Curing Behavior of Sucrose with p-Toluenesulfonic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Thermal Analysis
2.4. Measurement of Insoluble Matter Rate
2.5. FT−IR Analysis
2.6. Dynamic Viscoelastic Analysis
3. Results and Discussion
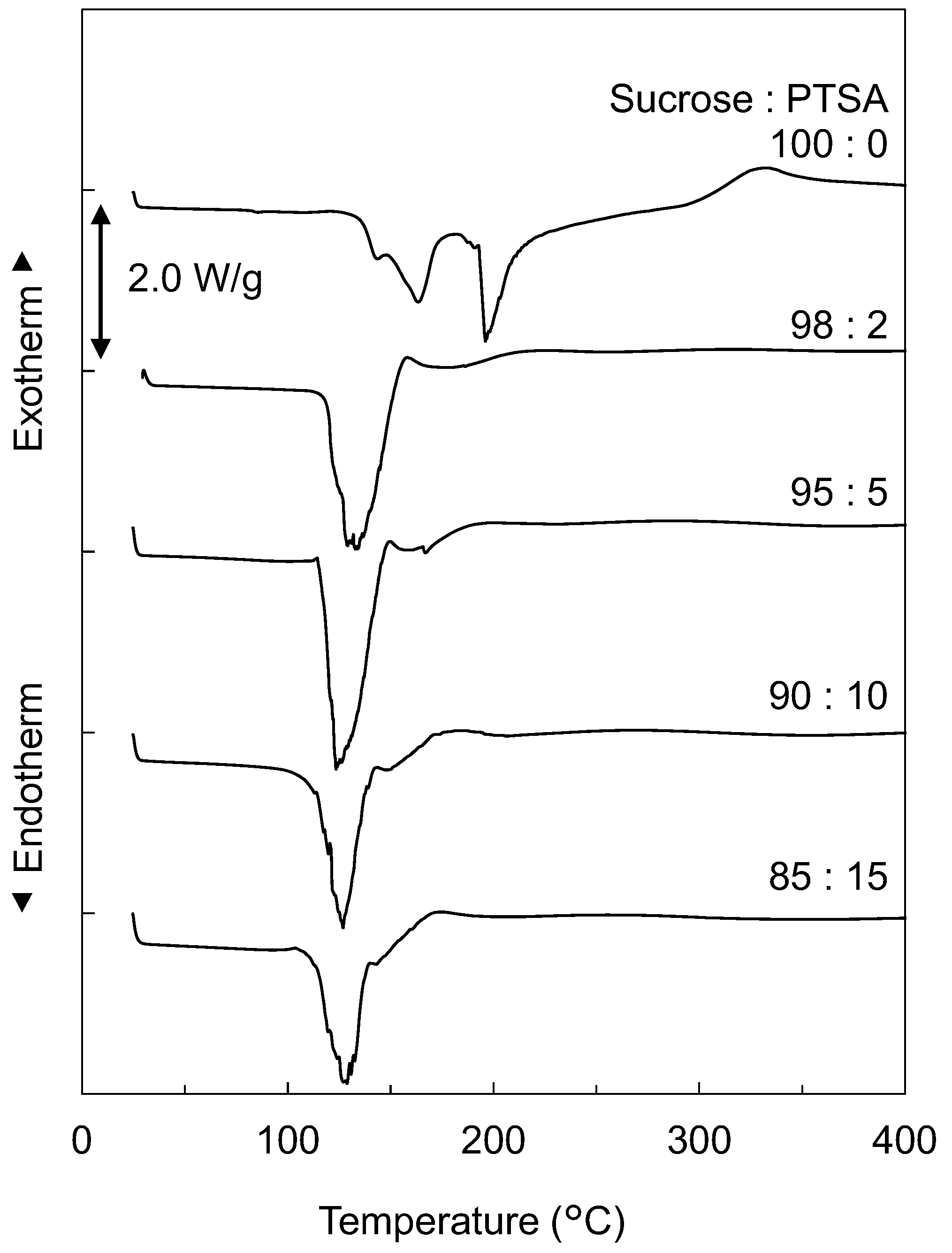
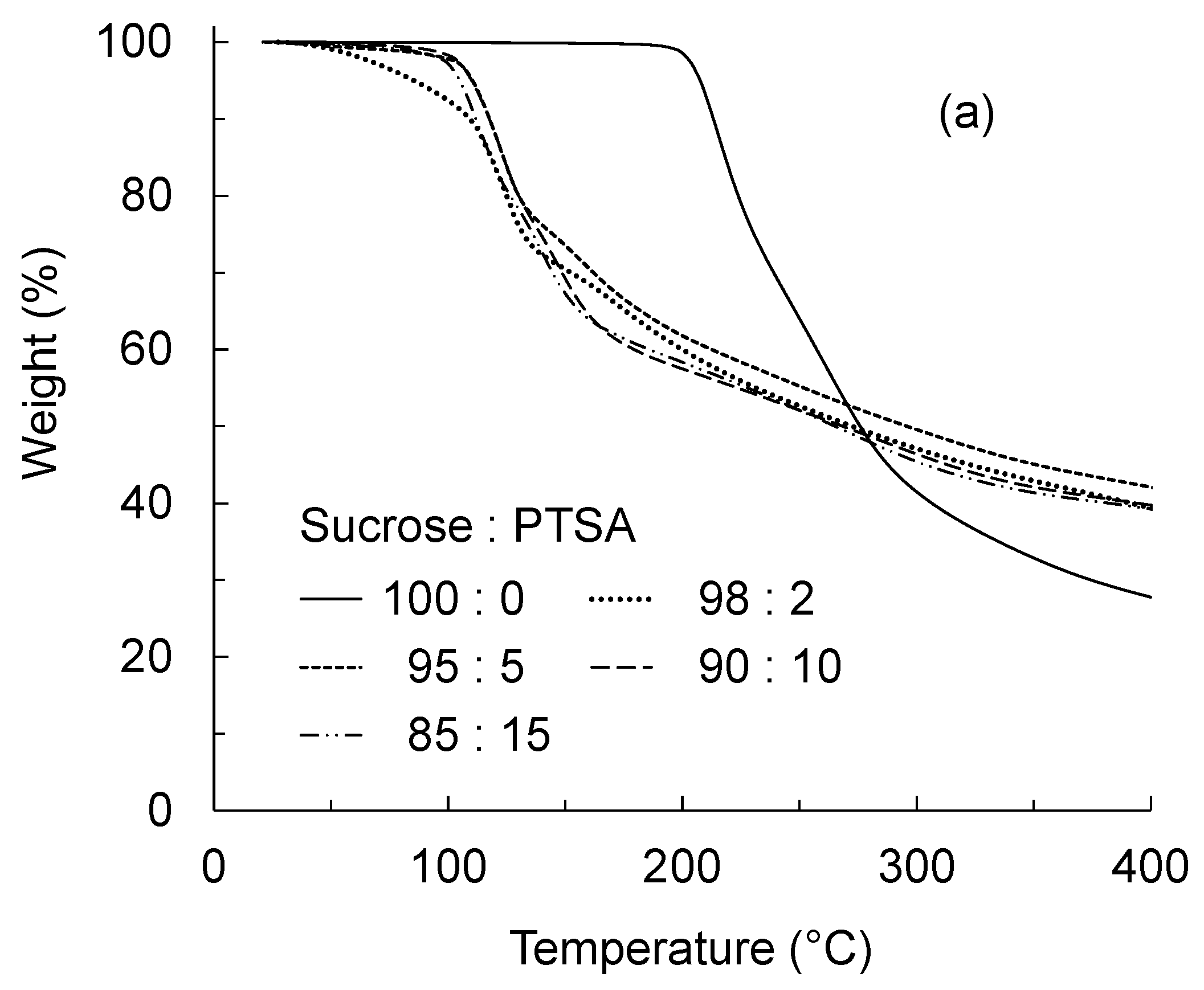
3.1. Thermal Properties of the Dried Mixtures of Suc-PTSA
3.2. Insoluble Matter Rates of the Heated Mixtures against Boiling Water
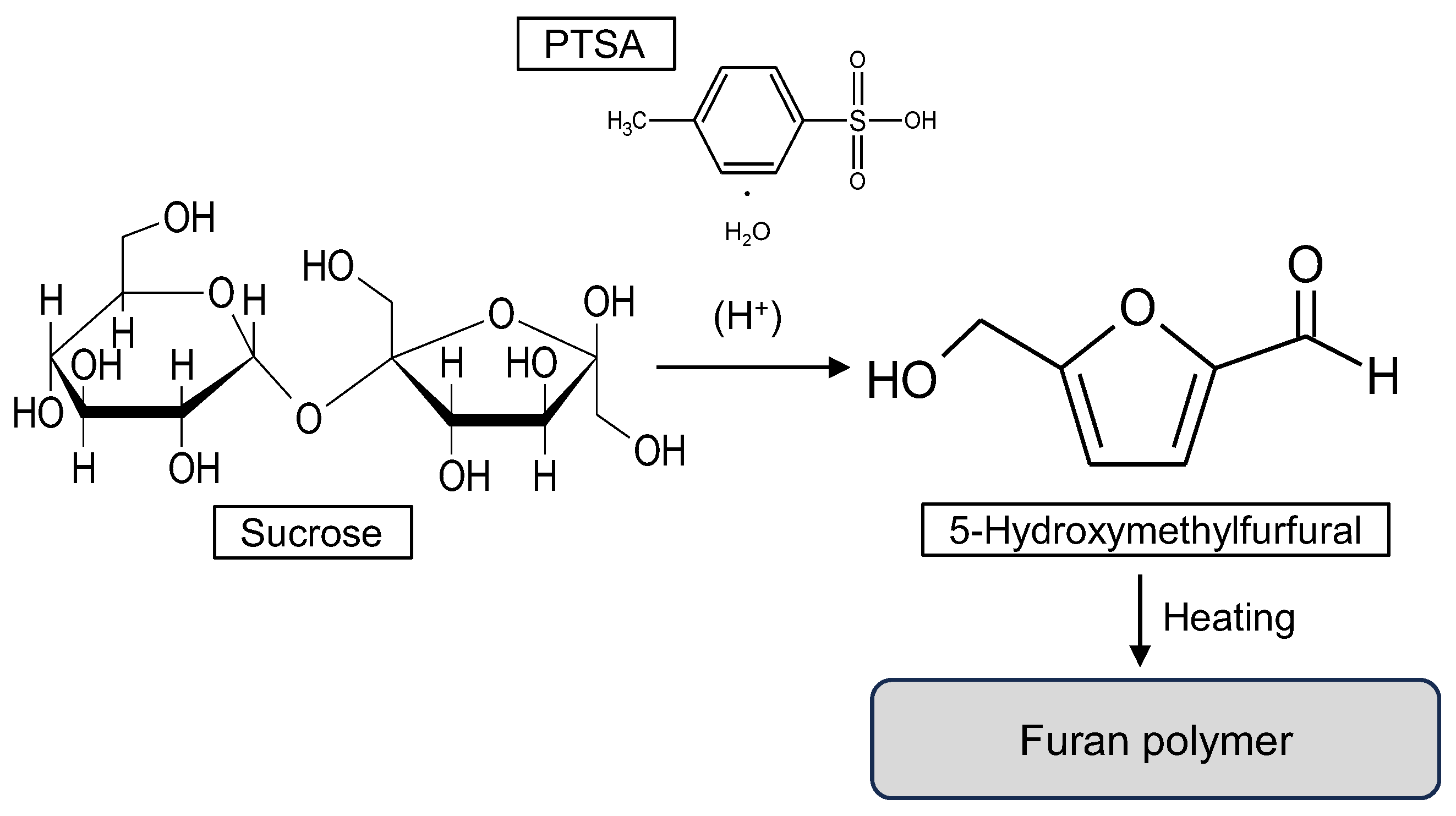
3.3. Chemical Changes and Expected Curing Reaction System of Suc-PTSA
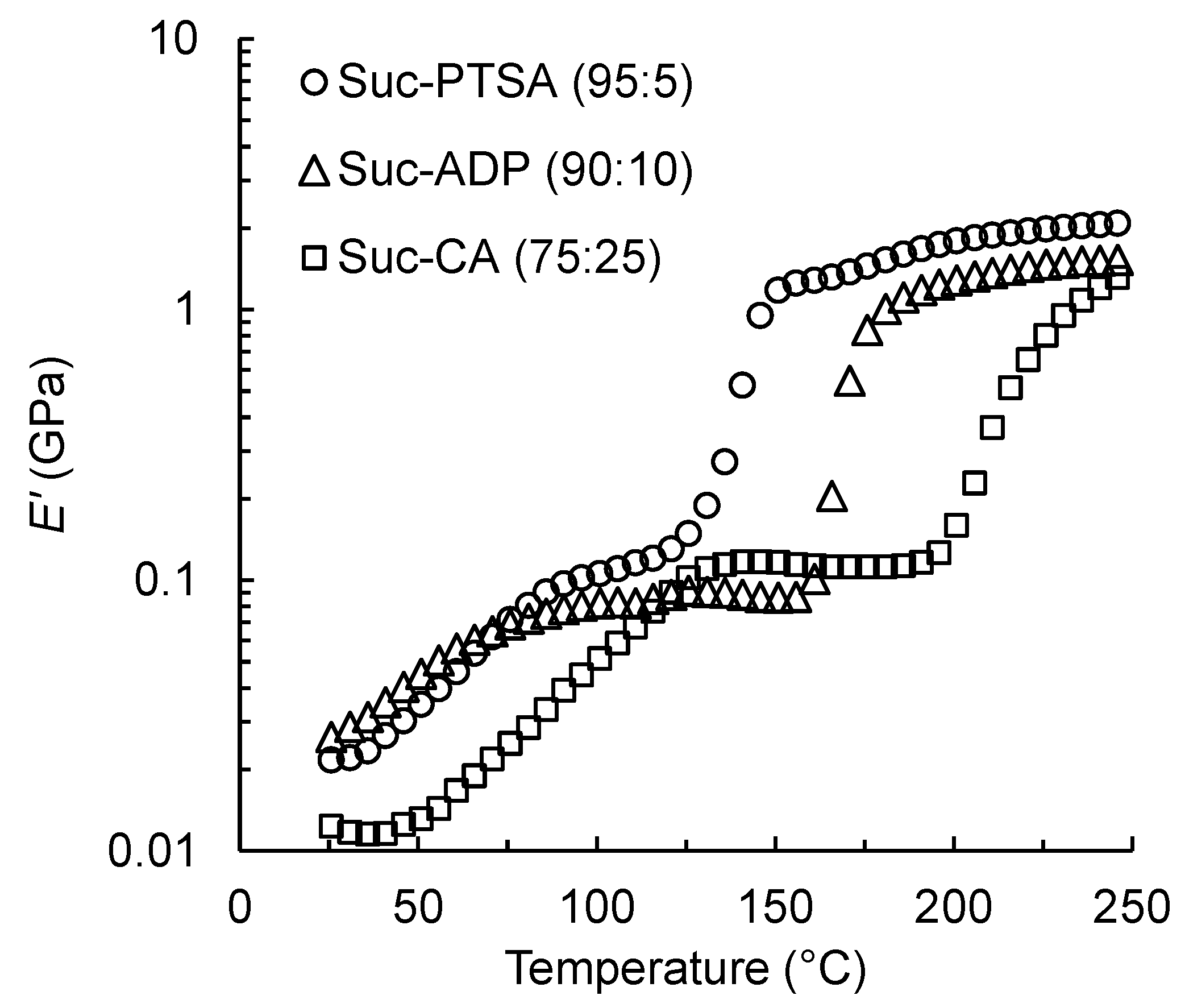
3.4. Dynamic Viscoelasticity of Suc-PTSA
4. Conclusions
- The thermal properties of Suc-PTSA were significantly lower than those of sucrose only. Suc-PTSA exhibited an endothermic peak and marked weight loss at 120–130 °C.
- Based on the results of the insoluble matter rate, the effective mixture ratio and heating conditions were 95:5 and 180 °C for 10 min. Then, 90% of the sucrose was changed into an insoluble substance in boiling water.
- With the addition of PTSA and heating, sucrose was almost completely decomposed. Sucrose was converted to a highly water-resistant substance through the formation of furan rings and carbonyl groups.
- The E′ of the Suc-PTSA solution began to increase from 110 °C to 145 °C, and the curing proceeded moderately. Compared to Suc-ADP and Suc-CA, Suc-PTSA was found to increase E′ at lower temperatures.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Panwar, N.L.; Kaushik, S.C.; Kothari, S. Role of renewable energy sources in environmental protection: A review. Renew. Sustain. Energy Rev. 2011, 15, 1513–1524. [Google Scholar] [CrossRef]
- Tursi, A. A review on biomass: Importance, chemistry, classification, and conversion. Biofuel Res. J. 2019, 6, 962–979. [Google Scholar] [CrossRef]
- Aziz, T.; Haq, F.; Farid, A.; Kiran, M.; Faisal, S.; Ullah, A.; Ullah, N.; Bokhari, A.; Mubashir, M.; Chuah, L.F.; et al. Challenges associated with cellulose composite material: Facet engineering and prospective. Environ. Res. 2023, 223, 962–979. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Aziz, T.; Sun, H.; Ullah, A.; Ali, A.; Cheng, L.; Ullah, R.; Khan, F.U. Advances and Applications of Cellulose Bio-Composites in Biodegradable Materials. J. Polym. Environ. 2023, 31, 2273–2284. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Global Forest Sector Outlook 2050 Assessing Future Demand and Sources of Timber for A Sustainable Economy. 2022. Available online: https://www.fao.org/3/cc2265en/cc2265en.pdf (accessed on 1 September 2023).
- Li, R.J.; Gutierrez, J.; Chung, Y.L.; Frank, C.W.; Billington, S.L.; Sattely, E.S. A lignin-epoxy resin derived from biomass as an alternative to formaldehyde-based wood adhesives. Green Chem. 2018, 20, 1459–1466. [Google Scholar] [CrossRef]
- Pizzi, A. Recent Developments in Eco-Efficient Bio-Based Adhesives for Wood Bonding: Opportunities and Issues. J. Adhes. Sci. Technol. 2006, 20, 829–846. [Google Scholar] [CrossRef]
- Ferdosian, F.; Pan, Z.; Gao, G.; Zhao, B. Bio-based adhesives and evaluation for wood composites application. Polymers 2017, 9, 70. [Google Scholar] [CrossRef]
- Yang, I.; Kuo, M.; Myers, D.J.; Pu, A. Comparison of protein-based adhesive resins for wood composites. J. Wood Sci. 2006, 52, 503–508. [Google Scholar] [CrossRef]
- Pan, Z.; Cathcart, A.; Wang, D. Thermal and chemical treatments to improve adhesive property of rice bran. Ind. Crops Prod. 2005, 22, 233–240. [Google Scholar] [CrossRef]
- El Mansouri, N.E.; Pizzi, A.; Salvado, J. Lignin-based polycondensation resins for wood adhesives. J. Appl. Polym. Sci. 2007, 103, 1690–1699. [Google Scholar] [CrossRef]
- Heinrich, L.A. Future opportunities for bio-based adhesives—Advantages beyond renewability. Green Chem. 2019, 21, 1866–1888. [Google Scholar] [CrossRef]
- Umemura, K.; Hayashi, S.; Tanaka, S.; Kanayama, K. Changes in Physical and Chemical Properties of Sucrose by the Addition of Ammonium Dihydrogen Phosphate. J. Adhes. Soc. Jpn. 2017, 53, 112–117. [Google Scholar] [CrossRef]
- Umemura, K.; Sugihara, O.; Kawai, S. Investigation of a new natural adhesive composed of citric acid and sucrose for particleboard. J. Wood Sci. 2013, 59, 203–208. [Google Scholar] [CrossRef]
- Plat, T.; Linhardt, R.J. Syntheses and applications of sucrose-based esters. J. Surfactants Deterg. 2001, 4, 415–421. [Google Scholar] [CrossRef]
- Samateh, M.; Marwaha, S.; James, J.K.; Nanda, V.; John, G. Sucralose hydrogels: Peering into the reactivity of sucralose versus sucrose under lipase catalyzed trans-esterification. Carbohydr. Res. 2022, 521, 108647. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Hayashi, S.; Xu, W.; Wu, Z.; Tanaka, S.; Sun, S.; Zhang, M.; Kanayama, K.; Umemura, K. A novel eco-friendly wood adhesive composed by sucrose and ammonium dihydrogen phosphate. Polymers 2018, 10, 1251. [Google Scholar] [CrossRef] [PubMed]
- Van Putten, R.J.; van der Waal, J.C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef]
- Rosatella, A.A.; Simeonov, S.P.; Frade, R.F.M.; Afonso, C.A.M. 5-Hydroxymethylfurfural (HMF) as a building block platform: Biological properties, synthesis and synthetic applications. Green Chem. 2011, 13, 754–793. [Google Scholar] [CrossRef]
- Tong, X.; Ma, Y.; Li, Y. Biomass into chemicals: Conversion of sugars to furan derivatives by catalytic processes. Appl. Catal. A 2011, 385, 1–13. [Google Scholar] [CrossRef]
- Sajid, M.; Bai, Y.; Liu, D.; Zhao, X. Conversion of Glucose to 5-Hydroxymethylfurfural by Co-catalysis of p-Toluenesulfonic Acid (pTSA) and Chlorides: A Comparison Based on Kinetic Modeling. Waste Biomass Valorization 2021, 12, 3271–3286. [Google Scholar] [CrossRef]
- Mulk, S.; Sajid, M.; Wang, L.; Liu, F.; Pan, G. Catalytic conversion of sucrose to 5-hydroxymethylfurfural in green aqueous and organic medium. J. Environ. Chem. Eng. 2022, 10, 106613. [Google Scholar] [CrossRef]
- Follensbee, R.A.; Koutsky, J.A.; Christiansen, A.W.; Myers, G.E.; Geimer, R.L. Development of dynamic mechanical methods to characterize the cure state of phenolic resole resins. J. Appl. Polym. Sci. 1993, 47, 1481–1496. [Google Scholar] [CrossRef]
- Beckett, S.T.; Francesconi, M.G.; Geary, P.M.; Mackenzie, G.; Maulny, A.P.E. DSC study of sucrose melting. Carbohydr. Res. 2006, 341, 2591–2599. [Google Scholar] [CrossRef] [PubMed]
- Ajandouz, E.; Puigserver, A. Nonenzymatic browning reaction of essential amino acids: Effect of pH on caramelization and maillard reaction kinetics. J. Agric. Food Chem. 1999, 47, 1786–1793. [Google Scholar] [CrossRef] [PubMed]
- Gintner, Z.; Végh, A.; Ritlop, B. Determination of sucrose content of cariogenic diets by thermal analysis. J. Therm. Anal. 1989, 35, 1399–1404. [Google Scholar] [CrossRef]
- Eggleston, G.; Trask-Morrell, B.J.; Vercellotti, J.R. Use of Differential Scanning Calorimetry and Thermogravimetric Analysis to Characterize the Thermal Degradation of Crystalline Sucrose and Dried Sucrose-Salt Residues. J. Agric. Food Chem. 1996, 44, 3319–3325. Available online: https://pubs.acs.org/sharingguidelines (accessed on 9 May 2023). [CrossRef]
- Kačuráková, M.; Mathlouthi, M. FTIR and laser-Raman spectra of oligosaccharides in water: Characterization of the glycosidic bond. Carbohydr. Res. 1996, 284, 145–157. [Google Scholar] [CrossRef]
- Sekkal, M.; Dincqb, V.; Legrandb, P.; Huvenneb, J.P. Investigation of the glycosidic linkages in several oligosaccharides using FT-IR and FT Raman spectroscopies. J. Mol. Struct. 1995, 349, 349–352. [Google Scholar] [CrossRef]
- Patil, S.K.R.; Heltzel, J.; Lund, C.R.F. Comparison of structural features of humins formed catalytically from glucose, fructose, and 5-hydroxymethylfurfuraldehyde. Energy Fuels 2012, 26, 5281–5293. [Google Scholar] [CrossRef]
- Bilba, K.; Ouensanga, A.; Analytical, J. Fourier transform infrared spectroscopic study degradation of sugar cane bagasse. J. Anal. Appl. Pyrolysis 1996, 38, 61–73. [Google Scholar] [CrossRef]
- Rathod, P.V.; Mujmule, R.B.; Chung, W.J.; Jadhav, A.R.; Kim, H. Efficient Dehydration of Glucose, Sucrose, and Fructose to 5-Hydroxymethylfurfural Using Tri-cationic Ionic Liquids. Catal. Lett. 2019, 149, 672–687. [Google Scholar] [CrossRef]
- Beta, I.A.; Jobic, H.; Geidel, E.; Bö Hlig, H.; Hunger, B. Inelastic neutron scattering and infrared spectroscopic study of furan adsorption on alkali-metal cation-exchanged faujasites. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2001, 57, 1393–1403. [Google Scholar] [CrossRef]
- Zhao, Z.; Sakai, S.; Wu, D.; Chen, Z.; Zhu, N.; Gui, C.; Zhang, M.; Umemura, K.; Yong, Q. Investigation of synthesis mechanism, optimal hot-pressing conditions, and curing behavior of sucrose and ammonium dihydrogen phosphate adhesive. Polymers 2020, 12, 216. [Google Scholar] [CrossRef] [PubMed]
- Weigang, F.; Charlie, V.; Yves, Q.; Florence, P. 5-Hydroxymethylfurfural (HMF) in Organic Synthesis: A Review of its Recent Applications Towards Fine Chemicals. Curr. Org. Synth. 2019, 16, 583–614. [Google Scholar]
- Ristova, M.; Pejov, L.; Žugić, M.; Šoptrajanov, B. Experimental IR, Raman and ab initio molecular orbital study of the 4-methylbenzenesulfonate anion. J. Mol. Struct. 1999, 483, 647–651. [Google Scholar] [CrossRef]
- Pejov, L.; Ristova, M.; Šoptrajanov, B. Quantum chemical study of p-toluenesulfonic acid, p-toluenesulfonate anion and the water-p-toluenesulfonic acid complex. Comparison with experimental spectroscopic data. Spectrochim. Acta A Mol. Bioml. 2011, 79, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Umemura, K. Curing Behavior and Bonding Performance of Wood Adhesives under High-Pressure Steam. Wood Res. Bull. Wood Res. Inst. Kyoto Univ. 1997, 84, 130–173. Available online: http://hdl.handle.net/2433/53201 (accessed on 21 July 2023).
- Kim, G.M.; Nieh, S.L.; Meacham, M.R. Study on the curing of phenol-formaldehyde resol resins by dynamic mechanical analysis. Ind. Eng. Chem. Res. 1991, 30, 798–803. [Google Scholar] [CrossRef]
- Umemura, K.; Sugihara, O.; Kawai, S. Investigation of a new natural adhesive composed of citric acid and sucrose for particleboard II: Effects of board density and pressing temperature. J. Wood Sci. 2015, 61, 40–44. [Google Scholar] [CrossRef]




| Mixture Ratios Sucrose: p-Toluene Sulfonic Acid (PTSA) | pH (50 wt%) | Drying | Heating | ||
|---|---|---|---|---|---|
| Temperature (°C) | Time (h) | Temperature (°C) | Time (min) | ||
| 100:0 | 5.79 | 80 | 12 | 120, 140, 160, 180, 200 | 10 |
| 98:2 | 0.84 | ||||
| 95:5 | 0.54 | ||||
| 90:10 | 0.29 | ||||
| 85:15 | 0.18 | ||||
| Sucrose:PTSA | |||||
|---|---|---|---|---|---|
| 100:0 | 98:2 | 95:5 | 90:10 | 85:15 | |
| Average | 7.24 | 3.06 | 2.90 | 2.53 | 2.40 |
| SD (n = 5) | 0.04 | 0.07 | 0.06 | 0.05 | 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakai, S.; Chen, S.; Matsuo-Ueda, M.; Umemura, K. Curing Behavior of Sucrose with p-Toluenesulfonic Acid. Polymers 2023, 15, 4592. https://doi.org/10.3390/polym15234592
Sakai S, Chen S, Matsuo-Ueda M, Umemura K. Curing Behavior of Sucrose with p-Toluenesulfonic Acid. Polymers. 2023; 15(23):4592. https://doi.org/10.3390/polym15234592
Chicago/Turabian StyleSakai, Shunsuke, Shuoye Chen, Miyuki Matsuo-Ueda, and Kenji Umemura. 2023. "Curing Behavior of Sucrose with p-Toluenesulfonic Acid" Polymers 15, no. 23: 4592. https://doi.org/10.3390/polym15234592
APA StyleSakai, S., Chen, S., Matsuo-Ueda, M., & Umemura, K. (2023). Curing Behavior of Sucrose with p-Toluenesulfonic Acid. Polymers, 15(23), 4592. https://doi.org/10.3390/polym15234592






