and brain delivery property of biodegradable polymersomes conjugated with OX26. J. Control. Release 2008, 128, 120–127. [Google Scholar] [CrossRef]
- Sisson, A.L.; Ekinci, D.; Lendlein, A. The contemporary role of ε-caprolactone chemistry to create advanced polymer architectures. Polymer 2013, 54, 4333–4350. [Google Scholar] [CrossRef]
- Karimi, M.; Heuchel, M.; Weigel, T.; Schossig, M.; Hofmann, D.; Lendlein, A. Formation and size distribution of pores in poly (ɛ-caprolactone) foams prepared by pressure quenching using supercritical CO2. J. Supercrit. Fluids 2012, 61, 175–190. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer-Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Zhuravlev, E.; Schmelzer, J.W.; Wunderlich, B.; Schick, C. Kinetics of nucleation and crystallization in poly (ɛ-caprolactone)(PCL). Polymer 2011, 52, 1983–1997. [Google Scholar] [CrossRef]
- Penczek, S.; Cypryk, M.; Duda, A.; Kubisa, P.; Słomkowski, S. Living ring-opening polymerizations of heterocyclic monomers. Prog. Polym. Sci. 2007, 32, 247–282. [Google Scholar] [CrossRef]
- Schmitz, L.A.; McCollum, A.M.; Rheingold, A.L.; Green, D.B.; Fritsch, J.M. Synthesis and structures of aluminum ion-pair complexes that act as L-and racemic-lactide ring opening polymerization initiators. Polyhedron 2018, 147, 94–105. [Google Scholar] [CrossRef]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef]
- Lorenzo, A.; Muller, A.; Lin, M.C.; Chen, H.L.; Jeng, U.S.; Priftis, D.; Pitsikalis, M.; Hadjichristidis, N. Influence of macromolecular architecture on the crystallization of (PCL2)-b-(PS2) 4-miktoarm star block copolymers in comparison to linear PCL-b-PS diblock copolymer analogues. Macromolecules 2009, 42, 8353–8364. [Google Scholar] [CrossRef]
- Tunca, U.; Ozyurek, Z.; Erdogan, T.; Hizal, G. Novel miktofunctional initiator for the preparation of an ABC-type miktoarm star polymer via a combination of controlled polymerization techniques. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 4228–4236. [Google Scholar] [CrossRef]
- Chen, W.Q.; Wei, H.; Li, S.L.; Feng, J.; Nie, J.; Zhang, X.Z.; Zhuo, R.X. Fabrication of star-shaped, thermo-sensitive poly (N-isopropylacrylamide)-cholic acid-poly (ɛ-caprolactone) copolymers and their self-assembled micelles as drug carriers. Polymer 2008, 49, 3965–3972. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, H.; Shi, G.; Wang, Y.; Pan, C.Y. Synthesis of ABCD 4-miktoarm star polymers by combination of RAFT, ROP, and “Click Chemistry”. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 6641–6653. [Google Scholar] [CrossRef]
- Sinha, V.; Bansal, K.; Kaushik, R.; Kumria, R.; Trehan, A. Poly-ϵ-caprolactone microspheres and nanospheres: An overview. Int. J. Pharm. 2004, 278, 1–23. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Wei, K.; Zhao, N.; Zhang, S.; Chen, J. Drug distribution within poly (ɛ-caprolactone) microspheres and in vitro release. J. Mater. Process. Technol. 2009, 209, 348–354. [Google Scholar] [CrossRef]
- Annuryanti, F.; Domínguez-Robles, J.; Anjani, Q.K.; Adrianto, M.F.; Larrañeta, E.; Thakur, R.R.S. Fabrication and Characterisation of 3D-Printed Triamcinolone Acetonide-Loaded Polycaprolactone-Based Ocular Implants. Pharmaceutics 2023, 15, 243. [Google Scholar] [CrossRef]
- Hinderer, S.; Layland, S.L.; Schenke-Layland, K. ECM and ECM-like materials-Biomaterials for applications in regenerative medicine and cancer therapy. Adv. Drug Deliv. Rev. 2016, 97, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Luu, Y.K.; Chang, C.; Fang, D.; Hsiao, B.S.; Chu, B.; Hadjiargyrou, M. Incorporation and controlled release of a hydrophilic antibiotic using poly (lactide-co-glycolide)-based electrospun nanofibrous scaffolds. J. Control. Release 2004, 98, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef]
- Hsu, K.H.; Fang, S.P.; Lin, C.L.; Liao, Y.-S.; Yoon, Y.-K.; Chauhan, A. Hybrid electrospun polycaprolactone mats consisting of nanofibers and microbeads for extended release of dexamethasone. Pharm. Res. 2016, 33, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Jassal, M.; Sengupta, S.; Bhowmick, S. Functionalization of electrospun poly (caprolactone) fibers for pH-controlled delivery of doxorubicin hydrochloride. J. Biomater. Sci. Polym. Ed. 2015, 26, 1425–1438. [Google Scholar] [CrossRef]
- Lu, C.; Liu, L.; Guo, S.R.; Zhang, Y.; Li, Z.; Gu, J. Micellization and gelation of aqueous solutions of star-shaped PEG–PCL block copolymers consisting of branched 4-arm poly (ethylene glycol) and polycaprolactone blocks. Eur. Polym. J. 2007, 43, 1857–1865. [Google Scholar] [CrossRef]
- Lu, C.; Guo, S.r.; Zhang, Y.; Yin, M. Synthesis and aggregation behavior of four types of different shaped PCL-PEG block copolymers. Polym. Int. 2006, 55, 694–700. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Nottelet, B.; Coudane, J. Robust & thermosensitive poly (ethylene glycol)-poly (ε-caprolactone) star block copolymer hydrogels. Polym. Degrad. Stab. 2017, 137, 173–183. [Google Scholar]
- Jeong, J.C.; Lee, J.; Cho, K. Effects of crystalline microstructure on drug release behavior of poly (ε-caprolactone) microspheres. J. Control. Release 2003, 92, 249–258. [Google Scholar] [CrossRef]
- Ahmed, F.; Discher, D. Controlled release from polymersome vesicles blended with PEO-PLA or related hydrolysable copolymer. J. Control. Release 2004, 96, 37–53. [Google Scholar] [CrossRef]
- Aliabadi, H.M.; Mahmud, A.; Sharifabadi, A.D.; Lavasanifar, A. Micelles of methoxy poly (ethylene oxide)-b-poly (ɛ-caprolactone) as vehicles for the solubilization and controlled delivery of cyclosporine A. J. Control. Release 2005, 104, 301–311. [Google Scholar] [CrossRef]
- Park, E.K.; Kim, S.Y.; Lee, S.B.; Lee, Y.M. Folate-conjugated methoxy poly (ethylene glycol)/poly (ɛ-caprolactone) amphiphilic block copolymeric micelles for tumor-targeted drug delivery. J. Control. Release 2005, 109, 158–168. [Google Scholar] [CrossRef]
- Quaglia, F.; Ostacolo, L.; De Rosa, G.; La Rotonda, M.I.; Ammendola, M.; Nese, G.; Maglio, G.; Palumbo, R.; Vauthier, C. Nanoscopic core-shell drug carriers made of amphiphilic triblock and star-diblock copolymers. Int. J. Pharm. 2006, 324, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle uptake: The phagocyte problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef] [PubMed]
- Stylianopoulos, T.; Jain, R.K. Design considerations for nanotherapeutics in oncology. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1893–1907. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mao, H.; Kawazoe, N.; Chen, G. Insight into the interactions between nanoparticles and cells. Biomater. Sci. 2017, 5, 173–189. [Google Scholar] [CrossRef]
- Kasinathan, N.; Amirthalingam, M.; Reddy, N.D.; Jagani, H.V.; Volety, S.M.; Rao, J.V. In-situ implant containing PCL-curcumin nanoparticles developed using design of experiments. Drug Deliv. 2016, 23, 1007–1015. [Google Scholar] [CrossRef]
- Chen, S.C.; Yang, M.H.; Chung, T.W.; Jhuang, T.S.; Yang, J.D.; Chen, K.C.; Chen, W.J.; Huang, Y.F.; Jong, S.B.; Tsai, W.C. Preparation and characterization of hyaluronic acid-polycaprolactone copolymer micelles for the drug delivery of radioactive iodine-131 labeled lipiodol. BioMed Res. Int. 2017, 2017. [Google Scholar] [CrossRef]
- Ke, X.; Ng, V.W.L.; Ono, R.J.; Chan, J.M.; Krishnamurthy, S.; Wang, Y.; Hedrick, J.L.; Yang, Y.Y. Role of non-covalent and covalent interactions in cargo loading capacity and stability of polymeric micelles. J. Control. Release 2014, 193, 9–26. [Google Scholar] [CrossRef]
- Khoee, S.; Hassanzadeh, S.; Goliaie, B. Effects of hydrophobic drug–polyesteric core interactions on drug loading and release properties of poly (ethylene glycol)–polyester–poly (ethylene glycol) triblock core–shell nanoparticles. Nanotechnology 2007, 18, 175602. [Google Scholar] [CrossRef]
- Shuai, X.; Ai, H.; Nasongkla, N.; Kim, S.; Gao, J. Micellar carriers based on block copolymers of poly (ε-caprolactone) and poly (ethylene glycol) for doxorubicin delivery. J. Control. Release 2004, 98, 415–426. [Google Scholar] [CrossRef]
- Zuo, C.; Peng, J.; Cong, Y.; Dai, X.; Zhang, X.; Zhao, S.; Zhang, X.; Ma, L.; Wang, B.; Wei, H. Fabrication of supramolecular star-shaped amphiphilic copolymers for ROS-triggered drug release. J. Colloid Interface Sci. 2018, 514, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Huynh, L.; Neale, C.; Pomès, R.; Allen, C. Computational approaches to the rational design of nanoemulsions, polymeric micelles, and dendrimers for drug delivery. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 20–36. [Google Scholar] [CrossRef]
- Nagarajan, R.; Barry, M.; Ruckenstein, E. Unusual selectivity in solubilization by block copolymer micelles. Langmuir 1986, 2, 210–215. [Google Scholar] [CrossRef]
- Loverde, S.M.; Klein, M.L.; Discher, D.E. Nanoparticle shape improves delivery: Rational coarse grain molecular dynamics (rCG-MD) of taxol in worm-like PEG-PCL micelles. Adv. Mater. 2012, 24, 3823–3830. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Guan, X.; Cao, H.; Su, T.; Cao, J.; Chen, Y.; Cai, M.; He, B.; Gu, Z.; Luo, X. Characteristic of core materials in polymeric micelles effect on their micellar properties studied by experimental and dpd simulation methods. Int. J. Pharm. 2015, 492, 152–160. [Google Scholar] [CrossRef]
- Qi, P.; Bu, Y.; Xu, J.; Qin, B.; Luan, S.; Song, S. pH-responsive release of paclitaxel from hydrazone-containing biodegradable micelles. Colloid Polym. Sci. 2017, 295, 1–12. [Google Scholar] [CrossRef]
- Tiwari, A.; Prabaharan, M. An amphiphilic nanocarrier based on guar gum-graft-poly (ε-caprolactone) for potential drug-delivery applications. J. Biomater. Sci. Polym. Ed. 2010, 21, 937–949. [Google Scholar] [CrossRef]
- Poustchi, F.; Amani, H.; Ahmadian, Z.; Niknezhad, S.V.; Mehrabi, S.; Santos, H.A.; Shahbazi, M.A. Combination therapy of killing diseases by injectable hydrogels: From concept to medical applications. Adv. Healthc. Mater. 2021, 10, 2001571. [Google Scholar] [CrossRef]
- Gong, C.Y.; Shi, S.; Peng, X.Y.; Kan, B.; Yang, L.; Huang, M.J.; Luo, F.; Zhao, X.; Wei, Y.-Q.; Qian, Z.-Y. Biodegradable thermosensitive injectable PEG-PCL-PEG hydrogel for bFGF antigen delivery to improve humoral immunity. Growth Factors 2009, 27, 377–383. [Google Scholar] [CrossRef]
- Kost, B.; Brzeziński, M.; Socka, M.; Baśko, M.; Biela, T. Biocompatible polymers combined with cyclodextrins: Fascinating materials for drug delivery applications. Molecules 2020, 25, 3404. [Google Scholar] [CrossRef] [PubMed]
- Kajdič, S.; Planinšek, O.; Gašperlin, M.; Kocbek, P. Electrospun nanofibers for customized drug-delivery systems. J. Drug Deliv. Sci. Technol. 2019, 51, 672–681. [Google Scholar] [CrossRef]
- Hsiung, E.; Celebioglu, A.; Chowdhury, R.; Kilic, M.E.; Durgun, E.; Altier, C.; Uyar, T. Antibacterial nanofibers of pullulan/tetracycline-cyclodextrin inclusion complexes for Fast-Disintegrating oral drug delivery. J. Colloid Interface Sci. 2022, 610, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, D.G.; Liu, Y.; Liu, Y.N. Progress of Electrospun Nanofibrous Carriers for Modifications to Drug Release Profiles. J. Funct. Biomater. 2022, 13, 289. [Google Scholar] [CrossRef] [PubMed]
- Nikpou, P.; Soleimani Rad, J.; Mohammad Nejad, D.; Samadi, N.; Roshangar, L.; Navali, A.M.; Shafaei, H.; Nozad Charoudeh, H.; Danandeh Oskoei, N.; Soleimani Rad, S. Indirect coculture of stem cells with fetal chondrons using PCL electrospun nanofiber scaffolds. Artif. Cells Nanomed. Biotechnol. 2017, 45, 283–290. [Google Scholar] [CrossRef]
- Zhang, H.; Xia, J.; Pang, X.; Zhao, M.; Wang, B.; Yang, L.; Wan, H.; Wu, J.; Fu, S. Magnetic nanoparticle-loaded electrospun polymeric nanofibers for tissue engineering. Mater. Sci. Eng. C 2017, 73, 537–543. [Google Scholar] [CrossRef]
- Nakajima, I.; Tsukimura, T.; Ono, T.; Shiga, T.; Shitara, H.; Togawa, T.; Sakuraba, H.; Miyaoka, Y. In Vivo Delivery of Therapeutic Molecules by Transplantation of Genome-Edited Induced Pluripotent Stem Cells. bioRxiv 2023, 2003, 522057. [Google Scholar]
- Zhou, F.; Song, Z.; Wen, Y.; Xu, H.; Zhu, L.; Feng, R. Transdermal delivery of curcumin-loaded supramolecular hydrogels for dermatitis treatment. J. Mater. Sci. Mater. Med. 2019, 30, 1–11. [Google Scholar] [CrossRef]
- Bonacucina, G.; Cespi, M.; Mencarelli, G.; Giorgioni, G.; Palmieri, G.F. Thermosensitive self-assembling block copolymers as drug delivery systems. Polymers 2011, 3, 779–811. [Google Scholar] [CrossRef]
- Ni, P.; Ding, Q.; Fan, M.; Liao, J.; Qian, Z.; Luo, J.; Li, X.; Luo, F.; Yang, Z.; Wei, Y. Injectable thermosensitive PEG–PCL–PEG hydrogel/acellular bone matrix composite for bone regeneration in cranial defects. Biomaterials 2014, 35, 236–248. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, M.; He, J.; Ni, P. Injectable hydrogels by inclusion complexation between a three-armed star copolymer (mPEG-acetal-PCL-acetal-) 3 and α-cyclodextrin for pH-triggered drug delivery. RSC Adv. 2016, 6, 40858–40868. [Google Scholar] [CrossRef]
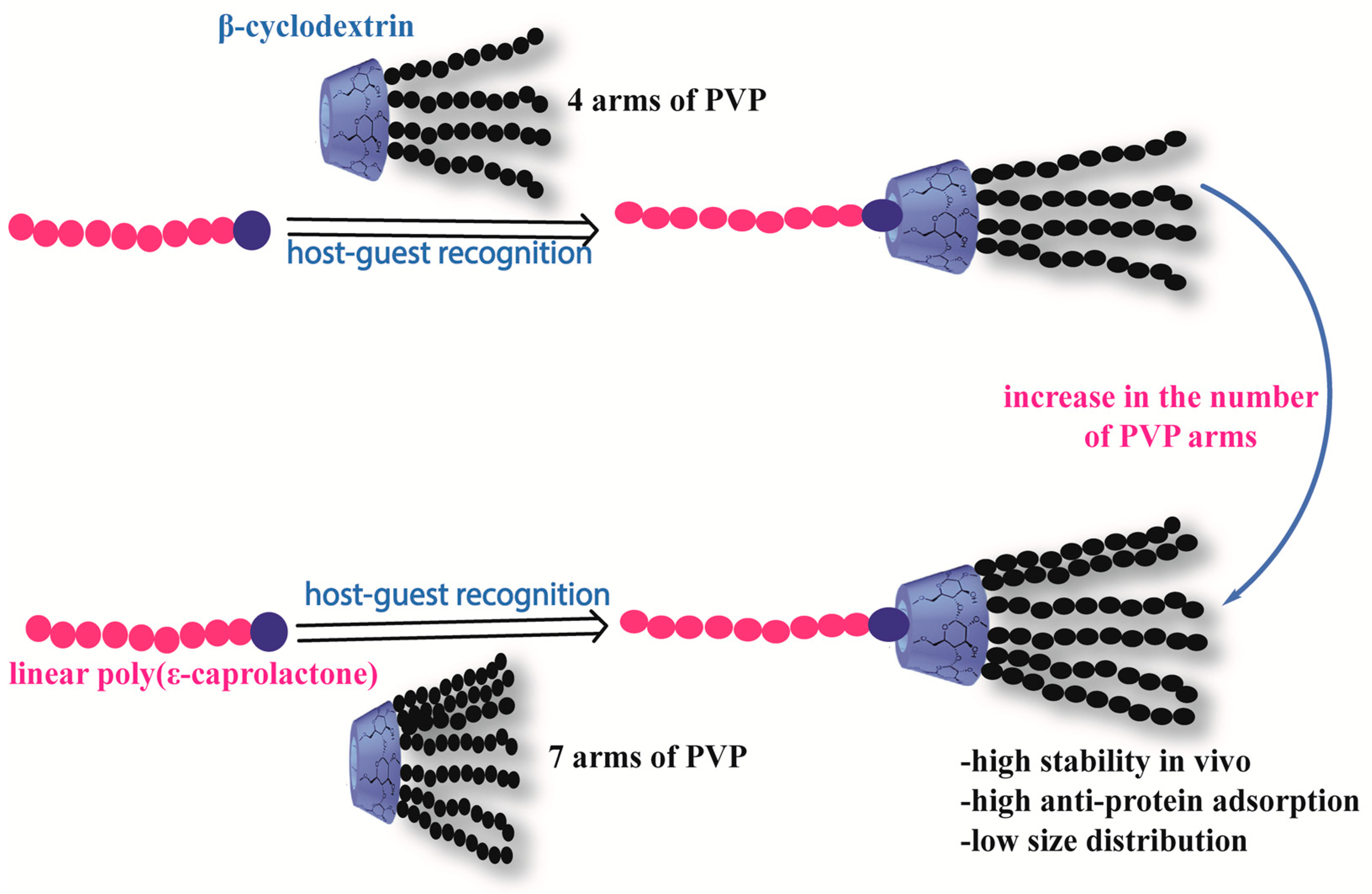
- Wei, X.; Lv, X.; Zhao, Q.; Qiu, L. Thermosensitive β-cyclodextrin modified poly (ε-caprolactone)-poly (ethylene glycol)-poly (ε-caprolactone) micelles prolong the anti-inflammatory effect of indomethacin following local injection. Acta Biomater. 2013, 9, 6953–6963. [Google Scholar] [CrossRef] [PubMed]
- Tangsangasaksri, M. Design, Synthesis, and Characterization of Linear and Branched Semifluorinated Polymers for Therapeutic and Diagnostic Drug Delivery Applications; The University of Wisconsin-Madison: Madison, WI, USA, 2020. [Google Scholar]
- Qi, J.; Huang, J.; Yan, Y. Vesicles Displaying Aggregation Induced Emission: Fabrication and Applications. Curr. Opin. Colloid Interface Sci. 2022, 63, 101640. [Google Scholar] [CrossRef]
- Guo, W.X.; Hu, L.F.; Feng, Y.H.; Chen, B.Z.; Guo, X.D. Advances in self-assembling of pH-sensitive polymers: A mini review on dissipative particle dynamics. Colloids Surf. B Biointerfaces 2022, 210, 112202. [Google Scholar] [CrossRef]
- Jiao, W.; Yang, H.; Wu, Z.; Liu, J.; Zhang, W. Self-assembled block polymer aggregates in selective solution: Controllable morphology transitions and their applications in drug delivery. Expert Opin. Drug Deliv. 2020, 17, 947–961. [Google Scholar] [CrossRef]
- Zhao, Y. Facile synthesis and topological transformation of multicomponent miktoarm star copolymers. Macromol. Rapid Commun. 2019, 40, 1800571. [Google Scholar] [CrossRef]
- Rodrigues, P.R.; Vieira, R.P. Advances in atom-transfer radical polymerization for drug delivery applications. Eur. Polym. J. 2019, 115, 45–58. [Google Scholar] [CrossRef]
- Huang, J.; Xiao, Z.; Liang, H.; Lu, J. Star graft copolymer via grafting-onto strategy using a comb!ination of reversible addition-fragmentation chain transfer arm-first technique and aldehyde-aminooxy click reaction. Polym. Int. 2014, 63, 1122–1128. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, S.; Lu, G.; Huang, X. Constructing well-defined star graft copolymers. Polym. Chem. 2013, 4, 1289–1299. [Google Scholar] [CrossRef]
- Kurmaz, S.; Pyryaev, A. Synthesis of N-vinyl-2-pyrrolidone-based branched copolymers via crosslinking free-radical copolymerization in the presence of a chain-transfer agent. Polym. Sci. Ser. B 2010, 52, 1–8. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, W.; Li, W.; Pan, R.; Lin, X. A review: Multidimensional internal plasticization of molecular structure in energetic polymer. J. Appl. Polym. Sci. 2023, 140, e53428. [Google Scholar] [CrossRef]
- Yong, H.W.; Kakkar, A. Nanoengineering Branched Star Polymer-Based Formulations: Scope, Strategies, and Advances. Macromol. Biosci. 2021, 21, 2100105. [Google Scholar] [CrossRef] [PubMed]
- Skandalis, A.; Sentoukas, T.; Giaouzi, D.; Kafetzi, M.; Pispas, S. Latest advances on the synthesis of linear abc-type triblock terpolymers and star-shaped polymers by raft polymerization. Polymers 2021, 13, 1698. [Google Scholar] [CrossRef] [PubMed]
- Karmegam, V.; Kuruppu, S.S.; Udamulle Gedara, C.M.; Biewer, M.C.; Stefan, M.C. Enhanced DOX loading in star-like benzyl functionalized polycaprolactone micelles. J. Polym. Sci. 2021, 59, 3040–3052. [Google Scholar] [CrossRef]
- Youssouf, L.; Bhaw-Luximon, A.; Diotel, N.; Catan, A.; Giraud, P.; Gimié, F.; Koshel, D.; Casale, S.; Bénard, S.; Meneyrol, V. Enhanced effects of curcumin encapsulated in polycaprolactone-grafted oligocarrageenan nanomicelles, a novel nanoparticle drug delivery system. Carbohydr. Polym. 2019, 217, 35–45. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Mendonça, P.V.; Simões, S.; Serra, A.C.; Coelho, J.F. Amphiphilic well-defined degradable star block copolymers by combination of ring-opening polymerization and atom transfer radical polymerization: Synthesis and application as drug delivery carriers. J. Polym. Sci. 2021, 59, 211–229. [Google Scholar] [CrossRef]
- Maji, P.; Naskar, K. Styrenic block copolymer-based thermoplastic elastomers in smart applications: Advances in synthesis, microstructure, and structure-property relationships-A review. J. Appl. Polym. Sci. 2022, 139, e52942. [Google Scholar] [CrossRef]
- Harn, Y.-W.; He, Y.; Wang, Z.; Chen, Y.; Liang, S.; Li, Z.; Li, Q.; Zhu, L.; Lin, Z. Synthesis of amphiphilic and double hydrophilic star-like block copolymers and the dual pH-responsiveness of unimolecular micelle. Macromolecules 2020, 53, 8286–8295. [Google Scholar] [CrossRef]
- Muniandy, A.; Lee, C.S.; Lim, W.H.; Pichika, M.R.; Mak, K.K. Hyperbranched poly (glycerol esteramide): A biocompatible drug carrier from glycerol feedstock and dicarboxylic acid. J. Appl. Polym. Sci. 2021, 138, 50126. [Google Scholar] [CrossRef]
- Liffland, S.; Hillmyer, M.A. Enhanced mechanical properties of aliphatic polyester thermoplastic elastomers through star block architectures. Macromolecules 2021, 54, 9327–9340. [Google Scholar] [CrossRef]
- Lu, C.; Wen, T.; Zheng, M.; Liu, D.; Quan, G.; Pan, X.; Wu, C. Poly (Ethylene Glycol) crosslinked multi-armed poly (L-lysine) with encapsulating capacity and antimicrobial activity for the potential treatment of infection-involved multifactorial diseases. Pharmaceutics 2020, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Richbourg, N.R.; Peppas, N.A. Solute diffusion and partitioning in multi-arm poly (ethylene glycol) hydrogels. J. Mater. Chem. B 2023, 11, 377–388. [Google Scholar] [CrossRef] [PubMed]
- El yousfi, R.; Achalhi, N.; Benahmed, A.; El Idrissi, A. Synthesis, characterization of multi-arm copolymers and linear blocks based on PEG and PCL: Effect of topology on dye adsorption. Mater. Today Proc. 2023, 72, 3650–3661. [Google Scholar] [CrossRef]
- Xie, W.; Jiang, N.; Gan, Z. Effects of Multi-Arm Structure on Crystallization and Biodegradation of Star-Shaped Poly (ε-caprolactone). Macromol. Biosci. 2008, 8, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Celik, A.; Kemikli, N.; Ozturk, R.; Muftuoglu, A.E.; Yilmaz, F. Synthesis, characterization and thermal properties of a novel star polymer consisting of poly (ε-caprolactone) arms emanating from an octa-functional porphyrazine core. React. Funct. Polym. 2009, 69, 705–713. [Google Scholar] [CrossRef]
- Cui, Y.; Ma, X.; Tang, X.; Luo, Y. Synthesis, characterization, and thermal stability of star-shaped poly (ε-caprolactone) with phosphazene core. Eur. Polym. J. 2004, 40, 299–305. [Google Scholar] [CrossRef]
- Gou, P.F.; Zhu, W.P.; Xu, N.; Shen, Z.Q. Synthesis and characterization of well-defined cyclodextrin-centered seven-arm star poly (ε-caprolactone) s and amphiphilic star poly (ε-caprolactone-b-ethylene glycol) s. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 6455–6465. [Google Scholar] [CrossRef]
- Yuan, W.; Yuan, J.; Huang, X.; Tang, X. Synthesis, characterization, and in vitro degradation of star-shaped P (ε-caprolactone)-b-poly (l-lactide)-b-poly (d, l-lactide-co-glycolide) from hexakis [p-(hydroxymethyl) phenoxy] cyclotriphosphazene initiator. J. Appl. Polym. Sci. 2007, 104, 2310–2317. [Google Scholar] [CrossRef]
- Xu, J.; Shi, W. Synthesis and crystallization kinetics of silsesquioxane-based hybrid star poly (ε-caprolactone). Polymer 2006, 47, 5161–5173. [Google Scholar] [CrossRef]
- Doganci, M.D. Effects of star-shaped PCL having different numbers of arms on the mechanical, morphological, and thermal properties of PLA/PCL blends. J. Polym. Res. 2021, 28, 11. [Google Scholar] [CrossRef]
- Karavelidis, V.; Karavas, E.; Giliopoulos, D.; Papadimitriou, S.; Bikiaris, D. Evaluating the effects of crystallinity in new biocompatible polyester nanocarriers on drug release behavior. Int. J. Nanomed. 2011, 6, 3021–3032. [Google Scholar]
- Vollrath, A.; Kretzer, C.; Beringer-Siemers, B.; Shkodra, B.; Czaplewska, J.A.; Bandelli, D.; Stumpf, S.; Hoeppener, S.; Weber, C.; Werz, O. Effect of crystallinity on the properties of polycaprolactone nanoparticles containing the dual FLAP/mPEGS-1 Inhibitor BRP-187. Polymers 2021, 13, 2557. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, Y.; Liu, L.; Sun, Q.; Shuai, X.; Zhu, W.; Chen, Y. Amphiphilic toothbrushlike copolymers based on poly (ethylene glycol) and poly (ε-caprolactone) as drug carriers with enhanced properties. Biomacromolecules 2010, 11, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Vora, A.; Singh, K.; Webster, D.C. A new approach to 3-miktoarm star polymers using a combination of reversible addition–fragmentation chain transfer (RAFT) and ring opening polymerization (ROP) via “Click” chemistry. Polymer 2009, 50, 2768–2774. [Google Scholar] [CrossRef]
- Maglio, G.; Nicodemi, F.; Conte, C.; Palumbo, R.; Tirino, P.; Panza, E.; Ianaro, A.; Ungaro, F.; Quaglia, F. Nanocapsules based on linear and Y-shaped 3-miktoarm star-block PEO-PCL copolymers as sustained delivery system for hydrophilic molecules. Biomacromolecules 2011, 12, 4221–4229. [Google Scholar] [CrossRef]
- Huang, C.F.; Huang, Y.S.; Lai, K.Y. Synthesis and self-assembly of poly (N-octyl benzamide)-μ-poly (ε-caprolactone) miktoarm star copolymers displaying uniform nanofibril morphology. Polymer 2019, 178, 121582. [Google Scholar] [CrossRef]
- Ning, Z.; Jiang, N.; Gan, Z. Four-armed PCL-b-PDLA diblock copolymer: 1. Synthesis, crystallization and degradation. Polym. Degrad. Stab. 2014, 107, 120–128. [Google Scholar] [CrossRef]
- Christodoulou, E.; Klonos, P.A.; Tsachouridis, K.; Zamboulis, A.; Kyritsis, A.; Bikiaris, D.N. Synthesis, crystallization, and molecular mobility in poly (ε-caprolactone) copolyesters of different architectures for biomedical applications studied by calorimetry and dielectric spectroscopy. Soft Matter 2020, 16, 8187–8201. [Google Scholar] [CrossRef]
- Deokar, M.D.; Garnaik, B.; Sivaram, S. Toughening Poly (l-lactide) Blends: Effectiveness of Sequence-Controlled Six-Arm Star-Branched Block Copolymers of Poly (l-lactide) and Poly (ε-caprolactone). ACS Omega 2022, 7, 9118–9129. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Camargo, R.A.; Saenz, G.; Laurichesse, S.; Casas, M.T.; Puiggali, J.; Avérous, L.; Müller, A.J. Nucleation, Crystallization, and Thermal Fractionation of Poly (ε-Caprolactone)-Grafted-Lignin: Effects of Grafted Chains Length and Lignin Content. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 1736–1750. [Google Scholar] [CrossRef]
- Fang, H.; Guymon, C.A. Recent advances to decrease shrinkage stress and enhance mechanical properties in free radical polymerization: A review. Polym. Int. 2022, 71, 596–607. [Google Scholar] [CrossRef]
- Liu, M.; Blankenship, J.R.; Levi, A.E.; Fu, Q.; Hudson, Z.M.; Bates, C.M. Miktoarm Star Polymers: Synthesis and Applications. Chem. Mater. 2022, 34, 6188–6209. [Google Scholar] [CrossRef]
- Pięta, M.; Purohit, V.B.; Pietrasik, J.; Plummer, C.M. Disulfide-containing monomers in chain-growth polymerization. Polym. Chem. 2023, 14, 7–31. [Google Scholar] [CrossRef]
- Wiltshire, J.T.; Qiao, G.G. Selectively degradable core cross-linked star polymers. Macromolecules 2006, 39, 9018–9027. [Google Scholar] [CrossRef]
- Du, J.; Chen, Y. PCL star polymer, PCL-PS heteroarm star polymer by ATRP, and core-carboxylated PS star polymer thereof. Macromolecules 2004, 37, 3588–3594. [Google Scholar] [CrossRef]
- Dai, S.; Wang, M.; Zhuang, Z.; Ning, Z. Crystallization and Alkaline Degradation Behaviors of Poly (l-Lactide)/4-Armed Poly (ε-Caprolactone)-Block-Poly (d-Lactide) Blends with Different Poly (d-Lactide) Block Lengths. Polymers 2020, 12, 2195. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Bronich, T.K.; Kabanov, A.V.; Rauh, R.D.; Roovers, J. Synthesis and evaluation of a star amphiphilic block copolymer from poly (ε-caprolactone) and poly (ethylene glycol) as a potential drug delivery carrier. Bioconjugate Chem. 2005, 16, 397–405. [Google Scholar] [CrossRef]
- Glaied, O.; Delaite, C.; Dumas, P. Synthesis of A2B star block copolymers from a heterotrifunctional initiator. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 1796–1806. [Google Scholar] [CrossRef]
- Yoon, K.; Kang, H.C.; Li, L.; Cho, H.; Park, M.-K.; Lee, E.; Bae, Y.H.; Huh, K.M. Amphiphilic poly (ethylene glycol)-poly (ε-caprolactone) AB 2 miktoarm copolymers for self-assembled nanocarrier systems: Synthesis, characterization, and effects of morphology on antitumor activity. Polym. Chem. 2015, 6, 531–542. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Y.; Sun, J.; Wang, S.; Zhao, Y.; Wu, Z. Chemoenzymatic synthesis of Y-shaped amphiphilic block copolymers and their micellization behavior. Polym. Int. 2009, 58, 752–761. [Google Scholar] [CrossRef]
- Erdogan, T.; Ozyurek, Z.; Hizal, G.; Tunca, U. Facile synthesis of AB2-type miktoarm star polymers through the combination of atom transfer radical polymerization and ring-opening polymerization. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 2313–2320. [Google Scholar] [CrossRef]
- Moquin, A.; Sharma, A.; Cui, Y.; Lau, A.; Maysinger, D.; Kakkar, A. Asymmetric AB3 Miktoarm Star Polymers: Synthesis, Self-Assembly, and Study of Micelle Stability Using AF4 for Efficient Drug Delivery. Macromol. Biosci. 2015, 15, 1744–1754. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.M.; Li, L.D.; Shang, L.; Zhou, Q.H.; Lin, J. Preparation of pH-sensitive micelles from miktoarm star block copolymers by ATRP and their application as drug nanocarriers. React. Funct. Polym. 2016, 107, 28–34. [Google Scholar] [CrossRef]
- Sathesh, V.; Chen, J.-K.; Chang, C.-J.; Aimi, J.; Chen, Z.-C.; Hsu, Y.-C.; Huang, Y.-S.; Huang, C.-F. Synthesis of Poly (ε-caprolactone)-Based Miktoarm Star Copolymers through ROP, SA ATRC, and ATRP. Polymers 2018, 10, 858. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Figg, C.A.; Carmean, R.N.; Bentz, K.C.; Mukherjee, S.; Savin, D.A.; Sumerlin, B.S. Tuning hydrophobicity to program block copolymer assemblies from the inside out. Macromolecules 2017, 50, 935–943. [Google Scholar] [CrossRef]
- Discher, D.E.; Ahmed, F. Polymersomes. Annu. Rev. Biomed. Eng. 2006, 8, 323–341. [Google Scholar] [CrossRef]
- Kedracki, D.; Abraham, J.N.; Prado, E.; Nardin, C. Self-Assembly of Biohybrid Polymers. Macromol. Self-Assem. 2016, 193–229. [Google Scholar]
- Yin, H.; Kang, S.W.; Bae, Y.H. Polymersome formation from AB2 type 3-miktoarm star copolymers. Macromolecules 2009, 42, 7456–7464. [Google Scholar] [CrossRef]
- Ji, X.; Dong, S.; Wei, P.; Xia, D.; Huang, F. A novel diblock copolymer with a supramolecular polymer block and a traditional polymer block: Preparation, controllable self-assembly in water, and application in controlled release. Adv. Mater. 2013, 25, 5725–5729. [Google Scholar] [CrossRef]
- Zhu, M.-m.; Song, F.; Nie, W.C.; Wang, X.L.; Wang, Y.Z. A facile chemoenzymatic synthesis of amphiphilic miktoarm star copolymers from a sugar core and their potential for anticancer drug delivery. Polymer 2016, 93, 159–166. [Google Scholar] [CrossRef]
- Brandt, J.V.; Piazza, R.D.; dos Santos, C.C.; Vega-Chacón, J.; Amantéa, B.E.; Pinto, G.C.; Junior, M.J.; Marques, R.F.C. Synthesis of core@ shell nanoparticles functionalized with folic acid-modified PCL-co-PEGMA copolymer for methotrexate delivery. Nano-Struct. Nano-Objects 2021, 25, 100675. [Google Scholar] [CrossRef]
- Shalaby, K.S.; Soliman, M.E.; Bonacucina, G.; Cespi, M.; Palmieri, G.F.; Sammour, O.A.; El Shamy, A.A.; Illum, L.; Casettari, L. Nanoparticles based on linear and star-shaped poly (ethylene glycol)-poly (ε-caprolactone) copolymers for the delivery of antitubulin drug. Pharm. Res. 2016, 33, 2010–2024. [Google Scholar] [CrossRef] [PubMed]
- Aghajanzadeh, M.; Zamani, M.; Rostamizadeh, K.; Sharafi, A.; Danafar, H. The role of miktoarm star copolymers in drug delivery systems. J. Macromol. Sci. Part A 2018, 55, 559–571. [Google Scholar] [CrossRef]
- Soliman, G.M.; Sharma, R.; Choi, A.O.; Varshney, S.K.; Winnik, F.M.; Kakkar, A.K.; Maysinger, D. Tailoring the efficacy of nimodipine drug delivery using nanocarriers based on A2B miktoarm star polymers. Biomaterials 2010, 31, 8382–8392. [Google Scholar] [CrossRef]
- Lin, W.; Nie, S.; Zhong, Q.; Yang, Y.; Cai, C.; Wang, J.; Zhang, L. Amphiphilic miktoarm star copolymer (PCL) 3-(PDEAEMA-b-PPEGMA) 3 as pH-sensitive micelles in the delivery of anticancer drug. J. Mater. Chem. B 2014, 2, 4008–4020. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, R.; Ghaemy, M. pH-responsive ABC type miktoarm star terpolymers: Synthesis via combination of click reaction and SET-LRP, characterization, self-assembly, and controlled drug release. Polymer 2015, 66, 179–191. [Google Scholar] [CrossRef]
- Poree, D.E.; Giles, M.D.; Lawson, L.B.; He, J.; Grayson, S.M. Synthesis of amphiphilic star block copolymers and their evaluation as transdermal carriers. Biomacromolecules 2011, 12, 898–906. [Google Scholar] [CrossRef]
- Ochs, J.; Alegría, A.; González de San Román, E.; Grayson, S.M.; Barroso-Bujans, F. Synthesis of Macrocyclic Poly (glycidyl phenyl ether) with an Inverted-Dipole Microstructure via Ring Closure of Two-Arm Linear Precursors Obtained by Initiation with t-BuP4/Water. Macromolecules 2020, 53, 10005–10014. [Google Scholar] [CrossRef]
- Kosakowska, K.A.; Casey, B.K.; Albert, J.N.; Wang, Y.; Ashbaugh, H.S.; Grayson, S.M. Synthesis and self-assembly of amphiphilic star/linear–dendritic polymers: Effect of core versus peripheral branching on reverse micelle aggregation. Biomacromolecules 2018, 19, 3177–3189. [Google Scholar] [CrossRef]
- Lo, Y.L.; Chen, G.J.; Feng, T.H.; Li, M.H.; Wang, L.F. Synthesis and characterization of S-PCL-PDMAEMA for co-delivery of pDNA and DOX. RSC Adv. 2014, 4, 11089–11098. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, J.; Wang, Q.; Zhong, Z.; Zhuo, R. Miktoarm copolymers bearing one poly (ethylene glycol) chain and several poly (ε-caprolactone) chains on a hyperbranched polyglycerol core. Macromolecules 2010, 43, 6671–6677. [Google Scholar] [CrossRef]
- Yuan, Y.-Y.; Wang, J. Temperature-induced morphological change of ABC 3-miktoarm star terpolymer assemblies in aqueous solution. Colloids Surf. B Biointerfaces 2011, 85, 81–85. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, X.; Peng, H.; Zhang, M.; Zhang, X.; Liu, Z.; Ma, L.; Wei, H. Optimization of amphiphilic miktoarm star copolymers for anticancer drug delivery. ACS Biomater. Sci. Eng. 2018, 4, 2903–2910. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Zhang, C.; Zhang, L.; Sun, H.; Song, C.; Wang, C.; Kong, D. Doxorubicin-loaded micelles based on multiarm star-shaped PLGA-PEG block copolymers: Influence of arm numbers on drug delivery. J. Mater. Sci. Mater. Med. 2016, 27, 1–15. [Google Scholar] [CrossRef]
- Xie, C.; Zhang, P.; Zhang, Z.; Yang, C.; Zhang, J.; Wu, W.; Jiang, X. Drug-loaded pseudo-block copolymer micelles with a multi-armed star polymer as the micellar exterior. Nanoscale 2015, 7, 12572–12580. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.; Yu, D.; Zhong, Z.; Liang, Q.; Jing, X. Enzymatic degradation of poly (ε-caprolactone)/poly (DL-lactide) blends in phosphate buffer solution. Polymer 1999, 40, 2859–2862. [Google Scholar] [CrossRef]
- Castilla-Cortázar, I.; Más-Estellés, J.; Meseguer-Dueñas, J.M.; Ivirico, J.E.; Marí, B.; Vidaurre, A. Hydrolytic and enzymatic degradation of a poly (ε-caprolactone) network. Polym. Degrad. Stab. 2012, 97, 1241–1248. [Google Scholar] [CrossRef]
- Lee, C.W.; Kimura, Y.; Chung, J.-D. Mechanism of enzymatic degradation of poly (butylene succinate). Macromol. Res. 2008, 16, 651–658. [Google Scholar] [CrossRef]
- Mochizuki, M.; Hirano, M.; Kanmuri, Y.; Kudo, K.; Tokiwa, Y. Hydrolysis of polycaprolactone fibers by lipase: Effects of draw ratio on enzymatic degradation. J. Appl. Polym. Sci. 1995, 55, 289–296. [Google Scholar] [CrossRef]
- Gan, Z.; Liang, Q.; Zhang, J.; Jing, X. Enzymatic degradation of poly (ε-caprolactone) film in phosphate buffer solution containing lipases. Polym. Degrad. Stab. 1997, 56, 209–213. [Google Scholar] [CrossRef]
- Li, S.; Pignol, M.; Gasc, F.; Vert, M. Synthesis, characterization, and enzymatic degradation of copolymers prepared from ε-caprolactone and β-butyrolactone. Macromolecules 2004, 37, 9798–9803. [Google Scholar] [CrossRef]
- Ponsart, S.; Coudane, J.; Saulnier, B.; Morgat, J.L.; Vert, M. Biodegradation of [3H] Poly (ε-caprolactone) in the Presence of Active Sludge Extracts. Biomacromolecules 2001, 2, 373–377. [Google Scholar] [CrossRef]
- Khatiwala, V.K.; Shekhar, N.; Aggarwal, S.; Mandal, U. Biodegradation of poly (ε-caprolactone)(PCL) film by Alcaligenes faecalis. J. Polym. Environ. 2008, 16, 61–67. [Google Scholar] [CrossRef]
- Yoshioka, T.; Kamada, F.; Kawazoe, N.; Tateishi, T.; Chen, G. Structural changes and biodegradation of PLLA, PCL, and PLGA sponges during in vitro incubation. Polym. Eng. Sci. 2010, 50, 1895–1903. [Google Scholar] [CrossRef]
- Sekosan, G.; Vasanthan, N. Morphological changes of annealed poly-ε-caprolactone by enzymatic degradation with lipase. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 202–211. [Google Scholar] [CrossRef]
- Blackwell, C.J.; Haernvall, K.; Guebitz, G.M.; Groombridge, M.; Gonzales, D.; Khosravi, E. Enzymatic degradation of star poly (ε-caprolactone) with different central units. Polymers 2018, 10, 1266. [Google Scholar] [CrossRef]
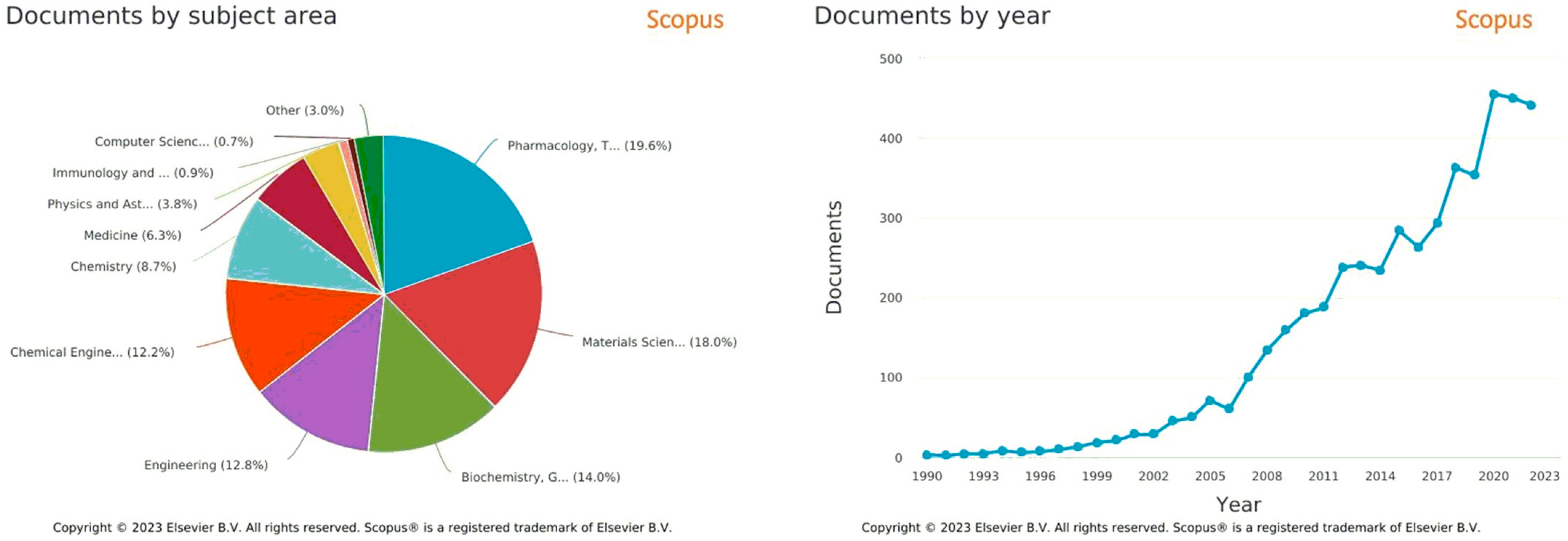
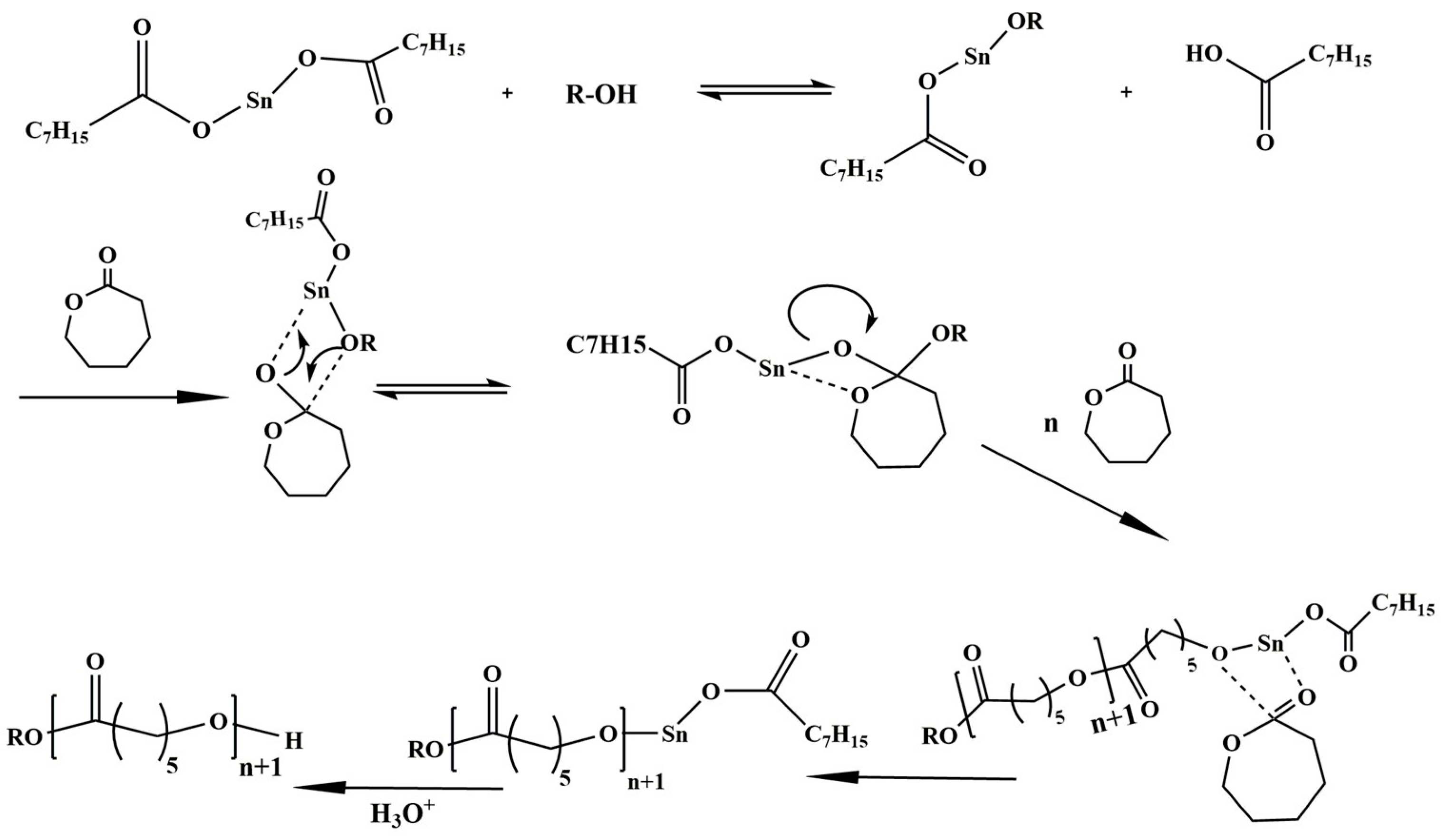
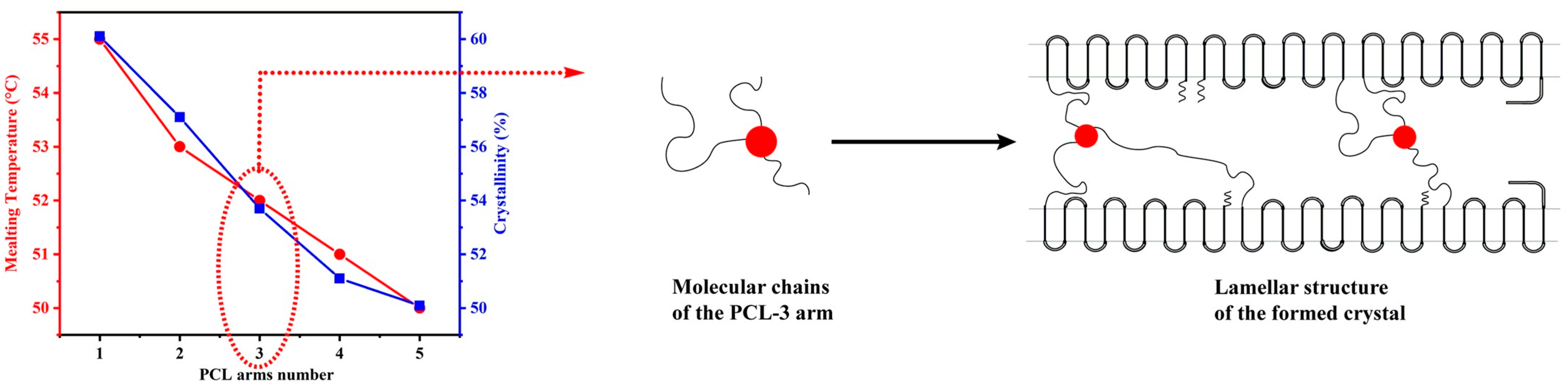
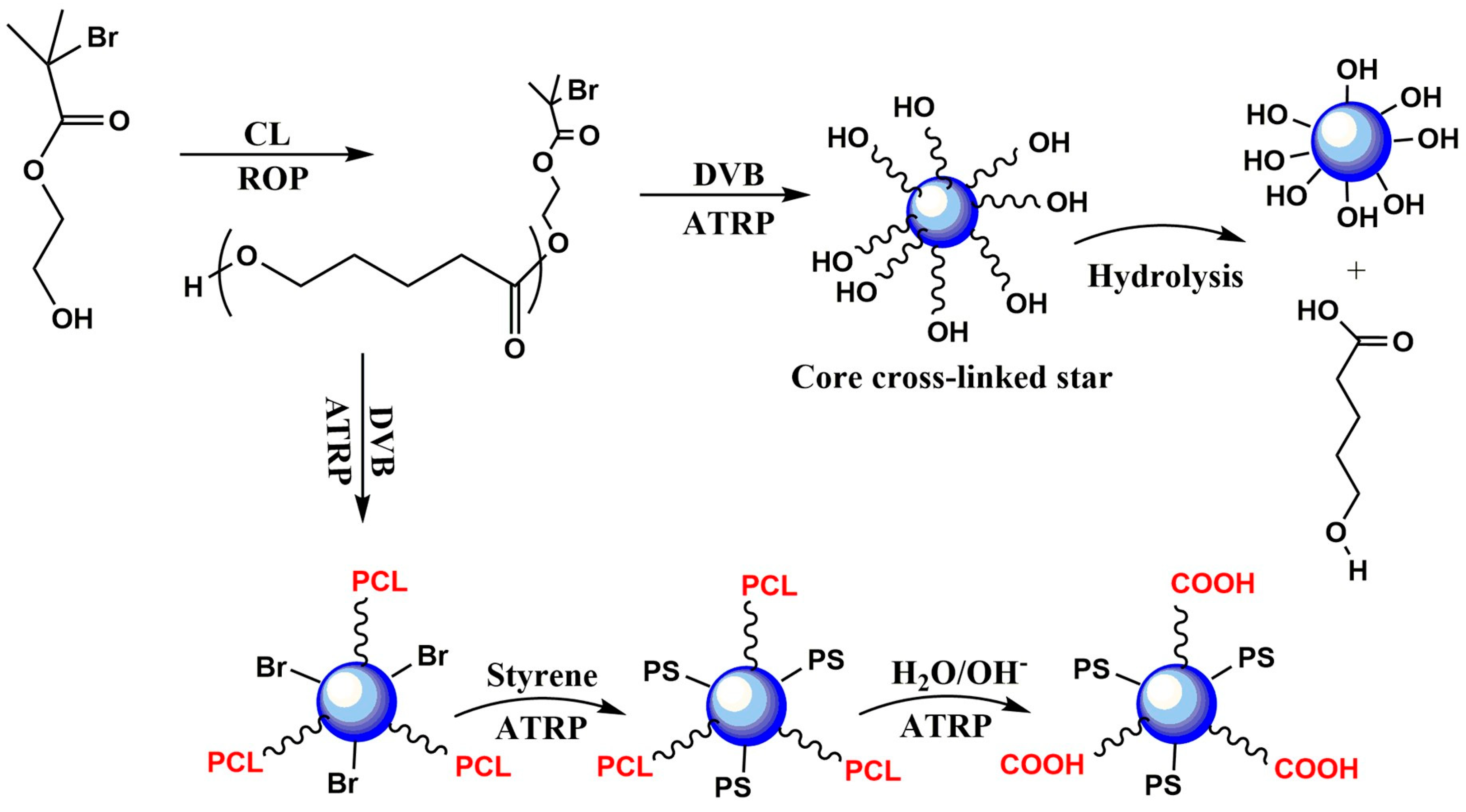
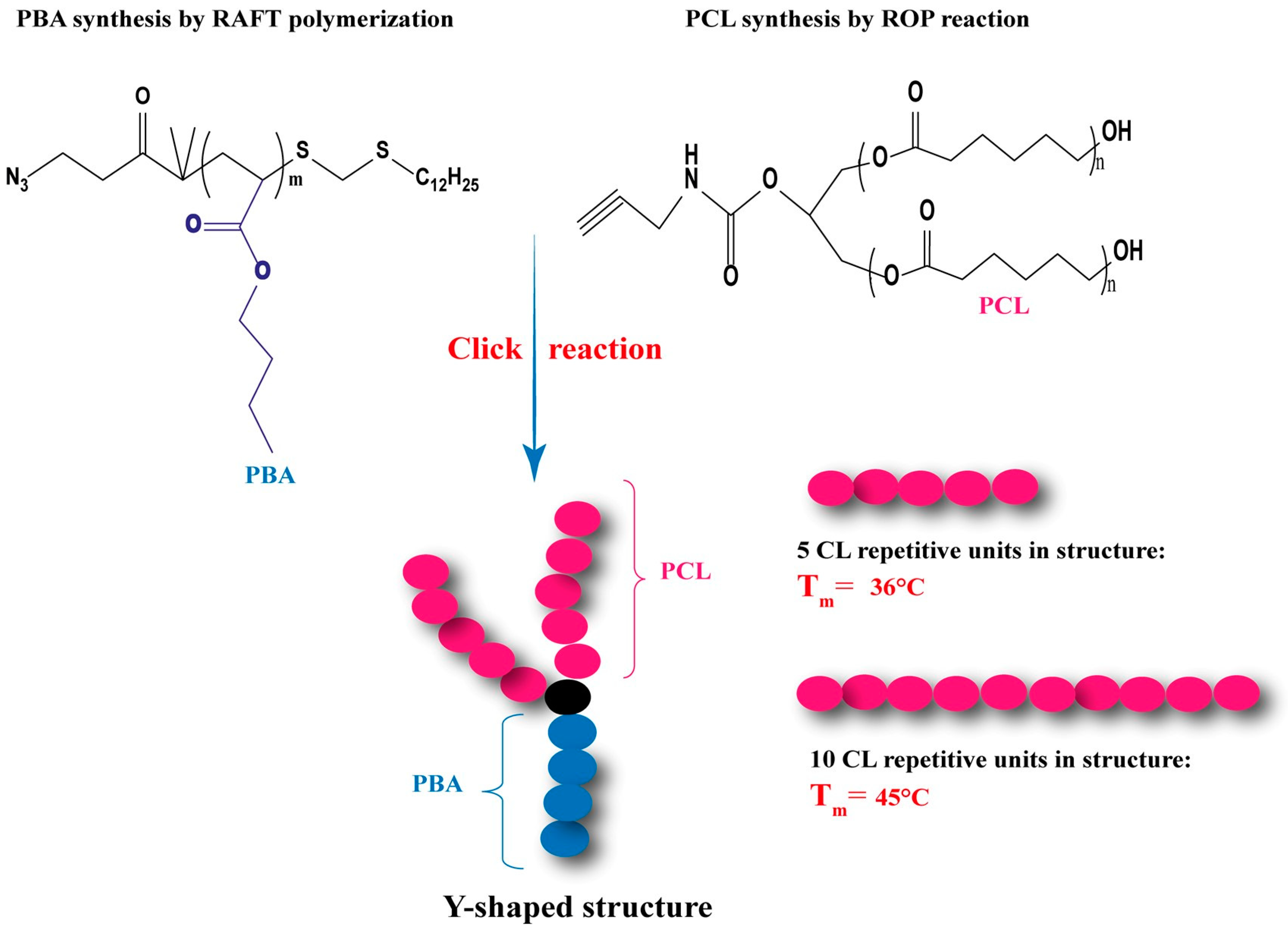
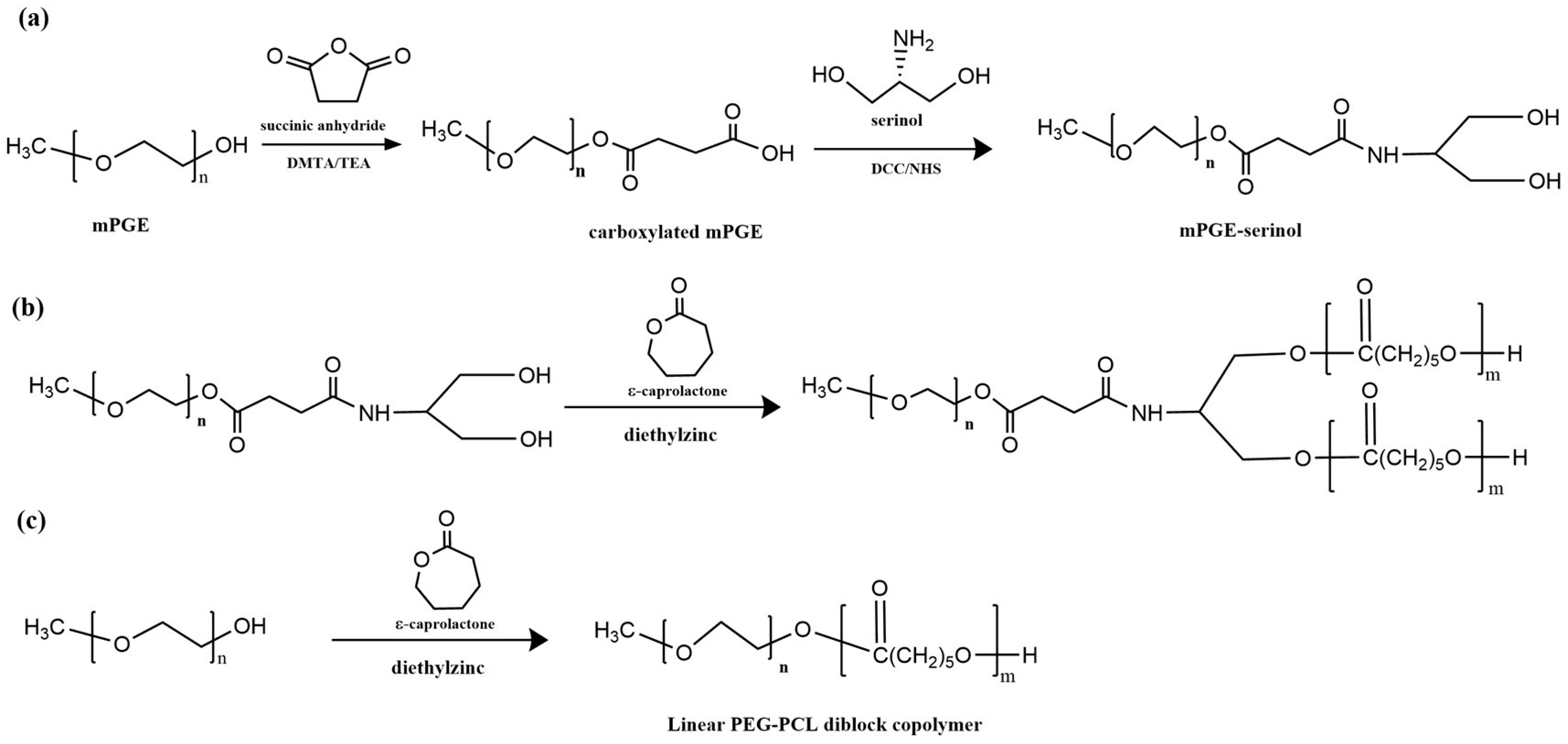
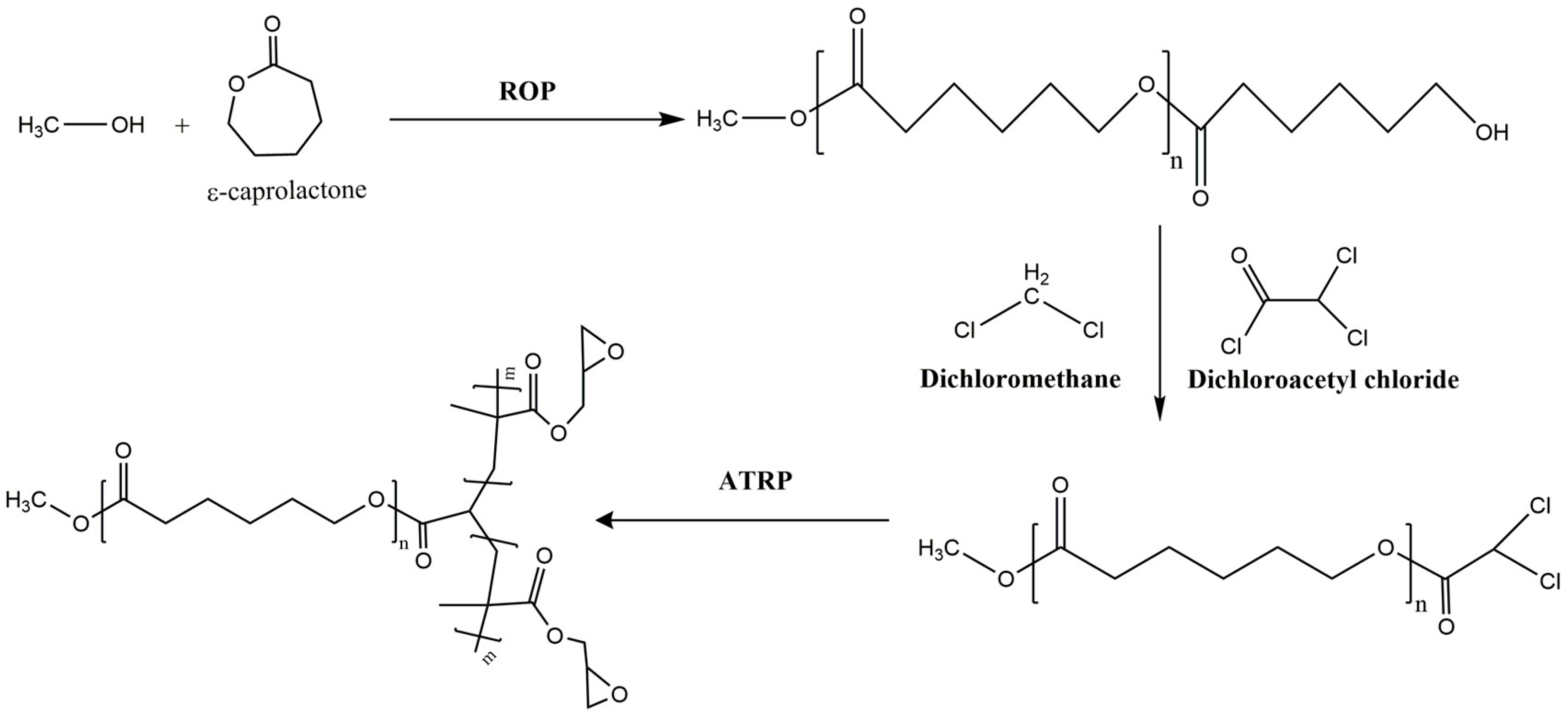

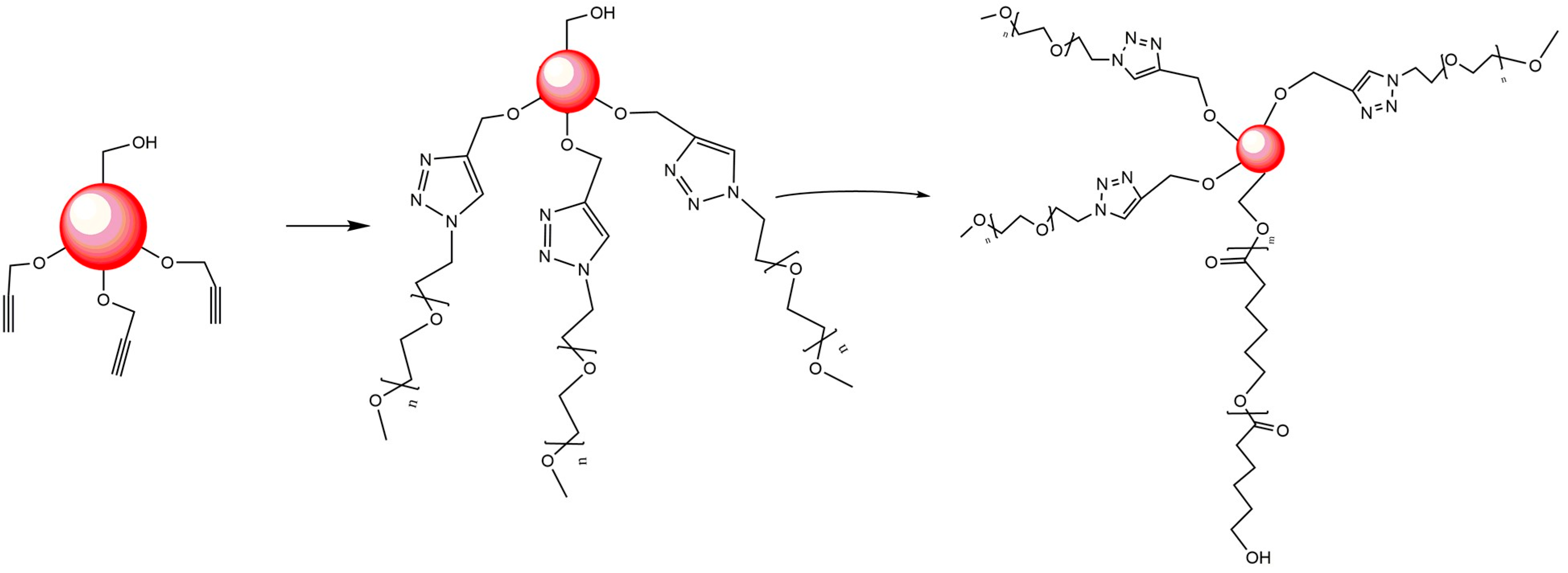




| Mode | Polymers | Drug | Nanoformulation | Therapeutic Target | Biological Evaluation | Ref. |
|---|---|---|---|---|---|---|
| Electrospun | PCL | Dexamethasone | Nanofibers | Biomedical applications, particularly in the eye. | Although the results are encouraging, further in vitro studies and finally in vivo animal studies are needed to determine the comfort and retention. | [123] |
| Electrospun | PCL | Tetracycline/βcyclodextrin | Nanofibers | Controlled release systems and their clinical application. | Strong adhesion and reduced demineralization of dentin. | [124] |
| High antimicrobial activity against A.a and P.g. | ||||||
| Electrospun | PCL | ampicillin | Nanofibers | Zero-order drug release kinetics. | [125] | |
| Electrospun | PCL | Seeded with IPFP and chondrones | Nanofibers | Restore the functional cartilage in articular disorders. | A positive effect on the differentiation of IPFP APCs into chondrogenic cells. | [126] |
| Electrospun | PCL-PEG | Fe3O4 NPs | Magnetic composite membrane (PCEC/Fe3O4 nanofibers) | Preventing tumor recurrence and improving dermal wound healing after an excision of malignant tumor in the skin. | In vitro cell culture of NIH 3T3 cells on the PCEC/Fe3O4 membranes showed that the PCEC/Fe3O4 fibers might be a suitable scaffold for cell adhesion. | [127] |
| MTT analysis also demonstrated that the membranes possessed lower cytotoxicity. | ||||||
| Electrospun | PCL-PEG | PCL fibers embedded in a PEG-fibrinogen hydrogel | Sufficient cell-approachable bio-signaling cues, which may synergistically facilitate the control of stem cell fates for regenerative therapies. | A novel nanocomposite that promoted the active interactions with stem cells and exerted excellent fibrogenic commitment in vitro. | [128] | |
| Hydrogel films | PCL-PEG | Curcumin | Sol-gel | Safe candidate for in situ gel-forming controlled drug delivery system. | No toxic response or histopathological changes were observed. | [129] |
| In vivo gel-formation, degradation test showed that a complete degradation occurred after 21 days. | ||||||
| Hydrogel films | PCL-PEG | proteins (BSA and HRP) | Sol-gel | In-situ gel depot at body temperature providing drug release control. | The in vivo gel-formation and degradation studies indicated that copolymer hydrogels can sustain at least 45 days. | [130] |
| Hydrogel films | PCL-PEG | Sol-gel | Facilitate the bone regeneration in the non-load-bearing cranial repair process by combining the advantages of the intrinsic osteoinductive ABM granules and the injectable thermosensitive PECE hydrogel. | In vivo bone regeneration performance was carried out in white rabbits for 4, 12, and 20 weeks. | [131] | |
| Hydrogel films | (PEG-PCL)3 | cyclodextrin | Injectable hydrogels | Treatment of joint disease. | In vitro drug release showed that DOX∙HCl was released in a controlled, pH-dependent manner. | [132] |
| Nanoparticles | β-cyclodextrin-PCL | Camptothecin/DOX | Micelles | Sustained release of hydrophobic drugs via local administration in clinical situations | IND-M was able to release the drug over an extended period in vitro and exhibited a significant therapeutic effect in pharmacodynamic studies in vivo (HepG2 cells). | [133] |
| Nanoparticles | 12-arm PEG-PCL | Docetaxel | Micelles | In vitro cytotoxicity (HeLa cells) study indicated a reduced cytotoxicity. | [113] |
| Polymers | Type | Arms | TC,PCL | Tm | Tg | XC% | Molten State Morphology | Year | Ref. |
|---|---|---|---|---|---|---|---|---|---|
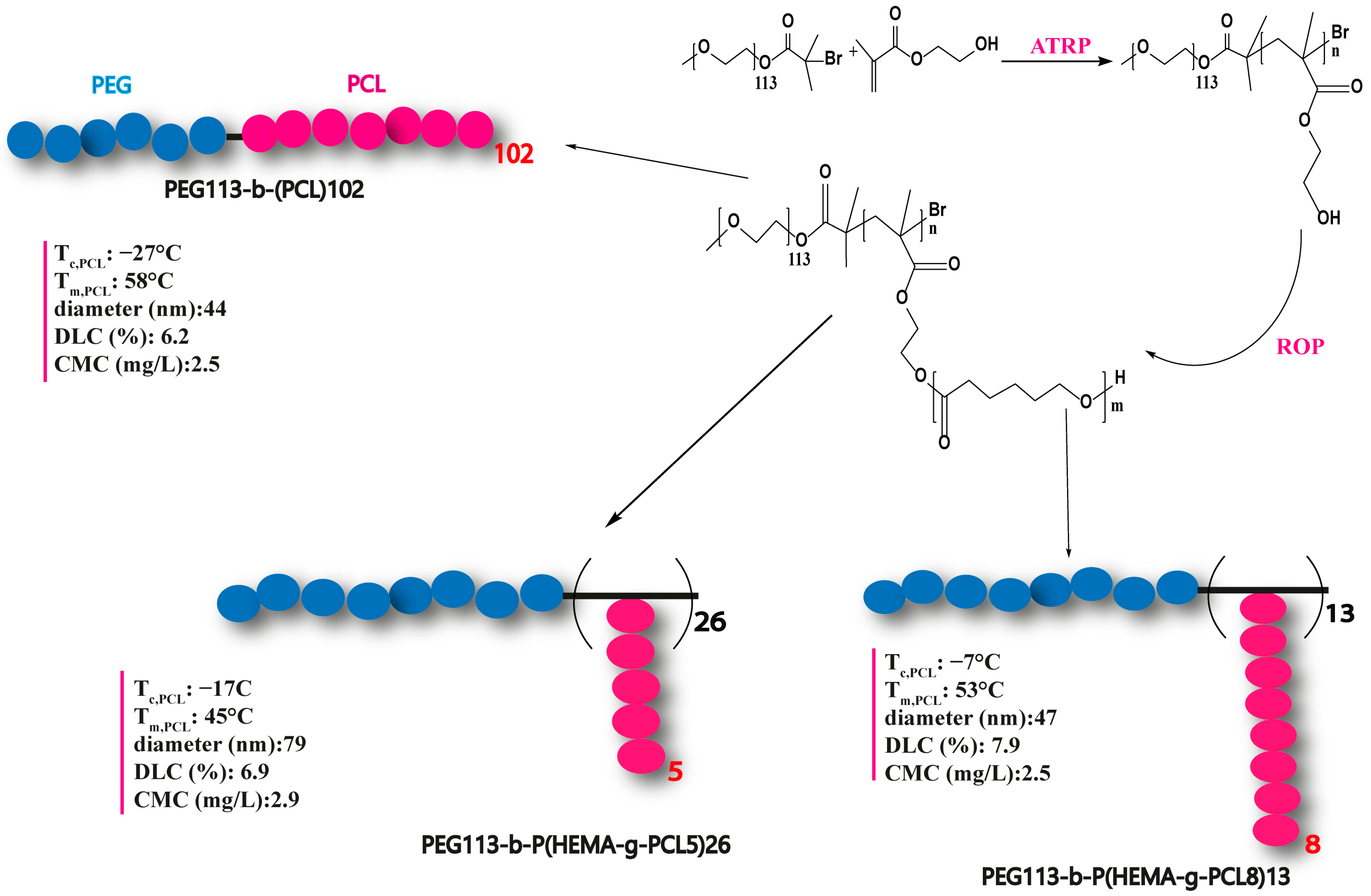
| -PEG113-b- PCL102 -PEG113-b-(HEMA-g-PCL8)13 -PEG113-b-(HEMA-g-PCL5)26 | -Linear -Multiarms -Multiarms | 1 13 26 | 27.6 −7 −17 | 58.6 53.1 45.1 | 2010 | [165] | |||
| Star-shaped PCLs similar molecular weights (Mn ¼ 10 300) | Star shaped | 1 2 3 4 5 | – – – – – | 57.5 55.1 54.9 53.1 52.9 | 61 57 55 53 50 | 2008 | [156] | ||
| -PPEGA-b-(PCL-5)2 -PPEGA-b-(PCL-10)2 | Multiarms | 2 2 | – – | 32.5 45 | 2009 | [166] | |||
| -PCL40-b-PS59 -(PCL2)39-b-(PS2)61 | AB A2B2 | 1 4 | 27.7 17.2 | 55.7 54.5 | 102.5 103.1 | 32 17 | Lamellar PCL cylinders. | 2009 | [85] |
| PEO2k-PCL4.3k PEO2k-(PCL2.1k)2 | AB AB2 | Linear 2 | 34.4 30.5 | 53.5 47.4 | 47 40 | [167] | |||
| -PCL -PBA -μ-(PBA)2(PCL)4 | – – μ-A2B4 | 6 | 25 – −7.8 | 49.9 – 44.8 | −56 22 −49.5PCL 26.3PBA | 66 – 26 | – – Uniform nanofiber | 2019 | [168] |
| -4a-PCL5k-b-PDLA 1k | 4armed | −4 −4 | 23 80 | 50 140 | 66 13 | Dense spherulites. No spherulites. | 2014 | [169] | |
| -4a-PCL5k-b-PDLA 10k | |||||||||
| LPCL4.8k 3SPCL3.7k 4SPCL9.4k 6SPCL13.9k | Linear 3armed 4armed 6armed | 1 3 4 6 | – 55.3 54.5 52.4 | 58 – – – | 65.3 57.8 56.3 54 | 2020 | [162] | ||
| PCL62K 3S-PCL99K 4S-PCL18K | Linear Star Star | 1 3 4 | 29 31 28 | 54 54 51 | −70 −68 −66 | 47 47 51 | 2020 | [170] | |
| 6s-PCL-b-PLA (Mn=66.1k) 6s-PLA-b-PCL (Mn=66.3K) | 6 armed 6 armed | 6 6 | 50.6PCL149PLA | −55PCL35.5PLA | Phase separation. No phase separation. | 2022 | [171] | ||
| 120PLA | −57PCL 36PLA | ||||||||
| -lignin-g-PCL1 -lignin-g-PCL29 -lignin-g-PCL37 | Grafted arms | 1 29 37 | 21.5 16.7 7.1 | 49.7 47.9 58liging 44.5PCL | 43 34 22 | High lamellar thickening. – Limited lamellar thickening. | 2015 | [172] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Yousfi, R.; Brahmi, M.; Dalli, M.; Achalhi, N.; Azougagh, O.; Tahani, A.; Touzani, R.; El Idrissi, A. Recent Advances in Nanoparticle Development for Drug Delivery: A Comprehensive Review of Polycaprolactone-Based Multi-Arm Architectures. Polymers 2023, 15, 1835. https://doi.org/10.3390/polym15081835
El Yousfi R, Brahmi M, Dalli M, Achalhi N, Azougagh O, Tahani A, Touzani R, El Idrissi A. Recent Advances in Nanoparticle Development for Drug Delivery: A Comprehensive Review of Polycaprolactone-Based Multi-Arm Architectures. Polymers. 2023; 15(8):1835. https://doi.org/10.3390/polym15081835
Chicago/Turabian StyleEl Yousfi, Ridouan, Mohamed Brahmi, Mohammed Dalli, Nafea Achalhi, Omar Azougagh, Abdesselam Tahani, Rachid Touzani, and Abderrahmane El Idrissi. 2023. "Recent Advances in Nanoparticle Development for Drug Delivery: A Comprehensive Review of Polycaprolactone-Based Multi-Arm Architectures" Polymers 15, no. 8: 1835. https://doi.org/10.3390/polym15081835
APA StyleEl Yousfi, R., Brahmi, M., Dalli, M., Achalhi, N., Azougagh, O., Tahani, A., Touzani, R., & El Idrissi, A. (2023). Recent Advances in Nanoparticle Development for Drug Delivery: A Comprehensive Review of Polycaprolactone-Based Multi-Arm Architectures. Polymers, 15(8), 1835. https://doi.org/10.3390/polym15081835









