Mechanistic Insights into Anion-Induced Electrochromism of Ru(II)-Based Metallo-Supramolecular Polymer
Abstract
1. Introduction
2. Experiment
2.1. Materials
2.2. Synthesis
2.2.1. The Preparation of PolyRu Powder
2.2.2. The Preparation of PolyRu Films
2.3. Measurements
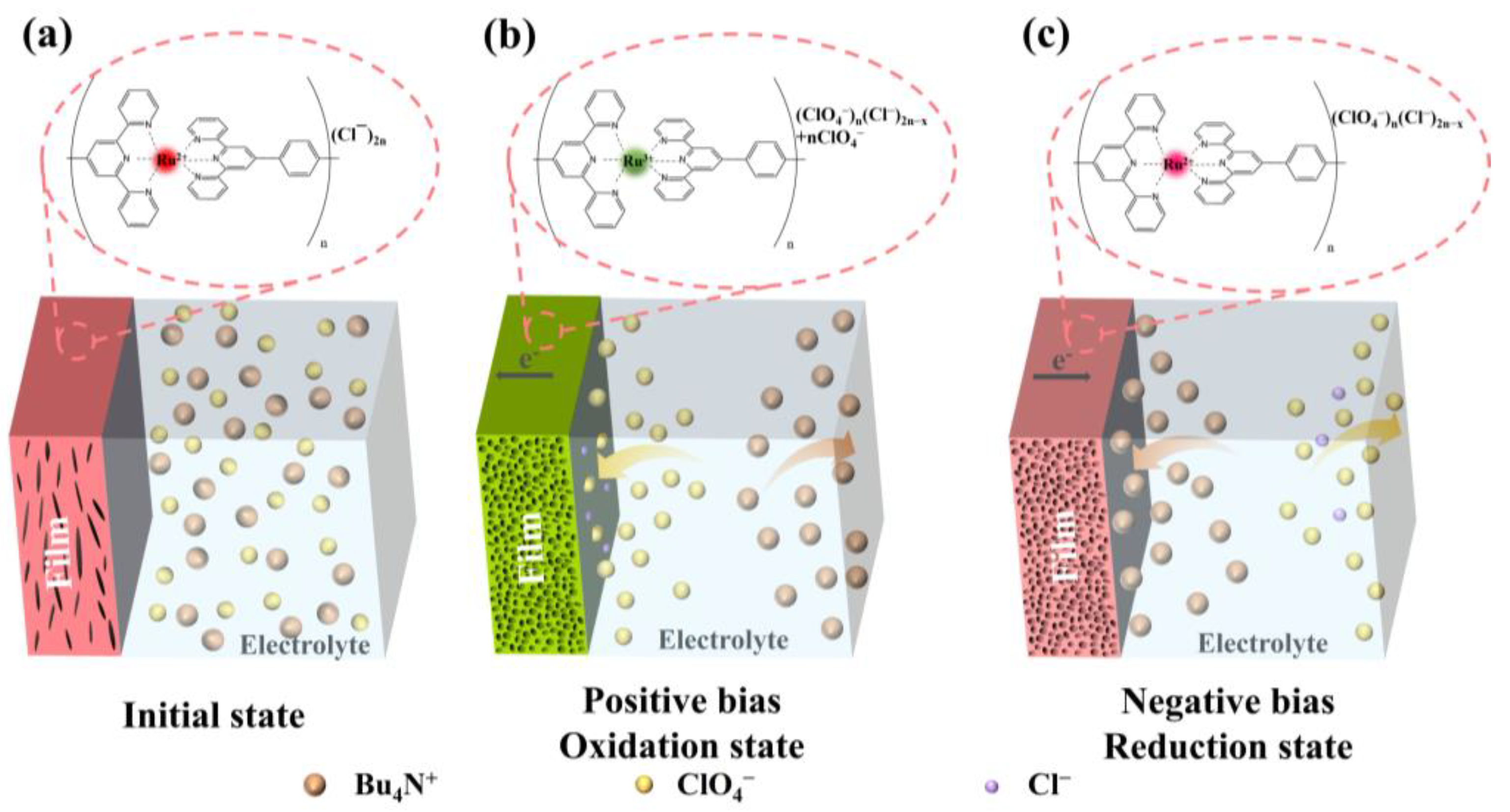
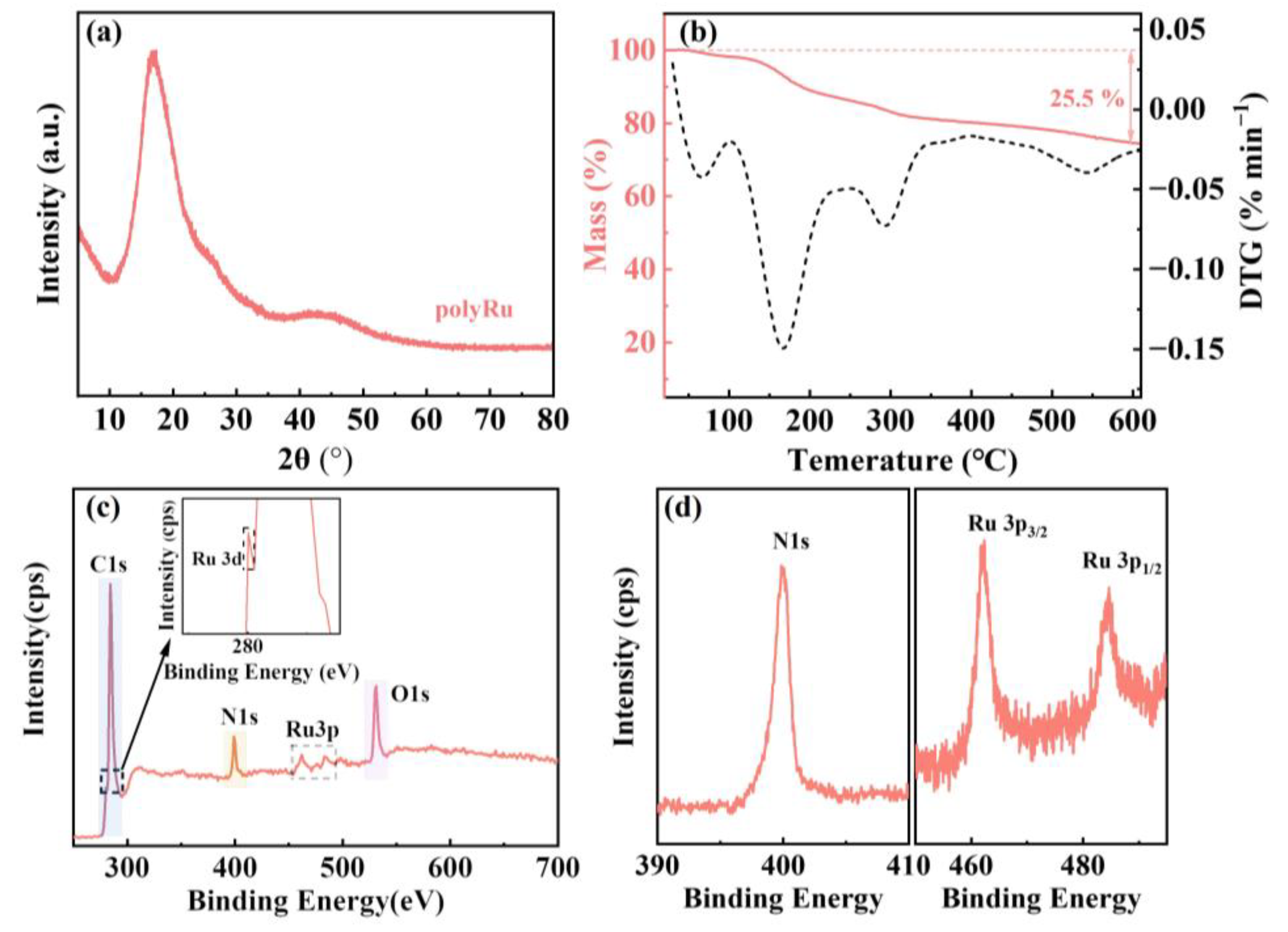
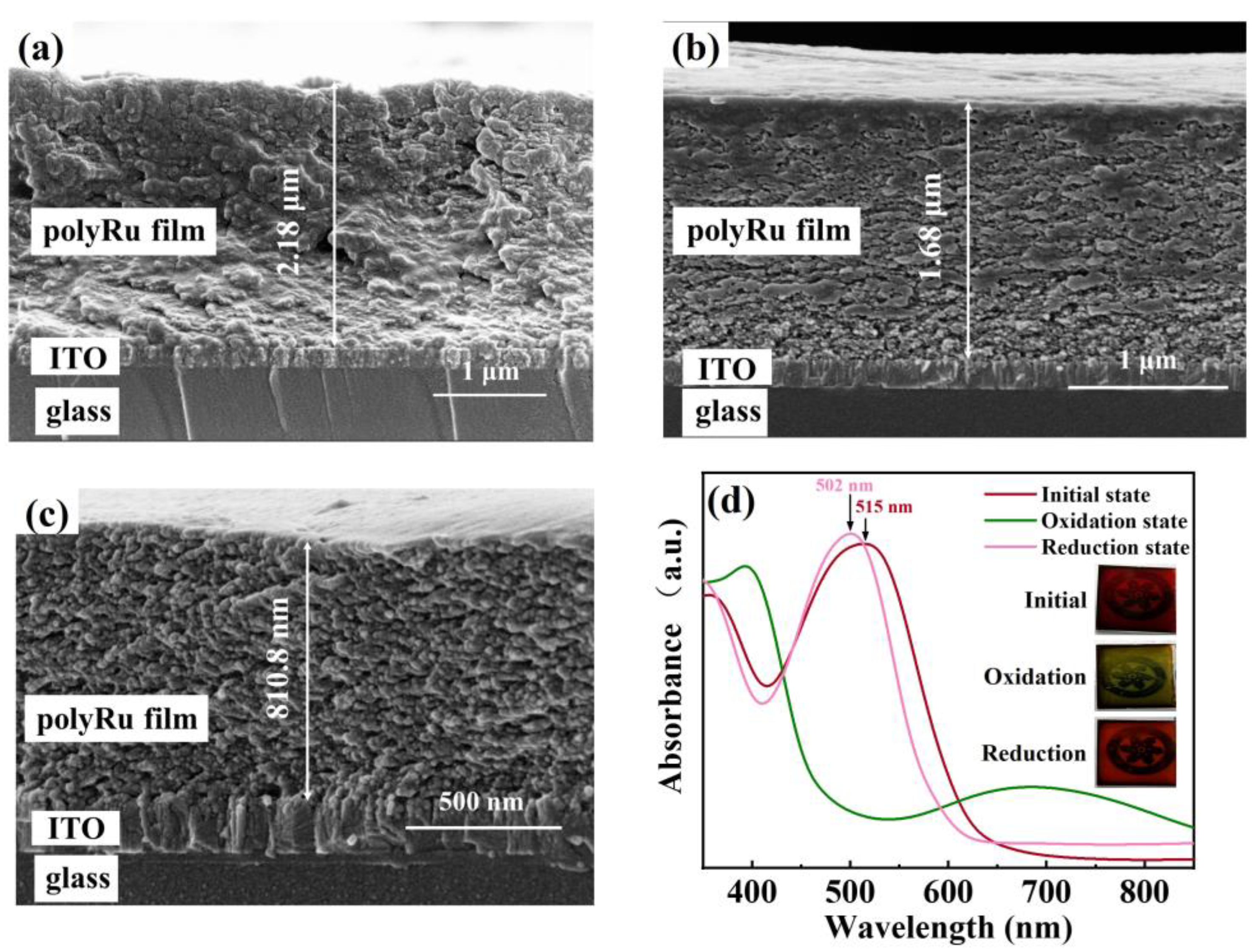
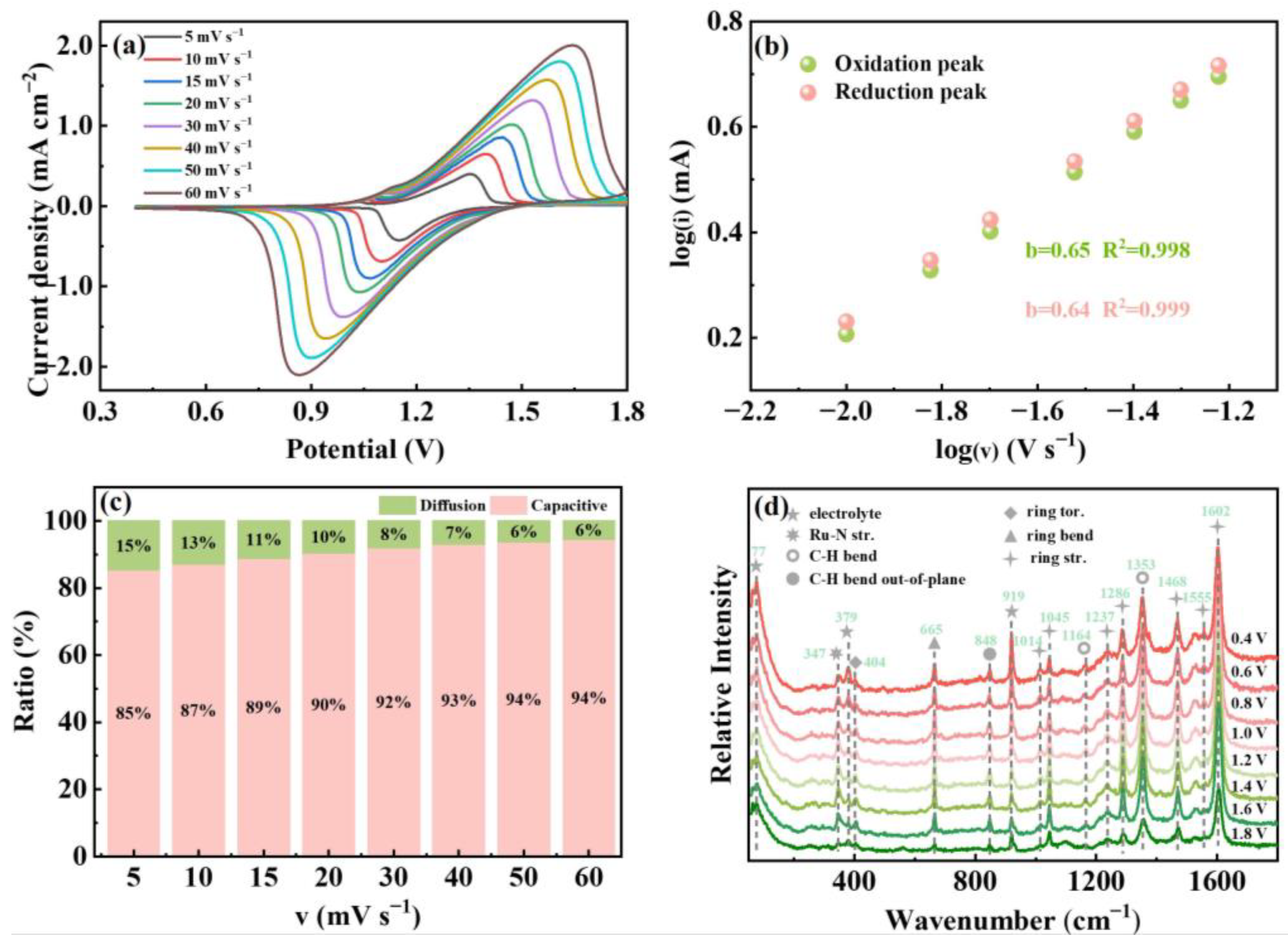
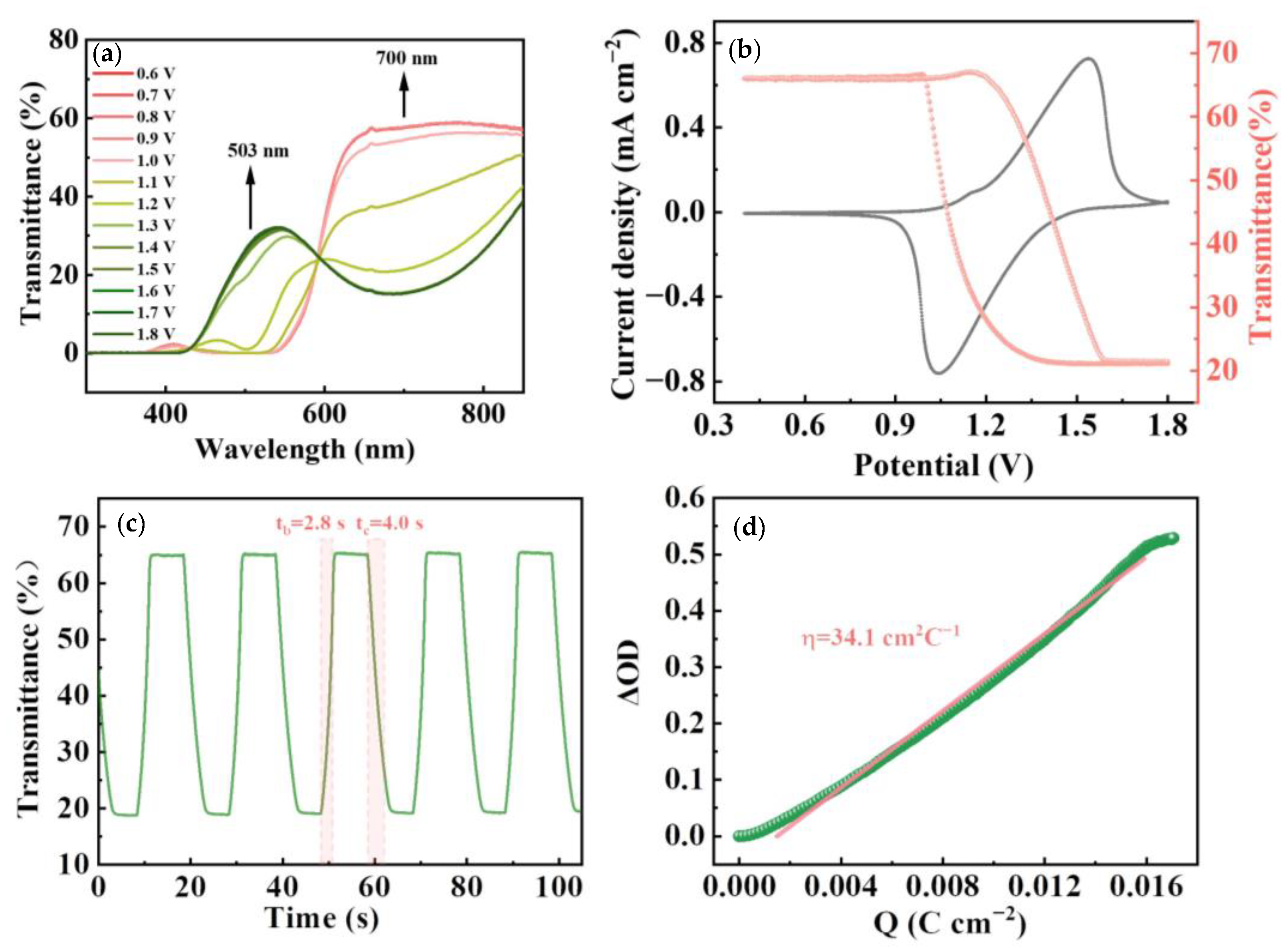
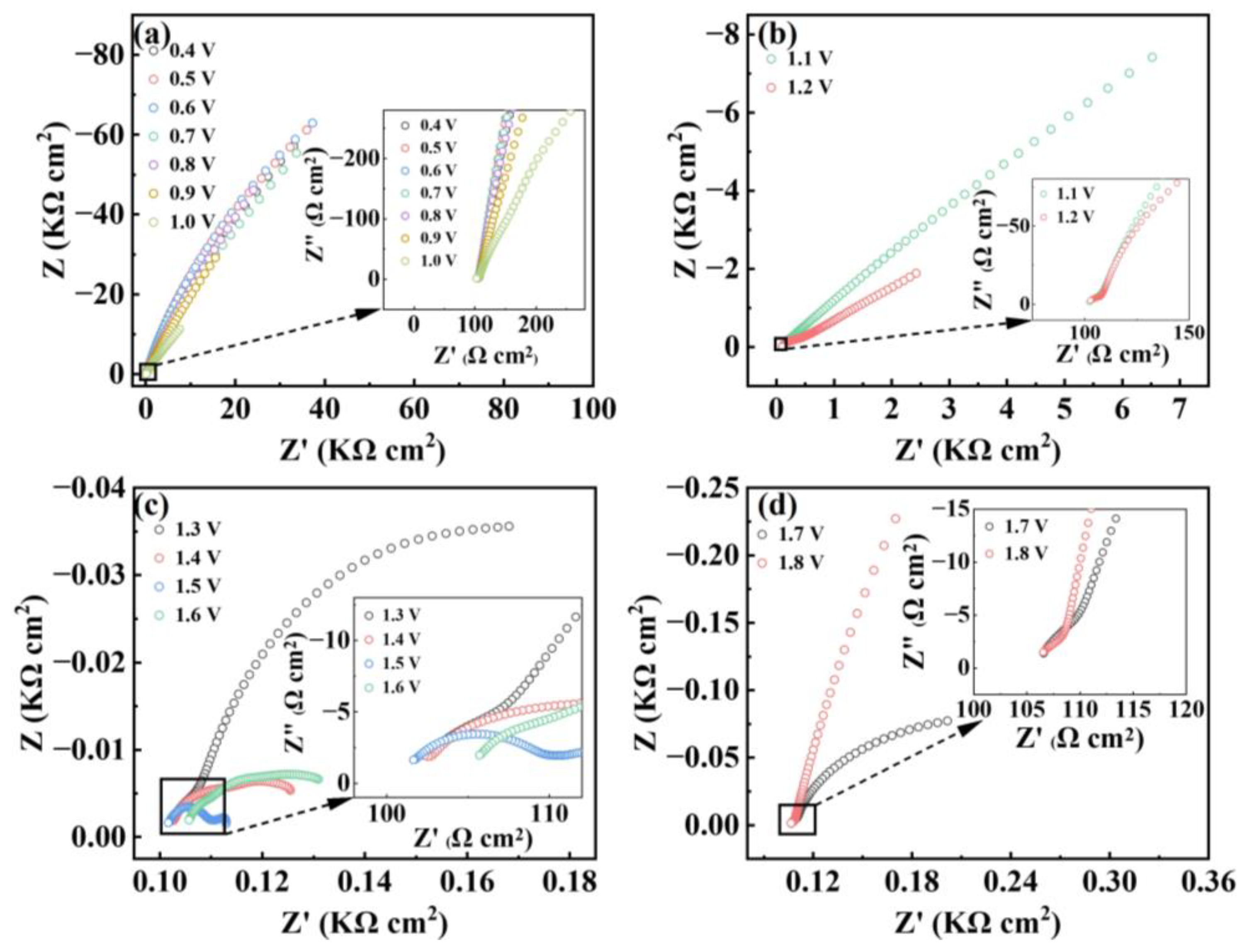
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, J.W.; Cho, N.; Woo, H.C.; Oh, B.M.; Almutlaq, J.; Bakr, O.M.; Kim, S.-H.; Lee, C.-L.; Kim, J.H. Investigation of high contrast and reversible luminescence thermochromism of the quantum confined Cs4PbBr6 perovskite solid. Nanoscale 2019, 11, 5754–5759. [Google Scholar] [CrossRef] [PubMed]
- Sekine, A. In-situ crystal structure analysis and control of photochromism with dual-mode photoreactive soft crystals. J. Photochem. Photobiol. C Photochem. Rev. 2022, 51, 100480. [Google Scholar] [CrossRef]
- Li, E.; Jie, K.; Liu, M.; Sheng, X.; Zhu, W.; Huang, F. Vapochromic crystals: Understanding vapochromism from the perspective of crystal engineering. Chem. Soc. Rev. 2020, 49, 1517–1544. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, M. Stimuli-responsive metallo-supramolecular polymer films: Design, synthesis and device fabrication. J. Mater. Chem. C 2014, 2, 9331–9341. [Google Scholar] [CrossRef]
- Santra, D.C.; Mondal, S.; Yoshida, T.; Ninomiya, Y.; Higuchi, M. Ru(II)-Based Metallo-Supramolecular Polymer with Tetrakis(N-methylbenzimidazolyl)bipyridine for a Durable, Nonvolatile, and Electrochromic Device Driven at 0.6 V. ACS Appl. Mater. Interfaces 2021, 13, 31153–31162. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Sun, W.; Maleski, K.; Shuck, C.E.; Li, K.; Urbankowski, P.; Hantanasirisakul, K.; Wang, X.; Kent, P.; et al. Intercalation-Induced Reversible Electrochromic Behavior of Two-Dimensional Ti3C2Tx MXene in Organic Electrolytes. ChemElectroChem 2021, 8, 151–156. [Google Scholar] [CrossRef]
- Bai, Z.; Li, R.; Ping, L.; Fan, Q.; Lu, Z.; Hou, C.; Zhang, Q.; Li, Y.; Li, K.; Ling, X.; et al. Photo-induced self-reduction enabling ultralow threshold voltage energy-conservation electrochromism. Chem. Eng. J. 2023, 452, 139645. [Google Scholar] [CrossRef]
- Bi, S.; Jin, W.; Han, X.; Cao, X.; He, Z.; Asare-Yeboah, K.; Jiang, C. Ultra-fast-responsivity with sharp contrast integrated flexible piezo electrochromic based tactile sensing display. Nano Energy 2022, 102, 107629. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, B.; Chen, F.; Han, Y.; Zhang, W.; Wu, X.; Li, R.; Jiang, Q.; Jia, X.; Zhang, R. Electrochromic Materials Based on Ions Insertion and Extraction. Adv. Opt. Mater. 2022, 10, 2101783. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, W.; Tam, B.; Zhang, H.; Zhou, Y.; Yong, L.; Cheng, W. Pseudocapacitive porous amorphous vanadium pentoxide with enhanced multicolored electrochromism. Chem. Eng. J. 2023, 452, 139655. [Google Scholar] [CrossRef]
- Kim, J.; Lee, K.H.; Lee, S.; Park, S.; Chen, H.; Kim, S.K.; Yim, S.; Song, W.; Lee, S.S.; Yoon, D.H.; et al. Minimized optical scattering of MXene-derived 2D V2O5 nanosheet-based electrochromic device with high multicolor contrast and accuracy. Chem. Eng. J. 2023, 453, 139973. [Google Scholar] [CrossRef]
- Zhao, L.; Jiang, C.; Chao, J.; Cai, Z.; Chen, Y.; Liang, X.; Zhong, G.; Hu, B.; Miao, L.; Liao, W. Rational design of nickel oxide/cobalt hydroxide heterostructure with configuration towards high-performance electrochromic-supercapacitor. Appl. Surf. Sci. 2023, 609, 155279. [Google Scholar] [CrossRef]
- Lee, J.T.; Das, D.; Davis, G.A., Jr.; Hati, S.; Ramana, C.V.; Sardar, R. Inorganic–Organic Interfacial Electronic Effects in Ligand-Passivated WO3–x Nanoplatelets Induce Tunable Plasmonic Properties for Smart Windows. ACS Appl. Nano Mater. 2022, 5, 9970–9980. [Google Scholar] [CrossRef]
- Ma, Q.; Chen, J.; Zhang, H.; Su, Y.; Jiang, Y.; Dong, S. Dual-Function Self-Powered Electrochromic Batteries with Energy Storage and Display Enabled by Potential Difference. ACS Energy Lett. 2022, 8, 306–313. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, M.; Mei, Z.; Diao, X. Novel Prussian White@MnO2-Based Inorganic Electrochromic Energy Storage Devices with Integrated Flexibility, Multicolor, and Long Life. ACS Appl. Mater. Interfaces 2022, 14, 48833–48843. [Google Scholar] [CrossRef] [PubMed]
- Andersson Ersman, P.; Boda, U.; Petsagkourakis, I.; Åhlin, J.; Posset, U.; Schott, M.; Brooke, R. Reflective and Complementary Transmissive All-Printed Electrochromic Displays Based on Prussian Blue. Adv. Eng. Mater. 2022, 25, 2201299. [Google Scholar] [CrossRef]
- Halder, S.; Roy, S.; Chakraborty, C. Multicolored and durable electrochromism in water soluble naphthalene and perylene based diimides. Sol. Energy Mater. Sol. Cells 2022, 234, 111429. [Google Scholar] [CrossRef]
- Sun, F.; Zhang, H.; Cai, J.; Su, F.; Tian, Y.; Liu, Y.J. Selenophene, thiophene, and furan functionalized π-extended viologen derivatives for tunable all-in-one ECDs. Sol. Energy Mater. Sol. Cells 2023, 250, 112106. [Google Scholar] [CrossRef]
- Xie, X.; Yu, J.; Li, Z.; Wu, Z.; Chen, S. Self-healable PEDOT-based all-organic films with excellent electrochromic performances. New J. Chem. 2022, 46, 21167–21175. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, Q.; Luo, X.; Ma, H.; Zheng, W.; Yu, J.; Zhang, Z.; Zhang, K.; Qu, K.; Yang, R.; et al. Low-Cost Fabrication of High-Performance Fluorinated Polythiophene-Based Vis–NIR Electrochromic Devices toward Deformable Display and Camouflage. Chem. Mater. 2022, 34, 9923–9933. [Google Scholar] [CrossRef]
- Yoshida, T.; Bera, M.K.; Narayana, Y.S.L.V.; Mondal, S.; Abe, H.; Higuchi, M. Electrochromic Os-based metallo-supramolecular polymers: Electronic state tracking by in situ XAFS, IR, and impedance spectroscopies. RSC Adv. 2020, 10, 24691–24696. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, L.-Y.; Chang, T.-H.; Lu, H.-C.; Wang, Y.-C.; Lu, Y.-A.; Ho, K.-C.; Higuchi, M. A panchromatic electrochromic device composed of Ru(ii)/Fe(ii)-based heterometallo-supramolecular polymer. J. Mater. Chem. C 2019, 7, 7554–7562. [Google Scholar] [CrossRef]
- Cai, G.; Chen, J.; Xiong, J.; Lee-Sie Eh, A.; Wang, J.; Higuchi, M.; Lee, P.S. Molecular Level Assembly for High-Performance Flexible Electrochromic Energy-Storage Devices. ACS Energy Lett. 2020, 5, 1159–1166. [Google Scholar] [CrossRef]
- Chen, W.-H.; Chang, T.-H.; Hu, C.-W.; Ting, K.-M.; Liao, Y.-C.; Ho, K.-C. An electrochromic device composed of metallo-supramolecular polyelectrolyte containing Cu(I) and polyaniline-carbon nanotube. Sol. Energy Mater. Sol. Cells 2014, 126, 219–226. [Google Scholar] [CrossRef]
- Lu, H.-C.; Hsiao, L.-Y.; Kao, S.-Y.; Seino, Y.; Santra, D.C.; Ho, K.-C.; Higuchi, M. Durable Electrochromic Devices Driven at 0.8 V by Complementary Chromic Combination of Metallo-Supramolecular Polymer and Prussian Blue Analogues for Smart Windows with Low-Energy Consumption. ACS Appl. Electron. Mater. 2021, 3, 2123–2135. [Google Scholar] [CrossRef]
- Han, F.S.; Higuchi, M.; Kurth, D.G. Metallosupramolecular polyelectrolytes self-assembled from various pyridine ring-substituted bisterpyridines and metal ions: Photophysical, electrochemical, and electrochromic properties. J. Am. Chem. Soc. 2008, 130, 2073–2081. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Yoshida, T.; Maji, S.; Ariga, K.; Higuchi, M. Transparent Supercapacitor Display with Redox-Active Metallo-Supramolecular Polymer Films. ACS Appl. Mater. Interfaces 2020, 12, 16342–16349. [Google Scholar] [CrossRef]
- Roy, S.; Chakraborty, C. Nanostructured metallo-supramolecular polymer-based gel-type electrochromic devices with ultrafast switching time and high colouration efficiency. J. Mater. Chem. C 2019, 7, 2871–2879. [Google Scholar] [CrossRef]
- Eguchi, M.; Momotake, M.; Inoue, F.; Oshima, T.; Maeda, K.; Higuchi, M. Inert Layered Silicate Improves the Electrochemical Responses of a Metal Complex Polymer. ACS Appl. Mater. Interfaces 2017, 9, 35498–35503. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Cui, J.; Lv, X.; Feng, M.; Ouyang, M.; Chen, Z.; Wright, D.S.; Zhang, C. Hydrogen-Bonding Induced Crosslinked Polymer Network for Highly Stable Electrochromic Device and a Construction Strategy for Black-Bilayer Electrochromic Film. Small 2023, 19, 2303359. [Google Scholar] [CrossRef]
- Klein, J.; Alarslan, F.; Steinhart, M.; Haase, M. Cerium-Modified Mesoporous Antimony Doped Tin Oxide as Intercalation-Free Charge Storage Layers for Electrochromic Devices. Adv. Funct. Mater. 2022, 33, 2210167. [Google Scholar] [CrossRef]
- Zhang, J.; Hsu, C.-Y.; Higuchi, M. Anion Effects to Electrochromic Properties of Ru-based Metallo-supramolecular Polymers. J. Photopolym. Sci. Technol. 2014, 27, 297–300. [Google Scholar] [CrossRef][Green Version]
- Pai, S.; Schott, M.; Niklaus, L.; Posset, U.; Kurth, D.G. A study of the effect of pyridine linkers on the viscosity and electrochromic properties of metallo-supramolecular coordination polymers. J. Mater. Chem. C 2018, 6, 3310–3321. [Google Scholar] [CrossRef]
- Griffin, J.M.; Forse, A.C.; Tsai, W.-Y.; Taberna, P.-L.; Simon, P.; Grey, C.P. In situ NMR and electrochemical quartz crystal microbalance techniques reveal the structure of the electrical double layer in supercapacitors. Nat. Mater. 2015, 14, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, M.; Kaizu, Y. Syntheses, absorption spectra, electrochemistry and MLCT excited-state properties of [Ru(imin)n(bpy)3−n] (PF6)2 (n = 0−3, imin = 2-(N-methylformimidoyl)pyridine) complexes. Inorg. Chim. Acta 1996, 247, 155–159. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Hu, C.-W.; Sato, T.; Zhang, J.; Moriyama, S.; Higuchi, M. Multi-colour electrochromic properties of Fe/Ru-based bimetallo-supramolecular polymers. J. Mater. Chem. C 2013, 1, 3408–3413. [Google Scholar] [CrossRef]
- Brezesinski, T.; Wang, J.; Tolbert, S.H.; Dunn, B. Ordered mesoporous α-MoO3 with iso-oriented nanocrystalline walls for thin-film pseudocapacitors. Nat. Mater. 2010, 9, 146–151. [Google Scholar] [CrossRef]
- Yang, P.; Sun, P.; Du, L.; Liang, Z.; Xie, W.; Cai, X.; Huang, L.; Tan, S.; Mai, W. Quantitative Analysis of Charge Storage Process of Tungsten Oxide that Combines Pseudocapacitive and Electrochromic Properties. J. Phys. Chem. C 2015, 119, 16483–16489. [Google Scholar] [CrossRef]
- Laschuk, N.O.; Ebralidze, I.I.; Easton, E.B.; Zenkina, O.V. Systematic Design of Electrochromic Energy Storage Devices Based on Metal–Organic Monolayers. ACS Appl. Energy Mater. 2021, 4, 3469–3479. [Google Scholar] [CrossRef]
- Hansen, P.W.; Jensen, P.W. Vibrational studies on bis-terpyridine-ruthenium(II) complexes. Spectrochim. Acta Pt. A Mol. Spectrosc. 1994, 50, 169–183. [Google Scholar] [CrossRef]
- Ricci, A.M.; Tognalli, N.; de la Llave, E.; Vericat, C.; Méndez De Leo, L.P.; Williams, F.J.; Scherlis, D.; Salvarezza, R.; Calvo, E.J. Electrochemistry of Os(2,2′-bpy)2ClPyCH2NHCOPh tethered to Au electrodes by S–Au and C–Au junctions. Phys. Chem. Chem. Phys. 2011, 13, 5336–5345. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, G.; Pande, G.K.; Choi, J.H.; Park, J.S. Enhanced electrochromic properties of terpyridine-attached asymmetric viologen with high transmittance and switching stability. Sol. Energy Mater. Sol. Cells 2020, 216, 110714. [Google Scholar] [CrossRef]
- Ahmad, R.; Laschuk, N.O.; Ebralidze, I.I.; Zenkina, O.V.; Easton, E.B. Probing the Influence of Counter Electrode Structure on Electrochromic-Device Operating Potentials and Performance Using Electrochemical Impedance Spectroscopy. ChemElectroChem 2021, 8, 2193–2204. [Google Scholar] [CrossRef]
- Laschuk, N.O.; Easton, E.B.; Zenkina, O.V. Reducing the resistance for the use of electrochemical impedance spectroscopy analysis in materials chemistry. RSC Adv. 2021, 11, 27925–27936. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, X.; Zhang, Z.; Cao, Z.; Rogachev, A.A.; Yarmolenko, M.A.; Chen, T.; Cao, H.; Zhang, H. Mechanistic Insights into Anion-Induced Electrochromism of Ru(II)-Based Metallo-Supramolecular Polymer. Polymers 2023, 15, 4735. https://doi.org/10.3390/polym15244735
Fu X, Zhang Z, Cao Z, Rogachev AA, Yarmolenko MA, Chen T, Cao H, Zhang H. Mechanistic Insights into Anion-Induced Electrochromism of Ru(II)-Based Metallo-Supramolecular Polymer. Polymers. 2023; 15(24):4735. https://doi.org/10.3390/polym15244735
Chicago/Turabian StyleFu, Xiaofang, Zhuohui Zhang, Zhenhu Cao, Alexandr Alexandrovich Rogachev, Maxim Anatolievich Yarmolenko, Tao Chen, Hongtao Cao, and Hongliang Zhang. 2023. "Mechanistic Insights into Anion-Induced Electrochromism of Ru(II)-Based Metallo-Supramolecular Polymer" Polymers 15, no. 24: 4735. https://doi.org/10.3390/polym15244735
APA StyleFu, X., Zhang, Z., Cao, Z., Rogachev, A. A., Yarmolenko, M. A., Chen, T., Cao, H., & Zhang, H. (2023). Mechanistic Insights into Anion-Induced Electrochromism of Ru(II)-Based Metallo-Supramolecular Polymer. Polymers, 15(24), 4735. https://doi.org/10.3390/polym15244735







