Ag2O-Containing Biocidal Interpolyelectrolyte Complexes on Glass Surfaces—Adhesive Properties of the Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
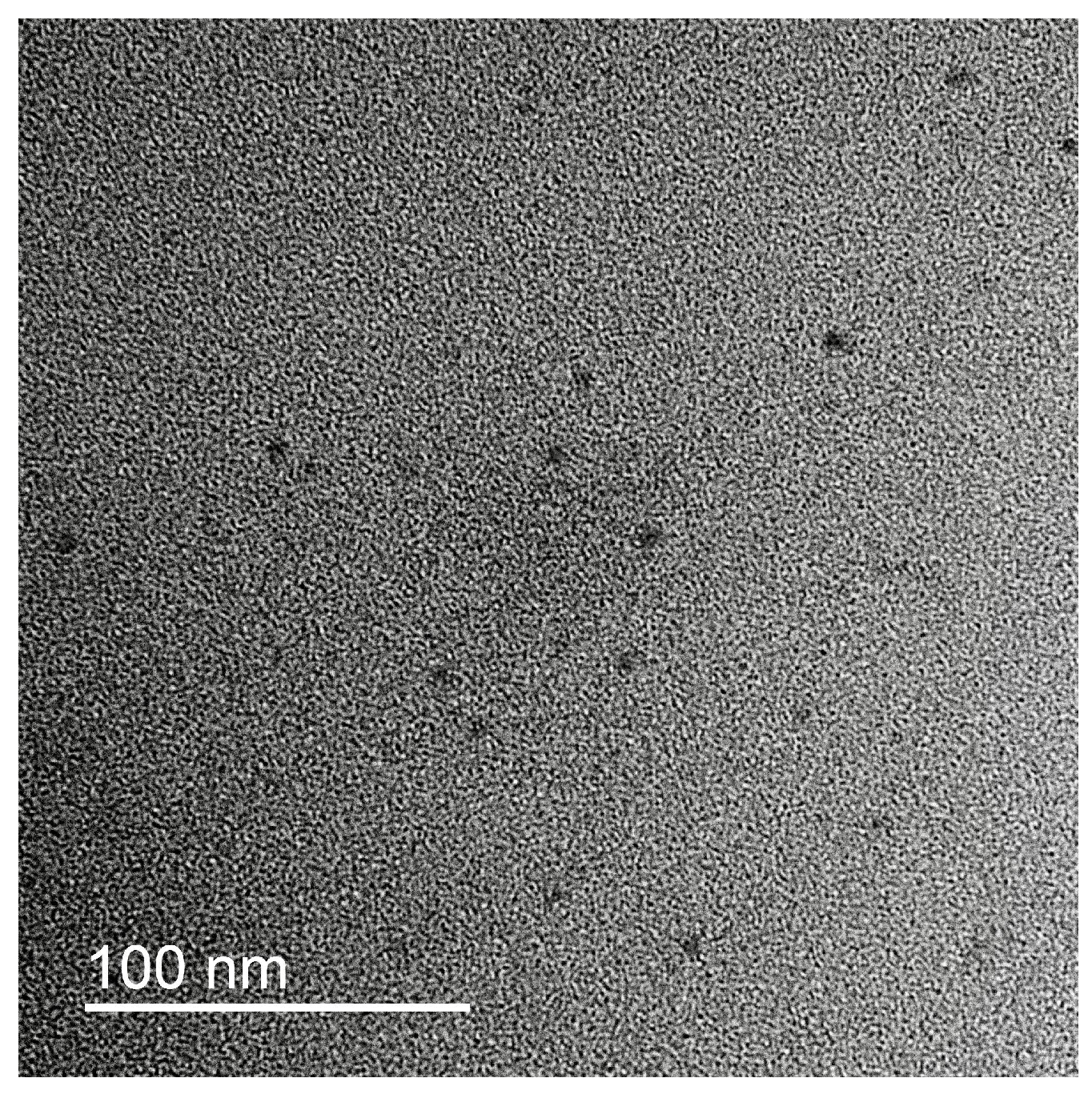
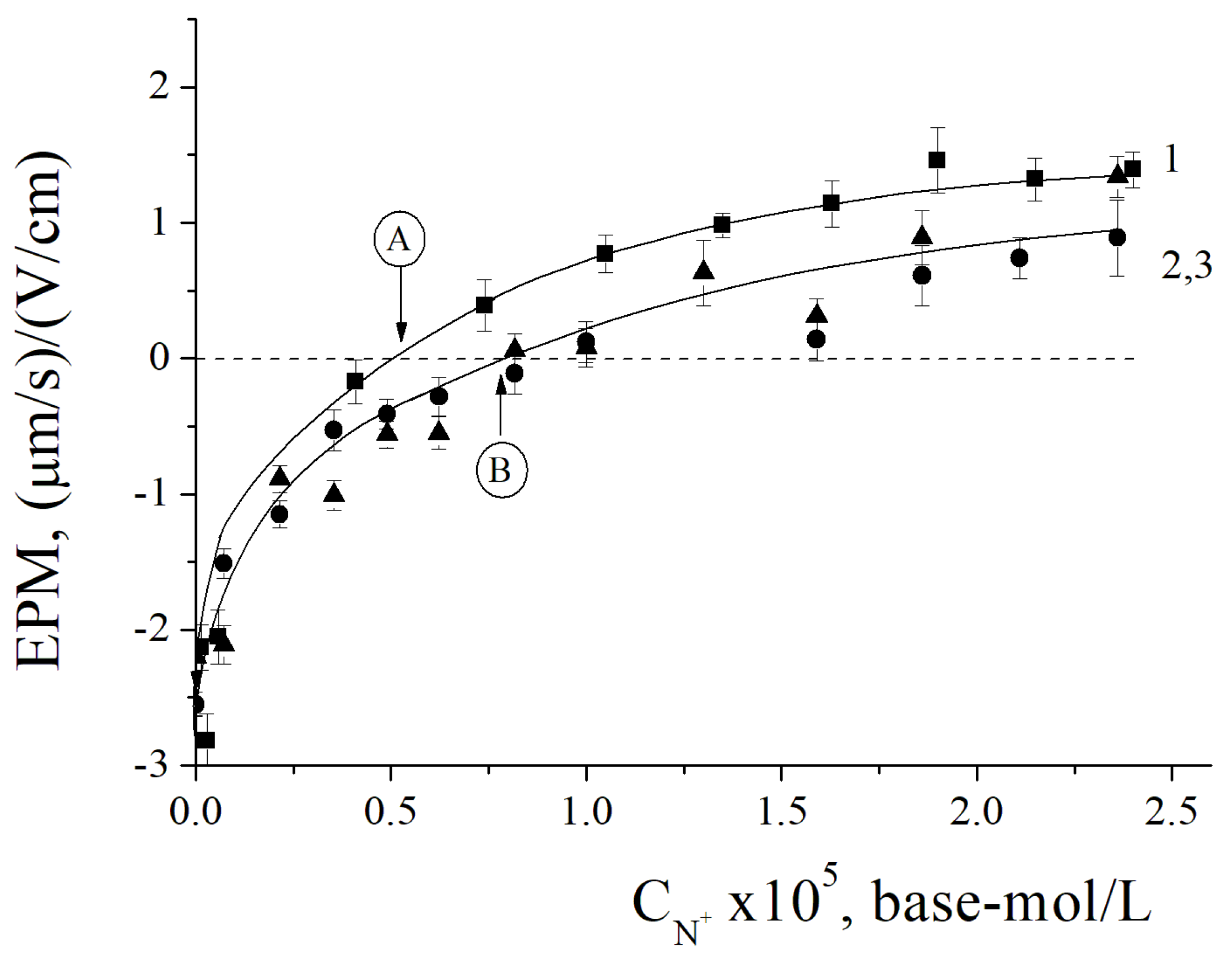
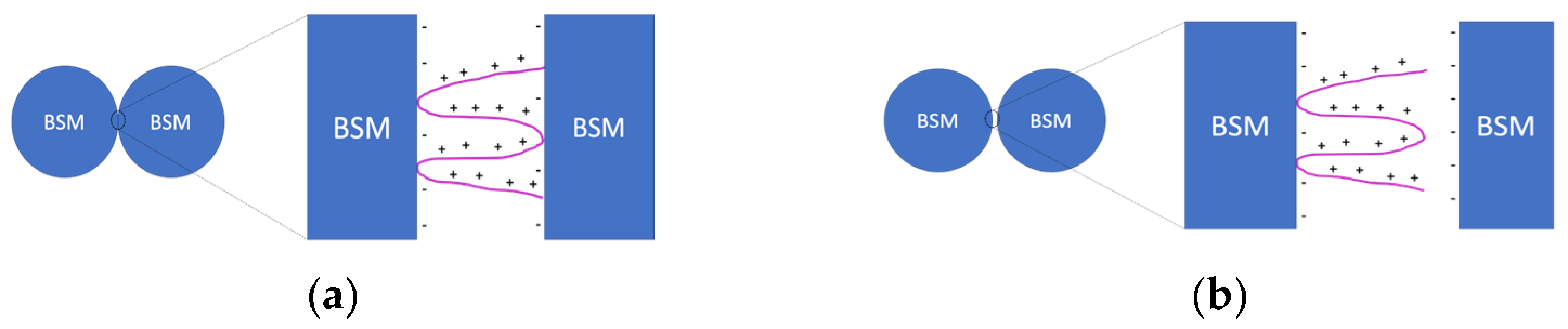
3.1. IPEC/Ag2O Complexes Characterization
3.2. Biocidal Properties of IPEC/Ag2O
3.3. Shelf-Life of IPEC/Ag2O Complexes
3.4. Formation of Polymer Coatings on Glass Surface
3.5. Mechanical Properties of PDADMAC and IPEC Coatings
3.6. Mechanical Properties of PDADMAC and IPEC Coatings on Submicron-Scale
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tarashi, S.; Siadat, S.D.; Fateh, A. Nontuberculous Mycobacterial Resistance to Antibiotics and Disinfectants: Challenges Still Ahead. Biomed Res Int. 2022, 2022, 8168750. [Google Scholar] [CrossRef]
- Boyce, J.M. Quaternary ammonium disinfectants and antiseptics: Tolerance, resistance and potential impact on antibiotic resistance. Antimicrob. Resist. Infect. Control 2023, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Protano, C.; Cammalleri, V.; Romano Spica, V.; Valeriani, F.; Vitali, M. Hospital environment as a reservoir for cross transmission: Cleaning and disinfection procedures. Ann. Ig. 2019, 31, 436–448. [Google Scholar] [PubMed]
- Slaughter, R.J.; Watts, M.; Vale, J.A.; Grieve, J.R.; Schep, L.J. The clinical toxicology of sodium hypochlorite. Clin Toxicol. 2019, 57, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Rutala, W.A.; Weber, D.J. Disinfection and Sterilization in Health Care Facilities: An Overview and Current Issues. Infect. Dis. Clin. North Am. 2021, 35, 575–607. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, L.; Guo, A.; Zhang, X.; Liu, W.; Ruan, Y. Formation of Multispecies Biofilms and Their Resistance to Disinfectants in Food Processing Environments: A Review. J. Food Prot. 2021, 84, 2071–2083. [Google Scholar] [CrossRef] [PubMed]
- Mc Carlie, S.; Boucher, C.E.; Bragg, R.R. Molecular basis of bacterial disinfectant resistance. Drug Resist. Updates 2020, 48, 100672. [Google Scholar] [CrossRef] [PubMed]
- Bharti, B.; Li, H.; Ren, Z.; Zhu, R.; Zhu, Z. Recent advances in sterilization and disinfection technology: A review. Chemosphere 2022, 308, 136404. [Google Scholar] [CrossRef]
- Duze, S.T.; Marimani, M.; Patel, M. Tolerance of Listeria monocytogenes to biocides used in food processing environments. Food Microbiol. 2021, 97, 103758. [Google Scholar] [CrossRef]
- Weber, D.J.; Rutala, W.A.; Sickbert-Bennett, E.E.; Kanamori, H.; Anderson, D. Prevention Epicenters Program. Continuous room decontamination technologies. Am. J. Infect. Control. 2019, 47S, A72–A78. [Google Scholar] [CrossRef]
- Arzani, F.A.; Dos Santos, J.H.Z. Biocides and techniques for their encapsulation: A review. Soft Matter. 2022, 18, 5340–5358. [Google Scholar] [CrossRef] [PubMed]
- Tiedje, J.M.; Fu, Y.; Mei, Z.; Schäffer, A.; Dou, Q.; Amelung, W.; Elsner, M.; Adu-Gyamfi, J.; Heng, L.; Virta, M.; et al. Antibiotic resistance genes in food production systems support One Health opinions. Curr. Opin. Environ. Sci. 2023, 34, 100492. [Google Scholar] [CrossRef]
- Ben, Y.; Fu, C.; Hu, M.; Liu, L.; Wong, M.H.; Zheng, C. Human health risk assessment of antibiotic resistance associated with antibiotic residues in the environment: A review. Environ. Res. 2019, 169, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Carrascosa, C.; Raheem, D.; Ramos, F.; Saraiva, A.; Raposo, A. Microbial Biofilms in the Food Industry-A Comprehensive Review. Int. J. Environ. Res. Public Health 2021, 18, 2014. [Google Scholar] [CrossRef] [PubMed]
- Pinho, A.C.; Piedade, A.P. Polymeric Coatings with Antimicrobial Activity: A Short Review. Polymers 2020, 12, 2469. [Google Scholar] [CrossRef] [PubMed]
- Weththimuni, M.L.; Chobba, M.B.; Sacchi, D.; Messaoud, M.; Licchelli, M. Durable Polymer Coatings: A Comparative Study of PDMS-Based Nanocomposites as Protective Coatings for Stone Materials. Chemistry 2022, 4, 60–76. [Google Scholar] [CrossRef]
- Song, F.; Zhang, L.; Chen, R.; Liu, Q.; Liu, J.; Yu, J.; Liu, P.; Duan, J.; Wang, J. Bioinspired Durable Antibacterial and Antifouling Coatings Based on Borneol Fluorinated Polymers: Demonstrating Direct Evidence of Antiadhesion. ACS Appl. Mater. Interfaces 2021, 13, 33417–33426. [Google Scholar] [CrossRef]
- Pigareva, V.A.; Senchikhin, I.N.; Bolshakova, A.V.; Sybachin, A.V. Modification of Polydiallyldimethylammonium Chloride with Sodium Polystyrenesulfonate Dramatically Changes the Resistance of Polymer-Based Coatings towards Wash-Off from Both Hydrophilic and Hydrophobic Surfaces. Polymers 2022, 14, 1247. [Google Scholar] [CrossRef]
- Pigareva, V.A.; Bolshakova, A.V.; Marina, V.I.; Sybachin, A.V. Water-Soluble Interpolyelectrolyte Complex Based on Poly(diallyldimethylammonium chloride) and Sodium Polyacrylate as a Component for Creating Stable Biocidal Coatings. Colloid J. 2023, 85, 433–441. [Google Scholar] [CrossRef]
- Wang, L.; Xin, M.; Li, M.; Liu, W.; Mao, Y. Effect of the Structure of Chitosan Quaternary Phosphonium Salt and Chitosan Quaternary Ammonium Salt on the Antibacterial and Antibiofilm Activity. Int. J. Biol. Macromol. 2023, 242, 124877. [Google Scholar] [CrossRef]
- Lan, T.; Guo, Q.; Shen, X. Polyethyleneimine and Quaternized Ammonium Polyethyleneimine: The Versatile Materials for Combating Bacteria and Biofilms. J. Biomater. Sci. Polym. Ed. 2019, 30, 1243–1259. [Google Scholar] [CrossRef] [PubMed]
- Kašparová, P.; Zmuda, M.; Vaňková, E.; Maťátková, O.; Masák, J. Low-Molecular Weight Chitosan Enhances Antibacterial Effect of Antibiotics and Permeabilizes Cytoplasmic Membrane of Staphylococcus Epidermidis Biofilm Cells. Folia Microbiol. 2021, 66, 983–996. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Yu, D.; Eom, S.-H.; Kim, S.-H.; Oh, J.; Jung, W.; Kim, Y.-M. Synergistic Antibacterial Effects of Chitosan-Caffeic Acid Conjugate against Antibiotic-Resistant Acne-Related Bacteria. Mar. Drugs 2017, 15, 167. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.; Yang, J.; Sun, W.; Su, X.; Chen, J.; Chen, X.; Pei, S. Design, Synthesis and Antibacterial Evaluation of a Novel Class of Tetrahydrobenzothiophene Derivatives. RSC Med. Chem. 2023, 14, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Urakawa, R.; Sakai, T.; Konishi, K. Bactericidal Effect of MA-T Against Escherichia Coli in Davis Minimal Medium in the Presence of Organic Materials, Compared to Perchlorous Acid as a Control. BPB Rep. 2023, 6, 122–125. [Google Scholar] [CrossRef]
- Pigareva, V.A.; Stepanova, D.A.; Bolshakova, A.V.; Marina, V.I.; Osterman, I.A.; Sybachin, A.V. Hyperbranched Kaustamin as an antibacterial for surface treatment. Mend. Comm. 2022, 32, 561–563. [Google Scholar] [CrossRef]
- Kaczor, P.; Bazan, P.; Kuciel, S. Bioactive Polyoxymethylene Composites: Mechanical and Antibacterial Characterization. Materials 2023, 16, 5718. [Google Scholar] [CrossRef]
- Hidayat, M.I.; Adlim, M.; Suhartono, S.; Hayati, Z.; Bakar, N.H.H.A. Comparison of antibacterial properties between chitosan stabilized silver and zinc oxide nanoparticles immobilized on white silica beads. S. Afr. J. Chem. Eng. 2023, 45, 111–119. [Google Scholar] [CrossRef]
- Dhir, S.; Dutt, R.; Singh, R.P.; Chauhan, M.; Virmani, T.; Kumar, G.; Alhalmi, A.; Aleissa, M.S.; Rudayni, H.A.; Al-Zahrani, M. Amomum subulatum Fruit Extract Mediated Green Synthesis of Silver and Copper Oxide Nanoparticles: Synthesis, Characterization, Antibacterial and Anticancer Activities. Processes 2023, 11, 2698. [Google Scholar] [CrossRef]
- Alharthi, A.F.; Gouda, M.; Khalaf, M.M.; Elmushyakhi, A.; Abou Taleb, M.F.; Abd El-Lateef, H.M. Cellulose-Acetate-Based Films Modified with Ag2O and ZnS as Nanocomposites for Highly Controlling Biological Behavior for Wound Healing Applications. Materials 2023, 16, 777. [Google Scholar] [CrossRef]
- Yılmaz, G.E.; Göktürk, I.; Ovezova, M.; Yılmaz, F.; Kılıç, S.; Denizli, A. Antimicrobial Nanomaterials: A Review. Hygiene 2023, 3, 269–290. [Google Scholar] [CrossRef]
- Corrêa, J.M.; Mori, M.; Sanches, H.L.; da Cruz, A.D.; Poiate, E.; Poiate, I.A. Silver nanoparticles in dental biomaterials. Int. J. Biomater. 2015, 2015, 485275. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Donia, D.T.; Sabbatella, G.; Antiochia, R. Silver nanoparticles in polymeric matrices for fresh food packaging. J. King Saud. Univ. Sci. 2016, 28, 273–279. [Google Scholar] [CrossRef]
- Smirnova, V.V.; Chausov, D.N.; Serov, D.A.; Kozlov, V.A.; Ivashkin, P.I.; Pishchalnikov, R.Y.; Uvarov, O.V.; Vedunova, M.V.; Semenova, A.A.; Lisitsyn, A.B.; et al. A Novel Biodegradable Composite Polymer Material Based on PLGA and Silver Oxide Nanoparticles with Unique Physicochemical Properties and Biocompatibility with Mammalian Cells. Materials 2021, 14, 6915. [Google Scholar] [CrossRef] [PubMed]
- Dirain, C.O.; Silva, R.C.; Antonelli, P.J. Prevention of biofilm formation by polyquaternary polymer. Int. J. Pediatr. Otorhinolaryngol. 2016, 88, 57–162. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, R.L.O.; Sarra, G.; Lincopan, N.; Petri, D.F.S.; Aliaga, J.; Marques, M.M.; Dias, R.B.; Coto, N.P.; Sugaya, N.N.; Paula, C.R. Preparation, Antimicrobial Properties, and Cytotoxicity of Acrylic Resins Containing Poly(diallyldimethylammonium chloride). Int. J. Prosthodont. 2021, 34, 635–641. [Google Scholar] [CrossRef]
- Dillard, D.A.; Pocius, A.V. The Mechanics of Adhesion; Elsevier: Amsterdam, The Netherlands, 2002; pp. 499–534. [Google Scholar]
- Benedek, I.; Feldstein, M.M. (Eds.) Fundamentals of Pressure Sensitivity, Handbook of Pressure-Sensitive Adhesives and Products; CRC Press—Taylor & Francis Group: Boca Raton, FL, USA, 2009; pp. 1–62. [Google Scholar]
- Izumrudov, V.A.; Paraschuk, V.V.; Sybachin, A.V. Controlled phase separations in solutions of polyelectrolyte complexes: Potential for gene delivery. J. Drug Deliv. Sci. Technol. 2006, 16, 267–274. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Pigareva, V.A.; Marina, V.I.; Bolshakova, A.V.; Berkovich, A.K.; Kuznetsova, O.A.; Semenova, A.A.; Yushina, Y.K.; Bataeva, D.S.; Grudistova, M.A.; Sybachin, A.V. Biocidal Coatings against Gram-Positive Bacteria from Linear and Branched Polycations: The Decisive Role of the Diffusion Coefficients of Macromolecules. Coatings 2023, 13, 1076. [Google Scholar] [CrossRef]
- Kusaia, V.S.; Kozhunova, E.Y.; Stepanova, D.A.; Pigareva, V.A.; Sybachin, A.V.; Zezin, S.B.; Bolshakova, A.V.; Shchelkunov, N.M.; Vavaev, E.S.; Lyubin, E.V.; et al. Synthesis of Magneto-Controllable Polymer Nanocarrier Based on Poly(N-isopropylacrylamide-co-acrylic Acid) for Doxorubicin Immobilization. Polymers 2022, 14, 5440. [Google Scholar] [CrossRef]
- Neuman, K.C.; Block, S.M. Optical Trapping. Rev. Sci. Instrum. 2004, 75, 2787. [Google Scholar] [CrossRef] [PubMed]
- Kanikireddy, V.; Varaprasad, K.; Jayaramudu, T.; Karthikeyan, C.; Sadiku, R. Carboxymethyl cellulose-based materials for infection control and wound healing: A review. Int J Biol Macromol. 2020, 164, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Bhanjana, G.; Heydarifard, S.; Dilbaghi, N.; Nazhad, M.M.; Kumar, V.; Kim, K.H.; Kumar, S. Enhanced antibacterial profile of nanoparticle impregnated cellulose foam filter paper for drinking water filtration. Carbohyd. Polym. 2018, 202, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Pergushov, D.V.; Müller, A.H.E.; Schacher, F.H. Micellar interpolyelectrolyte complexes. Chem. Soc. Rev. 2012, 41, 6888–6901. [Google Scholar] [CrossRef] [PubMed]
- Yaroslavov, A.A.; Efimova, A.A.; Sybachin, A.V.; Izumrudov, V.A.; Samoshin, V.V.; Potemkin, I.I. Stability of anionic liposome-cationic polymer complexes in water-salt media. Colloid J. 2011, 73, 430–435. [Google Scholar] [CrossRef]
- Panova, I.; Drobyazko, A.; Spiridonov, V.; Sybachin, A.; Kydralieva, K.; Jorobekova, S.; Yaroslavov, Y. Humics-based inter-polyelectrolyte complexes for antierosion protection of soil: Model investigation. Land Degrad. Dev. 2019, 30, 337–347. [Google Scholar] [CrossRef]
- Hernández-Rivas, M.; Guzmán, E.; Fernández-Peña, L.; Akanno, A.; Greaves, A.; Léonforte, F.; Ortega, F.; Rubio, R.G.; Luengo, G.S. Deposition of Synthetic and Bio-Based Polycations onto Negatively Charged Solid Surfaces: Effect of the Polymer Cationicity, Ionic Strength, and the Addition of an Anionic Surfactant. Colloids Interfaces 2020, 4, 33. [Google Scholar] [CrossRef]
- Pham, T.D.; Kobayashi, M.; Adachi, Y. Adsorption of Polyanion onto Large Alpha Alumina Beads with Variably Charged Surface. Adv. Phys. Chem. 2014, 2014, 460942. [Google Scholar] [CrossRef]
- Kohay, H.; Bilkis, I.I.; Mishael, Y.G. Effect of polycation charge density on polymer conformation at the clay surface and consequently on pharmaceutical binding. J. Colloid Interface Sci. 2019, 552, 517–527. [Google Scholar] [CrossRef]
- Izumrudov, V.A.; Mussabayeva, B.K.; Kassymova, Z.S.; Klivenko, A.N.; Orazzhanova, L.K. Interpolyelectrolyte complexes: Advances and prospects of application. Russ. Chem. Rev. 2019, 88, 1046–1062. [Google Scholar] [CrossRef]
- Sofronova, A.A.; Evstafyeva, D.B.; Izumrudov, V.A.; Muronetz, V.I.; Semenyuk, P.I. Protein-polyelectrolyte complexes: Molecular dynamics simulations and experimental study. Polymer 2017, 113, 39–45. [Google Scholar] [CrossRef]
- Motelica-Heino, M.; Predoi, M.V.; Ciobanu, S.C.; Iconaru, S.L.; Predoi, D. Studies of New Layer Formation on the Surface of Zinc Doped Hydroxyapatite/Chitosan Composite Coatings in Biological Medium. Coatings 2023, 13, 472. [Google Scholar] [CrossRef]
- Kosior, D.; Morga, M.; Maroni, P.; Cieśla, M.; Adamczyk, Z. Formation of Poly-l-Lysine Monolayers on Silica: Modeling and Experimental Studies. J. Phys. Chem. C 2020, 124, 4571–4581. [Google Scholar] [CrossRef]
- Dobrynin, A.V.; Deshkovski, A.; Rubinstein, M. Adsorption of Polyelectrolytes at Oppositely Charged Surfaces. Macromolecules 2001, 34, 3421–3436. [Google Scholar] [CrossRef]
- Akintola, J.; Chen, Y.; Digby, Z.A.; Schlenoff, J.B. Antifouling Coatings from Glassy Polyelectrolyte Complex Films. ACS Appl. Mater. Interfaces 2023, 15, 50058–50068. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, G.R.; Kudaka, A.M.; Netto, R.A.; Delarmelina, C.; Duarte, M.C.T.; Lona, L.M.F. Activity of sodium alginate/poly(diallyldimethylammonium chloride) polyelectrolyte film for packaging applications. Int. J. Biol. Macromol. 2023, 244, 125388. [Google Scholar] [CrossRef]
- Grigoras, A.G. Natural and synthetic polymeric antimicrobials with quaternary ammonium moieties: A review. Environ. Chem. Lett. 2021, 19, 3009–3022. [Google Scholar] [CrossRef]
- Misin, V.M.; Zezin, A.A.; Klimov, D.I.; Sybachin, A.V.; Yaroslavov, A.A. Biocidal Polymer Formulations and Coatings. Polym. Sci. Ser. B 2021, 63, 459–469. [Google Scholar] [CrossRef]


| Sample | MIC, mg/mL |
|---|---|
| PDADMAC | >0.4 |
| IPEC | >0.4 |
| IPEC/Ag2O | 0.1 |
| Coating | Lateral Peak Stress, Pa | Perpendicular Peak Stress, Pa |
|---|---|---|
| PDADMAC | 99,000 ± 10,000 | 52,000 ± 13,000 |
| IPEC | 139,000 ± 43,000 | 56,000 ± 8000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pigareva, V.A.; Paltsev, O.S.; Marina, V.I.; Lukianov, D.A.; Moiseenko, A.V.; Shchelkunov, N.M.; Fedyanin, A.A.; Sybachin, A.V. Ag2O-Containing Biocidal Interpolyelectrolyte Complexes on Glass Surfaces—Adhesive Properties of the Coatings. Polymers 2023, 15, 4690. https://doi.org/10.3390/polym15244690
Pigareva VA, Paltsev OS, Marina VI, Lukianov DA, Moiseenko AV, Shchelkunov NM, Fedyanin AA, Sybachin AV. Ag2O-Containing Biocidal Interpolyelectrolyte Complexes on Glass Surfaces—Adhesive Properties of the Coatings. Polymers. 2023; 15(24):4690. https://doi.org/10.3390/polym15244690
Chicago/Turabian StylePigareva, Vladislava A., Oleg S. Paltsev, Valeria I. Marina, Dmitrii A. Lukianov, Andrei V. Moiseenko, Nikita M. Shchelkunov, Andrey A. Fedyanin, and Andrey V. Sybachin. 2023. "Ag2O-Containing Biocidal Interpolyelectrolyte Complexes on Glass Surfaces—Adhesive Properties of the Coatings" Polymers 15, no. 24: 4690. https://doi.org/10.3390/polym15244690
APA StylePigareva, V. A., Paltsev, O. S., Marina, V. I., Lukianov, D. A., Moiseenko, A. V., Shchelkunov, N. M., Fedyanin, A. A., & Sybachin, A. V. (2023). Ag2O-Containing Biocidal Interpolyelectrolyte Complexes on Glass Surfaces—Adhesive Properties of the Coatings. Polymers, 15(24), 4690. https://doi.org/10.3390/polym15244690








