A Practical Tool for the Assessment of Polymer Biodegradability in Marine Environments Guides the Development of Truly Biodegradable Plastics
Abstract
1. Introduction
2. Materials and Methods
2.1. General Methods
2.2. Effects of Inoculum Preconditioning and Pre-Exposure
2.3. Effect of Particle Size and Shape
2.4. Application to Commercial and Customized Plastics
2.5. Quality Assurance
2.6. Assessment Criteria for the Classification of Marine Biodegradability
2.7. Statistical Methods
3. Results and Discussion
3.1. Effect of Inoculum Preconditioning and Pre-Exposure
3.2. Use of the Logistic Model to Estimate Biodegradability
3.3. Effect of Particle Size and Shape
3.4. Application to Commercial and Customized Plastics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Type | Product Description | Supplier Composition | Source | Polymer Base | Other Components |
|---|---|---|---|---|---|
| Reference materials | PHB powder (ID019) | PHB | Helian Polymers | PHB | |
| PHB pellets (ID020) | PHB | Helian Polymers | PHB | ||
| PHBV pellets (ID022) | PHBV | Helian Polymers | PHBV | ||
| Commercial materials | Conventional PE carrier bag (ID017) | PE | Pampols | PE | none |
| Home compostable bag-GreenMaker (ID016) | PBAT + PLA + starch | GreenMaker | Polyester | none | |
| Home compostable bag-Mater Bi (ID045) | MaterBi + starch + biodegradable polymers | BioBag | Polyester | none | |
| Industrial compostable bag (ID015) | Starch + biodegradable polymers | EcoPack | Polyester | none | |
| Industrial compostable bag (ID072) | MaterBi + corn starch + plant-based polymers | Saplex | Polyester | Unidentified ester | |
| Industrial compostable bag (ID073) | Potato starch | Vileda | Polyester | Unidentified ester | |
| “Bio” net-bag (black) (ID079) | PLA | EcoPlas | Polyester | Talcum | |
| Customized materials | 1st generation Glaukos polymers (GL09, GL12) | - | Glaukos | Bio-based poly-condensate | |
| 2nd generation Glaukos polymers (GL18, GL19, GL20) | - | Glaukos | Bio-based poly-condensate | ||
| Conventional coating (IC-Y) | - | I-Coats | Polyester-acrylic | none | |
| Alternative coating (IC-B) | - | I-Coats | Polyurethane | Unidentified ester |
Appendix B
| Comparison | C+ | GL18 | ID073 | ID015 | ID072 |
|---|---|---|---|---|---|
| Fixed slope vs. variable slope | |||||
| F (degrees of freedom) | 189.7 (1, 25) | 50.6 (1, 25) | 158.1 (1, 25) | 10.3 (1, 25) | 35.5 (1, 25) |
| p | <0.0001 | <0.0001 | <0.0001 | 0.0036 | <0.0001 |
| preferred | Variable slope | Variable slope | Variable slope | Variable slope | Variable slope |
| Variable slope vs. asymmetric | |||||
| F (degrees of freedom) | 7.60 (1, 24) | 16.17 (1, 24) | 14.38 (1, 24) | 1.466 (1, 24) | 0.7859 (1, 24) |
| p | 0.011 | 0.0005 | 0.0009 | 0.2378 | 0.3841 |
| AIC | 4.7 | 11.4 | 10.2 | −1.33 | −2.09 |
| preferred | Asymmetric | Asymmetric | Asymmetric | Variable slope | Variable slope |
| Material | Treatment | b | BODL (mg L−1) |
|---|---|---|---|
| PHB powder (ID019) | sieved <20 µm | 0.52 (0.373, 0.754) | 62.6 (59.63, 65.61) |
| PHB powder (ID019) | sieved 20–63 µm | 0.47 (0.365, 0.621) | 67.5 (64.75, 70.29) |
| PHB powder (ID019) | sieved 63–250 µm | 0.57 (0.398, 0.945) | 65.2 (61.91, 68.62) |
| PHB pellets (ID020) | ground and sieved <250 µm | 0.10 (0.085, 0.107) | 67.2 (64.57, 70.06) |
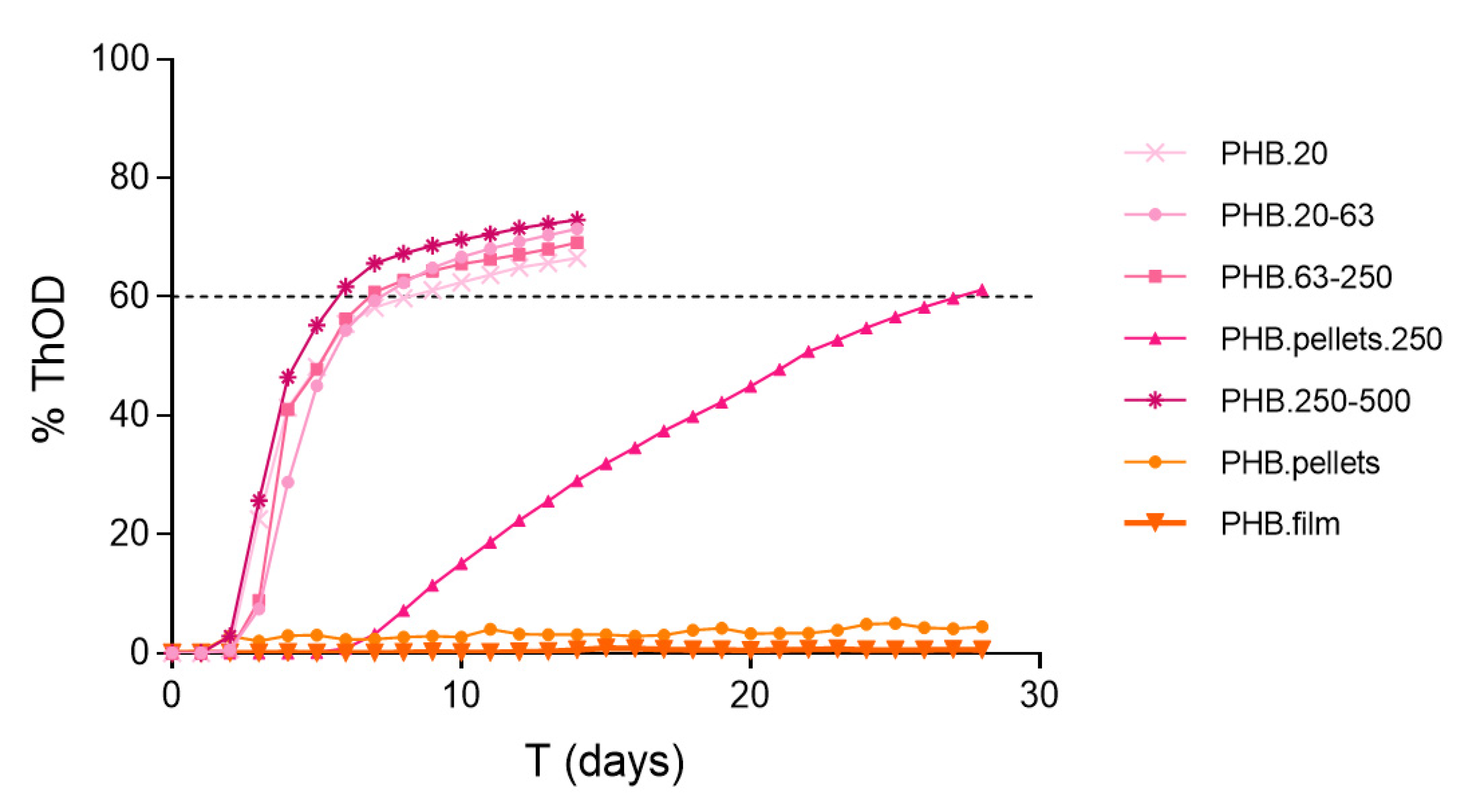
Appendix C
References
- Kühn, S.; Bravo Rebolledo, E.; van Franeker, J.A. Deleterious Effects of Litter on Marine Life. In Anthropogenic Marine Litter; Springer: Berlin/Heidelberg, Germany, 2015; pp. 75–116. [Google Scholar]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. A European Strategy for Plastics in a Circular Economy: COM/2018/028 Final; European Commission: Brussels, Belgium, 2018. [Google Scholar]
- Subach, D.J. Biodegradable Polymers. Chemist 1997, 74, 7–9. [Google Scholar] [CrossRef]
- George, A.; Sanjay, M.R.; Srisuk, R.; Parameswaranpillai, J.; Siengchin, S. A Comprehensive Review on Chemical Properties and Applications of Biopolymers and Their Composites. Int. J. Biol. Macromol. 2020, 154, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological Degradation of Plastics: A Comprehensive Review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef] [PubMed]
- Jian, J.; Xiangbin, Z.; Xianbo, H. An Overview on Synthesis, Properties and Applications of Poly(Butylene-Adipate-Co-Terephthalate)–PBAT. Adv. Ind. Eng. Polym. Res. 2020, 3, 19–26. [Google Scholar] [CrossRef]
- Witt, U.; Einig, T.; Yamamoto, M.; Kleeberg, I.; Deckwer, W.D.; Müller, R.J. Biodegradation of Aliphatic-Aromatic Copolyesters: Evaluation of the Final Biodegradability and Ecotoxicological Impact of Degradation Intermediates. Chemosphere 2001, 44, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Owonubi, S.J.; Mokhena, T.C.; Mochane, M.J.; Fasiku, V.O.; Aderibigbe, B.A.; Mukwevho, E.; Sadiku, E.R. Blends and Composites of Polyhydroxyalkanoates and Their Applications. Polyhydroxyalkanoates Biosynth. Chem. Struct. Appl. 2018, 161, 239–262. [Google Scholar] [CrossRef]
- Alexander, H. Tullo The Biodegradable Polymer PBAT Is Hitting the Big Time. 2021. Available online: https://cen.acs.org/business/biobased-chemicals/biodegradable-polymer-PBAT-hitting-big/99/i34 (accessed on 10 January 2023).
- Bagheri, A.R.; Laforsch, C.; Greiner, A.; Agarwal, S. Fate of So-Called Biodegradable Polymers in Seawater and Freshwater. Glob. Chall. 2017, 1, 1700048. [Google Scholar] [CrossRef]
- López-Ibáñez, S.; Beiras, R. Is a Compostable Plastic Biodegradable in the Sea? A Rapid Standard Protocol to Test Mineralization in Marine Conditions. Sci. Total Environ. 2022, 831, 154860. [Google Scholar] [CrossRef]
- Kliem, S.; Kreutzbruck, M.; Bonten, C. Review on the Biological Degradation of Polymers in Various Environments. Materials 2020, 13, 4586. [Google Scholar] [CrossRef]
- Sanchez-Safont, E.L.; Cabedo, L.; Gamez-Perez, J. Cellulose-Reinforced Biocomposites Based on PHB and PHBV for Food Packaging Applications. In Sustainable Food Packaging Technology; Wiley: Hoboken, NJ, USA, 2021; pp. 225–261. [Google Scholar]
- ASTM-D7081-05; Standard Specification for Non-Floating Biodegradable Plastics in the Marine Environment. (Withdrawn 2014). ASTM Standard: West Conshohocken, PA, USA, 2005.
- ASTM-D6691-17; Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials in the Marine Environment by a Defined Microbial Consortium or Natural Sea Water Inoculum. ASTM Standard: West Conshohocken, PA, USA, 2017.
- ISO 18830:2016; Plastics—Determination of Aerobic Biodegradation of Non-Floating Plastic Materials in a Seawater/Sandy Sediment Interface—Method by Measuring the Oxygen Demand in Closed Respirometer 2016. International Organization for Standardization: Geneva, Switzerland, 2016.
- ISO 22404:2019; Plastics—Determination of the Aerobic Biodegradation of Non-Floating Materials Exposed to Marine Sediment—Method by Analysis of Evolved Carbon Dioxide 2019. International Organization for Standardization: Geneva, Switzerland, 2019.
- ISO 22766:2020; Plastics—Determination of the Degree of Disintegration of Plastic Materials in Marine Habitats under Real Field Conditions 2020. International Organization for Standardization: Geneva, Switzerland, 2020.
- ISO 23977-2:2020; Determination of the Aerobic Biodegradation of Plastic Materials Exposed to Seawater. Method by Measuring the Oxygen Demand in Closed Respirometer. International Organization for Standardization: Geneva, Switzerland, 2020.
- ISO 23977-1:2020; Plastics—Determination of the Aerobic Biodegradation of Plastic Materials Exposed to Seawater—Part 1: Method by Analysis of Evolved Carbon Dioxide. International Organization for Standardization: Geneva, Switzerland, 2020.
- D7473-12; ASTM Standard Test Method for Weight Attrition on Plastic Materials in the Marine Environment by Open System Aquarium Incubations. ASTM Standard: West Conshohocken, PA, USA, 2012.
- D7991-15; ASTM Standard Test Method for Determining Aerobic Biodegradation of Plastics Buried in Sandy Marine Sediment under Controlled Laboratory Conditions. ASTM Standard: West Conshohocken, PA, USA, 2015.
- ISO 16221:2001; Water Quality—Guidance for Determination of Biodegradability in the Marine Environment. International Organization for Standardization: Geneva, Switzerland, 2001.
- Redfield, A.C. The Biological Control of Chemical Factors in the Environment. Am. Sci. 1958, 46, 230A–221. [Google Scholar]
- Sullivan, A.B.; Snyder, D.M.; Rounds, S.A. Controls on Biochemical Oxygen Demand in the Upper Klamath River, Oregon. Chem. Geol. 2010, 269, 12–21. [Google Scholar] [CrossRef]
- Martin, T.J.; Snape, J.R.; Bartram, A.; Robson, A.; Acharya, K.; Davenport, R.J. Environmentally Relevant Inoculum Concentrations Improve the Reliability of Persistent Assessments in Biodegradation Screening Tests. Environ. Sci. Technol. 2017, 51, 3065–3073. [Google Scholar] [CrossRef]
- OECD. Test No. 306: Biodegradability in Seawater; Group (New York); OECD: Paris, France, 1992; pp. 1–8. [Google Scholar]
- OECD. Test No. 301: Ready Biodegradability; OECD: Paris, France, 1992. [Google Scholar] [CrossRef]
- ISO 14851:2019; Determination of the Ultime Aerobic Biodegradability of Plastic Materials in an Aqueus Medium. Method by Measuring the Oxygen Demand in a Close Respirometer 2019. International Organization for Standardization: Geneva, Switzerland, 2019.
- OSPAR. OSPAR Guidelines for Completing the Harmonised Offshore Chemical Notification Format (HOCNF); OSPAR: Londn, UK, 2020; pp. 1–46. [Google Scholar]
- Krzan, A.; Hemjinda, S.; Miertus, S.; Corti, A.; Chiellini, E. Standardization and Certification in the Area of Environmentally Degradable Plastics. Polym. Degrad. Stab. 2006, 91, 2819–2833. [Google Scholar] [CrossRef]
- Motulsky, H. Intuitive Biostatistics. A Nonmathematical Guide to Statistical Thinking, 4th ed.; Oxford University Press: New York, NY, USA, 2018. [Google Scholar]
- Madbouly, S.A.; Schrader, J.A.; Srinivasan, G.; Liu, K.; McCabe, K.G.; Grewell, D.; Graves, W.R.; Kessler, M.R. Biodegradation Behavior of Bacterial-Based Polyhydroxyalkanoate (PHA) and DDGS Composites. Green Chem. 2014, 16, 1911–1920. [Google Scholar] [CrossRef]
- Tsuji, H.; Suzuyoshi, K. Environmental Degradation of Biodegradable Polyesters 2. Poly(ε-Caprolactone), Poly[(R)-3-Hydroxybutyrate], and Poly(L-Lactide) Films in Natural Dynamic Seawater. Polym. Degrad. Stab. 2002, 75, 357–365. [Google Scholar] [CrossRef]
- Doi, Y.; Kanesawa, Y.; Tanahashi, N.; Kumagai, Y. Biodegradation of Microbial Polyesters in the Marine Environment. Polym. Degrad. Stab. 1992, 36, 173–177. [Google Scholar] [CrossRef]
- Mabrouk, M.M.; Sabry, S.A. Degradation of Poly(3-Hydroxybutyrate) and Its Copolymer Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) by a Marine Streptomyces sp. SNG9. Microbiol. Res. 2001, 156, 323–335. [Google Scholar] [CrossRef]
- Sung, C.C.; Tachibana, Y.; Suzuki, M.; Hsieh, W.C.; Kasuya, K.I. Identification of a Poly(3-Hydroxybutyrate)-Degrading Bacterium Isolated from Coastal Seawater in Japan as Shewanella sp. Polym. Degrad. Stab. 2016, 129, 268–274. [Google Scholar] [CrossRef]
- Orts, W.J.; Nobes, G.A.R.; Kawada, J.; Nguyen, S.; Yu, G.E.; Ravenelle, F. Poly(Hydroxyalkanoates): Biorefinery Polymers with a Whole Range of Applications. The Work of Robert H. Marchessault. Can. J. Chem. 2008, 86, 628–640. [Google Scholar] [CrossRef]
- Bucci, D.Z.; Tavares, L.B.B.; Sell, I. PHB Packaging for the Storage of Food Products. Polym. Test. 2005, 24, 564–571. [Google Scholar] [CrossRef]
- Simmons, H.; Tiwary, P.; Colwell, J.E.; Kontopoulou, M. Improvements in the Crystallinity and Mechanical Properties of PLA by Nucleation and Annealing. Polym. Degrad. Stab. 2019, 166, 248–257. [Google Scholar] [CrossRef]
- Wu, J.H.; Hu, T.G.; Wang, H.; Zong, M.H.; Wu, H.; Wen, P. Electrospinning of PLA Nanofibers: Recent Advances and Its Potential Application for Food Packaging. J. Agric. Food Chem. 2022, 70, 8207–8221. [Google Scholar] [CrossRef] [PubMed]



| % C+ | Category |
|---|---|
| >60 | Readily biodegradable |
| 20 < x ≤ 60 | Moderately biodegradable |
| 5 < x ≤ 20 | Slightly biodegradable |
| ≤5 | Non-biodegradable |
| Material | %C+ | Biodegradability | b | BODL (mg L−1) |
|---|---|---|---|---|
| PE bag (ID017) | 1.2% | None | n.c. | 1.3 (n.c.) |
| Home-compostable bag (ID016) | 17.6% | Slightly | 1.40 (1.20, 1.62) | 22.2 (21.2, 23.5) |
| Home-compostable bag (ID045) | 7.8% | Slightly | 0.85 (0.68, 1.04) | 19.8 (16.5, 27.6) |
| Industrial-compostable bag (ID015) | 5.4% | Slightly | 1.75 (1.36, 2.23) | 7.6 (7.3, 8.0) |
| Industrial-compostable bag (ID072) | 13.4% | Slightly | 1.50 (1.32, 1.69) | 27.4 (23.7, 33.9) |
| Industrial-compostable bag (ID073) | 15.6% | Slightly | 1.84 (1.69, 2.01) | 21.6 (21.2, 22.0) |
| Compostable net (ID079) | 0.8% | None | 2.81 (0.98, n.c.) | 1.2 (1.1, 1.4) |
| Conventional coating (IC-Y) | 2.3% | None | 2.21 (1.59, 3.10) | 3.5 (3.4, 3.8) |
| Alternative coating (IC-B) | 42.0% | Moderate | 1.65 (1.49, 1.83) | 54.2 (52.7, 55.9) |
| GL09 | 0.5% | None | n.c. | 0.1 (n.c.) |
| GL12 | 16.5% | Slightly | 2.73 (2.33, 3.19) | 20.7 (20.1, 21.4) |
| GL18 | 21.4% | Moderate | 2.24 (1.82, 2.74) | 29.2 (27.8, 31.1) |
| GL19 | 10.0% | Slightly | 1.82 (1.50, 2.18) | 18.2 (15.6, 23.2) |
| GL20 | 13.1% | Slightly | 1.71 (1.25, 2.23) | 27.8 (20.5, 64.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beiras, R.; López-Ibáñez, S. A Practical Tool for the Assessment of Polymer Biodegradability in Marine Environments Guides the Development of Truly Biodegradable Plastics. Polymers 2023, 15, 974. https://doi.org/10.3390/polym15040974
Beiras R, López-Ibáñez S. A Practical Tool for the Assessment of Polymer Biodegradability in Marine Environments Guides the Development of Truly Biodegradable Plastics. Polymers. 2023; 15(4):974. https://doi.org/10.3390/polym15040974
Chicago/Turabian StyleBeiras, Ricardo, and Sara López-Ibáñez. 2023. "A Practical Tool for the Assessment of Polymer Biodegradability in Marine Environments Guides the Development of Truly Biodegradable Plastics" Polymers 15, no. 4: 974. https://doi.org/10.3390/polym15040974
APA StyleBeiras, R., & López-Ibáñez, S. (2023). A Practical Tool for the Assessment of Polymer Biodegradability in Marine Environments Guides the Development of Truly Biodegradable Plastics. Polymers, 15(4), 974. https://doi.org/10.3390/polym15040974






