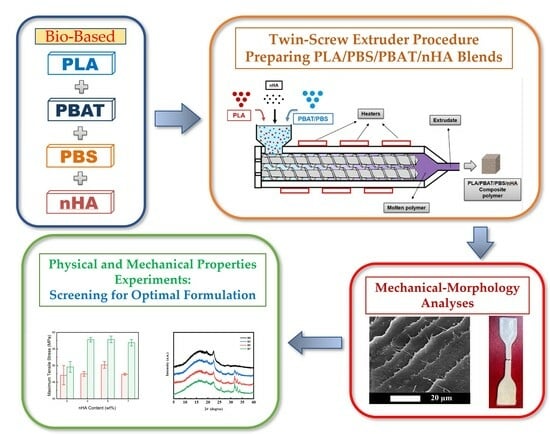
Bio-Based PLA/PBS/PBAT Ternary Blends with Added Nanohydroxyapatite: A Thermal, Physical, and Mechanical Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Composite Blends
2.3. Analysis and Testing
2.3.1. Fourier-Transform Infrared Spectroscopy (FTIR)
2.3.2. X-ray Diffraction Analysis
2.3.3. Differential Scanning Calorimeter Measurements
2.3.4. Mechanical Testing
2.3.5. Surface Morphology
2.3.6. TEM Measurements
2.3.7. Thermogravimetric Analysis (TGA)
2.3.8. Water Absorption Measurements
3. Results and Discussion
3.1. Tensile Strength Test Results for PLA/PBS/PBAT Blends
3.2. FTIR Measurement Results for PLA/PBS/PBAT/nHA Blends
3.3. XRD Measurement Results for PLA/PBS/PBAT/nHA Blends
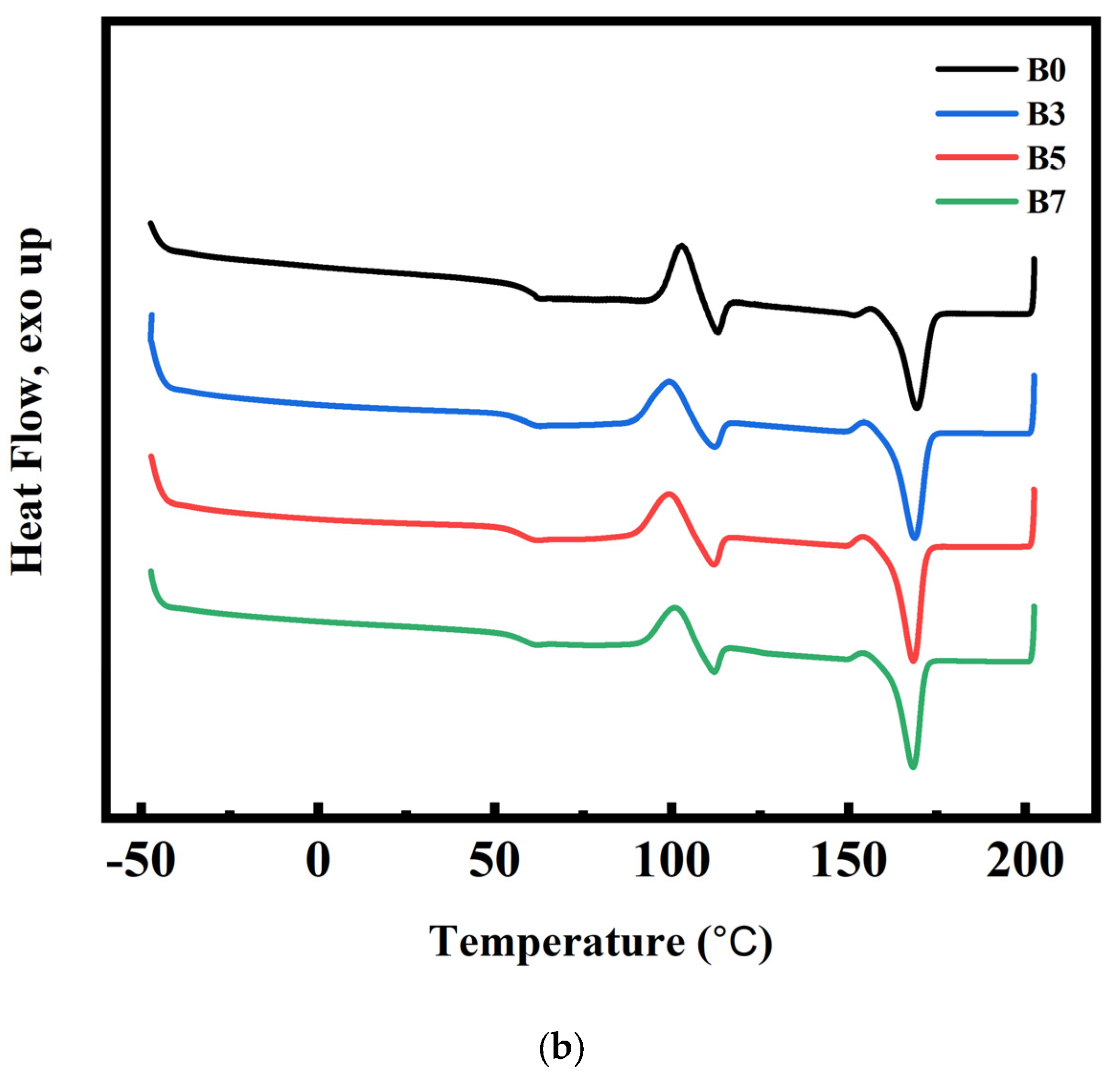
3.4. DSC and Crystallization Analysis Results for PLA/PBS/PBAT/nHA Blends
3.5. Nonisothermal Crystallization Behavior of PLA/PBS/PBAT/nHA Blends
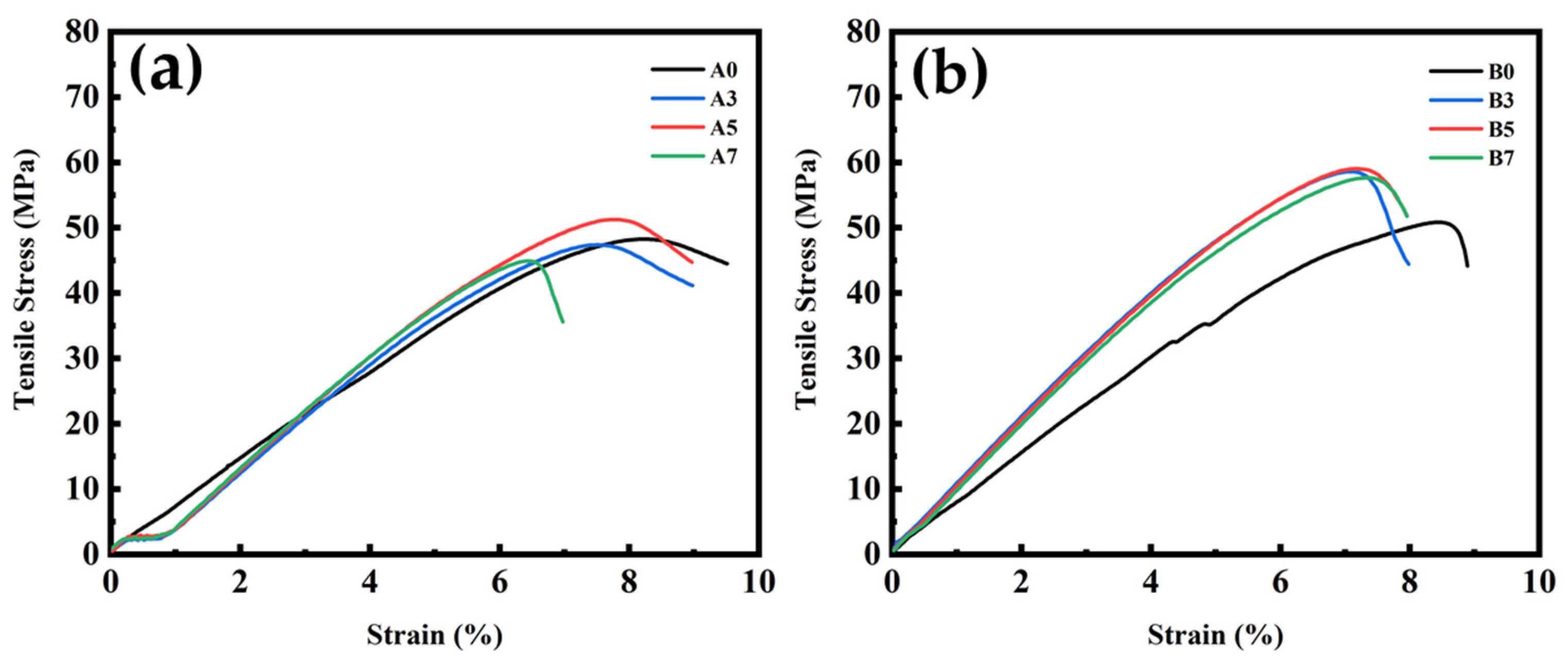
3.6. Tensile and Impact Strength Test Results for PLA/PBS/PBAT/nHA Blends
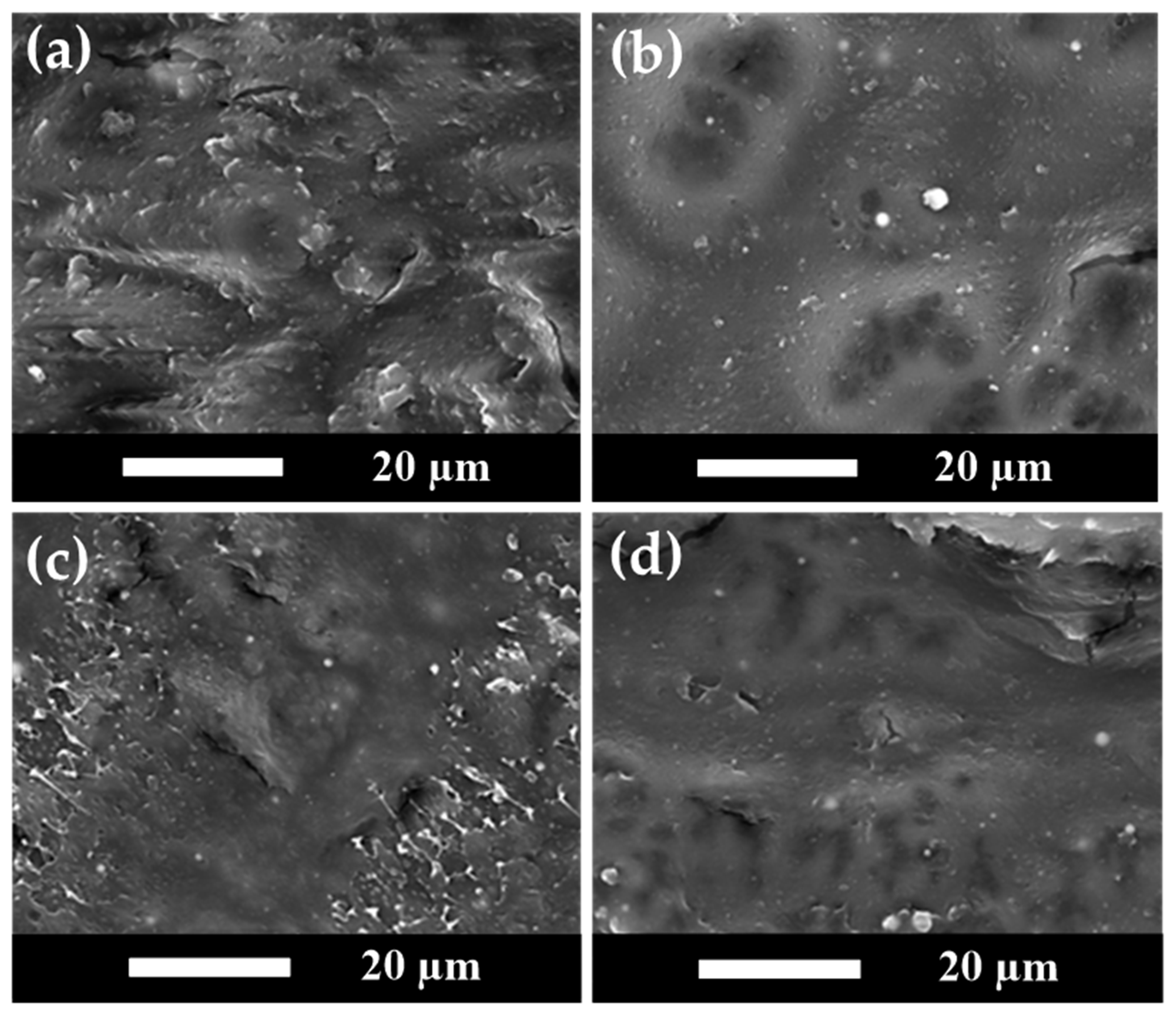
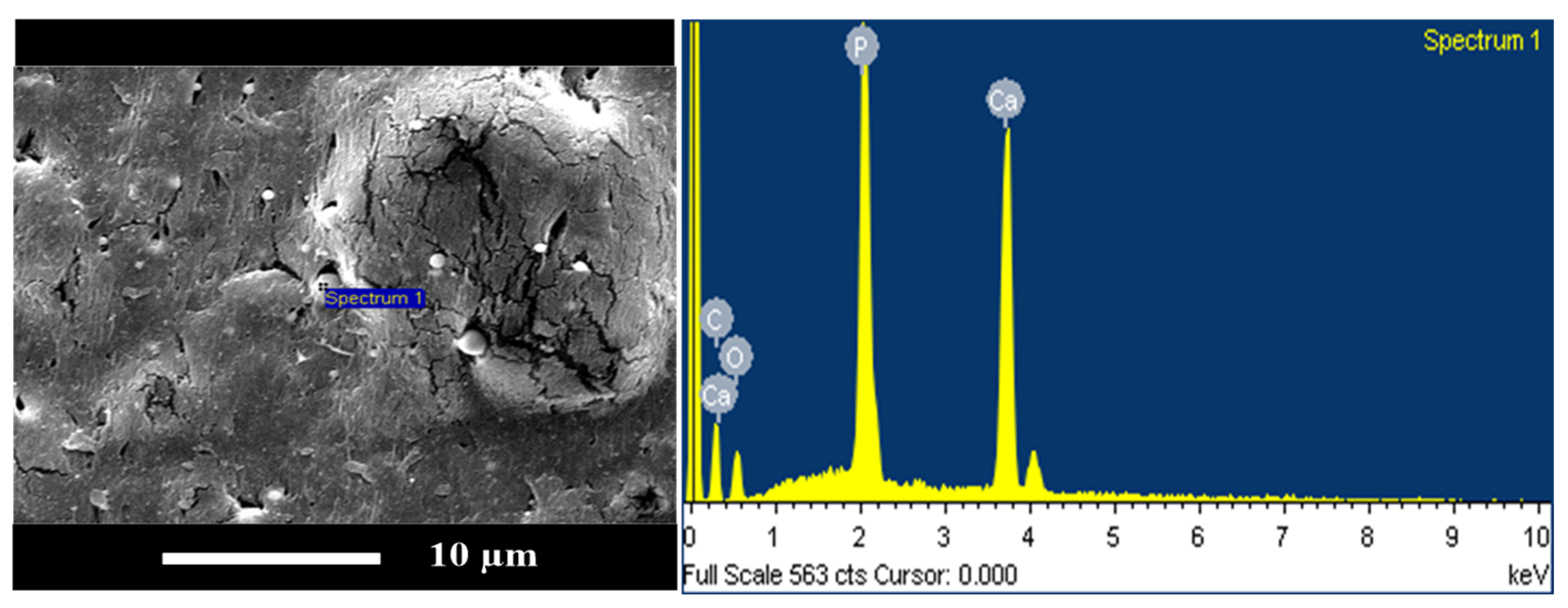
3.7. SEM Analysis Results for PLA/PBS/PBAT/nHA Blends
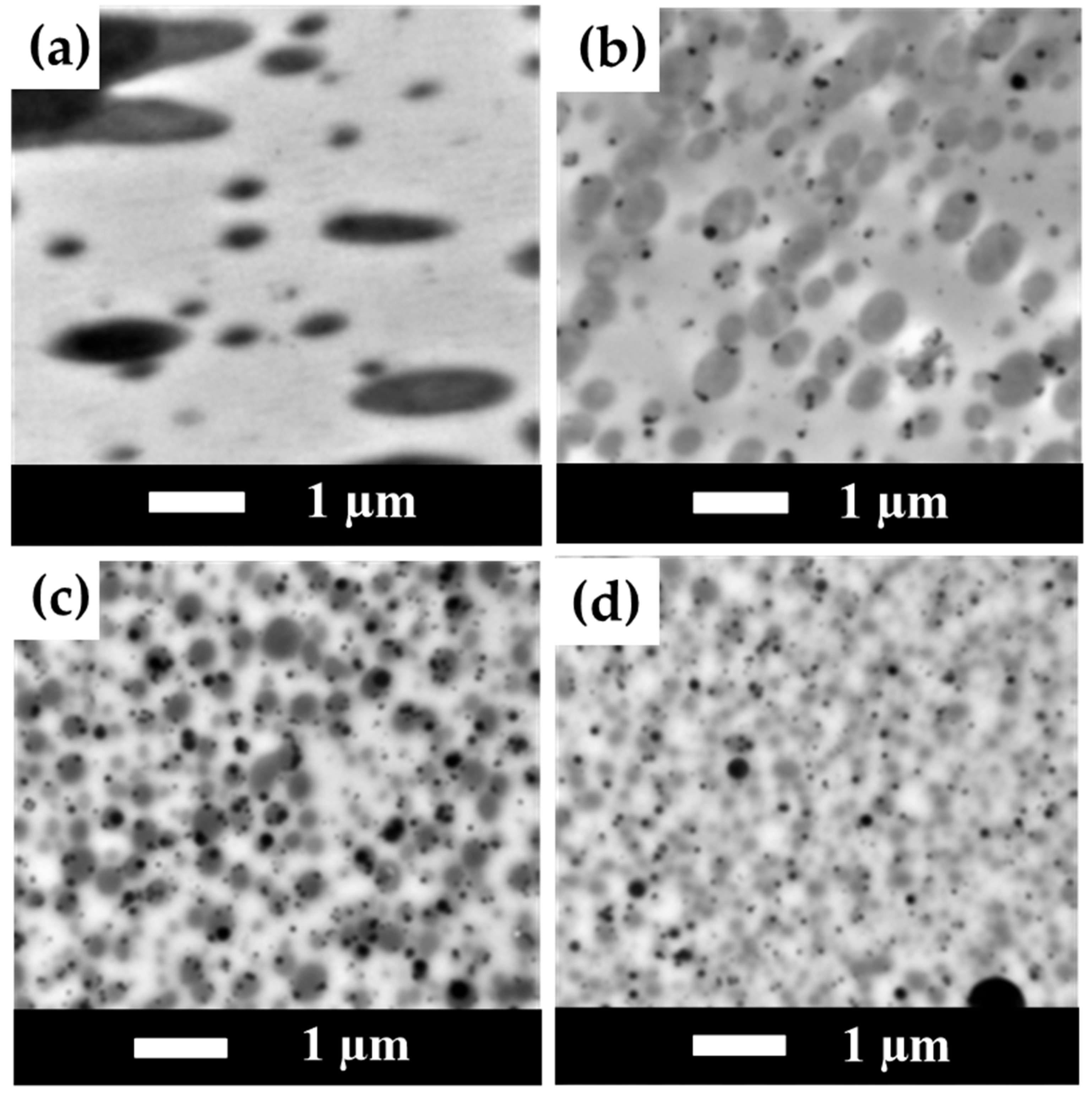
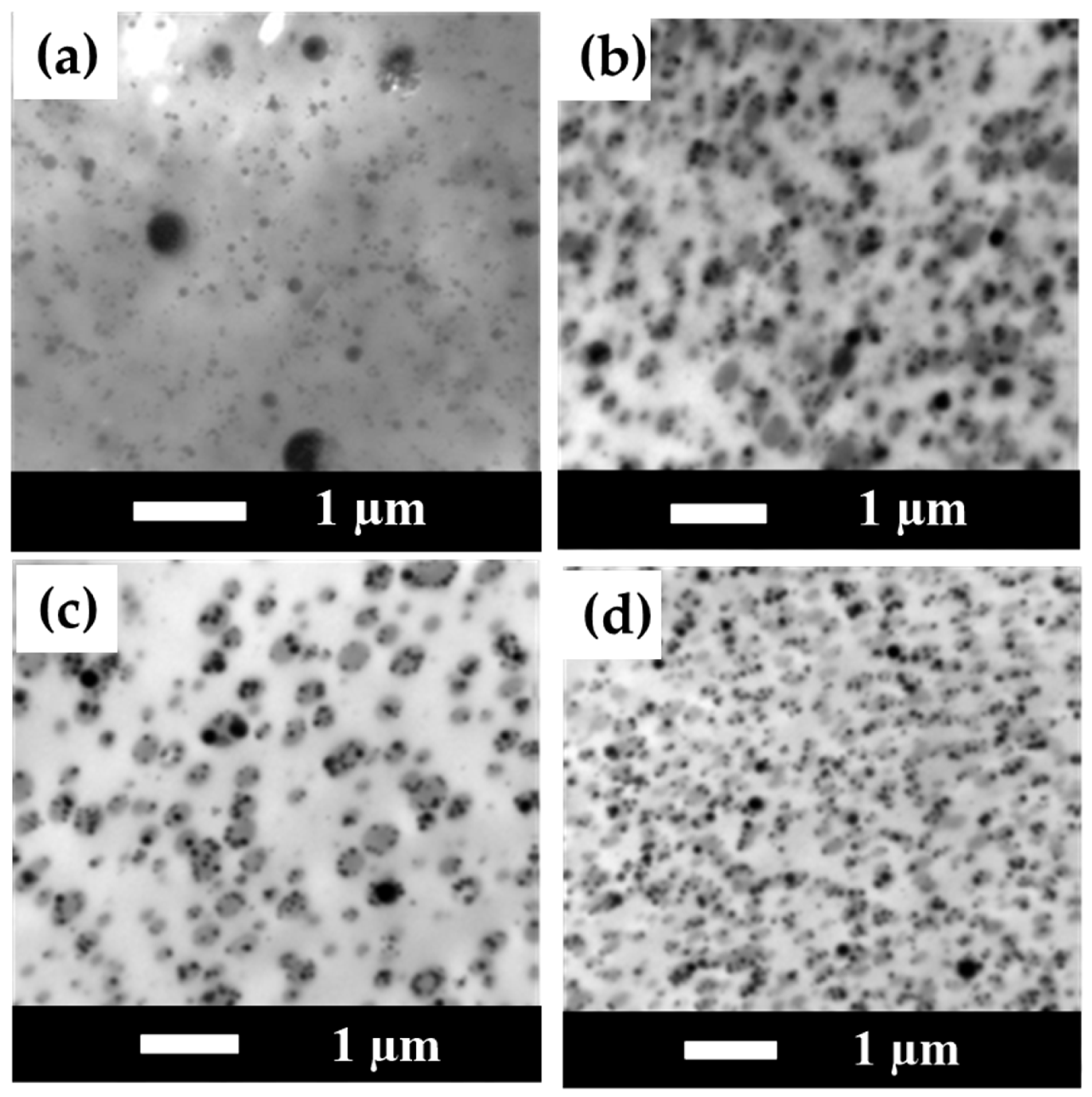
3.8. TEM Measurement Results for PLA/PBS/PBAT/nHA Blends
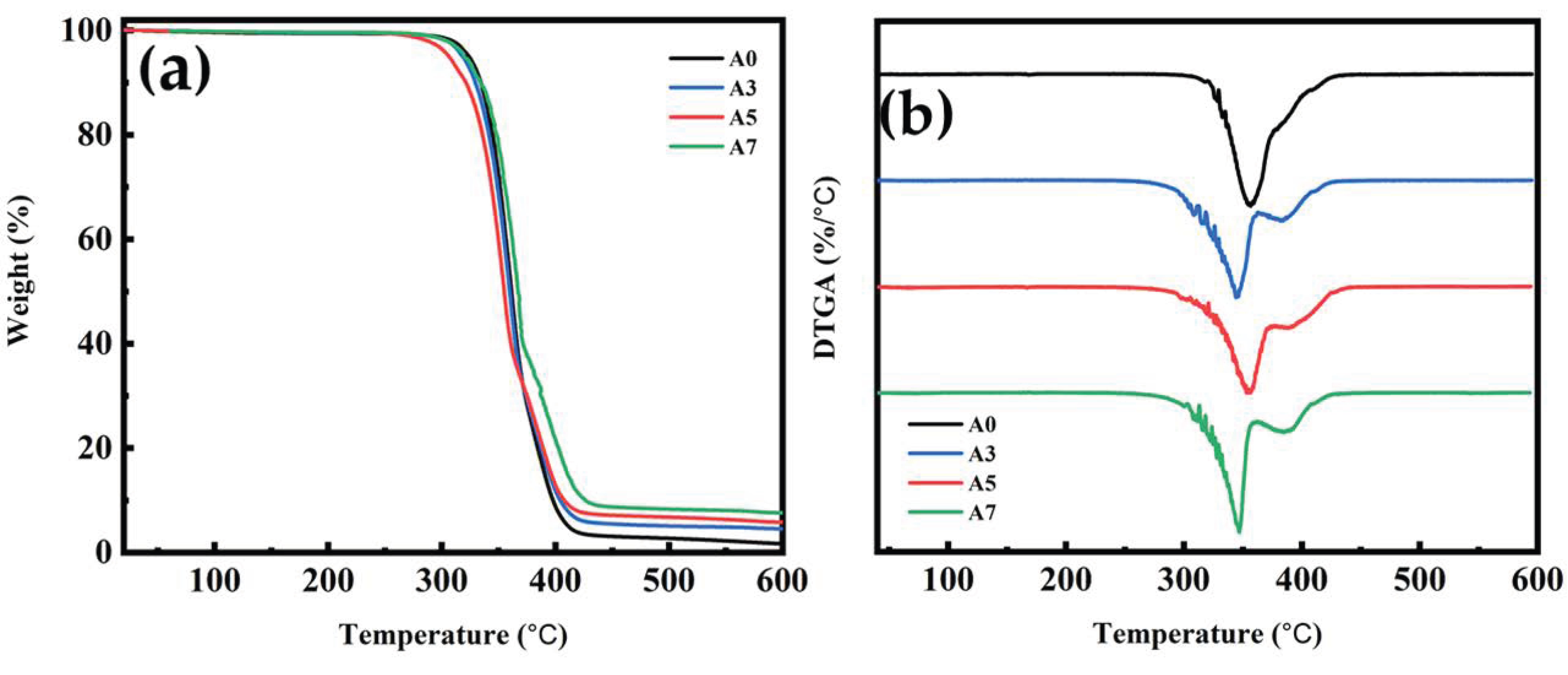
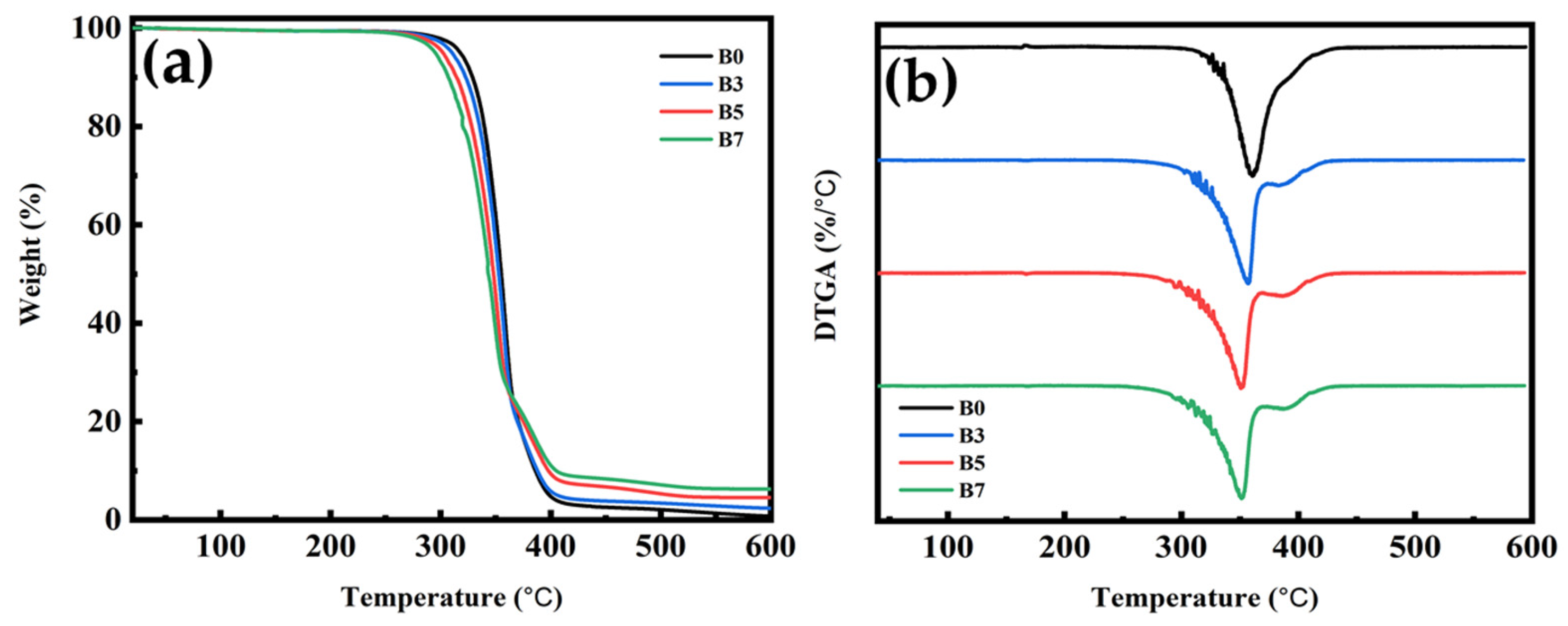
3.9. TGA for PLA/PBS/PBAT/nHA Blends
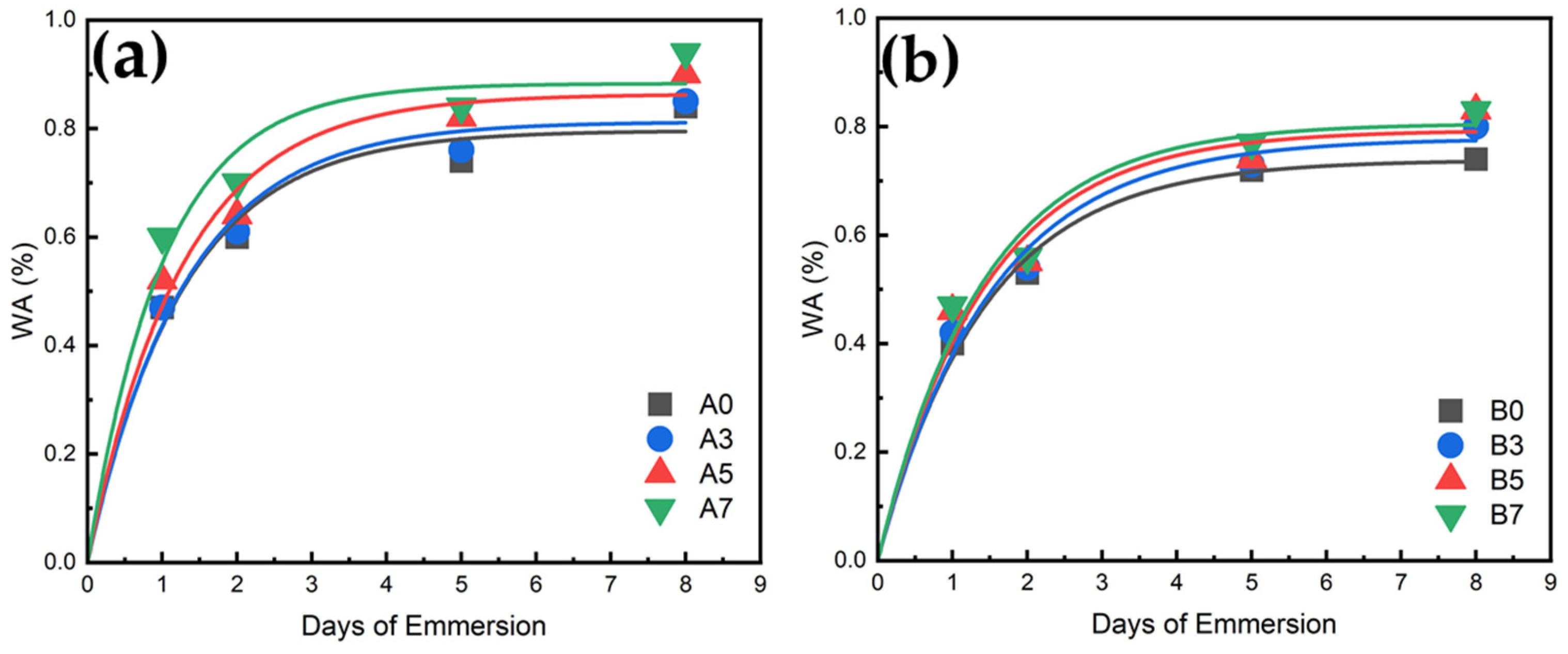
3.10. Water Absorption Measurement Results for PLA/PBS/PBAT/nHA Blends
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, A.P.; Appel, E.A.; Ashby, P.D.; Baker, B.M.; Franco, E.; Gu, L.; Haynes, K.; Joshi, N.S.; Kloxin, A.M.; Kouwer, P.H.J.; et al. The living interface between synthetic biology and biomaterial design. Nat. Mater. 2022, 21, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Eldeeb, A.E.; Salah, S.; Elkasabgy, N.A. Biomaterials for tissue engineering applications and current updates in the field: A comprehensive review. AAPS PharmSciTech 2022, 23, 267. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, S.; Hashmi, F.A.; Imran, I. Recent progress in development and applications of biomaterials. Mater. Today Proc. 2022, 62, 385–391. [Google Scholar] [CrossRef]
- Williams, D.F. Challenges with the development of biomaterials for sustainable tissue engineering. Front. Bioeng. Biotechnol. 2019, 7, 127. [Google Scholar] [CrossRef] [PubMed]
- Pollock, J.R.; Moore, M.L.; Hogan, J.S.; Haglin, J.M.; Brinkman, J.C.; Doan, M.K.; Chhabra, A. Orthopaedic group practice size is increasing. Sports Med. Arthrosc. Rehabil. Ther. Technol. 2021, 3, e1937–e1944. [Google Scholar] [CrossRef] [PubMed]
- Szpalski, C.; Wetterau, M.; Barr, J.; Warren, S.M. Bone tissue engineering: Current strategies and techniques—Part I: Scaffolds. Tissue Eng. Part B Rev. 2012, 18, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J.E. Scaffold design for bone regeneration. J. Nanosci. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef]
- Lee, S.S.; Du, X.; Kim, I.; Ferguson, S.J. Scaffolds for bone-tissue engineering. Matter 2022, 5, 2722–2759. [Google Scholar] [CrossRef]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone grafts: Which is the ideal biomaterial? J. Clin. Periodontol. 2019, 46 (Suppl. S21), 92–102. [Google Scholar] [CrossRef]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone—Tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Ravoor, J.; Thangavel, M.; Elsen, R.S. Comprehensive review on design and manufacturing of bioscaffolds for bone reconstruction. ACS Appl. Bio Mater. 2021, 4, 8129–8158. [Google Scholar] [CrossRef] [PubMed]
- Bahraminasab, M.; Janmohammadi, M.; Arab, S.; Talebi, A.; Nooshabadi, V.T.; Koohsarian, P.; Nourbakhsh, M.S. Bone scaffolds: An incorporation of biomaterials, cells, and biofactors. ACS Biomater. Sci. Eng. 2021, 7, 5397–5431. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold fabrication technologies and structure/function properties in bone tissue engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Szczesny, G.; Kopec, M.; Politis, D.J.; Kowalewski, Z.L.; Łazarski, A.; Szolc, T. A review on biomaterials for orthopaedic surgery and traumatology: From past to present. Materials 2022, 15, 3622. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable materials for bone repair and tissue engineering applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Ma, J.-X.; Xu, L.; Gu, X.-S.; Ma, X.-L. Biodegradable materials for bone defect repair. Mil. Med. Res. 2020, 7, 54. [Google Scholar] [CrossRef]
- Modrák, M.; Trebunová, M.; Balogová, A.F.; Hudák, R.; Živcák, J. Biodegradable materials for tissue engineering: Development, classification and current applications. J. Funct. Biomater. 2023, 14, 159. [Google Scholar] [CrossRef]
- Yu, H.; Liu, H.; Shen, Y.; Ao, Q. Synthetic biodegradable polymer materials in the repair of tumor-associated bone defects. Front. Bioeng. Biotechnol. 2023, 11, 1096525. [Google Scholar] [CrossRef]
- Bernardo, M.P.; da Silva, B.C.R.; Hamouda, A.E.I.; de Toledo, M.A.S.; Schalla, C.; Rütten, S.; Goetzke, R.; Mattoso, L.H.C.; Zenke, M.; Sechi, A. PLA/Hydroxyapatite scaffolds exhibit in vitro immunological inertness and promote robust osteogenic differentiation of human mesenchymal stem cells without osteogenic stimuli. Sci. Rep. 2022, 12, 2333. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, H.; Wang, X.; Yu, X.; Zhou, W.; Peng, S. Super tough poly(lactic acid) blends: A comprehensive review. RSC Adv. 2020, 10, 13316–13368. [Google Scholar] [CrossRef]
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M.-C. Poly(lactic acid) blends: Processing, properties and applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef] [PubMed]
- De Matos Costa, A.R.; Crocitti, A.; de Carvalho, L.H.; Carroccio, S.C.; Cerruti, P.; Santagata, G. Properties of biodegradable films based on poly(butylene Succinate) (PBS) and poly(butylene Adipate-co-Terephthalate) (PBAT) blends. Polymers 2020, 12, 2317. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Xue, K.; Liu, Z.; Xu, Z.; Zhao, L. The essential role of PBS on PBAT foaming under supercritical CO2 toward green engineering. J. CO2 Util. 2022, 60, 101965. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, L.; Zhou, J.; Fan, X.; Xie, H.; Zhang, H.; Zhang, G.; Shi, X. Influence of polylactide (PLA) stereocomplexation on the microstructure of PLA/PBS blends and the cell morphology of their microcellular foams. Polymers 2020, 12, 2362. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Kopitzky, R.; Tolga, S.; Kabasci, S. Polylactide (PLA) and its blends with poly(butylene succinate) (PBS): A brief review. Polymers 2019, 11, 1193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Q.; Shi, J.; Ye, H.; Zhou, Q. Distinctive tensile properties of the blends of poly(l-lactic acid) (PLLA) and poly(butylene succinate) (PBS). J. Polym. Environ. 2018, 26, 1737–1744. [Google Scholar] [CrossRef]
- Ou-Yang, Q.; Guo, B.; Xu, J. Preparation and characterization of poly(butylene succinate)/polylactide blends for fused deposition modeling 3D printing. ACS Omega 2018, 3, 14309–14317. [Google Scholar] [CrossRef]
- Yu, P.; Mi, H.-Y.; Huang, A.; Geng, L.-H.; Chen, B.-Y.; Kuang, T.-R.; Mou, W.-J.; Peng, X.-F. Effect of Poly(butylenes succinate) on poly(lactic acid) foaming behavior: Formation of open cell structure. Ind. Eng. Chem. Res. 2015, 54, 6199–6207. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, H.; Liu, Y.; Yang, Y. Blends of poly(butylene adipate-co-terephthalate) (PBAT) and stereocomplex polylactide with improved rheological and mechanical properties. RSC Adv. 2020, 10, 10482–10490. [Google Scholar] [CrossRef]
- Kang, Y.; Chen, P.; Shi, X.; Zhang, G.; Wang, C. Preparation of open-porous stereocomplex PLA/PBAT scaffolds and correlation between their morphology, mechanical behavior, and cell compatibility. RSC Adv. 2018, 8, 12933–12943. [Google Scholar] [CrossRef] [PubMed]
- Canales, D.A.; Pinones, N.; Saavedra, M.; Loyo, C.; Palza, H.; Peponi, L.; Leones, A.; Baier, R.V.; Boccaccini, A.R.; Grünelwald, A.; et al. Fabrication and assessment of bifunctional electrospun poly(L-lactic acid) scaffolds with bioglass and zinc oxide nanoparticles for bone tissue engineering. Int. J. Biol. Macromol. 2023, 228, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Kaliva, M.; Georgopoulou, A.; Dragatogiannis, D.A.; Charitidis, C.A.; Chatzinikolaidou, M.; Vamvakaki, M. Biodegradable chitosan-graft-poly(l-lactide) copolymers for bone tissue engineering. Polymers 2020, 12, 316. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Song, Z.; Li, J.; Zhang, L. Micromorphology, mechanical, crystallization and permeability properties analysis of HA/PBAT/PLA (HA, hydroxyapatite; PBAT, poly(butylene adipate-co-butylene terephthalate); PLA, polylactide) degradability packaging films. Polym. Int. 2020, 69, 301–307. [Google Scholar] [CrossRef]
- Dos Santos Silva, A.; Rodrigues, B.V.M.; Oliveira, F.C.; Carvalho, J.O.; de Vasconcellos, L.M.R.; de Araújo, J.C.R.; Marciano, F.R.; Lobo, A.O. Characterization and in vitro and in vivo assessment of poly(butylene adipate-co-terephthalate)/nano-hydroxyapatite composites as scaffolds for bone tissue engineering. J. Polym. Res. 2019, 26, 53. [Google Scholar] [CrossRef]
- Santana-Melo, G.F.; Rodrigues, B.V.M.; da Silva, E.; Ricci, R.; Marciano, F.R.; Webster, T.J.; Vasconcellosa, L.M.R.; Lobo, A.O. Electrospun ultrathin PBAT/nHAp fibers influenced the in vitro and in vivo osteogenesis and improved the mechanical properties of neoformed bone. Colloids Surf. B 2017, 155, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, J.; Jia, G.; Jiang, Y.; Liu, Q.; Yang, X.; Pan, S. Improving osteogenesis of PLGA/HA porous scaffolds based on dual delivery of BMP-2 and IGF-1 via a polydopamine coating. RSC Adv. 2017, 7, 56732–56742. [Google Scholar] [CrossRef]
- Khan, M.A.; Hussain, Z.; Liaqat, U.; Liaqat, M.A.; Zahoor, M. Preparation of PBS/PLLA/HAP composites by the solution casting method: Mechanical properties and biocompatibility. Nanomaterials 2020, 10, 1778. [Google Scholar] [CrossRef]
- Gui, X.; Peng, W.; Xu, X.; Su, Z.; Liu, G.; Zhou, Z.; Liu, M.; Li, Z.; Song, G.; Zhou, C.; et al. Synthesis and application of nanometer hydroxyapatite in biomedicine. Nanotechnol. Rev. 2022, 11, 2154–2168. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, B.; Li, M.; Li, J.; Zhang, C.; Han, Y.; Wang, L.; Wang, K.; Zhou, C.; Liu, L.; et al. 3D printing of PLA/n-HA composite scaffolds with customized mechanical properties and biological functions for bone tissue engineering. Compos. B Eng. 2021, 224, 109192. [Google Scholar] [CrossRef]
- Mou, Z.-L.; Zhao, L.-J.; Zhang, Q.-A.; Zhang, J.; Zhang, Z.-Q. Preparation of porous PLGA/HA/collagen scaffolds with supercritical CO2 and application in osteoblast cell culture. J. Supercrit. Fluids 2011, 58, 398–406. [Google Scholar] [CrossRef]
- Yan, D.; Wang, Z.; Guo, Z.; Ma, Y.; Wang, C.; Tan, H.; Zhang, Y. Study on the properties of PLA/PBAT composite modified by nanohydroxyapatite. J. Mater. Res. Technol. 2020, 9, 11895–11904. [Google Scholar] [CrossRef]
- Park, J.-W.; Hwang, J.-U.; Back, J.-H.; Jang, S.-W.; Kim, H.-J.; Kim, P.-S.; Shin, S.; Kim, T. High strength PLGA/Hydroxyapatite composites with tunable surface structure using PLGA direct grafting method for orthopedic implants. Compos. B Eng. 2019, 178, 107449. [Google Scholar] [CrossRef]
- Lowe, B.; Hardy, J.G.; Walsh, L.J. Optimizing nanohydroxyapatite nanocomposites for bone tissue engineering. ACS Omega 2020, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Velasco, M.A.; Narváez-Tovar, C.A.; Garzόn-Alvarado, D.A. Design, materials, and mechanobiology of biodegradable scaffolds for bone tissue engineering. BioMed Res. Int. 2015, 2015, 729076. [Google Scholar] [CrossRef] [PubMed]
- Shelton, T.J.; Delman, C.; McNary, S.; Taylor, J.R.; Marder, R.A. Aging decreases the ultimate tensile strength of bone-partellar tendon-bone allografts. Arthroscopy 2021, 37, 2173–2180. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.C.S.; de Mesquita, F.A.S.; de Carvalho, L.H.; Alves, T.S.; Folkersma, R.; Araújo, R.S.d.R.M.; Oliveira, A.D.; Barbosa, R. Preparation and characterization of polymeric films based on PLA, PBAT and corn starch and babassu mesocarp starch by flat extrusion. Mater. Res. Express 2021, 8, 035305. [Google Scholar] [CrossRef]
- Gheisari, H.; Karamian, E.; Abdellahi, M. A novel hydroxyapatite–Hardystonite nanocomposite ceramic. Ceram. Int. 2015, 41, 5967–5975. [Google Scholar] [CrossRef]
- Rasheed, M.; Jawaid, M.; Parveez, B.; Bhat, A.H.; Alamery, S. Morphology, structural, thermal, and tensile properties of bamboo microcrystalline cellulose/poly(Lactic Acid)/poly(Butylene Succinate) Composites. Polymers 2021, 13, 465. [Google Scholar] [CrossRef]
- Solechan, S.; Suprihanto, A.; Widyanto, S.A.; Triyono, J.; Fitriyana, D.F.; Siregar, J.P.; Cionita, T. Characterization of PLA/PCL/Nano-Hydroxyapatite (nHA) biocomposites prepared via cold isostatic pressing. Polymers 2023, 15, 559. [Google Scholar] [CrossRef]
- Qiu, T.Y.; Song, M.; Zhao, L.G. Testing, characterization and modelling of mechanical behaviour of poly (lactic-acid) and poly (butylene succinate) blends. Mech. Adv. Mater. Mod. Process. 2016, 2, 7. [Google Scholar] [CrossRef]
- Qahtani, M.; Wu, F.; Misra, M.; Gregori, S.; Mielewski, D.F.; Mohanty, A.K. Experimental design of sustainable 3D-printed poly(Lactic acid)/biobased poly(butylene succinate) blends via fused deposition modeling. ACS Sustain. Chem. Eng. 2019, 7, 14460–14470. [Google Scholar] [CrossRef]
- Righetti, M.C.; Di Lorenzo, M.L.; Cinelli, P.; Gazzano, M. Temperature dependence of the rigid amorphous fraction of poly(butylene succinate). RSC Adv. 2021, 11, 25731–25737. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Pei, H.; Zhou, X.; Xie, X. Reactive compatibilization and properties of low-cost and high-performance PBAT/thermoplastic starch blends. Eur. Polym. J. 2021, 143, 110198. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, D.; Yu, S.; Zhou, H.; Peng, S. Recent advances in compatibility and toughness of poly(lactic acid)/poly(butylene succinate) blends. e-Polymers 2021, 21, 793–810. [Google Scholar] [CrossRef]
- Wang, B.; Jin, Y.; Kang, K.; Yang, N.; Weng, Y.; Huang, Z.; Men, S. Investigation on compatibility of PLA/PBAT blends modified by epoxy-terminated branched polymers through chemical micro-crosslinking. e-Polymers 2020, 20, 39–54. [Google Scholar] [CrossRef]
- Zhou, Y.; Qiu, S.; Waterhouse, G.I.N.; Zhang, K.; Xu, J. Enhancing the properties of PBAT/PLA composites with novel phosphorus-based ionic liquid compatibilizers. Mater. Today Commun. 2021, 27, 102407. [Google Scholar] [CrossRef]
- De Luna, M.S.; Filippone, G. Effects of nanoparticles on the morphology of immiscible polymer blends—Challenges and opportunities. Eur. Polym. J. 2016, 79, 198–218. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, B.; Qin, C.; Li, Q.; Liu, C.; Shen, C.; Wang, Y. Nonisothermal melt and cold crystallization behaviors of biodegradable poly(lactic acid)/Ti3C2Tx MXene nanocomposites. J. Therm. Anal. Calorim. 2022, 147, 2239–2251. [Google Scholar] [CrossRef]
- Mata-Padilla, J.M.; Avila-Orta, C.A.; Almendarez-Camarillo, A.; Martinez-Colunga, J.G.; Hernandez-Hernandez, E.; Cruz-Delgado, V.J.; Gonzalez-Morones, P.; Solis-Rosales, S.G.; Gonzalez-Calderon, J.A. Non-isothermal crystallization behavior of isotactic polypropylene/copper nanocomposites. J. Therm. Anal. Calorim. 2021, 143, 2919–2932. [Google Scholar] [CrossRef]
- Gao, H.; Qiang, T. Fracture surface morphology and impact strength of cellulose/PLA composites. Materials 2017, 10, 624. [Google Scholar] [CrossRef]
- Pivsa-Art, W.; Pivsa-Art, S. Effect of talc on mechanical characteristics and fracture toughness of poly(lactic acid)/poly(butylene succinate) blend. J. Polym. Environ. 2019, 27, 1821–1827. [Google Scholar] [CrossRef]
- Mao, H.-I.; Hsu, T.-S.; Chen, C.-W.; Huang, K.-W.; Rwei, S.-P. Sunthesis and characteristics of poly(ethylene terephthalate) with EO-PO-EO triblock copolymers: A thermal and mechanical property study. J. Appl. Polym. Sci. 2022, 139, e51065. [Google Scholar] [CrossRef]
- Zhao, T.; Yu, J.; Zhang, X.; Han, W.; Zhang, S.; Pan, H.; Zhang, Q.; Yu, X.; Bian, J.; Zhang, H. Thermal, crystallization, and mechanical properties of polylactic acid (PLA)/poly(butylene succinate) (PBS) blends. Polym. Bull. 2023, 1–24. [Google Scholar] [CrossRef]
- Ecker, J.V.; Haider, A.; Burzic, I.; Huber, A.; Eder, G.; Hild, S. Mechanical properties and water absorption behaviour of PLA and PLA/wood composites prepared by 3D printing and injection moulding. Rapid Prototyp. J. 2019, 25, 672–678. [Google Scholar] [CrossRef]
- Avci, A.; Eker, A.A.; Bodur, M.S.; Candan, Z. Water absorption characterization of boron compounds-reinforced PLA/flax fiber sustainable composite. Int. J. Biol. Macromol. 2023, 233, 123546. [Google Scholar] [CrossRef] [PubMed]



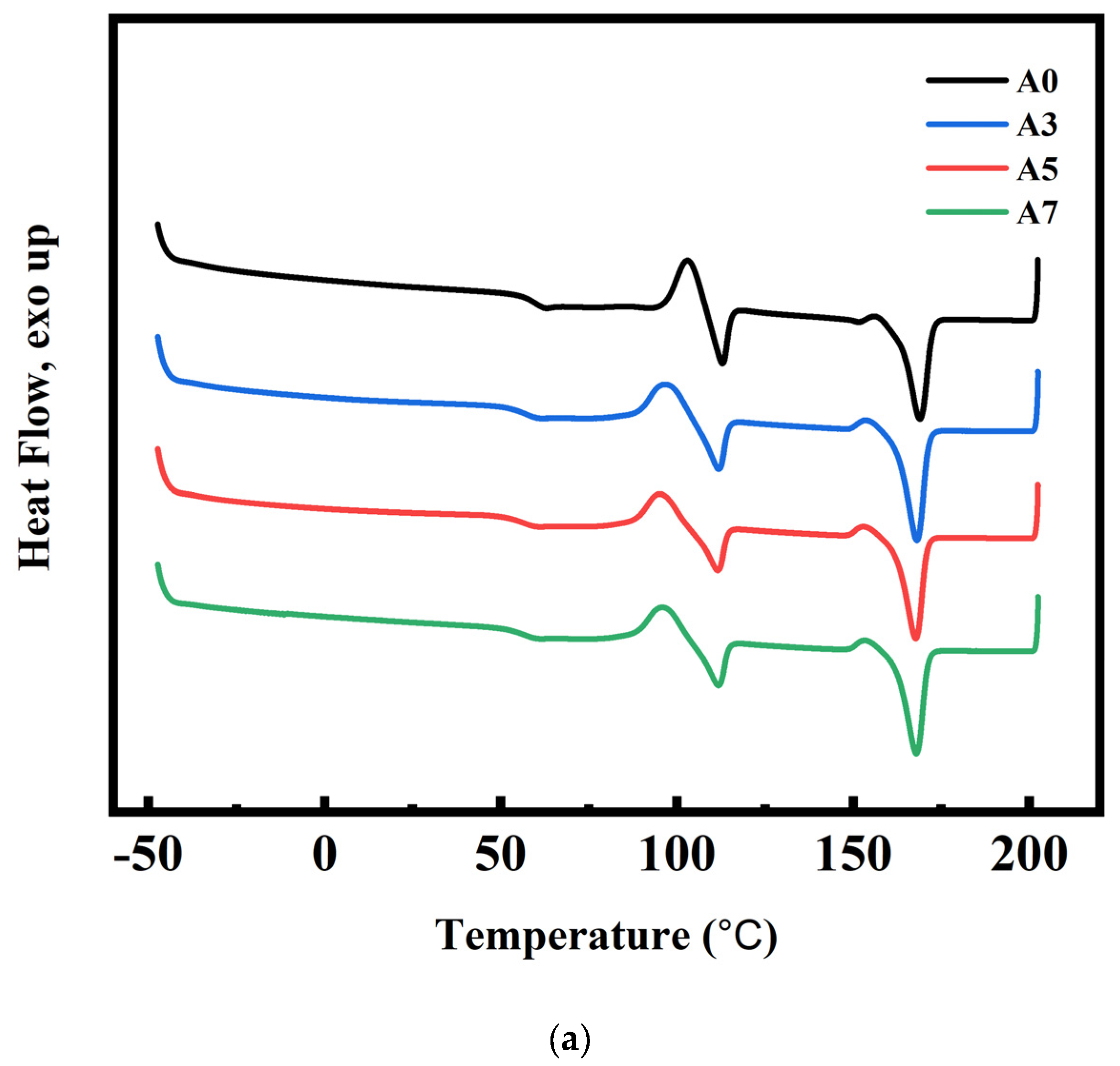


 : sample series A;
: sample series A;  : sample series B).
: sample series B).
 : sample series A;
: sample series A;  : sample series B).
: sample series B).
| Physical Properties | PLA | PBS | PBAT |
|---|---|---|---|
| Density (g/cm3) | 1.24 | 1.26 | 1.25–1.27 |
| Melt flow index (g/10 min, at 190 °C, 2.16 kg) | 7 | 5 | 2.7–4.9 |
| Melting temperature, Tm (°C) | 155–170 | 115 | 110–120 |
| Tensile yield strength (MPa) | 60 | 39 | 36 |
| Tensile modulus (GPa) | 3.5 | 0.58 | – |
| Tensile elongation (%) | 6.0 | 350 | 560 |
| Specimen Code | PLA (wt%) | PBS (wt%) | PBAT (wt%) | nHA (wt%) |
|---|---|---|---|---|
| Sample with 60 wt% PLA | ||||
| PLA60 | 60 | 25 | 15 | 0 |
| Sample series A | ||||
| A0 | 70 | 20 | 10 | 0 |
| A3 | 70 | 20 | 10 | 3 |
| A5 | 70 | 20 | 10 | 5 |
| A7 | 70 | 20 | 10 | 7 |
| Sample series B | ||||
| B0 | 80 | 15 | 5 | 0 |
| B3 | 80 | 15 | 5 | 3 |
| B5 | 80 | 15 | 5 | 5 |
| B7 | 80 | 15 | 5 | 7 |
| Sample of PLA/PBS/PBAT Blends | Maximum Tensile Stress (MPa) | Elongation at Maximum Tensile Stress (%) | Tensile Modulus (MPa) |
|---|---|---|---|
| PLA60 | 37.6 ± 1.8 | 7.0 ± 0.5 | 584.8 ± 71.9 |
| A0 | 47.1 ± 2.9 | 7.9 ± 0.4 | 642.0 ± 109.1 |
| B0 | 49.6 ± 1.6 | 8.0 ± 0.6 | 686.1 ± 91.3 |
| Specimen Code | Tg,PLA (°C) | Tcc (°C) | Tm,PLA (°C) | ΔHcc (J g−1) | ΔHm (J g−1) | Xc (%) |
|---|---|---|---|---|---|---|
| A0 | 62 | 103 | 169 | 12.0 | 36.3 | 20.1 |
| A3 | 63 | 100 | 169 | 13.0 | 41.9 | 24.8 |
| A5 | 63 | 99 | 168 | 14.9 | 45.2 | 26.0 |
| A7 | 61 | 99 | 168 | 13.5 | 45.0 | 27.0 |
| B0 | 62 | 103 | 169 | 15.7 | 37.9 | 20.1 |
| B3 | 63 | 101 | 169 | 18.6 | 41.3 | 20.6 |
| B5 | 63 | 102 | 169 | 15.6 | 40.2 | 22.3 |
| B7 | 63 | 102 | 169 | 14.8 | 38.5 | 21.5 |
| Specimen Code | Cooling Rate (°C min−1) | |||
|---|---|---|---|---|
| 2.5 | 5 | 7.5 | 10 | |
| ∆Hhc (J g−1) | ||||
| A0 | 0 | 0 | 0 | 0 |
| A3 | 7.80 | 1.08 | 0.63 | 0.37 |
| A5 | 9.88 | 1.70 | 0.45 | 0.41 |
| A7 | 10.90 | 2.72 | 0.91 | 0.50 |
| B0 | 10.12 | 3.46 | 0.74 | 0.55 |
| B3 | 11.96 | 3.31 | 0.94 | 0.46 |
| B5 | 10.09 | 2.56 | 0.84 | 0.36 |
| B7 | 10.91 | 2.71 | 0.94 | 0.48 |
| Specimen Code | Maximum Tensile Stress (MPa) | Elongation at Maximum Tensile Stress (%) | Tensile Modulus (MPa) | Impact Strength (kJ m−2) |
|---|---|---|---|---|
| A0 | 47.1 ± 2.9 | 7.9 ± 0.4 | 642.0 ± 109.1 | 4.83 ± 0.19 |
| A3 | 47.5 ± 0.7 | 7.5 ± 0.3 | 887.8 ± 57.2 | 4.32 ± 0.17 |
| A5 | 50.2 ± 1.0 | 7.4 ± 0.4 | 905.5 ± 64.1 | 4.64 ± 0.21 |
| A7 | 47.4 ± 0.3 | 6.7 ± 0.3 | 707.5 ± 15.0 | 4.20 ± 0.07 |
| B0 | 49.6 ± 1.6 | 8.0 ± 0.6 | 686.1 ± 91.3 | 3.36 ± 0.20 |
| B3 | 57.8 ± 0.7 | 7.1 ± 0.2 | 1039.4 ± 37.5 | 3.21 ± 0.16 |
| B5 | 57.9 ± 1.0 | 7.3 ± 0.2 | 1062.2 ± 97.8 | 3.42 ± 0.17 |
| B7 | 56.7 ± 1.0 | 7.3 ± 0.1 | 962.6 ± 17.4 | 3.19 ± 0.25 |
| Specimen Code | T5% (°C) | T50% (°C) | Tmax (°C) | WR (%) at 600 °C |
|---|---|---|---|---|
| A0 | 325 | 361 | 361 | 1.6 |
| A3 | 318 | 360 | 360 | 4.5 |
| A5 | 306 | 354 | 355 | 5.7 |
| A7 | 319 | 367 | 366 | 7.6 |
| B0 | 317 | 355 | 371 | 0.7 |
| B3 | 310 | 353 | 369 | 2.3 |
| B5 | 302 | 348 | 362 | 4.5 |
| B7 | 295 | 343 | 352 | 6.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.-H.; Chen, C.-W.; Mao, H.-I.; Dai, C.-A.; Su, C.-S.; Tsai, J.-C.; Lin, F.-H. Bio-Based PLA/PBS/PBAT Ternary Blends with Added Nanohydroxyapatite: A Thermal, Physical, and Mechanical Study. Polymers 2023, 15, 4585. https://doi.org/10.3390/polym15234585
Chen P-H, Chen C-W, Mao H-I, Dai C-A, Su C-S, Tsai J-C, Lin F-H. Bio-Based PLA/PBS/PBAT Ternary Blends with Added Nanohydroxyapatite: A Thermal, Physical, and Mechanical Study. Polymers. 2023; 15(23):4585. https://doi.org/10.3390/polym15234585
Chicago/Turabian StyleChen, Pei-Hua, Chin-Wen Chen, Hsu-I Mao, Chi-An Dai, Chie-Shaan Su, Jung-Chin Tsai, and Feng-Huei Lin. 2023. "Bio-Based PLA/PBS/PBAT Ternary Blends with Added Nanohydroxyapatite: A Thermal, Physical, and Mechanical Study" Polymers 15, no. 23: 4585. https://doi.org/10.3390/polym15234585
APA StyleChen, P.-H., Chen, C.-W., Mao, H.-I., Dai, C.-A., Su, C.-S., Tsai, J.-C., & Lin, F.-H. (2023). Bio-Based PLA/PBS/PBAT Ternary Blends with Added Nanohydroxyapatite: A Thermal, Physical, and Mechanical Study. Polymers, 15(23), 4585. https://doi.org/10.3390/polym15234585