Strategy Based on Michael Addition Reaction for the Development of Bioinspired Multilayered and Multiphasic 3D Constructs
Abstract
1. Introduction
2. Materials and Methods
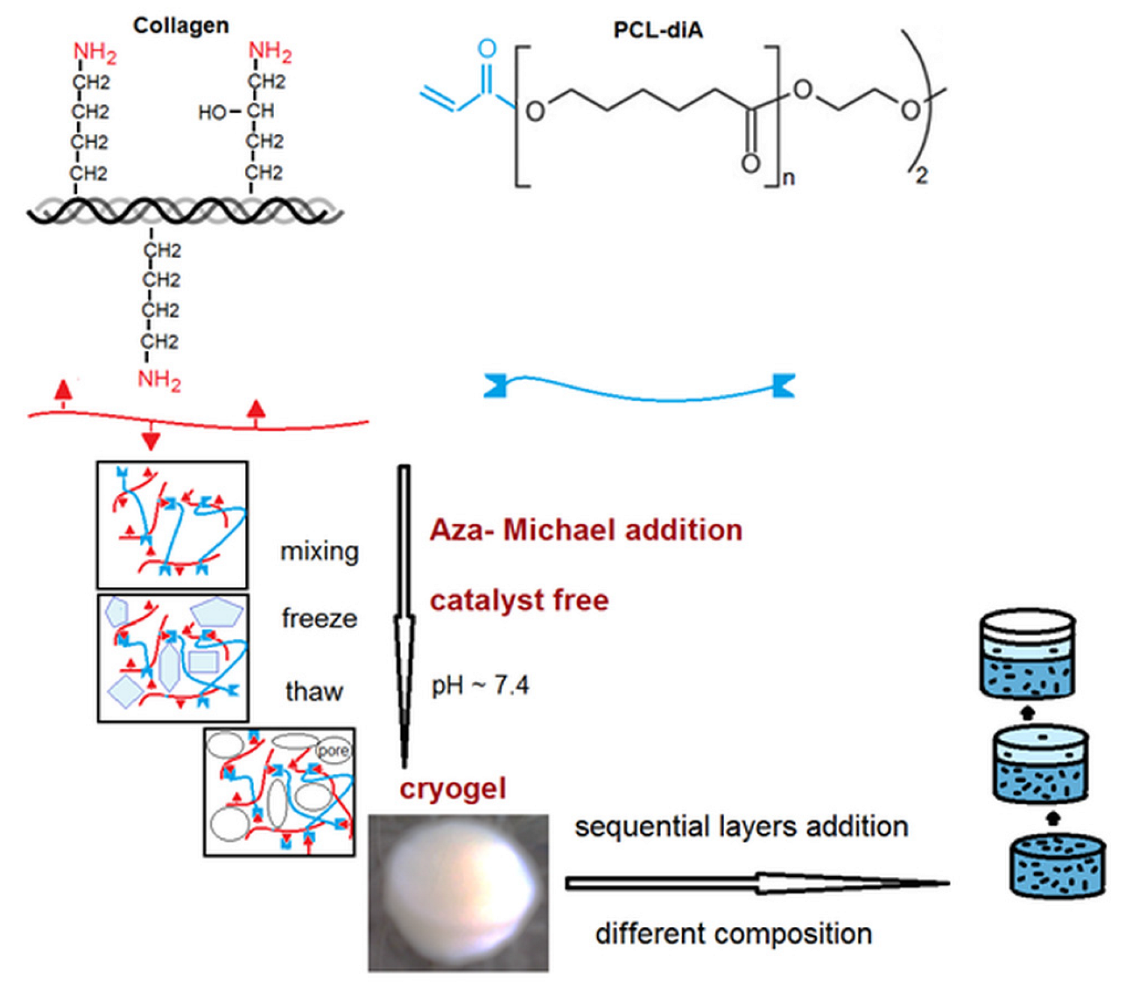
2.1. Preparation of Cryogels
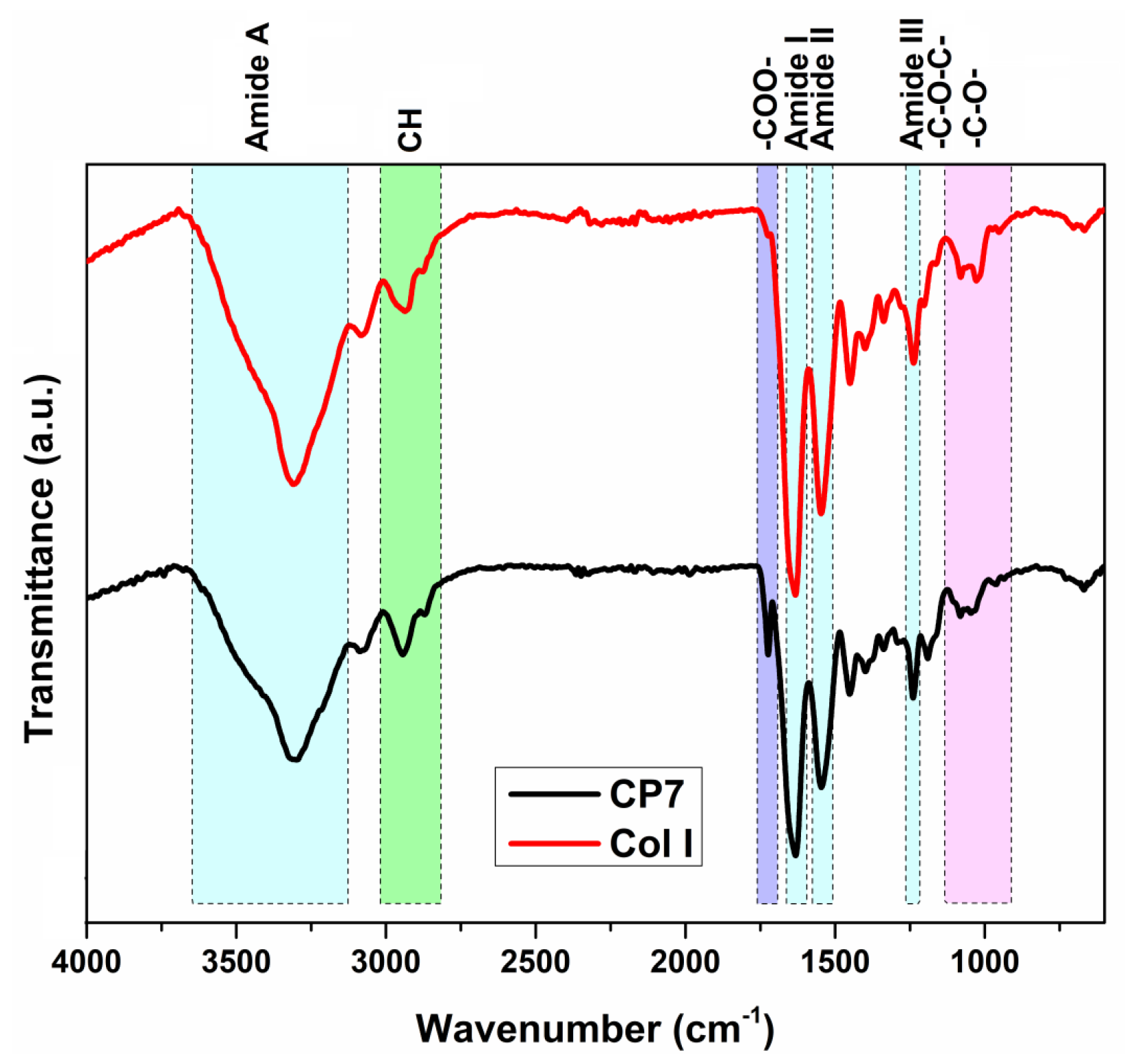
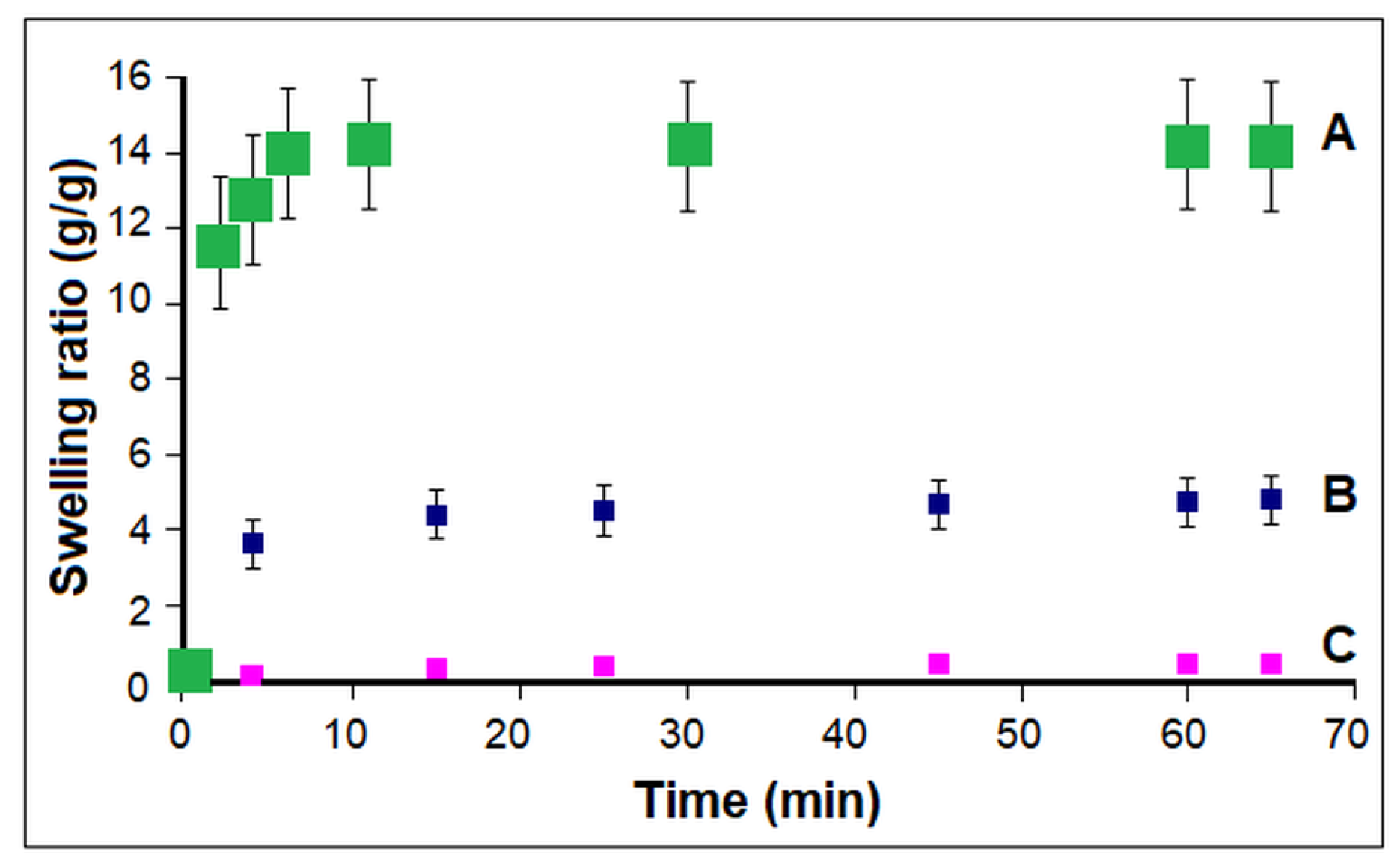
2.2. Characterization
2.3. In Vitro Biocompatibility Assessment (MTS Assay)
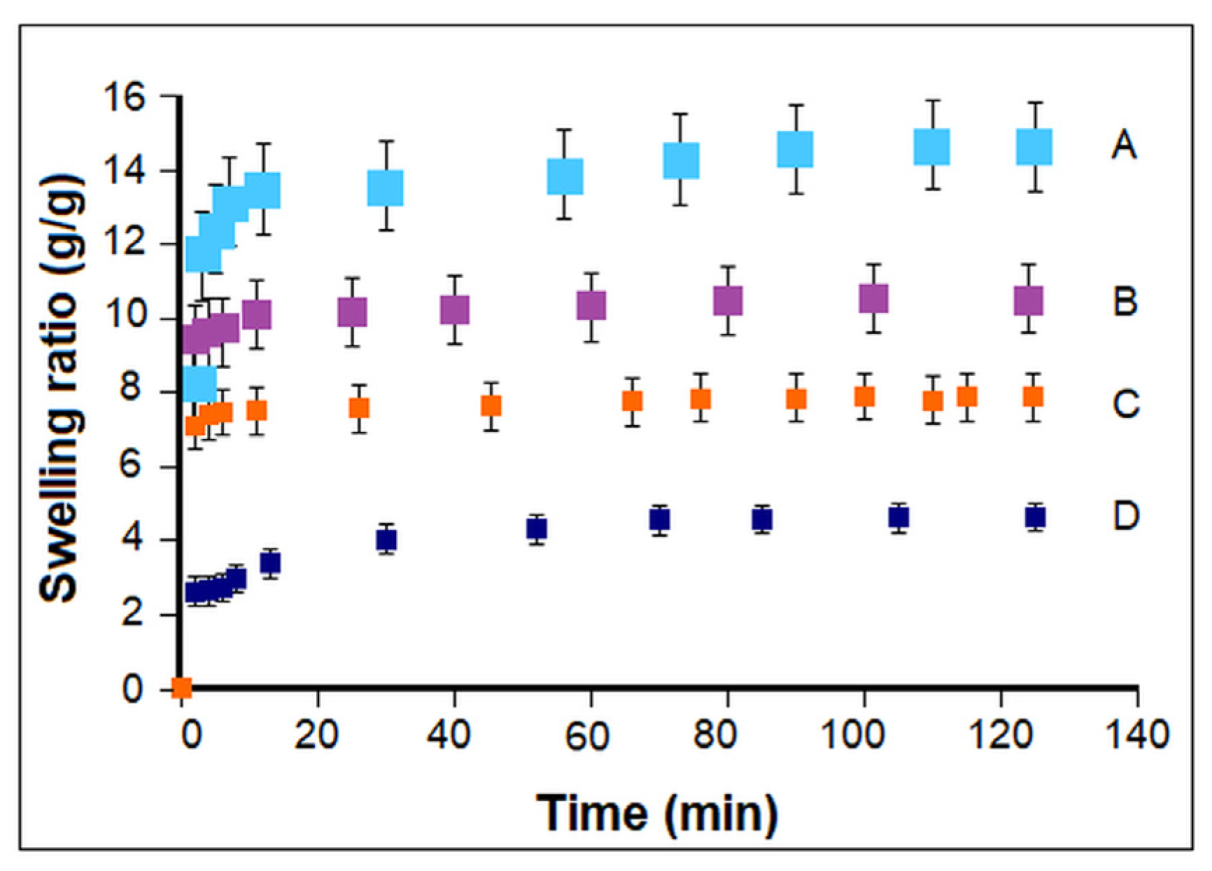
3. Results
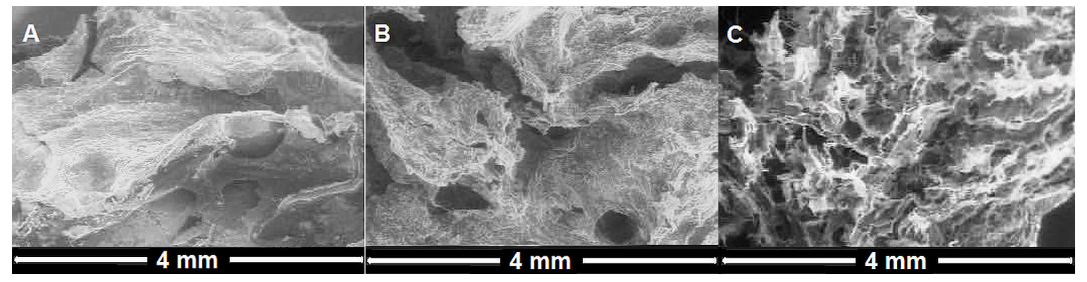
3.1. Influence of Polymerization Conditions on Collagen–PCL Construct Properties
3.2. Multilayered Structures Obtained by Aza-Michael Addition
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Żylińska, B.; Silmanowicz, P.; Sobczyńska-Rak, A.; Jarosz, Ł.; Szponder, T. Treatment of Articular Cartilage Defects: Focus on Tissue Engineering. Vivo 2018, 32, 1289–1300. [Google Scholar] [CrossRef]
- Kwon, H.; Brown, W.E.; Lee, C.A.; Wang, D.; Paschos, N.; Hu, J.C.; Athanasiou, K.A. Surgical and tissue engineering strategies for articular cartilage and meniscus repair. Nat. Rev. Rheumatol. 2019, 15, 550–570. [Google Scholar] [CrossRef]
- Wenying, W.; Honglian, D. Articular cartilage and osteochondral tissue engineering techniques: Recent advances and challenges. Bioact. Mater. 2021, 6, 4830–4855. [Google Scholar]
- Yousefi, A.-M.; Hoque, E.; Prasad, R.G.S.V.; Uth, N. Current strategies in multiphasic scaffold design for osteochondral tissue engineering: A review. J. Biomed. Mater. Res. Part A 2015, 103, 2460–2481. [Google Scholar] [CrossRef]
- Kon, E.; Filardo, G.; Perdisa, F.; Venieri, G.; Marcacci, M. Clinical results of multilayered biomaterials for osteochondral re-generation. J. Exp. Orthop. 2014, 1, 10. [Google Scholar] [CrossRef]
- Boushell, M.K.; Hung, C.T.; Hunziker, E.B.; Strauss, E.J.; Lu, H.H. Current strategies for integrative cartilage repair. Connect. Tissue Res. 2017, 58, 393–406. [Google Scholar] [CrossRef]
- Cui, W.; Wang, Q.; Chen, G.; Zhou, S.; Chang, Q.; Zuo, Q.; Ren, K.; Fan, W. Repair of articular cartilage defects with tissue-engineered osteochondral composites in pigs. J. Biosci. Bioeng. 2011, 111, 493–500. [Google Scholar] [CrossRef]
- Kon, E.; Filardo, G.; Brittberg, M.; Busacca, M.; Condello, V.; Engebretsen, L.; Marlovits, S.; Niemeyer, P.; Platzer, P.; Posthumus, M.; et al. A multilayer biomaterial for osteochondral regeneration shows superiority vs. microfractures for the treatment of osteochondral lesions in a multicentre randomized trial at 2 years. Knee Surg. Sport. Traumatol. Arthrosc. 2018, 26, 2704–2715. [Google Scholar] [CrossRef]
- Fuentes-Mera, L.; Camacho, A.; Engel, E.; Pérez-Silos, V.; Lara-Arias, J.; Marino-Martínez, I.; Peña-Martínez, V. Therapeutic potential of articular cartilage regeneration using tissue engineering based on multiphase Designs. In Cartilage Tissue Engineering and Regeneration Techniques; Nikolopoulos, D.D., Safos, G.K., Dimitrios, K., Eds.; IntechOpen: London, UK, 2019; pp. 1–28. [Google Scholar]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef]
- Lee, E.J.; Kasper, F.K.; Mikos, A.G. Biomaterials for tissue engineering. Ann Biomed Eng. 2014, 42, 323–337. [Google Scholar] [CrossRef]
- Cao, Z.; Dou, C.; Dong, S. Scaffolding Biomaterials for Cartilage Regeneration. J. Nanomater. 2014, 2014, 489128. [Google Scholar] [CrossRef]
- Nooeaid, P.; Salih, V.; Beier, J.P.; Boccaccini, A.R. Osteochondral tissue engineering: Scaffolds, stem cells and applications. J. Cell. Mol. Med. 2012, 16, 2247–2270. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Duan, P.; Liu, X.; Wang, H.; Cao, L.; He, Y.; Dong, J.; Ding, J. Effect of porosities of bilayered porous scaffolds on spontaneous osteochondral repair in cartilage tissue engineering. Regen. Biomater. 2015, 2, 9–19. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Okay, O. Cryogels on the basis of natural and synthetic polymers: Preparation, properties and areas of imple-mentation. Russ. Chem. Rev. 2002, 71, 489–511. [Google Scholar] [CrossRef]
- Savina, I.N.; Ingavle, G.C.; Cundy, A.B.; Mikhalovsky, S.V. A simple method for the production of large volume 3D macroporous hydrogels for advanced biotechnological, medical and environmental applications. Sci. Rep. 2016, 6, 21154. [Google Scholar] [CrossRef]
- Bölgen, N.; Yang, Y.; Korkusuz, P.; Güzel, E.; El Haj, A.J.; Pişkin, E. 3D ingrowth of bovine articular chondrocytes in biodegradable cryogel scaffolds for cartilage tissue engineering. J. Tissue Eng. Regen. Med. 2011, 5, 770–779. [Google Scholar] [CrossRef]
- Gupta, A.; Bhat, S.; Jagdale, P.R.; Chaudhari, B.P.; Lidgren, L.; Gupta, K.C.; Kumar, A. Evaluation of three-dimensional chitosan -agarose- gelatin cryogel scaffold for the repair of subchondral cartilage defects: An in vivo study in a rabbit model. Tissue Eng. Part A 2014, 20, 3101–3111. [Google Scholar] [CrossRef]
- Wartenberg, A.; Weisser, J.; Schnabelrauch, M. Glycosaminoglycan-Based Cryogels as Scaffolds for Cell Cultivation and Tissue Regeneration. Molecules 2021, 26, 5597. [Google Scholar] [CrossRef]
- Nikhil, A.; Kumar, A. Evaluating potential of tissue-engineered cryogels and chondrocyte derived exosomes in articular cartilage repair. Biotechnol. Bioeng. 2022, 119, 605–625. [Google Scholar] [CrossRef]
- Simionescu, B.C.; Neamtu, A.; Balhui, C.; Danciu, M.; Ivanov, D.; David, G. Macroporous structures based on biodegradable polymers-candidates for biomedical application. J. Biomed. Mater. Res. Part A 2013, 101, 2689–2698. [Google Scholar] [CrossRef]
- Simionescu, B.C.; Drobota, M.; Timpu, D.; Vasiliu, T.; Constantinescu, C.A.; Rebleanu, D.; Calin, M.; David, G. Biopoly-mers/poly(ε-caprolactone)/polyethylenimine functionalized nanohydroxyapatite hybrid cryogel: Synthesis, characterization and application in gene delivery. Mater. Sci. Eng. C 2017, 81, 167–176. [Google Scholar] [CrossRef]
- Timpu, D.; Sacarescu, L.; Vasiliu, T.; Dinu, M.V.; David, G. Surface cationic functionalized nano-hydroxyapatite—Preparation, characterization, effect of coverage on properties and related applications. Eur. Polym. J. 2020, 132, 109759. [Google Scholar] [CrossRef]
- Rulev, A.Y. Aza-Michael reaction: Achievements and prospects. Russ. Chem. Rev. 2011, 80, 197–218. [Google Scholar] [CrossRef]
- Dispinar, T.; Van Camp, W.; De Cock, L.J.; De Geest, B.G.; Du Prez, F.E. Redox-Responsive Degradable PEG Cryogels as Potential Cell Scaffolds in Tissue Engineering. Macromol. Biosci. 2012, 12, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, H. Superelastic and pH-responsive degradable dendrimer cryogels prepared by cryo-aza-Michael addition reaction. Sci. Rep. 2018, 8, 7155. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Siddiqui, N.; Asawa, S.; Birru, B.; Baadhe, R.; Rao, S. PCL-Based Composite Scaffold Matrices for Tissue Engineering Applications. Mol. Biotechnol. 2018, 60, 506–532. [Google Scholar] [CrossRef] [PubMed]
- Diaconescu, R.; Simionescu, B.C.; David, G. Control and prediction of degradation of biopolymer based hydrogels with poly(ɛ-caprolactone) subunits. Int. J. Biol. Macromol. 2014, 71, 147–154. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, H.; Sun, S.; Zhou, T.; Wu, J.; Wan, Y. Designed composites for mimicking compressive mechanical properties of articular cartilage matrix. J. Mech. Behav. Biomed. Mater. 2014, 36, 32–46. [Google Scholar] [CrossRef]
- Kon, E.; Delcogliano, M.; Filardo, G.; Fini., M.; Giavaresi, G.; Francioli, S.; Martin, I.; Pressato, D.; Arcangeli, E.; Quarto, R.; et al. Orderly osteochondral regeneration in a sheep model using a novel nano-composite multilayered biomaterial. J. Orthop. Res. 2010, 28, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Delcogliano, M.; Filardo, G.; Busacca, M.; Di Martino, A.; Marcacci, M. Novel nano-composite multilayered biomaterial for osteochondral regeneration: A pilot clinical trial. Am. J. Sports Med. 2011, 39, 1180–1190. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.; Dinda, A.K.; Gupta, A.; Koul, V. Polycaprolactone diacrylate crosslinked biodegradable semi-interpenetrating networks of polyacrylamide and gelatin for controlled drug delivery. Biomed. Mater. 2010, 5, 065014. [Google Scholar] [CrossRef] [PubMed]
- de Campos, V.B.; Mello, M.L.S. Collagen type I amide I band infrared spectroscopy. Micron 2011, 42, 283–289. [Google Scholar] [CrossRef]
- Stani, C.; Vaccari, L.; Mitri, E.; Birarda, G. FTIR investigation of the secondary structure of type I collagen: New insight into the amide III band. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 229, 118006. [Google Scholar] [CrossRef]
- Sylvester, M.; Yannas, I.; Salzman, E.; Forbes, M. Collagen banded fibril structure and the collagen-platelet reaction. Thromb. Res. 1989, 55, 135–148. [Google Scholar] [CrossRef]
- Liu, X.; Dan, N.; Dan, W. Preparation and characterization of an advanced collagen aggregate from porcine acellular dermal matrix. Int. J. Biol. Macromol. 2016, 88, 179–188. [Google Scholar] [CrossRef]
- Albu, M.G.; Deselnicu, V.; Ioannidis, I.; Deselnicu, D.; Chelaru, C. Chemical functionalization and stabilization of type I collagen with organic tanning agents. Korean J. Chem. Eng. 2015, 32, 354–361. [Google Scholar] [CrossRef]
- David, G.; Cristea, M.; Balhui, C.; Timpu, D.; Doroftei, F.; Simionescu, B.C. Effect of crosslinking methods on structure and properties of poly(e-caprolactone) stabilized hydrogels containing biopolymers. Biomacromolecules 2012, 13, 2263–2272. [Google Scholar] [CrossRef]
- Rehakova, M.; Bakos, D.; Vizarova, K.; Soldan, M.; Jurickova, M. Properties of collagen and hyaluronic acid composite materials and their modification by chemical crosslinking. J. Biomed. Mater. Res. 1996, 30, 369–372. [Google Scholar] [CrossRef]
- Available online: https://www.knieprobleme.at/upload/399856_Majo%20Regen%20Pr~C3B6sentation.pdf (accessed on 14 March 2023).
- Davis, S.; Roldo, M.; Blunn, G.; Tozzi, G.; Roncada, T. Influence of the Mechanical Environment on the Regeneration of Osteochondral Defects. Front. Bioeng. Biotechnol. 2021, 9, 603408. [Google Scholar] [CrossRef] [PubMed]
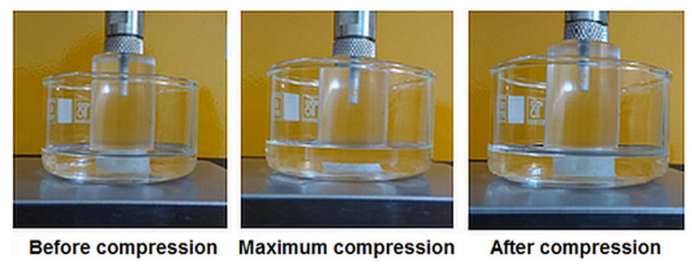
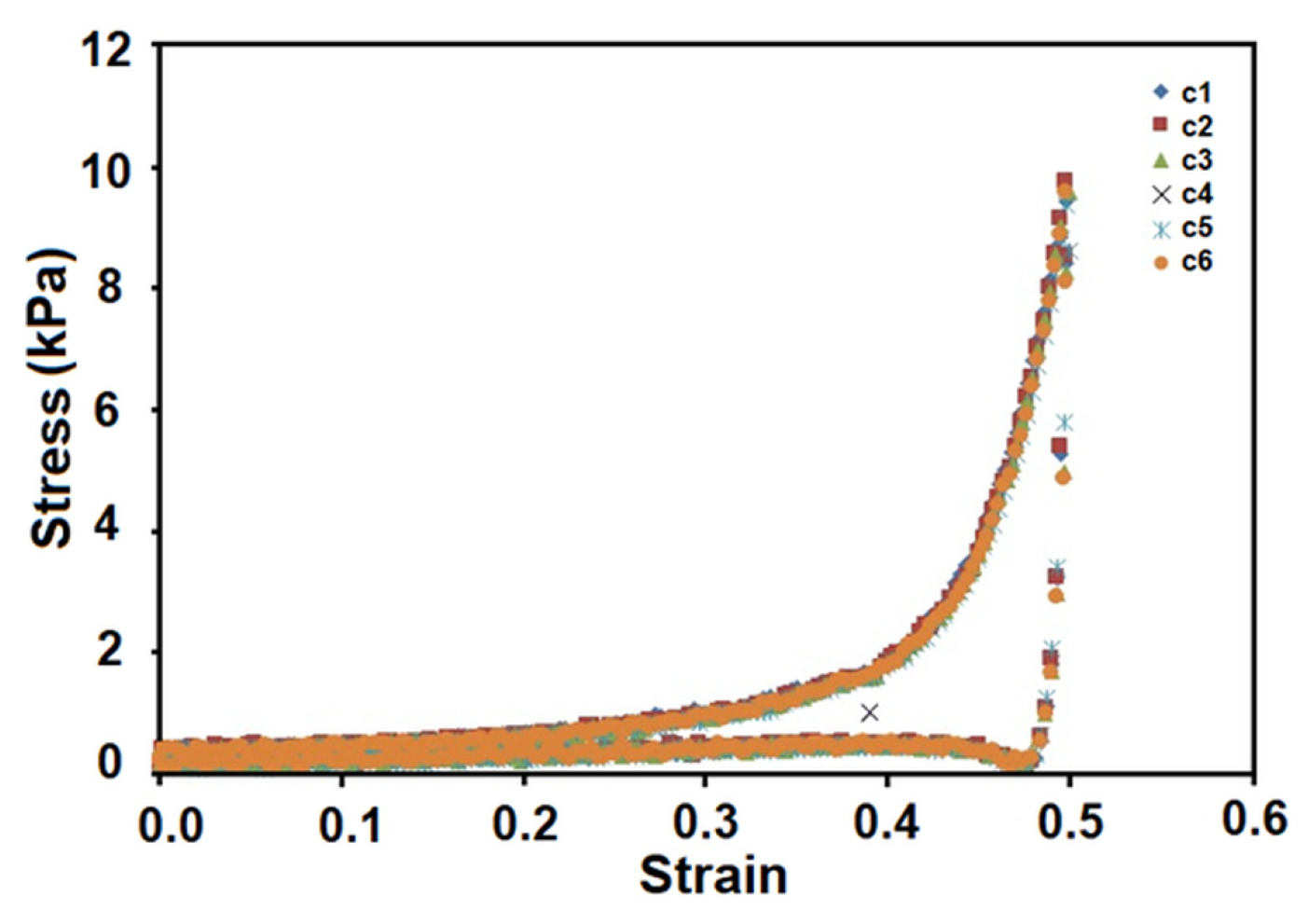

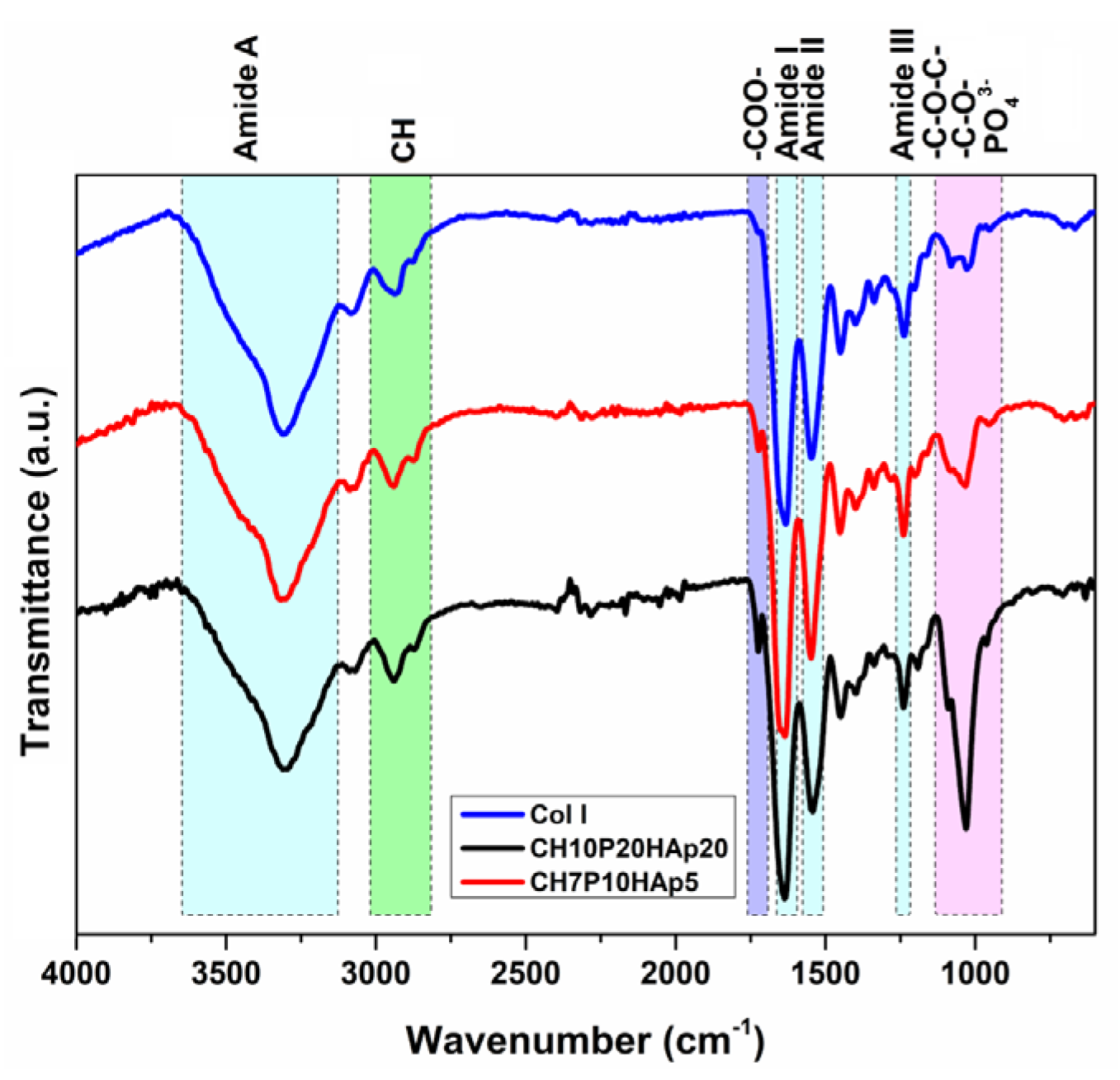
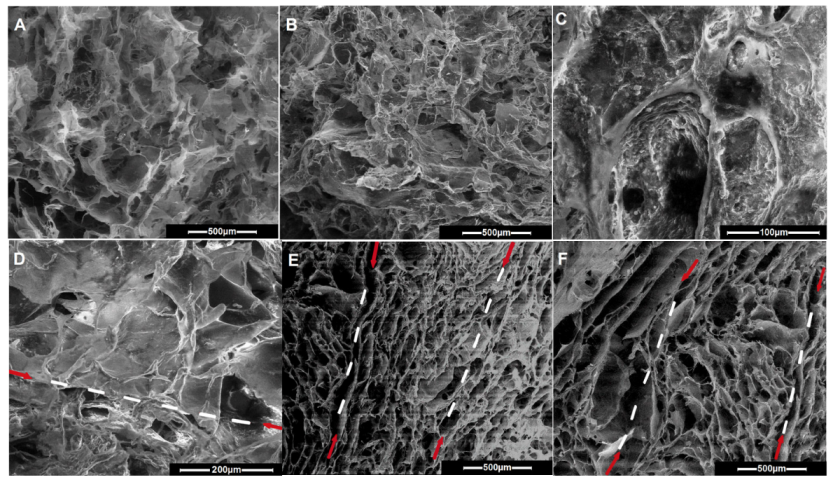

| Entry | Code | DMSHA a | PCL-diA b | HApLPEI c | Col d | Dispersion Concentration e |
|---|---|---|---|---|---|---|
| (wt.%) | (wt.%) | (wt.%) | (wt.%) | (wt.%) | ||
| 1 | CP7 | - | 7 | - | 93.4 | 1.25 |
| 2 | CP7 | - | 7 | - | 93.4 | 2.40 |
| 3 | CH5P15HAp20 | 5 | 15 | 20 | 69.0 | 2.90 |
| 4 | CH5P10HAp5 | 5 | 10 | 5 | 82.5 | 2.30 |
| 5 | CH10P20HAp20 | 10 | 20 | 20 | 63.1 | 2.45 |
| 6 | CH7P10HAp5 | 7 | 10 | 5 | 80.9 | 2.30 |
| 7 | CH5P10 | 5 | 10 | - | 86.6 | 2.40 |
| 8 | CH10P15HAp5 | 10 | 15 | 5 | 75.3 | 2.30 |
| 9 | CH7P10 | 7 | 10 | - | 85.0 | 2.25 |
| Top Layer (Entry 7) | Middle Layer (Entry 6) | Bottom Layer (Entry 5) | ||||
|---|---|---|---|---|---|---|
| Element | wt. (%) | at. (%) | wt. (%) | at. (%) | wt. (%) | at. (%) |
| C | 40.7 | 46.1 | 41.3 | 46.9 | 43.7 | 49.9 |
| N | 28.0 | 27.2 | 26.2 | 25.6 | 21.5 | 21.1 |
| O | 31.3 | 21.6 | 32.0 | 27.3 | 32.9 | 28.2 |
| P | 0.2 | 0.1 | 0.7 | 0.3 | ||
| Ca | 0.3 | 0.1 | 1.2 | 0.4 | ||
| Composition/Architecture | ESR | E (kPa) |
|---|---|---|
| CP7/1 | 4.8 ± 0.08 | - |
| CP7/2 | 14.0 ± 0.3 | 3.5 ± 0.02 |
| CH5P15HAp20 | 5.6 ± 0.2 | 9.6 ± 0.7 |
| BL | 7.6 ± 0.1 | 8.9 ± 0.5 |
| CH10P20HAp20 | 4.6 ± 0.2 | 10.0 ± 0.6 |
| TL1 | 10.5 ± 0.3 | 8.1 ± 0.1 |
| TL2 | 9.1 ± 0.3 | 7.3 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olaru, M.; Simionescu, N.; Doroftei, F.; David, G. Strategy Based on Michael Addition Reaction for the Development of Bioinspired Multilayered and Multiphasic 3D Constructs. Polymers 2023, 15, 1635. https://doi.org/10.3390/polym15071635
Olaru M, Simionescu N, Doroftei F, David G. Strategy Based on Michael Addition Reaction for the Development of Bioinspired Multilayered and Multiphasic 3D Constructs. Polymers. 2023; 15(7):1635. https://doi.org/10.3390/polym15071635
Chicago/Turabian StyleOlaru, Mihaela, Natalia Simionescu, Florica Doroftei, and Geta David. 2023. "Strategy Based on Michael Addition Reaction for the Development of Bioinspired Multilayered and Multiphasic 3D Constructs" Polymers 15, no. 7: 1635. https://doi.org/10.3390/polym15071635
APA StyleOlaru, M., Simionescu, N., Doroftei, F., & David, G. (2023). Strategy Based on Michael Addition Reaction for the Development of Bioinspired Multilayered and Multiphasic 3D Constructs. Polymers, 15(7), 1635. https://doi.org/10.3390/polym15071635






