In Vitro and Anti-Inflammatory Activity Evaluation Nanofibers from a Breath Mask and Filter Based on Polyurethane and Polyvinylidene Fluoride
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
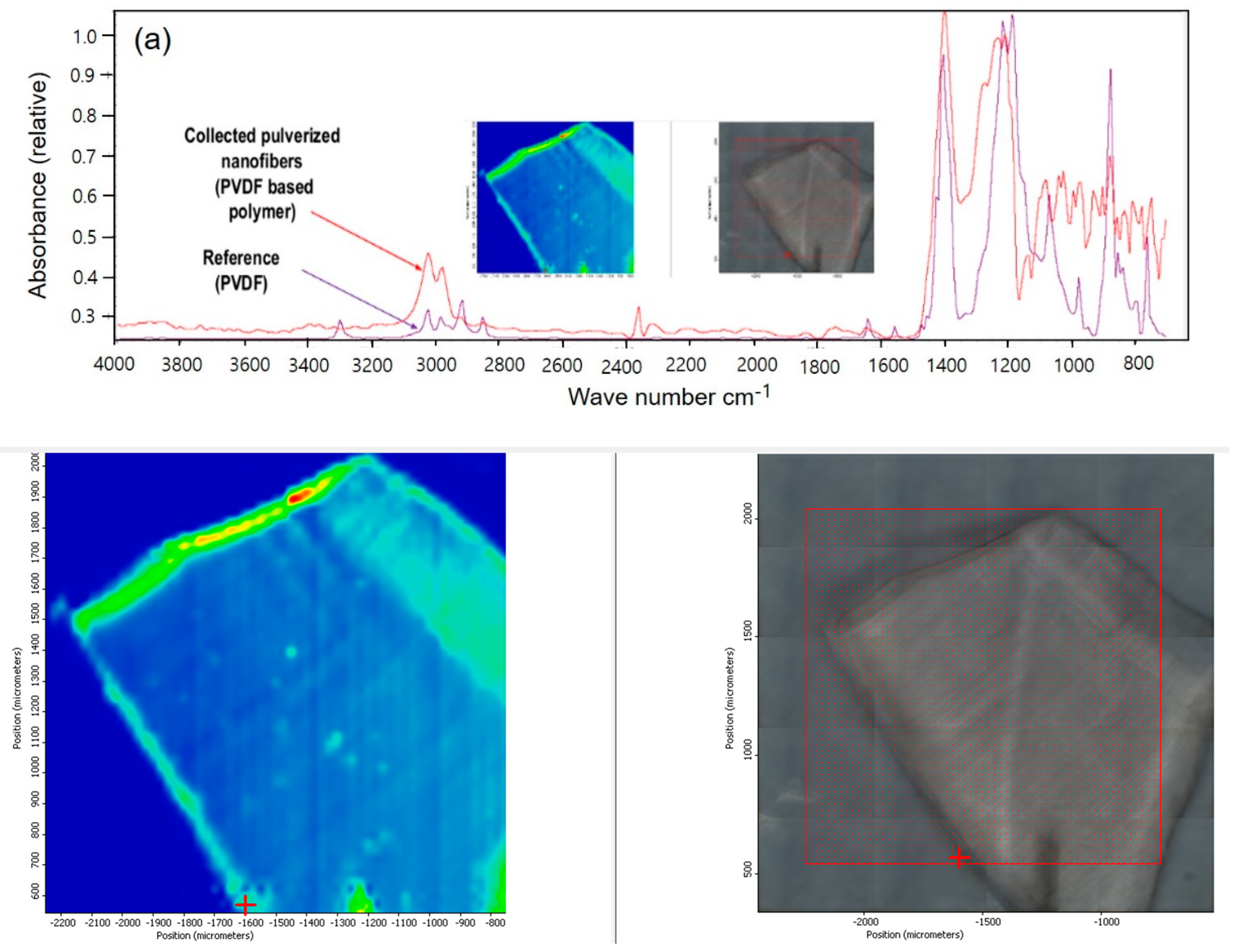
2.2. Fourier-Transform Infrared Spectroscopy
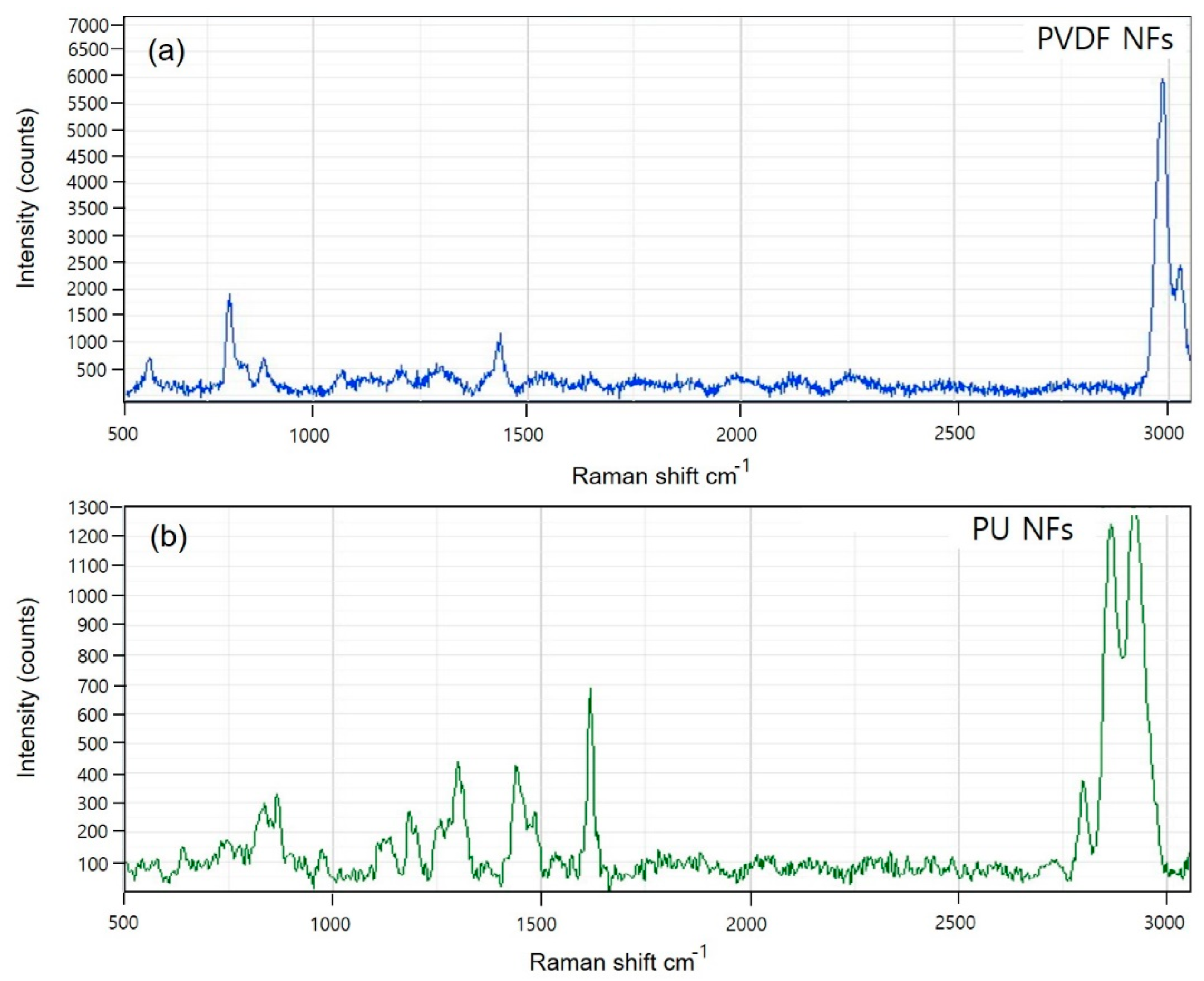
2.3. Raman Spectroscopy
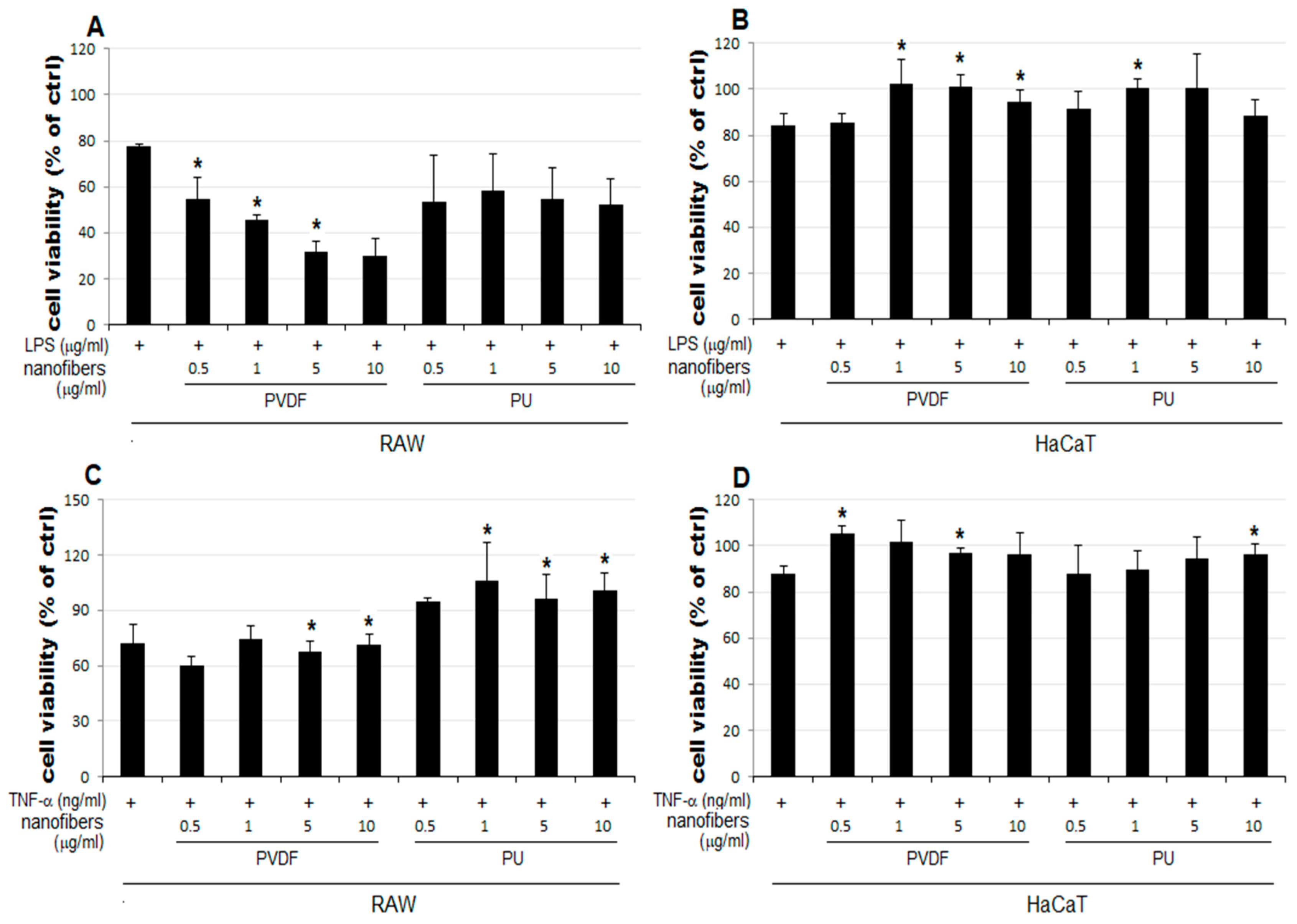
2.4. Cell Viability
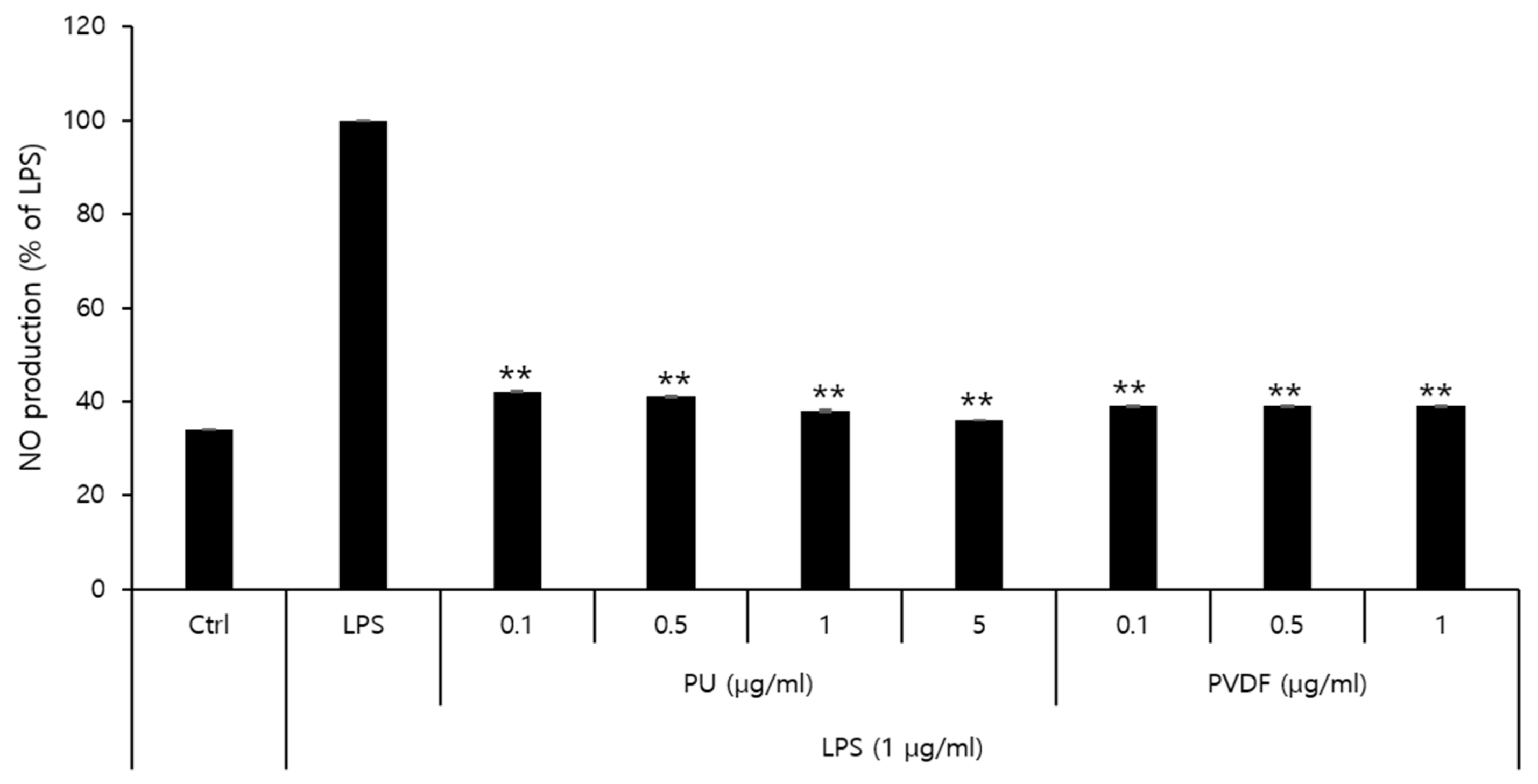
2.5. Inhibitory Effects on Nitric Oxide (NO) Production
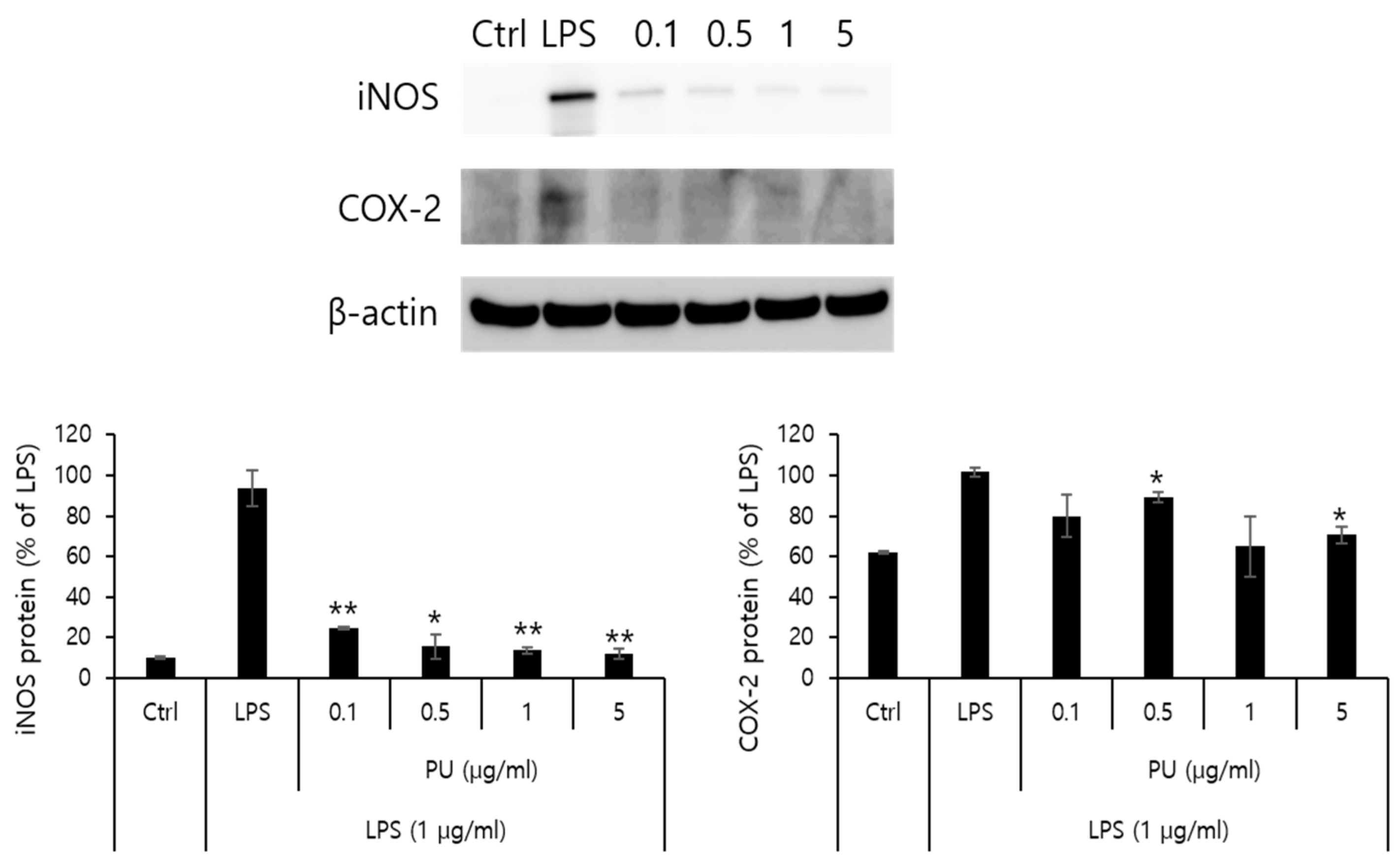
2.6. Western Blot
3. Results and Discussion
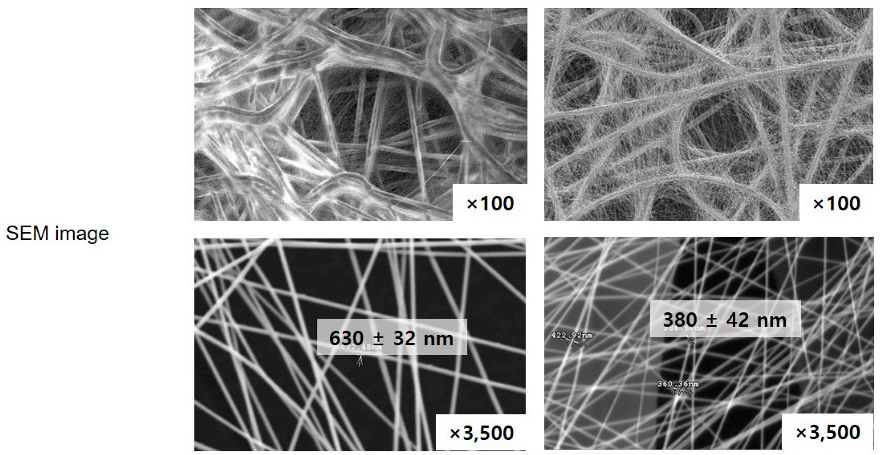
3.1. Spectroscopic Investigation of Electrospun PVDF and PU Nanofibers
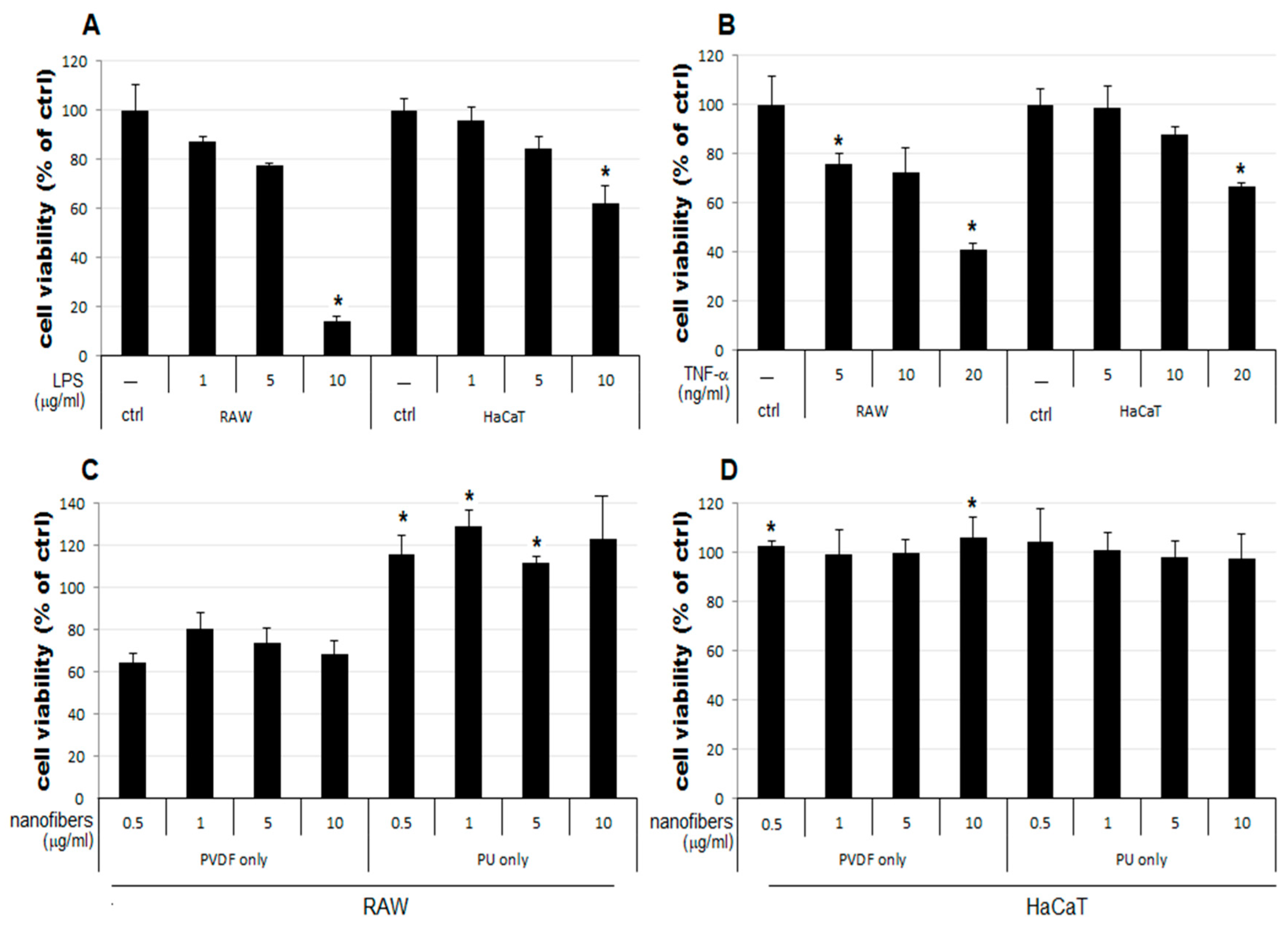
3.2. Anti-Inflammatory Activity Investigation of PVDF and PU NFs
- The cell viability decreases to 64–80% in RAW cells treated only with PVDF NFs (Figure 4C). For RAW cells, the simultaneous treatment with LPS, TNF-α, and PVDF NFs led to a fall in cell viability. A significant reduction is observed upon simultaneous treatment with LPS and PVDF NFs in a concentration-dependent manner (Figure 4A).
- The cell viability is at an excessive level in RAW cells treated with only PU NFs. In contrast, the simultaneous treatment with LPS and PU NFs does not increase cell viability (Figure 4A). Cells treated with TNF-α and PU NFs simultaneously show a significant increase in cell viability compared to those treated with only TNF-α (Figure 4B).
3.3. Inhibitory Effects of PVDF and PU NFs on Nitric Oxide Production
3.4. Inhibitory Effects of PU NFs on iNOS and COX-2 Protein Expression
4. Conclusions
- The cell viability is unaffected in HaCaT cells treated with only NFs (Figure 4D). The cell viability increased in cells simultaneously treated with LPS or TNF-α and PU or PVDF NFs.
- The treatment with PU and PVDF NFs was effective in inhibiting the expression of inflammatory mediators in RAW 264.7 cells.
- The LPS-induced iNOS expression in RAW 264.7 cells decreased after treatment with PU NFs at 0.1, 0.5, 1, and 5 μg/mL, indicating a high level of inhibition in a concentration-dependent manner. The inhibition of COX-2 expression was observed at equal concentrations, whereas the level of inhibition was higher compared to cells treated with LPS.
Supplementary Materials
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tiwari, J.N.; Tiwari, R.N.; Kim, K.S. Zero-dimensional, one-dimensional, two-dimensional and three-dimensional nanostructured materials for advanced electrochemical energy devices. Prog. Mater. Sci. 2012, 57, 724–803. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2010, 110, 2574. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, W.; Sun, L.; Aifantis, K.E.; Yu, B.; Fan, Y.; Feng, Q.; Cui, F.; Watari, F. Effects of physicochemical properties of nanomaterials on their toxicity. J. Biomed. Mater. Res. Part A 2015, 103, 2499–2507. [Google Scholar] [CrossRef] [PubMed]
- Nowrouzi, A.; Meghrazi, K.; Golmohammadi, T.; Golestani, A.; Ahmadian, S.; Shafiezadeh, M.; Shajary, Z.; Khaghani, S.; Amiri, A.N. Cytotoxicity of subtoxic AgNP in human hepatoma cell line (HepG2) after long-term exposure. Iran. Biomed. J. 2010, 14, 23–32. [Google Scholar]
- De Berardis, B.; Civitelli, G.; Condello, M.; Lista, P.; Pozzi, R.; Arancia, G.; Meschini, S. Exposure to ZnO nanoparticles induces oxidative stress and cytotoxicity in human colon carcinoma cells. Toxicol. Appl. Pharmacol. 2010, 246, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, V.; Samal, S.S.; Manoharan, N. Safety and risk associated with nanoparticles—A review. J. Miner. Mater. Charact. Eng. 2010, 9, 455–459. [Google Scholar] [CrossRef]
- Yang, L.; Watts, D.J. Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles. Toxicol. Lett. 2005, 158, 122–132. [Google Scholar] [CrossRef]
- Mostafalou, S.; Mohammadi, H.; Ramazani, A.; Abdollahi, M. Different biokinetics of nanomedicines linking to their toxicity; an overview. Daru 2013, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Tucker, N.; Stanger, J.J.; Staiger, M.P.; Razzaq, H.; Hofman, K. The History of the Science and Technology of Electrospinning from 1600 to 1995. J. Eng. Fibers Fabr. 2012, 7 (Suppl. 2). [Google Scholar] [CrossRef]
- Aman Mohammadi, M.; Hosseini, S.M.; Yousefi, M. Application of electrospinning technique in development of intelligent food packaging: A short review of recent trends. Food Sci. Nutr. 2020, 8, 4656–4665. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Lee, K.Y.; Lee, S.J.; Park, K.E.; Park, W.H. Plasma-Treated Poly (Lactic-Co-Glycolic Acid) Nanofibers for Tissue Engineering. Macromol. Res. 2007, 15, 238–243. [Google Scholar] [CrossRef]
- Duan, M.; Sun, J.; Huang, Y.; Jiang, H.; Hu, Y.; Pang, J.; Wu, C. Electrospun gelatin/chitosan nanofibers containing curcumin for multifunctional food packaging. Food Sci. Hum. Wellness 2023, 12, 614–621. [Google Scholar] [CrossRef]
- Devarayan, K.; Kim, B.S. Reversible and universal pH sensing cellulose nanofibers for health monitor. Sens. Actuators B Chem. 2015, 209, 281–286. [Google Scholar] [CrossRef]
- Saallah, S.; Naim, M.N.; Lenggoro, I.W.; Mokhtar, M.N.; Abu Bakar, N.F.A.; Gen, M. Immobilisation of cyclodextrin glucanotransferase into polyvinyl alcohol (PVA) nanofibres via electrospinning. Biotechnol. Rep. 2016, 10, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.J.; Yoon, K.J.; Kim, K.O. Glucose-responsive poly (vinyl alcohol)/β-cyclodextrin hydrogel with glucose oxidase immobilization. J. Mater. Sci. 2019, 54, 12806–12817. [Google Scholar] [CrossRef]
- Kharaghani, D.; Gitigard, P.; Ohtani, H.; Kim, K.O.; Ullah, S.; Saito, Y.; Khan, M.Q.; Kim, I.S. Design and characterization of dual drug delivery based on in-situ assembled PVA/PAN core-shell nanofibers for wound dressing application. Sci. Rep. 2019, 9, 12640. [Google Scholar] [CrossRef]
- Kim, G.J.; Kim, K.O. Novel glucose-responsive of the transparent nanofiber hydrogel patches as a wearable biosensor via electrospinning. Sci. Rep. 2020, 10, 18858. [Google Scholar] [CrossRef]
- Sheikh, F.A.; Ju, H.W.; Moon, B.M.; Lee, O.J.; Kim, J.; Park, H.J.; Kim, D.W.; Kim, D.; Jang, J.E.; Khang, G. Hybrid Scaffolds Based on PLGA and Silk for Bone Tissue Engineering. J. Tissue Eng. Regen. Med. 2016, 10, 209–221. [Google Scholar] [CrossRef]
- Kim, K.O.; Kim, G.J.; Kim, J.H. A cellulose/β-cyclodextrin nanofiber patch as a wearable epidermal glucose sensor. RSC Adv. 2019, 9, 22790–22794. [Google Scholar] [CrossRef] [PubMed]
- Sefat, F.; McKean, R.; Deshpande, P.; Ramachandran, C.; Hill, C.J.; Sangwan, V.S.; Ryan, A.J.; MacNeil, S. Production, Sterilisation and Storage of Biodegradable Electrospun PLGA Membranes for Delivery of Limbal Stem Cells to the Cornea. Procedia Eng. 2013, 59, 101–116. [Google Scholar] [CrossRef]
- Kim, K.O.; Kim, B.S. Immobilization of glucose oxidase on a PVA/PAA nanofiber matrix reduces the effect of the hematocrit levels on a glucose biosensor. J. Fiber Sci. Technol. 2017, 73, 27–33. [Google Scholar] [CrossRef]
- El-Shanshory, A.A.; Agwa, M.M.; Abd-Elhamid, A.I.; Soliman, H.M.A.; Mo, X.; Kenawy, E.R. Metronidazole topically immobilized electrospun nanofibrous scaffold: Novel secondary intention wound healing accelerator. Polymers 2022, 14, 454. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Yoo, J.J.; Lim, G.J.; Atala, A.; Stitzel, J. In vitro evaluation of electrospun nanofiber scaffolds for vascular graft application. J. Biomed. Mater. Res. A 2007, 83, 999–1008. [Google Scholar] [CrossRef]
- Gomes, S.R.; Rodrigues, G.; Martins, G.G.; Roberto, M.A.; Mafra, M.; Henriques, C.M.R.; Silva, J.C. In vitro and in vivo evaluation of electrospun nanofibers of PCL, chitosan and gelatin: A comparative study. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 46, 348–358. [Google Scholar] [CrossRef]
- Nakkala, J.R.; Yao, Y.; Zhai, Z.; Duan, Y.; Zhang, D.; Mao, Z.; Lu, L.; Gao, C. Dimethyl itaconate-loaded nanofibers rewrite macrophage polarization, reduce inflammation, and enhance repair of myocardic infarction. Small 2021, 17, e2006992. [Google Scholar] [CrossRef]
- Sadeghi-Soureh, S.; Jafari, R.; Gholikhani-Darbroud, R.; Pilehvar-Soltanahmadi, Y. Potential of chrysin-loaded PCL/gelatin nanofibers for modulation of macrophage functional polarity towards anti-inflammatory/pro-regenerative phenotype. J. Drug Deliv. Sci. Technol. 2020, 58, 101802. [Google Scholar] [CrossRef]
- ASTM D3776/D3776M; Standard Test Methods for Mass per Unit Area (Weight) of Woven Fabric. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM D 737-18; Standard Test Method for Air Permeability of Textile Fabrics. ASTM International: West Conshohocken, PA, USA, 2023.
- ASTM D1777-96; Standard Test Method for Thickness of Textile Materials. ASTM International: West Conshohocken, PA, USA, 2019.
- AATCC 127; Water Resistance: Hydrostatic Pressure Test. Howes: New York, NY, USA, 2008; Volume 85.
- Wilson, V.G. Growth and differentiation of HaCaT keratinocytes. Epidermal Cells. Methods Protoc. 2014, 33–41. [Google Scholar]
- Kong, L.; Smith, W.; Hao, D. Overview of RAW264. 7 for osteoclastogensis study: Phenotype and stimuli. J. Cell. Mol. Med. 2019, 23, 3077–3087. [Google Scholar] [CrossRef]
- Park, J.; Kim, H.D.; Lee, S.H.; Kwak, C.H.; Chang, Y.C.; Lee, Y.C.; Chung, T.W.; Magae, J.; Kim, C.H. Ascochlorin induces caspase-independent necroptosis in LPS-stimulated RAW 264.7 macrophages. J. Ethnopharmacol. 2019, 239, 111898. [Google Scholar] [CrossRef]
- Twentyman, P.R.; Luscombe, M. A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br. J. Cancer 1987, 56, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Elashmawi, I.S. Effect of LiCl filler on the structure and morphology of PVDF films. Mater. Chem. Phys. 2008, 107, 96–100. [Google Scholar] [CrossRef]
- Ye, Y.U.N.; Jiang, Y.; Wu, Z.; Zeng, H. Phase transitions of poly (vinylidene fluoride) under electric fields. Integr. Ferroelectr. 2006, 80, 245–251. [Google Scholar] [CrossRef]
- Li, J.C.; Wang, C.L.; Zhong, W.L.; Zhang, P.L.; Wang, Q.H.; Webb, J.F. Vibrational mode analysis of β-phase poly (vinylidene fluoride). Appl. Phys. Lett. 2002, 81, 2223–2225. [Google Scholar] [CrossRef]
- Patton, T.C. Alkyd Resin Technology; Interscience: New York, NY, USA, 1962; p. 1. [Google Scholar]
- Boccaccio, T.; Bottino, A.; Capannelli, G.; Piaggio, P. Characterization of PVDF membranes by vibrational spectroscopy. J. Membr. Sci. 2002, 210, 315–329. [Google Scholar] [CrossRef]
- Podgórski, R.; Wojasiński, M.; Ciach, T. Nanofibrous materials affect the reaction of cytotoxicity assays. Sci. Rep. 2022, 12, 9047. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.o. In Vitro and Anti-Inflammatory Activity Evaluation Nanofibers from a Breath Mask and Filter Based on Polyurethane and Polyvinylidene Fluoride. Polymers 2023, 15, 4650. https://doi.org/10.3390/polym15244650
Kim Ko. In Vitro and Anti-Inflammatory Activity Evaluation Nanofibers from a Breath Mask and Filter Based on Polyurethane and Polyvinylidene Fluoride. Polymers. 2023; 15(24):4650. https://doi.org/10.3390/polym15244650
Chicago/Turabian StyleKim, Kyu oh. 2023. "In Vitro and Anti-Inflammatory Activity Evaluation Nanofibers from a Breath Mask and Filter Based on Polyurethane and Polyvinylidene Fluoride" Polymers 15, no. 24: 4650. https://doi.org/10.3390/polym15244650
APA StyleKim, K. o. (2023). In Vitro and Anti-Inflammatory Activity Evaluation Nanofibers from a Breath Mask and Filter Based on Polyurethane and Polyvinylidene Fluoride. Polymers, 15(24), 4650. https://doi.org/10.3390/polym15244650