New Quaternary Ammonium Derivatives Based on Citrus Pectin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthetic Procedure
2.3. Methods
2.3.1. Chemical Structure Characterization
2.3.2. Conductometric Titration
2.3.3. Capillary Viscometry Measurements
2.3.4. DLS Measurements
2.3.5. Fluorescence Measurements
2.3.6. AFM
2.3.7. Antimicrobial Activity
3. Results and Discussion
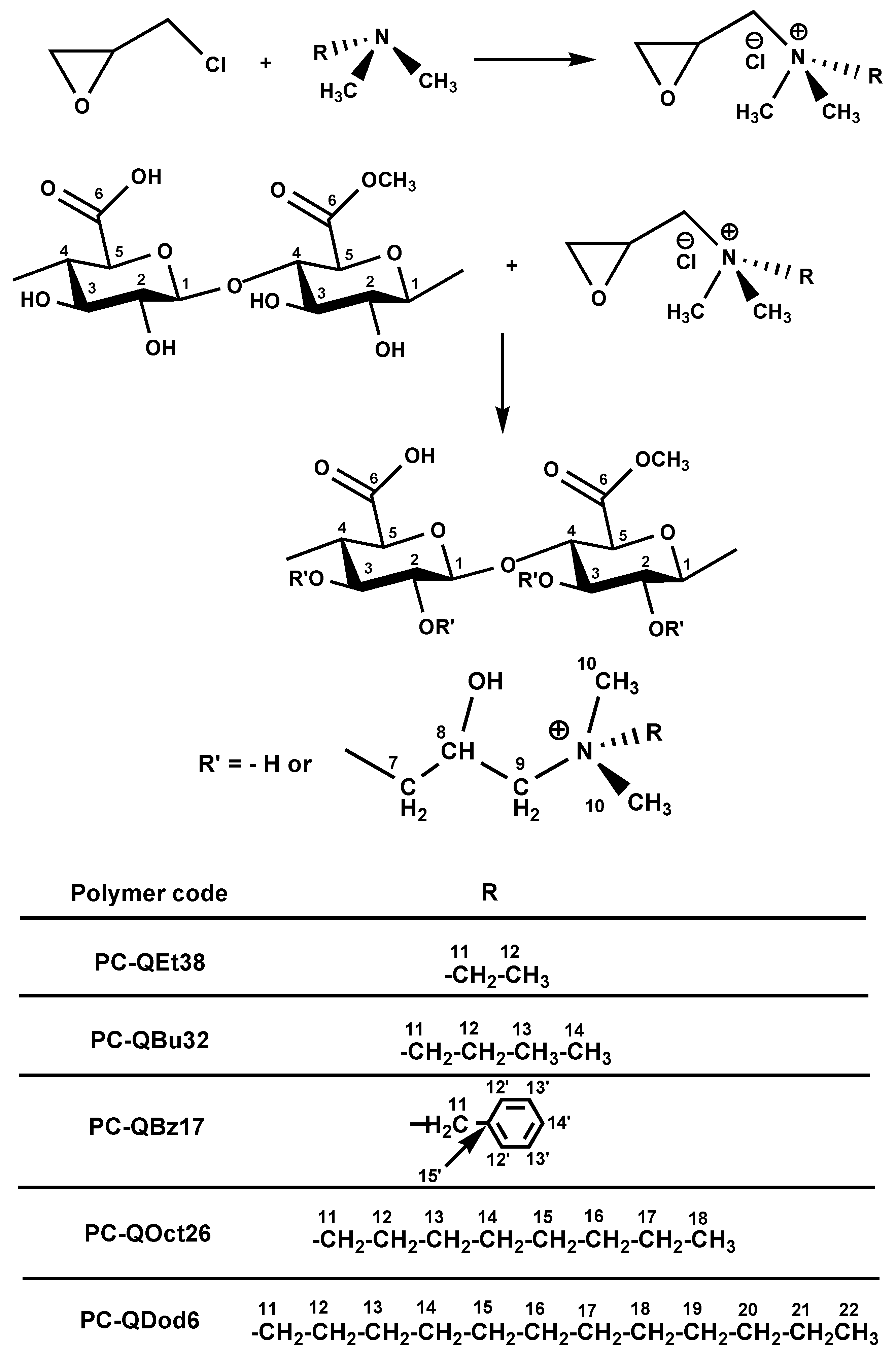
3.1. Synthesis of Quaternary Ammonium Derivatives of Pectin
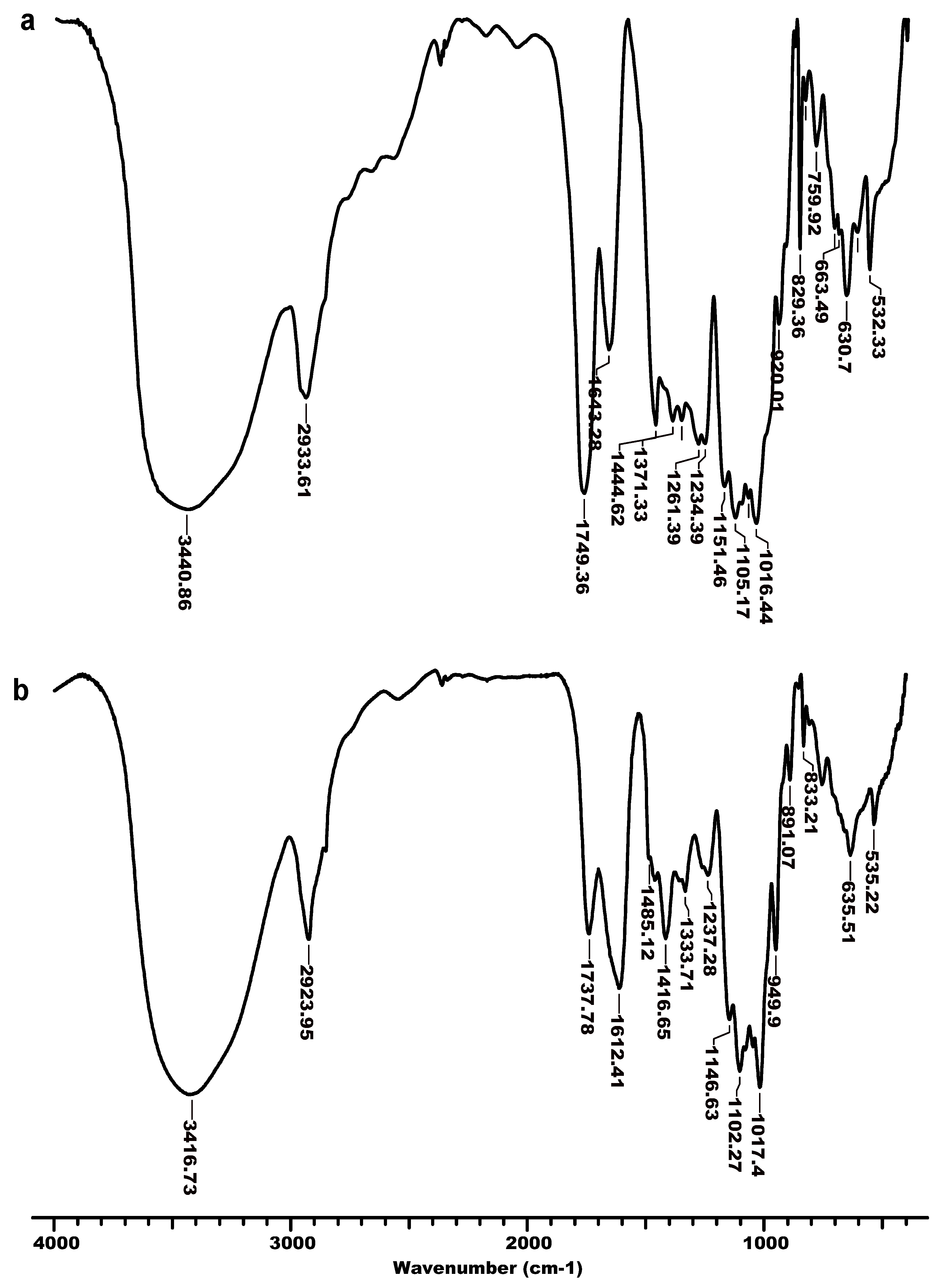
3.2. FTIR Analysis
3.3. 1H and 13C NMR Analysis
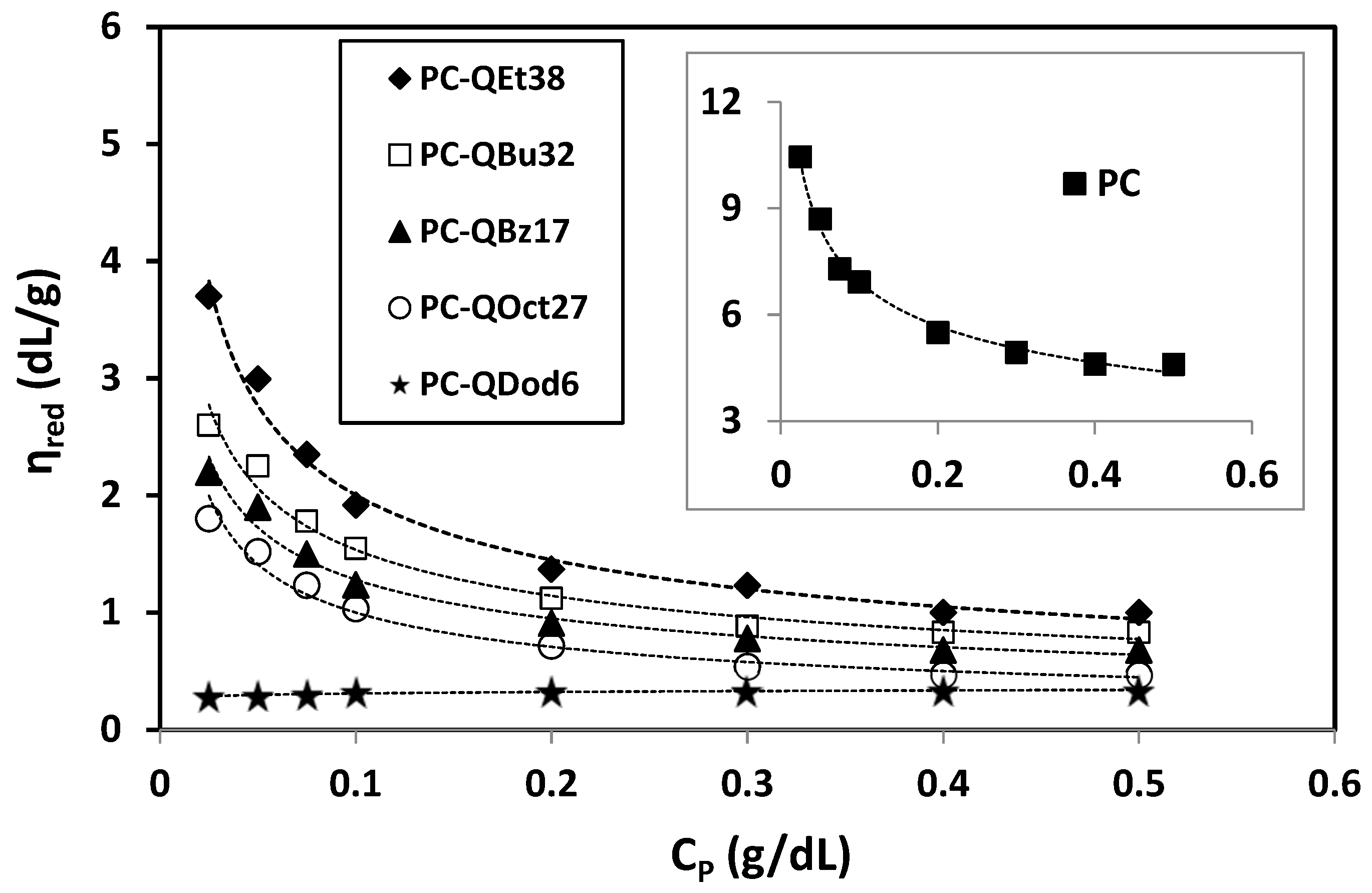
3.4. Viscosity Method
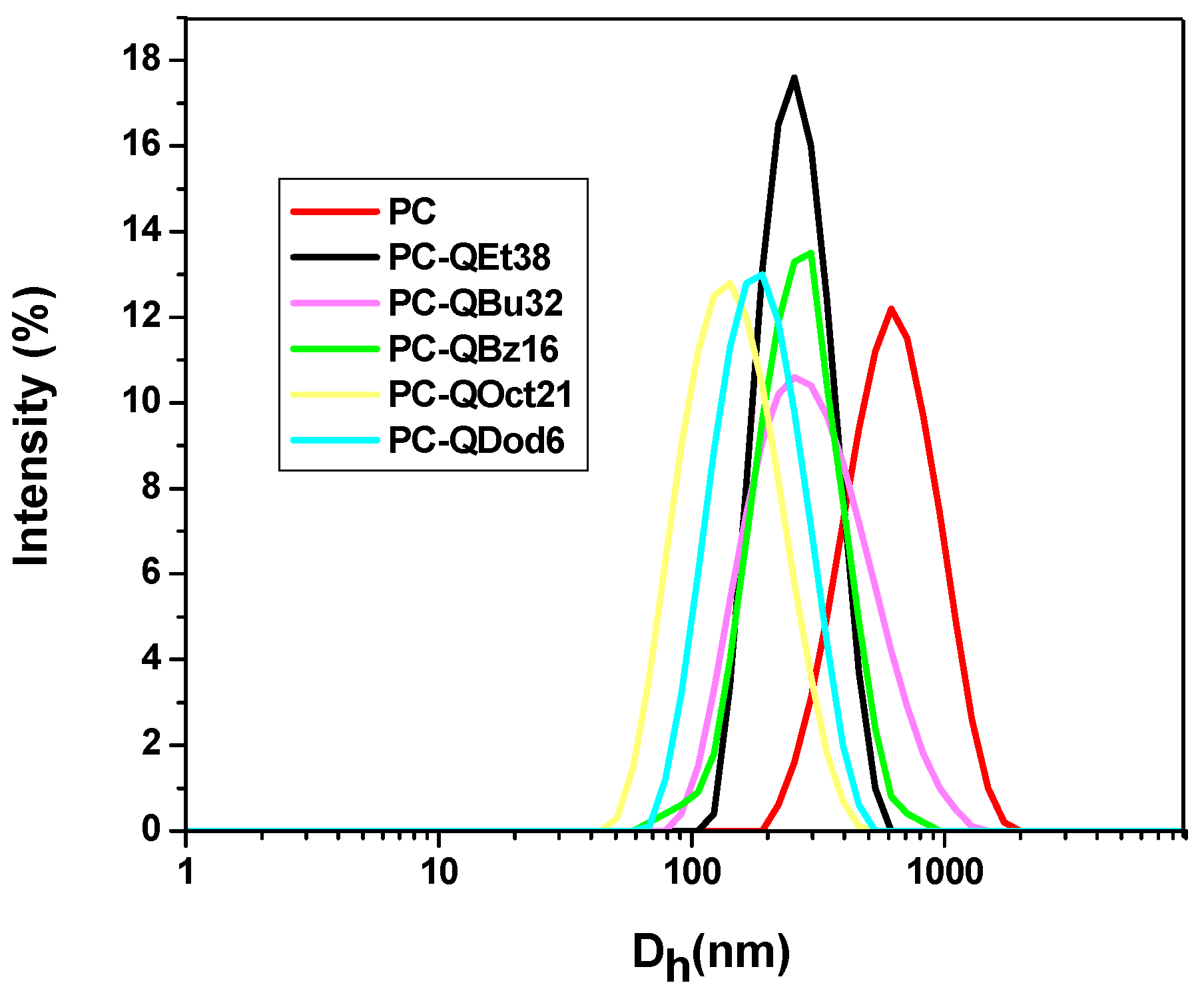
3.5. DLS Measurements
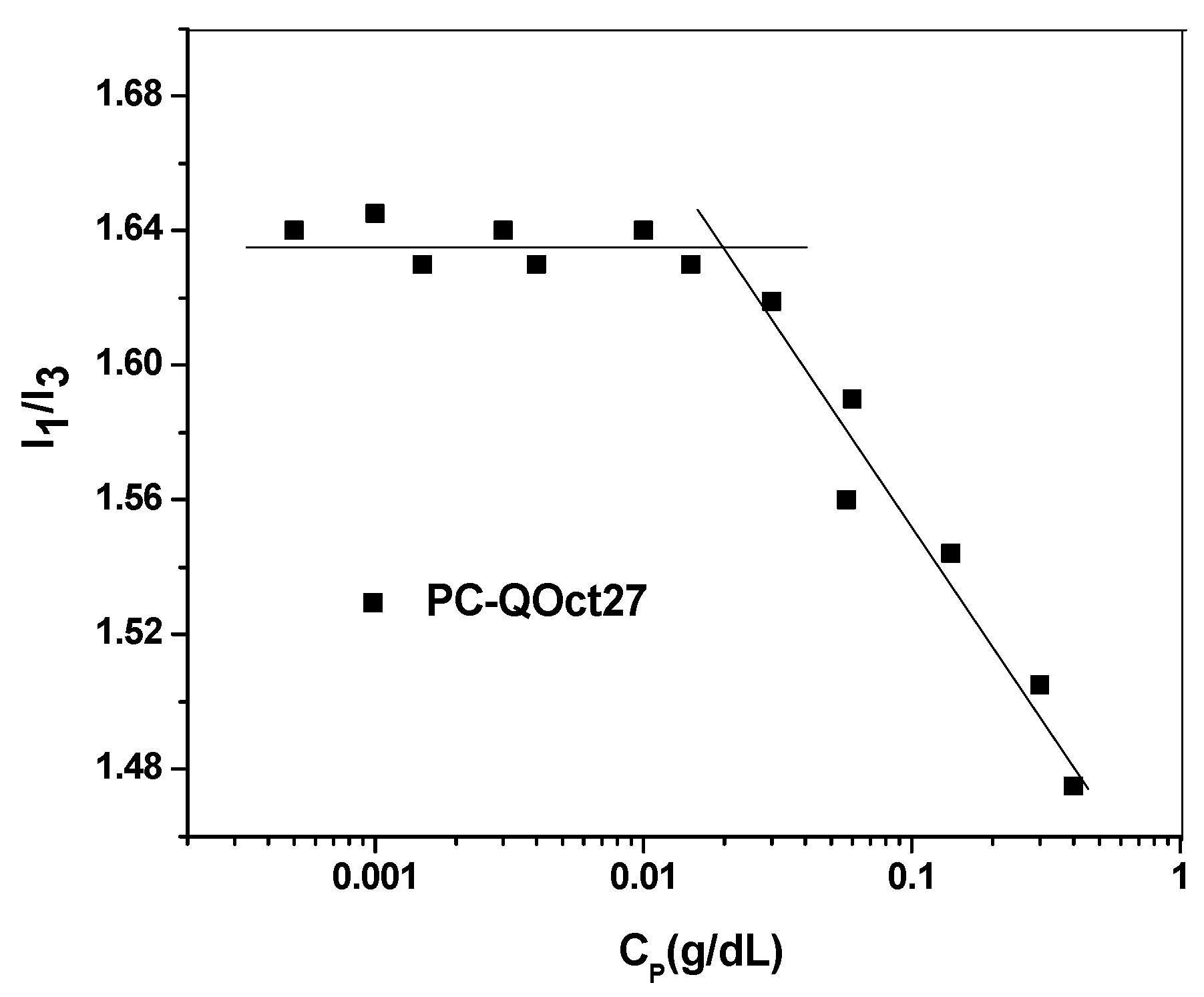
3.6. Fluorescence Studies
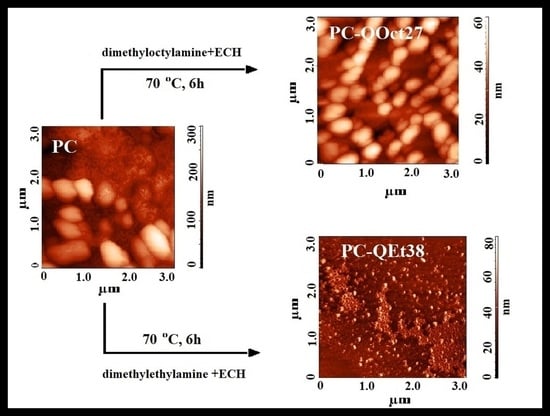
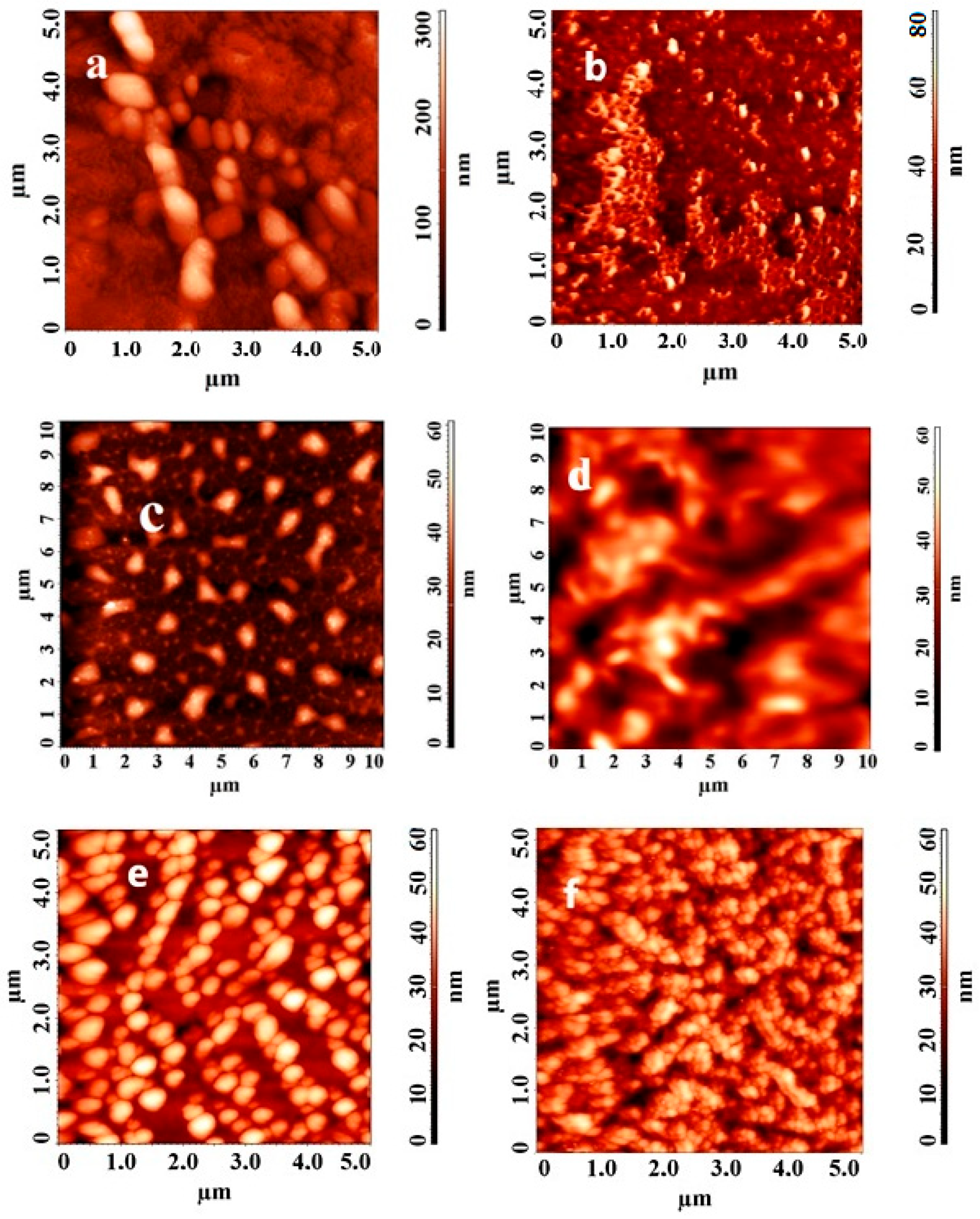
3.7. AFM Technique
3.8. Antimicrobial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Li, D.-Q.; Li, J.; Dong, H.-L.; Li, X.; Zhang, J.-Q.; Ramaswamy, S.; Xu, F. Pectin in biomedical and drug delivery applications: A review. Int. J. Biol. Macromol. 2021, 185, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Belkheiri, A.; Forouhar, A.; Ursu, A.V.; Dubessay, P.; Pierre, G.; Delattre, C.; Djelveh, G.; Abdelkafi, S.; Hamdami, N.; Michaud, P. Extraction, Characterization, and Applications of Pectins from Plant By-Products. Appl. Sci. 2021, 11, 6596. [Google Scholar] [CrossRef]
- Freitas, C.M.P.; Coimbra, J.S.R.; Souza, V.G.L.; Sousa, R.C.S. Structure and Applications of Pectin in Food, Biomedical, and Pharmaceutical Industry: A Review. Coatings 2021, 11, 922. [Google Scholar] [CrossRef]
- Morello, G.; De Iaco, G.; Gigli, G.; Polini, A.; Gervaso, F. Chitosan and Pectin Hydrogels for Tissue Engineering and In Vitro Modeling. Gels 2023, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Munarin, F.; Tanzi, M.C.; Petrini, P. Advances in biomedical applications of pectin gels. Int. J. Biol. Macromol. 2012, 51, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Katav, T.; Liu, L.; Traitel, T.; Goldbart, R.; Wolfson, M.; Kost, J. Modified pectin-based carrier for gene delivery: Cellular barriers in gene delivery course. J. Control. Release 2008, 130, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Alvarado, K.; Chacón-Cerdas, R.; Starbird-Perez, R. Pectin Microspheres: Synthesis Methods, Properties, and Their Multidisciplinary Applications. Chemistry 2022, 4, 121–136. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, P.; Zhang, H. Pectin in cancer therapy: A review. Trends Food Sci. Technol. 2015, 44, 258–271. [Google Scholar] [CrossRef]
- Khramova, D.S.; Vityazev, F.V.; Saveliev, N.Y.; Burkov, A.A.; Beloserov, V.S.; Martinson, E.A.; Litvinets, S.G.; Popov, S.V. Pectin gelling in acidic gastric condition increases rheological properties of gastric digesta and reduces glycaemic response in mice. Carbohydr. Polym. 2019, 205, 456–464. [Google Scholar] [CrossRef]
- Zhu, R.-G.; Sun, Y.D.; Li, T.P.; Chen, G.; Peng, X.; Duan, W.-B.; Zheng, Z.-Z.; Shi, S.-L.; Xu, J.-G.; Liu, Y.-H.; et al. Comparative effects of hawthorn (Crataegus pinnatifida Bunge) pectin and pectin hydrolyzates on the cholesterol homeostasis of hamsters fed high-cholesterol diets. Chem.-Biol. Interact. 2015, 238, 42–47. [Google Scholar] [CrossRef]
- Pirsa, S.; Hafezi, K. Hydrocolloids: Structure, preparation method, and application in food industry. Food Chem. 2023, 399, 133967. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.; Hulle, N.R.S. Citrus pectins: Structural properties, extraction methods, modifications and applications in food systems—A review. Appl. Food Res. 2022, 2, 100215. [Google Scholar] [CrossRef]
- Mellinas, C.; Valdés, A.; Ramos, M.; Burgos, N.; Garrigós, M.C.; Jiménez, A. Active edible films: Current state and future trends. J. Appl. Polym. Sci. 2016, 133, 42631. [Google Scholar] [CrossRef]
- González-Henríquez, C.M.; Sarabia-Vallejos, M.A.; Rodriguez-Hernandez, J. Polymers for additive manufacturing and 4D-printing: Materials, methodologies, and biomedical applications. Prog. Polym. Sci. 2019, 94, 57–116. [Google Scholar] [CrossRef]
- Chen, J.; Liu, W.; Liu, C.M.; Li, T.; Liang, R.H.; Luo, S.J. Pectin modifications: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1684–1698. [Google Scholar] [CrossRef] [PubMed]
- Würfel, H.; Geitel, K.; Qi, H.; Heinze, T. Chemical modification of pectin and polygalacturonic acid: A critical review. BioResources 2021, 16, 8457–8488. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Li, J.; Zhou, Y.; Li, D.-Q.; Du, G.-M. Chemical cross-linking approach for prolonging diclofenac sodium release from pectin-based delivery system. Int. J. Biol. Macromol. 2019, 137, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Nie, H.; Chen, Y.; Xiang, Z.-Y.; Li, J.-B. Amide pectin: A carrier material for colon-targeted controlled drug release. J. Appl. Polym. Sci. 2016, 133, 43697. [Google Scholar] [CrossRef]
- Fan, L.; Cao, M.; Gao, S.; Wang, W.; Peng, K.; Tan, C.; Wen, F.; Tao, S.; Xie, W. Preparation and characterization of a quaternary ammonium derivative of pectin. Carbohydr. Polym. 2012, 88, 707–712. [Google Scholar] [CrossRef]
- de Almeida, W.S.; da Silva, D.A. Does polysaccharide quaternization improve biological activity? Int. J. Biol. Macromol. 2021, 182, 1419–1436. [Google Scholar] [CrossRef]
- Imkovic, I.; Uhliariková, I.; Yadav, M.P.; Mendichi, R. Branched arabinan obtained from sugar beet pulp by quaternization under acidic conditions. Carbohydr. Polym. 2010, 82, 815–821. [Google Scholar] [CrossRef]
- Qin, C.; Yang, G.; Wu, S.; Zhang, H.; Zhu, C. Synthesis, physicochemical characterization, antibacterial activity, and biocompatibility of quaternized hawthorn pectin. Int. J. Biol. Macromol. 2022, 213, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Chintakunta, R.; Buaron, N.; Kahn, N.; Moriah, A.; Lifshiz, R.; Goldbart, R.; Traitel, T.; Tyler, B.; Brem, H.; Kost, J. Synthesis, characterization, and self-assembly with plasmid DNA of a quaternary ammonium derivative of pectic galactan and its fluorescent labeling for bioimaging applications. Carbohydr. Polym. 2016, 150, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Vityazev, F.V.; Fedyuneva, M.I.; Golovchenko, V.V.; Patova, O.A.; Ipatova, E.U.; Durnev, E.A.; Martinson, E.A.; Litvinets, S.G. Pectin-silica gels as matrices for controlled drug release in gastrointestinal tract. Carbohydr. Polym. 2017, 157, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Gao, S.; Wang, L.; Wu, P.; Cao, M.; Zheng, H.; Xie, W.; Zhou, J. Synthesis and anticoagulant activity of pectin sulfates. J. Appl. Polym. Sci. 2012, 124, 2171–2178. [Google Scholar] [CrossRef]
- Li, D.-Q.; Wang, S.-Y.; Meng, Y.-J.; Li, J.-F.; Li, J. An injectable, self-healing hydrogel system from oxidized pec-tin/chitosan/γ-Fe2O3. Int. J. Biol. Macromol. 2020, 164, 4566–4574. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Yao, B.; Gao, M.; Sun, X.; Gou, D.; Hu, J.; Zhou, Y.; Liu, Y. Effects of pectin structure and crosslinking method on the properties of crosslinked pectin nanofibers. Carbohydr. Polym. 2017, 157, 766–774. [Google Scholar] [CrossRef]
- Babaladimath, G.; Badalamoole, V. Magnetic nanoparticles embedded in pectin-based hydrogel for the sustained release of diclofenac sodium. Polym. Int. 2018, 67, 983–992. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, L.; Feng, X. Introduction of 3-(trimethylammonium chloride)-2-hydroxypropyls onto starch chains for improving the grafting efficiency and sizing property of starch-g-poly(acrylic acid). Starch-Stärke 2016, 68, 742–752. [Google Scholar] [CrossRef]
- Šimkovic, I.; Yadav, M.P.; Zalibera, M.; Hicks, K.B. Chemical modification of corn fiber with ion-exchanging groups. Carbohydr. Polym. 2009, 76, 250–254. [Google Scholar] [CrossRef]
- Gong, Y.; Yuan, J.; Pei, Y.; Liu, S.; Luo, X. One-step quaternization and macromolecular reconstruction to prepare micro-/nano-porous cellulose beads from homogeneous solution for low-concentration amoxicillin removal. Carbohydr. Polym. 2023, 315, 120985. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Xing, L.; Ouyang, J.; Li, Z.; Wang, X. Adsorption Properties of Anionic Dyes on Quaternized Microcrystalline Cellulose. ACS Omega 2023, 8, 5617–5624. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, J.F.S.; Rasteiro, M.G.; Neto, C.P.; Ferreira, P.J.T. Effect of cationization pretreatment on the properties of cationic Eucalyptus micro/nanofibrillated cellulose. Int. J. Biol. Macromol. 2022, 201, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.L.; Sun, R.C.; Liu, C.F.; Lin, L.; He, B.H. Synthesis and characterization of novel cationic SCB hemicelluloses with a low degree of substitution. Carbohydr. Polym. 2007, 67, 347–357. [Google Scholar] [CrossRef]
- Fröhlich, A.C.; Bazzo, G.C.; Stulzer, H.K.; Parize, A.L. Synthesis and physico-chemical characterization of quaternized and sulfated xylan-derivates with enhanced microbiological and antioxidant properties. Biocatal. Agric. Biotechnol. 2022, 43, 102416. [Google Scholar] [CrossRef]
- Wang, J.; Guo, C.; Yue, T.; Yuan, Y.; Liu, X.; Kennedy, J.F. Cationization of Ganoderma lucidum polysaccharides in concentrated alkaline solutions as gene carriers. Carbohydr. Polym. 2012, 88, 966–972. [Google Scholar] [CrossRef]
- Thomas, J.J.; Rekha, M.R.; Sharma, C.P. Dextran-glycidyltrimethyl ammonium chloride conjugate/DNA nanoplex: A potential non-viral and haemocompatible gene delivery system. Int. J. Pharm. 2010, 389, 195–206. [Google Scholar] [CrossRef]
- Kumar, V.; Goyal, P.; Sharma, P. Preparation of quaternary ammonium compound of tamarind kernel powder. Trends Carbohydr. Res. 2012, 4, 47–53. [Google Scholar]
- Zhang, S.; Huang, S.; Lu, L.; Song, X.; Li, P.; Wang, F. Curdlan sulfate-O-linked quaternized chitosan nanoparticles: Potential adjuvants to improve the immunogenicity of exogenous antigens via intranasal vaccination. Int. J. Nanomed. 2018, 13, 2377–2394. [Google Scholar] [CrossRef]
- Fusteş-Dămoc, I.; Măluţan, T.; Mija, A. Chitosan as a Polyfunctional Crosslinker for a Renewable-Based Resorcinol Diglycidyl Ether. ACS Sustain. Chem. Eng. 2023, 11, 7605–7616. [Google Scholar] [CrossRef]
- Lee, J.K.; Kim, I.W.; Kim, T.S.; Choi, J.H.; Kim, J.H.; Park, S.H. Immunological activities of cationic methylan derivatives. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 319–321. [Google Scholar] [CrossRef]
- Wang, K.; Gao, S.; Shen, C.; Liu, J.; Li, S.; Chen, J.; Ren, X.; Yuan, Y. Preparation of cationic konjac glucomannan in NaOH/urea aqueous solution. Carbohydr. Polym. 2018, 181, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Qiu, L.; Tan, W.; Gu, G.; Guo, Z. Novel 1,2,3-triazolium-functionalized inulin derivatives: Synthesis, free radical-scavenging activity, and antifungal activity. RSC Adv. 2017, 7, 42225–42232. [Google Scholar] [CrossRef]
- Tyagi, R.; Sharma, P.; Nautiyal, R.; Lakhera, A.K.; Kumar, V. Synthesis of quaternised guar gum using Taguchi L(16) orthogonal array. Carbohydr. Polym. 2020, 237, 116136. [Google Scholar] [CrossRef] [PubMed]
- Novac, O.; Lisa, G.; Profire, L.; Tuchilus, C.; Popa, M.I. Antibacterial quaternized gellan gum based particles for controlled release of ciprofloxacin with potential dermal applications. Mater. Sci. Eng. C 2014, 35, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Quelemes, P.V.; de Araújo, A.R.; Plácido, A.; Delerue-Matos, C.; Maciel, J.S.; Bessa, L.J.; Ombredane, A.S.; Joanitti, G.A.; dos, S. Soares, M.J.; Eaton, P.; et al. Quaternized cashew gum: An anti-staphylococcal and biocompatible cationic polymer for biotechnological applications. Carbohydr. Polym. 2017, 157, 567–575. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, A.K.A.; Ribeiro, F.O.S.; de Oliveira, T.M.; de Araújo, A.R.; Dias, J.N.; Albuquerque, P.; Silva-Pereira, I.; de Jesus Oliveira, A.C.; Quelemes, P.V.; Leite, J.R.S.A.; et al. Quaternization of angico gum and evaluation of anti-staphylococcal effect and toxicity of their derivatives. Int. J. Biol. Macromol. 2020, 150, 1175–1183. [Google Scholar] [CrossRef]
- Nichifor, M.; Stanciu, M.C.; Simionescu, B.C. New cationic hydrophilic and amphiphilic polysaccharides synthesized by one pot procedure. Carbohydr. Polym. 2010, 82, 965–975. [Google Scholar] [CrossRef]
- Stanciu, M.C.; Nichifor, M.; Ailiesei, G.L. Bile salts adsorption on dextran-based hydrogels. Int. J. Biol. Macromol. 2021, 190, 270–283. [Google Scholar] [CrossRef]
- Stanciu, M.C.; Nichifor, M.; Prisacaru, A.-I. Adsorption of Sodium Cholate on Cationic Dextran Gels: Comparison of Isotherm Binding Models. Mater. Plast. 2020, 57, 181–192. [Google Scholar] [CrossRef]
- Stanciu, M.C.; Nichifor, M. Influence of dextran hydrogel characteristics on adsorption capacity for anionic dyes. Carbohyd. Polym. 2018, 199, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Stanciu, M.C.; Nichifor, M. Adsorption of anionic dyes on a cationic amphiphilic dextran hydrogel: Equilibrium, kinetic, and thermodynamic studies. Colloid Polym. Sci. 2019, 297, 45–57. [Google Scholar] [CrossRef]
- Shi, M.; Xu, Y.; Li, S.; Wang, L.; Gu, J.; Zhang, Y.-X. The Development of a Polysaccharide-Based Hydrogel Encapsulating Tobramycin-Loaded Gelatine Microspheres as an Antibacterial System. Gels 2023, 9, 219. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.A.; Binhamad, H.A.S. Isolation and characterisation of Pectin. In Pectin: Technological and Physiological Properties; Kontogiorgos, V., Ed.; Springer: Cham, Switzerland, 2020; pp. 61–82. [Google Scholar] [CrossRef]
- Popescu, I.; Lupei, M.; Constantin, M. Double cross-linked pectin beads stable in physiological environment as potential support for biomedical applications. J. Polym. Res. 2021, 28, 424. [Google Scholar] [CrossRef]
- CLSI. Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts, CLSI Guideline M44, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, CLSI Supplement M100, 32nd ed.; Clinical and laboratory Standards Institute: Wayne, PA, USA, 2022. [Google Scholar]
- Monfregola, L.; Leone, M.; Vittoria, V.; Amodeo, P.; De Luca, S. Chemical modification of pectin: Environmental friendly process for new potential material development. Polym. Chem. 2011, 2, 800–804. [Google Scholar] [CrossRef]
- Han, Q.; Wu, Z.; Huang, B.; Sun, L.; Ding, C.; Yuan, S.; Zhang, Z.; Chen, Y.; Hu, C.; Zhou, L.; et al. Extraction, antioxidant and antibacterial activities of Broussonetia papyrifera fruits polysaccharides. Int J Biol Macromol. 2016, 92, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Mal, D.; Singh, R.P. Cationic starch: An effective flocculating agent. Carbohydr. Polym. 2005, 59, 417–423. [Google Scholar] [CrossRef]
- Anastassopoulou, J.D. Mass and FT-IR Spectra of Quaternary Ammonium Surfactants. In Chemistry and Properties of Biomolecular Systems; Topics in Molecular Organization and Engineering; Rizzarelli, E., Theophanides, T., Eds.; Kluwer Academic Publishers: New York, NY, USA, 1991; Volume 8, pp. 1–9. [Google Scholar]
- Synystsya, A.; Copikova, J.; Matejka, P.; Machovic, V. Fourrier transform Raman and infrared spectroscopy of pectins. Carbohydr. Polym. 2003, 54, 97–106. [Google Scholar] [CrossRef]
- Pacheco, M.T.; Villamiel, M.; Moreno, R.; Moreno, F.J. Structural and Rheological Properties of Pectins Extracted from Industrial Sugar Beet By-Products. Molecules 2019, 24, 392. [Google Scholar] [CrossRef]
- Binhamad, H. Extraction, Characterisation and Properties of Polysaccharides from Novel Sources. Ph.D. Thesis, University of Huddersfield, Huddersfield, UK, 2018. [Google Scholar]
- Perrone, P.; Hewage, C.M.; Thomson, A.R.; Bailey, K.; Sadler, I.H.; Fry, S.C. Patterns of methyl and O-acetyl esterification in spinach pectins: New complexity. Phytochemistry 2002, 60, 67–77. [Google Scholar] [CrossRef]
- Winning, H.; Viereck, N.; Nørgaard, L.; Larsen, J.; Engelsen, S.B. Quantification of the degree of blockiness in pectins using 1H NMR spectroscopy and chemometrics. Food Hydrocoll. 2007, 21, 256–266. [Google Scholar] [CrossRef]
- Rosenbohm, C.; Lundt, I.; Christensen, T.; Young, N. Chemically methylated and reduced pectins: Preparation, characterisation by 1H NMR spectroscopy, enzymatic degradation, and gelling properties. Carbohydr. Res. 2003, 338, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.R.; Feitosa, J.P.; Ricardo, N.; Brito, E.S. Isolation and Characterization of Pumpkin Pectin for Drug Encapsulation. Congrès Lat. Am. Biomatér. (COLAOB) 2012, 22, 25. [Google Scholar]
- Marcon, M.; Carneiro, P.; Wosiacki, G.; Beleski-Carneiro, E.; Petkowicz, C. Pectins from apple pomace–characterization by 13C and 1H NMR spectroscopy. Ann. Magn. Reson. 2005, 4, 56–63. [Google Scholar]
- Synytsya, A.; Copııkova, J.; Marounek, M.; Mlcochova, P.; Sihelnııkova, L.; Skoblya, S. N-octadecylpectinamide, a hydrophobic sorbent based on modification of highly methoxylated citrus pectin. Carbohydr. Polym. 2004, 56, 169–179. [Google Scholar] [CrossRef]
- Yu, H.; Huang, Y.; Ying, H.; Xiao, C. Preparation and characterization of a quaternary ammonium derivative of konjac glucomannan. Carbohydr. Polym. 2007, 69, 29–40. [Google Scholar] [CrossRef]
- Song, Y.; Sun, Y.; Zhang, X. Homogeneous quaternization of cellulose in NaOH/urea aqueous solutions as gene carriers. Biomacromolecules 2008, 9, 2259–2264. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-x.; Wu, X.-l.; Xue, D.-h.; Xu, K.; Tan, Y.; Du, X.-B. Preparation and characterization of cationic corn starch with a high degree of substitution in dioxane-THF-water media. Carbohydr. Res. 2009, 344, 851–855. [Google Scholar] [CrossRef]
- Yoo, S.-H.; Fishman, M.L.; Hotchkiss, A.T.; Lee, H.G. Viscometric behavior of high-methoxy and low-methoxy pectin solutions. Food Hydrocoll. 2006, 20, 62–67. [Google Scholar] [CrossRef]
- Ding, F.; Shi, X.; Li, X.; Cai, J.; Duan, B.; Du, Y. Homogeneous synthesis and characterization of quaternized chitin in NaOH/urea aqueous solution. Carbohydr. Polym. 2012, 87, 422–426. [Google Scholar] [CrossRef]
- Sundeen, K.E. Pectin: The Miracle Molecule. Master’s Thesis, University of Pennsylvania, Philadelphia, PA, USA, 2009. [Google Scholar]
- Lima, A.; Soldi, V.; Borsali, R. Dynamic Light Scattering and Viscosimetry of Aqueous Solutions of Pectin, Sodium Alginate and their Mixtures: Effects of Added Salt, Concentration, Counterions, Temperature and Chelating Agent Polym. J. Braz. Chem. Soc. 2009, 20, 1705–1714. [Google Scholar] [CrossRef]
- Nichifor, M.; Stanciu, M.C.; Simionescu, B.C. Fluorescence Study of Intermolecular Interactions in Diluted Aqueous Solutions of Some Cationic Amphiphilic Polysaccharides. J. Macromol. Sci., Part B 2010, 49, 983–993. [Google Scholar] [CrossRef]
- Pieczywek, P.M.; Kozioł, A.; Płaziński, W.; Cybulska, J.; Zdunek, A. Resolving the nanostructure of sodium carbonate extracted pectins (DASP) from apple cell walls with atomic force microscopy and molecular dynamics. Food Hydrocoll. 2020, 104, 105726. [Google Scholar] [CrossRef]
- Round, A.N.; Rigby, N.M.; MacDougall, A.J.; Morris, V.J. A new view of pectin structure revealed by acid hydrolysis and atomic force microscopy. Carbohydr Res. 2010, 345, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Morris, V.J.; Gromer, A.; Kirby, A.R.; Bongaerts, R.J.M.; Gunning, A.P. Using AFM and force spectroscopy to determine pectin structure and (bio) functionality. Food Hydrocoll. 2011, 25, 230–237. [Google Scholar] [CrossRef]
- Tuchilus, C.G.; Nichifor, M.; Mocanu, G.; Stanciu, M.C. Antimicrobial of chemically modified dextran derivatives. Carbohydr. Polym. 2017, 161, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Tuchilus, C.G.; Belei, D.; Coroaba, A.; Nichifor, M.; Stanciu, M.C. Hydrophobically Modified Dextran Esters As Potential External Biocides. Farmacia 2022, 70, 617–627. [Google Scholar] [CrossRef]
- Ciriminna, R.; Fidalgo, A.; Meneguzzo, F.; Presentato, A.; Scurria, A.; Nuzzo, D.; Alduina, R.; Ilharco, L.M.; Pagliaro, M. Pectin: A Long-Neglected Broad-Spectrum Antibacterial. ChemMedChem 2020, 15, 2228. [Google Scholar] [CrossRef]
- Presentato, A.; Scurria, A.; Albanese, L.; Lino, C.; Sciortino, M.; Pagliaro, M.; Zabini, F.; Meneguzzo, F.; Alduina, R.; Nuzzo, D.; et al. Superior Antibacterial Activity of Integral Lemon Pectin Extracted via Hydrodynamic Cavitation. ChemistryOpen 2020, 9, 628. [Google Scholar] [CrossRef]


| Type of Bond | Polymer Code | |||||
|---|---|---|---|---|---|---|
| PC | PC-QEt38 | PC-QBut32 | PC-QBz17 | PC-QOct27 | PC-QDod6 | |
| O–H | 3440 | 3416 | 3416 | 3409 | 3401 | 3417 |
| C–H(aliphatic) | 2933 | 2923 | 2923 | 2936 | 2927 | 2923 |
| C=Oester | 1749 | 1738 | 1741 | 1742 | 1741 | 1742 |
| C=Oacid (ionic form) | 1643 | 1612 | 1612 | 1608 | 1611 | 1609 |
| CH3–N+ | - | 1485 | 1485 | 1483 | 1480 | 1485 |
| CH3–(CH2)n–CH2– N+ | - | - | - | - | 721 | 719 |
| C–N | - | 1416 | 1416 | 1413 | 1414 | 1413 |
| C–O (glycosidic) | 1105 | 1084 | 1084 | 1100 | 1099 | 1102 |
| C–C (pyranoid ring) | 1016 | 1017 | 1017 | 1021 | 1020 | 1021 |
| C–H (aromatic) | - | - | - | 1040/953/737 | - | - |
| Polymer Code | PC | PC-QEt38 | PC-QBu32 | PC-QBz17 | PC-QOct27 | PC-QDod6 | |
|---|---|---|---|---|---|---|---|
| DM (%) | FTIR | 62.7 | 46.4 | 48.1 | 52.2 | 50.1 | 53.4 |
| 1H NMR | 64.3 | - | - | - | - | - | |
| conductometric titration | 64.9 | - | - | - | - | - | |
| DS (mol %) | % N | - | 38 | 32 | 17 | 27 | 6 |
| 1H NMR | - | 38 | 32 | 17 | 27 | 5.6 | |
| Polymer Code | PC | PC-QEt38 | PC-QBu32 | PC-QBz17 | PC-QOct27 | PC-QDod6 |
|---|---|---|---|---|---|---|
| Dh (nm) | 561 | 263 | 302 | 352 | 221 | 150 |
| ζ-potential (mV) | −29.8 | −4.4 | −1.0 | −0.8 | −2.6 | −10.1 |
| [η] (mL/g) | 328.6 | 111.3 | 93.9 | 80.6 | 63.2 | 39.3 |
| Polymeric Code | S. aureus ATCC 25923 | E. coli ATCC 25922 | P. aeruginosa ATCC 27853 | C. albicans ATCC 90028 |
|---|---|---|---|---|
| PC | 11 | - | - | 11 |
| PC-QEt38 | 12 | - | - | 14 |
| PC-QBu32 | 14 | - | - | 12 |
| PC-QBz17 | 17 | - | - | 15 |
| PC-QOct27 | 19 | - | - | 16 |
| PC-QDod6 | 12 | - | - | 11 |
| Ciprofloxacin | 34 | 35 | 33 | - |
| Nystatin | - | - | - | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanciu, M.-C.; Nichifor, M.; Ailiesei, G.-L.; Popescu, I.; Hitruc, G.-E.; Ghimici, L.; Tuchilus, C.G. New Quaternary Ammonium Derivatives Based on Citrus Pectin. Polymers 2023, 15, 4492. https://doi.org/10.3390/polym15234492
Stanciu M-C, Nichifor M, Ailiesei G-L, Popescu I, Hitruc G-E, Ghimici L, Tuchilus CG. New Quaternary Ammonium Derivatives Based on Citrus Pectin. Polymers. 2023; 15(23):4492. https://doi.org/10.3390/polym15234492
Chicago/Turabian StyleStanciu, Magdalena-Cristina, Marieta Nichifor, Gabriela-Liliana Ailiesei, Irina Popescu, Gabriela-Elena Hitruc, Luminita Ghimici, and Cristina G. Tuchilus. 2023. "New Quaternary Ammonium Derivatives Based on Citrus Pectin" Polymers 15, no. 23: 4492. https://doi.org/10.3390/polym15234492
APA StyleStanciu, M.-C., Nichifor, M., Ailiesei, G.-L., Popescu, I., Hitruc, G.-E., Ghimici, L., & Tuchilus, C. G. (2023). New Quaternary Ammonium Derivatives Based on Citrus Pectin. Polymers, 15(23), 4492. https://doi.org/10.3390/polym15234492