Mass Spectrometry Insight for Assessing the Destiny of Plastics in Seawater
Abstract
1. Introduction
2. Materials and Methods
2.1. Plastic Samples
2.2. Sample Treatment
2.3. IR Measurements
2.4. Metal Analysis
2.5. Data Analysis
3. Results and Discussion
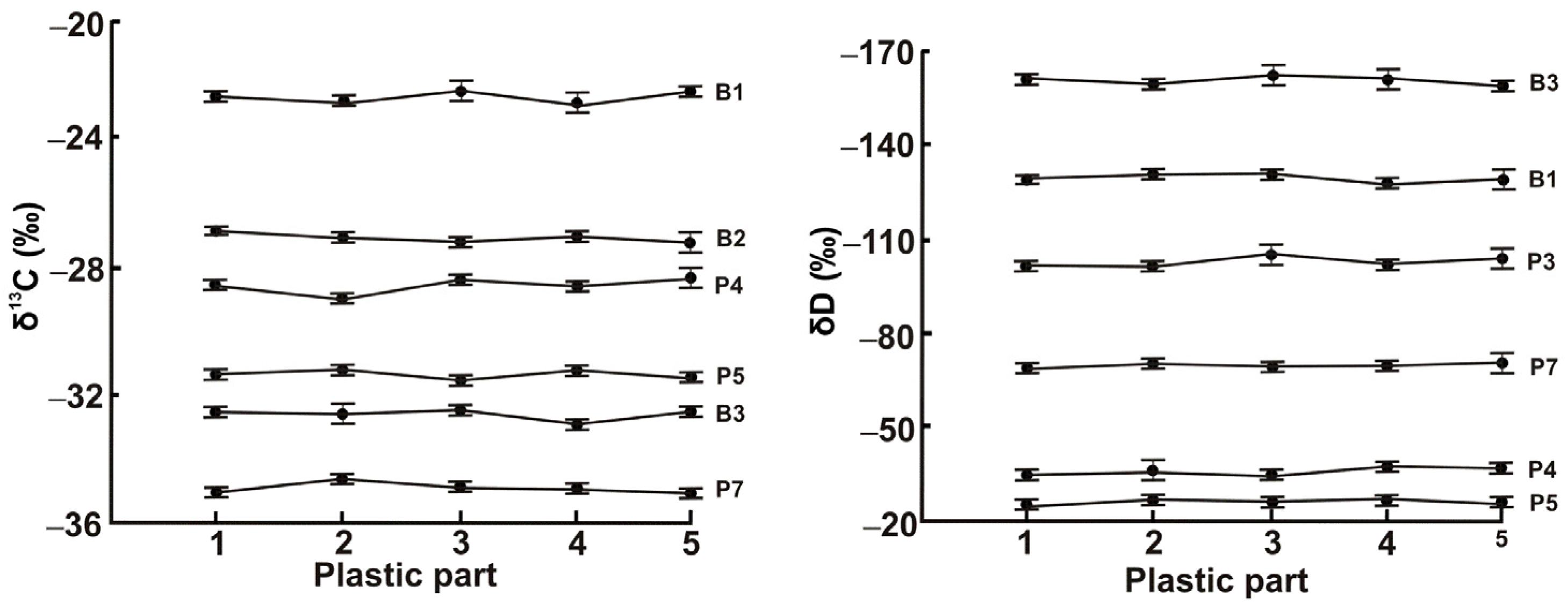
3.1. Optimization of IRMS Measurements
3.2. Identification of Plastic Material
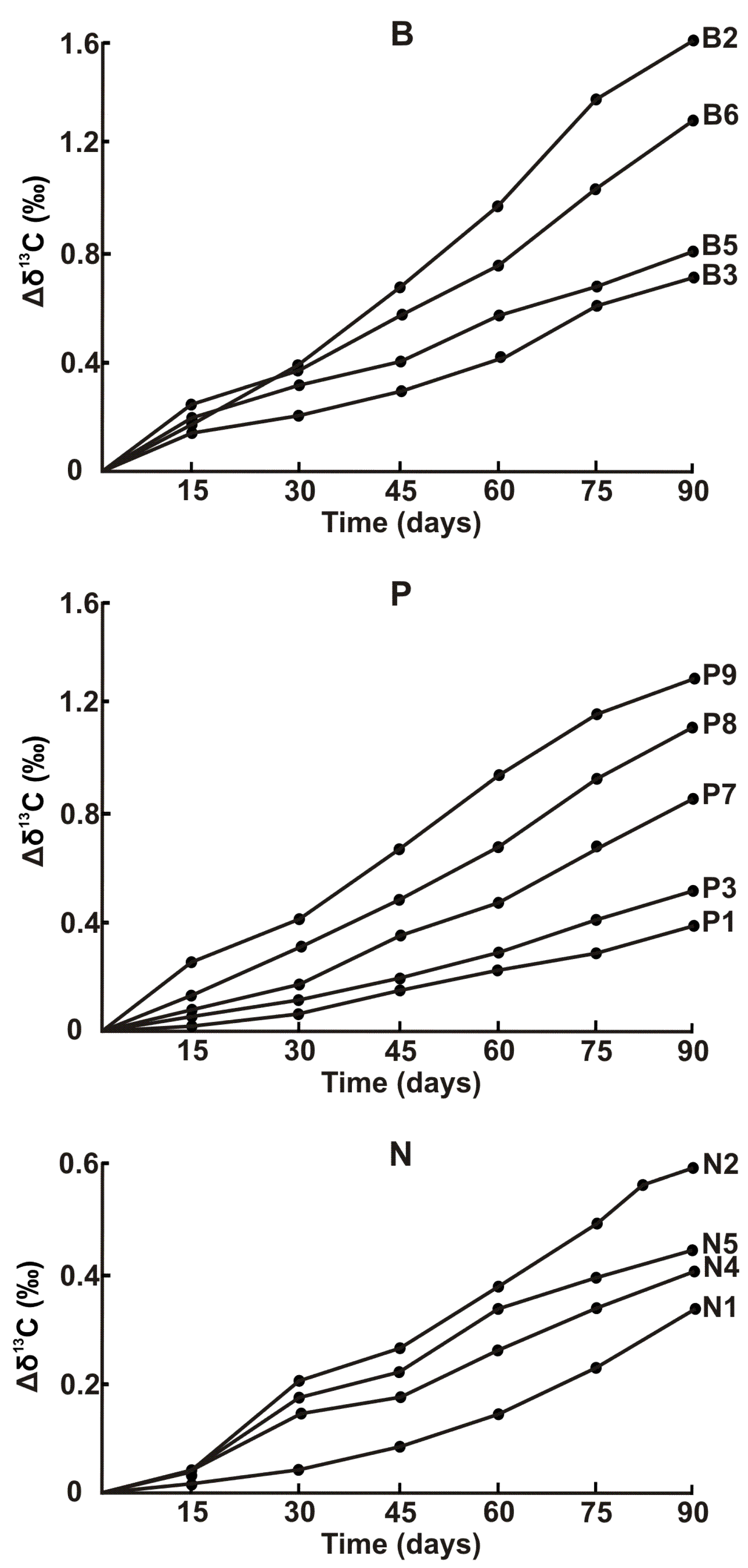
3.3. Seawater-Induced Degradation
3.4. Metal Contamination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hale, R.C.; Seeley, M.E.; La Guardia, M.J.; Mai, L.; Zeng, E.Y. A global perspective on microplastics. J. Geophys. Res. Oceans 2020, 125, e2018JC014719. [Google Scholar] [CrossRef]
- Iroegbu, A.O.C.; Ray, S.S.; Mbarane, V.; Bordado, J.C.; Sardinha, J.P. Plastic pollution: A perspective on matters arising: Challenges and opportunities. ACS Omega 2021, 6, 19343–19355. [Google Scholar] [CrossRef]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A detailed review study on potential effects of microplastics and additives of concern on human health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef]
- Huang, D.; Tao, J.; Cheng, M.; Deng, R.; Chen, S.; Yin, L.; Li, R. Microplastics and nanoplastics in the environment: Macroscopic transport and effects on creatures. J. Hazard. Mat. 2021, 407, 124399. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.E.; Hamann, M.; Kroon, F.J. Bioaccumulation and biomagnification of microplastics in marine organisms: A review and meta-analysis of current data. PLoS ONE 2020, 15, e0240792. [Google Scholar] [CrossRef] [PubMed]
- Koelmans, A.A.; Redondo-Hasselerharm, P.E.; Nor, N.H.M.; de Ruijter, V.N.; Mintenig, S.M.; Kooi, M. Risk assessment of microplastic particles. Nat. Rev. Mat. 2022, 7, 138–152. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mat. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Giaganini, G.; Cifelli, M.; Biagini, D.; Ghimenti, S.; Corti, A.; Castelvetro, V.; Domenici, V.; Lomonaco, T. Multi-analytical approach to characterize the degradation of different types of microplastics: Identification and quantification of released organic compounds. Molecules 2023, 28, 1382. [Google Scholar] [CrossRef]
- Godoy, V.; Blázquez, G.; Calero, M.; Quesada, L.; Martín-Lara, M.A. The potential of microplastics as carriers of metals. Environ. Pollut. 2019, 255, 113363. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Zhu, X.; Xu, H.; Chen, F.; Ma, J.; Pan, K. Copper adsorption to microplastics and natural particles in seawater: A comparison of kinetics, isotherms, and bioavailability. Environ. Sci. Technol. 2021, 55, 13923–13931. [Google Scholar]
- Monikh, F.A.; Hansen, S.F.; Vijver, M.G.; Kentin, E.; Nielsen, M.B.; Baun, A.; Syberg, K.; Lynch, I.; Valsami-Jones, E.; Peijnenburg, W.J.G.M. Can current regulations account for intentionally produced nanoplastics? Environ. Sci. Technol. 2022, 56, 3836–3839. [Google Scholar] [CrossRef] [PubMed]
- Rosenboom, J.G.; Langer, R.; Traverso, G. Bioplatics for a circular economy. Nat. Rev. Mat. 2022, 7, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Censi, V.; Saiano, F.; Bongiorno, D.; Indelicato, S.; Napoli, A.; Piazzese, D. Bioplastics: A new analytical challenge. Front. Chem. 2022, 10, 971792. [Google Scholar] [CrossRef] [PubMed]
- Ivleva, N.P. Chemical analysis of microplastics and nanoplastics: Challenges, advanced methods, and perspectives. Chem. Rev. 2021, 121, 11886–11936. [Google Scholar] [CrossRef]
- Huppertsberg, S.; Knepper, T.P. Instrumental analysis of microplastic–sbenefits and challenges. Anal. Bioanal. Chem. 2018, 410, 6343–6352. [Google Scholar] [CrossRef]
- Silva, A.B.; Bastos, A.S.; Justino, C.I.L.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T.A.P. Microplastics in the environment: Challenges in analytical chemistry–A review. Anal. Chim. Acta 2018, 1017, 1–19. [Google Scholar] [CrossRef]
- Mai, L.; Bao, L.-J.; Shi, L.; Wong, C.S.; Zeng, E.Y. A review of methods for measuring microplastics in aquatic environments. Environ. Sci. Pollut. Res. 2018, 25, 11319–11332. [Google Scholar] [CrossRef]
- Zarfl, C. Promising techniques and open challenges for microplastic identification and quantification in environmental matrices. Anal Bioanal. Chem. 2019, 411, 3743–3756. [Google Scholar] [CrossRef]
- Miller, E.; Sedlak, M.; Lin, D.; Box, C.; Holleman, C.; Rochman, C.M.; Sutton, R. Recommended best practices for collecting, analyzing, and reporting microplastics in environmental media: Lessons learned from comprehensive monitoring of San Francisco Bay. J. Hazard Mat. 2021, 409, 124770. [Google Scholar] [CrossRef]
- Kuznetsova, O.V.; Timerbaev, A.R. Marine sediment analysis–A review of advanced approaches and practices focused on contaminants. Anal. Chim. Acta 2022, 1209, 339640. [Google Scholar] [CrossRef]
- Pipkin, W.; Belganeh, R.; Robberson, W.; Allen, H.L.; Cook, A.-M.; Watanabe, A. Identification of microplastics in environmental monitoring using pyrolysis–GC–MS analysis. LCGC Eur. 2021, 34, 308–314. [Google Scholar]
- Fries, E.; Dekiff, J.H.; Willmeyer, J.M.; Nuelle, T.; Ebert, M.; Remy, D. Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy. Environ. Sci. Proc. Impacts 2013, 15, 1949–1956. [Google Scholar] [CrossRef]
- Faure, F.; Demars, C.; Wieser, O.; Kunz, M.; Felippe de Alencastro, L. Plastic pollution in Swiss surface waters: Nature and concentrations, interaction with pollutants. Environ. Chem. 2015, 12, 582–591. [Google Scholar] [CrossRef]
- Cai, H.; Xu, E.G.; Du, F.; Li, R.; Kiu, J.; Shi, H. Analysis of environmental nanoplastics: Progress and challenges. Chem. Eng. J. 2021, 410, 128208. [Google Scholar] [CrossRef]
- Sanchís, J.; Jimenez-Lamana, J.; Abad, E.; Szpunar, J.; Farré, M. Occurrence of cerium-, titanium-, and silver-bearing nanoparticles in the Besòs and Ebro rivers. Environ. Sci. Technol. 2020, 54, 3969–3978. [Google Scholar] [CrossRef] [PubMed]
- de Vega, R.G.; Lockwood, T.E.; Xu, X.; Gonzalez de Vega, C.; Scholz, J.; Horstmann, M.; Doble, P.A.; Clases, D. Analysis of Ti- and Pb-based particles in the aqueous environment of Melbourne (Australia) via single particle ICP-MS. Anal. Bioanal. Chem. 2022, 414, 5671–5681. [Google Scholar] [CrossRef]
- Berto, D.; Rampazzo, F.; Gion, C.; Noventa, S.; Ronchi, F.; Traldi, U.; Giorgi, G.; Cicero, A.M.; Giovanardi, O. Preliminary study to characterize plastic polymers using elemental analyser/isotope ratio mass spectrometry (EA/IRMS). Chemosphere 2017, 176, 47–56. [Google Scholar] [CrossRef]
- Kuznetsova, O.V.; Keppler, B.K.; Timerbaev, A.R. Analysis of engineered nanoparticles in seawater using ICP-MS-based technology: From negative to positive samples. Molecules 2023, 28, 994. [Google Scholar] [CrossRef] [PubMed]
- Birch, Q.T.; Potter, P.M.; Pinto, P.X.; Dionysiou, D.D.; Al-Abed, S.R. Isotope ratio mass spectrometry and spectroscopic techniques for microplastics characterization. Talanta 2021, 224, 121743. [Google Scholar] [CrossRef]
- Jones, K.; Koens, F.; Simpson, T. Background survey of polyethylene in the Australian Capital Territory–A demonstration of variability in isotopic abundance values and their application to forensic casework. Sci. Justice 2018, 58, 276–281. [Google Scholar] [CrossRef]
- Lai, Y.; Dong, L.; Sheng, X.; Li, Q.; Li, P.; Hao, Z.; Yu, S.; Liu, J. Swelling-Induced Fragmentation and Polymer Leakage of Nanoplastics in Seawater. Environ. Sci. Technol. 2022, 56, 17694–17701. [Google Scholar] [CrossRef] [PubMed]
- McWilliams, M.; Liboiron, M.; Wiersma, Y. Rocky shoreline protocols miss microplastics in marine debris surveys (Fogo Island, Newfoundland and Labrador). Marine Poll. Bull. 2017, 129, 480–486. [Google Scholar] [CrossRef]
- Napper, I.E.; Thompson, R.C. Environmental deterioration of biodegradable, oxo-biodegradable, compostable, and conventional plastic carrier bags in the sea, soil, and open-air over a 3-year period. Environ. Sci. Technol. 2019, 53, 4775–4783. [Google Scholar] [CrossRef] [PubMed]
- Walsh, A.N.; Mazzotta, M.G.; Nelson, T.F.; Reddy, C.M.; Ward, C.P. Synergy between sunlight, titanium dioxide, and microbes enhances cellulose diacetate degradation in the ocean. Environ. Sci. Technol. 2022, 56, 13810–13819. [Google Scholar] [CrossRef] [PubMed]
- Gunaalan, K.; Fabbri, E.; Capolupo, M. The hidden threat of plastic leachates: A critical review on their impacts on aquatic organisms. Water Res. 2020, 184, 116170. [Google Scholar] [CrossRef]
- Suzuki, Y.; Akamatsu, F.; Nakashita, R.; Korenaga, T.A. Novel method to discriminate between plant- and petroleum-derived plastics by stable carbon isotope analysis. Chem. Lett. 2010, 39, 998–999. [Google Scholar] [CrossRef]
- Pittroff, M.; Müller, Y.K.; Witzig, C.S.; Scheurer, M.; Storck, F.R.; Zumbülte, N. Microplastic analysis in drinking water based on fractionated filtration sampling and Raman microspectroscopy. Environ. Sci. Pollut. Res. 2021, 28, 59439–59451. [Google Scholar] [CrossRef]
- Ourgaud, M.; Phuong, N.N.; Papillon, L.; Panagiotopoulos, C.; Galgani, F.; Schmidt, N.; Fauvelle, V.; Brach-Papa, C.; Sempéré, R. Identification and quantification of microplastics in the marine environment using the laser direct infrared (LDIR) technique. Environ. Sci. Technol. 2022, 56, 9999–10009. [Google Scholar] [CrossRef]
| Sample | δ13C (‰) | Assigned Composition a | Respective δ13C (‰) |
|---|---|---|---|
| N1 | −26.91 ± 0.51 | PP | −28.0 ± 1.5 [27] |
| N2 | −28.27 ± 0.53 | PE or PP | −28.0 ± 1.5 [27] |
| N3 | −27.58 ± 0.62 | PP | −28.0 ± 1.5 [27] |
| N4 | −28.48 ± 0.50 | PVC | −28.5 ± 1.5 [29] |
| N5 | −28.80 ± 0.91 | PE | −29.3 ± 1.1 [27] |
| N6 | −28.89 ± 0.77 | PE | −29.3 ± 1.1 [27] |
| N7 | −38.79 ± 1.27 | PTFE | −40.2 ± 1.2 [29] |
| N8 | −27.51 ± 0.56 | PP | −28.0 ± 1.5 [27] |
| N9 | −27.06 ± 0.77 | PP | −28.0 ± 1.5 [27] |
| N10 | −29.07 ± 1.11 | PE | −29.3 ± 1.1 [27] |
| N11 | −26.96 ± 1.14 | PP | −28.0 ± 1.5 [27] |
| Sample | δ13C (‰) | Mass (g) | Mass Loss (%) | ||
|---|---|---|---|---|---|
| Initial | After 3 Months | Initial | After 3 Months | ||
| B1 | −22.42 ± 0.42 | −21.04 ± 0.59 | 0.39343 | 0.39187 | 0.40 |
| B2 | −26.07 ± 0.71 | −24.44 ± 1.13 | 0.41561 | 0.41322 | 0.58 |
| B3 | −30.51 ± 0.44 | −29.78 ± 0.41 | 0.68275 | 0.68251 | 0.04 |
| B4 | −28.92 ± 0.36 | −27.61 ± 0.77 | 0.23454 | 0.23411 | 0.18 |
| B5 | −15.43 ± 0.47 | −14.61 ± 0.53 | 1.23363 | 1.23140 | 0.18 |
| B6 | −25.62 ± 1.01 | −24.38 ± 0.61 | 0.46944 | 0.46825 | 0.25 |
| Sample | Cd | Co | Cu | Cr | Fe | Mn | Mo | Ni | Pb | Ti | V | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N1 | 0.10 ± 0.01 | 5.4 ± 0.2 | 22 ± 1 | 38 ± 3 | 91 ± 8 | 6.0 ± 0.5 | 0.80 ± 0.03 | 9.7 ± 0.5 | 3.6 ± 0.3 | 0.6 ± 0.03 | 0.30 ± 0.01 | 14 ± 1 |
| N2 | 0.10 ± 0.01 | 3.3 ± 0.2 | 14 ± 1. | 5.2 ± 0.5 | 77 ± 6 | 6.2 ± 0.5 | 0.30 ± 0.02 | 5.5 ± 03 | 1.4 ± 0.1 | 0.8 ± 0.04 | 0.20 ± 0.01 | 18 ± 1 |
| N3 | 0.02 ± 0.001 | 2.9 ± 0.2 | 11 ± 1 | 4.6 ± 0.4 | 89 ± 8 | 5.1 ± 0.4 | 0.30 ± 0.01 | 3.7 ± 0.2 | 1.6 ± 0.2 | 0.3 ± 0.01 | 0.10 ± 0.01 | 14 ± 1 |
| N4 | 0.70 ± 0.02 | 1.3 ± 0.1 | 6.8 ± 0.3 | 5.3 ± 0.5 | 92 ± 9 | 4.9 ± 0.4 | 0.20 ± 0.02 | 3.5 ± 0.2 | 1.4 ± 0.1 | 0.25 ± 0.03 | 0.10 ± 0.01 | 15 ± 1 |
| N5 | 0.03 ± 0.002 | 0.20 ± 0.01 | 2.4 ± 0.2 | 44 ± 3 | 79 ± 8 | 8.1 ± 0.6 | 0.30 ± 0.02 | 5.5 ± 0.4 | 2.4 ± 0.1 | 66 ± 5 | 0.20 ± 0.01 | 17 ± 2 |
| N6 | 0.02 ± 0.001 | 1.1 ± 0.05 | 5.5 ± 0.4 | 4.9 ± 0.5 | 90 ± 6 | 6.2 ± 0.5 | 0.20 ± 0.01 | 4.5 ± 0.3 | 0.50 ± 0.03 | 0.6 ± 0.04 | 0.20 ± 0.01 | 15 ± 1 |
| N7 | 0.10 ± 0.005 | 1.3 ± 0.1 | 4.6 ± 0.3 | 5.1 ± 0.4 | 102 ± 9 | 56 ± 5 | 1.6 ± 0.1 | 5.2 ± 0.4 | 5.9 ± 0.4 | 3.2 ± 0.2 | 0.50 ± 0.04 | 16 ± 1 |
| N8 | 0.10 ± 0.003 | 1.1 ± 0.05 | 6.2 ± 0.4 | 11 ± 1 | 98 ± 8 | 2.4 ± 0.2 | 0.40 ± 0.03 | 6.6 ± 0.5 | 0.30 ± 0.02 | 0.2 ± 0.01 | 0.10 ± 0.01 | 19 ± 1 |
| N9 | 0.10 ± 0.004 | 2.2 ± 0.2 | 47 ± 4 | 6.1 ± 0.4 | 105 ± 9 | 9.1 ± 0.8 | 0.40 ± 0.02 | 88 ± 8 | 1.2 ± 0.1 | 8.2 ± 0.6 | 0.90 ± 0.02 | 22 ± 1 |
| N10 | 3.2 ± 0.3 | 2.6 ± 0.1 | 77 ± 6 | 13 ± 1 | 126 ± 8 | 55 ± 4 | 1.3 ± 0.05 | 27 ± 2 | 68 ± 5 | 10 ± 1 | 4.3 ± 0.3 | 18 ± 1 |
| N11 | 0.20 ± 0.01 | 5.3 ± 0.2 | 90 ± 8 | 8.0 ± 0.6 | 99 ± 8 | 8.2 ± 0.7 | 0.80 ± 0.03 | 13 ± 1 | 8.7 ± 0.7 | 1.2 ± 0.1 | 0.30 ± 0.02 | 24 ± 1 |
| Sample | Time a | Cd | Co | Cu | Cr | Fe | Mn | Mo | Ni | Pb | Ti | V | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B1 | 0 | 0.03 ± 0.001 | 0.10 ± 0.01 | 1.7 ± 0.1 | 2.0 ± 0.1 | 11 ± 0.2 | 1.0 ± 0.02 | 0.10 ± 0.01 | 1.3 ± 0.1 | 0.60 ± 0.03 | 0.20 ± 0.01 | 0.10 ± 0.01 | 3.1 ± 0.3 |
| 6 h | 0.40 ± 0.03 | 1.0 ± 0.1 | 27 ± 4 | 22 ± 0.5 | 2860 ± 20 | 397 ± 25 | 1.3 ± 0.03 | 12 ± 0.5 | 13 ± 0.1 | 3300 ± 230 | 7.6 ± 0.3 | 93 ± 7 | |
| 3 m | 0.50 ± 0.03 | 5.1 ± 0.4 | 39 ± 3 | 26 ± 2 | 3390 ± 240 | 510 ± 42 | 3.4 ± 0.4 | 49 ± 4 | 28 ± 2 | 4400 ± 270 | 15 ± 1 | 161 ± 13 | |
| B2 | 0 | 2.2 ± 0.2 | 3.3 ± 0.2 | 57 ± 4 | 30 ± 2 | 144 ± 9 | 59 ± 5 | 0.30 ± 0.02 | 7.0 ± 0.1 | 60 ± 5 | 0.9 ± 0.1 | 0.4 ± 0.02 | 19 ± 2 |
| 6 h | 25 ± 0.6 | 26 ± 3 | 841 ± 32 | 757 ± 9 | 8330 ± 2100 | 1800 ± 140 | 1.7 ± 0.1 | 43 ± 21 | 600 ± 27 | 117 ± 3 | 13 ± 3 | 696 ± 24 | |
| 3 m | 28 ± 2 | 42 ± 3 | 1330 ± 110 | 1500 ± 110 | 8890 ± 770 | 2640 ± 200 | 3.6 ± 3 | 78 ± 6 | 624 ± 70 | 157 ± 13 | 19 ± 2 | 862 ± 66 | |
| B3 | 0 | 0.03 ± 0.001 | 0.10 ± 0.01 | 4.7 ± 0.3 | 1.2 ± 0.05 | 14 ± 1 | 5.1 ± 0.2 | 0.10 ± 0.01 | 2.1 ± 0.2 | 1.4 ± 0.1 | 3.2 ± 0.3 | 0.10 ± 0.01 | 10 ± 0.5 |
| 6 h | 1.2 ± 0.1 | 6.1 ± 0.4 | 97 ± 2 | 13 ± 0.5 | 755 ± 41 | 112 ± 12 | 1.1 ± 0.1 | 27 ± 1 | 16 ± 0.2 | 83 ± 2 | 2.3 ± 0.1 | 14 ± 1 | |
| 3 3 m | 1.3 ± 0.1 | 14 ± 1 | 184 ± 14 | 19 ± 1 | 814 ± 59 | 185 ± 14 | 4.6 ± 0.3 | 57 ± 5 | 40 ± 3 | 116 ± 9 | 3.8 ± 0.3 | 16 ± 1 | |
| B4 | 0 | 0.02 ± 0.001 | 0.20 ± 0.01 | 4.1 ± 0.4 | 1.1 ± 0.05 | 18 ± 2 | 3.0 ± 0.2 | 0.10 ± 0.01 | 4.6 ± 0.4 | 5.0 ± 0.3 | 1.1 ± 0.1 | 0.10 ± 0.01 | 1.0 ± 0.05 |
| 6 h | 1.2 ± 0.1 | 6.4 ± 0.2 | 432 ± 6 | 54 ± 4 | 2720 ± 250 | 56 ± 4 | 2.5 ± 0.1 | 61 ± 3 | 168 ± 7 | 13 ± 0.2 | 3.5 ± 0.6 | 2.5 ± 0.2 | |
| 3 m | 1.3 ± 0.1 | 9.5 ± 0.8 | 576 ± 47 | 103 ± 9 | 4320 ± 260 | 150 ± 11 | 6.6 ± 0.5 | 94 ± 8 | 196 ± 17 | 21 ± 1 | 7.3 ± 0.5 | 11 ± 1 | |
| P1 | 0 | 0.01 ± 0.001 | 0.1 ± 0.01 | 3.1 ± 0.2 | 1.3 ± 0.1 | 22 ± 2 | 2.0 ± 0.2 | 0.01 ± 0.001 | 2.4 ± 0.1 | 6.0 ± 0.4 | 0.30 ± 0.02 | 0.10 ± 0.01 | 3.0 ± 0.2 |
| 6 h | 0.40 ± 0.05 | 0.9 ± 0.1 | 71 ± 1 | 21 ± 2 | 530 ± 61 | 25 ± 2 | 0.20 ± 002 | 45 ± 3 | 116 ± 2.3 | 5.1 ± 0.6 | 2.0 ± 0.2 | 179 ± 2 | |
| 3 m | 0.50 ± 0.03 | 1.8 ± 0.2 | 117 ± 12 | 47 ± 4 | 772 ± 62 | 29 ± 2 | 0.30 ± 0.02 | 74 ± 5 | 139 ± 10 | 11 ± 1 | 3.3 ± 0.2 | 190 ± 14 | |
| P2 | 0 | 0.01 ± 0.001 | 0.01 ± 0.001 | 4.0 ± 0.3 | 0.30 ± 0.02 | 23 ± 2 | 2.0 ± 0.2 | 0.10 ± 0.01 | 3.4 ± 0.2 | 1.6 ± 0.1 | 0.20 ± 0.02 | 0.10 ± 0.01 | 6.0 ± 0.4 |
| 6 h | 0.30 ± 0.02 | 0.80 ± 0.10 | 49 ± 1 | 8.6 ± 0.5 | 295 ± 25 | 38 ± 3 | 2.0 ± 0.3 | 37 ± 2 | 42 ± 3 | 2.0 ± 0.1 | 1.7 ± 0.1 | 188 ± 4 | |
| 3 m | 0.35 ± 0.03 | 1.2 ± 0.1 | 55 ± 4 | 9.7 ± 0.9 | 411 ± 37 | 50 ± 4 | 2.6 ± 0.2 | 44 ± 3 | 61 ± 5 | 2.7 ± 0.2 | 2.1 ± 0.1 | 203 ± 16 | |
| P3 | 0 | 0.01 ± 0.001 | 0.01 ± 0.001 | 2.0 ± 0.2 | 0.20 ± 0.02 | 13 ± 1 | 1.0 ± 0.1 | 0.02 ± 0.001 | 2.0 ± 0.2 | 3.0 ± 0.2 | 0.05 ± 0.003 | 0.05 ± 0.001 | 11 ± 0.4 |
| 6 h | 0.30 ± 0.02 | 0.70 ± 0.10 | 58 ± 2 | 8.3 ± 0.3 | 225 ± 19 | 36 ± 4 | 2.0 ± 0.1 | 41 ± 2 | 40 ± 1 | 2.5 ± 0.1 | 1.8 ± 0.1 | 156 ± 4 | |
| 3 m | 0.35 ± 0.03 | 0.90 ± 0.04 | 66 ± 5 | 11 ± 1 | 330 ± 24 | 44 ± 3 | 2.6 ± 0.2 | 55 ± 4 | 67 ± 6 | 3.1 ± 0.2 | 2.2 ± 0.2 | 199 ± 18 | |
| P4 | 0 | 0.01 ± 0.001 | 0.20 ± 0.01 | 4.5 ± 0.3 | 2.3 ± 0.2 | 20 ± 2 | 1.4 ± 0.1 | 0.01 ± 0.001 | 1.4 ± 0.1 | 4.0 ± 0.3 | 0.10 ± 0.01 | 0.10 ± 0.01 | 5.0 ± 0.5 |
| 6 h | 0.30 ± 0.03 | 3.2 ± 0.4 | 58 ± 2 | 19 ± 1 | 231 ± 23 | 26 ± 3 | 1.3 ± 0.06 | 34 ± 1 | 37 ± 1 | 2.0 ± 0.02 | 1.2 ± 0.05 | 567 ± 7 | |
| 3 m | 0.40 ± 0.01 | 6.8 ± 0.4 | 69 ± 5 | 22 ± 1 | 329 ± 22 | 38 ± 2 | 1.8 ± 0.1 | 44 ± 3 | 52 ± 4 | 2.7 ± 0.2 | 2.5 ± 0.2 | 830 ± 65 | |
| P5 | 0 | 0.01 ± 0.001 | 0.01 ± 0.001 | 1.5 ± 0.1 | 0.20 ± 0.02 | 10 ± 1 | 1.1 ± 0.1 | 0.01 ± 0.001 | 1.1 ± 0.02 | 3.0 ± 0.1 | 0.05 ± 0.003 | 0.05 ± 0.001 | 3.0 ± 0.5 |
| 6 h | 0.10 ± 0.02 | 0.50 ± 0.10 | 15.0 ± 0.7 | 7.3 ± 0.2 | 109 ± 12 | 9.1 ± 0.8 | 0.70 ± 0.05 | 14 ± 1 | 128 ± 7 | 0.70 ± 0.02 | 0.60 ± 0.02 | 57 ± 2 | |
| 3 m | 0.20 ± 0.02 | 1.0 ± 0.1 | 24 ± 2 | 14 ± 1 | 124 ± 10 | 10 ± 1 | 1.3 ± 0.1 | 23 ± 1 | 220 ± 19 | 1.1 ± 0.05 | 1.8 ± 0.1 | 92 ± 8 | |
| P6 | 0 | 0.02 ± 0.001 | 0.10 ± 0.01 | 5 ± 0.3 | 0.20 ± 0.02 | 13 ± 1 | 1.2 ± 0.1 | 0.10 ± 0.01 | 2.0 ± 0.2 | 4.0 ± 0.2 | 0.50 ± 0.03 | 0.05 ± 0.001 | 9 ± 1 |
| 6 h | 0.60 ± 0.01 | 4.4 ± 0.6 | 106 ± 9 | 22 ± 1 | 612 ± 47 | 37 ± 2 | 2.7 ± 0.1 | 69 ± 7 | 25 ± 1 | 4.2 ± 0.6 | 1.7 ± 0.01 | 478 ± 21 | |
| 3 m | 0.70 ± 0.03 | 11 ± 0.5 | 204 ± 15 | 28 ± 2 | 887 ± 66 | 58 ± 5 | 4.1 ± 0.4 | 102 ± 8 | 41 ± 3 | 8.1 ± 0.4 | 3.0 ± 0.2 | 1050 ± 90 | |
| P7 | 0 | 0.10 ± 0.01 | 0.30 ± 0.02 | 5.7 ± 0.4 | 3.0 ± 0.1 | 14 ± 1 | 5.6 ± 0.5 | 0.10 ± 0.01 | 3.0 ± 0.2 | 6.0 ± 0.2 | 0.30 ± 0.02 | 0.2 ± 0.02 | 13 ± 1 |
| 6 h | 0.50 ± 0.10 | 3.0 ± 0.2 | 171 ± 6 | 32 ± 2 | 728 ± 55 | 50 ± 4 | 2.7 ± 0.1 | 63 ± 3 | 45 ± 2 | 3.7 ± 0.2 | 2.7 ± 0.1 | 223 ± 4 | |
| 3 m | 0.60 ± 0.1 | 4.8 ± 0.3 | 333 ± 24 | 75 ± 6 | 1170 ± 100 | 77 ± 6 | 5.6 ± 0.4 | 96 ± 7 | 72 ± 5 | 4.9 ± 0.3 | 3.8 ± 0.2 | 280 ± 27 | |
| B5 | 0 | 0.02 ± 0.001 | 0.01 ± 0.001 | 0.30 ± 0.03 | 24 ± 2 | 11 ± 1 | 0.02 ± 0.001 | 0.01 ± 0.001 | 0.05 ± 0.002 | 48 ± 2 | 0.50 ± 0.04 | 0.05 ± 0.001 | 39 ± 3 |
| 6 h | 0.30 ± 0.03 | 0.10 ± 0.001 | 7.8 ± 0.6 | 972 ± 83 | 155 ± 6 | 0.50 ± 0.02 | 0.10 ± 0.02 | 1.0 ± 0.03 | 8550 ± 920 | 13 ± 0.4 | 0.60 ± 0.10 | 1820 ± 40 | |
| 3 m | 0.40 ± 0.01 | 0.11 ± 0.01 | 11 ± 1 | 1130 ± 110 | 189 ± 14 | 0.60 ± 0.03 | 0.11 ± 0.01 | 1.3 ± 0.1 | 9540 ± 810 | 14 ± 1 | 0.70 ± 0.05 | 2010 ± 180 | |
| P8 | 0 | 0.30 ± 0.02 | 0.20 ± 0.02 | 0.7 ± 0.04 | 3.0 ± 0.2 | 112 ± 10 | 28 ± 2 | 0.30 ± 0.03 | 0.80 ± 0.03 | 11 ± 0.3 | 22 ± 1 | 0.40 ± 0.02 | 77 ± 4 |
| 6 h | 8.5 ± 0.3 | 2.4 ± 0.4 | 13.8 ± 0.6 | 82 ± 3 | 6330 ± 980 | 1060 ± 90 | 3.8 ± 0.2 | 33 ± 1 | 249 ± 9 | 343 ± 10 | 10 ± 0.4 | 20,600 ± 1500 | |
| 3 m | 9.2 ± 0.6 | 2.9 ± 0.2 | 16.1 ± 1.5 | 99 ± 8 | 7020 ± 520 | 1130 ± 100 | 5.1 ± 0.3 | 44 ± 3 | 297 ± 22 | 411 ± 25 | 13 ± 0.5 | 23,600 ± 2000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuznetsova, O.V.; Shtykov, S.N.; Timerbaev, A.R. Mass Spectrometry Insight for Assessing the Destiny of Plastics in Seawater. Polymers 2023, 15, 1523. https://doi.org/10.3390/polym15061523
Kuznetsova OV, Shtykov SN, Timerbaev AR. Mass Spectrometry Insight for Assessing the Destiny of Plastics in Seawater. Polymers. 2023; 15(6):1523. https://doi.org/10.3390/polym15061523
Chicago/Turabian StyleKuznetsova, Olga V., Sergey N. Shtykov, and Andrei R. Timerbaev. 2023. "Mass Spectrometry Insight for Assessing the Destiny of Plastics in Seawater" Polymers 15, no. 6: 1523. https://doi.org/10.3390/polym15061523
APA StyleKuznetsova, O. V., Shtykov, S. N., & Timerbaev, A. R. (2023). Mass Spectrometry Insight for Assessing the Destiny of Plastics in Seawater. Polymers, 15(6), 1523. https://doi.org/10.3390/polym15061523






