Improvement of Selectivity of RALEX-CM Membranes via Modification by Ceria with a Functionalized Surface
Abstract
1. Introduction
2. Materials and Methods
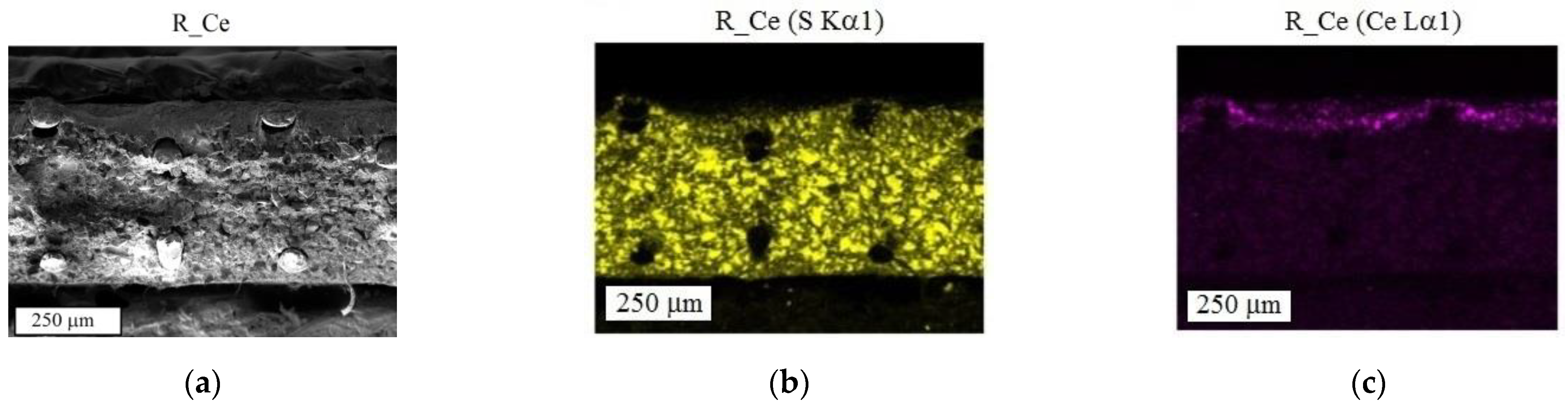
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strathmann, H.; Grabowski, A.; Eigenberger, G. Ion-Exchange Membranes in the Chemical Process Industry. Ind. Eng. Chem. Res. 2013, 52, 10364–10379. [Google Scholar] [CrossRef]
- Ran, J.; Wu, L.; He, Y.; Yang, Z.; Wang, Y.; Jiang, C.; Ge, L.; Bakangura, E.; Xu, T. Ion exchange membranes: New developments and applications. J. Membr. Sci. 2017, 522, 267–291. [Google Scholar] [CrossRef]
- Hagesteijn, K.F.L.; Jiang, S.; Ladewig, B.P. A review of the synthesis and characterization of anion exchange membranes. J. Mater. Sci. 2018, 53, 11131–11150. [Google Scholar] [CrossRef]
- Al-Amshawee, S.; Yunus, M.Y.B.M.; Azoddein, A.A.M.; Hassell, D.G.; Dakhil, I.H.; Hasan, H.A. Electrodialysis desalination for water and wastewater: A review. Chem. Eng. J. 2020, 380, 122231. [Google Scholar] [CrossRef]
- Gurreri, L.; Tamburini, A.; Cipollina, A.; Micale, G. Electrodialysis Applications in Wastewater Treatment for Environmental Protection and Resources Recovery: A Systematic Review. Prog. Perspect. Membr. 2020, 10, 146. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Huang, C. Electrodialysis-based separation technologies: A critical review. AIChE J. 2008, 54, 3147–3159. [Google Scholar] [CrossRef]
- Chandra, A.; Bhuvanes, E.; Chattopadhyay, S. A critical analysis on ion transport of organic acid mixture through an anion-exchange membrane during electrodialysis. Chem. Eng. Res. Des. 2022, 178, 13–24. [Google Scholar] [CrossRef]
- Cao, G.; Alam, M.M.; Juthi, A.Z.; Zhang, Z.; Wang, Y.; Jiang, C.; Xu, T. Electro-desalination: State-of-the-art and prospective. Adv. Membr. 2023, 3, 100058. [Google Scholar] [CrossRef]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356, 6343. [Google Scholar] [CrossRef]
- Golubenko, D.V.; Pourcelly, G.; Yaroslavtsev, A.B. Permselectivity and ion-conductivity of grafted cation-exchange membranes based on UV-oxidized polymethylpenten and sulfonated polystyrene. Separ. Purif. Technol. 2018, 207, 329–335. [Google Scholar] [CrossRef]
- Campione, A.; Gurreri, L.; Ciofalo, M.; Micale, G.; Tamburini, A.; Cipollina, A. Electrodialysis for water desalination: A critical assessment of recent developments on process fundamentals, models and applications. Desalination 2018, 434, 121–160. [Google Scholar] [CrossRef]
- Biesheuvel, P.M.; Porada, S.; Elimelech, M.; Dykstra, J.E. Tutorial review of reverse osmosis and electrodialysis. J. Membr. Sci. 2022, 647, 120221. [Google Scholar] [CrossRef]
- Nikonenko, V.; Nebavsky, A.; Mareev, S.; Kovalenko, A.; Urtenov, M.; Pourcelly, G. Modelling of ion transport in electromembrane systems: Impacts of membrane bulk and surface heterogeneity. Appl. Sci. 2019, 9, 25. [Google Scholar] [CrossRef]
- Fan, H.; Yip, N.Y. Elucidating conductivity-permselectivity tradeoffs in electrodialysis and reverse electrodialysis by structure-property analysis of ion-exchange membranes. J. Membr. Sci. 2019, 573, 668–681. [Google Scholar] [CrossRef]
- Huang, Y.; Fan, H.; Yip, N.Y. Influence of electrolyte on concentration-induced conductivity-permselectivity tradeoff of ion-exchange membranes. J. Membr. Sci. 2023, 668, 121184. [Google Scholar] [CrossRef]
- Kononenko, N.; Nikonenko, V.; Grande, D.; Larchet, C.; Dammak, L.; Fomenko, M.; Volfkovich, Y. Porous structure of ion exchange membranes investigated by various techniques. Adv. Colloid Interface Sci. 2017, 246, 196–216. [Google Scholar] [CrossRef] [PubMed]
- Zyryanova, S.; Mareev, S.; Gil, V.; Korzhova, E.; Pismenskaya, N.; Sarapulova, V.; Rybalkina, O.; Boyko, E.; Larchet, C.; Dammak, L.; et al. How Electrical Heterogeneity Parameters of Ion-Exchange Membrane Surface Affect the Mass Transfer and Water Splitting Rate in Electrodialysis. Int. J. Mol. Sci. 2020, 21, 973. [Google Scholar] [CrossRef]
- Yaqub, M.; Lee, W. Zero-liquid discharge (ZLD) technology for resource recovery from wastewater: A review. Sci. Total Environ. 2019, 681, 551–563. [Google Scholar] [CrossRef]
- Verma, A.; Johnson, G.H.; Corbin, D.R.; Shiflett, M.B. Separation of Lithium and Cobalt from LiCoO2: A Unique Critical Metals Recovery Process Utilizing Oxalate Chemistry. ACS Sustain. Chem. Eng. 2020, 8, 6100–6108. [Google Scholar] [CrossRef]
- Zante, G.; Masmoudi, A.; Barillon, R.; Trébouet, D.; Boltoeva, M. Separation of lithium, cobalt and nickel from spent lithium-ion batteries using TBP and imidazolium-based ionic liquids. J. Ind. Eng. Chem. 2020, 82, 269–277. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Q.; Wang, Y.; Yun, R.; Xiang, X. Recent advances in magnesium/lithium separation and lithium extraction technologies from salt lake brine. Separ. Purif. Technol. 2021, 256, 117807. [Google Scholar] [CrossRef]
- Siekierka, A. Lithium and magnesium separation from brines by hybrid capacitive deionization. Desalination 2022, 527, 115569. [Google Scholar] [CrossRef]
- Feng, Q.; Wen, S.; Zhao, W.; Chen, Y. Effect of calcium ions on adsorption of sodium oleate onto cassiterite and quartz surfaces and implications for their flotation separation. Separ. Purif.Technol. 2018, 200, 300–306. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Yang, X.; Sun, H.; Wu, Y.; Shao, L. Monovalent Cation Exchange Membranes with Janus Charged Structure for Ion Separation. Engineering, 2022, in press. [CrossRef]
- Wang, W.; Zhang, Y.; Tan, M.; Xue, C.; Zhou, W.; Bao, H.; Lau, C.H.; Yang, X.; Ma, J.; Shao, L. Recent advances in monovalent ion selective membranes towards environmental remediation and energy harvesting. Sep. Purif. Technol. 2022, 297, 121520. [Google Scholar] [CrossRef]
- Ge, L.; Wu, B.; Yu, D.B.; Mondal, A.N.; Hou, L.X.; Ul Afsar, N.; Li, Q.H.; Xu, T.T.; Miao, J.B.; Xu, T.W. Monovalent cation perm-selective membranes (MCPMs): New developments and perspectives. Chin. J. Chem. Eng. 2017, 25, 1606–1615. [Google Scholar] [CrossRef]
- Luo, T.; Abdu, S. ; Wessling, M. Selectivity of ion exchange membranes: A review. J. Membr. Sci. 2018, 555, 429–454. [Google Scholar] [CrossRef]
- Gurreri, L.; Filingeri, A.; Ciofalo, M.; Cipollina, A.; Tedesco, M.; Tamburini, A.; Micale, G. Electrodialysis with asymmetrically profiled membranes: Influence of profiles geometry on desalination performance and limiting current phenomena. Desalination 2021, 506, 115001. [Google Scholar] [CrossRef]
- Roghmans, F.; Evdochenko, E.; Martí-Calatayud, M.C.; Garthe, M.; Tiwari, R.; Walther, A.; Wessling, M. On the permselectivity of cation-exchange membranes bearing an ion selective coating. J. Membr. Sci. 2020, 600, 117854. [Google Scholar] [CrossRef]
- Golubenko, D.V.; Yaroslavtsev, A.B. Effect of current density, concentration of ternary electrolyte and type of cations on the monovalent ion selectivity of surface-sulfonated graft anion-exchange membranes: Modelling and experiment. J. Membr. Sci. 2021, 635, 119466. [Google Scholar] [CrossRef]
- Yaroslavtsev, A.B.; Stenina, I.A.; Voropaeva, E.Y.; Ilyina, A.A. Ion transfer in composite membranes based on MF-4SC incorporating nanoparticles of silica, zirconia, and polyaniline. Polym. Adv. Technol. 2009, 20, 566–570. [Google Scholar] [CrossRef]
- Yurova, P.A.; Malakhova, V.R.; Gerasimova, E.V.; Stenina, I.A.; Yaroslavtsev, A.B. Nafion/surface modified ceria hybrid membranes for proton exchange fuel cell application. Polymers 2021, 13, 2513. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xing, Y.; Li, Y.; Fu, Z.; Li, Z.; Li, H. Double-Layer Expanded Polytetrafluoroethylene Reinforced Membranes with Cerium Oxide Radical Scavengers for Highly Stable Proton Exchange Membrane Fuel Cells. ACS Appl. Energy Mater. 2022, 5, 8743–8755. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, B.; Li, S.; Jin, B.; Yue, Q.; Wang, Z. Cerium oxide doped nanocomposite membranes for reverse osmosis desalination. Chemosphere 2019, 218, 974–983. [Google Scholar] [CrossRef]
- Lakhotia, S.R.; Mukhopadhyay, M.; Kumari, P. Cerium oxide nanoparticles embedded thin-film nanocomposite nanofiltration membrane for water treatment. Sci. Rep. 2018, 8, 4976. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, P.; Tal, A.A.; Skallberg, A.; Brommesson, C.; Hu, Z.; Boyd, R.D.; Olovsson, W.; Fairley, N.; Abrikosov, I.A.; Zhang, X. Cerium oxide nanoparticles with antioxidant capabilities and gadolinium integration for MRI contrast enhancement. Sci. Rep. 2018, 8, 6999. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, C.; Zhai, X.; Luo, F.; Du, Y.; Yan, C. Antibacterial mechanism and activity of cerium oxide nanoparticles. Sci. China Mater. 2019, 62, 1727–1739. [Google Scholar] [CrossRef]
- Dammak, L.; Fouilloux, J.; Bdiri, M.; Larchet, C.; Renard, E.; Baklouti, L.; Sarapulova, V.; Kozmai, A.; Pismenskaya, N. A Review on Ion-Exchange Membrane Fouling during the Electrodialysis Process in the Food Industry, Part 1: Types, Effects, Characterization Methods, Fouling Mechanisms and Interactions. Membranes 2021, 11, 789. [Google Scholar] [CrossRef]
- Apel, P.Y.; Velizarov, S.; Volkov, A.V.; Eliseeva, T.V.; Nikonenko, V.V.; Parshina, A.V.; Pismenskaya, N.D.; Popov, K.I.; Yaroslavtsev, A.B. Fouling and Membrane Degradation in Electromembrane and Baromembrane Processes. Membr. Membr. Technol. 2022, 4, 69–92. [Google Scholar] [CrossRef]
- Kurian, M. Cerium oxide based materials for water treatment—A review. J. Envir. Chem. Eng. 2020, 8, 104439. [Google Scholar] [CrossRef]
- Golubenko, D.V.; Manin, A.D.; Wang, Y.; Xu, T.; Yaroslavtsev, A.B. The way to increase the monovalent ion selectivity of FujiFilm® anion-exchange membranes by cerium phosphate modification for electrodialysis desalination. Desalination 2022, 531, 115719. [Google Scholar] [CrossRef]
- Zaton, M.; Prélot, B.; Donzel, N.; Rozière, J.; Jones, D.J. Migration of Ce and Mn ions in PEMFC and its impact on PFSA membrane degradation. J. Electrochem. Soc. 2018, 165, F3281–F3289. [Google Scholar] [CrossRef]
- Weissbach, T.; Peckham, T.J.; Holdcroft, S. CeO2, ZrO2 and YSZ as mitigating additives against degradation of proton exchange membranes by free radicals. J. Membr. Sci. 2016, 498, 94–104. [Google Scholar] [CrossRef]
- Rui, Z.; Liu, J. Understanding of free radical scavengers used in highly durable proton exchange membranes. Prog. Nat. Sci. Mat. Int. 2020, 30, 732–742. [Google Scholar] [CrossRef]
- Novikova, S.A.; Volodina, E.I.; Pis’menskaya, N.D.; Veresov, A.G.; Stenina, I.A.; Yaroslavtsev, A.B. Ionic Transport in Cation-Exchange Membranes MK-40 Modified with Zirconium Phosphate. Russ. J. Electrochem. 2005, 41, 1070–1076. [Google Scholar] [CrossRef]
- Yurova, P.A.; Stenina, I.A.; Yaroslavtsev, A.B. A Comparative Study of the Transport Properties of Homogeneous and Heterogeneous Cation-Exchange Membranes Doped with Zirconia Modified with Phosphoric Acid Groups. Pet. Chem. 2018, 58, 1144–1153. [Google Scholar] [CrossRef]
- Gough, D.A.; Leypoldt, J.K. Membrane-Covered, Rotated Disc Electrode. Anal. Chem. 1979, 51, 439–444. [Google Scholar] [CrossRef]
- Yurova, P.A.; Tabachkova, N.Y.; Stenina, I.A.; Yaroslavtsev, A.B. Properties of ceria nanoparticles with surface modified by acidic groups. J. Nanopart. Res. 2020, 22, 318. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; John Wiley & Sons Marquette University: New York, NY, USA, 1978. [Google Scholar]
- Babilas, D.; Muszyński, J.; Milewski, A.; Leśniak-Ziółkowska, K.; Dydo, P. Electrodialysis enhanced with disodium EDTA as an innovative method for separating Cu(II) ions from zinc salts in wastewater. Chem. Eng. J. 2021, 408, 127908. [Google Scholar] [CrossRef]
- Stenina, I.A.; Yaroslavtsev, A.B. Ionic Mobility in Ion-Exchange Membranes. Membranes 2021, 11, 198. [Google Scholar] [CrossRef]
- Golubenko, D.V.; Karavanova, Y.A.; Melnikov, S.S.; Achoh, A.R.; Pourcelly, G.; Yaroslavtsev, A.B. An approach to increase the permselectivity and monovalent ion selectivity of cation-exchange membranes by introduction of amorphous zirconium phosphate nanoparticles. J. Membr. Sci. 2018, 563, 777–784. [Google Scholar] [CrossRef]
- Nikonenko, V.V.; Pismenskaya, N.D.; Belova, E.I.; Sistat, P.; Huguet, P.; Pourcelly, G.; Larchet, C. Intensive current transfer in membrane systems: Modelling, mechanisms and application in electrodialysis. Adv. Colloid Interface Sci. 2010, 160, 101–123. [Google Scholar] [CrossRef] [PubMed]
- Pismenskaya, N.D.; Pokhidnia, E.V.; Pourcelly, G.; Nikonenko, V.V. Can the electro-chemical performance of heterogeneous ion-exchange membranes be better than that of homogeneous membranes? J. Membr. Sci. 2018, 566, 54–68. [Google Scholar] [CrossRef]
- Afsar, N.U.; Shehzad, M.A.; Irfan, M.; Emmanuel, K.; Sheng, F.; Xu, T.; Ren, X.; Ge, L.; Xu, T. Cation exchange membrane integrated with cationic and anionic layers for selective ion separation via electrodialysis. Desalination 2019, 458, 25–33. [Google Scholar] [CrossRef]
- Wang, W.; Liu, R.; Tan, M.; Sun, H.; Niu, Q.J.; Xu, T.; Nikonenko, V.; Zhang, Y. Evaluation of the ideal selectivity and the performance of selectrodialysis by using TFC ion exchange membranes. J. Membr. Sci. 2019, 582, 236–245. [Google Scholar] [CrossRef]
- Evdochenko, E.; Kamp, J.; Femmer, R.; Xu, Y.; Nikonenko, V.; Wessling, M. Unraveling the effect of charge distribution in a polyelectrolyte multilayer nanofiltration membrane on its ion transport properties. J. Membr. Sci. 2020, 611, 118045. [Google Scholar] [CrossRef]
- White, N.; Misovich, M.; Alemayehu, E.; Yaroshchuk, A.; Bruening, M.L. Highly selective separations of multivalent and monovalent cations in electrodialysis through Nafion membranes coated with polyelectrolyte multilayers. Polymer 2016, 103, 478–485. [Google Scholar] [CrossRef]
- Malik, M.S.; Qaiser, A.A.; Arif, M.A. Structural and electrochemical studies of heterogeneous ion exchange membranes based on polyaniline-coated cation exchange resin particles. RSC Adv. 2016, 6, 115046–115054. [Google Scholar] [CrossRef]
- Belashova, E.D.; Melnik, N.A.; Pismenskaya, N.D.; Shevtsova, K.A.; Nebavsky, A.V.; Lebedev, K.A.; Nikonenko, V.V. Overlimiting mass transfer through cation-exchange membranes modified by Nafion film and carbon nanotubes. Electrochim. Acta 2012, 59, 412–423. [Google Scholar] [CrossRef]





| Membrane | ω(H2O), ±0.5% | IEC, ±0.05 mmol/g | θc, ±1° | t+, ±0.1% | Limiting Current Density, mA/cm2 |
|---|---|---|---|---|---|
| RALEX-CM | 31.3 | 1.34 | 88 | 93.8 | 11.13 |
| R_Ce | 24.8 | 0.98 | 100 | 93.9 | 11.97 |
| R_Ce_HP | 26.3 | 1.22 | 83 | 94.4 | 13.08 |
| R_Ce_NaP | 26.6 | 1.13 | 81 | 94.7 | 11.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stenina, I.; Yurova, P.; Achoh, A.; Zabolotsky, V.; Wu, L.; Yaroslavtsev, A. Improvement of Selectivity of RALEX-CM Membranes via Modification by Ceria with a Functionalized Surface. Polymers 2023, 15, 647. https://doi.org/10.3390/polym15030647
Stenina I, Yurova P, Achoh A, Zabolotsky V, Wu L, Yaroslavtsev A. Improvement of Selectivity of RALEX-CM Membranes via Modification by Ceria with a Functionalized Surface. Polymers. 2023; 15(3):647. https://doi.org/10.3390/polym15030647
Chicago/Turabian StyleStenina, Irina, Polina Yurova, Aslan Achoh, Victor Zabolotsky, Liang Wu, and Andrey Yaroslavtsev. 2023. "Improvement of Selectivity of RALEX-CM Membranes via Modification by Ceria with a Functionalized Surface" Polymers 15, no. 3: 647. https://doi.org/10.3390/polym15030647
APA StyleStenina, I., Yurova, P., Achoh, A., Zabolotsky, V., Wu, L., & Yaroslavtsev, A. (2023). Improvement of Selectivity of RALEX-CM Membranes via Modification by Ceria with a Functionalized Surface. Polymers, 15(3), 647. https://doi.org/10.3390/polym15030647








