Assembling Complex Macromolecules and Self-Organizations of Biological Relevance with Cu(I)-Catalyzed Azide-Alkyne, Thio-Bromo, and TERMINI Double “Click” Reactions †
Abstract
1. Introduction
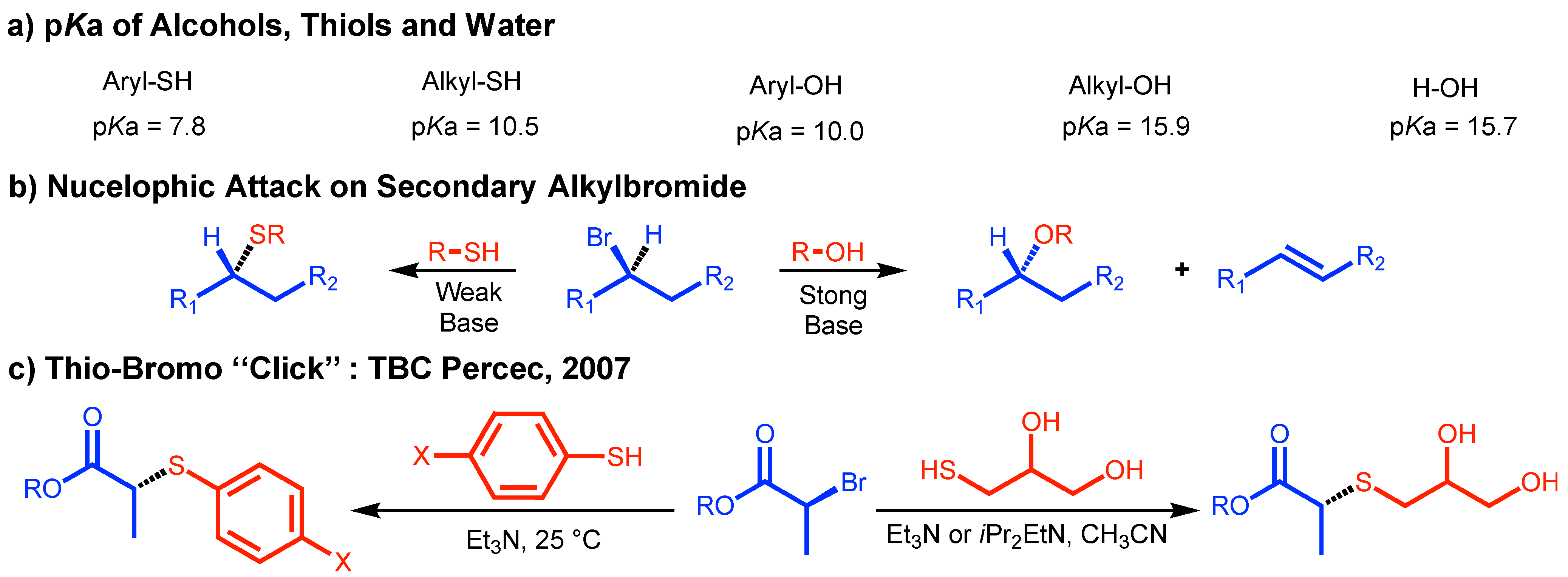
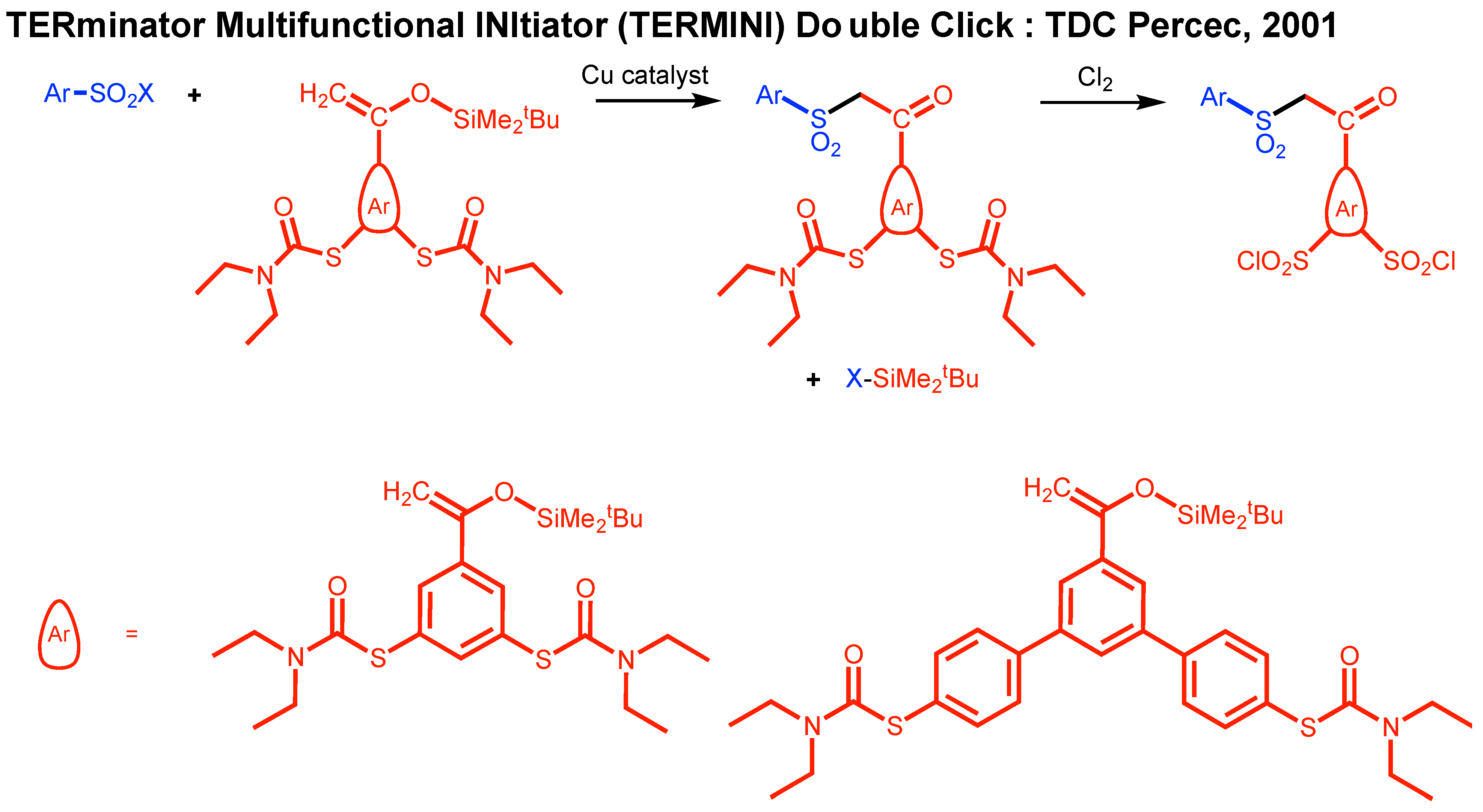
2. A Brief Discussion of the Development of CuAAC, TBC, and TDC Concepts
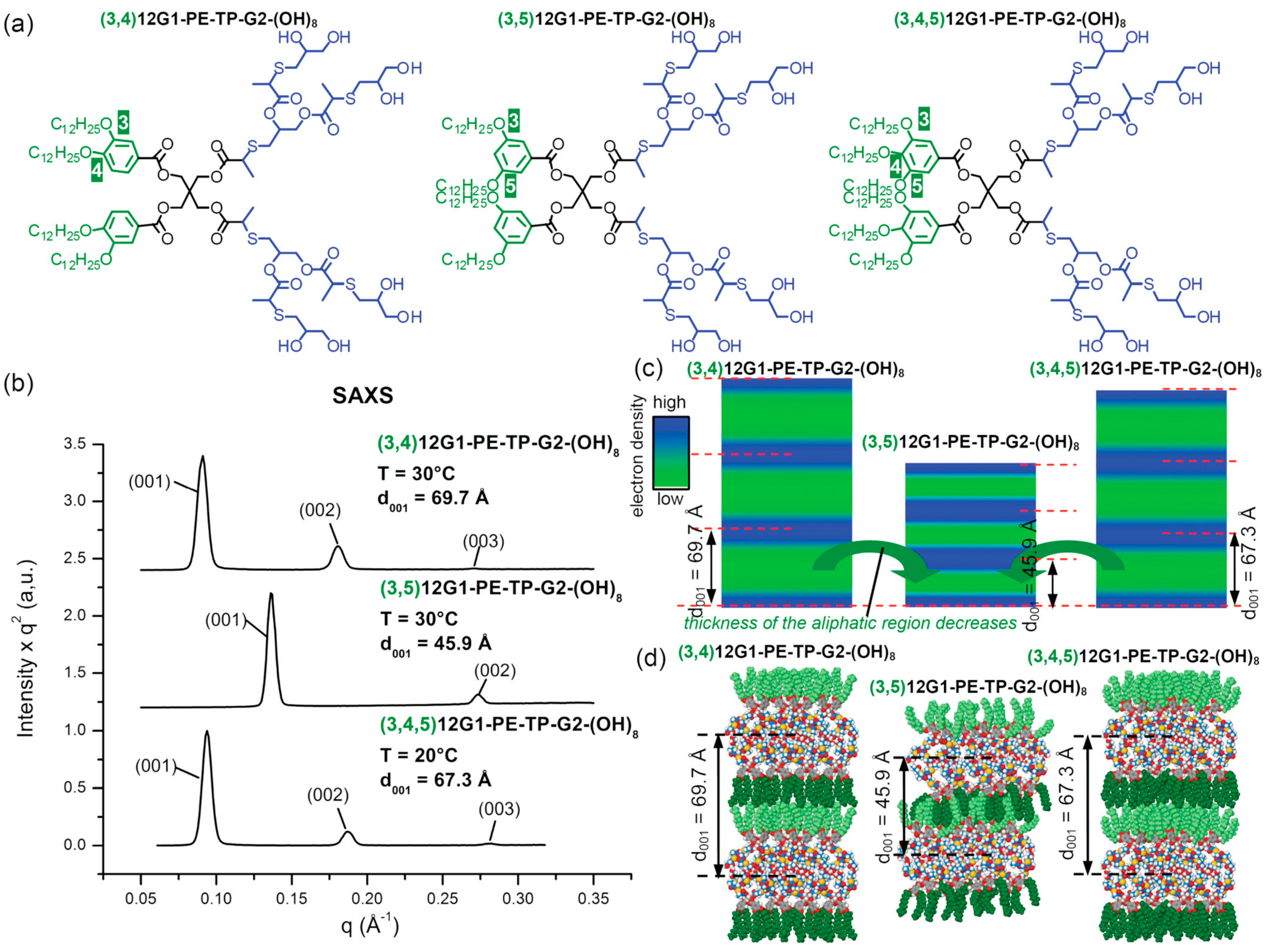
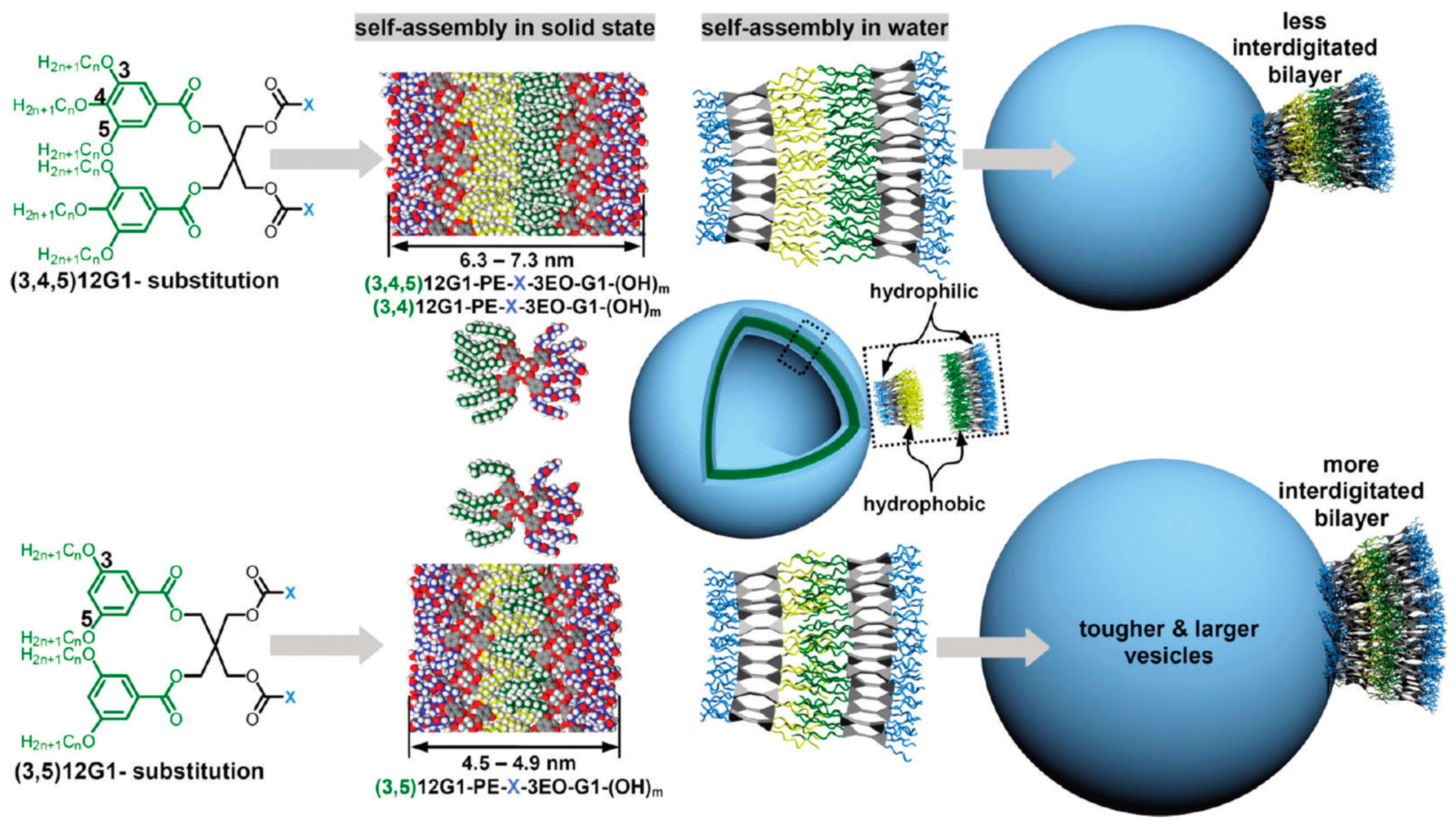
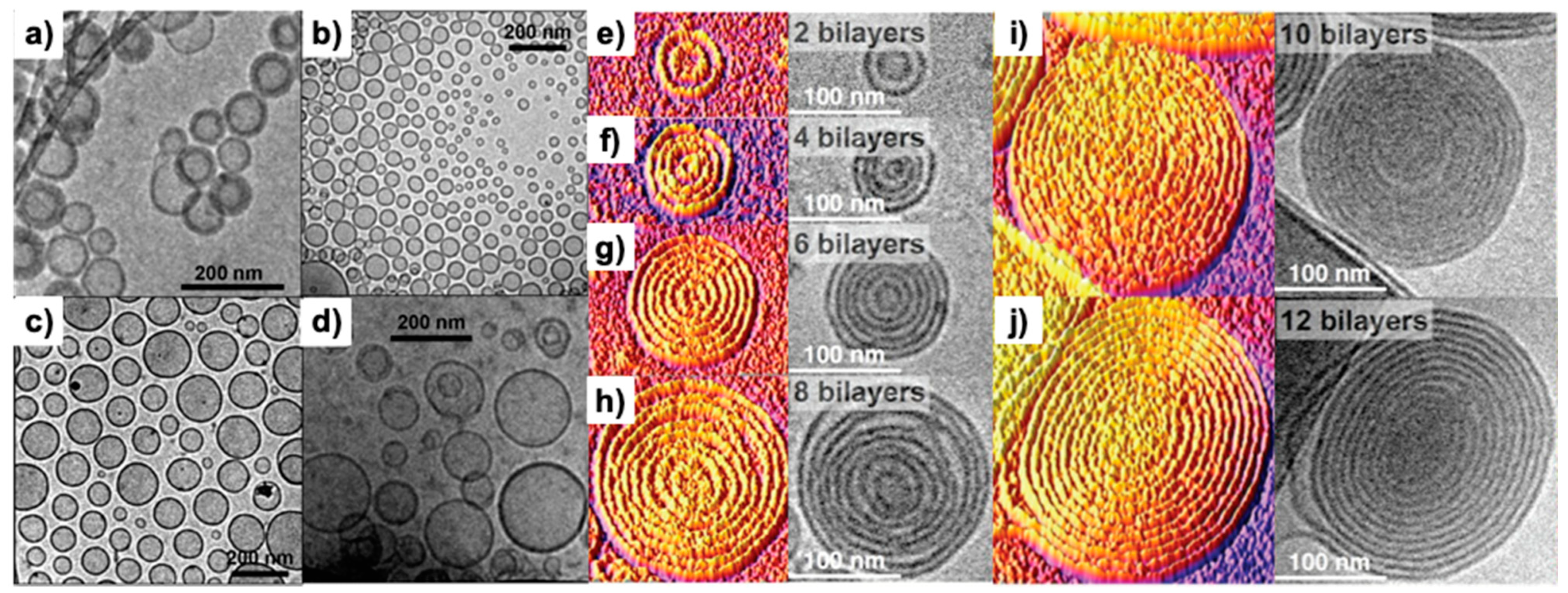
3. Dendrimersomes and Glycodendrimersomes as Mimics of Biological Membranes
4. Perfecting SET-LRP with the Aid of TBC
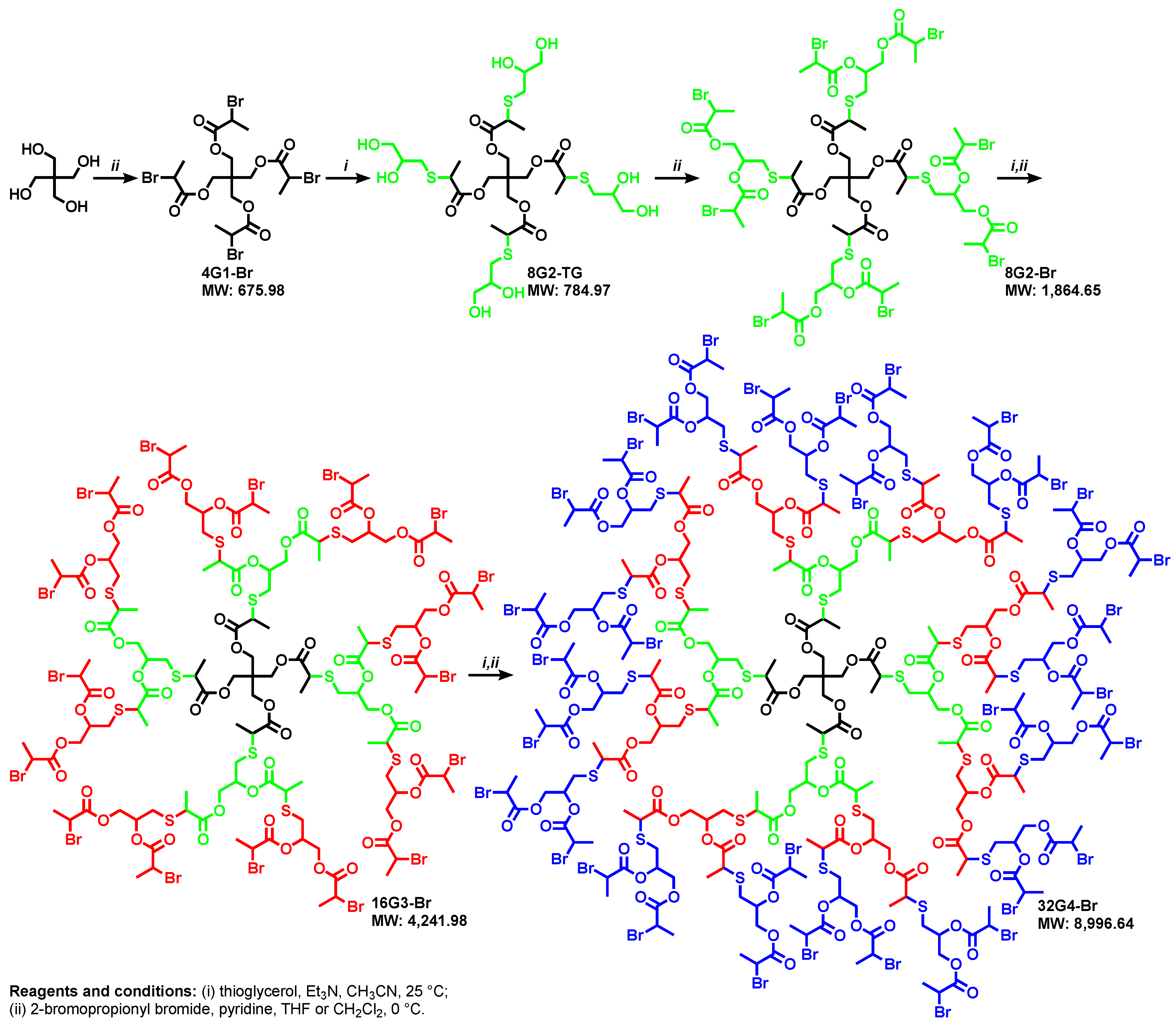
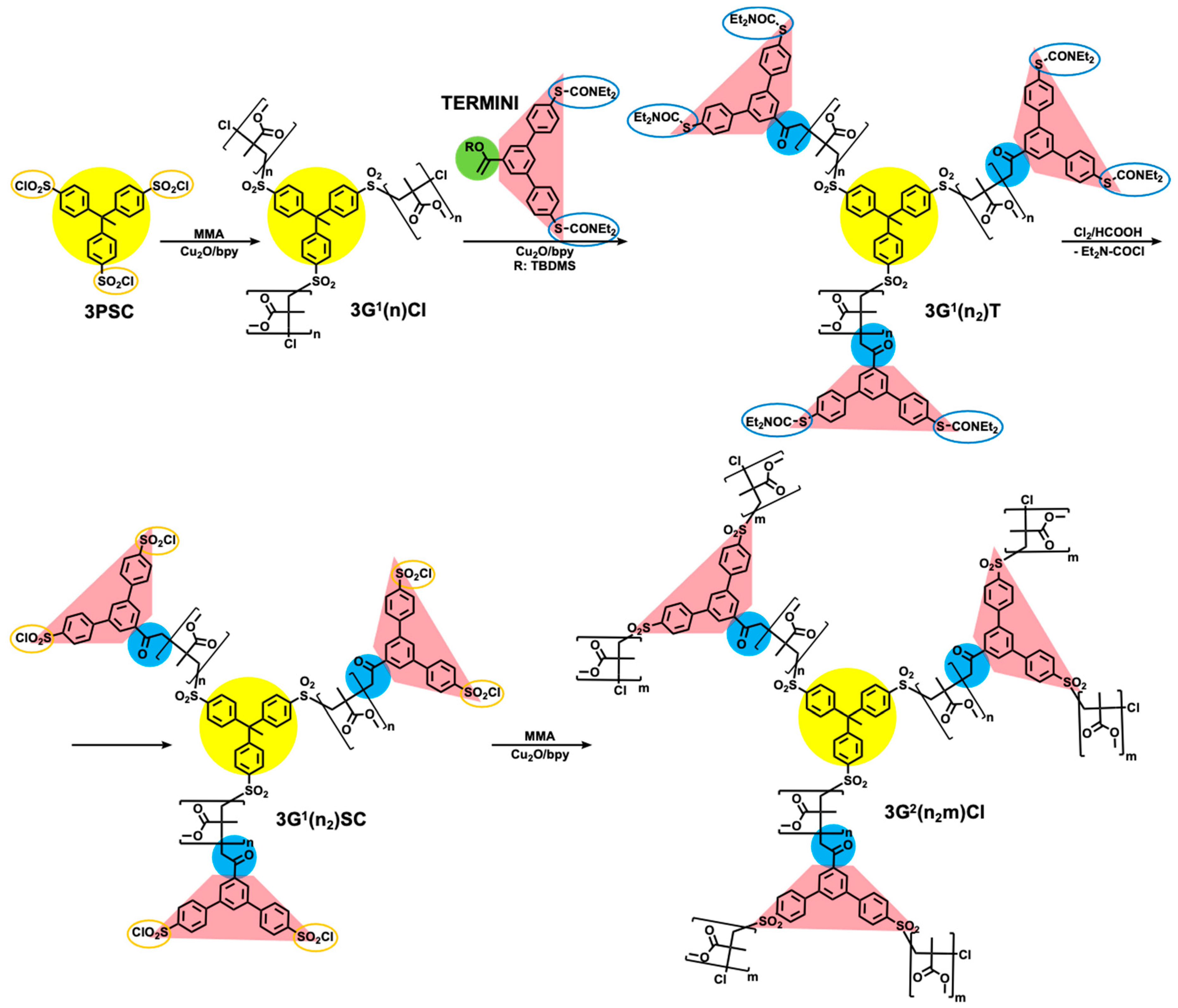
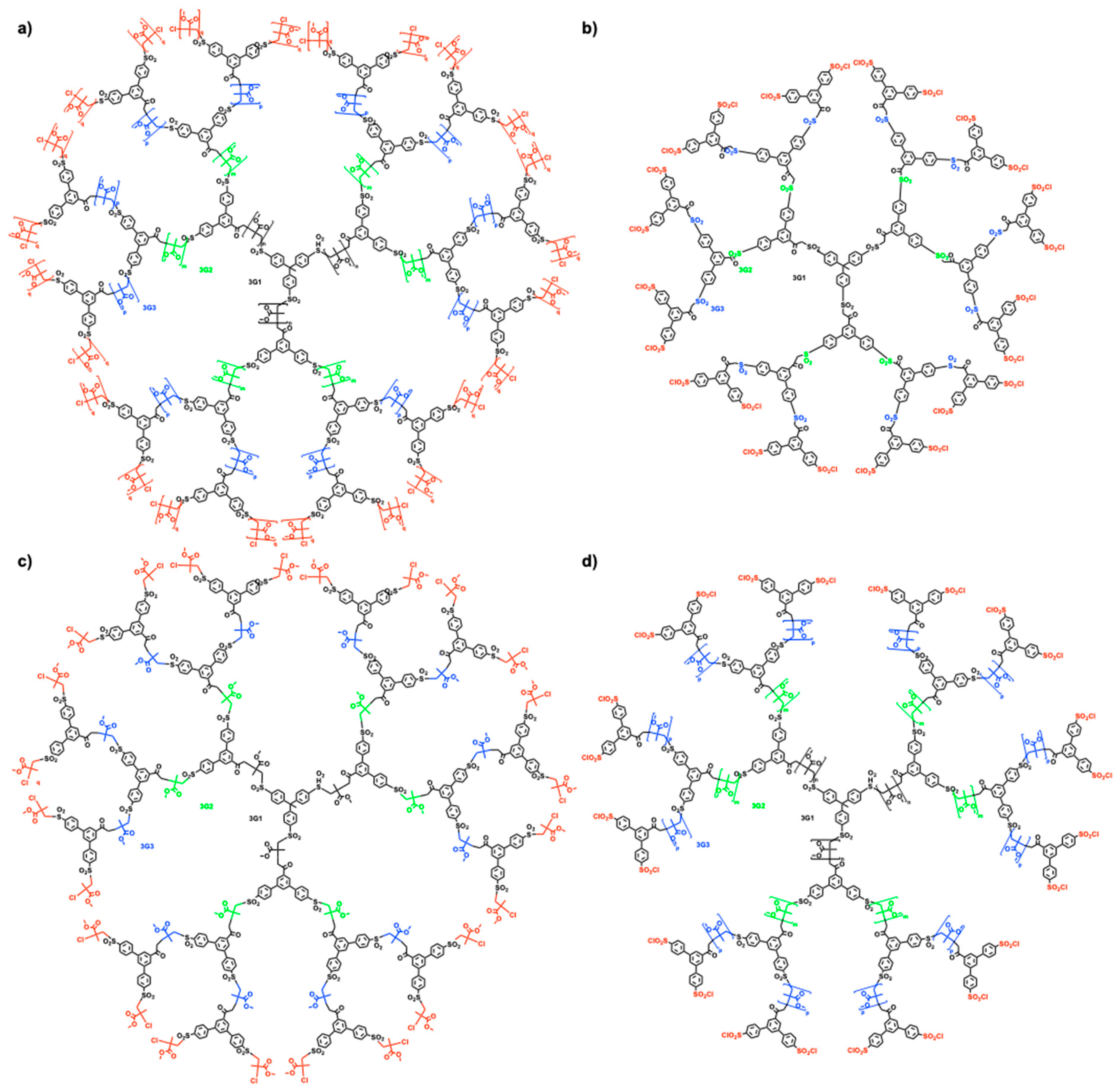
5. Divergent Synthesis of Dendrimers by TBC
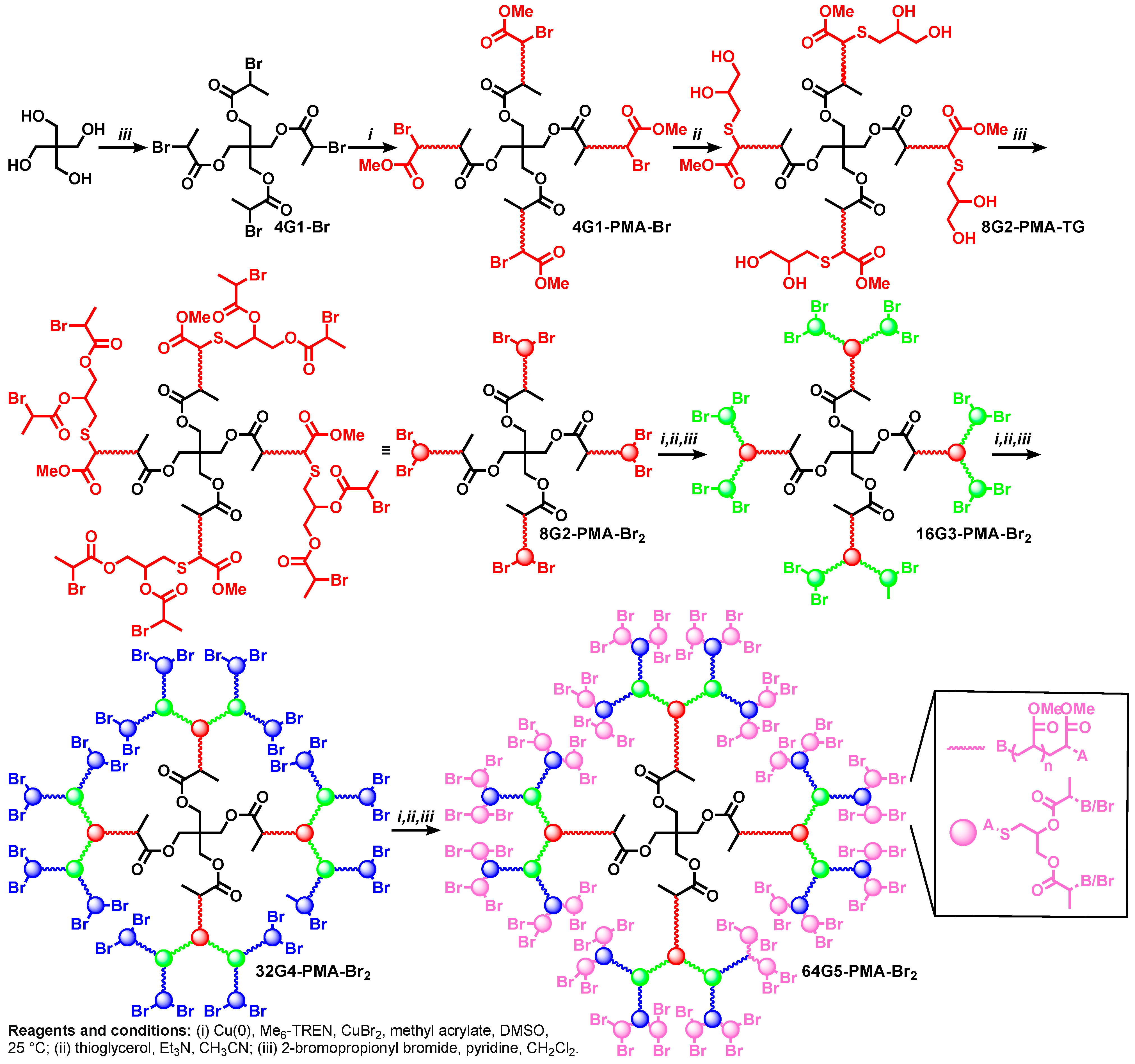
6. Divergent Synthesis of Dendritic Macromolecules from Commercial Monomers by TBC
7. Modular-Orthogonal Assembly of Amphiphilic Janus Dendrimers by TBC
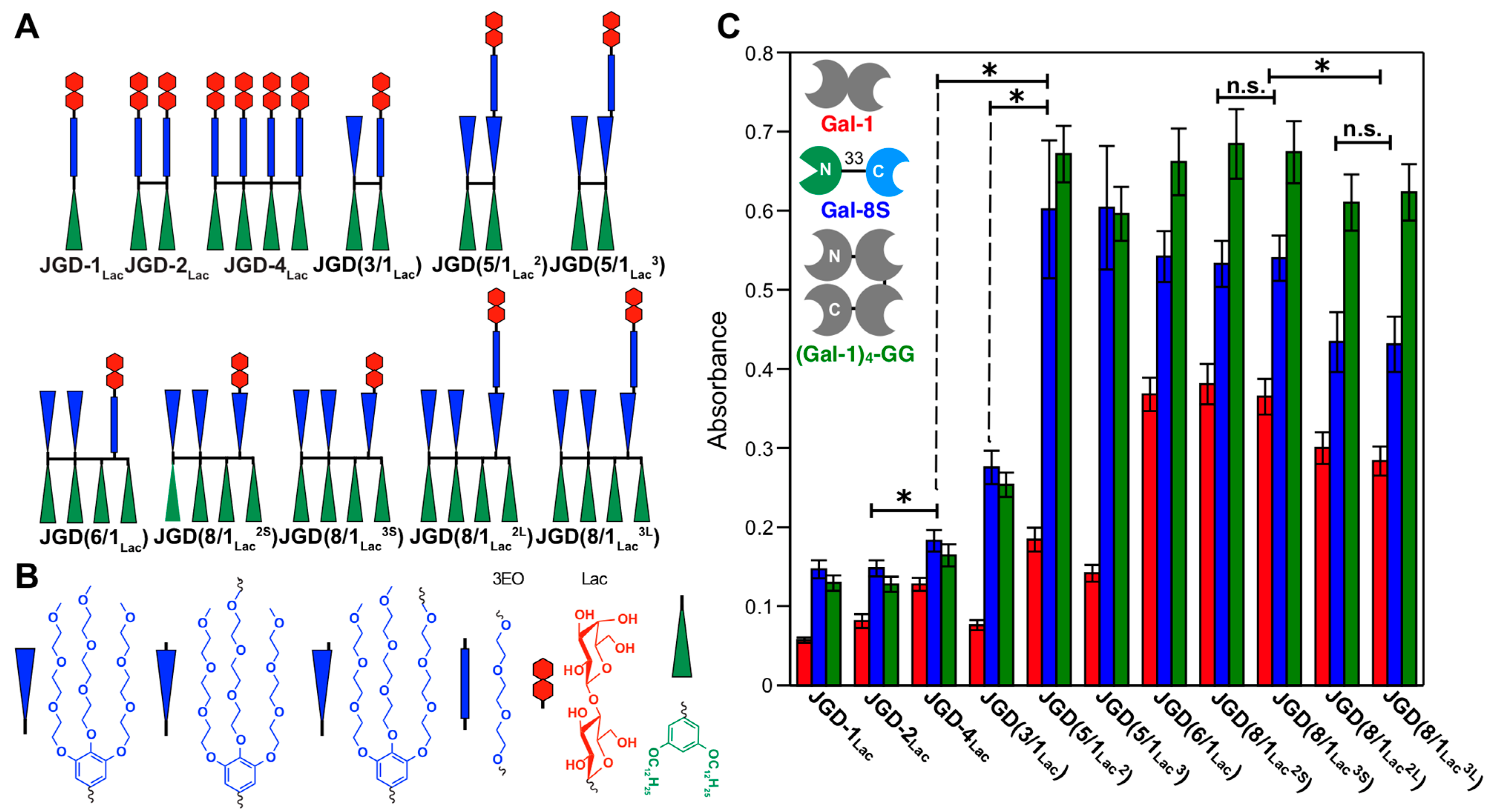
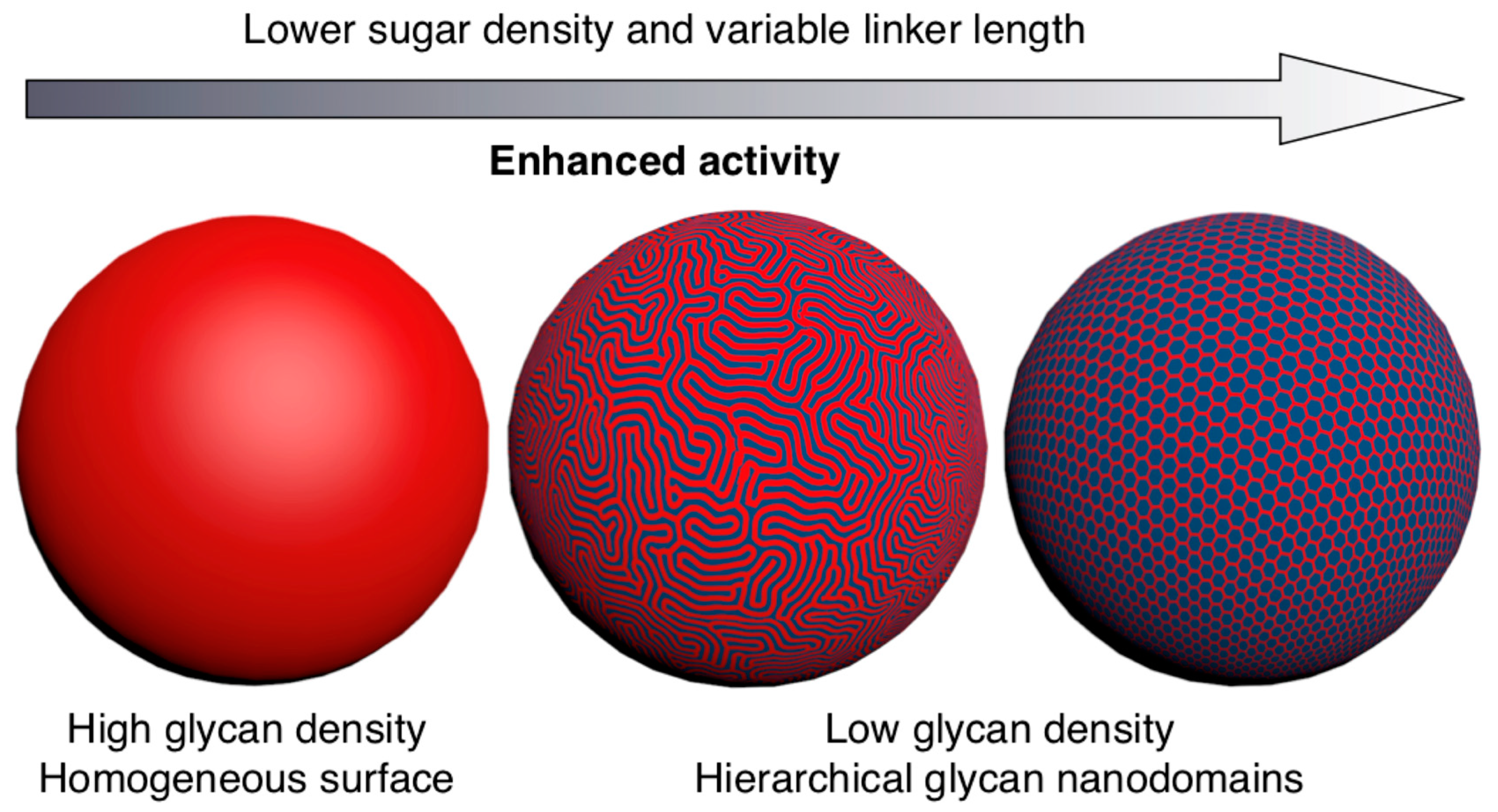
8. Modular-Orthogonal CuAAC Synthesis of Amphiphilic Janus Glycodendrimers
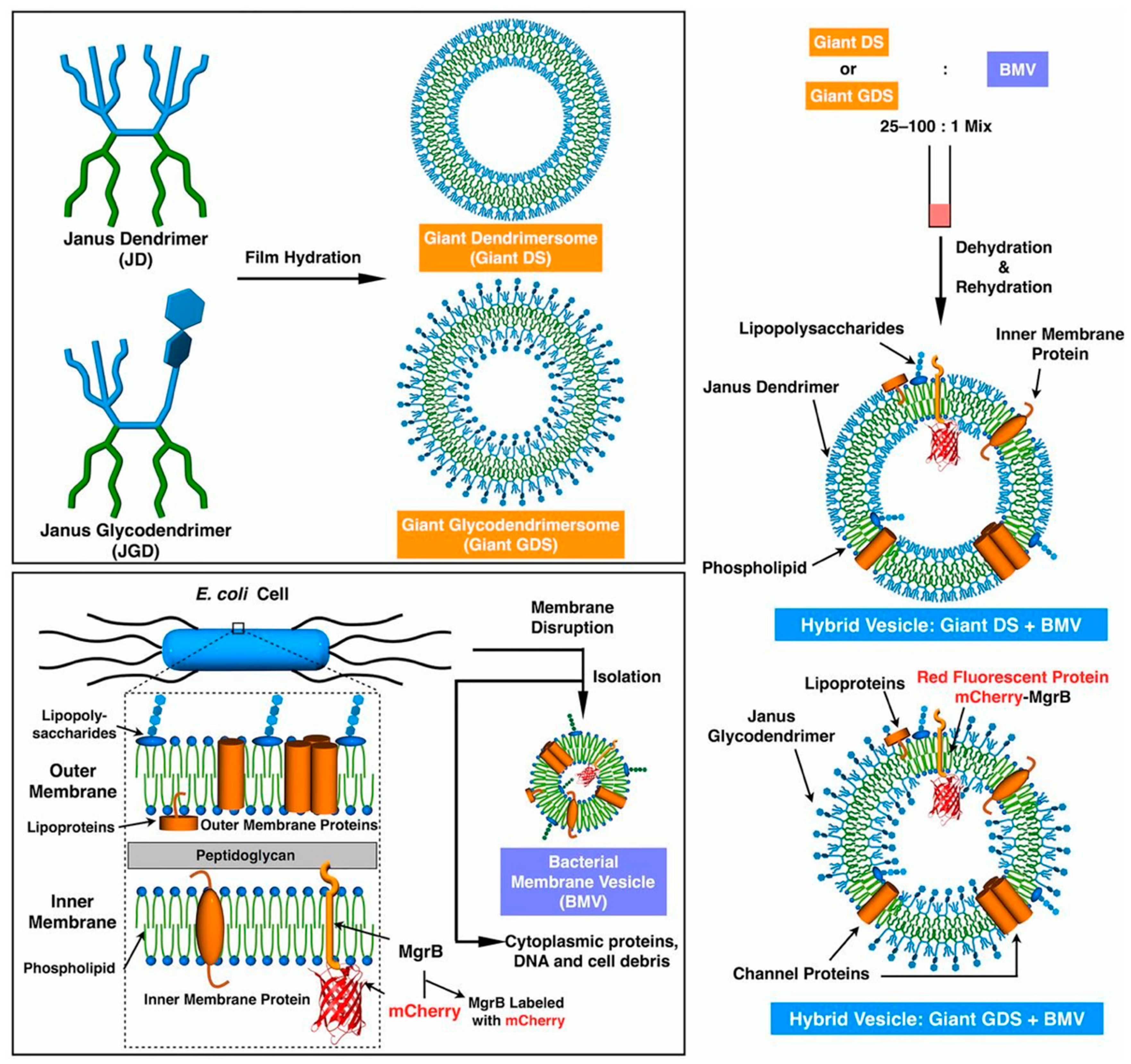
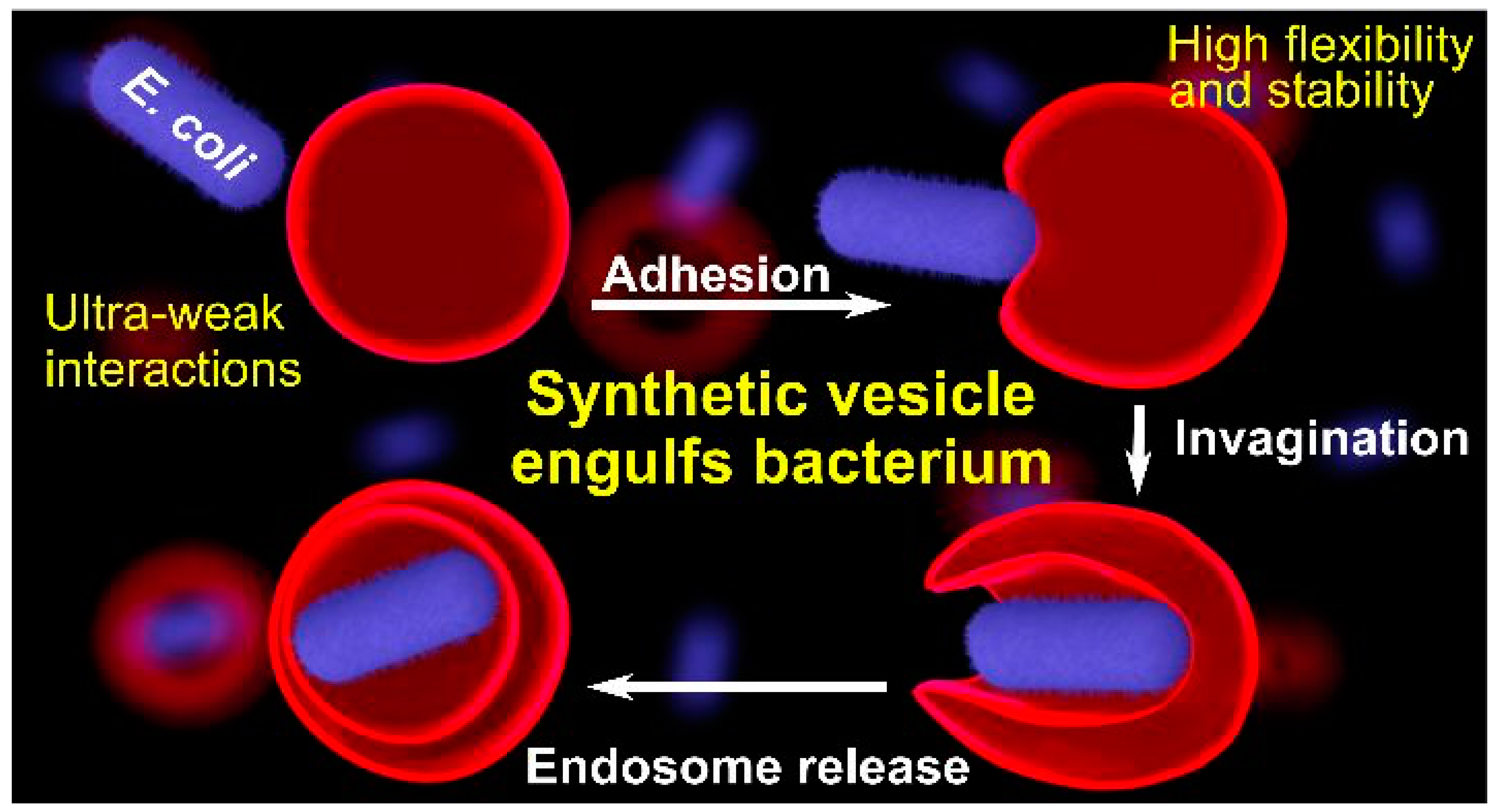
9. Hybrids of Dendrimersomes/Glycodendrimersomes with Bacteria and Human Cell Membranes and Dendrimersomes Engulf Living Bacteria via Endocytosis
10. Divergent Synthesis of Dendritic Macromolecules from Commercial Methyl Methacylate by DTC
11. Organizing Frontiers in Macromolecular and Supramolecular Science Symposia together with Bogdan C. Simionescu
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saxon, E.; Bertozzi, C.R. Cell Surface Engineering by a Modified Staudinger Reaction. Science 2000, 287, 2007–2010. [Google Scholar] [CrossRef] [PubMed]
- Saxon, E.; Armstrong, J.I.; Bertozzi, C.R. A “Traceless” Staudinger Ligation for the Chemoselective Synthesis of Amide Bonds. Org. Lett. 2000, 2, 2141–2143. [Google Scholar] [CrossRef] [PubMed]
- Nilson, B.L.; Kiessling, L.L.; Raines, R.T. Staudinger Ligation: A Peptide from a Thioester and Azide. Org. Lett. 2000, 2, 1939–1941. [Google Scholar] [CrossRef] [PubMed]
- Percec, V.; Xiao, Q. The Legacy of Hermann Staudinger: Covalently Linked Macromolecules. Chem 2020, 6, 2855–2861. [Google Scholar] [CrossRef]
- Percec, V.; Xiao, Q. From Organic Chemistry to Chemical Biology via Macromolecules with Hermann Staudinger. Giant 2020, 4, 100036. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Tornoe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on Solid Phase: (1,2,3)-Triazoles by Regiospecific Copper (I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Gree, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation”: Of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Huisgen, R.; Szeimies, G.; Moebius, L. Kinetik der Additionen Organischer Azide an CC-Mehrfachbindungen. Chem. Ber. 1967, 100, 2494–2507. [Google Scholar] [CrossRef]
- Agard, N.J.; Prescher, J.A.; Bertozzi, C.R.A. A Strain-Promoted [3+2] Azide-Alkyne Cycloaddition for Covalent Modification of Biomolecules in Living Systems. J. Am. Chem. Soc. 2004, 126, 15046–15047. [Google Scholar] [CrossRef]
- Percec, V.; Wilson, D.A.; Leowanawat, P.; Wilson, C.J.; Hughes, A.D.; Kaucher, M.S.; Hammer, D.A.; Levine, D.H.; Kim, A.J.; Bates, F.S.; et al. Self-Assembly of Janus Dendrimers into Uniform Dendrimersomes and other Complex Architectures. Science 2010, 328, 1009–1014. [Google Scholar] [CrossRef]
- Percec, V.; Leowanawat, P.; Sun, H.-J.; Kulikov, O.; Nusbaum, C.J.; Tran, T.M.; Bertin, A.; Wilson, D.A.; Peterca, M.; Zhang, S.; et al. Modular Synthesis of Amphiphilic Janus Glycodendrimers and their Self-Assembly into Glycodendrimersomes and other Complex Architectures with Bioactivity to Biomedically Relevant Lectins. J. Am. Chem. Soc. 2013, 135, 9055–9077. [Google Scholar] [CrossRef]
- Lligadas, G.; Percec, V. Synthesis of Perfectly Bifunctional Polyacrylates by Single-Electron Transfer Living Radical Polymerization. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 4684–4695. [Google Scholar] [CrossRef]
- Rosen, B.M.; Lligadas, G.; Hahn, C.; Percec, V. Synthesis of Dendrimers through Divergent Iterative Thio-Bromo “Click” Chemistry. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 3931–3939. [Google Scholar] [CrossRef]
- Rosen, B.M.; Lligadas, G.; Hahn, C.; Percec, V. Synthesis of Dendritic Macromolecules through Divergent Iterative Thio-Bromo “Click” Chemistry and SET-LRP. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 3940–3948. [Google Scholar] [CrossRef]
- Percec, V.; Bera, T.K.; De, B.D.; Sanai, Y.; Smith, J.; Holerca, M.N.; Barboiu, B.; Grubbs, R.B.; Frechet, J.M.J. Synthesis of Functional Aromatic Multisulfonyl Chlorides and their Masked Precursors. J. Org. Chem. 2001, 66, 2104–2117. [Google Scholar] [CrossRef] [PubMed]
- Percec, V.; Barboiu, B.; Grigoras, C.; Bera, T.K. Universal Iterative Strategy for the Divergent Synthesis of Dendritic Macromolecules from Conventional Monomers by a Combination of Living Radical Polymerization and Irreversible TERminator Multifunctional INItiator (TERMINI). J. Am. Chem. Soc. 2003, 125, 6503–6516. [Google Scholar] [CrossRef] [PubMed]
- Meldal, M.; Tornoe, W. Cu-Catalyzed Azide-Alkyne Cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef]
- Kolb, H.C.; Sharpless, K.B. The Growing Impact of Click Chemistry on Drug Delivery. Drug. Discov. Today 2003, 8, 1128–1137. [Google Scholar] [CrossRef]
- Binder, W.H.; Sachsenhofer, R. Click Chemistry in Polymer and Materials Science. Macromol. Rapid Commun. 2007, 28, 15–54. [Google Scholar] [CrossRef]
- Lutz, J.F. 1,3-Dipolar Cycloadditions of Azides and Alkynes: A Universal Ligation Tool in Polymer and Materials Science. Angew. Chem. Int. Ed. 2007, 46, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Iha, R.K.; Wooley, K.L.; Nystrom, A.M.; Burke, D.J.; Kade, M.J.; Hawker, C.J. Applications of Orthogonal “Click” Chemistries in the Synthesis of Functional Soft Materials. Chem. Rev. 2009, 109, 5620–5686. [Google Scholar] [CrossRef] [PubMed]
- Sherman, S.E.; Xiao, Q.; Percec, V. Mimicking Complex Biological Membranes and their Programmable Glycan Ligands with Dendrimersomes and Glycodendrimersomes. Chem. Rev. 2017, 117, 6538–6631. [Google Scholar] [CrossRef] [PubMed]
- Sletten, E.M.; Bertozzi, C.R. From Mechanism to Mouse: A Mechanism of Two Bioorthogonal Reactions. Acc. Chem. Res. 2011, 44, 666–676. [Google Scholar] [CrossRef]
- Percec, V.; Guliashvili, T.; Ladislaw, J.S.; Wistrand, A.; Stjerndahl, A.; Sienkowska, M.J.; Monteiro, M.J.; Sahoo, S. Ultrafast Synthesis of Ultrahigh Molar Mass Polymers by Metal-Catalyzed Living Radical Polymerization of Acrylates, Methacrylates and Vinyl Chloride Mediated by SET at 25 °C. J. Am. Chem. Soc. 2006, 128, 14156–14165. [Google Scholar] [CrossRef]
- Rosen, B.M.; Percec, V. Single Electron-Transfer and Single-Electron Transfer Degenerative Chain Transfer Living Radical Polymerization. Chem. Rev. 2009, 109, 5069–5119. [Google Scholar] [CrossRef]
- Lligadas, G.; Grama, S.; Percec, V. Single-Electron Transfer Living Radical Polymerization Platform to Practice, Develop and Invent. Biomacromolecules 2017, 18, 2981–3008. [Google Scholar] [CrossRef]
- Lligadas, G.; Grama, S.; Percec, V. Recent Developments in the Synthesis of Biomacromolecules and their Conjugates by Single Electron Transfer- Living Radical Polymerization. Biomacromolecules 2017, 18, 1039–1063. [Google Scholar] [CrossRef]
- Percec, V.; Barboiu, B.; Kim, H.-J. Arenesulfonyl Halides: A Universal Class of Functional Initiators for Metal-Catalyzed Living Radical Polymerization of Styrene(s), Methacrylates, and Acrylates. J. Am. Chem. Soc. 1998, 120, 305–316. [Google Scholar] [CrossRef]
- Percec, V.; Grigoras, C.; Kim, H.-J. Toward Self-Assembling Dendritic Macromolecules from Conventional Monomers by a Combination of Living Radical Polymerization and Irreversible terminator Multifunctional Initiator. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 505–513. [Google Scholar] [CrossRef]
- Percec, V.; Grigoras, C.; Bera, T.K.; Barboiu, B.; Bissel, P. Accelerated Iterative Strategy for the Divergent Synthesis of Dendritic Macromolecules Using a Combination of Living Radical Polymerization and an Irreversible Terminator Multifunctional Initiator. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 4894–4906. [Google Scholar] [CrossRef]
- Percec, V.; Barboiu, B.; Bera, T.K.; van der Sluis, M.; Grubbs, R.B.; Frechet, J.M.J. Designing Functional Aromatic Multisulfonyl Chloride Initiators for Complex Organic Synthesis by Living Radical Polymerization. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 4776–4791. [Google Scholar] [CrossRef]
- Bangham, A.D.; Horne, R.W. Negative Staining of Phospholipids and their Structural Modification by Surface-Active Agents as observed in the Electron Microscope. J. Mol. Biol. 1964, 8, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Chonn, A. Large Unilamellar Liposomes with Low Uptake into the Reticuloendothelial System. Febs Lett. 1987, 223, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Drug Delivery Systems: Entering the Mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef]
- Discher, B.M.; Won, Y.-Y.; Ege, D.S.; Lee, J.C.-M.; Bates, F.S.; Discher, D.E.; Hammer, D.A. Polymersomes: Tough Vesicles Made from Diblock Copolymers. Science 1999, 284, 1143–1146. [Google Scholar] [CrossRef]
- Miura, Y.; Hoshino, Y.; Seto, H. Glycopolymer Nanotechnology. Chem. Rev. 2016, 116, 1673–1692. [Google Scholar] [CrossRef]
- Turnbull, W.B.; Stoddart, J.F. Design and Synthesis of Glycodendrimers. Rev. Mol. Biol. 2002, 90, 231–255. [Google Scholar] [CrossRef]
- Jayaraman, N.; Maiti, K.; Naresh, K. Multivalent Glycoliposomes and Micelles to Study Carbohydrate-Protein and Carbohydrate-Carbohydrate Interactions. Chem. Soc. Rev. 2013, 42, 464–4656. [Google Scholar] [CrossRef]
- Lligadas, G.; Ladislaw, J.S.; Guliashvili, T.; Percec, V. Functionally Terminated Poly(Methyl Acrylate) by SET-LRP Initiated with CHBr3 and CHI3. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 278–288. [Google Scholar] [CrossRef]
- Jiang, X.; Rosen, B.M.; Percec, V. Mimicking “Nascent” Cu(0) Mediated SET-LRP of Methyl Acrylate in DMSO Leads to Complete Conversion in Several Minutes. J. Polym. Sci. Part A Polym Chem. 2010, 48, 403–409. [Google Scholar] [CrossRef]
- Fleischmann, S.; Rosen, B.M.; Percec, V. SET-LRP of Acrylates in Air. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 1190–1196. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Percec, V. Dramatic Acceleration of SET-LRP of Methyl Acrylate during Catalysis with Activated Cu(0) Wire. J. Polym. Chem. Part A Polym. Chem. 2010, 48, 5109–5119. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Rosen, B.M.; Percec, V. Improving the Initiation Efficiency in the Single Electron Transfer Living Radical Polymerization of Methyl Acrylate with Electronic Chain-End Mimics. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 1235–1247. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Percec, V. Acid Dissolution of Copper Oxides as a Method for the Activation of Cu(0) Wire Catalyst for SET-LRP. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 4241–4252. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Percec, V. SET-LRP of Methyl Acrylate Catalyzed with Activated Cu(0) Wire in Methanol in the Presence of Air. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 4756–4765. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Levere, M.E.; Percec, V. TREN vs. Me6-TREN as Ligands in SET-LRP of Methyl Acrylate. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 35–46. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Levere, M.E.; Percec, V. SET-LRP of Methyl Acrylate to Complete Conversion with Zero Termination. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 860–873. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Levere, M.E.; Kulis, J.; Monteiro, M.J.; Percec, V. Analysis of the Cu(0)-Catalyzed Polymerization of Methyl Acrylate in Disproportionating and Nondisproportionating Solvents. Macromolecules 2012, 45, 4606–4622. [Google Scholar] [CrossRef]
- Samanta, S.R.; Levere, M.E.; Percec, V. SET-LRP of Hydophobic and Hydrophilic Acrylates in Trifluoroethanol. Polym. Chem. 2013, 4, 3212–3224. [Google Scholar] [CrossRef]
- Samanta, S.R.; Anastasaki, A.; Waldron, C.; Haddleton, D.M.; Percec, V. SET-LRP of Hydrophobic and Hydrophilic Acrylates in Tetrafluoropropanol. Polym. Chem. 2013, 4, 5555–5562. [Google Scholar] [CrossRef]
- Samanta, S.R.; Cai, R.; Percec, V. SET-LRP of Semifluorinated Acrylates and Methacrylates. Polym. Chem. 2014, 5, 5479–5491. [Google Scholar] [CrossRef]
- Samanta, S.R.; Nikolaou, V.; Keller, S.; Monteiro, M.J.; Wilson, D.A.; Haddleton, D.M.; Percec, V. Aqueous SET-LRP Catalyzed with “In Situ” Generated Cu(0) Demonstrates Surface Mediated Activation and Bimolecular Termination. Polym. Chem. 2015, 6, 2084–2097. [Google Scholar] [CrossRef]
- Enayati, M.; Jezorek, R.L.; Monteiro, M.J.; Percec, V. Ultrafast SET-LRP of Hydrophobic Acrylates in Multiphase Alcohol-Water Mixtures. Polym. Chem. 2016, 7, 3608–3621. [Google Scholar] [CrossRef]
- Enayati, M.; Jezorek, R.L.; Percec, V. A Multiple-Stage Activation of the Catalytically Inhomogeneous Cu(0) Wire Used in SET-LRP. Polym. Chem. 2016, 7, 4549–4558. [Google Scholar] [CrossRef]
- Enayati, M.; Jezorek, R.L.; Monteiro, M.J.; Percec, V. Ultrafast SET-LRP in Biphasic Mixtures of the Non-Disproportionating Solvent Acetonitrile with Water. Polym. Chem. 2016, 7, 5930–5942. [Google Scholar] [CrossRef]
- Enayati, M.; Smail, R.B.; Grama, S.; Jezorek, R.L.; Monteiro, M.J.; Percec, V. The Synergistic Effect during Biphasic SET-LRP in Ethanol-Nonpolar Solvent-Water Mixtures. Polym. Chem. 2016, 7, 7230–7241. [Google Scholar] [CrossRef]
- Smail, R.B.; Jezorek, R.L.; Lejnieks, J.; Enayati, M.; Grama, S.; Monteiro, M.J.; Percec, V. Acetone–Water Biphasic Mixtures as Solvents for Ultrafast SET-LRP of Hydrophobic Acrylates. Polym. Chem. 2017, 8, 3102–3123. [Google Scholar] [CrossRef]
- Moreno, A.; Grama, S.; Liu, T.; Galià, M.; Lligadas, G.; Percec, V. SET-LRP Mediated by TREN in Biphasic Water–Organic Solvent Mixtures Provides the Most Economical and Efficient Process. Polym. Chem. 2017, 8, 7559–7574. [Google Scholar] [CrossRef]
- Lligadas, G.; Enayati, M.; Grama, S.; Smail, R.; Sherman, S.E.; Percec, V. Ultrafast SET-LRP with Peptoid Cytostatic Drugs as Monofunctional and Bifunctional Initiators. Biomacromolecules 2017, 18, 2610–2622. [Google Scholar] [CrossRef]
- Moreno, A.; Liu, T.; Ding, L.; Buzzacchera, I.; Galià, M.; Möller, M.; Wilson, C.J.; Lligadas, G.; Percec, V. SET-LRP in Biphasic Mixtures of Fluorinated Alcohols with Water. Polym. Chem. 2018, 9, 2313–2327. [Google Scholar] [CrossRef]
- Moreno, A.; Jezorek, R.L.; Liu, T.; Galià, M.; Lligadas, G.; Percec, V. Macromonomers, Telechelics and More Complex Architectures of PMA by a Combination of Biphasic SET-LRP and Biphasic Esterification. Polym Chem. 2018, 9, 1885–1899. [Google Scholar] [CrossRef]
- Moreno, A.; Liu, T.; Galià, M.; Lligadas, G.; Percec, V. Acrylate-Macromonomers and Telechelics of PBA by Merging Biphasic SET-LRP of BA, Chain Extension with MA and Biphasic Esterification. Polym Chem. 2018, 9, 1961–1971. [Google Scholar] [CrossRef]
- Moreno, A.; Galià, M.; Lligadas, G.; Percec, V. SET-LRP in Biphasic Mixtures of the Non-Disproportionating Solvent Hexafluoroisopropanol with Water. Biomacromolecules 2018, 19, 4480–4491. [Google Scholar] [CrossRef]
- Feng, X.; Maurya, D.S.; Bensabeh, N.; Moreno, A.; Oh, T.; Luo, Y.; Lejnieks, J.; Galià, M.; Miura, Y.; Monteiro, M.J.; et al. Replacing Cu(II)Br2 with Me6-TREN in Biphasic Cu(0)/TREN Catalyzed SET-LRP Reveals the Mixed-Ligand Effect. Biomacromolecules 2020, 21, 250–261. [Google Scholar] [CrossRef]
- Maurya, D.S.; Malik, A.; Feng, X.; Bensabeh, N.; Lligadas, G.; Percec, V. Me6-TREN/TREN Mixed-Ligand Effect during SET-LRP in the Catalytically Active DMSO Revitalizes TREN into an Excellent Ligand. Biomacromolecules 2020, 21, 1902–1919. [Google Scholar] [CrossRef]
- Lu, D.; Jia, Z.; Monteiro, M.J. Synthesis of Alkyne Functional Cyclic Polymers by One-Pot Thiol–Ene Cyclization. Polym. Chem. 2013, 4, 2080–2089. [Google Scholar] [CrossRef]
- Gavrilov, M.; Zerk, T.J.; Bernhardt, P.V.; Percec, V.; Monteiro, M.J. SET-LRP of NIPAM in Water via In Situ Reduction of Cu(II) to Cu(0) with NaBH4. Polym. Chem. 2016, 7, 933–939. [Google Scholar] [CrossRef]
- Gavrilov, M.; Jia, Z.; Percec, V.; Monteiro, M.J. Quantitative End-Group Functionalization of PNIPAM from Aqueous SET-LRP via in Situ Reduction of Cu(II) with NaBH4. Polym. Chem. 2016, 7, 4802–4809. [Google Scholar] [CrossRef]
- Moreno, A.; Garcia, D.; Galià, M.; Ronda, J.C.; Cádiz, V.; Lligadas, G.; Percec, V. SET-LRP in the Neoteric Ethyl Lactate Alcohol. Biomacromolecules 2017, 18, 3447–3456. [Google Scholar] [CrossRef]
- Bensabeh, N.; Ronda, J.C.; Galià, M.; Cádiz, V.; Lligadas, G.; Percec, V. SET-LRP of the Hydrophobic Biobased Menthyl Acrylate. Biomacromolecules 2018, 19, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Bensabeh, N.; Moreno, A.; Roig, A.; Monoghan, O.R.; Ronda, J.C.; Cádiz, V.; Galià, M.; Howdle, S.M.; Lligadas, G.; Percec, V. Polyacrylates Derived from Biobased Ethyl Lactate Solvent via SET-LRP. Biomacromolecules 2019, 20, 2135–2147. [Google Scholar] [CrossRef]
- Moreno, A.; Ronda, J.C.; Cádiz, V.; Galià, M.; Lligadas, G.; Percec, V. SET-LRP from Programmed Difunctional Initiators Encoded with Double Single-Cleavage and Double Dual-Cleavage Groups. Biomacromolecules 2019, 20, 3200–3210. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.; Ronda, J.C.; Cádiz, V.; Galià, M.; Lligadas, G.; Percec, V. pH-Responsive Micellar Nanoassemblies from Water-Soluble Telechelic Homopolymers Endcoding Acid-Labile Middle-Chain Groups in Their Hydrophobic Sequence-Defined Initiator Residue. ACS Macro Lett. 2019, 8, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Bensabeh, N.; Moreno, A.; Roig, A.; Rahimzadeh, M.; Rahimi, K.; Ronda, J.C.; Cádiz, V.; Galià, M.; Percec, V.; Rodriguez-Emmenegger, C.; et al. Photoinduced Upgrading of Lactic Acid-Based Solvents to Block Copolymer Surfactants. ACS Sustain. Chem. Eng. 2020, 8, 1276–1284. [Google Scholar] [CrossRef]
- Soeriyadi, A.H.; Boyer, C.; Nyström, F.; Zetterlund, P.B.; Whittaker, M.R. High-Order Multiblock Copolymers via Iterative Cu(0)-Mediated Radical Polymerizations (SET-LRP): Toward Biological Precision. J. Am. Chem. Soc. 2011, 133, 11128–11131. [Google Scholar] [CrossRef]
- Enayati, M.; Abbaspourrad, A. Glass Surface Modification via Cu(0)-Mediated Living Radical Polymerization of Fluorinated and Non-Fluorinated Acrylates. Polym. Chem. 2017, 8, 7457–7468. [Google Scholar] [CrossRef]
- Enayati, M.; Abbaspourrad, A. Facile Preparation of Superhydrophobic and Oleophobic Surfaces via the Combination of Cu(0)-Mediated Reversible-Deactivation Radical Polymerization and Click Chemistry. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 1684–1694. [Google Scholar] [CrossRef]
- Xu, J.; Tao, L.; Boyer, C.; Lowe, A.B.; Davis, T.P. Combining Thio-Bromo Click Chemistry and Raft Polymerization: A Powerful Tool for Preparing Functionalized Multiblock and Hyperbranched Polymers. Macromolecules 2010, 43, 20–24. [Google Scholar] [CrossRef]
- Prohl, M.; Englert, C.; Gottschaldt, M.; Brendel, J.C.; Schubert, U.S. RAFT Polymerization and Thio-Bromo Substitution: An Efficient Way towards Well-Defined Glycopolymers. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3617–3626. [Google Scholar] [CrossRef]
- Kumar, S.; Deike, S.; Binder, W.H. One-Pot Synthesis of Thermoresponsive Amyloidogenic Peptide–Polymer Conjugates via Thio–Bromo “Click” Reaction of RAFT Polymers. Macromol. Rapid Commun. 2018, 39, 1700507. [Google Scholar] [CrossRef]
- Döhler, D.; Kaiser, J.; Binder, W.H. Supramolecular H-bonded Three-Arm Star Polymers by Efficient Combination of RAFT Polymerization and Thio-Bromo “Click” Reaction. Polymer 2017, 122, 148–158. [Google Scholar] [CrossRef]
- Tang, D.; Dai, W.; Zhang, J.; Zhou, X.; Zhao, Y. Facile Synthesis of Sual-Responsive Thioether-Bridging Graft Copolymers by Combination of Controlled Polymerization and Thio-Bromo Click Reaction. Polymer 2018, 157, 59–66. [Google Scholar] [CrossRef]
- Biewend, M.; Neumann, S.; Michael, P.; Binder, W.H. Synthesis of Polymer-Linked Copper(I) Bis(N-Heterocyclic Carbene) Complexes of Linear and Chain Extended Architecture. Polym. Chem. 2019, 10, 1078–1088. [Google Scholar] [CrossRef]
- Hobbs, C.E.; Vasireddy, M. Combining ATRP and ROMP with Thio-Bromo, Copper-Catalyzed, and Strain-Promoted Click Reactions for Brush Copolymer Synthesis Starting from a Single Initiator/Monomer/Click Partner. Macromol. Chem. Phys. 2019, 220, 1800497. [Google Scholar] [CrossRef]
- Foster, O.; Soeriyadi, A.H.; Whittaker, M.H.; Davis, T.P.; Boyer, C. Synthesis of Block Copolymers via Atom Transfer Radical Polymerization and ‘Click Chemistry’ Grafted from Pre-Functionalized Polypropylene Surfaces Using Gamma Irradiation. Polym. Chem. 2012, 3, 2102–2111. [Google Scholar] [CrossRef]
- Yao, Q.; Gutierrez, D.C.; Hoang, N.H.; Kim, D.; Wang, R.; Hobbs, C.E.; Zhu, L. Efficient Codelivery of Paclitaxel and Curcumin by Novel Bottlebrush Copolymer-based Micelles. Mol. Pharm. 2017, 14, 2378–2389. [Google Scholar] [CrossRef]
- Grubb, J.; Carosio, F.; Vasireddy, M.; Moncho, S.; Brothers, E.N.; Hobbs, C.E. Ring Opening Metathesis Polymerization (ROMP) and Thio-Bromo “Click” Chemistry Approach toward the Preparation of Flame-Retardant Polymers. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 645–652. [Google Scholar] [CrossRef]
- Boyer, C.; Bousquet, A.; Rondolo, J.; Whittaker, M.R.; Stenzel, M.H.; Davis, T.P. Glycopolymer Decoration of Gold Nanoparticles Using a LbL Approach. Macromolecules 2010, 43, 3775–3784. [Google Scholar] [CrossRef]
- Xu, L.Q.; Zhang, B.; Wang, R.; Chen, Y.; Neoh, K.-G.; Kang, E.-T.; Fu, G.D. Fluorescent Nanoparticles from Self-Assembly of b-Cyclodextrin-Functionalized Fluorene Copolymers for Organic Molecule Sensing and Cell Labeling. Polym. Chem. 2012, 3, 2444–2450. [Google Scholar] [CrossRef]
- Zhang, Q.; Anastasaki, A.; Li, G.-Z.; Haddleton, A.; Wilson, P.; Haddleton, D.M. Multiblock Sequence-Controlled Glycopolymers via Cu(0)-LRP Following Efficient Thiol–Halogen, Thiol–Epoxy and CuAAC Reactions. Polym. Chem. 2014, 5, 3876–3883. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Li, Y.-G.; Mu, H.-L.; Pan, L.; Li, Y.-S. Efficient Synthesis of Diverse Well-Defined Functional Polypropylenes with High Molecular Weights and High Functional Group Contents via Thiol–Halogen Click Chemistry. Polym. Chem. 2015, 6, 1150–1158. [Google Scholar] [CrossRef]
- Chen, S.; Ströhl, D.; Binder, W.H. Orthogonal Modification of Polymers via Thio–Bromo “Click” Reaction and Supramolecular Chemistry: An Easy Method Toward Head-to-Tail Self-Assembled Supramolecular Polymers. ACS Macro Lett. 2015, 4, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Kothapalli, V.A.; Shetty, M.; de los Santos, C.; Hobbs, C.E. Thio-bromo “Click,” Post-Polymerization Strategy for Functionalizing Ring Opening Metathesis Polymerization (ROMP)-Derived Materials. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 179–185. [Google Scholar] [CrossRef]
- Subnaik, S.I.; Hobbs, C.E. Flow-Facilitated Ring Opening Metathesis Polymerization (ROMP) and Post-Polymerization Modification Reactions. Polym. Chem. 2019, 10, 4524–4528. [Google Scholar] [CrossRef]
- Dai, Y.; Hu, Z.; Wang, X.; Liu, X.; Li, Y.; Shi, Y.; Chen, Y. Synthesis of Fully Degradable Cationic Polymers with Various Topological Structures via Postpolymerization Modification by Using Thio-Bromo “Click” Reaction. Polym. Chem. 2021, 12, 2592–2597. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, P.; Zhang, J.; Hu, J.; Zhao, Y. Facile Synthesis of Monocyclic, Dumbbell-Shaped and Jellyfish-Like Copolymers Using a Telechelic Multisite Hexablock Copolymer. Polym. Chem. 2022, 13, 4963–4965. [Google Scholar] [CrossRef]
- Kumar, S.; Hause, G.; Binder, W.H. Thio-Bromo “Click” Reaction Derived Polymer–Peptide Conjugates for Their Self-Assembled Fibrillar Nanostructures. Macromol. Biosci. 2020, 20, 2000048. [Google Scholar] [CrossRef]
- Kumar, S.; Hause, G.; Binder, W.H. Bifunctional Peptide–Polymer Conjugate-Based Fibers via a One-Pot Tandem Disulfide Reduction Coupled to a Thio-Bromo “Click” Reaction. ACS Omega 2020, 5, 19020–19028. [Google Scholar] [CrossRef]
- Truong, V.X.; Donderwinkel, I.; Frith, J.E. Bioorthogonal Hydrogels by Thiol–Halide Click Crosslinking with Fast Gelation Time and Tunable Stability in Aqueous Media. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 1872–1876. [Google Scholar] [CrossRef]
- Kyriakou, N.; Merlet, R.B.; Willott, J.D.; Nijmeijer, A.; Winnubst, L.; Pizzoccaro-Zilamy, M.-A. New Method toward a Robust Covalently Attached Cross-Linked Nanofiltration Membrane. ACS Appl. Mater. Interfaces 2020, 12, 47948–47956. [Google Scholar] [CrossRef]
- Beyer, V.P.; Cattoz, B.; Strong, A.; Phillips, D.J.; Schwarz, A.; Becer, C.R. Fast Track Access to Multi-Block Copolymers via Thiol-Bromo Click Reaction of Telechelic Dibromo Polymers. Polym. Chem. 2019, 10, 4259–4270. [Google Scholar] [CrossRef]
- Fan, X.; Wang, Z.; Yuan, D.; Sun, Y.; Lib, Z.; He, C. Novel Linear-Dendritic-Like Amphiphilic Copolymers: Synthesis and Self-Assembly Characteristics. Polym. Chem. 2014, 5, 4069–4075. [Google Scholar] [CrossRef]
- Fan, X.; Hu, Z.; Wang, G. Facile Synthesis of Polyester Dendrimer via Combining Thio-bromo “Click” Chemistry and ATNRC. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 1762–1768. [Google Scholar] [CrossRef]
- Chen, S.; Schulz, M.; Lechner, B.-D.; Appiah, C.; Binder, W.H. One-Pot Synthesis and Self-Assembly of Supramolecular Dendritic Polymers. Polym. Chem. 2015, 6, 7988–7994. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, H.-J.; Hughes, A.D.; Draghici, B.; Lejnieks, J.; Leowanawat, P.; Bertin, A.; De Leon, L.; Kulikov, O.; Chen, Y.; et al. “Single-Single” Amphiphilic Janus Dendrimners Self-Assemble into Uniform Dendrimersiomes with Predictable Size. ACS Nano 2014, 8, 1554–1565. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, H.-J.; Hughes, A.D.; Moussodia, R.-O.; Bertin, A.; Chen, Y.; Pochan, D.J.; Heiney, P.A.; Klein, M.L.; Percec, V. Self-Assembly of Amphiphilic Janus Dendrimers into Uniform Onion-Like Dendrimersomes with Predictable Size and Number of Internalized Bilayers. Proc. Natl. Acad. Sci. USA 2014, 111, 9058–9063. [Google Scholar] [CrossRef]
- Xiao, Q.; Rubien, J.D.; Wang, Z.; Reed, E.H.; Hammer, D.A.; Sahoo, D.; Heiney, P.A.; Yadavalli, S.S.; Goulian, M.; Wilner, S.E.; et al. Self-Sorting and Coassembly of Fluorinated, Hydrogenated and Hybrid Janus Dendrimers into Dendrimersomes. J. Am. Chem. Soc. 2016, 138, 12655–12663. [Google Scholar] [CrossRef]
- Xiao, Q.; Wang, Z.; Williams, D.; Leowanawat, P.; Peterca, M.; Sherman, S.E.; Zhang, S.; Hammer, D.A.; Heiney, P.A.; King, S.R.; et al. Why Do Membranes of Some Unhealthy Cells Adopt a Cubic Architecture? ACS Cent. Sci. 2016, 2, 943–953. [Google Scholar] [CrossRef]
- Xiao, Q.; Sherman, S.E.; Wilner, S.E.; Zhou, X.; Dazen, C.; Baumgart, T.; Reed, E.H.; Hammer, D.A.; Klein, M.L.; Percec, V. Janus Dendrimersomes Coassembled from Fluorinated, Hydrogenated and Hybrid Janus Dendrimers as Models for Fusion and Fission of Biological Membranes. Proc. Natl. Acad. Sci. USA 2017, 114, E7045–E7053. [Google Scholar] [CrossRef]
- Wilner, S.E.; Xiao, Q.; Peterca, M.; Graber, Z.T.; Sherman, S.E.; Percec, V.; Baumgart, T. Dendrimersomes Exhibit Lamellar to Sponge Phase Transitions. Langmuir 2018, 34, 5527–5534. [Google Scholar] [CrossRef]
- Wilner, S.E.; Xiao, Q.; Percec, V.; Baumgart, T. Dumbell-Shaped Janus Dendrimersomes Exhibit Lamellar to Sponge Phase Transitions. Biophysical. J. 2018, 114, 272a–273a. [Google Scholar] [CrossRef]
- Yadavalli, S.S.; Xiao, Q.; Sherman, S.E.; Hasley, W.D.; Klein, M.L.; Goulian, M.; Percec, V. Bioactive Cell-Like Hybrids from Dendrimersomes with a Human Cell Membrane and its Components. Proc. Natl. Acad. Sci. USA 2019, 116, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Buzzachera, L.; Xian, Q.; Han, H.; Rahimi, K.; Li, S.; Kostina, N.Y.; Toebes, B.J.; Wilner, S.E.; Moeller, M.; Rodriguez-Emmenegger, C.; et al. Screening Libraries of Amphiphilic Janus Dendrimers Based on Natural Phenolic Acids to Discover Monodisperse Unilamellar Dendrimersomes. Biomacromolecules 2019, 20, 712–727. [Google Scholar] [CrossRef] [PubMed]
- Torre, P.; Xiao, Q.; Buzzachera, I.; Sherman, S.E.; Rahimi, K.; Kostina, N.Y.; Rodriguez-Emmenegger, C.; Moeller, M.; Wilson, C.J.; Klein, M.L.; et al. Encapsulation of Hydrophobic Components in Dendrimersomes and Decoration of their Surface with Proteins and Nucleic Acids. Proc. Natl. Acad. Sci. USA 2019, 116, 15378–15385. [Google Scholar] [CrossRef]
- Kostina, N.Y.; Rahimi, K.; Xiao, Q.; Haraszti, T.; Dedisch, S.; Spatz, J.P.; Schwaneberg, U.; Klein, M.L.; Percec, V.; Moeller, M.; et al. Membrane-Mimetic Dendrimersomes Engulf Living Bacteria via Endocytosis. NanoLetters 2019, 19, 5732–5738. [Google Scholar] [CrossRef]
- Li, S.; Xia, B.; Javed, G.; Hasley, W.D.; Melendez-Davila, A.; Liu, M.; Kerzner, M.; Agarwal, S.; Xiao, Q.; Torre, P.; et al. Direct Visualization of Vesicle Disassembly and Reassembly Using Photocleavable Dendrimers Elucidates Cargo Release Mechanisms. ACS Nano 2020, 14, 7398–7411. [Google Scholar] [CrossRef]
- Kostina, N.Y.; Wagner, A.M.; Haraszti, T.; Rahimi, K.; Xiao, Q.; Klein, M.L.; Percec, V.; Rodriguez-Emmenegger, C. Unravelling Topology-Induced Shape Transformations in Dendrimersomes. Soft Matter 2021, 17, 254–267. [Google Scholar] [CrossRef]
- Xiao, Q.; Revira-Martinez, N.; Raab, C.; Good, M.C.; Klein, M.L.; Percec, V. Co-Assembly of Loposomes with Amphiphilic Janus dendrimers Conjugated to Mono-and Tris-Nitrilotriacetic Acid Enhances Protein Revruitment. Giant 2022, 9, 100089. [Google Scholar] [CrossRef]
- Joseph, A.; Wagner, A.M.; Garay-Sarmiento, M.; Alexanuyan, M.; Haraszti, T.; Soder, D.; Georgiov, V.N.; Dimova, R.; Percec, V.; Rodriguez-Emmenegger, C. Zwitterionic Dendrimersomes: A Closer Xenobiotic Mimic of Cell Membranes. Adv. Mater. 2022, 34, 2206288. [Google Scholar] [CrossRef]
- Peterca, M.; Percec, V.; Leowanawat, P.; Bertin, A. Predicting the Size and Properties of Dendrimersomes from the Lamellar Structure of their Amphiphilic Janus Dendrimers. J. Am. Chem. Soc. 2011, 133, 20507–20520. [Google Scholar] [CrossRef]
- Zhang, S.; Mousodia, R.-O.; Sun, H.-J.; Leowanawat, P.; Muncan, A.; Nusbaum, C.D.; Chelling, K.M.; Klein, M.N.; Andre, A.; Roy, R.; et al. Mimicking Biological Membranes with Programmable Glycan Ligands Self-Assembled from Amphiphilic Janus Glycodendrimers. Angew. Chem. Int. Ed. 2014, 53, 10899–10903. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Mousodia, R.-O.; Murzeau, C.; Sun, H.-J.; Klein, M.L.; Vertesy, S.; Andre, S.; Roy, R.; Gabius, H.-J.; Percec, V. Dissecting Moleculkar Aspects of Cell Interractions Using Glycodendrimersomes with Programmable Glycan Presentation and Engineered Human Lectins. Angew. Chem. Int. Ed. 2015, 54, 4036–4040. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Mousodia, R.-O.; Vertesy, S.; Andre, S.; Klein, M.L.; Gabius, H.-J.; Percec, V. Unraveling Functional Significance of Natural Variations of a Human Galectin by Glycvodendrimersomes with Programmable Glycan Surface. Proc. Natl. Acad. Sci. USA 2015, 112, 5585–5590. [Google Scholar] [CrossRef]
- Zhang, S.; Xiao, S.; Sherman, S.E.; Muncan, A.; Ramos Vicente, A.D.M.; Wang, Z.; Hammer, D.A.; Eilliams, D.; Chen, Y.; Pochan, D.J.; et al. Glycodendrimersomes from Sequence-Defined Janus Glycodendrimers Reveal High Activity and Sensor Capacity for the Agglutination of Natural Variants of Human Lectins. J. Am. Chem. Soc. 2015, 137, 13334–13344. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Zhang, S.; Wang, Z.; Sherman, S.E.; Mousodia, R.-O.; Peterca, M.; Muncan, A.; Williams, D.A.; Hammer, D.A.; Vertesy, S.; et al. Onion-Like Glycodfendrimersomes from Sequence-Defined Janus Glycodendrimers and Influence of Architecture on Reactivity of a Lectin. Proc. Natl. Acad. Sci. USA 2016, 113, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Yadavalli, S.S.; Zhang, S.; Sherman, S.E.; Fiorin, E.; da Silva, L.; Wilson, D.A.; Hammer, D.A.; Andre, S.; Gabius, H.-J.; et al. Bioactive Cell-Like Hybrids Co-Assembled from (Glyco)Dendrimersomes with Bacterial Membranes. Proc. Natl. Acad. Sci. USA 2016, 113, E1134–E1141. [Google Scholar] [CrossRef]
- Kopitz, J.; Xiao, Q.; Ludwig, A.-K.; Romero, A.; Michalak, M.; Sherman, S.E.; Zhou, X.; Dazen, C.; Vertesy, S.; Kaltner, H.; et al. Programmable Glycan-Presentation of Glycodendrimersomes and Cells react with Engineered Human Lectins to Unveil Cell-Surface Sugar Functionality. Angew. Chem. Int. Ed. 2017, 56, 14677–14681. [Google Scholar] [CrossRef]
- Xiao, Q.; Ludwig, A.-K.; Romano, C.; Buzzacchera, I.; Sherman, S.E.; Vetro, M.; Vertesy, S.; Kaltner, H.; Reed, E.H.; Moeller, M.; et al. Exploring Functional Pairing Between Surface Glycoconjugates and Hyuman Galectins using Programmable Glycodendrimersomes. Proc. Natl. Acad. Sci. USA 2018, 115, E2509–E2518. [Google Scholar] [CrossRef]
- Ludwig, A.-K.; Michalak, M.; Xioao, Q.; Gilles, U.; Medrano, F.J.; Ma, H.; Fitz Gerarg, F.G.; Hasley, W.D.; Davila, A.M.; Liu, M.; et al. Design-Functionality Relationship for Adhesion/Growth-Regukatory Galectins. Proc. Natl. Acad. Sci. USA 2019, 116, 2837–2842. [Google Scholar] [CrossRef]
- Rodriguez-Emmenegger, C.; Xiao, Q.; Kostina, N.Y.; Sherman, S.E.; Rahimi, K.; Partridge, B.E.; Reveron Perez, A.M.; Buzzacchera, I.; Han, H.; Kerzner, M.; et al. Encoding Biological recognition in. a Bicomponent Cell-Membrane Mimic. Proc. Natl. Acad. Sci. USA 2019, 116, 5376–5382. [Google Scholar] [CrossRef]
- Xiao, Q.; Delbianco, M.; Sherman, S.E.; Reveron Perez, A.M.; Bharate, P.; Pardo-Vargas, A.; Rodriguez-Emmenegger, C.; Kostina, N.Y.; Rahimi, K.; Soeder, S.; et al. Nanovesicles Displaying Functional Linear and Branched Oligomannose Self-Assembled from Sequence-defined Janus Glycodendrimers. Proc. Natl. Acad. Sci. USA 2020, 117, 11931–11939. [Google Scholar] [CrossRef] [PubMed]
- Kostina, N.Y.; Soeder, D.; Haraszti, T.; Xiao, Q.; Rahimi, K.; Partridge, P.E.; Klein, M.L.; Percec, V.; Rodriguez-Emmenegger, C. Enhanced Concanavalin A Binding to Mannose Nanoarrays in Glycodendrimersomes Revealed Cooperative Interactions. Angew. Chem. Int. Ed. 2021, 60, 8352–8360. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.V.; Romero, A.; Xiao, Q.; Ludwig, A.-K.; Jogula, S.; Shilova, N.V.; Singh, T.; Gabba, A.; Javed, G.; Zhang, D.; et al. Probing Sulfatide-Tissue Recognition with Functionalized Glycodendrimersomes. iScience 2021, 24, 101919. [Google Scholar] [CrossRef] [PubMed]
- Percec, V.; Asgarzadeh, F. Metal-catalyzed living radical graft copolymerization of olefins initiated from the structural defects of poly(vinyl chloride). J. Polym. Sci. Part A Polym. Chem. 2001, 39, 1120–1135. [Google Scholar] [CrossRef]
- Percec, V.; Dulcey, A.E.; Balagurusamy, V.S.K.; Miura, Y.; Smidrkal, J.; Peterca, M.; Nummelin, A.; Edlund, U.; Hudson, S.D.; Heiney, P.A.; et al. Self-Assembly of Amphiphilic Dendritic Dipeptides into Helical Pores. Nature 2004, 430, 764–768. [Google Scholar] [CrossRef]
- Percec, V.; Dulcey, A.E.; Peterca, M.; Ilies, M.; Nummelin, S.; Sienkowska, M.J.; Heiney, P.A. Principles of Self-Assembluy of Helical Pores from Dendritic Dipeptides. Proc. Natl. Acad. Sci. USA 2006, 103, 2518–2523. [Google Scholar] [CrossRef]
- Kaucher, M.S.; Peterca, M.; Dulcey, A.E.; Kim, A.J.; Vinogradov, S.A.; Hammer, D.A.; Heiney, P.A.; Percec, V. Selective Transport of Water by Porous Dendritic Dipeptides. J. Am. Chem. Soc. 2007, 129, 11698–11699. [Google Scholar] [CrossRef]
- Percec, V.; Heck, J.; Lee, M.; Ungar, G.; Alvarezcastillo, A. Poly(2-vinyloxyethyloxy 3,4,5-tris [4-(N-Dodecyloxy)benzyloxy)benzoate] a Self-Assembling Polymer Similar to Tobacco Mosaic Virus. J. Mater. Chem. 1992, 2, 1033–1039. [Google Scholar] [CrossRef]
- Percec, V.; Xiao, Q. Helical Self-Organizations and Emerging Functions in Architectures, Biological and Synthetic Macromolecules. Bull. Chem. Soc. Jpn. 2021, 94, 900–928. [Google Scholar] [CrossRef]
- Percec, V. Merging Macromolecular and Supramolecular Chemistry into Bioinspired Synthesis of Complex Systems. Isr. J. Chem. 2020, 60, 48–66. [Google Scholar] [CrossRef]
- Percec, V.; Xiao, Q. Helical Chirality of Supramoleculat Columns and Spheres Self-Organizes Complex Liquid Crystals, Crystals and Quasicrystals. Isr. J. Chem. 2021, 61, 530–556. [Google Scholar] [CrossRef]
- Balagurusamy, V.S.K.; Ungar, G.; Johansson, G. Rational Design of the First Spherical Supramolecular Dendrimers Self-Organized in a Novel Thermotropic Liquid-Crystal Phase and the Determination of their Shaoe by X-ray Analysis. J. Am. Chem. Soc. 1997, 119, 1539–1555. [Google Scholar] [CrossRef]
- Hudson, S.D.; Jung, H.T.; Percec, V.; Cho, W.D.; Johansson, G.; Ungar, G.; Balagurusamy, V.S.K. Direct Visualization of Individual Cylindrical and Spherical Supramolecular Dendrimers. Science 1997, 278, 449–452. [Google Scholar] [CrossRef]
- Ungar, G.; Liu, Y.S.; Zeng, X.B.; Percec, V.; Cho, W.D. Giant Supramolecular Liquid Crystals. Science 2003, 199, 1208–1211. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.B.; Ungar, G.; Liu, Y.S.; Dulcey, A.E.; Hobbs, J.K. Supramolecular Dendritic Liquid Quasicrystals. Nature 2004, 428, 157–160. [Google Scholar] [CrossRef]
- Ahn, C.H.; Ungar, G.; Yeardley, D.J.P.; Moller, M.; Sheiko, S.S. Controling Polymer Shape through the Self-Assembly of Dendritic Side Groups. Nature 1998, 391, 161–164. [Google Scholar]
- Rosen, B.M.; Wilson, C.J.; Wilson, D.A.; Peterca, M.; Imam, M.R.; Percec, V. Dendron-Mediated Self-Assembly, Disassembly, and Self-Organization of Complex Systems. Chem. Rev. 2009, 109, 6275–6540. [Google Scholar] [CrossRef]
- Sun, H.J.; Zhang, S.D.; Percec, V. From Structure to Function via Complex Supramolecular Dendrimer Systyems. Chem. Soc. Rev. 2015, 44, 3900–3923. [Google Scholar] [CrossRef]
- Percec, V.; Glodde, M.; Bera, T.K.; Miura, Y.; Shiyanowskaya, I.; Singer, K.D.; Balagurusamy, V.S.K.; Heiney, P.A.; Schnell, I.; Rapp, A.; et al. Self-Organization of Supramolecular Helical Dendrimers into Complex Electronic Materials. Nature 2002, 419, 384–387. [Google Scholar] [CrossRef]
- Percec, V.; Bae, J.Y.; Hill, D.H. Aryl Mesylates in Metal-Catalyzed Homo-Coupling and Cross-Coupling Reactions. 2. Suzuki-Type Nickel-Catalyzed Cross-Coupling of Aryl Arenesolfonates and Aryl Mesylkates via Aryl Boronic Acids. J. Org. Chem. 1995, 60, 1060–1065. [Google Scholar] [CrossRef]
- Percec, V.; Golding, G.M.; Smidrkal, J.; Weichold, O. NiCl2(dppe)-Catalyzed Cross-Coupling of Aryl Mesylates, Arenesulfonates and Aryl Halides with Aryl Boronic Acid. J. Org. Chem. 2004, 69, 3447–3452. [Google Scholar] [CrossRef] [PubMed]
- Rosen, B.M.; Quasdorf, K.W.; Wilson, D.A.; Zhang, N.; Resmerita, A.M.; Gard, N.K.; Percec, V. Nickel-Catalyzed Cross-Coupling Involving Carbon-Oxygen Bonds. Chem. Rev. 2011, 111, 1346–1416. [Google Scholar] [CrossRef]
- Zhang, N.; Samantha, S.R.; Rosen, B.M.; Percec, V. Single-Electron Transfer in Radical and Radical-Mediated Organic, Materials and Polymer Synthesis. Chem. Rev. 2014, 114, 5848–5958. [Google Scholar] [CrossRef] [PubMed]
- Percec, V.; Barboiu, B. Living Radical Polymerization of Styrene Initiated with Arenesulfonyl Chlorides and Catalyzed by CuI(BPY)Cl. Macromolecules 1995, 28, 7970–7972. [Google Scholar] [CrossRef]
- Fleischmann, S.; Percec, V. SET-LRP of Methyl Methacrylate Initiated with CCl4 in the Presence and Absence of Air. J. Polym. Sci. Part A Polym. Chem. 2009, 48, 2243–2250. [Google Scholar] [CrossRef]
- Jiang, X.; Fleischmann, S.; Nguyen, N.H.; Rosen, B.H.; Percec, V. Cooperative and synergoistic Effects in SET-LRP of Methyl Acrylate. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 5591–5605. [Google Scholar] [CrossRef]
- Simionescu, C.I.; Percec, V.; Dumitrescu, S. Polymerization of Acetylenic Derivatives. 30. Isomers of Polyphenylacetylene. J. Polym. Sci. Part A Polym. Chem 1977, 15, 2497–2509. [Google Scholar] [CrossRef]
- Zhang, D.; Atochina-Vasserman, E.N.; Maurya, D.S.; Huang, N.; Xiao, Q.; Ona, N.; Liu, M.; Shahnawaz, H.; Ni, H.; Kim, K.; et al. One-Component Multifunctional Sequence-Defined Ionizable Amphiphilic Janus Dendrimer Delivery Systems for mRNA. J. Am. Chem. Soc. 2021, 143, 12315–12327. [Google Scholar] [CrossRef]
- Zhang, D.; Atochina-Vasserman, E.N.; Maurya, D.S.; Liu, M.; Xiao, Q.; Lu, J.; Lauri, G.; Ona, N.; Reagan, E.K.; Ni, H.; et al. Targeted Delivery of mRNA with One-Component Ionizable Amphiphilic Janus Dendrimers. J. Am. Chem. Soc. 2021, 143, 17975–17982. [Google Scholar] [CrossRef]
- Zhang, D.; Atochina-Vasserman, E.N.; Lu, J.; Maurya, D.S.; Xiao, Q.; Liu, M.; Adamson, J.; Ona, N.; Reagan, E.K.; Ni, H.; et al. The Unexpected Importance of the Primary Structure of the Hydrophobic Part of One-Component Ionizable Amphiphilic Janus Dendrimers in Targeted mRNA Delivery Activity. J. Am. Chem. Soc. 2022, 144, 4746–4753. [Google Scholar] [CrossRef] [PubMed]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno, A.; Lligadas, G.; Adamson, J.; Maurya, D.S.; Percec, V. Assembling Complex Macromolecules and Self-Organizations of Biological Relevance with Cu(I)-Catalyzed Azide-Alkyne, Thio-Bromo, and TERMINI Double “Click” Reactions. Polymers 2023, 15, 1075. https://doi.org/10.3390/polym15051075
Moreno A, Lligadas G, Adamson J, Maurya DS, Percec V. Assembling Complex Macromolecules and Self-Organizations of Biological Relevance with Cu(I)-Catalyzed Azide-Alkyne, Thio-Bromo, and TERMINI Double “Click” Reactions. Polymers. 2023; 15(5):1075. https://doi.org/10.3390/polym15051075
Chicago/Turabian StyleMoreno, Adrian, Gerard Lligadas, Jasper Adamson, Devendra S. Maurya, and Virgil Percec. 2023. "Assembling Complex Macromolecules and Self-Organizations of Biological Relevance with Cu(I)-Catalyzed Azide-Alkyne, Thio-Bromo, and TERMINI Double “Click” Reactions" Polymers 15, no. 5: 1075. https://doi.org/10.3390/polym15051075
APA StyleMoreno, A., Lligadas, G., Adamson, J., Maurya, D. S., & Percec, V. (2023). Assembling Complex Macromolecules and Self-Organizations of Biological Relevance with Cu(I)-Catalyzed Azide-Alkyne, Thio-Bromo, and TERMINI Double “Click” Reactions. Polymers, 15(5), 1075. https://doi.org/10.3390/polym15051075







