ESBR Nanocomposites Filled with Monodisperse Silica Modified with Si747: The Effects of Amount and pH on Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Modified Silica
2.3. Preparation of Silica/ESBR Master Batches
2.4. Preparation of Silica/ESBR Compounds and Vulcanizates
2.5. Characterization
3. Results
3.1. Characterization of Pure and Modified Silica
3.1.1. SEM and DLS Results of Pure Silica
3.1.2. Silane Coupling Agent Si747
3.1.3. FTIR Spectra of Pure and Modified Silica
3.1.4. TGA Analysis of Pure and Modified Silica
3.2. Characterization of ESBR/Silica Compounds
3.2.1. Vulcanization Properties of ESBR/Silica Compounds
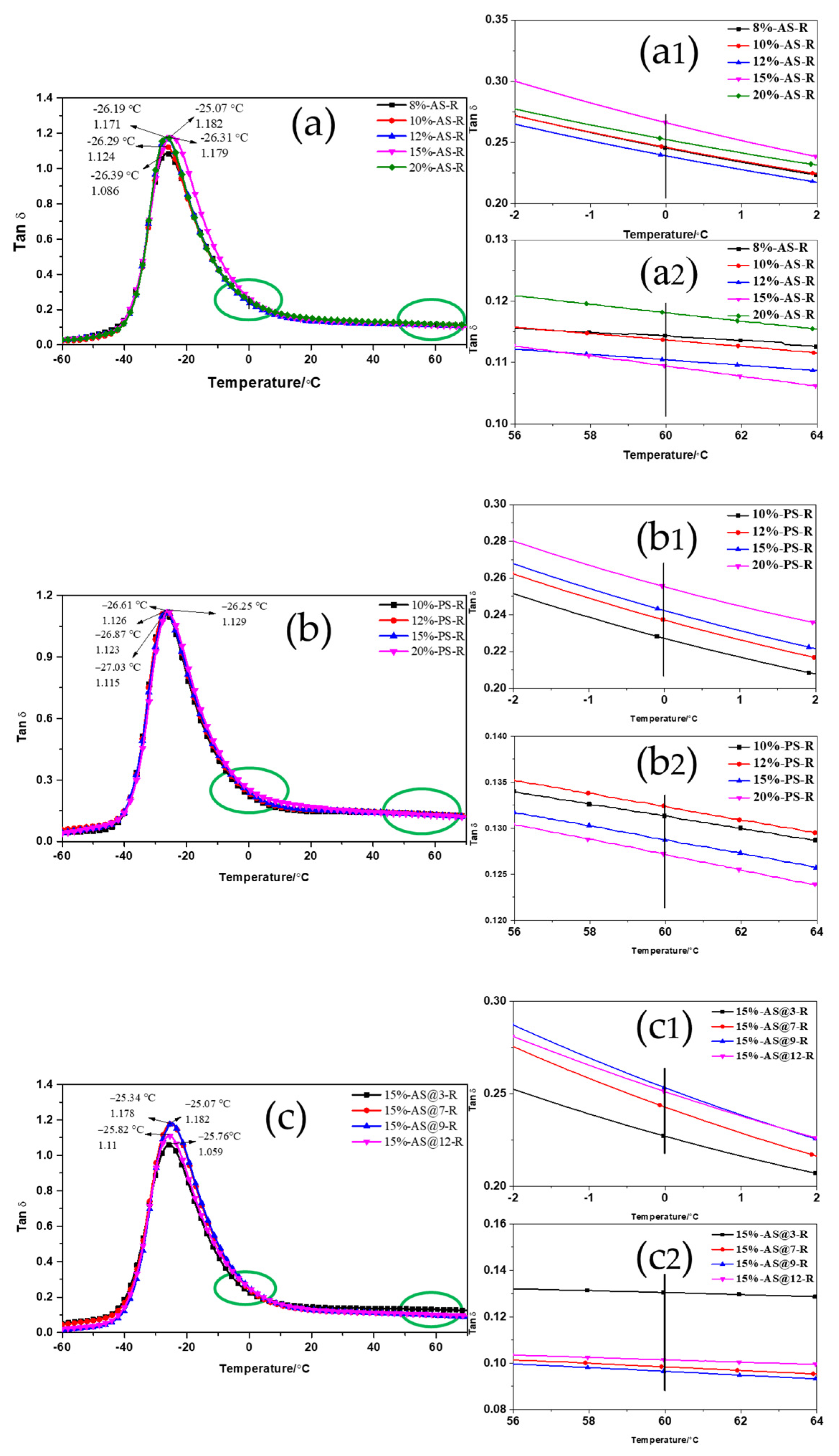
3.2.2. Dynamic Mechanical Properties of ESBR/Silica Compounds
3.3. Characterization of ESBR/Silica Vulcanizates
3.3.1. Dynamic Viscoelastic Properties of ESBR/Silica Vulcanizates
3.3.2. Physical Mechanical Properties of ESBR/Silica Vulcanizates
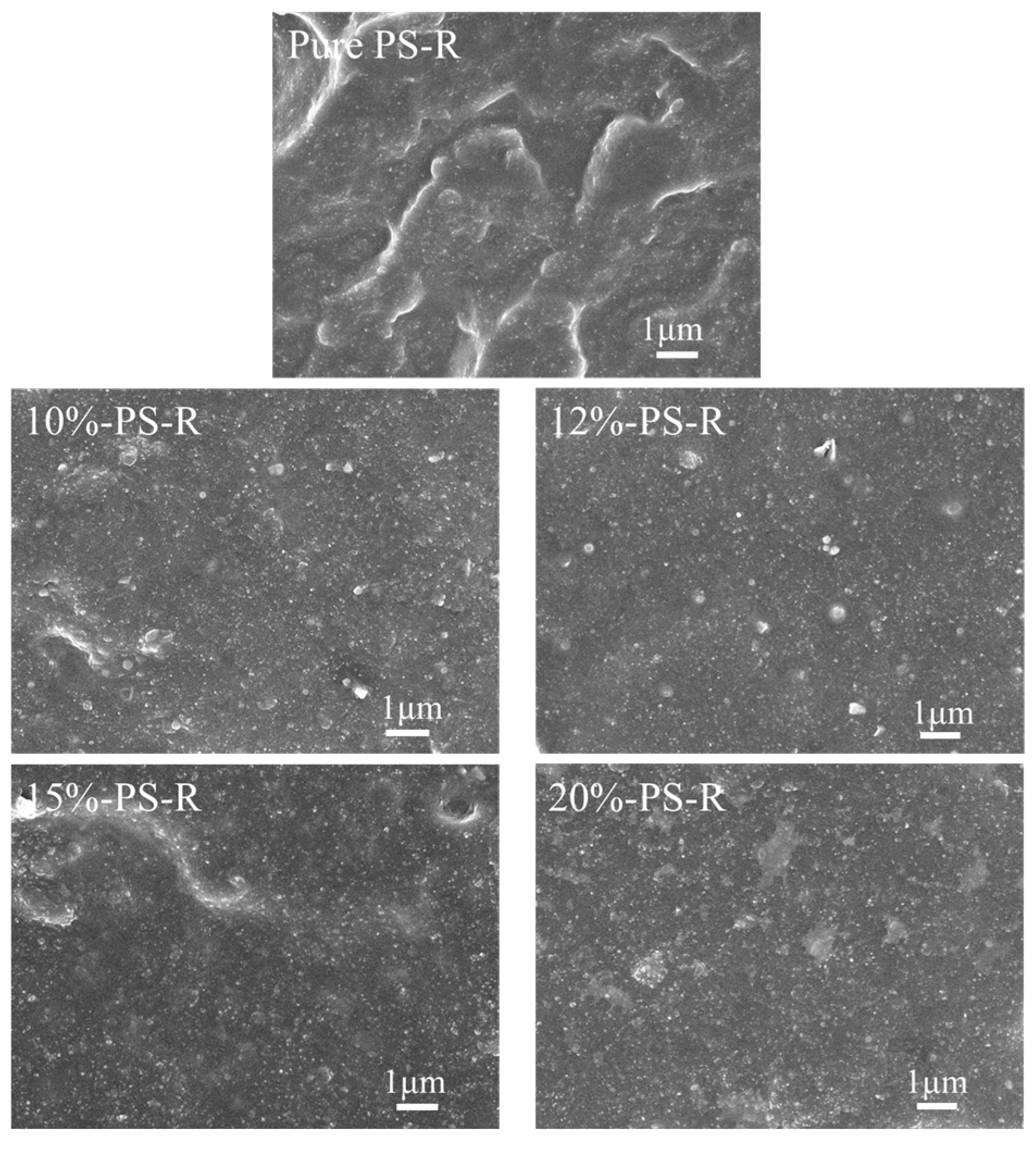
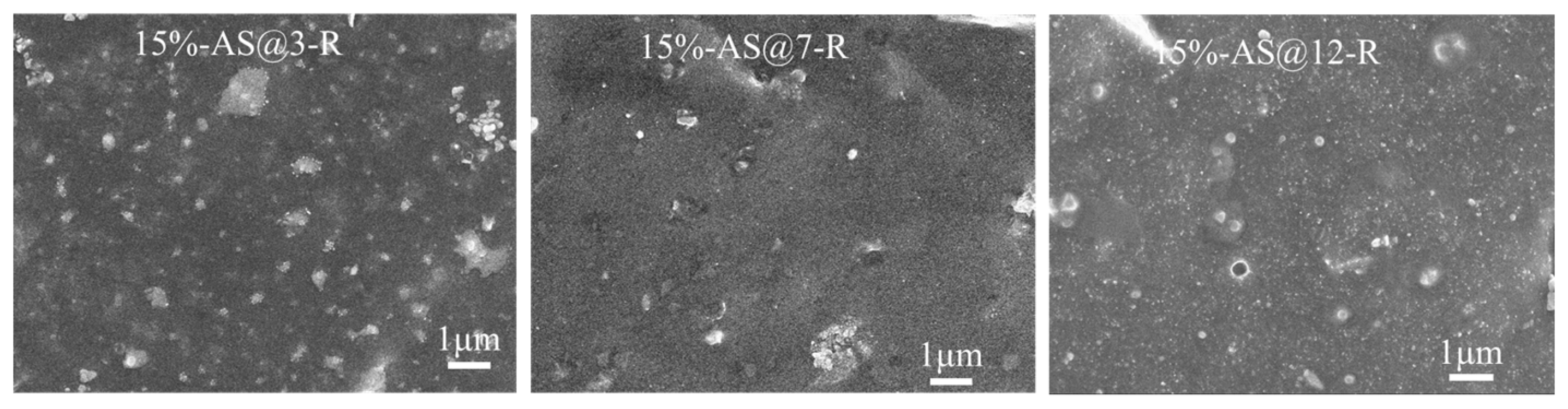
3.3.3. Micromorphology of ESBR/Silica Vulcanizates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sengloyluan, K.; Sahakaro, K.; Dierkes, W.K.; Noordermeer, J.W.M. Silica-reinforced tire tread compounds compatibilized by using epoxidized natural rubber. Eur. Polym. J. 2014, 51, 69–79. [Google Scholar] [CrossRef]
- Prasertsri, S.; Rattanasom, N. Fumed and precipitated silica reinforced natural rubber composites prepared from latex system: Mechanical and dynamic properties. Polym. Test. 2012, 31, 593–605. [Google Scholar] [CrossRef]
- Sarkawi, S.S.; Dierkes, W.K.; Noordermeer, J.W.M. Elucidation of filler-to-filler and filler-to-rubber interactions in silica-reinforced natural rubber by TEM Network Visualization. Eur. Polym. J. 2014, 54, 118–127. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Lodha, V.; Dasgupta, S.; Mukhopadhyay, R.; Guha, A.; Sarkar, P.; Saha, T.; Bhowmick, A.K. Influence of highly dispersible silica filler on the physical properties, tearing energy, and abrasion resistance of tire tread compound. J. Appl. Polym. Sci. 2019, 136, 47560. [Google Scholar] [CrossRef]
- Sun, C.Z.; Wen, S.P.; Ma, H.W.; Li, Y.; Chen, L.; Wang, Z.; Yuan, B.B.; Liu, L. Improvement of Silica Dispersion in Solution Polymerized Styrene–Butadiene Rubber via Introducing Amino Functional Groups. Ind. Eng. Chem. Res. 2019, 58, 1454–1461. [Google Scholar] [CrossRef]
- Kaewsakul, W.; Sahakaro, K.; Dierkes, W.K.; Noordermeer, J.W.M. Optimization of mixing conditions for silica-reinforced natural rubber tire tread compounds. Rubber Chem. Technol. 2012, 85, 277–294. [Google Scholar] [CrossRef]
- Qu, L.L.; Wang, L.J.; Xie, X.M.; Yu, G.Z.; Bu, S.H. Contribution of silica–rubber interactions on the viscoelastic behaviors of modified solution polymerized styrene butadiene rubbers (M-S-SBRs) filled with silica. RSC Adv. 2014, 4, 64354–64363. [Google Scholar] [CrossRef]
- Zou, Y.K.; He, J.W.; Tang, Z.H.; Zhu, L.X.; Luo, Y.F.; Liu, F. Effect of multifunctional samarium lysine dithiocarbamate on curing properties, static and dynamic mechanical properties of SBR/silica composites. RSC Adv. 2016, 6, 269–280. [Google Scholar] [CrossRef]
- Ye, N.; Zheng, J.C.; Ye, X.; Xue, J.J.; Han, D.L.; Xu, H.S.; Wang, Z.; Zhang, L.Q. Performance enhancement of rubber composites using VOC-Free interfacial silica coupling agent. Compos. Part B 2020, 202, 108301. [Google Scholar] [CrossRef]
- Tian, Q.F.; Zhang, C.H.; Tang, Y.; Liu, Y.L.; Niu, L.Y.; Ding, T.; Li, X.H.; Zhang, Z.J. Preparation of hexamethyl disilazane-surface functionalized nano-silica by controlling surface chemistry and its “agglomeration-collapse” behavior in solution polymerized styrene butadiene rubber/butadiene rubber composites. Compos. Sci. Technol. 2021, 201, 108482. [Google Scholar] [CrossRef]
- Yang, J.S.; Xian, B.; Li, H.X.; Zhang, L.Q.; Han, D.L. Preparation of silica/natural rubber masterbatch using solution compounding. Polymer 2022, 244, 124661. [Google Scholar] [CrossRef]
- Li, Y.; Han, B.Y.; Liu, L.; Zhang, F.Z.; Zhang, L.Q.; Wen, S.P.; Lu, Y.H.; Yang, H.B.; Shen, J. Surface modification of silica by two-step method and properties of solution styrene butadiene rubber (SSBR) nanocomposites filled with modified silica. Compos. Sci. Technol. 2013, 88, 69–75. [Google Scholar] [CrossRef]
- Wahba, L.; D’Arienzo, M.; Donetti, R.; Hanel, T.; Scotti, R.; Tadiello, L.; Morazzoni, F. In situ sol–gel obtained silica–rubber nanocomposites: Influence of the filler precursors on the improvement of the mechanical properties. RSC Adv. 2013, 3, 5832–5844. [Google Scholar] [CrossRef]
- Sittiphan, T.; Prasassarakich, P.; Poompradub, S. Styrene grafted natural rubber reinforced by in situ silica generated via sol–gel technique. Mater. Sci. Eng. B 2014, 181, 39–45. [Google Scholar] [CrossRef]
- Poompradub, S.; Thirakulrati, M.; Prasassarakich, P. In situ generated silica in natural rubber latex via the sol–gel technique and properties of the silica rubber composites. Mater. Chem. Phy. 2014, 144, 122–131. [Google Scholar] [CrossRef]
- Prasertsri, S.; Rattanasom, N. Mechanical and damping properties of silica/natural rubber composites prepared from latex system. Polym. Test. 2011, 30, 515–526. [Google Scholar] [CrossRef]
- Xia, L.J.; Song, J.H.; Wang, H.; Kan, Z. Silica nanoparticles reinforced natural rubber latex composites: The effects of silica dimension and polydispersity on performance. J. Appl. Polym. Sci. 2019, 136, 1–11. [Google Scholar] [CrossRef]
- Ngeow, Y.W.; Heng, J.Y.Y.; Williams, D.R.; Davies, R.T.; Lawrence, K.M.E.; Chapman, A.V. TEM observation of silane coupling agent in silica-filled rubber tyre compound. J. Rubb. Res. 2019, 22, 1–12. [Google Scholar] [CrossRef]
- Sae-oui, P.; Sirisinha, C.; Hatthapanit, K.; Thepsuwan, U. Comparison of reinforcing efficiency between Si-69 and Si-264 in an efficient vulcanization system. Polym. Test. 2005, 24, 439–446. [Google Scholar] [CrossRef]
- Ahn, B.; Kim, D.; Kim, K.; Kim, I.J.; Kim, H.J.; Kang, C.H.; Lee, J.-Y.; Kim, W. Effect of the functional group of silanes on the modification of silica surface and the physical properties of solution styrene-butadiene rubber/silica composites. Compos. Interface 2019, 26, 585–596. [Google Scholar] [CrossRef]
- Luginsland, H.D.; Röben, C. The Development of Sulphur-Functional Silanes as Coupling Agents in Silica-Reinforced Rubber Compounds. Their Historical Development over Several Decades. Int. Polym. Sci. Tech. 2016, 43, 734–737. [Google Scholar] [CrossRef]
- Gui, Y.; Zheng, J.C.; Ye, X.; Han, D.L.; Xi, M.M.; Zhang, L.Q. Preparation and performance of silica/SBR masterbatches with high silica loading by latex compounding method. Compos. Part B-Eng. 2016, 85, 130–139. [Google Scholar] [CrossRef]
- Li, Y.; Han, B.Y.; Wen, S.P.; Lu, Y.L.; Yang, H.B.; Zhang, L.Q.; Liu, L. Effect of the temperature on surface modification of silica and properties of modified silica filled rubber composites. Compos. Part A-Appl. S 2014, 62, 52–59. [Google Scholar] [CrossRef]
- Zheng, J.C.; Han, D.L.; Ye, X.; Wu, X.H.; Wu, Y.P.; Wang, Y.Q.; Zhang, L.Q. Chemical and physical interaction between silane coupling agent with long arms and silica and its effect on silica/natural rubber composites. Polymer 2018, 135, 200–210. [Google Scholar] [CrossRef]
- Bel-Hassen, R.; Boufi, S.; Salon, M.-C.B.; Abdelmouleh, M.; Belgacem, M.N. Adsorption of silane onto cellulose fibers. II. The effect of pH on silane hydrolysis, condensation, and adsorption behavior. J. Appl. Polym. Sci. 2008, 108, 1958–1968. [Google Scholar] [CrossRef]
- Pantoja, M.; Velasco, F.; Broekema, D.; Abenojar, J.; del Real, J.C. The Influence of pH on the Hydrolysis Process of γ-Methacryloxypropyltrimethoxysilane, Analyzed by FT-IR, and the Silanization of Electrogalvanized Steel. J. Adhes. Sci. Technol. 2010, 24, 1131–1143. [Google Scholar] [CrossRef]
- Rostami, M.; Mohseni, M.; Ranjbar, Z. Investigating the effect of pH on the surface chemistry of an amino silane treated nano silica. Pigm. Resin Technol. 2011, 40, 363–373. [Google Scholar] [CrossRef]
- Yokoi, T.; Wakabayashi, J.; Otsuka, Y.; Fan, W.; Iwama, M.; Watanabe, R.; Aramaki, K.; Shimojima, A.; Tatsumi, T.; Okubo, T. Mechanism of Formation of Uniform-Sized Silica Nanospheres Catalyzed by Basic Amino Acids. Chem. Mater. 2009, 21, 3719–3729. [Google Scholar]
- Osterholtz, F.D.; Pohl, E.R. Kinetics of the hydrolysis and condensation of organofunctional alkoxysilanes: A review. J. Adhes. Sci. Technol. 1992, 6, 127–149. [Google Scholar] [CrossRef]
- Beari, F.; Brand, M.; Jenkner, P.; Lehnert, R.; Metternich, H.J.; Monkiewicz, J.; Siesler, H.W. Organofunctional alkoxysilanes in dilute aqueous solution: New accounts on the dynamic structural mutability. J. Organom. Chem. 2001, 625, 208–216. [Google Scholar] [CrossRef]
- Daniels, M.W.; Sefcik, J.; Lorraine, F.; Francis, L.F.; McCormick, A.V. Reactions of a Trifunctional Silane Coupling Agent in the Presence of Colloidal Silica Sols in Polar Media. J. Colloid Interf. Sci. 1999, 219, 351–356. [Google Scholar]
- Yoshida, T.; Tanabe, T.; Hirano, M.; Muto, S. FT-IR study on the effect of oh content on the damage process in silica glasses irradiated by hydrogen. Nucl. Instrum. Methods Phys. Res. 2004, 218, 202–208. [Google Scholar] [CrossRef]
- Zheng, J.C.; Ye, X.; Han, D.L.; Zhao, S.H.; Wu, X.H.; Wu, Y.P.; Dong, D.; Wang, Y.P.; Zhang, L.Q. Silica Modified by Alcohol Polyoxyethylene Ether and Silane Coupling Agent Together to Achieve High Performance Rubber Composites Using the Latex Compounding Method. Polymers 2018, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.M.; Song, L.X.; Lu, A.; Jiang, T.; Yu, F.M.; Wang, X.C. Influence of immobilized rubber on the non-linear viscoelasticity of filled silicone rubber with different interfacial interaction of silica. RSC Adv. 2016, 6, 15155–15166. [Google Scholar] [CrossRef]
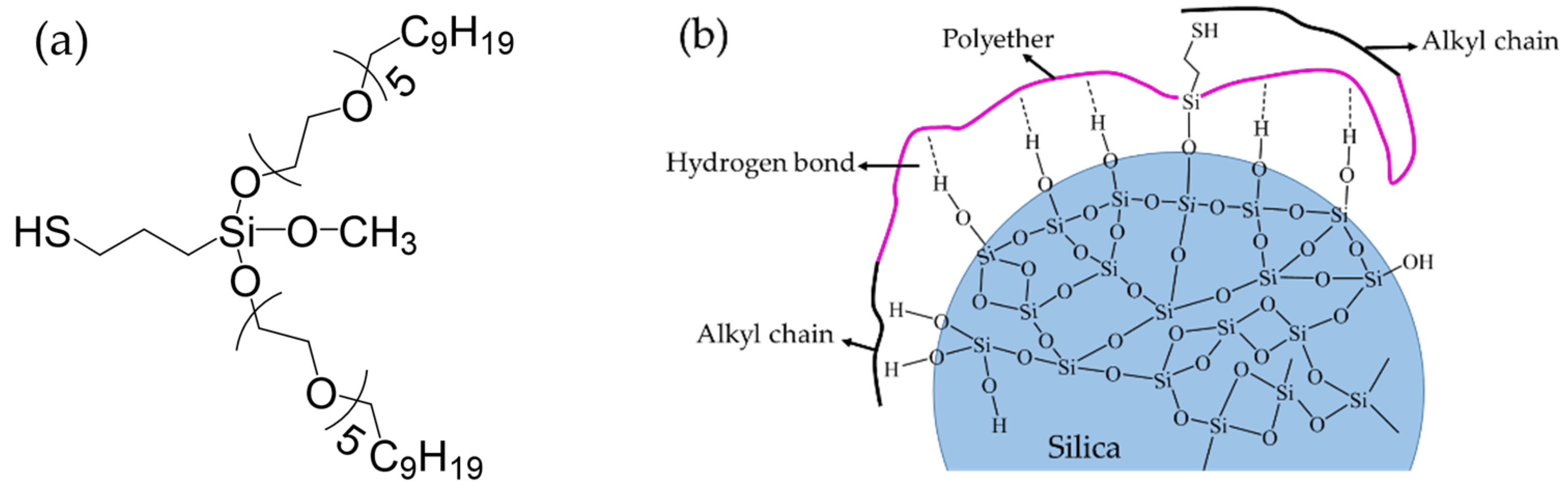


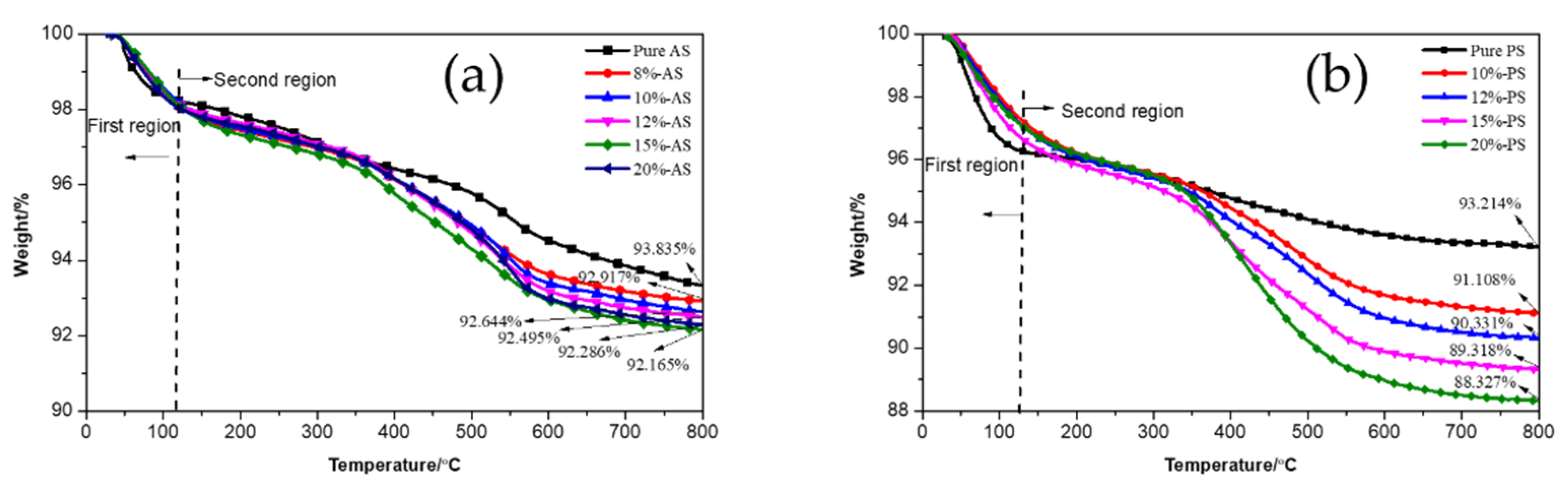
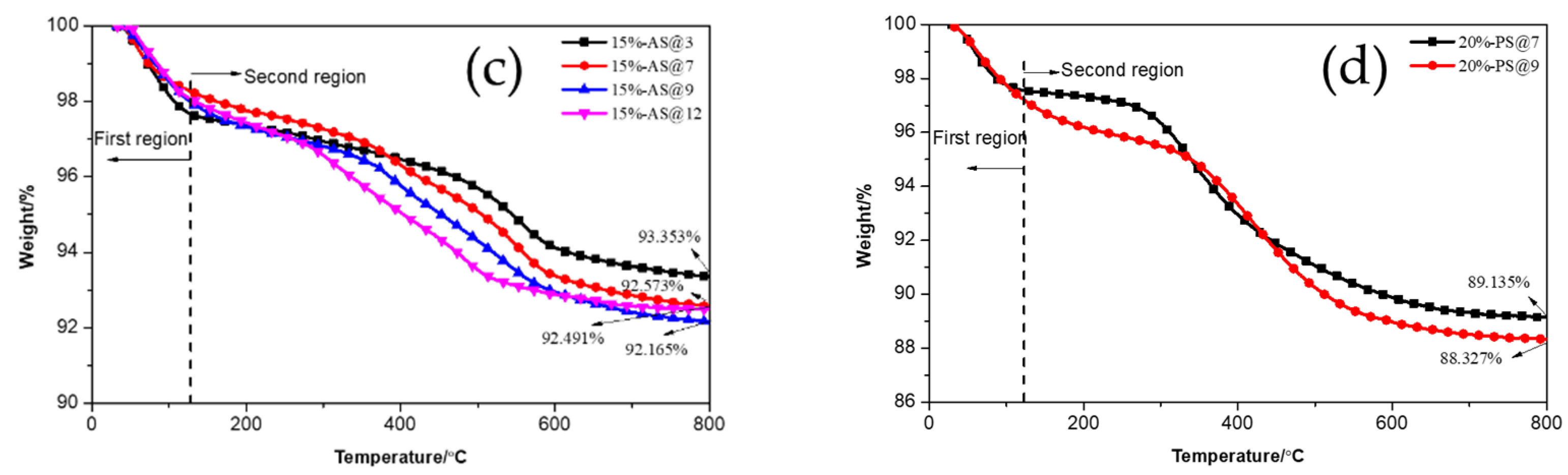
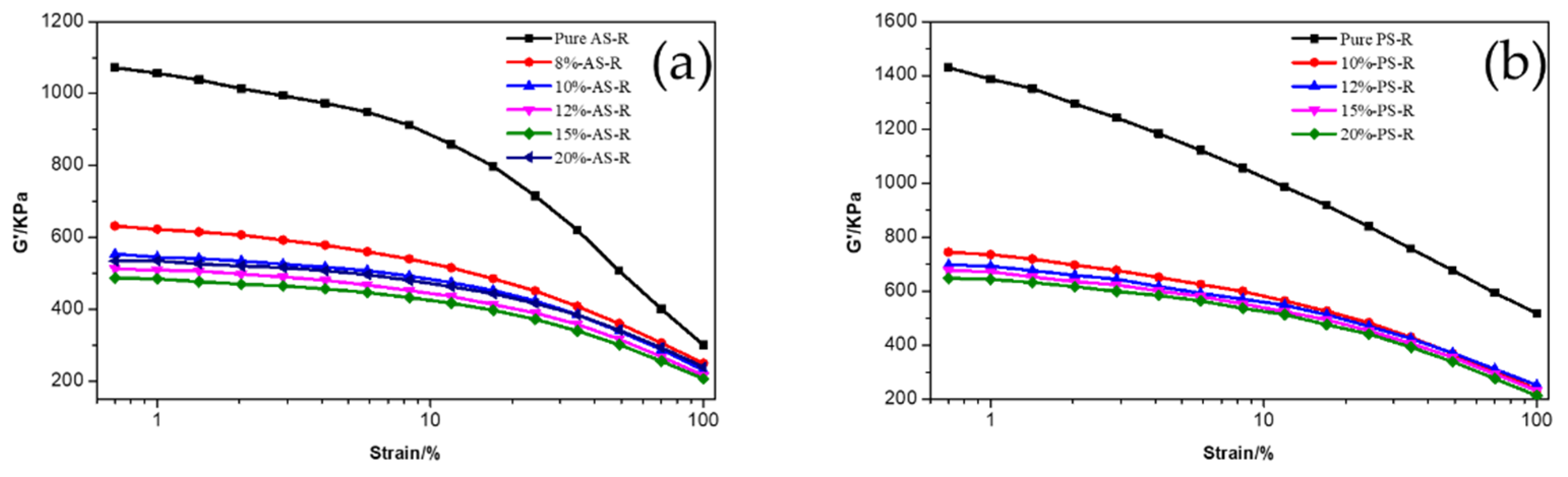



| Material | Content/phr 1 |
|---|---|
| Dried ESBR 2 | 100 |
| AS | 50 |
| PS | 50 |
| Si747 | Variable 3 |
| Zinc oxide 4 | 3 |
| Stearic acid 5 | 1 |
| N-tert-butylbenzothiazole-2-sulfonamide 6 | 1.5 |
| Diphenyl guanidine 7 | 1.5 |
| Sulfur 8 | 1.75 |
| Sample | Pure AS | 8%-AS | 10%-AS | 12%-AS | 15%-AS | 20%-AS |
| RI 1/cm−1 | 0.242 | 0.1432 | 0.118 | 0.0844 | 0.0596 | 0.0896 |
| Sample | Pure PS | - | 10%-PS | 12%-PS | 15%-PS | 20%-PS |
| RI/cm−1 | 0.0684 | - | 0.0653 | 0.0500 | 0.0459 | 0.0404 |
| Sample | 15%-AS@3 | 15%-AS@7 | 15%-AS@9 | 15%-AS@12 | 20%-PS@7 | 20%-PS@9 |
| RI/cm−1 | 0.05961 | 0.03934 | 0.0243 | 0.04442 | 0.40098 | 0.27502 |
| Sample | Compound Types | ts2/min | t90/min | CRI/min−1 | ML/dN·m | MH/dN·m | MH–ML/dN·m |
|---|---|---|---|---|---|---|---|
| Pure ESBR | - | 1.04 | 4.66 | 27.62 | 0.29 | 4.13 | 3.84 |
| ESBR/AS compounds | Pure AS-R | 5.57 | 10.94 | 18.62 | 2.96 | 18.13 | 15.17 |
| 8%-AS-R | 1.77 | 6.43 | 21.46 | 1.85 | 13.81 | 11.96 | |
| 10%-AS-R | 1.49 | 5.29 | 26.32 | 1.75 | 13.78 | 12.03 | |
| 12%-AS-R | 1.43 | 4.01 | 38.76 | 1.72 | 13.94 | 12.22 | |
| 15%-AS-R | 1.14 | 3.43 | 43.67 | 1.70 | 14.37 | 12.67 | |
| 20%-AS-R | 0.91 | 3.09 | 45.87 | 1.51 | 13.28 | 11.77 | |
| 15%-AS@3-R | 2.48 | 6.30 | 26.18 | 2.40 | 10.63 | 8.23 | |
| 15%-AS@7-R | 1.31 | 3.98 | 37.45 | 2.18 | 11.21 | 9.03 | |
| 15%-AS@9-R | 1.14 | 3.43 | 43.67 | 1.70 | 14.37 | 12.67 | |
| 15%-AS@12-R | 1.00 | 2.85 | 54.05 | 2.31 | 13.48 | 11.17 | |
| ESBR/PS compounds | Pure PS-R | 2.09 | 7.02 | 20.28 | 2.83 | 17.18 | 14.35 |
| 10%-PS-R | 1.59 | 3.52 | 51.81 | 2.18 | 14.27 | 12.09 | |
| 12%-PS-R | 1.45 | 3.33 | 53.19 | 2.04 | 14.43 | 12.39 | |
| 15%-PS-R | 1.19 | 2.69 | 66.67 | 1.94 | 14.54 | 12.7 | |
| 20%-PS-R | 0.93 | 2.39 | 68.49 | 1.68 | 14.66 | 12.98 | |
| 20%-PS@7-R | 1.51 | 3.72 | 45.25 | 1.89 | 13.02 | 11.13 | |
| 20%-PS@9-R | 0.93 | 2.39 | 68.49 | 1.68 | 14.66 | 12.98 |
| Sample | Pure ESBR | Pure AS-R | 8%-AS-R | 10%-AS-R | 12%-AS-R | 15%-AS-R | 20%-AS-R |
|---|---|---|---|---|---|---|---|
| Shore A/° | 41 | 63 | 56 | 55 | 54 | 53 | 54 |
| Tensile strength/MPa | 1.90 ± 0.05 | 4.46 ± 0.06 | 10.88 ± 0.12 | 11.67 ± 0.15 | 12.42 ± 0.22 | 15.70 ± 0.52 | 13.33 ± 0.41 |
| Modulus at 100% elongation/MPa | 0.63 ± 0.02 | 1.41 ± 0.04 | 1.16 ± 0.03 | 1.11 ± 0.03 | 0.99 ± 0.02 | 1.09 ± 0.03 | 1.10 ± 0.03 |
| Modulus at 300% elongation/MPa | 1.13 ± 0.02 | 2.22 ± 0.05 | 2.63 ± 0.06 | 2.78 ± 0.06 | 2.82 ± 0.07 | 3.75 ± 0.08 | 3.64 ± 0.07 |
| Reinforcing index | 1.79 | 1.57 | 2.27 | 2.50 | 2.91 | 3.44 | 3.31 |
| Elongation at break/% | 520 ± 13 | 476 ± 12 | 583 ± 15 | 672 ± 22 | 687 ± 23 | 716 ± 28 | 664 ± 21 |
| Sample | Pure ESBR | Pure PS-R | 10%-PS-R | 12%-PS-R | 15%-PS-R | 20%-PS-R |
|---|---|---|---|---|---|---|
| Shore A/° | 41 | 68 | 61 | 59 | 60 | 61 |
| Tensile strength/MPa | 1.90 ± 0.05 | 8.57 ± 0.08 | 10.69 ± 0.10 | 11.45 ± 0.15 | 11.95 ± 0.15 | 13.68 ± 0.20 |
| Modulus at 100% elongation/MPa | 0.63 ± 0.02 | 3.49 ± 0.06 | 1.55 ± 0.03 | 1.39 ± 0.04 | 1.41 ± 0.04 | 1.43 ± 0.03 |
| Modulus at 300% elongation/MPa | 1.13 ± 0.02 | - | 6.23 ± 0.08 | 7.02 ± 0.08 | 7.55 ± 0.08 | 8.20 ± 0.09 |
| Reinforcing index | 1.79 | - | 4.02 | 5.05 | 5.35 | 5.73 |
| Elongation at break/% | 520 ± 13 | 271 ± 8 | 374 ± 9 | 380 ± 9 | 438 ± 10 | 441 ± 10 |
| Sample | 15%-AS@3-R | 15%-AS@7-R | 15%-AS@9-R | 15%-AS@12-R | 20%-PS@7-R | 20%-PS@9-R |
|---|---|---|---|---|---|---|
| Shore A/° | 60 | 54 | 53 | 57 | 62 | 61 |
| Tensile strength/MPa | 11.47 ± 0.12 | 13.06 ± 0.22 | 15.70 ± 0.52 | 13.90 ± 0.25 | 12.76 ± 0.20 | 13.68 ± 0.20 |
| Modulus at 100% elongation/MPa | 1.46 ± 0.03 | 1.21 ± 0.03 | 1.09 ± 0.03 | 1.10 ± 0.03 | 1.54 ± 0.04 | 1.43 ± 0.03 |
| Modulus at 300% elongation/MPa | 3.30 ± 0.06 | 3.60 ± 0.05 | 3.75 ± 0.08 | 3.15 ± 0.06 | 7.02 ± 0.08 | 8.20 ± 0.09 |
| Reinforcing index | 2.26 | 2.98 | 3.44 | 2.92 | 4.56 | 5.73 |
| Elongation at break/% | 496 ± 15 | 630 ± 25 | 716 ± 28 | 608 ± 23 | 434 ± 12 | 441 ± 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, L.; Tao, A.; Cui, J.; Sun, A.; Kan, Z.; Liu, S. ESBR Nanocomposites Filled with Monodisperse Silica Modified with Si747: The Effects of Amount and pH on Performance. Polymers 2023, 15, 981. https://doi.org/10.3390/polym15040981
Xia L, Tao A, Cui J, Sun A, Kan Z, Liu S. ESBR Nanocomposites Filled with Monodisperse Silica Modified with Si747: The Effects of Amount and pH on Performance. Polymers. 2023; 15(4):981. https://doi.org/10.3390/polym15040981
Chicago/Turabian StyleXia, Lijian, Anmin Tao, Jinyun Cui, Abin Sun, Ze Kan, and Shaofeng Liu. 2023. "ESBR Nanocomposites Filled with Monodisperse Silica Modified with Si747: The Effects of Amount and pH on Performance" Polymers 15, no. 4: 981. https://doi.org/10.3390/polym15040981
APA StyleXia, L., Tao, A., Cui, J., Sun, A., Kan, Z., & Liu, S. (2023). ESBR Nanocomposites Filled with Monodisperse Silica Modified with Si747: The Effects of Amount and pH on Performance. Polymers, 15(4), 981. https://doi.org/10.3390/polym15040981







