Polyaniline as a Nitrogen Source and Lignosulfonate as a Sulphur Source for the Preparation of the Porous Carbon Adsorption of Dyes and Heavy Metal Ions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Adsorbent Materials
2.3. Characterization
3. Results and Discussion
3.1. Effect of Time on Adsorption Performance and Adsorption Kinetic Study
- qe—equilibrium adsorption amount, mg/g;
- k1—adsorption rate constant, min−1;
- qt—adsorption amount at time t, mg/g.
- qe—equilibrium adsorption amount, mg/g;
- k2—adsorption rate constant in this model, g/mg/min;
- qt—adsorbed amount per unit mass of adsorbent at any adsorption time t, mg/g.
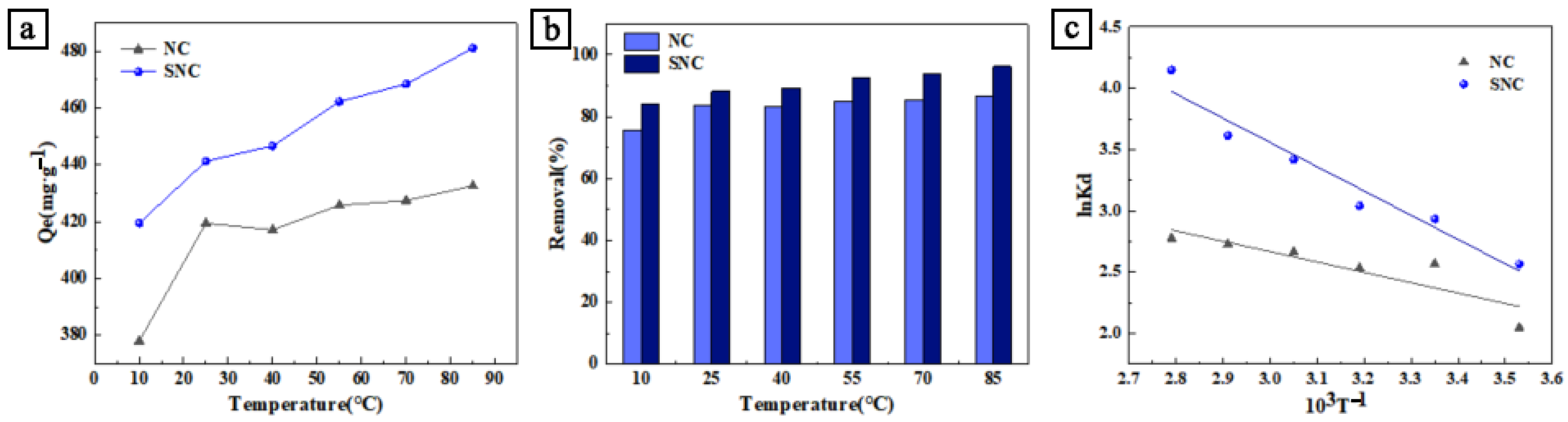
3.2. Effect of Concentration and Temperature on Adsorption Performance and Thermodynamic Study of Adsorption
- Qe—equilibrium adsorption amount, mg/g;
- Ce—equilibrium concentration, mg/L;
- KF—adsorption equilibrium constant;
- n—intensity factor.
- KL—Langmuir constant, L/mg;
- Qm is the maximum adsorption capacity per unit mass of adsorbent, mg/g.
- R—standard molar constant, 8.314 × 10−3 J/(mol·K);
- ΔG0—Gibbs free energy, kJ/mol;
- ΔS0—standard entropy change, kJ/mol;
- ΔH0—standard enthalpy change, kJ/mol;
- Kd—partition coefficient;
- m—mass of adsorbent, g;
- V—volume of dye solution, L.
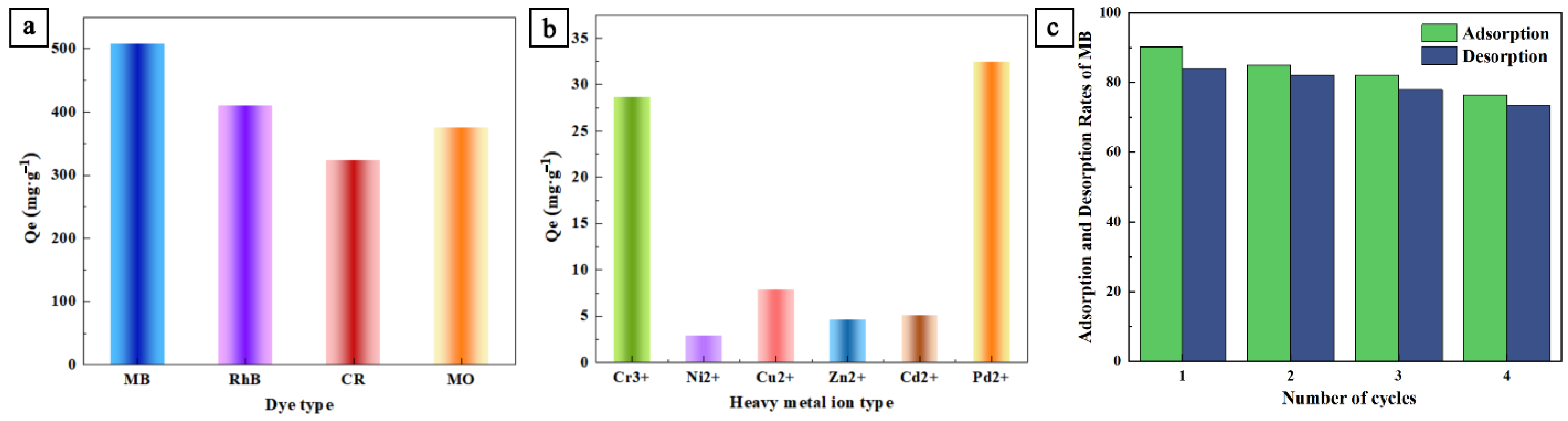
3.3. The Applicability of SNC to the Adsorption of Various Pollutants and Cyclic Performance
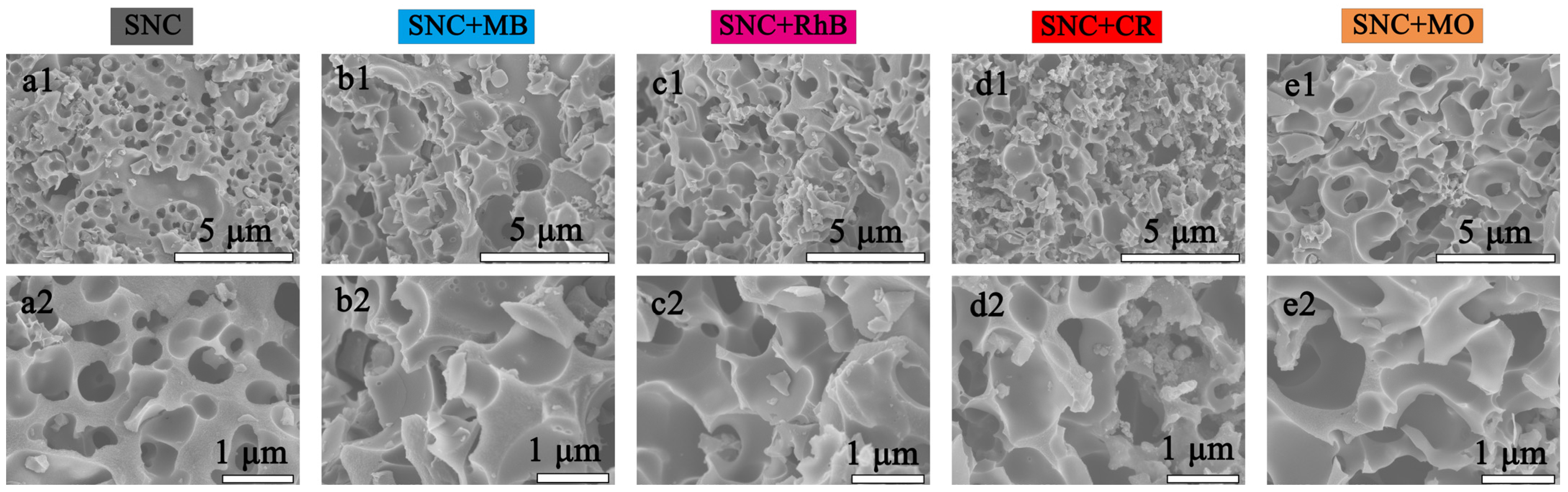
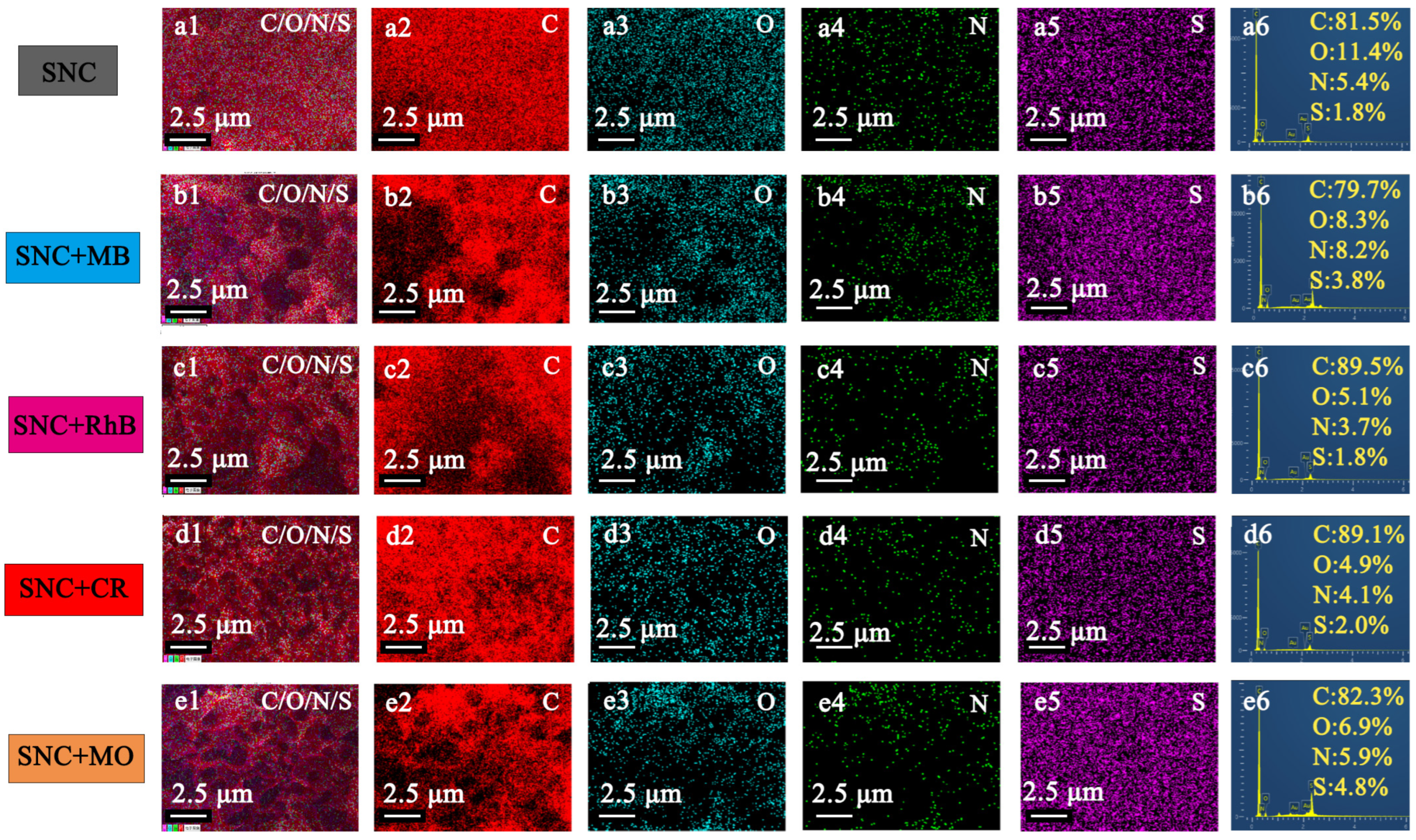
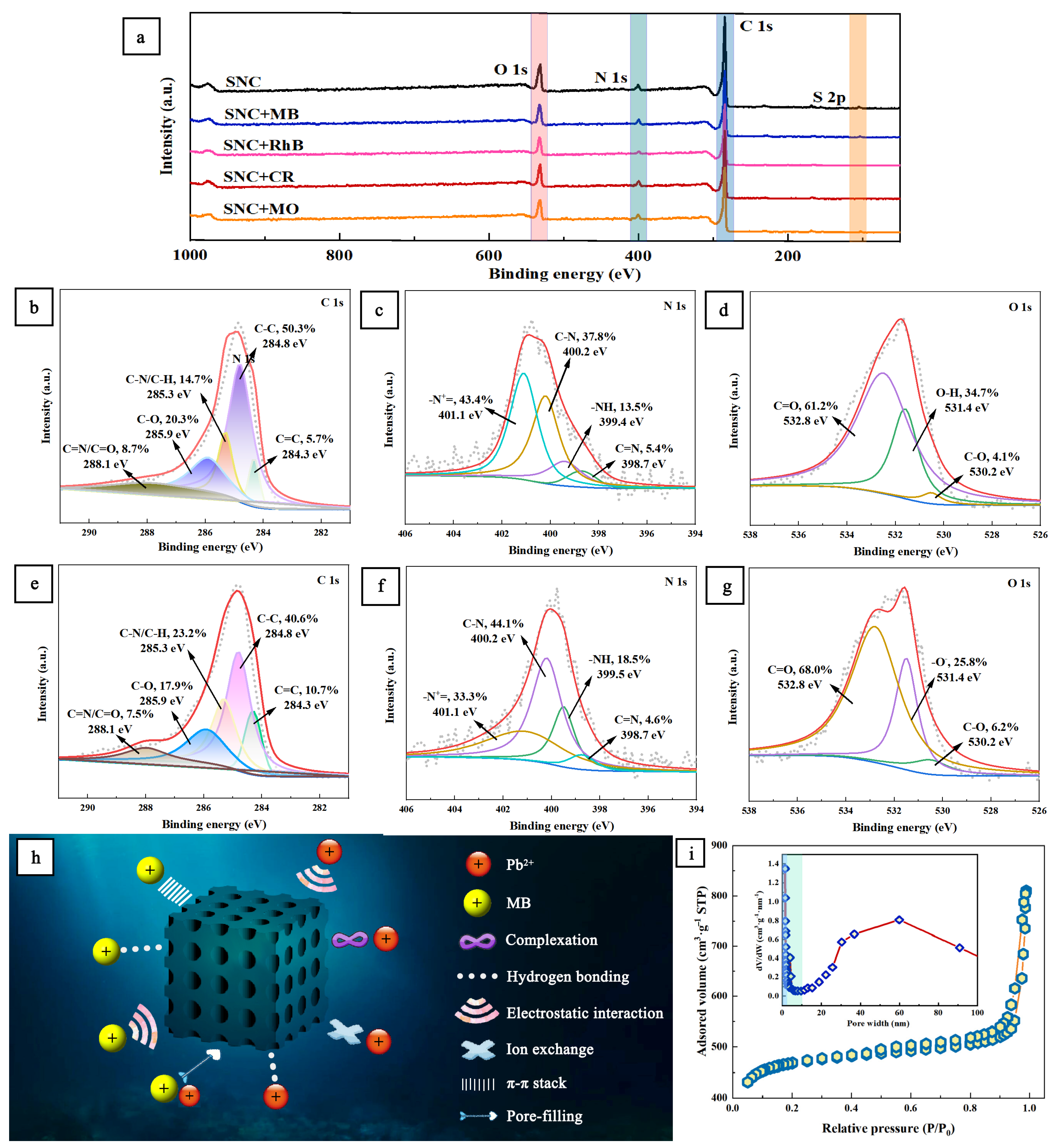
3.4. Adsorption Mechanism and Physical Properties of MB and Pb2+ by SNC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, W.H.; Xu, D.Y.; Liu, J.C. The forest resources input-output model: An application in China. Ecol. Indic. 2015, 51, 87–97. [Google Scholar] [CrossRef]
- Fang, Q.S.; Li, H.X. The concept delimitation, the value realization process, and the realization path of the capitalization of forest ecological resources. Nat. Resour. Forum 2021, 45, 424–440. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Lu, S.Y. Selective and efficient cleavage of lignin model compound into value-added aromatic chemicals with CuFe2O4 nanoparticles decorated on partially reduced graphene oxides via sunlight-assisted heterogeneous Fenton processes. J. Taiwan Inst. Chem. E 2016, 97, 264–271. [Google Scholar] [CrossRef]
- Liao, J.J.; Abd Latif, N.H.; Trache, D.; Brosse, N.; Hussin, M.H. Current advancement on the isolation, characterization and application of lignin. Int. J. Biol. Macromol. 2020, 162, 985–1024. [Google Scholar] [CrossRef] [PubMed]
- Tribot, A.; Amer, G.; Alio, M.A.; De Baynast, H.; Delattre, C.; Pons, A.; Mathias, J.D.; Callois, J.M.; Vial, C.; Michaud, P. Wood-lignin: Supply, extraction processes and use as bio-based material. Eur. Polym. J. 2019, 112, 228–240. [Google Scholar] [CrossRef]
- Duval, A.; Lawoko, M. A review on lignin-based polymeric, micro-and nano-structured materials. React. Funct. Polym. 2014, 85, 78–96. [Google Scholar] [CrossRef]
- El Hage, R.; Brosse, N.; Sannigrahi, P.; Ragauskas, A. Effects of process severity on the chemical structure of Miscanthus ethanol organosolv lignin. Polym. Degrad. Stabil. 2010, 95, 997–1003. [Google Scholar] [CrossRef]
- Zhang, W.L.; Yin, J.; Wang, C.W.; Zhao, L.; Jian, W.B.; Lu, K.; Lin, H.B.; Qiu, X.Q.; Alshareef, H.N. Lignin derived porous carbons: Synthesis methods and supercapacitor application. Small Methods 2021, 5, 2100896. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Jin, M.H.; Park, J.H.; Lee, Y.J.; Choi, Y.C. Flexible synthetic strategies for lignin-derived hierarchically porous carbon materials. ACS Sustain. Chem. Eng. 2018, 6, 10454–10462. [Google Scholar] [CrossRef]
- Chatterjee, S.; Saito, T. Lignin-Derived Advanced Carbon Materials. ChemSusChem 2015, 8, 3941–3958. [Google Scholar] [CrossRef]
- Qu, W.D.; Liang, C.; Zhao, Z.Z.; Hu, P.Y.; Ma, Z.Y. Simple, additive-free, extra pressure-free process to direct convert lignin into carbon foams. Int. J. Biol. Macromol. 2022, 209, 692–702. [Google Scholar] [CrossRef]
- Silva, C.F.L.E.; Lemoes, J.S.; Romani, R.F.; de Oliveira, W.G.; Leite, G.F. Activated carbon from residual lignin used for color removal. Water Air Soil Poll. 2022, 233, 177. [Google Scholar] [CrossRef]
- Cho, M.S.; Park, S.Y.; Hwang, J.Y.; Choi, H.J. Synthesis and electrical properties of polymer composites with polyaniline nanoparticles. Mat. Sci. Eng. C Mater. 2004, 24, 15–18. [Google Scholar] [CrossRef]
- Beygisangchin, M.; Abdul Rashid, S.; Shafie, S.; Sadrolhosseini, A.; Lim, H. Preparations, Properties, and Applications of Polyaniline and Polyaniline Thin Films—A Review. Polymers 2021, 13, 2003. [Google Scholar] [CrossRef]
- Nerkar, N.V.; Kondawar, S.B.; Brahme, S.K.; Kim, Y.H. Polyaniline/ZnO nanocomposites for the removal of methyl orange dye from waste water. Int. J. Mod. Phys. B 2018, 32, 1840085. [Google Scholar] [CrossRef]
- Hu, E.L.; Shang, S.M.; Tao, X.M.; Jiang, S.X.; Chiu, K.L. Regeneration and reuse of highly polluting textile dyeing effluents through catalytic ozonation with carbon aerogel catalysts. J. Clean. Prod. 2016, 137, 1055–1065. [Google Scholar] [CrossRef]
- Ali, H. Biodegradation of synthetic dyes—A Review. Water Air Soil Poll. 2010, 213, 251–273. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Zhang, S.B.; Li, W.Y.; Zhou, H.J.; Wang, G.Z.; Zhang, H.M. Metal (Co/Mo)-N bond anchor-doped N in porous carbon for electrochemical nitrogen reduction. Inorg. Chem. Front. 2021, 8, 1476–1481. [Google Scholar] [CrossRef]
- Kaminska, M.; Krusiec-Swidergol, B.; Pawelczyk, W.; Hartman-Petrycka, M.; Banys, A.; Jonderko, K.; Lebiedowska, A.; Koprowski, R.; Wilczynski, S. Application of the hyperspectral imaging method to assess the effectiveness of permanent makeup removal. Appl. Sci. 2023, 13, 2330. [Google Scholar] [CrossRef]
- Khurana, I.; Saxena, A.; Bharti; Khurana, J.M.; Rai, P.K. Removal of dyes using graphene-based composites: A review. Water Air Soil Poll. 2017, 228, 180. [Google Scholar] [CrossRef]
- Saeed, M.; Muneer, M.; ul Haq, A.; Akram, N. Photocatalysis: An effective tool for photodegradation of dyes—A review. Environ. Sci. Pollut. R 2022, 29, 293–311. [Google Scholar] [CrossRef]
- Zhuang, P.F.; Zhang, P.; Li, K.; Kumari, B.; Li, D.; Mei, X.F. Silver nanoclusters encapsulated into metal-organic frameworks for rapid removal of heavy metal ions from water. Molecules 2019, 24, 2442. [Google Scholar] [CrossRef]
- Li, Z.W.; Wang, L.; Qin, L.; Lai, C.; Wang, Z.H.; Zhou, M.; Xiao, L.H.; Liu, S.Y.; Zhang, M.M. Recent advances in the application of water-stable metal-organic frameworks: Adsorption and photocatalytic reduction of heavy metal in water. Chemosphere 2021, 285, 131432. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Jiang, X.P.; Liu, X.; Zhou, W.M.; Garba, Z.N.; Lawan, I.; Wang, L.W.; Yuan, Z.H. Adsorption of organic dyes from wastewater by metal-doped porous carbon materials. J. Clean. Prod. 2021, 284, 124773. [Google Scholar] [CrossRef]
- Guo, J.J.; Huo, J.J.; Liu, Y.; Wu, W.J.; Wang, Y.; Wu, M.H.; Liu, H.; Wang, G.X. Nitrogen-Doped Porous Carbon Supported Nonprecious Metal Single-Atom Electrocatalysts: From Synthesis to Application. Small Methods 2019, 3, 1900159. [Google Scholar] [CrossRef]
- Shi, X.F.; Wang, C.; Dong, B.B.; Kong, S.F.; Das, R.; Pan, D.; Guo, Z.H. Cu/N doped lignin for highly selective efficient removal of As(v) from polluted water. Int. J. Biol. Macromol. 2020, 161, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Li, P.H.; Wei, Y.M.; Wu, C.W.; Yang, C.; Jiang, B.; Wu, W.J. Lignin-based composites for high-performance supercapacitor electrode materials. RSC Adv. 2022, 12, 19485–19494. [Google Scholar] [CrossRef]
- Li, P.; Yang, C.; Wu, C.; Wei, Y.; Jiang, B.; Jin, Y.; Wu, W. Bio-Based Carbon Materials for High-Performance Supercapacitors. Nanomaterials 2022, 12, 2931. [Google Scholar] [CrossRef]
- Li, P.; Yang, C.; Wang, Y.; Su, W.; Wei, Y.; Wu, W. Adsorption Studies on the Removal of Anionic and Cationic Dyes from Aqueous Solutions Using Discarded Masks and Lignin. Molecules 2023, 28, 3349. [Google Scholar] [CrossRef]
- Li, P.; Yang, C.; Xu, X.; Miao, C.; He, T.; Jiang, B.; Wu, W. Preparation of Bio-Based Aerogel and Its Adsorption Properties for Organic Dyes. Gels 2022, 8, 755. [Google Scholar] [CrossRef]
- Vargas, A.M.M.; Cazetta, A.L.; Kunita, M.H.; Silva, T.L.; Almeida, V.C. Adsorption of methylene blue on activated carbon produced from flamboyant pods (Delonix regia): Study of adsorption isotherms and kinetic models. Chem. Eng. J. 2011, 168, 722–730. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Li, S.; Zhong, W.H.; Wei, W. Enhanced methylene blue adsorption onto activated reed-derived biochar by tannic acid. J. Mol. Liq. 2018, 268, 658–666. [Google Scholar] [CrossRef]
- Galán, J.; Rodríguez, A.; Gómez, J.M.; Allen, S.J.; Walker, G.M. Reactive dye adsorption onto a novel mesoporous carbon. Chem. Eng. J. 2013, 219, 62–68. [Google Scholar]
- Zhou, Y.; Li, Z.; Ji, L.; Wang, Z.; Cai, L.; Guo, J.; Song, W.; Wang, Y.; Piotrowski, A.M. Facile preparation of alveolate biochar derived from seaweed biomass with potential removal performance for cationic dye. J. Mol. Liq. 2022, 353, 118623. [Google Scholar] [CrossRef]
- Krishnani, K.K.; Meng, X.; Christodoulatos, C.; Boddu, V.M. Biosorption mechanism of nine different heavy metals onto biomatrix from rice husk. J. Hazard. Mater. 2008, 153, 1222–1234. [Google Scholar] [CrossRef]
- Wang, Q.; He, D.; Li, C.; Sun, Z.; Mu, J. Honeycomb-like cork activated carbon modified with carbon dots for high-efficient adsorption of Pb(Ⅱ) and rhodamine B. Ind. Crops Prod. 2023, 196, 116485. [Google Scholar] [CrossRef]
- Azimvand, J.; Didehban, K.; Mirshokraie, S.A. Safranin-O removal from aqueous solutions using lignin nanoparticle-g-polyacrylic acid adsorbent: Synthesis, properties, and application. Adsorpt. Sci. Technol. 2018, 36, 1422–1440. [Google Scholar] [CrossRef]
- Zhang, X.F.; Navarathna, C.M.; Leng, W.Q.; Karunaratne, T.; Thirumalai, R.V.K.G.; Kim, Y.; Pittman, C.U.; Mlsna, T.; Cai, Z.Y.; Zhang, J.L. Lignin-based few-layered graphene-encapsulated iron nanoparticles for water remediation. Chem. Eng. J. 2021, 417, 129199. [Google Scholar] [CrossRef]
- Yao, G.L.; Wang, K.; Wang, M.Y.; Shao, X.; Qiu, F.X.; Zhang, T. Magnetic FeS@Lignin-derived carbon nanocomposites as an efficient adsorbent for multistage collaborative selective recovery of tellurium (IV) from wastewater. J Environ. Chem. Eng. 2021, 9, 106135. [Google Scholar] [CrossRef]
- Saini, K.; Sahoo, A.; Biswas, B.; Kumar, A.; Bhaskar, T. Preparation and characterization of lignin-derived hard templated carbon (s): Statistical optimization and methyl orange adsorption isotherm studies. Bioresour. Technol. 2021, 342, 125924. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.Y.; Xu, J.; Kuang, Y.S.; Cheng, Z.; Wu, Q.Q.; Xie, J.X.; Wang, B.; Gao, W.H.; Zeng, J.S.; Li, J.; et al. Lignin-derived sulfonated porous carbon from cornstalk for efficient and selective removal of cationic dyes. Ind. Crops Prod. 2021, 159, 113071. [Google Scholar] [CrossRef]
- Liu, D.; Gu, W.Y.; Zhou, L.; Lei, J.Y.; Wang, L.Z.; Zhang, J.L.; Liu, Y.D. From biochar to functions: Lignin induced formation of Fe3C in carbon/Fe composites for efficient adsorption of tetracycline from wastewater. Sep. Purif. Technol. 2023, 304, 122217. [Google Scholar] [CrossRef]
- Fu, K.F.; Yue, Q.Y.; Gao, B.Y.; Sun, Y.Y.; Zhu, L.J. Preparation, characterization and application of lignin-based activated carbon from black liquor lignin by steam activation. Chem. Eng. J. 2013, 228, 1074–1082. [Google Scholar] [CrossRef]
- Han, X.B.; Li, R.; Miao, P.P.; Gao, J.; Hu, G.W.; Zhao, Y.; Chen, T. Design, synthesis and adsorption evaluation of bio-based lignin/chitosan beads for congo red removal. Materials 2022, 15, 2310. [Google Scholar] [CrossRef]
- Dai, K.; Zhao, G.L.; Kou, J.W.; Wang, Z.C.; Zhang, J.; Wu, J.L.; Yang, P.P.; Li, M.; Tang, C.L.; Zhuang, W.; et al. Magnetic mesoporous sodium citrate modified lignin for improved adsorption of calcium ions and methylene blue from aqueous solution. J. Environ. Chem. Eng. 2021, 9, 105180. [Google Scholar] [CrossRef]
- Du, B.Y.; Bai, Y.T.; Pan, Z.; Xu, J.Y.; Wang, Q.Y.; Wang, X.; Lv, G.J.; Zhou, J.H. pH fractionated lignin for the preparation of lignin-based magnetic nanoparticles for the removal of methylene blue dye. Sep. Purif. Technol. 2022, 295, 121302. [Google Scholar] [CrossRef]
- Yu, L.; Keffer, D.J.; Hsieh, C.T.; Scroggins, J.R.; Chen, H.; Dai, S.; Harper, D.P. Lignin-derived magnetic activated carbons for effective methylene blue removal. Ind. Eng. Chem. Res. 2022, 61, 11840–11850. [Google Scholar] [CrossRef]
- Du, B.Y.; Chai, L.F.; Li, W.; Wang, X.; Chen, X.H.; Zhou, J.H.; Sun, R.C. Preparation of functionalized magnetic graphene oxide/lignin composite nanoparticles for adsorption of heavy metal ions and reuse as electromagnetic wave absorbers. Sep. Purif. Technol. 2022, 297, 121509. [Google Scholar] [CrossRef]
- Wang, A.Q.; Zheng, Z.K.; Li, R.Q.; Hu, D.; Lu, Y.R.; Luo, H.X.; Yan, K. Biomass-derived porous carbon highly efficient for removal of Pb(II) and Cd(II). Green Energy Environ. 2019, 4, 414–423. [Google Scholar] [CrossRef]
- Kriaa, A.; Hamdi, N.; Srasra, E. Adsorption studies of methylene blue dye on tunisian activated lignin. Russ. J. Chem. Phys. A+ 2011, 85, 279–287. [Google Scholar] [CrossRef]
- Das, S.; Chatterjee, S.; Mondal, S.; Modak, A.; Chandra, B.K.; Das, S.; Nessim, G.D.; Majee, A.; Bhaumik, A. Thiadiazole containing N- and S-rich highly ordered periodic mesoporous organosilica for efficient removal of Hg(ii) from polluted water. Chem. Commun. 2020, 56, 3963–3966. [Google Scholar] [CrossRef] [PubMed]
- Ruidas, S.; Chowdhury, A.; Ghosh, A.; Ghosh, A.; Mondal, S.; Wonanke, A.D.D.; Addicoat, M.; Das, A.K.; Modak, A.; Bhaumik, A. Covalent organic framework as a metal-free photocatalyst for dye degradation and radioactive iodine adsorption. Langmuir 2023, 39, 4071–4081. [Google Scholar] [CrossRef] [PubMed]
- Naushad, M.; Alqadami, A.A.; AlOthman, Z.A.; Alsohaimi, I.H.; Algamdi, M.S.; Aldawsari, A.M. Adsorption kinetics, isotherm and reusability studies for the removal of cationic dye from aqueous medium using arginine modified activated carbon. J. Mol. Liq. 2019, 293, 111442. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, G.; Wen, J.; Li, X.; Zhu, J.; Wu, Z. Simultaneous removal of aqueous same ionic type heavy metals and dyes by a magnetic chitosan/polyethyleneimine embedded hydrophobic sodium alginate composite: Performance, interaction and mechanism. Chemosphere 2023, 318, 137869. [Google Scholar] [CrossRef]
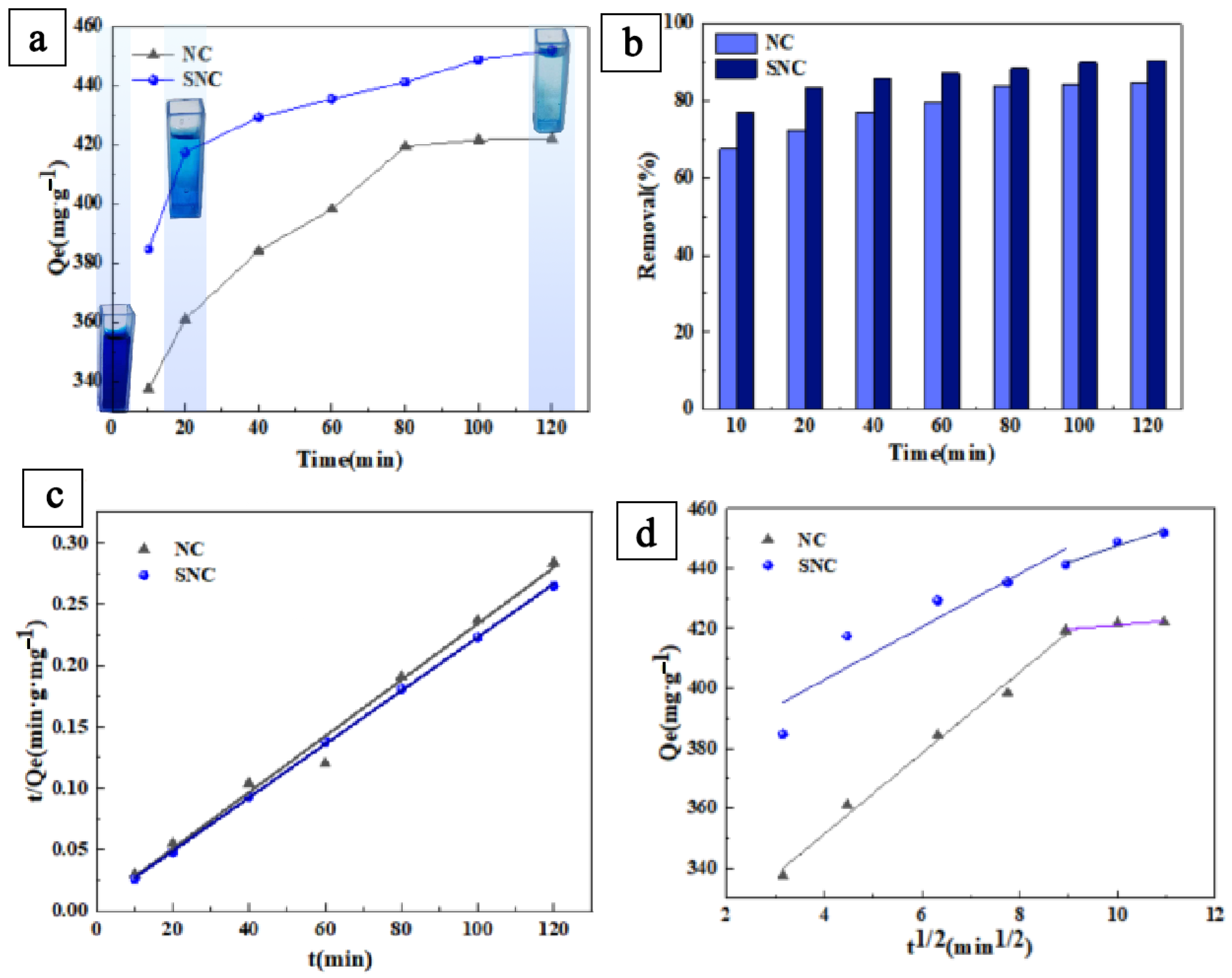
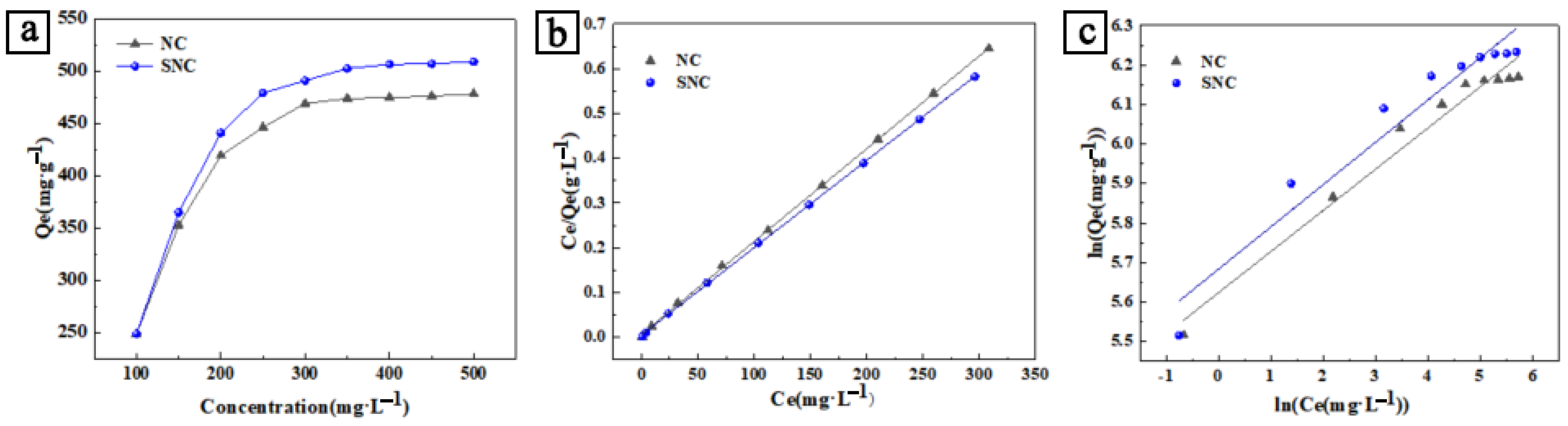
| Samples | Pseudo-First-Order Adsorption Kinetic Model | Pseudo-Second-Order Adsorption Kinetic Model | ||||
|---|---|---|---|---|---|---|
| (mg·g−1) | (min−1) | R2 | (mg·g−1) | (g·mg−1min−1) | R2 | |
| NC | 405.65 | 0.16 | 0.67 | 426.69 | 7.79 × 10−3 | 0.91 |
| SNC | 439.31 | 0.20 | 0.82 | 453.42 | 1.22 × 10−3 | 0.97 |
| Samples | (mg·g−1·min−0.5) | (mg·g−1) | (mg·g−1·min−0.5) | (mg·g−1) | ||
|---|---|---|---|---|---|---|
| NC | 13.51 | 297.29 | 0.99 | 1.36 | 407.53 | 0.89 |
| SNC | 8.85 | 367.38 | 0.86 | 5.40 | 393.58 | 0.96 |
| Samples | Langmuir Adsorption Isotherm Model | Freundlich Adsorption Isotherm Model | ||||
|---|---|---|---|---|---|---|
| (g·L−1) | (mg·g−1) | R2 | ) | R2 | ||
| NC | 8.85 × 10−3 | 615.72 | 0.91 | 73.02 | 3.19 | 0.81 |
| SNC | 7.92 × 10−3 | 671.78 | 0.91 | 67.49 | 2.97 | 0.82 |
| Samples | ||||||||
|---|---|---|---|---|---|---|---|---|
| 283 K | 298 K | 313 K | 328 K | 343 K | 358 K | |||
| NC | −5.22 | −5.87 | −6.51 | −7.16 | −7.81 | −8.46 | 7.02 | 43.24 |
| SNC | −5.88 | −7.07 | −8.25 | −9.44 | −10.62 | −11.80 | 16.45 | 78.92 |
| Lignin-Based Carbon Materials | Adsorption of Dye/Heavy Metal Ions | Adsorption Capacity (mg/g) | Ref. |
|---|---|---|---|
| Cork activated carbon | RhB | 1734.6 | [36] |
| Pb(Ⅱ) | 231.5 | ||
| Lignin nanoparticle-g-polyacrylic acid adsorbent | Safranin-O | 138.9 | [37] |
| Lignin-based few-layered graphene-encapsulated iron nanoparticles | As(III) | 214.7 | [38] |
| FeS@Lignin-derived carbon | Tellurium (IV) | 148.4 | [39] |
| Lignin-derived mordenite templated carbon | MO | 225.0 | [40] |
| Cu/N-doped lignin | As(V) | 253.5 | [26] |
| Lignin-derived sulfonated porous carbon | MB | 234.2 | [41] |
| Carbon-Fe3C/lignin composites | Cr(VI) | 164.0 | [42] |
| Black liquor lignin | MB | 92.5 | [43] |
| Bio-based lignin/chitosan adsorbent | CR | 173.0 | [44] |
| Magnetic mesoporous sodium citrate-modified lignin | Ca(II) | 339.4 | [45] |
| MB | 281.4 | ||
| Lignin-based magnetic nanoparticle adsorbent | MB | 234.3 | [46] |
| Lignin-derived magnetic activated carbons | MB | 220.2 | [47] |
| Carbon nanofibers from a blend of lignin | Pb(II) | 147.8 | [48] |
| Lignin-based porous carbon with layered graphene-like structure | Pb(II) | 250.5 | [49] |
| Activated carbon prepared from natural lignin | MB | 147.0 | [50] |
| Sodium lignosulfonate/polyaniline composite as the precursor, the activated high-temperature pyrolysis process is used to prepare porous carbon materials with oxygen, sulfur and nitrogen content | MB | 509.0 | This work |
| RhB | 410.2 | ||
| CR | 323.6 | ||
| MO | 375.4 | ||
| Cr(III) | 28.7 | ||
| Ni(II) | 2.9 | ||
| Cu(II) | 7.9 | ||
| Zn(II) | 4.7 | ||
| Cd(II) | 5.1 | ||
| Pb(II) | 32.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.; Li, P.; Su, W.; Yan, Z.; Wang, X.; Xu, S.; Wei, Y.; Wu, C. Polyaniline as a Nitrogen Source and Lignosulfonate as a Sulphur Source for the Preparation of the Porous Carbon Adsorption of Dyes and Heavy Metal Ions. Polymers 2023, 15, 4515. https://doi.org/10.3390/polym15234515
Wu W, Li P, Su W, Yan Z, Wang X, Xu S, Wei Y, Wu C. Polyaniline as a Nitrogen Source and Lignosulfonate as a Sulphur Source for the Preparation of the Porous Carbon Adsorption of Dyes and Heavy Metal Ions. Polymers. 2023; 15(23):4515. https://doi.org/10.3390/polym15234515
Chicago/Turabian StyleWu, Wenjuan, Penghui Li, Wanting Su, Zifei Yan, Xinyan Wang, Siyu Xu, Yumeng Wei, and Caiwen Wu. 2023. "Polyaniline as a Nitrogen Source and Lignosulfonate as a Sulphur Source for the Preparation of the Porous Carbon Adsorption of Dyes and Heavy Metal Ions" Polymers 15, no. 23: 4515. https://doi.org/10.3390/polym15234515
APA StyleWu, W., Li, P., Su, W., Yan, Z., Wang, X., Xu, S., Wei, Y., & Wu, C. (2023). Polyaniline as a Nitrogen Source and Lignosulfonate as a Sulphur Source for the Preparation of the Porous Carbon Adsorption of Dyes and Heavy Metal Ions. Polymers, 15(23), 4515. https://doi.org/10.3390/polym15234515