Evaluation of Chemical and Morphological Properties of Spruce Wood Stored in the Natural Environment
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
2.2.1. Chemical Analyses
2.2.2. pH Value
2.2.3. Chromatographic Analyses
2.2.4. Fibers Length and Width
3. Results and Discussion
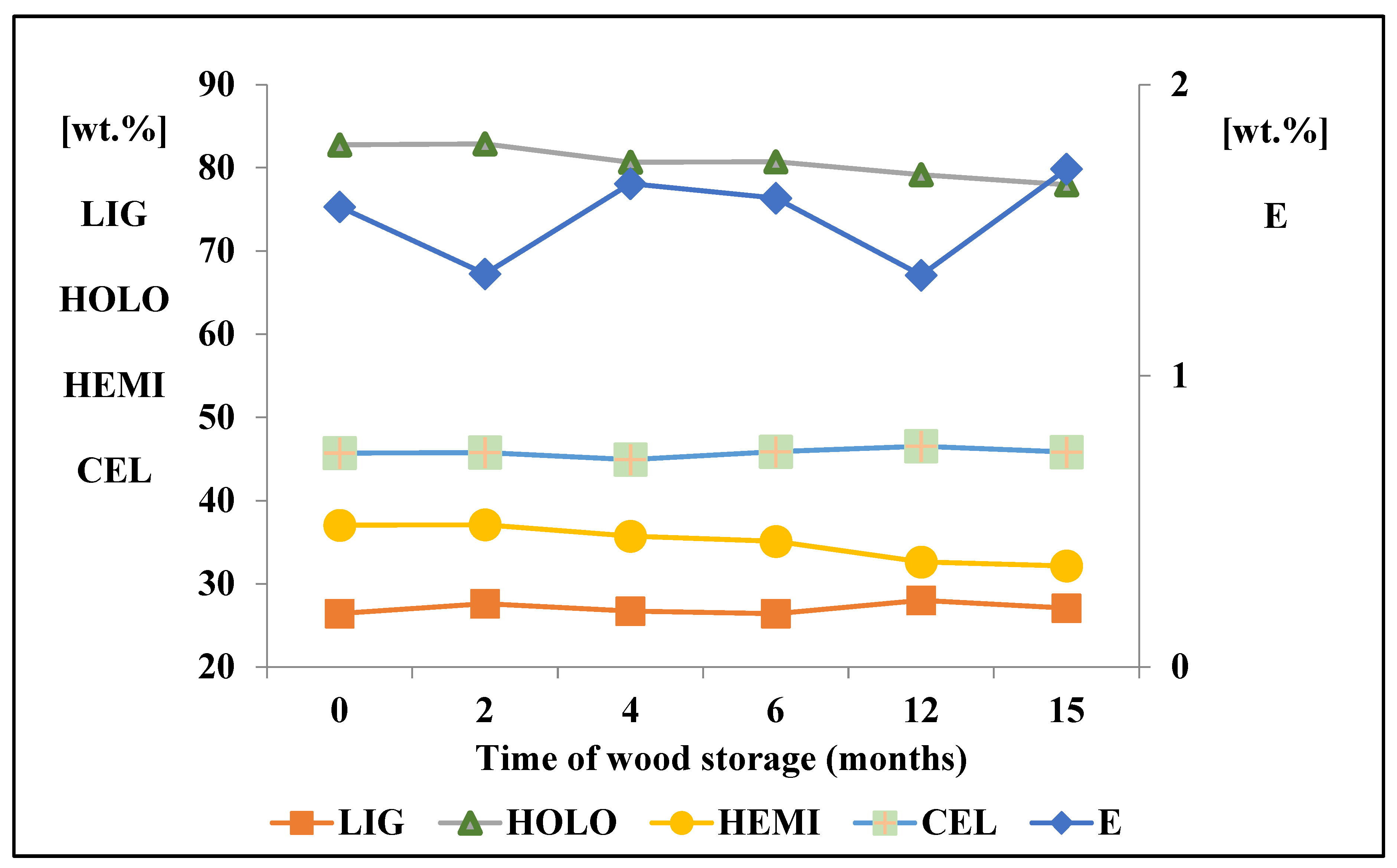
3.1. Changes in the Chemical Composition of Wood during Its Storage
3.2. Change in the pH Values of Wood during Its Storage
- Acetic acid, which is produced during the anaerobic fermentation of sugars found in wood. This process is called acetic fermentation and can lead to the formation of not only acetic acid but also ethanol.
- Formic acid, which is formed in wood through the action of fungi and bacteria. It is a strong acid that can cause metal corrosion.
- Malic acid, which is formed in wood during its storage as a result of metabolic processes that take place within the wood tissues.
3.3. Changes in the Content of Organic Acids in Wood during Its Storage
3.4. Changes in Molecular Weights and Average Degree of Polymerization of Cellulose during Wood Storage
3.5. Changes in the Content of Saccharides in Wood during Storage
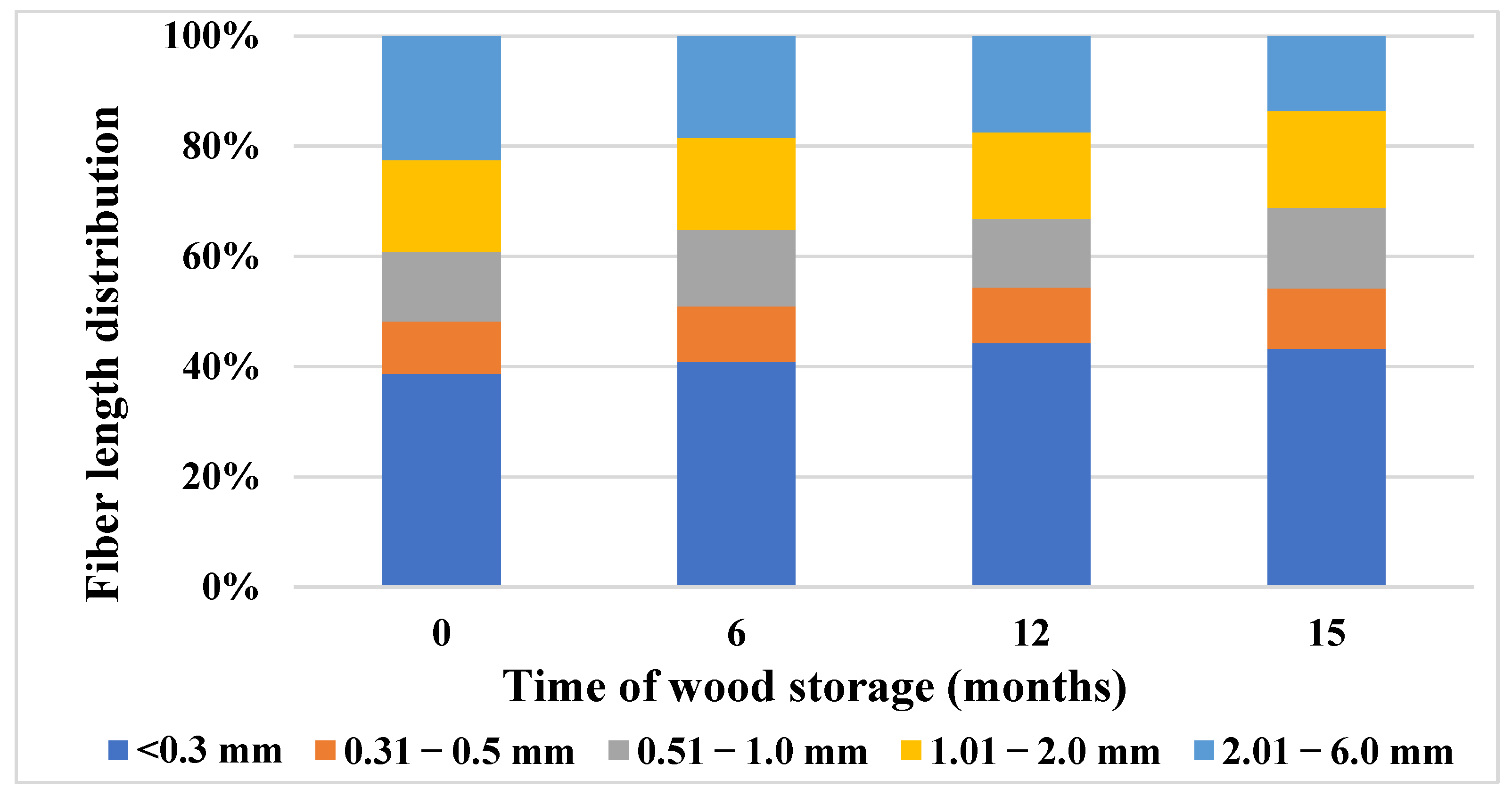
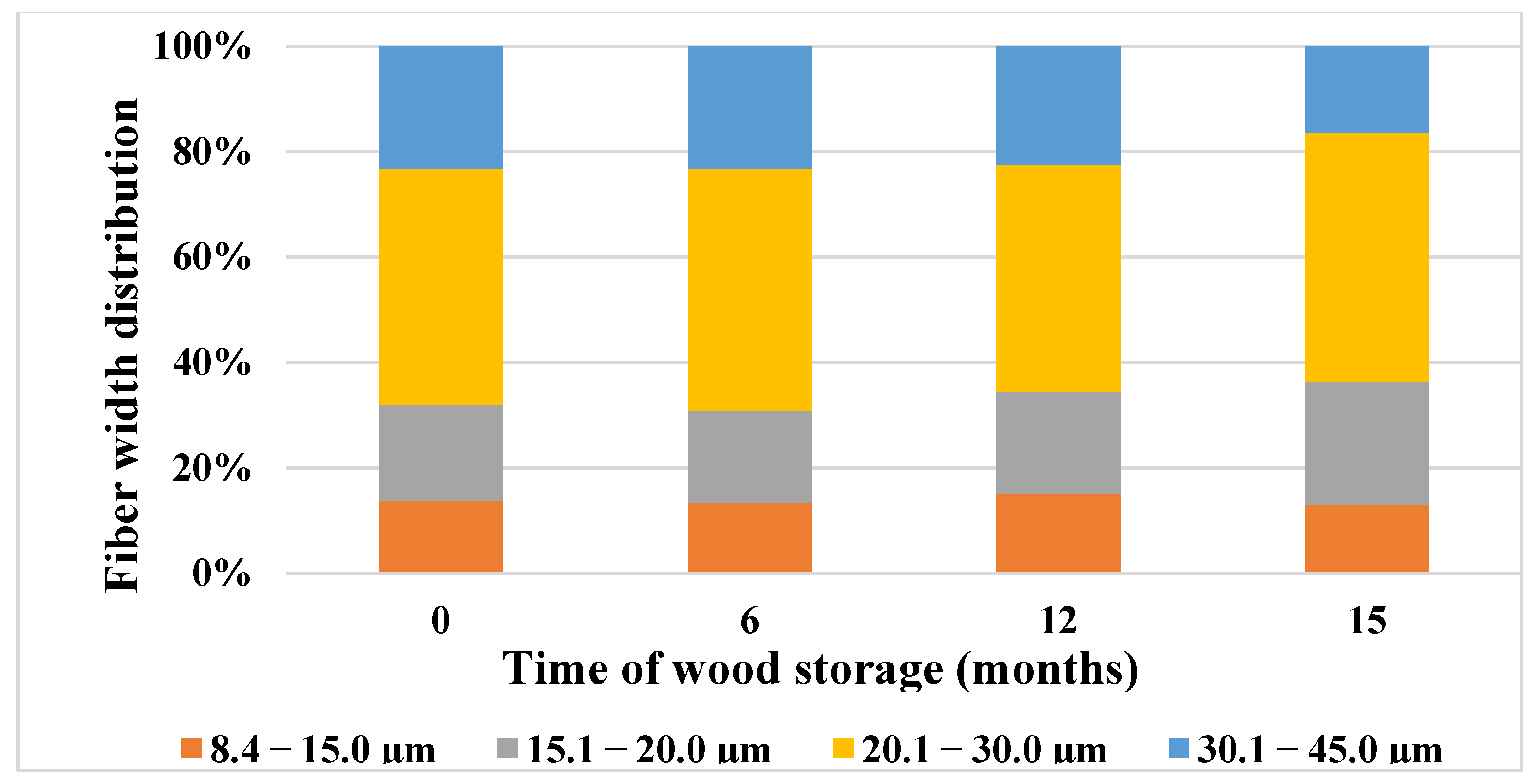
3.6. Changes in the Morphological Properties of Wood during 15 Months of Storage
4. Conclusions
- The quantity of extractives decreased by 14% after 2 months of storage. During storage, there was also observed an increase in the content of extractives due to the degradation of other wood components, primarily hemicelluloses.
- The content of cellulose, determined according to Seifert, and the content of lignin, changed minimally.
- Chemical analysis showed a decrease in the content of hemicelluloses by 13.21%, due to its easier degradation and less stability compared to cellulose.
- The pH value after 15 months of wood storage decreased by one degree compared to the pH of freshly harvested wood.
- Organic acids, mainly acetic and formic, were not detected in samples of spruce wood stored in outdoor conditions due to its high volatility.
- After 15 months of storage, a decrease in the cellulose DP by 9.18% was observed, which points to the degradation of cellulose due to the influence of various factors (e.g., humidity, temperature, microorganisms) in the external environment.
- Using the HPLC method, we found a decrease in the quantity of hemicelluloses after 15 months of wood storage, of 40.24%, compared to freshly harvested wood. From the point of view of hemicellulose-type saccharides, the greatest degradation occurred in the content of L-arabinose (by 71.99%), D-galactose (by 61.11%), and D-xylose (by 43.04%). The total decrease in the amount of D-glucose from cellulose was 16.52%.
- A sample taken immediately after harvesting contained a fine fiber fraction of 38.71%; after 6 months of storage this fraction increased by 5.28% and after 15 months of storage this increase was 10.5%.
- The fraction of fibers in the length class from 2.01 to 6.0 mm decreased during storage. Immediately after harvesting this share was 22.49%; after 6 months it decreased by 21.3% and after 15 months it decreased by 65.4%. The average fiber length decreased by 38.17% after 15 months of wood storage.
- The analysis of the fiber width showed that there was a decrease after 15 months of storage, by 4.8%. After 15 months of wood storage, we noted a decrease in the content of fibers in the width class from 30.1 to 45.0 μm, by 38.42%.
- There are some differences in the methods used—the determination of cellulose according to Seifert gave us different data compared to the determination of D-glucose via the HPLC method.
- Several changes in the chemical structure and morphology of the fibers were detected during 15 months of wood storage in an open forest warehouse. However, the quality of the raw material remains very good and we do not expect it to significantly affect the quality of such products as pulp, paper, or composite boards.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, G.; Jaakkola, P. Wood Chemistry and Isolation of Extractives from Wood; Literature Study for BIOTULI Project; Saimaa University of Applied Sciences: Lappeenranta, Finland, 2011; 47p. [Google Scholar]
- Srinivas, K.; Pandley, K.K. Photodegradation of thermally modified wood. J. Photochem. Photobiol. B 2012, 117, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Reinprecht, L. Wood Deterioration, Protection and Maintenance; John Wiley & Sons: Chichester, UK, 2016. [Google Scholar]
- Hill, C.A.S. Wood Modification—Chemical, Thermal and Other Processes; John Wiley & Sons Ltd.: Chichester, UK, 2006. [Google Scholar]
- Militz, H. Thermal Treatment of Wood: European Processes and Their Background; (IRG/WP 02-40241); International Research Group on Wood Preservation: Stockholm, Sweden, 2002. [Google Scholar]
- Sonderegger, W.; Kránitz, K.; Bues, C.T.; Niemz, P. Aging effects on physical and mechanical properties of spruce, fir and oak wood. J. Cult. Herit. 2015, 16, 883–889. [Google Scholar] [CrossRef]
- Huckfeldt, T.; Rehnbein, M. Bakterien und Pilze an Wasserbau Holz Institut für Holzqualität und Holzschäden; Dr. Rehbein und Dr. Huckfeldt GbR: Hamburg, Germany, 2017. [Google Scholar]
- Erikson, K.L.; Blanchette, R.A.; Ander, P. Microbial and Enzymatic Degradation of Wood and Wood Components; Springer Series in Wood Science; Springer: Berlin/Heidelberg, Germany, 1990. [Google Scholar]
- Kránitz, K.; Sonderegger, W.; Bues, C.T.; Niemz, P. Effects of aging on wood: A literature review. Wood Sci. Technol. 2016, 50, 7–22. [Google Scholar] [CrossRef]
- Kataoka, Y.; Kiguchi, M.; Williams, R.S.; Evans, P.D. Violet light causes photodegradation of wood beyond the zone affected by ultraviolet radiation. Holzforshung 2007, 61, 23–27. [Google Scholar] [CrossRef]
- Papp, E.A.; Csiha, C.; Makk, A.N.; Hofmann, T.; Csoka, L. Wettability of wood surface layer examined from chemical change perspective. Coatings 2020, 10, 257. [Google Scholar] [CrossRef]
- Csanády, E.; Magoss, E.; Tolvaj, L. Quality of Machined Wood Surfaces; Springer International Publishing: New York, NY, USA, 2015. [Google Scholar]
- Eugenio, M.E.; Ibarra, D.; Martin-Sanpedro, R.; Espinosa, E.; Bascón, I.; Rodríguez, A. Alternative Raw Materials for Pulp and Paper Production in the Concept of a Lignocellulosic Biorefinery. In Cellulose; Pascual, A., Martin, M., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Aremu, M.O.; Aperolola, S.O.; Dabonyan, O.O. Suitability of Nigerian corn husk and plantain stalk for pulp and paper production. Eur. Sci. J. 2015, 11, 146–152. [Google Scholar]
- Fahmy, Y.; Fahmy, T.Y.A.; Mobarekk, F.; El-Sakhawy, M.; Fadl, M.H. Agricultural Residues (Wastes) for Manufacture of Paper, Board, and Miscellaneous Products: Background Overview and Future Prospects. Int. J. ChemTech Res. 2017, 10, 424–448. [Google Scholar] [CrossRef]
- Brännvall, E. Increasing pulp yield in kraft cooking of softwoods by high initial effective alkali concetration (HIEAC) during impregnation leading to decreasing secondary peeling of cellulose. Holzforshung 2018, 72, 819–827. [Google Scholar] [CrossRef]
- Geffertová, J.; Geffert, A. Dimensional characteristics of the fibres selected clones of willow Salix viminalis—ULV, ORM, RAPP. Acta Fac. Xylologiae 2012, 54, 15–22. (In Slovak) [Google Scholar]
- Retulainen, E.; Niskanen, K.; Nilsen, N. Fibers and Bonds. Paper Physics; Fapet Oy: Helsinki, Finland, 1998; pp. 54–87. [Google Scholar]
- Buksnowitz, C.H.; Teischunger, A.; Grabner, M.; Müller, U.; Mahn, L. Tracheid length in Norway spruce (Picea abies (L.) Karst.) analysis of three databases regarding tree age, cambial age, tree height, inter-annual variation, radial distance to pith and log qualities. Wood Res. 2010, 55, 1–13. [Google Scholar]
- Burdon, R.D.; Kibblewhite, R.; Walker, J.C.F.; Megraw, R.A.; Evans, R.; Cown, D.J. Juvenile versus mature wood: A new concept, orthogonal to corewood versus outerwood, with special reference to Pinus radiata and P. taeda. For. Sci. 2004, 50, 399–415. [Google Scholar]
- Herman, M.; Dutilleul, P.; Avella-Shaw, T. Growth rate effects on temporal trajectories of ring width, wood density, and mean tracheid length in Norway spruce (Picea abies L. Karst.). Wood Fiber Sci. 1998, 30, 6–17. [Google Scholar]
- Mäkinen, H.; Saranpää, P.; Linder, S. Effect of growth rate on fibre characteristics in Norway spruce (Picea abies L. Karst.). Holzforschung 2002, 56, 449–460. [Google Scholar] [CrossRef]
- Mäkinen, H.; Saranpää, P.; Linder, S. Wood-density variation of Norway spruce in relation to nutrient optimization and fibre dimensions. Can. J. For. Res. 2002, 32, 185–194. [Google Scholar] [CrossRef]
- Dutilleul, P.; Herman, M.; Avella-Shaw, T. Growth rate effects on correlations among ring width, wood density, and mean tracheid lenght in Norway spruce (Picea abies). Can. J. For. Res. 1998, 28, 56–68. [Google Scholar] [CrossRef]
- Sirviö, J. Variation of cross-sectional properties within single Norway spruce tracheids. Wood Fiber Sci. 2000, 32, 311–331. [Google Scholar]
- Sirviö, J.; Kärenlampi, P. Two scales of variantion in Norway spruce tracheid properties. Wood Fiber Sci. 2001, 33, 16–25. [Google Scholar]
- Wimmer, R.; Grabner, M.A. Comparison of tree-ring features in Picea abies as correlated with climate. IAWA J. 2000, 21, 403–416. [Google Scholar] [CrossRef]
- Freitas, T.P.; Oliveira, J.T.S.; Vidaurre, G.B.; Rodrigues, B.P. Environmental effect on chemical composition of eucalyptus clones wood for pulp production. CERNE 2018, 24, 219–224. [Google Scholar] [CrossRef]
- Malkov, S.; Tikka, P.; Gullichsen, J. Towards complete impregnation of wood chips with aqueous solutions. Pap. Ja Puu 2001, 83, 468–473. [Google Scholar]
- Geffert, A.; Geffertová, J. Consequences of the degradative action of fungi on wood and pulp. Papír Celulóza 2007, 62, 282–285. (In Slovak) [Google Scholar]
- Allen, L.H.; Sithole, B.B.; Macleod, J.M.; Lapointe, C.L.; Mcphee, F.J. Importance of seasoning and barking in the kraft pulping of aspen. J. Pulp Pap. Sci. 1991, 17, J85–J91. [Google Scholar]
- Silverio, F.O.; Barbosa, L.C.; Maltha, C.R.; Fidencio, P.H.; Cruz, M.P.; Veloso, D.P.; Milanez, A.F. Effect of storage time on the composition and content of wood extractives in Eucalyptus cultivated in Brazil. Biores. Technol. 2008, 99, 4878. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Mishara, O.P.; Ganwar, A.; Chakrabarti, S.K.; Varadhan, R. Impact of wood storage on pulp and paper making properties. IPPTA Q. J. Indian Pulp Pap. Tech. Assoc. 2011, 23, 161–164. [Google Scholar]
- Pereira, M.; Sousa, G.; Anjos, O. Influence of Wood Storage Time in the Paper Properties of Eucalyptus globulus. In Proceedings of the Progress in Paper Physics Seminar, Espoo, Finland, 2–5 June 2008; pp. 247–250. [Google Scholar]
- Ramnath, L.; Sithole, B.; Govinden, R. The effects of wood storage on the chemical composition and indigenous microflora of Eucalyptus species used in the pulping industry. Bioresources 2017, 13, 86–103. [Google Scholar] [CrossRef]
- ISO 13061–1; Physical and Mechanical Properties of Wood—Test Methods for Small Clear Wood Specimens—Part 1: Determination of Moisture Content for Physical and Mechanical Tests. International Organization for Standardization: Geneva, Switzerland, 2014.
- ISO 13061–2; Physical and Mechanical Properties of Wood—Test Methods for Small Clear Wood Specimens—Part 2: Determination of Density for Physical and Mechanical Tests. International Organization for Standardization: Geneva, Switzerland, 2017.
- ASTM D1107-21; Standard Test Method for Ethanol-Toluene Solubility of Wood. ASTM International: West Conshohocken, PA, USA, 2021.
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; (NREL/TP-510-42618); National Renewable Energy Laboratory: Golden, CO, USA, 2012. [Google Scholar]
- Seifert, V.K. About a new method for rapid determination of pure cellulose. Papier 1956, 10, 301–306. (In German) [Google Scholar]
- Wise, L.E.; Murphy, M.; D’Addieco, A.A. Chlorite holocellulose, its fractionation and bearing on summative wood analysis and on studies on the hemicelluloses. Pap. Trade J. 1946, 122, 35–44. [Google Scholar]
- ISO 6588-1; Paper, Board and Pulps—Determination of pH of Aqueous Extracts—Part 1: Cold Extraction. International Organization for Standardization: Geneva, Switzerland, 2021.
- Geffert, A.; Geffertová, J.; Dudiak, M. Direct method of measuring the pH value of wood. Forests 2019, 10, 852. [Google Scholar] [CrossRef]
- Kačík, F.; Podzimek, Š.; Vizárová, K.; Kačíková, D.; Čabalová, I. Characterization of cellulose degradation during accelerated ageing by SECMALS, SEC-DAD, and A4F-MALS methods. Cellulose 2016, 23, 357–366. [Google Scholar] [CrossRef]
- Kačík, F.; Kačíková, D.; Jablonský, M.; Katuščák, S. Cellulose degradation in the process of newsprint paper ageing. Polym. Degrad. Stab. 2009, 94, 1509–1514. [Google Scholar] [CrossRef]
- Kačík, F.; Kačíková, D. Characteristics and Analysis of Cellulose and Its Derivatives; Technical University in Zvolen: Zvolen, Slovakia, 2007; 92p. (In Slovak) [Google Scholar]
- Bergström, D.; Matison, M. Efficient Forest Biomass Supply Chain Management for Biorefineries; Synthesis Report—Forest Refine 2012–2014; Department of Forest Biomaterials and Technology, Swedish University of Agricultural Sciences: Uppsala, Sweden, 2014; 116p. [Google Scholar]
- Holmbom, B. Extraction and utilisation of non-structural wood and bark components. In Biorefining of Forest Resources; Allén, R., Ed.; Puunjalostusinsinöörit ry—Forest Products Engineers: Espoo, Finland, 2011; pp. 178–224. [Google Scholar]
- Sikora, A.; Kačík, F.; Gaff, M.; Vondrová, V.; Bubeníková, T.; Kubovský, I. Impact of thermal modification on color and chemical changes of spruce and oak wood. J. Wood Sci. 2018, 64, 406–416. [Google Scholar] [CrossRef]
- Čabalová, I.; Kačík, F.; Zachar, M.; Dúbravský, R. Chemical changes of hardwoods at thermal loading by radiant heating. Acta Fac. Xylologiae 2016, 58, 43–50. [Google Scholar]
- Ahajji, A.; Diouf, P.N.; Aloui, F.; Elbakali, I.; Perrin, D.; Merlin, A.; George, B. Influence of heat treatment on antioxidant properties and colour stability of beech and spruce wood and their extractives. Wood Sci. Technol. 2009, 43, 69–83. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, X.; Yang, F.; Zhang, H.; Feng, X.; Zhang, J. Effect of thermal modification on the nano-mechanical properties of the wood cell wall and waterborne polyacrylic coating. Forests 2020, 11, 1247. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, X.; Liu, L.; Yu, Y.; Zheng, W.; Song, P. Probing Chemical Changes in Holocellulose and Lignin of Timbers in Ancient Buildings. Polymers 2019, 11, 809. [Google Scholar] [CrossRef]
- Popescu, C.M.; Hill, C.A.S. The water vapour adsorption–desorption behaviour of naturally aged Tilia cordata Mill. wood. Polym. Degrad. Stab. 2013, 98, 1804–1813. [Google Scholar] [CrossRef]
- Boonstra, M.; Acker, J.; Kegel, E.; Stevens, M. Optimisation of a two-stage heat treatment process: Durability aspects. Wood Sci. Technol. 2007, 41, 31–57. [Google Scholar] [CrossRef]
- Čabalová, I.; Bélik, M.; Kučerová, V.; Jurczyková, T. Chemical and morphological composition of Norway spruce wood (Picea abies, L.) in the dependence of its storage. Polymers 2021, 13, 1619. [Google Scholar] [CrossRef]
- Davim, J.P. Wood and Wood Products—Materials and Manufacturing Technology; Nova Novinka: Brighton, UK, 2013; 130p. [Google Scholar]
- Sundqvist, B.; Karlsson, O.; Westermark, U. Determination of formic-acid and acetic acid concentrations formed during hydrothermal treatment of birch wood and its relation to colour, strength and hardness. Wood Sci. Technol. 2006, 40, 549–556. [Google Scholar] [CrossRef]
- Nurmi, A.; Vuorinen, T.; Lappi, J. Chemical composition of Norway spruce heartwood, sapwood and transition zone wood. Wood Sci. Technol. 2013, 47, 319–335. [Google Scholar]
- Tsuchiya, K.; Tokiwa, Y. Influence of pH on the polymerization of Cellulose I studied by Molecular Dynamics Simulations. Langmuir 2018, 34, 9395–9403. [Google Scholar] [CrossRef]
- Gibson, L.T.; Watt, C. Acetic and formic acids emitted from wood samples and their effect on selected materials in museum environments. Chemistry. Corros. Sci. 2010, 52, 172–178. [Google Scholar] [CrossRef]
- Dupont, A.L.; Egasse, C.; Morin, A.; Vasseur, F. Comprehensive characterisation of cellulose-and lignocellulose-degradation products in aged papers: Capillary zone electrophoresis of low-molar mass organic acids, carbohydrates, and aromatic lignin derivatives. Carbohydr. Polym. 2007, 68, 1–16. [Google Scholar] [CrossRef]
- Risholm-Sundman, M.; Lundgren, M.; Vestin, E.; Herder, P. Emissions of acetic acid and other volatile organic compounds from different species of solid wood. Holz Roh Werkstoff 1998, 56, 125–129. [Google Scholar] [CrossRef]
- Ramalho, O.; Dupont, A.L.; Egasse, C.; Lattuati-Derieux, A. Emission rates of volatile organic compounds from paper. E-Preserv. Sci. 2009, 6, 53–59. [Google Scholar]
- Jablonský, M.; Botková, M.; Hroboňová, K. Accelerated ageing of wood-containing papers: Formation of weak acids and deterioration of tensile strength. Wood Res. 2012, 57, 419–434. [Google Scholar]
- Hunt, S.; Grau-Bove, J.; Schofield, E.; Gaisford, S. Effect of polyethylene glycol treatment on acetic acid emissions from wood. Forests 2021, 12, 1629. [Google Scholar] [CrossRef]
- Kraševec, I.; Menart, E.; Strlič, M.; Kralj Cigić, I. Validation of passive samplers for monitoring of acetic and formic acid in museum environments. Herit. Sci. 2021, 9, 19. [Google Scholar] [CrossRef]
- Smedemark, S.H.; Ryhl-Svendsen, M.; Schieweck, A. Quantification of formic acid and acetic acid emissions from heritage collections under indoor room conditions. Part I: Laboratory and field measurements. Herit. Sci. 2020, 8, 58. [Google Scholar] [CrossRef]
- Barbero-López, A.; Hossain, M.; Haapala, A. Antifungal activity of organic acids and their impact on wood decay resistance. Wood Fiber Sci. 2020, 52, 410–418. [Google Scholar] [CrossRef]
- Hamed, S.A.K.M.; Salem, M.Z.M.; Ali, H.M.; Ahmed, K.M.E.S. Investigating the impact of weathering and indoor aging on wood acidity using spectroscopic analyses. Bioresources 2020, 15, 6506–6525. [Google Scholar] [CrossRef]
- Straže, A.; Torkar, S.; Tišler, V.; Gorišek, Ž. Changes of ash wood pH-value during conventional drying. Zbornik Gozdarstva in Lesarstva 2003, 71, 107–124. [Google Scholar]
- Vojta, A.; Pažitný, A.; Ihnát, V.; Medo, P. Lower Quality Wood Processing Processes—Part II: Chemical Technologies; Research report VÚPC; Pulp and Paper Research Institute: Bratislava, Slovakia, 2018; 55p. (In Slovak) [Google Scholar]
- Xu, X.; Chen, S.; Lin, L.; Huang, L. The effects of temperature and pH on the degradation of cellulose. Polymers 2020, 12, 1635. [Google Scholar]
- Schubert, M.; Militz, H.; Mai, C. Influence of decay by white-rot fungi on the cellulose crystallinity of wood. Holzforschung 2017, 71, 21–27. [Google Scholar]
- Zakzeski, J.; Kremer, D.; Clouston, P.; Wolcott, M. Changes in the chemical composition of Norway spruce during storage. Wood Sci. Technol. 2019, 53, 467–481. [Google Scholar]
- Kärki, T.; Vaino, E.; Viitanen, H. Chemical changes in spruce and pine wood during storage in covered and uncovered piles. Wood Sci. Technol. 2004, 38, 363–374. [Google Scholar]
- Liu, H.; Lu, J.; Jiang, J.; Li, K.; Fang, G. Effect of storage time and temperature on the properties of radiata pine (Pinus radiata D. Don) Wood: Changes in chemical composition. Bioresources 2019, 14, 3188–3198. [Google Scholar]
- Vidholdová, Z.; Kačík, F.; Reinprecht, L.; Kučerová, V.; Luptáková, J. Changes in chemical structure of thermally modified spruce wood due to decaying fungi. J. Fungi 2022, 8, 739. [Google Scholar] [CrossRef]
- Sweet, M.S.; Winandy, J.E. Influence of degree of polymerization of cellulose and hemicellulose on strength loss in fire-retardant treated southern pine. Holzforschung 1999, 53, 311–317. [Google Scholar] [CrossRef]
- Čabalová, I.; Kačík, F.; Kačíková, D.; Oravec, M. The influence of radiant heating on chemical changes of spruce wood. Acta Fac. Xylologiae 2013, 55, 59–66. (In Slovak) [Google Scholar]
- Zachar, M.; Čabalová, I.; Kačíková, D.; Jurczyková, T. Effect of natural aging on oak wood fire resistance. Polymers 2021, 13, 2059. [Google Scholar] [CrossRef] [PubMed]
- Kačík, F.; Šmíra, P.; Kačíková, D.; Reinprecht, L.; Nasswettova, A. Chemical changes in fir wood from old buildings due to ageing. Cellul. Chem. Technol. 2014, 48, 79–88. [Google Scholar]
- Kačíková, D.; Kačík, F.; Čabalová, I.; Ďurkovič, J. Effects of thermal treatment on chemical, mechanical and colour traits in Norway spruce wood. Biores. Technol. 2013, 14, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Esteves, B.M.; Pereira, H.M. Wood modification by heat treatment a Review. Bioresources 2009, 4, 370–404. [Google Scholar] [CrossRef]
- Müller, J.; Varnai, A.; Johansson, E.; Olsson, E. Changes in chemical composition and saccharification during storage of willow and poplar chips. Biomass Bioenergy 2015, 81, 271–280. [Google Scholar] [CrossRef]
- Rasmussen, H.; Sørensen, H.R.; Meyer, A.S.; Pedersen, L.T. Effects of storage on the composition and structure of woody biomass for bioenergy. Biomass Bioenergy 2014, 63, 218–231. [Google Scholar] [CrossRef]
- Mader, P.P.; Cann, G.; Palmer, L. Effects of polluted atmospheres on organic acid composition in plant tissues. Plant Physiol. 1955, 30, 318–323. [Google Scholar] [CrossRef][Green Version]
- Malhotra, S.S.; Khan, A.A. Biochemical and Physiological Impact of Major Pollutants. In Air Pollution and Plant Life; Treshow, M., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 1984; pp. 113–157. [Google Scholar]
- Sroka-Bizoń, K.; Szymańska-Chargot, M.; Zdunek, A. Comparative study of Seifert method and HPLC for the determination of cellulose in plant samples. Pol. J. Environ. Stud. 2017, 26, 415–421. [Google Scholar]
- Antczak, A.; Michałuszko, A.; Kłosińska, T.; Droždžek, M. Determination of the structural substances content in the field maple wood (Acer campestre L.)—Comparison of the classical methods with instrumental. Ann. Wars. Univ. Life Sci. SGGW For. Wood Technol. 2013, 82, 11–17. [Google Scholar]
- Čabalová, I.; Zachar, M.; Kačík, F.; Tribulová, T. Impact of thermal loading on selected chemical and morphological properties of spruce ThermoWood. Bioresources 2019, 14, 387–400. [Google Scholar] [CrossRef]
- Sawoszczuk, T.; Wandelt, P.; Barański, A.; Łagan, J.M.; Łojewski, T.; Perlińska-Sipa, K. Degradation of Paper as Studied by Fiber Length Measurements After Hydrodynamical Treatment. In Proceedings of the International Conference Durability of Paper and Writing, Ljubljana, Slovenia, 16–22 November 2004; pp. 78–80. [Google Scholar]
- Harris, G. Comparison of northern softwood and southern pine fiber characteristics for groudwood publication paper. TAPPI 1993, 76, 55–61. [Google Scholar]
- Löönberg, B.; Bruun, H.; Lindquist, J. UV-microspectrophotometric study of wood and fibers. Paperi ja Puu 1991, 73, 848–851. [Google Scholar]
- Gerendiain, A.Z.; Peltola, H.; Pulkkinen, P.; Jaatinen, R.; Pappinen, A. Differences in fibre properties in cloned Norway spruce (Picea abies). Can. J. For. Res. 2008, 39, 1071–1082. [Google Scholar] [CrossRef]
- Baharoğlu, M.; Nemli, G.; Sarı, B.; Birtürk, T.; Bardak, S. Effects of anatomical and chemical properties of wood on the quality of particleboard, Compos. B Eng. 2013, 52, 282–285. [Google Scholar] [CrossRef]
- Ressel, J.B. Wood Yard Operations. In Handbook of Pulp; Sixta, H., Ed.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2006; 1352p. [Google Scholar]
| Storage Time/ Conditions | Average Air Humidity (%) | Average Air Temperature (°C) | Average Precipitation (mm) | Sample Moisture (%) | Sample Density (g·cm−3) |
|---|---|---|---|---|---|
| September–harvesting time | - | - | - | 9.35 | 0.420 |
| (pith: 0.388, perimeter: 0.452) | |||||
| from September to November | 88.22 | 8.63 | 0.87 | 9.29 | 0.423 |
| (max. 99.17, min. 69.09) | (max. 19.57, min. −1.52) | (max. 29.6, min. 0.87) | (pith: 0.403, perimeter: 0.448) | ||
| from November to January | 91.34 | −0.47 | 0.96 | 6.73 | 0.448 |
| (max. 99.17, min. 65.31) | (max. 11.334, min. −7.38) | (max. 15, min. 0) | (pith: 0.426, perimeter: 0.469) | ||
| from January to March | 72.12 | 3.45 | 0.94 | 6.82 | 0.443 |
| (max. 99.17, min. 38.13) | (max. 11.33, min −2.31) | (max. 15, min. 0) | (pith: 0.413, perimeter: 0.466) | ||
| from March to September | 71.68 | 17.30 | 1.59 | 7.79 | 0.430 |
| (max. 99.17, min. 43.25) | (max. 27.80, min. 1.47) | (max. 34.40, min. 0) | (pith: 0.396, perimeter: 0.464) | ||
| from September to December | 90.89 | 10.02 | 1.54 | 8.64 | 0.441 |
| (max. 100, min. 69.23) | (max. 19.46, min −0.28) | (max. 18.2, min 0) | (pith: 0.423, perimeter: 0.459) |
| Time of Wood Storage (Months) | pH Values | |
|---|---|---|
| Transverse Direction | Longitudinal Direction | |
| 0 | 5.32 ± 0.29 | 5.26 ± 0.22 |
| 2 | 4.25 ± 0.22 | 5.19 ± 0.17 |
| 4 | 4.43 ± 0.08 | 4.58 ± 0.26 |
| 6 | 4.59 ± 0.37 | 4.17 ± 0.06 |
| 12 | 4.89 ± 0.31 | 4.49 ± 0.34 |
| 15 | 4.28 ± 0.28 | 4.23 ± 0.58 |
| Time of Wood Storage (Months)/ Amount of Organic Acids | 0 | 2 | 4 | 6 | 12 | 15 |
|---|---|---|---|---|---|---|
| acetic acid (mL/L) | Ui | Ui | Ui | Ui | Ui | Ui |
| formic acid (mL/L) | Ui | Ui | Ui | Ui | Ui | Ui |
| Time of Wood Storage (Months)/ Characteristics | 0 | 2 | 4 | 6 | 12 | 15 |
|---|---|---|---|---|---|---|
| Mn | 14,037 ± 222 | 13,740 ± 48 | 13,550 ± 56 | 13,577 ± 29 | 12,858 ± 30 | 12,735 ± 47 |
| Mw | 139,542 ± 2142 | 136,971 ± 700 | 119,874 ± 741 | 120,197 ± 589 | 121,024 ± 170 | 126,622 ± 387 |
| PD | 9.94 ± 0.03 | 9.97 ± 0.02 | 8.85 ± 0.02 | 8.86 ± 0.02 | 9.41 ± 0.01 | 9.94 ± 0.01 |
| DP | 861 ± 13 | 846 ± 4 | 740 ± 5 | 742 ± 4 | 747 ± 1 | 782 ± 2 |
| Time of Wood Storage (Months)/ Saccharide Content (mg/g) | 0 | 2 | 4 | 6 | 12 | 15 | a Total Decrease (%) |
|---|---|---|---|---|---|---|---|
| GLC | 460.7 ± 3.5 | 470.1 ± 2.4 | 466.1 ± 2.0 | 479.1 ± 0.5 | 517.9 ± 0.9 | 379.9 ± 1.8 | 17.5 |
| XYL | 59.4 ± 1.5 | 60.7 ± 1.2 | 61.3 ± 1.1 | 65.2 ± 1.0 | 66.7 ± 1.2 | 33.8 ± 0.4 | 43.0 |
| GAL | 37.5 ± 0.7 | 37.0 ± 1.1 | 33.9 ± 1.5 | 39.12 ± 1.0 | 17.21 ± 0.8 | 14.6 ± 0.6 | 61.1 |
| ARA | 21.0 ± 1.8 | 13.0 ± 0.4 | 16.2 ± 0.5 | 13.9 ± 0.7 | 7.6 ± 0.2 | 5.9 ± 0.1 | 72.0 |
| MAN | 110.3 ± 3.0 | 110.7 ± 2.0 | 11.6 ± 0.2 | 102.3 ± 1.1 | 110.0 ± 3.4 | 78.0 ± 0.5 | 29.3 |
| SUM SACCH | 688.9 ± 6.0 | 691.5 ± 2.2 | 689.2 ± 3.0 | 699.6 ± 1.4 | 719.5 ± 1.3 | 512.2 ± 0.8 | 26.6 |
| GLC from HEMI | 36.8 ± 1.0 | 36.9 ± 0.7 | 37.2 ± 0.1 | 34.1 ± 0.4 | 36.7 ± 1.1 | 26.1 ± 0.2 | 29.3 |
| GLC from CEL | 423.9 ± 3.7 | 433.2 ± 3.0 | 428.9 ± 2.0 | 445.0 ± 0.3 | 481.2 ± 1.8 | 353.9 ± 2.0 | 16.5 |
| SUM HEMI | 264.9 ± 4.1 | 258.3 ± 2.0 | 260.3 ± 1.1 | 254.6 ± 1.6 | 238.3 ± 2.7 | 158.3 ± 1.5 | 40.2 |
| SUM PENT | 80.3 ± 2.0 | 73.7 ± 1.5 | 77.6 ± 0.8 | 79.1 ± 0.8 | 74.4 ± 0.8 | 39.7 ± 0.4 | 50.6 |
| SUM HEX | 608.5 ± 4.4 | 617.8 ± 2.3 | 611.6 ± 3.4 | 620.5 ± 1.2 | 645.1 ± 1.2 | 472.5 ± 0.7 | 22.4 |
| Ratio CEL/HEMI | 1.60 | 1.68 | 1.65 | 1.75 | 2.02 | 2.24 | - |
| Time of Wood Storage (Months) | 0 | 6 | 12 | 15 |
|---|---|---|---|---|
| average fiber length (mm) | 1.31 | 1.15 | 0.88 | 0.81 |
| average fiber width (µm) | 24.36 | 24.19 | 23.80 | 23.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čabalová, I.; Bélik, M.; Kučerová, V.; Jurczyková, T.; Bubeníková, T. Evaluation of Chemical and Morphological Properties of Spruce Wood Stored in the Natural Environment. Polymers 2023, 15, 4734. https://doi.org/10.3390/polym15244734
Čabalová I, Bélik M, Kučerová V, Jurczyková T, Bubeníková T. Evaluation of Chemical and Morphological Properties of Spruce Wood Stored in the Natural Environment. Polymers. 2023; 15(24):4734. https://doi.org/10.3390/polym15244734
Chicago/Turabian StyleČabalová, Iveta, Michal Bélik, Viera Kučerová, Tereza Jurczyková, and Tatiana Bubeníková. 2023. "Evaluation of Chemical and Morphological Properties of Spruce Wood Stored in the Natural Environment" Polymers 15, no. 24: 4734. https://doi.org/10.3390/polym15244734
APA StyleČabalová, I., Bélik, M., Kučerová, V., Jurczyková, T., & Bubeníková, T. (2023). Evaluation of Chemical and Morphological Properties of Spruce Wood Stored in the Natural Environment. Polymers, 15(24), 4734. https://doi.org/10.3390/polym15244734







