Assessing the Impact and Suitability of Dense Carbon Dioxide as a Green Solvent for the Treatment of PMMA of Historical Value
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
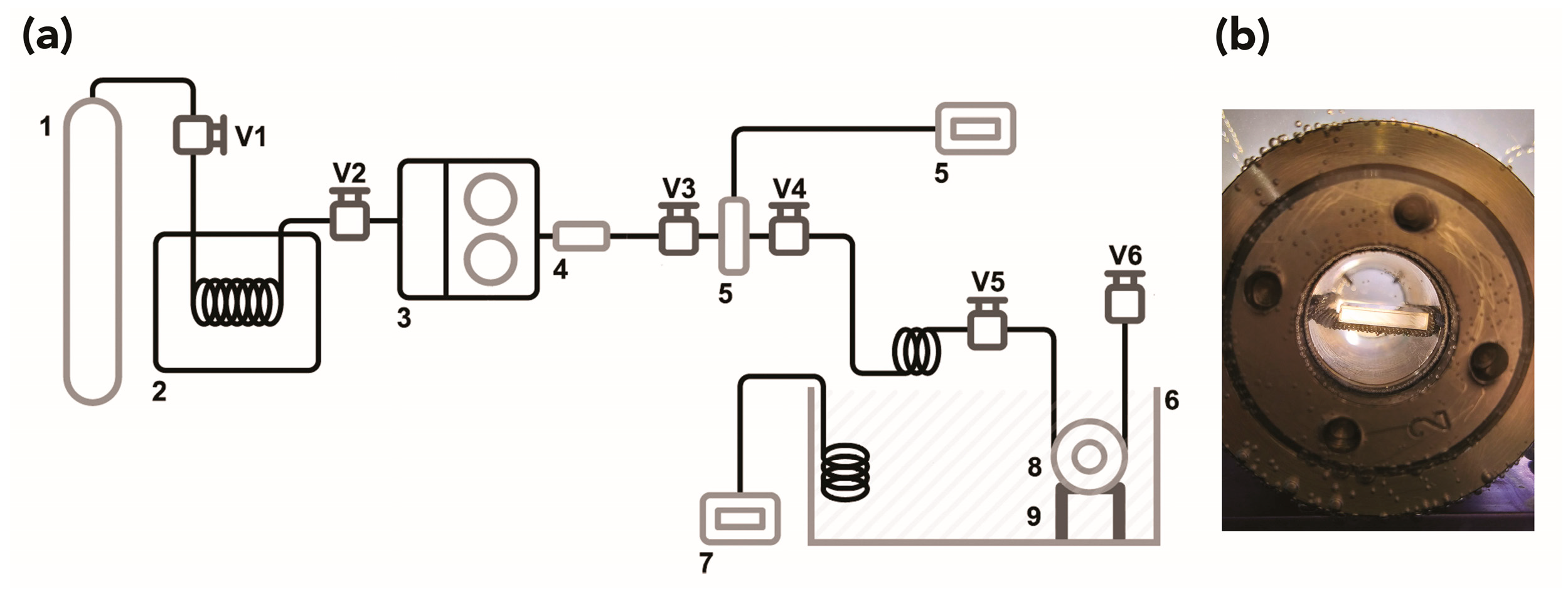
2.2. Description of the CO2 Apparatus and Experimental Conditions
2.3. Sample Characterization
2.3.1. Change in Mass
2.3.2. Change in Dimensions
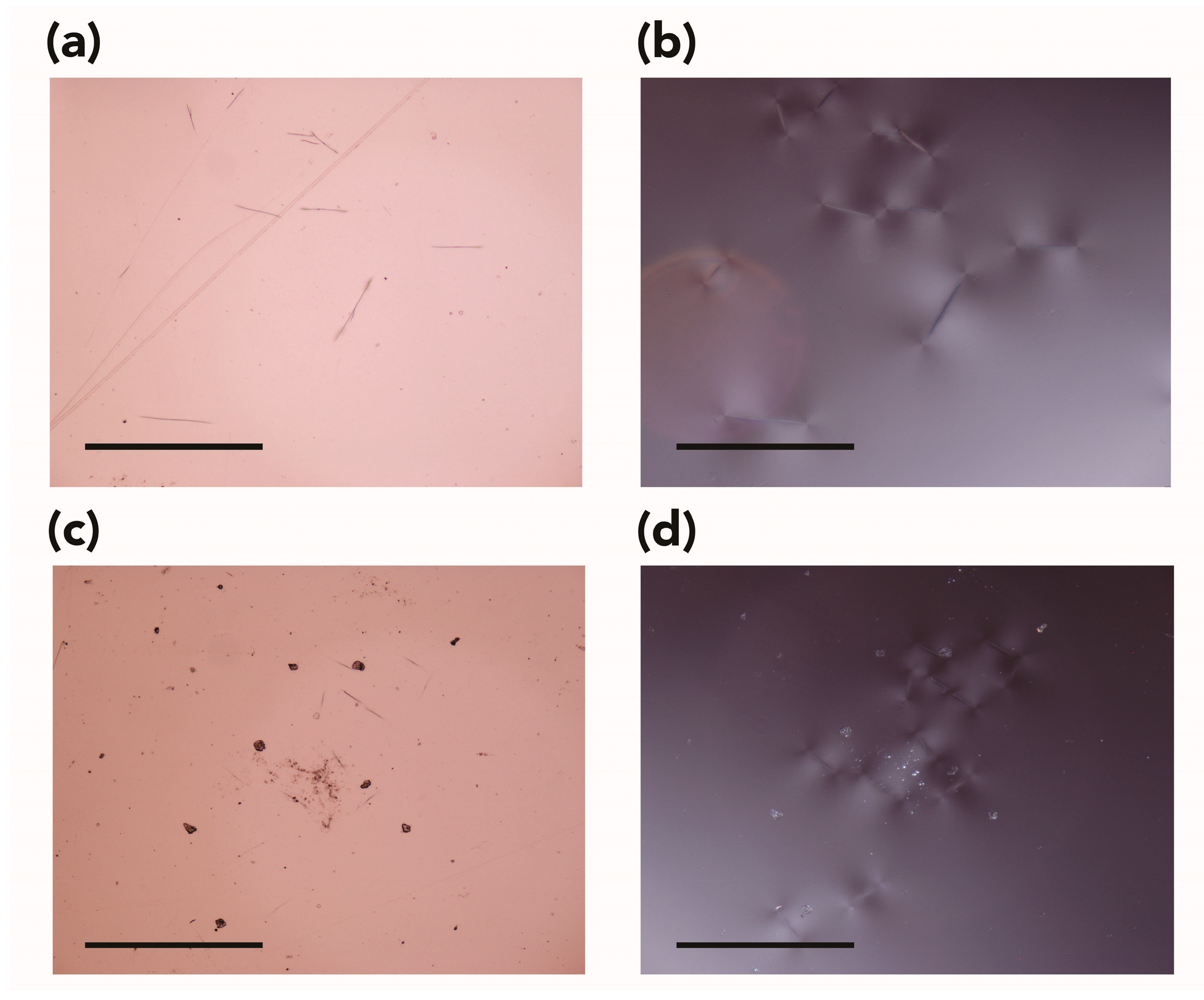
2.3.3. Imaging
2.3.4. Raman Spectroscopy (μ-Raman)
2.3.5. Attenuated Total Reflection Fourier Transform Infrared (ATR-FTIR) Spectroscopy
2.3.6. Surface Hardness
3. Results and Discussion
3.1. Post-Treatment Assessment
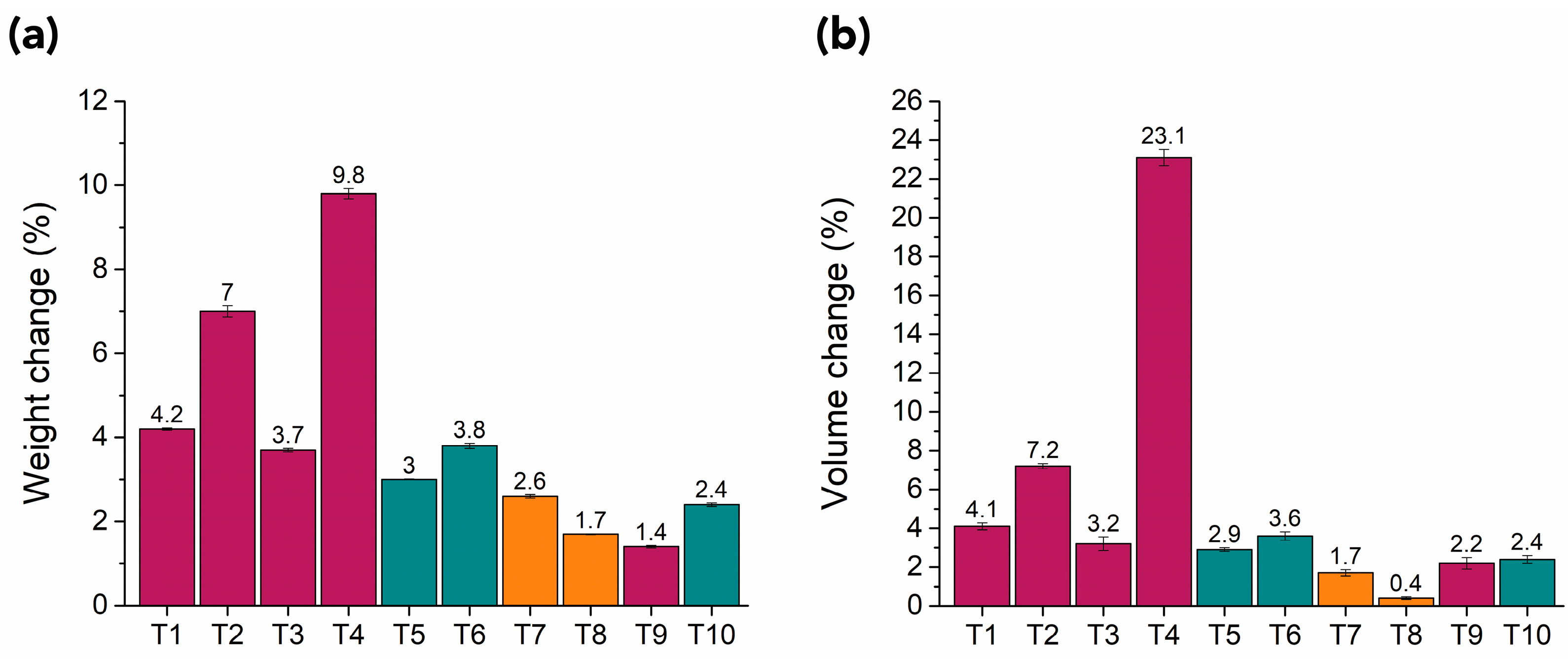
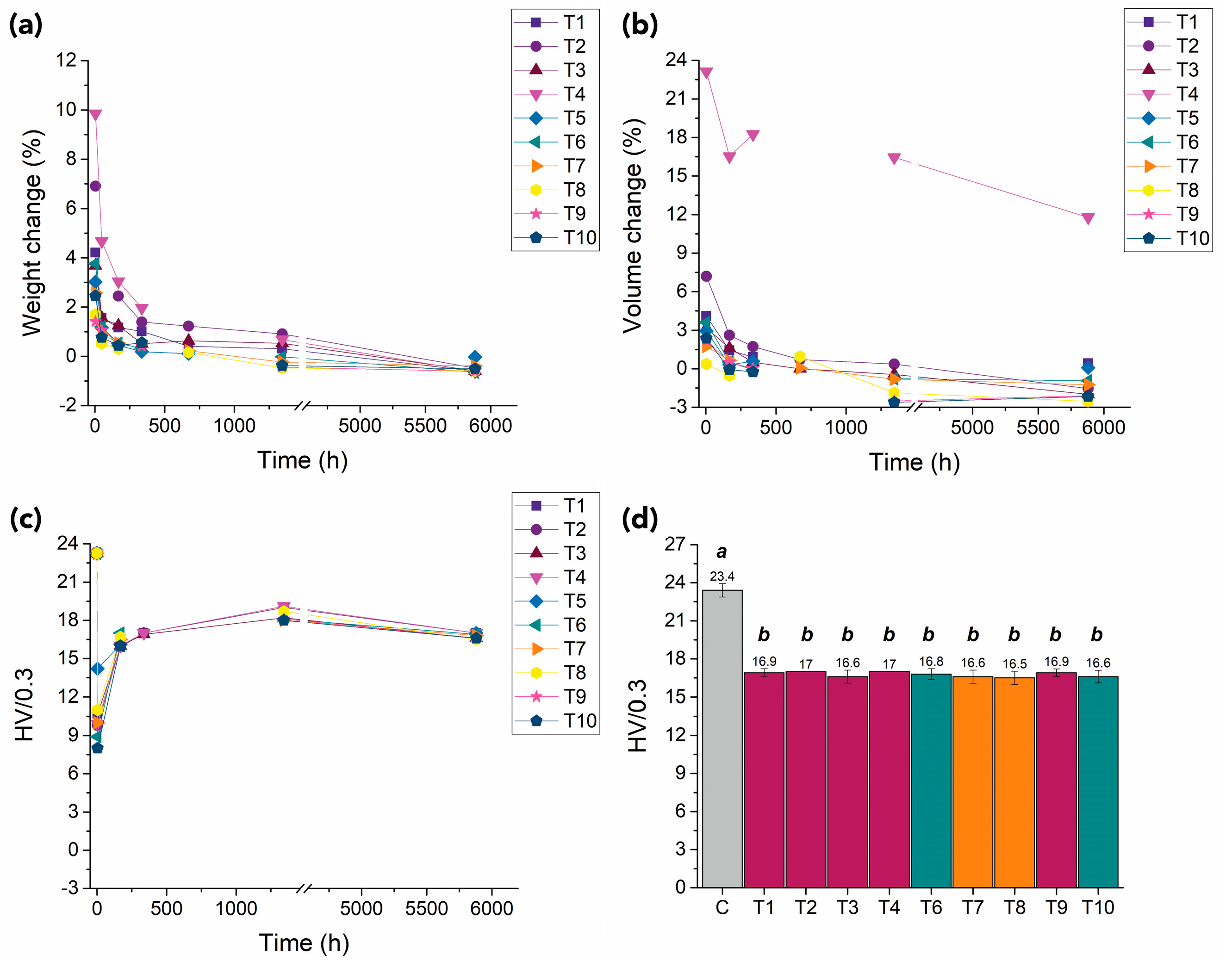
3.1.1. Appearance, Weight, Dimensions, and Visual Observations
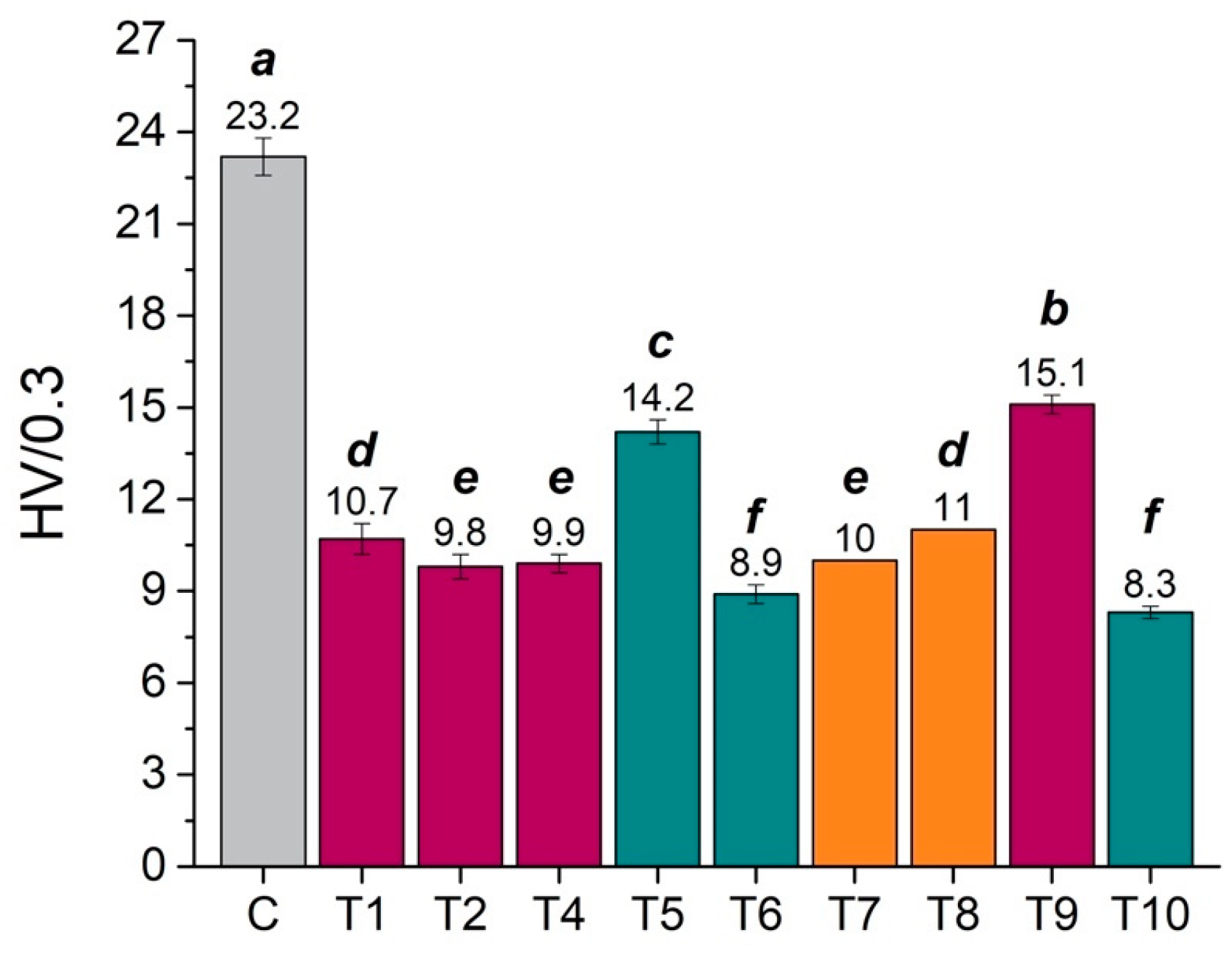
3.1.2. Mechanical Properties
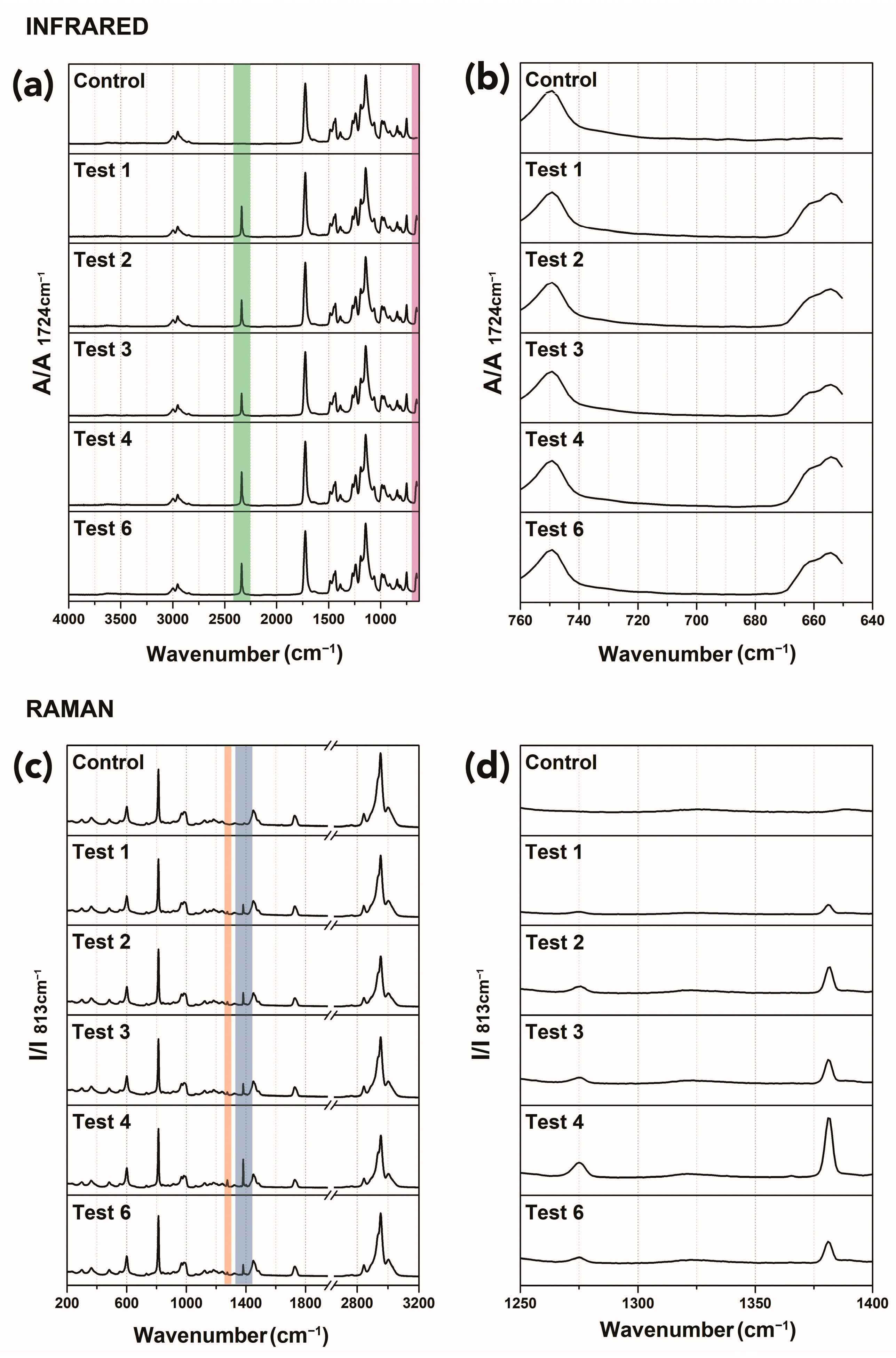
3.1.3. Spectroscopic Examinations
3.2. Long-Term Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quye, A.; Williamson, C. (Eds.) Plastics Collecting and Conserving; NMS Publishing Limited: Edinburgh, UK, 1999. [Google Scholar]
- Shashoua, Y. Conservation of Plastics—Materials Science, Degradation and Preservation; Elsevier: Oxford, UK, 2008. [Google Scholar]
- Van Oosten, T.; Lorne, A.; Béringuer, O. PUR Facts: Conservation of Polyurethane Foam in Art and Design; Amsterdam University Press: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Lavédrine, B.; Fournier, A.; Martin, G. (Eds.) Preservation of Plastic Artefacts in Museum Collections (POPART); Comité Des Travaux Historiques et Scientifiques (CTHS): Paris, France, 2012. [Google Scholar]
- Appelbaum, B. Criteria for Treatment: Reversibility. J. Am. Inst. Conserv. JAIC 1987, 26, 65–73. [Google Scholar] [CrossRef]
- Wei, W. International Research on the Conservation and Restoration of Face-Mounted Photographs. In Proceedings of the ICOM-CC 15th Triennial Meeting, New Delhi, India, 22–26 September 2008; Allied Publishers Pvt Ltd: New Delhi, India, 2008; Volume 1, pp. 709–715. [Google Scholar]
- Wei, W. Surface Micro-Roughness, Cleaning, and Perception. In Proceedings of the ICOM Committee for Conservation 16th Triennial Meeting, Lisbon, Portugal, 19–23 September 2011. [Google Scholar]
- Balcar, N.; Barabant, G.; Bollard, C.; Kuperholc, S.; Keneghan, B.; Laganà, A.; van Oosten, T.; Segel, K.; Shashua, Y. Studies in Cleaning Plastics. In Preservation of Plastic Artefacts in Museum Collections (POPART); Lavédrine, B., Fournier, A., Martin, G., Eds.; Comité des Travaux Historiques et Scientifiques (CTHS): Paris, France, 2012; pp. 225–269. [Google Scholar]
- Babo, S.; Ferreira, J.L.; Ramos, A.M.; Micheluz, A.; Pamplona, M.; Casimiro, M.H.; Ferreira, L.M.; Melo, M.J. Characterization and Long-Term Stability of Historical PMMA: Impact of Additives and Acrylic Sheet Industrial Production Processes. Polymers 2020, 12, 2198. [Google Scholar] [CrossRef] [PubMed]
- Micheluz, A.; Angelini, E.M.; Lopes, J.A.; Melo, M.J.; Pamplona, M. Discoloration of Historical Plastic Objects: New Insight into the Degradation of β-Naphthol Pigment Lakes. Polymers 2021, 13, 2278. [Google Scholar] [CrossRef] [PubMed]
- Krieg, T.; Mazzon, C.; Gómez-Sánchez, E. Material Analysis and a Visual Guide of Degradation Phenomena in Historical Synthetic Polymers as Tools to Follow Ageing Processes in Industrial Heritage Collections. Polymers 2021, 14, 121. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.; Ramos, A.M.; Callapez, M.E.; Friedel, R.; Réfrégiers, M.; Thoury, M.; Melo, M.J. Novel Markers to Early Detect Degradation on Cellulose Nitrate-Based Heritage at the Submicrometer Level Using Synchrotron UV–VIS Multispectral Luminescence. Sci. Rep. 2021, 11, 20208. [Google Scholar] [CrossRef]
- Lazzari, M.; Reggio, D. Some Applications of a Novel Surface Enhanced Raman Spectroscopy (SERS)-Based Strategy for the Detection of Art Materials. In Science and Digital Technology for Cultural Heritage; CRC Press: London, UK, 2019; pp. 333–337. ISBN 9780429345470. [Google Scholar]
- Reggio, D.; Saviello, D.; Lazzari, M.; Iacopino, D. Characterization of Contemporary and Historical Acrylonitrile Butadiene Styrene (ABS)-Based Objects: Pilot Study for Handheld Raman Analysis in Collections. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2020, 242, 118733. [Google Scholar] [CrossRef]
- Soares, I.; de Sá, S.F.; Ferreira, J.L. A First Approach into the Characterisation of Historical Plastic Objects by in Situ Diffuse Reflection Infrared Fourier Transform (DRIFT) Spectroscopy. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2020, 240, 118548. [Google Scholar] [CrossRef]
- Da Ros, S.; Curran, K.; del Gaudio, I.; Ormsby, B.; Townsend, J.H.; Cane, D.; Gili, A. Unveiling the Importance of Diffusion on the Deterioration of Cellulose Acetate Artefacts: The Profile of Plasticiser Loss as Assessed by Infrared Microscopy. In Proceedings of the Transcending Boundaries: Integrated Approaches to Conservation, ICOM-CC 19th Triennial Conference Preprints, Beijing, China, 17–21 May 2021. [Google Scholar]
- Angelini, E.; de Sá, S.F.; Soares, I.; Callapez, M.E.; Ferreira, J.L.; Melo, M.J.; Bacci, M.; Picollo, M. Application of Infrared Reflectance Spectroscopy on Plastics in Cultural Heritage Collections: A Comparative Assessment of Two Portable Mid-Fourier Transform Infrared Reflection Devices. Appl. Spectrosc. 2021, 75, 818–833. [Google Scholar] [CrossRef]
- Shashoua, Y. Modern plastics: Do they suffer from the cold? Stud. Conserv. 2004, 49, 91–95. [Google Scholar] [CrossRef]
- Shashoua, Y. Mesocycles in Conserving Plastics. Stud. Conserv. 2016, 61, 208–213. [Google Scholar] [CrossRef]
- Curran, K. A System Dynamics Approach to the Preventive Conservation of Modern Polymeric Materials in Collections. Stud. Conserv. 2018, 63, 342–344. [Google Scholar] [CrossRef]
- King, R.; Grau-Bové, J.; Curran, K. Plasticiser Loss in Heritage Collections: Its Prevalence, Cause, Effect, and Methods for Analysis. Herit. Sci. 2020, 8, 123. [Google Scholar] [CrossRef]
- Cappitelli, F.; Villa, F.; Sanmartín, P. Interactions of Microorganisms and Synthetic Polymers in Cultural Heritage Conservation. Int. Biodeterior. Biodegrad. 2021, 163, 105282. [Google Scholar] [CrossRef]
- Stuart, B.; Wong, S.; Goodall, R.; Beale, A.; Chu, C.; Nel, P.; Amin-Jones, H.; Thomas, P. Safe Storage? An Assessment of Polyethylene for the Storage of Heritage Objects. Stud. Conserv. 2022, 1–10. [Google Scholar] [CrossRef]
- Waentig, F. Plastics in Art; Michael Imhof Verlag: Petersberg, Germany, 2008. [Google Scholar]
- Lozano, M.V.C.; Sciutto, G.; Prati, S.; Mazzeo, R. Deep Eutectic Solvents: Green Solvents for the Removal of Degraded Gelatin on Cellulose Nitrate Cinematographic Films. Herit. Sci. 2022, 10, 114. [Google Scholar] [CrossRef]
- Kampasakali, E.; Fardi, T.; Pavlidou, E.; Christofilos, D. Towards Sustainable Museum Conservation Practices: A Study on the Surface Cleaning of Contemporary Art and Design Objects with the Use of Biodegradable Agents. Heritage 2021, 4, 115. [Google Scholar] [CrossRef]
- Kavda, S.; Dhopatkar, N.; Angelova, L.V.; Richardson, E.; Golfomitsou, S.; Dhinojwala, A. Surface Behaviour of PMMA: Is Gel Cleaning the Way to Go? Stud. Conserv. 2016, 61, 297–299. [Google Scholar] [CrossRef]
- Shashoua, Y.; Alterini, M.; Pastorelli, G.; Cone, L. From Microfibre Cloths to Poly(Vinyl Alcohol) Hydrogels—Conservation Cleaning of Plastics Heritage. J. Cult. Herit. 2021, 52, 38–43. [Google Scholar] [CrossRef]
- Angelova, L.V.; Sofer, G.; Bartoletti, A.; Ormsby, B. A Comparative Surface Cleaning Study of Op Structure, an Op Art PMMA Sculpture by Michael Dillon. J. Am. Inst. Conserv. 2022, 1–20. [Google Scholar] [CrossRef]
- Zhang, X.; Heinonen, S.; Levänen, E. Applications of Supercritical Carbon Dioxide in Materials Processing and Synthesis. RSC Adv. 2014, 4, 61137–61152. [Google Scholar] [CrossRef]
- Brunner, G. Gas Extraction; Topics in Physical Chemistry; Steinkopff: Heidelberg, Germany, 1994; Volume 4, ISBN 9783662073827. [Google Scholar]
- Fleming, O.S.; Kazarian, S.G. Polymer Processing with Supercritical Fluids. In Supercritical Carbon Dioxide: In Polymer Reaction Engineering; Kemmere, M.F., Meyer, T., Eds.; Weinheim Wiley-VCH: Hoboken, NJ, USA, 2006; pp. 205–238. ISBN 9783527310920. [Google Scholar]
- Nalawade, S.P.; Picchioni, F.; Janssen, L.P.B.M. Supercritical Carbon Dioxide as a Green Solvent for Processing Polymer Melts: Processing Aspects and Applications. Prog. Polym. Sci. 2006, 31, 19–43. [Google Scholar] [CrossRef]
- Aguiar-Ricardo, A.; Bonifácio, V.D.; Casimiro, T.; Correia, V.G.; Bonifácio, V.D.; Casimiro, T.; Correira, V. Supercritical Carbon Dioxide Design Strategies: From Drug Carriers to Soft Killers. Philos. Trans. Math. Phys. Eng. Sci. 2015, 373, 20150009. [Google Scholar] [CrossRef] [PubMed]
- Champeau, M.; Thomassin, J.-M.; Tassaing, T.; Jérôme, C. Drug Loading of Polymer Implants by Supercritical CO2 Assisted Impregnation: A Review. J. Control. Release 2015, 209, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Coelho, J.P.; Cristino, A.F.; Matos, P.G.; Rauter, A.P.; Nobre, B.P.; Mendes, R.L.; Barroso, J.G.; Mainar, A.; Urieta, J.S.; Fareleira, J.M.N.A.; et al. Extraction of Volatile Oil from Aromatic Plants with Supercritical Carbon Dioxide: Experiments and Modeling. Molecules 2012, 17, 10550–10573. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, H.; Wang, J.; Wang, J.; Wang, Z.; Li, J. Phytochemical Compositions and Antioxidant Activities of Essential Oils Extracted from the Flowers of Paeonia Delavayi Using Supercritical Carbon Dioxide Fluid. Molecules 2022, 27, 3000. [Google Scholar] [CrossRef]
- Chouaibi, M. Chapter 31—Supercritical Carbon Dioxide Extraction of Clove Essential Oil: Optimization and Characterization. In Clove (Syzygium aromaticum); Ramadan, M.F., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 531–540. ISBN 9780323851770. [Google Scholar]
- De Marco, I.; Riemma, S.; Iannone, R. Life Cycle Assessment of Supercritical CO2 Extraction of Caffeine from Coffee Beans. J. Supercrit. Fluids 2018, 133, 393–400. [Google Scholar] [CrossRef]
- Coelho, J.P.; Filipe, R.M.; Paula Robalo, M.; Boyadzhieva, S.; Cholakov, G.S.; Stateva, R.P. Supercritical CO2 Extraction of Spent Coffee Grounds. Influence of Co-Solvents and Characterization of the Extracts. J. Supercrit. Fluids 2020, 161, 104825. [Google Scholar] [CrossRef]
- McHardy, J.; Sawan, S.P. (Eds.) Supercritical Fluid Cleaning: Fundamentals, Technology, and Applications; Materials Science and Process Technology Series; Noyes Publications: Westwood, NJ, USA, 1998; ISBN 9780815514169. [Google Scholar]
- Banerjee, S.; Sutanto, S.; Kleijn, J.M.; van Roosmalen, M.J.E.; Witkamp, G.-J.; Stuart, M.A.C. Colloidal Interactions in Liquid CO2—A Dry-Cleaning Perspective. Adv. Colloid Interface Sci. 2012, 175, 11–24. [Google Scholar] [CrossRef]
- Lawandy, N.M.; Smuk, A.Y. Supercritical Fluid Cleaning of Banknotes. Ind. Eng. Chem. Res. 2014, 53, 530–540. [Google Scholar] [CrossRef]
- Ben Said, A.; Guinot, C.; Ruiz, J.-C.; Grandjean, A.; Dole, P.; Joly, C.; Chalamet, Y. Supercritical CO2 Extraction of Contaminants from Polypropylene Intended for Food Contact: Effects of Contaminant Molecular Structure and Processing Parameters. J. Supercrit. Fluids 2016, 110, 22–31. [Google Scholar] [CrossRef]
- Alassali, A.; Aboud, N.; Kuchta, K.; Jaeger, P.; Zeinolebadi, A. Assessment of Supercritical CO2 Extraction as a Method for Plastic Waste Decontamination. Polymers 2020, 12, 1347. [Google Scholar] [CrossRef] [PubMed]
- Clifford, T. Fundamentals of Supercritical Fluids; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Weingärtner, H.; Franck, E.U.; Weingärtner, H.; Franck, E.U. Supercritical Water as a Solvent. Angew. Chem. Int. Ed. 2005, 44, 2672–2692. [Google Scholar] [CrossRef] [PubMed]
- Kaye, B.; Cole-Hamilton, D.J.; Morphet, K. Supercritical Drying: A New Method for Conserving Waterlogged Archaeological Materials. Stud. Conserv. 2000, 45, 233. [Google Scholar] [CrossRef]
- Cretté, S.A.; Näsänen, L.M.; González-Pereyra, N.G.; Rennison, B. Conservation of Waterlogged Archaeological Corks Using Supercritical CO2 and Treatment Monitoring Using Structured-Light 3D Scanning. J. Supercrit. Fluids 2013, 79, 299–313. [Google Scholar] [CrossRef]
- Tello, H.; Unger, A. Pesticides “Green Chemistry” Finds Its Way into Conservation Science. ICOM-CC Work. Group Ethnogr. Conserv. Newsl. 2006, 27, 3–5. [Google Scholar]
- Kang, S.M.; Unger, A.; Morrell, J.J. The Effect of Supercritical Carbon Dioxide Extraction on Color Retention and Pesticide Reduction of Wooden Artifacts. J. Am. Inst. Conserv. 2004, 43, 151–160. [Google Scholar] [CrossRef]
- Selli, E.; Langè, E.; Mossa, A.; Testa, G.; Seves, A. Preservation Treatments of Aged Papers by Supercritical Carbon Dioxide. Macromol. Mater. Eng. 2000, 280–281, 71–75. [Google Scholar] [CrossRef]
- Dobrodskaya, T.V.; Egoyants, P.A.; Ikonnikov, V.K.; Romashenkova, N.D.; Sirotin, S.A.; Dobrusina, S.A.; Podgornaya, N.I. Treatment of Paper with Basic Agents in Alcohols and Supercritical Carbon Dioxide to Neutralize Acid and Prolong Storage Time. Russ. J. Appl. Chem. 2004, 77, 2017–2021. [Google Scholar] [CrossRef]
- Ikonnikov, V.K.; Dobrodskaya, T.V.; Romaschenkova, N.D.; Sirotin, S.A.; Dobrusina, S.A.; Podgornaya, N.I. Development of Technology for Large-Batch Treatment of Paper-Based Information Carriers with Carbon Dioxide Ensuring Their Long-Term Preservation. Russ. J. Phys. Chem. B 2010, 4, 1196–1206. [Google Scholar] [CrossRef]
- Wang, Y.J.; Tan, W.; Liu, C.Y.; Fang, Y.X. Deacidification of Paper in Supercritical Carbon Dioxide (CO2SCF) Solvent System with Magnesium Acetate and Calcium Hydroxide. Adv. Mater. Res. 2012, 347–353, 504–507. [Google Scholar] [CrossRef]
- Tan, W.; Cheng, L.F.; Fang, Y.X. Deacidification of Paper Using Supercritical Carbon Dioxide Containing Calcium Propionate or Magnesium Bicarbonate. Adv. Mater. Res. 2013, 781–784, 2637–2640. [Google Scholar] [CrossRef]
- Yanjuan, W.; Yanxiong, F.; Wei, T.; Chunying, L. Preservation of Aged Paper Using Borax in Alcohols and the Supercritical Carbon Dioxide System. J. Cult. Herit. 2013, 14, 16–22. [Google Scholar] [CrossRef]
- Teixeira, F.S.; dos Reis, T.A.; Sgubin, L.; Thomé, L.E.; Bei, I.W.; Clemencio, R.E.; Corrêa, B.; Salvadori, M.C. Disinfection of Ancient Paper Contaminated with Fungi Using Supercritical Carbon Dioxide. J. Cult. Herit. 2018, 30, 110–116. [Google Scholar] [CrossRef]
- Sousa, M.; Melo, M.J.; Casimiro, T.; Aguiar-Ricardo, A. The Art of CO2 for Art Conservation: A Green Approach to Antique Textile Cleaning. Green Chem. 2007, 9, 943. [Google Scholar] [CrossRef]
- Frade, C.S.C.; Cruz, P.; Lopes, E.; Sousa, M.M.; Hallett, J.; Santos, R.; Aguiar-Ricardo, A.; Casimiro, T. Cleaning Classical Persian Carpets with Silk and Precious Metal Thread: Conservation and Ethical Considerations. In Proceedings of the ICOM Committee for Conservation 16th Triennial Meeting, Lisbon, Portugal, 19–23 September 2011; Critério Artes Gráficas, Lda.. ICOM Committee for Conservation: Lisbon, Portugal, 2011. [Google Scholar]
- Aslanidou, D.; Tsioptsias, C.; Panayiotou, C. A Novel Approach for Textile Cleaning Based on Supercritical CO2 and Pickering Emulsions. J. Supercrit. Fluids 2013, 76, 83–93. [Google Scholar] [CrossRef]
- Aslanidou, D.; Karapanagiotis, I.; Panayiotou, C. Tuneable Textile Cleaning and Disinfection Process Based on Supercritical CO2 and Pickering Emulsions. J. Supercrit. Fluids 2016, 118, 128–139. [Google Scholar] [CrossRef]
- Aslanidou, D.; Karapanagiotis, I.; Panayiotou, C. Superhydrophobic, Superoleophobic Coatings for the Protection of Silk Textiles. Prog. Org. Coat. 2016, 97, 44–52. [Google Scholar] [CrossRef]
- Ikeda-Fukazawa, T.; Kita, D.; Nagashima, K. Raman Spectroscopic Study of CO2 Sorption Process in Poly Methyl Methacrylate. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 831–842. [Google Scholar] [CrossRef]
- Shieh, Y.-T.; Su, J.-H.; Manivannan, G.; Lee, P.H.C.; Sawan, S.P.; Spall, W.D. Interaction of Supercritical Carbon Dioxide with Polymers. I. Crystalline Polymers. J. Appl. Polym. Sci. 1996, 59, 695–705. [Google Scholar] [CrossRef]
- Shieh, Y.T.; Su, J.H.; Manivannan, G.; Lee, P.H.C.; Sawan, S.P.; Spall, W.D. Interaction of Supercritical Carbon Dioxide with Polymers. II. Amorphous Polymers. J. Appl. Polym. Sci. 1996, 59, 707–717. [Google Scholar] [CrossRef]
- Üzer, S.; Akman, U.; Hortaçsu, Ö. Polymer Swelling and Impregnation Using Supercritical CO2: A Model-Component Study towards Producing Controlled-Release Drugs. J. Supercrit. Fluids 2006, 38, 119–128. [Google Scholar] [CrossRef]
- Jiménez, A.; Thompson, G.L.; Matthews, M.A.; Davis, T.A.; Crocker, K.; Lyons, J.S.; Trapotsis, A. Compatibility of Medical-Grade Polymers with Dense CO2. J. Supercrit. Fluids 2007, 42, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Rindfleisch, F.; DiNoia, T.P.; McHugh, M.A. Solubility of Polymers and Copolymers in Supercritical CO2. J. Phys. Chem. 1996, 100, 15581–15587. [Google Scholar] [CrossRef]
- Kirby, C.F.; McHugh, M.A. Phase Behavior of Polymers in Supercritical Fluid Solvents. Chem. Rev. 1999, 99, 565–602. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.A. Solubility of Polymers in Supercritical Carbon Dioxide. In Green Chemistry Using Liquid and Supercritical Carbon Dioxide; DeSimone, J., Tumas, W., Eds.; Oxford University Press: New York, NY, USA, 2003; pp. 125–133. ISBN 9780195154832. [Google Scholar]
- Sadowski, G. Phase Behavior of Polymer Systems in High-Pressure Carbon Dioxide. In Supercritical Carbon Dioxide: In Polymer Reaction Engineering; Kemmere, M.F., Thierry, M., Eds.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; pp. 15–36. [Google Scholar]
- Kemmere, M.F.; Meyer, T. (Eds.) Supercritical Carbon Dioxide: In Polymer Reaction Engineering; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; ISBN 9783527310920. [Google Scholar]
- Di Maio, E.; Kiran, E. Foaming of Polymers with Supercritical Fluids and Perspectives on the Current Knowledge Gaps and Challenges. J. Supercrit. Fluids 2018, 134, 157–166. [Google Scholar] [CrossRef]
- Koros, W.J.; Smith, G.N.; Stannett, V. High-pressure Sorption of Carbon Dioxide in Solvent-cast Poly(Methyl Methacrylate) and Poly(Ethyl Methacrylate) Films. J. Appl. Polym. Sci. 1981, 26, 159–170. [Google Scholar] [CrossRef]
- Kamiya, Y.; Hirose, T.; Mizoguchi, K.; Naito, Y. Gravimetric Study of High-Pressure Sorption of Gases in Polymers. J. Polym. Sci. Part B Polym. Phys. 1986, 24, 1525–1539. [Google Scholar] [CrossRef]
- Wissinger, R.G.; Paulaitis, M.E. Swelling and Sorption in Polymer– CO2 Mixtures at Elevated Pressures. J. Polym. Sci. Part B Polym. Phys. 1987, 25, 2497–2510. [Google Scholar] [CrossRef]
- Chang, S.-H.; Park, S.-C.; Shim, J.-J. Phase Equilibria of Supercritical Fluid–Polymer Systems. J. Supercrit. Fluids 1998, 13, 113–119. [Google Scholar] [CrossRef]
- Kamiya, Y.; Mizoguchi, K.; Terada, K.; Fujiwara, Y.; Wang, J.-S. CO2 Sorption and Dilation of Poly(Methyl Methacrylate). Macromolecules 1998, 31, 472–478. [Google Scholar] [CrossRef]
- Webb, K.F.; Teja, A.S. Solubility and Diffusion of Carbon Dioxide in Polymers. Fluid Phase Equilibria 1999, 158–160, 1029–1034. [Google Scholar] [CrossRef]
- Gallyamov, M.O.; Vinokur, R.A.; Nikitin, L.N.; Said-Galiyev, E.E.; Khokhlov, A.R.; Schaumburg, K. Poly(Methyl Methacrylate) and Poly(Butyl Methacrylate) Swelling in Supercritical Carbon Dioxide and the Formation of a Porous Structure. Высoкoмoлекулярные Сoединения. Серия А 2002, 44, 12. [Google Scholar]
- Nikitin, L.N.; Said-Galiyev, E.E.; Vinokur, R.A.; Khokhlov, A.R.; Gallyamov, M.O.; Schaumburg, K. Poly(Methyl Methacrylate) and Poly(Butyl Methacrylate) Swelling in Supercritical Carbon Dioxide. Macromolecules 2002, 35, 934–940. [Google Scholar] [CrossRef]
- Liu, D.; Li, H.; Noon, M.S.; Tomasko, D.L. CO2-Induced PMMA Swelling and Multiple Thermodynamic Property Analysis Using Sanchez−Lacombe EOS. Macromolecules 2005, 38, 4416–4424. [Google Scholar] [CrossRef]
- Rajendran, A.; Bonavoglia, B.; Forrer, N.; Storti, G.; Mazzotti, M.; Morbidelli, M. Simultaneous Measurement of Swelling and Sorption in a Supercritical CO2−Poly(Methyl Methacrylate) System. Ind. Eng. Chem. Res. 2005, 44, 2549–2560. [Google Scholar] [CrossRef]
- Pantoula, M.; Panayiotou, C. Sorption and Swelling in Glassy Polymer/Carbon Dioxide Systems. J. Supercrit. Fluids 2006, 37, 254–262. [Google Scholar] [CrossRef]
- Pantoula, M.; von Schnitzler, J.; Eggers, R.; Panayiotou, C. Sorption and Swelling in Glassy Polymer/Carbon Dioxide Systems. J. Supercrit. Fluids 2007, 39, 426–434. [Google Scholar] [CrossRef]
- Tang, Q.; Yang, B.; Zhao, Y.; Zhao, L. Sorption and Diffusion of Sub/Supercritical Carbon Dioxide in Poly(Methyl Methacrylate). J. Macromol. Sci. Part B 2007, 46, 275–284. [Google Scholar] [CrossRef]
- Carlà, V.; Hussain, Y.; Grant, C.; Sarti, G.C.; Carbonell, R.G.; Doghieri, F. Modeling Sorption Kinetics of Carbon Dioxide in Glassy Polymeric Films Using the Nonequilibrium Thermodynamics Approach. Ind. Eng. Chem. Res. 2009, 48, 3844–3854. [Google Scholar] [CrossRef]
- Eslami, H.; Kesik, M.; Karimi-Varzaneh, H.A.; Müller-Plathe, F. Sorption and Diffusion of Carbon Dioxide and Nitrogen in Poly(Methyl Methacrylate). J. Chem. Phys. 2013, 139, 124902. [Google Scholar] [CrossRef]
- Li, R.; Zhang, Z.; Fang, T. Experimental Research on Swelling and Glass Transition Behavior of Poly(Methyl Methacrylate) in Supercritical Carbon Dioxide. J. Supercrit. Fluids 2016, 110, 110–116. [Google Scholar] [CrossRef]
- Gurina; Budkov; Kiselev Molecular Dynamics Study of the Swelling of Poly(Methyl Methacrylate) in Supercritical Carbon Dioxide. Materials 2019, 12, 3315. [CrossRef] [PubMed]
- Ushiki, I.; Hayashi, S.; Kihara, S.; Takishima, S. Solubilities and Diffusion Coefficients of Carbon Dioxide and Nitrogen in Poly(Methyl Methacrylate) at High Temperatures and Pressures. J. Supercrit. Fluids 2019, 152, 104565. [Google Scholar] [CrossRef]
- Sobornova, V.V.; Belov, K.V.; Dyshin, A.A.; Gurina, D.L.; Khodov, I.A.; Kiselev, M.G. Molecular Dynamics and Nuclear Magnetic Resonance Studies of Supercritical CO2 Sorption in Poly(Methyl Methacrylate). Polymers 2022, 14, 5332. [Google Scholar] [CrossRef] [PubMed]
- Kazarian, S.G.; Vincent, M.F.; Bright, F.V.; Liotta, C.L.; Eckert, C.A. Specific Intermolecular Interaction of Carbon Dioxide with Polymers. J. Am. Chem. Soc. 1996, 118, 1729–1736. [Google Scholar] [CrossRef]
- Kazarian, S.G.; Brantley, N.H.; West, B.L.; Vincent, M.F.; Eckert, C.A. In Situ Spectroscopy of Polymers Subjected to Supercritical CO2: Plasticization and Dye Impregnation. Appl. Spectrosc. 1997, 51, 491–494. [Google Scholar] [CrossRef]
- Chiou, J.S.; Barlow, J.W.; Paul, D.R. Plasticization of Glassy Polymers by CO2. J. Appl. Polym. Sci. 1985, 30, 2633–2642. [Google Scholar] [CrossRef]
- Goel, S.K.; Beckman, E.J. Plasticization of Poly(Methyl Methacrylate) (PMMA) Networks by Supercritical Carbon Dioxide. Polymer 1993, 34, 1410–1417. [Google Scholar] [CrossRef]
- Handa, Y.P.; Kruus, P.; O’Neill, M. High-Pressure Calorimetric Study of Plasticization of Poly(Methyl Methacrylate) by Methane, Ethylene, and Carbon Dioxide. J. Polym. Sci. Part B Polym. Phys. 1996, 34, 2635–2639. [Google Scholar] [CrossRef]
- Alessi, P.; Cortesi, A.; Kikic, I.; Vecchione, F. Plasticization of Polymers with Supercritical Carbon Dioxide: Experimental Determination of Glass-Transition Temperatures. J. Appl. Polym. Sci. 2003, 88, 2189–2193. [Google Scholar] [CrossRef]
- Watanabe, M.; Hashimoto, Y.; Kimura, T.; Kishida, A. Characterization of Engineering Plastics Plasticized Using Supercritical CO2. Polymers 2020, 12, 134. [Google Scholar] [CrossRef] [PubMed]
- Tsivintzelis, I.; Angelopoulou, A.G.; Panayiotou, C. Foaming of Polymers with Supercritical CO2: An Experimental and Theoretical Study. Polymer 2007, 48, 5928–5939. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, P.; Li, W. Fabrication of Functionally Graded Porous Polymer via Supercritical CO2 Foaming. Compos. Part B Eng. 2011, 42, 318–325. [Google Scholar] [CrossRef]
- Tsivintzelis, I.; Sanxaridou, G.; Pavlidou, E.; Panayiotou, C. Foaming of Polymers with Supercritical Fluids: A Thermodynamic Investigation. J. Supercrit. Fluids 2016, 110, 240–250. [Google Scholar] [CrossRef]
- Goel, S.K.; Beckman, E.J. Generation of Microcellular Polymeric Foams Using Supercritical Carbon Dioxide. I: Effect of Pressure and Temperature on Nucleation. Polym. Eng. Sci. 1994, 34, 1137–1147. [Google Scholar] [CrossRef]
- Feng, J.J.; Bertelo, C.A. Prediction of Bubble Growth and Size Distribution in Polymer Foaming Based on a New Heterogeneous Nucleation Model. J. Rheol. 2004, 48, 439–462. [Google Scholar] [CrossRef]
- Chen, X.; Feng, J.J.; Bertelo, C.A. Plasticization Effects on Bubble Growth during Polymer Foaming. Polym. Eng. Sci. 2006, 46, 97–107. [Google Scholar] [CrossRef]
- Taki, K. Experimental and Numerical Studies on the Effects of Pressure Release Rate on Number Density of Bubbles and Bubble Growth in a Polymeric Foaming Process. Chem. Eng. Sci. 2008, 63, 3643–3653. [Google Scholar] [CrossRef]
- Al-Enezi, S. CO2 Induced Foaming Behavior of Polystyrene near the Glass Transition. Int. J. Polym. Sci. 2017, 2017, 7804743. [Google Scholar] [CrossRef]
- Tomasko, D.L.; Li, H.; Liu, D.; Han, X.; Wingert, M.J.; Lee, L.J.; Koelling, K.W. A Review of CO2 Applications in the Processing of Polymers. Ind. Eng. Chem. Res. 2003, 42, 6431–6456. [Google Scholar] [CrossRef]
- Kiran, E. Supercritical Fluids and Polymers—The Year in Review—2014. J. Supercrit. Fluids 2016, 110, 126–153. [Google Scholar] [CrossRef]
- Morrissey, P.; Vesely, D. Accurate Measurement of Diffusion Rates of Small Molecules through Polymers. Polymer 2000, 41, 1865–1872. [Google Scholar] [CrossRef]
- Royer, J.R.; DeSimone, J.M.; Khan, S.A. Carbon Dioxide-Induced Swelling of Poly(Dimethylsiloxane). Macromolecules 1999, 32, 8965–8973. [Google Scholar] [CrossRef]
- Nikitin, L.N.; Gallyamov, M.O.; Vinokur, R.A.; Nikolaec, A.Y.; Said-Galiyev, E.E.; Khokhlov, A.R.; Jespersen, H.T.; Schaumburg, K. Swelling and Impregnation of Polystyrene Using Supercritical Carbon Dioxide. J. Supercrit. Fluids 2003, 26, 263–273. [Google Scholar] [CrossRef]
- Rodríguez, D.C.; Carrascal, D.; Solórzano, E.; Pérez, M.A.R.; Pinto, J. Analysis of the Retrograde Behavior in PMMA-CO2 Systems by Measuring the (Effective) Glass Transition Temperature Using Refractive Index Variations. J. Supercrit. Fluids 2021, 170, 105159. [Google Scholar] [CrossRef]
- Laganà, A.; van Oosten, T. Back to Transparency, Back to Life. Research into the Restoration of Broken Transparent Unsaturated Polyester and Poly(Methyl Methacrylate) Works of Art. In Proceedings of the ICOM-CC 16th Triennial Conference Preprints, Lisbon, Portugal, 19–23 September 2011; pp. 1–9. [Google Scholar]
- Laganà, A.; David, M.; Doutre, M.; de Groot, S.; van Keulen, H.; Madden, O.; Schilling, M.; van Bommel, M. Reproducing Reality. Recreating Bonding Defects Observed in Transparent Poly(Methyl Methacrylate) Museum Objects and Assessing Defect Formation. J. Cult. Herit. 2021, 48, 254–268. [Google Scholar] [CrossRef]
- Wissinger, R.G.; Paulaitis, M.E. Glass Transitions in Polymer / CO2 Mixtures at Elevated Pressures. J. Polym. Sci. Part B Polym. Phys. 1991, 29, 631–633. [Google Scholar] [CrossRef]
- Condo, P.D.; Johnston, K.P. Retrograde Vitrification of Polymers with Compressed Fluid Diluents: Experimental Confirmation. Macromolecules 1992, 25, 6730–6732. [Google Scholar] [CrossRef]
- Zhang, Z.; Handa, Y.P. CO2-Assisted Melting of Semicrystalline Polymers. Macromolecules 1997, 30, 8505–8507. [Google Scholar] [CrossRef]
- Jung, M.R.; Horgen, F.D.; Orski, S.V.; Rodriguez, C.V.; Beers, K.L.; Balazs, G.H.; Jones, T.T.; Work, T.M.; Brignac, K.C.; Royer, S.-J.; et al. Validation of ATR FT-IR to Identify Polymers of Plastic Marine Debris, Including Those Ingested by Marine Organisms. Mar. Pollut. Bull. 2018, 127, 704–716. [Google Scholar] [CrossRef]
- Yuan, Y.; Teja, A.S. Quantification of Specific Interactions between CO2 and the Carbonyl Group in Polymers via ATR-FTIR Measurements. J. Supercrit. Fluids 2011, 56, 208–212. [Google Scholar] [CrossRef]
- Lipschitz, I. The Vibrational Spectrum of Poly(Methyl Methacrylate): A Review. Polym. Plast. Technol. Eng. 1982, 19, 53–106. [Google Scholar] [CrossRef]
- Matsushita, A.; Ren, Y.; Matsukawa, K.; Inoue, H.; Minami, Y.; Noda, I.; Ozaki, Y. Two-Dimensional Fourier-Transform Raman and near-Infrared Correlation Spectroscopy Studies of Poly(Methyl Methacrylate) Blends: 1. Immiscible Blends of Poly(Methyl Methacrylate) and Atactic Polystyrene. Vib. Spectrosc. 2000, 24, 171–180. [Google Scholar] [CrossRef]
- Takahashi, M.; Yamamoto, Y.; Nawaby, A.V.; Handa, Y.P. Raman Spectroscopic Investigation of the Phase Behavior and Phase Transitions in a Poly(Methyl Methacrylate)-Carbon Dioxide System. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 2214–2217. [Google Scholar] [CrossRef]




| Test No. | Temperature (°C) | Pressure (MPa) | CO2 Density (g/mL) | CO2 Phase | Flux (mL/min) | Compression Time (min) | Exposure Time (min) | Depressurization Time (min) |
|---|---|---|---|---|---|---|---|---|
| 1 | 35 | 10 | 0.71281 | Supercritical | 10 | ~15 | 60 | 20 |
| 2 | 35 | 28 | 0.91864 | Supercritical | 10 | ~15 | 60 | 20 |
| 3 | 55 | 10 | 0.32507 | Supercritical | 10 | ~15 | 60 | 20 |
| 4 | 55 | 28 | 0.8357 | Supercritical | 10 | ~15 | 60 | 20 |
| 5 | 25 | 7 | 0.74303 | Liquid | 10 | ~15 | 60 | 20 |
| 6 | 25 | 10 | 0.81763 | Liquid | 10 | ~15 | 60 | 20 |
| 7 | 35 | 6.7 | 0.19798 | Vapor | 10 | ~15 | 60 | 20 |
| 8 | 25 | 6 | 0.19061 | Vapor | 10 | ~15 | 60 | 20 |
| 9 | 35 | 10 | 0.71281 | Supercritical | 10 | ~15 | 30 | 20 |
| 10 | 25 | 10 | 0.81763 | Liquid | 10 | ~15 | 30 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartoletti, A.; Soares, I.; Ramos, A.M.; Shashoua, Y.; Quye, A.; Casimiro, T.; Ferreira, J.L. Assessing the Impact and Suitability of Dense Carbon Dioxide as a Green Solvent for the Treatment of PMMA of Historical Value. Polymers 2023, 15, 566. https://doi.org/10.3390/polym15030566
Bartoletti A, Soares I, Ramos AM, Shashoua Y, Quye A, Casimiro T, Ferreira JL. Assessing the Impact and Suitability of Dense Carbon Dioxide as a Green Solvent for the Treatment of PMMA of Historical Value. Polymers. 2023; 15(3):566. https://doi.org/10.3390/polym15030566
Chicago/Turabian StyleBartoletti, Angelica, Inês Soares, Ana Maria Ramos, Yvonne Shashoua, Anita Quye, Teresa Casimiro, and Joana Lia Ferreira. 2023. "Assessing the Impact and Suitability of Dense Carbon Dioxide as a Green Solvent for the Treatment of PMMA of Historical Value" Polymers 15, no. 3: 566. https://doi.org/10.3390/polym15030566
APA StyleBartoletti, A., Soares, I., Ramos, A. M., Shashoua, Y., Quye, A., Casimiro, T., & Ferreira, J. L. (2023). Assessing the Impact and Suitability of Dense Carbon Dioxide as a Green Solvent for the Treatment of PMMA of Historical Value. Polymers, 15(3), 566. https://doi.org/10.3390/polym15030566







