Polyphenol Iongel Patches with Antimicrobial, Antioxidant and Anti-Inflammatory Properties
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Phenolic-Based Ionic Liquids
2.3. Preparation of Self-Assembly Iongel Materials
3. Characterization of Iongel Materials
3.1. Thermal Analysis
3.2. FTIR Spectroscopy
3.3. Rheological Behavior
3.4. Mechanical Properties
3.5. Ionic Conductivity
4. Biological Assays of Iongel Materials
4.1. Cytotoxicity
4.2. In Vitro Hemolysis and Agglutination Test
4.3. In Chemico Antioxidant Activity
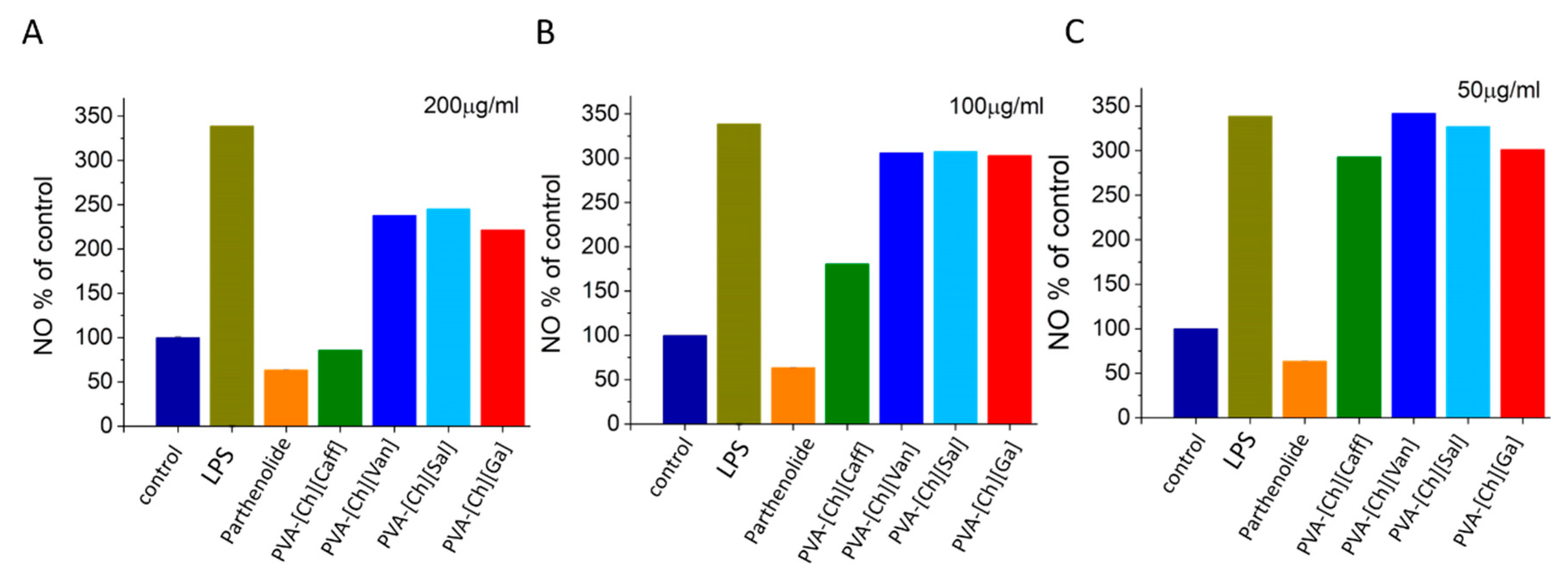
4.4. Anti-Inflammatory Assay
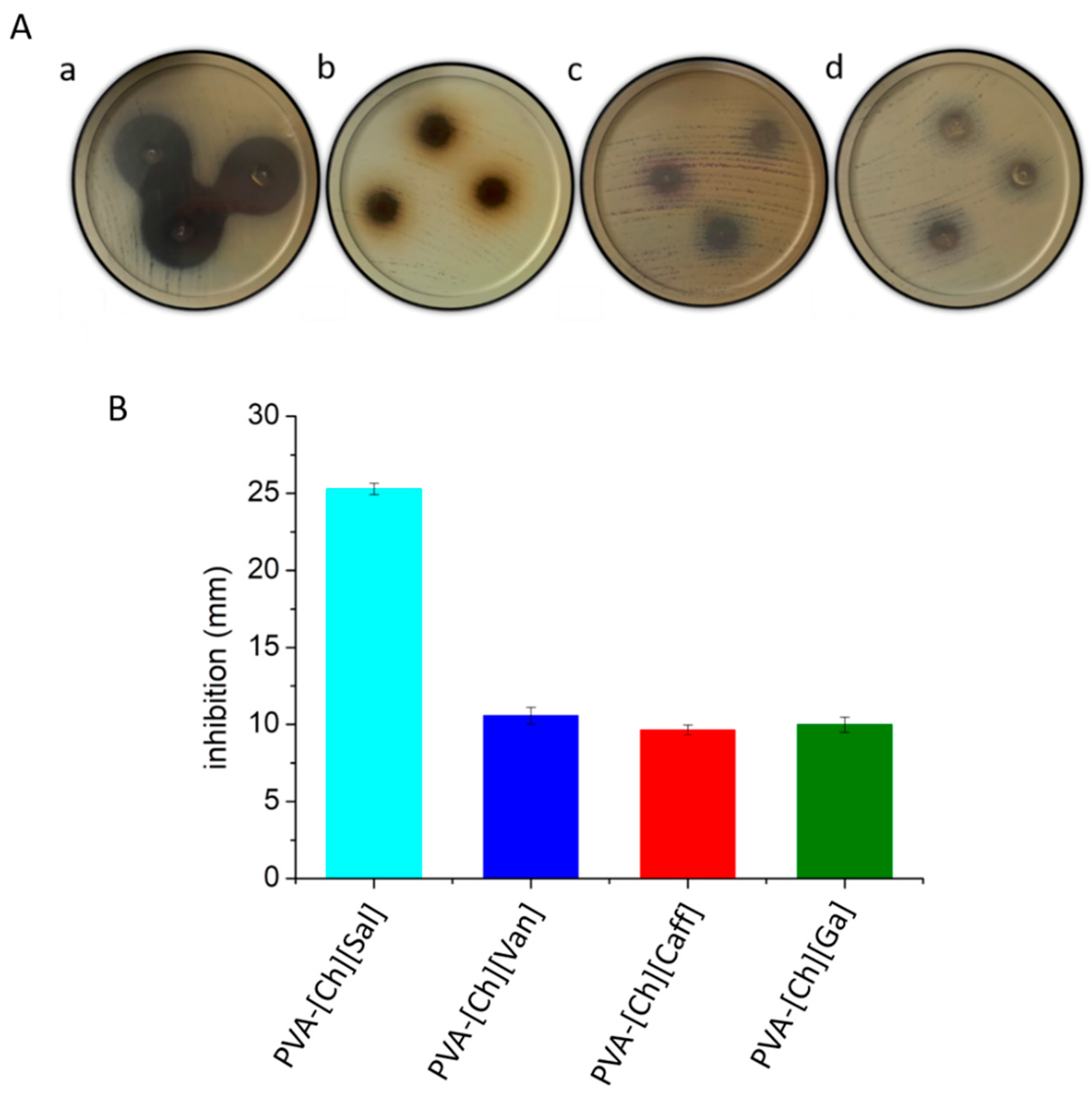
4.5. Antibacterial Activity
5. Results and Discussion
5.1. Preparation and Characterization of Iongel Materials
5.2. Biological Activity
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kost, B.; Brzezinski, M.; Socka, M.; Basko, M.; Biela, T. Biocompatible Polymers Combined with Cyclodextrins: Fascinating Materials for Drug Delivery Applications. Molecules 2020, 25, 3404. [Google Scholar] [CrossRef] [PubMed]
- Birajdar, M.S.; Joo, H.; Koh, W.G.; Park, H. Natural Bio-Based Monomers for Biomedical Applications: A Review. Biomater. Res. 2021, 25, 8. [Google Scholar] [CrossRef] [PubMed]
- Suarato, G.; Bertorelli, R.; Athanassiou, A. Borrowing from Nature: Biopolymers and Biocomposites as Smart Wound Care Materials. Front. Bioeng. Biotechnol. 2018, 6, 137. [Google Scholar] [CrossRef]
- Qi, X.; Xiang, Y.; Cai, E.; You, S.; Gao, T.; Lan, Y.; Deng, H.; Li, Z.P.; Hu, R.; Shen, J. All-in-One: Harnessing Multifunctional Injectable Natural Hydrogels for Ordered Therapy of Bacteria-Infected Diabetic Wounds. Chem. Eng. J. 2022, 439, 135691. [Google Scholar] [CrossRef]
- Tejiram, S.; Kavalukas, S.L.; Shupp, J.W.; Barbul, A. Wound Healing; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; Volume 1, ISBN 9780081006054. [Google Scholar]
- Adomėnienė, A.; Venskutonis, P.R. Dioscorea Spp.: Comprehensive Review of Antioxidant Properties and Their Relation to Phytochemicals and Health Benefits. Molecules 2022, 27, 2530. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; DiPietro, L.A. Critical Review in Oral Biology & Medicine: Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Hupkens, P.; Boxma, H.; Dokter, J. Tannic Acid as a Topical Agent in Burns: Historical Considerations and Implications for New Developments. Burns 1995, 21, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.G.; Dalvi, L.T.; Silva, J.M.C.; Lopes, G.K.B.; Alonso, A.; Hermes-Lima, M. The Antioxidant Effect of Tannic Acid on the in Vitro Copper-Mediated Formation of Free Radicals. Arch. Biochem. Biophys. 2005, 437, 1–9. [Google Scholar] [CrossRef]
- Ninan, N.; Forget, A.; Shastri, V.P.; Voelcker, N.H.; Blencowe, A. Antibacterial and Anti-Inflammatory PH-Responsive Tannic Acid-Carboxylated Agarose Composite Hydrogels for Wound Healing. ACS Appl. Mater. Interfaces 2016, 8, 28511–28521. [Google Scholar] [CrossRef]
- Euti, E.M.; Wolfel, A.; Picchio, M.L.; Romero, M.R.; Martinelli, M.; Minari, R.J.; Igarzabal, C.I.A. Controlled Thermoreversible Formation of Supramolecular Hydrogels Based on Poly(Vinyl Alcohol) and Natural Phenolic Compounds. Macromol. Rapid Commun. 2019, 40, 1900217. [Google Scholar] [CrossRef] [PubMed]
- Cencha, L.G.; Allasia, M.; Passeggi, M.C.G.; Gugliotta, L.M.; Minari, R.J. Formulation of Self-Crosslinkable Hybrid Acrylic/Casein Latex by Tannic Acid. Prog. Org. Coat. 2021, 159, 106413. [Google Scholar] [CrossRef]
- Luque, G.C.; Picchio, M.L.; Martins, A.P.S.; Dominguez-Alfaro, A.; Tomé, L.C.; Mecerreyes, D.; Minari, R.J. Elastic and Thermoreversible Iongels by Supramolecular PVA/Phenol Interactions. Macromol. Biosci. 2020, 20, 2000119. [Google Scholar] [CrossRef]
- Luque, G.C.; Picchio, M.L.; Martins, A.P.S.; Dominguez-Alfaro, A.; Ramos, N.; del Agua, I.; Marchiori, B.; Mecerreyes, D.; Minari, R.J.; Tomé, L.C. 3D Printable and Biocompatible Iongels for Body Sensor Applications. Adv. Electron. Mater. 2021, 20, 2100178. [Google Scholar] [CrossRef]
- Aguzin, A.; Luque, G.C.; Ronco, L.I.; Del Agua, I.; Guzmán-González, G.; Marchiori, B.; Gugliotta, A.; Tomé, L.C.; Gugliotta, L.M.; Mecerreyes, D.; et al. Gelatin and Tannic Acid Based Iongels for Muscle Activity Recording and Stimulation Electrodes. ACS Biomater. Sci. Eng. 2022, 8, 2598–2609. [Google Scholar] [CrossRef] [PubMed]
- Shekaari, H.; Zafarani-Moattar, M.T.; Mokhtarpour, M. Therapeutic Deep Eutectic Solvent-Based Ion-Gel as a Neoteric Drug Delivery Carrier for 5-Fluorouracil. J. Iran. Chem. Soc. 2022, 19, 4275–4286. [Google Scholar] [CrossRef]
- Qader, I.B.; Prasad, K. Recent Developments on Ionic Liquids and Deep Eutectic Solvents for Drug Delivery Applications. Pharm. Res. 2022, 39, 2367–2377. [Google Scholar] [CrossRef] [PubMed]
- Mokhtarpour, M.; Shekaari, H.; Shayanfar, A. Design and Characterization of Ascorbic Acid Based Therapeutic Deep Eutectic Solvent as a New Ion-Gel for Delivery of Sunitinib Malate. J. Drug Deliv. Sci. Technol. 2020, 56, 101512. [Google Scholar] [CrossRef]
- Joshi, N.; Rawat, K.; Solanki, P.R.; Bohidar, H.B. Biocompatible Laponite Ionogels Based Non-Enzymatic Oxalic Acid Sensor. Sens. Bio-Sens. Res. 2015, 5, 105–111. [Google Scholar] [CrossRef]
- De Anastro, A.F.; Porcarelli, L.; Hilder, M.; Berlanga, C.; Galceran, M.; Howlett, P.; Forsyth, M.; Mecerreyes, D. UV-Cross-Linked Ionogels for All-Solid-State Rechargeable Sodium Batteries. ACS Appl. Energy Mater. 2019, 2, 6960–6966. [Google Scholar] [CrossRef]
- Forsyth, M.; Porcarelli, L.; Wang, X.; Goujon, N.; Mecerreyes, D. Innovative Electrolytes Based on Ionic Liquids and Polymers for Next-Generation Solid-State Batteries. Acc. Chem. Res. 2019, 52, 686–694. [Google Scholar] [CrossRef]
- Sato, T.; Banno, K.; Maruo, T.; Nozu, R. New Design for a Safe Lithium-Ion Gel Polymer Battery. J. Power Sources 2005, 152, 264–271. [Google Scholar] [CrossRef]
- Qin, H.; Owyeung, R.E.; Sonkusale, S.R.; Panzer, M.J. Highly Stretchable and Nonvolatile Gelatin-Supported Deep Eutectic Solvent Gel Electrolyte-Based Ionic Skins for Strain and Pressure Sensing. J. Mater. Chem. C 2019, 7, 601–608. [Google Scholar] [CrossRef]
- Leleux, P.; Johnson, C.; Strakosas, X.; Rivnay, J.; Hervé, T.; Owens, R.M.; Malliaras, G.G. Ionic Liquid Gel-Assisted Electrodes for Long-Term Cutaneous Recordings. Adv. Healthc. Mater. 2014, 3, 1377–1380. [Google Scholar] [CrossRef]
- Tomé Liliana, C.; Luca, P.; Jason, B.; Forsyth Maria, M.D. Emerging Iongel Materials towards Applications in Energy and Bioelectronics. Mater. Horizons 2021, 8, 3239–3265. [Google Scholar] [CrossRef] [PubMed]
- Morais, E.S.; Silva, N.H.C.S.; Sintra, T.E.; Santos, S.A.O.; Neves, B.M.; Almeida, I.F.; Costa, P.C.; Correia-Sá, I.; Ventura, S.P.M.; Silvestre, A.J.D.; et al. Anti-Inflammatory and Antioxidant Nanostructured Cellulose Membranes Loaded with Phenolic-Based Ionic Liquids for Cutaneous Application. Carbohydr. Polym. 2019, 206, 187–197. [Google Scholar] [CrossRef] [PubMed]
- De Fernandes, I.A.A.; Maciel, G.M.; Ribeiro, V.R.; Rossetto, R.; Pedro, A.C.; Haminiuk, C.W.I. The Role of Bacterial Cellulose Loaded with Plant Phenolics in Prevention of UV-Induced Skin Damage. Carbohydr. Polym. Technol. Appl. 2021, 2, 100122. [Google Scholar] [CrossRef]
- Gomes, J.M.; Silva, S.S.; Fernandes, E.M.; Lobo, F.C.M.; Martín-Pastor, M.; Taboada, P.; Reis, R.L. Silk Fibroin/Cholinium Gallate-Based Architectures as Therapeutic Tools. Acta Biomater. 2022, 147, 168–184. [Google Scholar] [CrossRef]
- You, S.; Huang, Y.; Mao, R.; Xiang, Y.; Cai, E.; Chen, Y.; Shen, J.; Dong, W.; Qi, X. Together Is Better: Poly(Tannic Acid) Nanorods Functionalized Polysaccharide Hydrogels for Diabetic Wound Healing. Ind. Crops Prod. 2022, 186, 115273. [Google Scholar] [CrossRef]
- Fang, H.; Wang, J.; Li, L.; Xu, L.; Wu, Y.; Wang, Y.; Fei, X.; Tian, J.; Li, Y. A Novel High-Strength Poly(Ionic Liquid)/PVA Hydrogel Dressing for Antibacterial Applications. Chem. Eng. J. 2019, 365, 153–164. [Google Scholar] [CrossRef]
- Sintra, T.E.; Luís, A.; Rocha, S.N.; Ferreira, A.I.M.C.L.; Gonçalves, F.; Santos, L.M.N.B.F.; Neves, B.M.; Freire, M.G.; Ventura, S.P.M.; Coutinho, J.A.P. Enhancing the Antioxidant Characteristics of Phenolic Acids by Their Conversion into Cholinium Salts. ACS Sustain. Chem. Eng. 2015, 3, 2558–2565. [Google Scholar] [CrossRef]
- Kanaan, A.F.; Piedade, A.P.; de Sousa, H.C.; Dias, A.M.A. Semi-Interpenetrating Chitosan/Ionic Liquid Polymer Networks as Electro-Responsive Biomaterials for Potential Wound Dressings and Iontophoretic Applications. Mater. Sci. Eng. C 2021, 121, 111798. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Fu, R.; Duan, Z.; Zhu, C.; Deng, J.; Fan, D. Ionic Liquid-Based Non-Releasing Antibacterial, Anti-Inflammatory, High-Transparency Hydrogel Coupled with Electrical Stimulation for Infected Diabetic Wound Healing. Compos. Part B Eng. 2022, 236, 109804. [Google Scholar] [CrossRef]
- Gachuz, E.J.; Castillo-Santillán, M.; Juarez-Moreno, K.; Maya-Cornejo, J.; Martinez-Richa, A.; Andrio, A.; Compañ, V.; Mota-Morales, J.D. Electrical Conductivity of an All-Natural and Biocompatible Semi-Interpenetrating Polymer Network Containing a Deep Eutectic Solvent. Green Chem. 2020, 22, 5785–5797. [Google Scholar] [CrossRef]
- Toribio, L.A.; Doctoral, T. Mecanismos Moleculares Que Regulan La Activación Clásica y Alternativa En Los Macrófagos; Universitat de Barcelona: Barcelona, Spain, 2008. [Google Scholar]
- Thermo Fisher Scientific. CyQUANT TM XTT Cell Viability Assay. 2018. Available online: www.thermofisher.com/us/en/home/global/terms-and-conditions.html (accessed on 12 February 2023).
- Abcam. XTT Assay Kit (ab232856). 2020. Available online: https://www.abcam.com/ps/products/232/ab232856/documents/XTT-Assay-protocol-book-v1a-ab232856%20(website).pdf (accessed on 24 September 2022).
- Fischer, J.; Prosenc, M.H.; Wolff, M.; Hort, N.; Willumeit, R.; Feyerabend, F. Interference of Magnesium Corrosion with Tetrazolium-Based Cytotoxicity Assays. Acta Biomater. 2010, 6, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Ding, A.H.; Nathan, C.F.; Stuehr, D.J. Release of Reactive Nitrogen Intermediates and Reactive Oxygen Intermediates from Mouse Peritoneal Macrophages. Comparison of Activating Cytokines and Evidence for Independent Production. J. Immunol. 1988, 141, 2407–2412. [Google Scholar] [CrossRef]
- Chen, Y.C.; Kuo, Y.H.; Yang, N.C.; Liu, C.W.; Chang, W.T.; Hsu, C.L. Cytotoxic and Apoptotic Effects of Caffeate Derivatives on A549 Human Lung Carcinoma Cells. J. Chin. Med. Assoc. 2014, 77, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Von Petersdorff-Campen, K.; Daners, M.S. Hemolysis Testing In Vitro: A Review of Challenges and Potential Improvements. ASAIO J. 2022, 68, 3–13. [Google Scholar] [CrossRef]
- Wu, C.H.; Chen, T.L.; Chen, T.G.; Ho, W.P.; Chiu, W.T.; Chen, R.M. Nitric Oxide Modulates Pro- and Anti-Inflammatory Cytokines in Lipopolysaccharide-Activated Macrophages. J. Trauma 2003, 55, 540–545. [Google Scholar] [CrossRef]
- Meng, F.; Lowell, C.A. Lipopolysaccharide (LPS)-Induced Macrophage Activation and Signal Transduction in the Absence of Src-Family Kinases Hck, Fgr, and Lyn. J. Exp. Med. 1997, 185, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Predoi, G. Antioxidant Capacity Determination in Plants and Plant-Derived Products: A Review. Oxid. Med. Cell. Longev. 2016, 2016, 36. [Google Scholar] [CrossRef]
- Garcia, E.J.; Cadorin Oldoni, T.L.; de Alencar, S.M.; Reis, A.; Loguercio, A.D.; Miranda Grande, R.H. Antioxidant Activity by DPPH Assay of Potential Solutions to Be Applied on Bleached Teeth. Braz. Dent. J. 2012, 23, 22–27. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.B.; Biswas, M.; Khurshid Alam, A.H.M. In Vitro Antioxidant and Free Radical Scavenging Activity of Different Parts of Tabebuia Pallida Growing in Bangladesh. BMC Res. Notes 2015, 8, 1–9. [Google Scholar] [CrossRef]
- Shavandi, A.; Bekhit, A.E.D.A.; Saeedi, P.; Izadifar, Z.; Bekhit, A.A.; Khademhosseini, A. Polyphenol Uses in Biomaterials Engineering. Biomaterials 2018, 167, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sandhir, R.; Ojha, S. Evaluation of Antioxidant Activity and Total Phenol in Different Varieties of Lantana Camara Leaves. BMC Res. Notes 2014, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Vavrusova, M.; Skibsted, L.H. Antioxidant Activity and Calcium Binding of Isomeric Hydroxybenzoates. J. Food Drug Anal. 2018, 26, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, T.M.; Dinis-Oliveira, J.R.; Duarte, A.J.; Bastos, L.M.; Carvalho, F. Antioxidant Properties and Associated Mechanisms of Salicylates. Curr. Med. Chem. 2011, 18, 3252–3264. [Google Scholar] [CrossRef]
- Nikfarjam, N.; Ghomi, M.; Agarwal, T.; Hassanpour, M.; Sharifi, E.; Khorsandi, D.; Ali Khan, M.; Rossi, F.; Rossetti, A.; Nazarzadeh Zare, E.; et al. Antimicrobial Ionic Liquid-Based Materials for Biomedical Applications. Adv. Funct. Mater. 2021, 31, 2104148. [Google Scholar] [CrossRef]
- Ibsen, K.N.; Ma, H.; Banerjee, A.; Tanner, E.E.L.; Nangia, S.; Mitragotri, S. Mechanism of Antibacterial Activity of Choline-Based Ionic Liquids (CAGE). ACS Biomater. Sci. Eng. 2018, 4, 2370–2379. [Google Scholar] [CrossRef]
- Kuang, X.; Hao, H.; Dai, M.; Wang, Y.; Ahmad, I.; Liu, Z.; Yuan, Z. Serotypes and Antimicrobial Susceptibility of Salmonella Spp. Isolated from Farm Animals in China. Front. Microbiol. 2015, 6, 602. [Google Scholar] [CrossRef]
- Song, X.; Li, R.; Zhang, Q.; He, S.; Wang, Y. Antibacterial Effect and Possible Mechanism of Salicylic Acid Microcapsules against Escherichia Coli and Staphylococcus Aureus. Int. J. Environ. Res. Public Health 2022, 19, 12761. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luque, G.C.; Moya, M.; Picchio, M.L.; Bagnarello, V.; Valerio, I.; Bolaños, J.; Vethencourt, M.; Gamboa, S.-H.; Tomé, L.C.; Minari, R.J.; et al. Polyphenol Iongel Patches with Antimicrobial, Antioxidant and Anti-Inflammatory Properties. Polymers 2023, 15, 1076. https://doi.org/10.3390/polym15051076
Luque GC, Moya M, Picchio ML, Bagnarello V, Valerio I, Bolaños J, Vethencourt M, Gamboa S-H, Tomé LC, Minari RJ, et al. Polyphenol Iongel Patches with Antimicrobial, Antioxidant and Anti-Inflammatory Properties. Polymers. 2023; 15(5):1076. https://doi.org/10.3390/polym15051076
Chicago/Turabian StyleLuque, Gisela C., Melissa Moya, Matias L. Picchio, Vanessa Bagnarello, Idalia Valerio, José Bolaños, María Vethencourt, Sue-Hellen Gamboa, Liliana C. Tomé, Roque J. Minari, and et al. 2023. "Polyphenol Iongel Patches with Antimicrobial, Antioxidant and Anti-Inflammatory Properties" Polymers 15, no. 5: 1076. https://doi.org/10.3390/polym15051076
APA StyleLuque, G. C., Moya, M., Picchio, M. L., Bagnarello, V., Valerio, I., Bolaños, J., Vethencourt, M., Gamboa, S.-H., Tomé, L. C., Minari, R. J., & Mecerreyes, D. (2023). Polyphenol Iongel Patches with Antimicrobial, Antioxidant and Anti-Inflammatory Properties. Polymers, 15(5), 1076. https://doi.org/10.3390/polym15051076