Vinyl-Addition Homopolymeization of Norbornenes with Bromoalkyl Groups
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Monomers
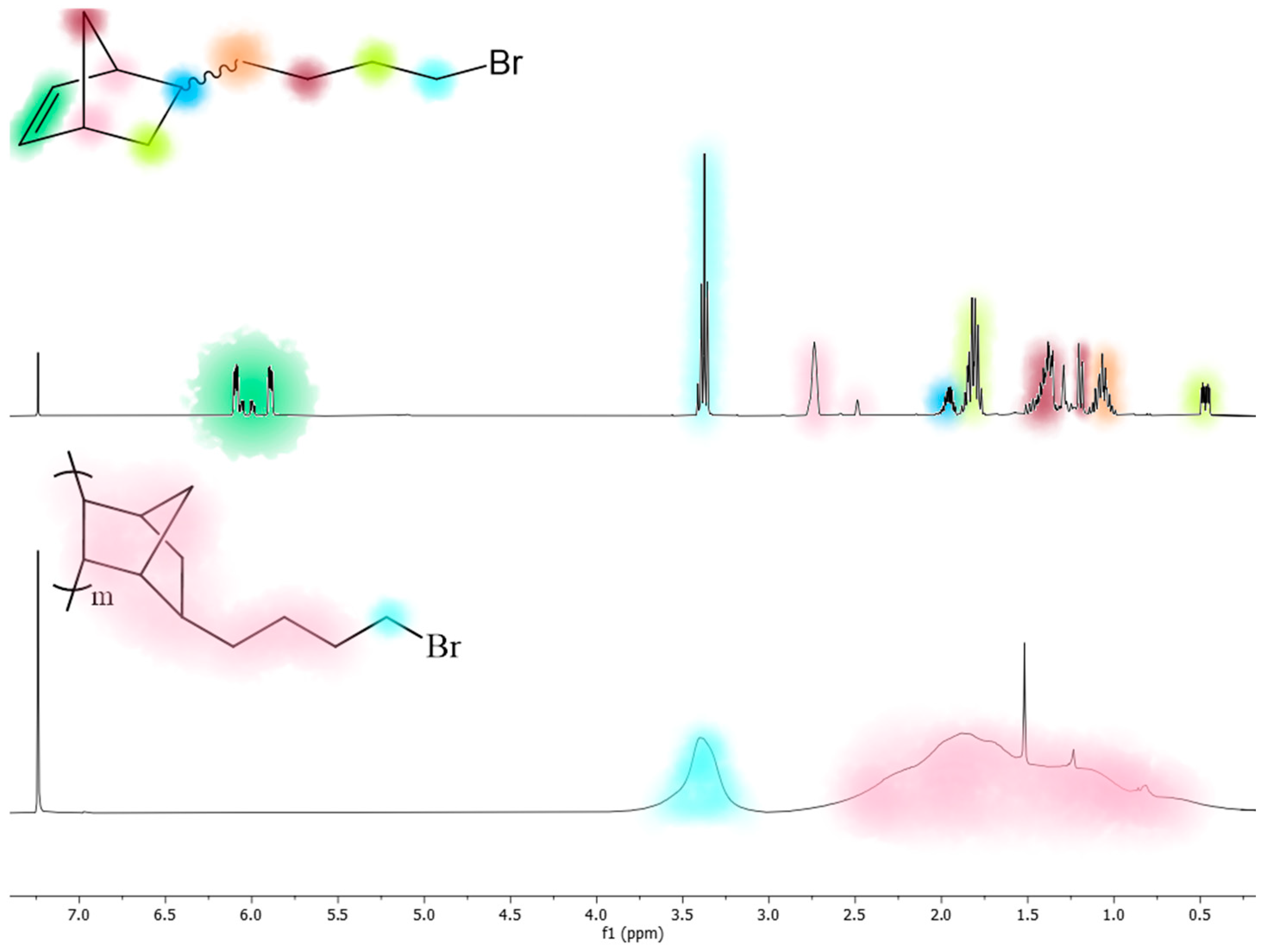
2.1.1. Synthesis of 5-(Bromomethyl)-2-norbornene (M1) [11]
2.1.2. Synthesis of 5-(2-Bromoethyl)-2-norbornene (M2) [11]
2.1.3. Synthesis of 5-(4-Bromobutyl)-2-norbornene (M4) [11]
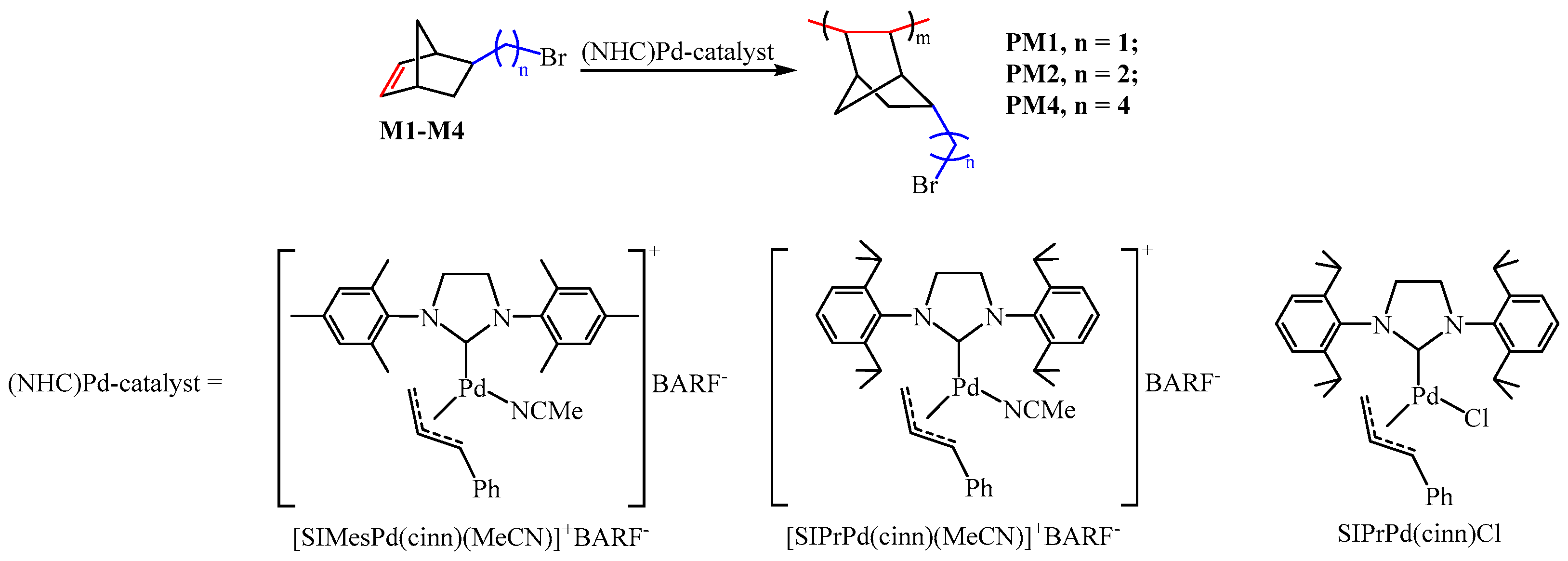
2.2. General Procedures of Vinyl-Addition Polymerization (PM1–PM4)
2.2.1. Polymerization in the Presence of the Single-Component Catalyst
2.2.2. Polymerization in the Presence of the Three-Component Catalytic System
2.3. Procedure for Copolymerization of M1–M4 Monomers
3. Results and Discussion
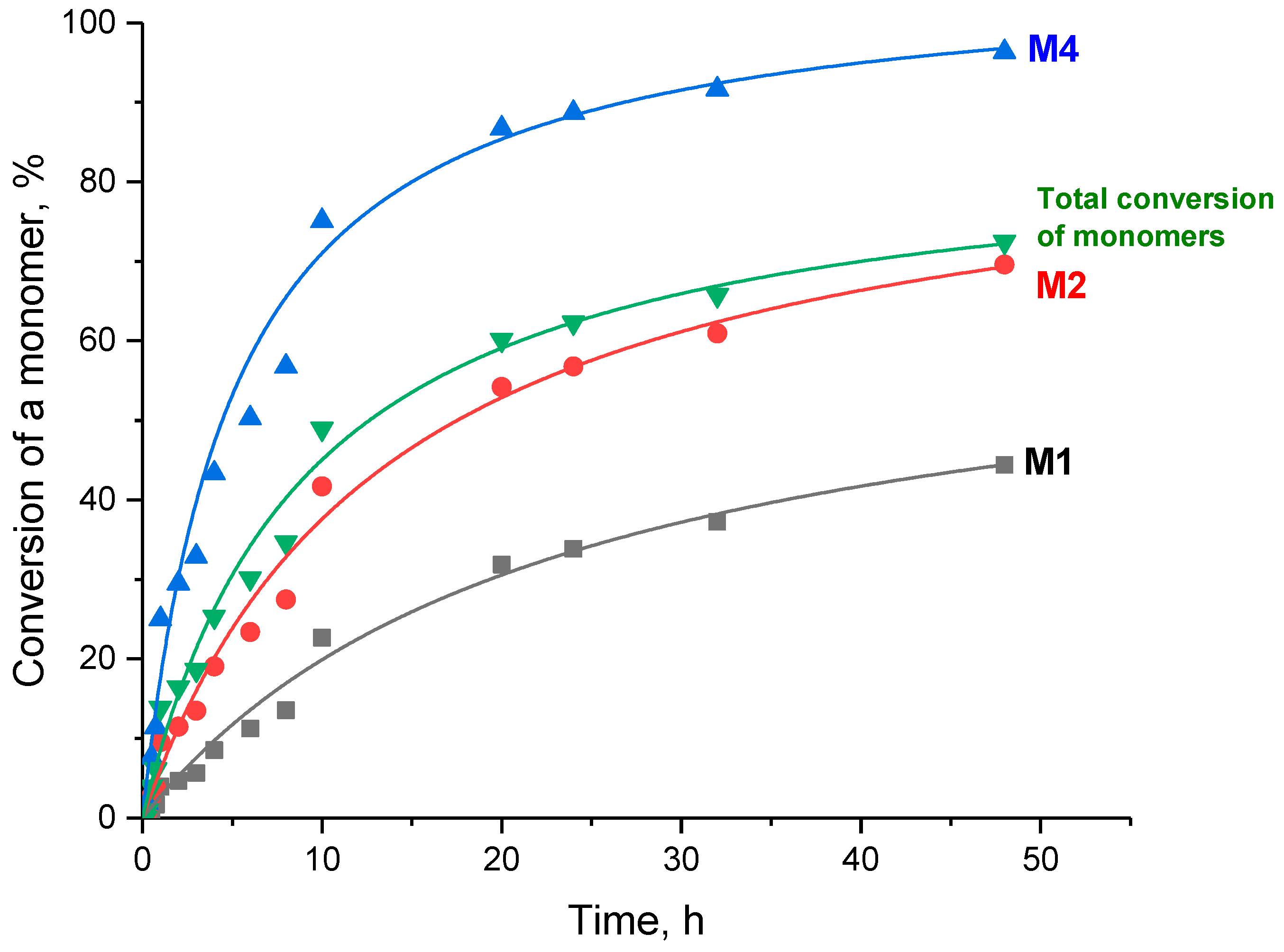
3.1. Vinyl-Addition Polymerization of 5-Bromoalkylnorbornenes
3.2. The Basic Physico-Chemical Properties of the Prepared Vinyl-Addition Homopolymers Based on 5-Bromoalkylnorbornenes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lehmann, M.L.; Tyler, L.; Self, E.C.; Yang, G.; Nanda, J.; Saito, T. Membrane design for non-aqueous redox flow batteries: Current status and path forward. Chem 2022, 8, 1611–1636. [Google Scholar] [CrossRef]
- Li, J.; Liu, C.; Ge, J.; Xing, W.; Zhu, J. Challenges and Strategies of Anion Exchange Membranes in Hydrogen-electricity Energy Conversion Devices. Chem. Eur. J. 2023, 29, e202203173. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xie, N.; Xue, J.; Li, M.; Zheng, C.; Zhang, J.; Qin, Y.; Yin, Y.; Dekel, D.R.; Guiver, M.D. Magnetic-field-oriented mixed-valence-stabilized ferrocenium anion-exchange membranes for fuel cells. Nat. Energy 2022, 7, 329–339. [Google Scholar] [CrossRef]
- Chen, N.; Lee, Y.M. Anion exchange polyelectrolytes for membranes and ionomers. Prog. Polym. Sci. 2021, 113, 101345. [Google Scholar] [CrossRef]
- Clemens, A.L.; Jayathilake, B.S.; Karnes, J.J.; Schwartz, J.J.; Baker, S.E.; Duoss, E.B.; Oakdale, J.S. Tuning Alkaline Anion Exchange Membranes through Crosslinking: A Review of Synthetic Strategies and Property Relationships. Polymers 2023, 15, 1534. [Google Scholar] [CrossRef]
- Shah, P.N.; Park, H.; Tee, H.M.; Dietrich, C.; Kohl, P.A. In situ amination of anion conducting solid polymer electrolyte membranes. Mater. Adv. 2023, 4, 2322–2331. [Google Scholar] [CrossRef]
- Lehmann, M.; Leonard, D.; Zheng, J.; He, L.; Tang, X.; Chen, X.C.; Lim, K.H.; Maurya, S.; Kim, Y.S.; Saito, T. Quaternized Polynorbornene Random Copolymers for Fuel Cell Devices. ACS Appl. Energy Mater. 2023, 6, 1822–1833. [Google Scholar] [CrossRef]
- Wang, W.; Cao, D.-F.; Sun, X.-W.; Pan, L.; Ma, Z.; Li, Y.-S. The Influence of Various Cationic Group on Polynorbornene Based Anion Exchange Membranes with Hydrophobic Large Steric Hindrance Arylene Substituent. Chin. J. Polym. Sci. 2023, 41, 278–287. [Google Scholar] [CrossRef]
- Bermeshev, M.V.; Chapala, P.P. Addition polymerization of functionalized norbornenes as a powerful tool for assembling molecular moieties of new polymers with versatile properties. Prog. Polym. Sci. 2018, 84, 1–46. [Google Scholar] [CrossRef]
- Hsu, J.H.; Peltier, C.R.; Treichel, M.; Gaitor, J.C.; Li, Q.; Girbau, R.; Macbeth, A.J.; Abruña, H.D.; Noonan, K.J.T.; Coates, G.W.; et al. Direct Insertion Polymerization of Ionic Monomers: Rapid Production of Anion Exchange Membranes. Angew. Chem. Int. Ed. 2023, 62, e202304778. [Google Scholar] [CrossRef]
- Martínez-Arranz, S.; Albéniz, A.C.; Espinet, P. Versatile Route to Functionalized Vinylic Addition Polynorbornenes. Macromolecules 2010, 43, 7482–7487. [Google Scholar] [CrossRef]
- García-Loma, R.; Albéniz, A.C. Vinylic Addition Polynorbornene in Catalysis. Asian J. Org. Chem. 2019, 8, 304–315. [Google Scholar] [CrossRef]
- Suslov, D.S.; Bykov, M.V.; Kravchenko, O.V. Norbornene Addition Polymerization with Catalysts Based on Transition Metal Compounds: 2008–2018. Polym. Sci. Ser. C 2019, 61, 145–173. [Google Scholar] [CrossRef]
- Bermesheva, E.V.; Medentseva, E.I.; Khrychikova, A.P.; Wozniak, A.I.; Guseva, M.A.; Nazarov, I.V.; Morontsev, A.A.; Karpov, G.O.; Topchiy, M.A.; Asachenko, A.F.; et al. Air-Stable Single-Component Pd-Catalysts for Vinyl-Addition Polymerization of Functionalized Norbornenes. ACS Catal. 2022, 12, 15076–15090. [Google Scholar] [CrossRef]
- Bermesheva, E.V.; Wozniak, A.I.; Andreyanov, F.A.; Karpov, G.O.; Nechaev, M.S.; Asachenko, A.F.; Topchiy, M.A.; Melnikova, E.K.; Nelyubina, Y.V.; Gribanov, P.S.; et al. Polymerization of 5-Alkylidene-2-norbornenes with Highly Active Pd–N-Heterocyclic Carbene Complex Catalysts: Catalyst Structure–Activity Relationships. ACS Catal. 2020, 10, 1663–1678. [Google Scholar] [CrossRef]
- Bermesheva, E.V.; Nazarov, I.V.; Kataranova, K.D.; Khrychikova, A.P.; Zarezin, D.P.; Melnikova, E.K.; Asachenko, A.F.; Topchiy, M.A.; Rzhevskiy, S.A.; Bermeshev, M.V. Cocatalyst versus precatalyst impact on the vinyl-addition polymerization of norbornenes with polar groups: Looking at the other side of the coin. Polym. Chem. 2021, 12, 6355–6362. [Google Scholar] [CrossRef]
- Nazarov, I.V.; Khrychikova, A.P.; Medentseva, E.I.; Bermesheva, E.V.; Borisov, I.L.; Yushkin, A.A.; Volkov, A.V.; Wozniak, A.I.; Petukhov, D.I.; Topchiy, M.A.; et al. CO2-selective vinyl-addition polymers from nadimides: Synthesis and performance for membrane gas separation. J. Membr. Sci. 2023, 677, 121624. [Google Scholar] [CrossRef]
- Jung, I.G.; Seo, J.; Chung, Y.K.; Shin, D.M.; Chun, S.-H.; Son, S.U. Polymerization of carboxylic ester functionalized norbornenes catalyzed by (η3-allyl)palladium complexes bearing N-heterocyclic carbene ligands. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 3042–3052. [Google Scholar] [CrossRef]
- Lee, D.J.; Choi, W.H.; Kim, M.; Kim, C.K.; Lee, I.M. Copolymerization of Functional Norbornenes Catalyzed by Pd Complexes: Is it Living? Bull. Korean Chem. Soc. 2020, 41, 697–701. [Google Scholar] [CrossRef]
- Lee, D.J.; Kim, M.; Kim, C.K.; Lee, I.M. Synthesis of Novel Palladium Complexes Containing β-Diketonate and NHC Ligands and their Catalytic Ability for Addition Polymerizations of the Functional Norbornenes. Bull. Korean Chem. Soc. 2019, 40, 710–718. [Google Scholar] [CrossRef]
- Alentiev, D.A.; Nikiforov, R.Y.; Rudakova, M.A.; Zarezin, D.P.; Topchiy, M.A.; Asachenko, A.F.; Alentiev, A.Y.; Bolshchikov, B.D.; Belov, N.A.; Finkelshtein, E.S.; et al. Polynorbornenes bearing ether fragments in substituents: Promising membrane materials with enhanced CO2 permeability. J. Membr. Sci. 2022, 648, 120340. [Google Scholar] [CrossRef]
- Crosbie, D.; Stubbs, J.; Sundberg, D. Catalytic Emulsion Polymerization of Olefins: Ab-Initio Polymerization of a Family of Norbornene-Derived Monomers. Macromolecules 2008, 41, 2445–2450. [Google Scholar] [CrossRef]
- Jung, I.G.; Lee, Y.T.; Choi, S.Y.; Choi, D.S.; Kang, Y.K.; Chung, Y.K. Polymerization of 5-norbornene-2-methyl acetate catalyzed by air-stable cationic (η3-substituted allyl) palladium complexes of N-heterocyclic carbene. J. Organomet. Chem. 2009, 694, 297–303. [Google Scholar] [CrossRef]
- Lee, E.J.; Won, W.K.; Lee, B.; Kye, Y.H.; Lee, I.M. Novel Pd Catalysts with β-Diketiminates for Homopolymerization of Functionalized Norbornene Derivatives in Water/Organic Mixed Solvents. Bull. Korean Chem. Soc. 2013, 34, 2720–2724. [Google Scholar] [CrossRef]
- Wozniak, A.I.; Bermesheva, E.V.; Borisov, I.L.; Volkov, A.V.; Petukhov, D.I.; Gavrilova, N.N.; Shantarovich, V.P.; Asachenko, A.F.; Topchiy, M.A.; Finkelshtein, E.S.; et al. Switching on/switching off solubility controlled permeation of hydrocarbons through glassy polynorbornenes by the length of side alkyl groups. J. Membr. Sci. 2022, 641, 119848. [Google Scholar] [CrossRef]
- Yevlampieva, N.P.; Bermeshev, M.V.; Vezo, O.S.; Bermesheva, E.V.; Voznyak, A.I.; Kim, R.O. Synthesis and Molecular Properties of Additive Poly(5-ethylidene-2-norbornene). Polym. Sci. Ser. A 2019, 61, 134–141. [Google Scholar] [CrossRef]
- Askadskii, A.A. Computational Materials Science of Polymers; Cambridge International Science Publishing Ltd.: Cambridge, UK, 2003; p. 16. [Google Scholar]
- Wilks, B.R.; Chung, W.J.; Ludovice, P.J.; Rezac, M.E.; Meakin, P.; Hill, A.J. Structural and free-volume analysis for alkyl-substituted palladium-catalyzed poly(norbornene): A combined experimental and Monte Carlo investigation. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 215–233. [Google Scholar] [CrossRef]
- Marion, N.; Navarro, O.; Mei, J.; Stevens, E.D.; Scott, N.M.; Nolan, S.P. Modified (NHC)Pd(allyl)Cl (NHC = N-Heterocyclic Carbene) Complexes for Room-Temperature Suzuki−Miyaura and Buchwald−Hartwig Reactions. J. Am. Chem. Soc. 2006, 128, 4101–4111. [Google Scholar] [CrossRef] [PubMed]
- Viciu, M.S.; Zinn, F.K.; Stevens, E.D.; Nolan, S.P. Telomerization of Amines Mediated by Cationic N-Heterocyclic Carbene (NHC) Palladium Complexes. Organometallics 2003, 22, 3175–3177. [Google Scholar] [CrossRef]
- Kolychev, E.L.; Asachenko, A.F.; Dzhevakov, P.B.; Bush, A.A.; Shuntikov, V.V.; Khrustalev, V.N.; Nechaev, M.S. Expanded ring diaminocarbene palladium complexes: Synthesis, structure, and Suzuki–Miyaura cross-coupling of heteroaryl chlorides in water. Dalton Trans. 2013, 42, 6859–6866. [Google Scholar] [CrossRef]
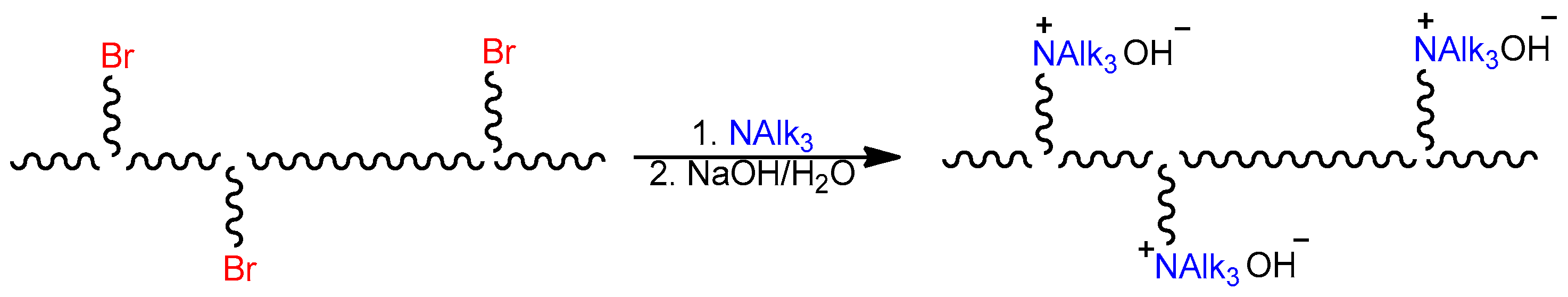





| Monomer (M) | M/Pd Molar Ratio | C(M), M | Reaction Time, h | T, °C | Solvent | Yield, % | Mw∙10−3 | Mn∙10−3 | Mw/Mn |
|---|---|---|---|---|---|---|---|---|---|
| M1 | 750 | 1 | 24 | 45 | CH2Cl2 | Traces | - | - | - |
| M2 | 1500 | 0.2 | 2.5 | 30 | CH2Cl2 | 77 | - a | - a | - a |
| 1500 | 0.2 | 340 | 45 | CH2Cl2 | 0 b | - | - | - | |
| 3000 | 0.2 | 2.5 | 30 | CH2Cl2 | 72 | - a | - a | - a | |
| 4500 | 0.2 | 2.5 | 30 | CH2Cl2 | 68 | - a | - a | - a | |
| 6000 | 0.2 | 2.5 | 30 | CH2Cl2 | 66 | - a | - a | - a | |
| 3000 | 0.2 | 2.5 | 30 | PhCl | 48 | - a | - a | - a | |
| 3000 | 0.2 | 2.5 | 30 | CHCl3 | 44 | - a | - a | - a | |
| 3000 | 0.2 | 2.5 | 30 | Toluene | Traces | - a | - a | - a | |
| M4 | 1500 | 0.2 | 500 | 45 | CH2Cl2 | 0 b | - | - | - |
| 4500 | 0.16 | 1.5 | 45 | CH2Cl2 | 43 | 1662 | 969 | 1.7 | |
| 6000 | 0.16 | 1.5 | 45 | CH2Cl2 | 39 | 1549 | 975 | 1.6 | |
| 3000 | 0.12 | 2.5 | 45 | CH2Cl2 | 56 | 1712 | 766 | 2.2 | |
| 4500 | 0.12 | 2.5 | 45 | CH2Cl2 | 46 | 1902 | 908 | 2.1 | |
| 6000 | 0.12 | 2.5 | 45 | CH2Cl2 | 42 | 1834 | 1070 | 1.7 | |
| 3000 | 0.08 | 2 | 45 | CH2Cl2 | 62 | 1629 | 637 | 2.6 | |
| 4500 | 0.08 | 2 | 45 | CH2Cl2 | 55 | 1731 | 1016 | 1.7 | |
| 6000 | 0.08 | 2 | 45 | CH2Cl2 | 50 | 1711 | 922 | 1.9 |
| Monomer (M) | M/Pd Molar Ratio | C(M), M | Reaction Time, h | T, °C | Solvent | Yield, % | Mw∙10−3 | Mn∙10−3 | Mw/Mn |
|---|---|---|---|---|---|---|---|---|---|
| M1 | 500 | 0.5 | 24 | 45 | CH2Cl2 | 55 | 80 | 40 | 2.0 |
| 1000 | 1 | 18 | 45 | CH2Cl2 | Traces b | ||||
| 1000 | 0.5 | 24 | 45 | CH2Cl2 | 35 | 243 | 87 | 2.8 | |
| 1500 | 1 | 18 | 45 | CH2Cl2 | 40 | 311 | 124 | 2.5 | |
| 1500 | 0.2 | 6 | 45 | Toluene | Traces | 48 | 15.7 | 3.1 | |
| 1500 | 0.2 | 6 | 45 | CH2Cl2 | Traces | 72 | 20 | 3.6 | |
| 2000 | 0.5 | 18 | 45 | CH2Cl2 | 11 | 98 | 38 | 2.5 | |
| 2000 | 1 | 18 | 45 | CH2Cl2 | 19 | 302 | 106 | 2.9 | |
| 3000 | 1 | 24 | 45 | CH2Cl2 | 22 | 372 | 112 | 3.3 | |
| 4500 | 1 | 24 | 45 | CH2Cl2 | 18 | 293 | 109 | 2.7 | |
| M2 | 1500 | 0.2 | 24 | 45 | CH2Cl2 | 48 | 815 | 216 | 3.8 |
| 3000 | 0.2 | 24 | 45 | CH2Cl2 | 17 | 567 | 196 | 2.9 | |
| 4500 | 0.2 | 24 | 45 | CH2Cl2 | Traces | 455 | 158 | 2.9 | |
| 6000 | 0.2 | 24 | 45 | CH2Cl2 | Traces | 407 | 104 | 3.9 | |
| 1500 | 0.2 | 2.3 | 45 | Toluene | 62 | 916 | 629 | 1.5 | |
| 3000 | 0.2 | 2.3 | 45 | Toluene | 15 | - c | - c | - c | |
| M4 | 1500 | 0.2 | 1.5 | 45 | CH2Cl2 | 68 | 1571 | 642 | 2.4 |
| 3000 | 0.2 | 1.5 | 45 | CH2Cl2 | 53 | 1930 | 1245 | 1.6 | |
| 4500 | 0.2 | 1.5 | 45 | CH2Cl2 | 40 | 2027 | 1393 | 1.5 | |
| 6000 | 0.2 | 1.5 | 45 | CH2Cl2 | 32 | 1890 | 1318 | 1.4 | |
| 1500 | 0.15 | 2.5 | 45 | CH2Cl2 | 72 d | - f | - f | - f | |
| 1500 | 0.1 | 2.5 | 45 | CH2Cl2 | 76 e | - f | - f | - f | |
| 1500 | 0.2 | 2 | 45 | Toluene | 69 | 2013 | 1179 | 1.7 | |
| 3000 | 0.2 | 2 | 45 | Toluene | 43 | 1439 | 966 | 1.5 |
| Polymer | Acetone | CH2Cl2 | DMSO | DMF | CHCl3 | C6H6 | Et2O | THF | n-Hexane | PhCH3 | CH3CN | PhCl |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PM1 | − | − | − | − | + | − | − | + | − | − | − | x |
| PM2 | − | − | − | − | + | − | − | + | − | − | − | x |
| PM4 | − | + | − | − | + | − | − | + | − | − | − | + |
| Polymer | Density, g/cm3 | FFV, % | Tg, °C |
|---|---|---|---|
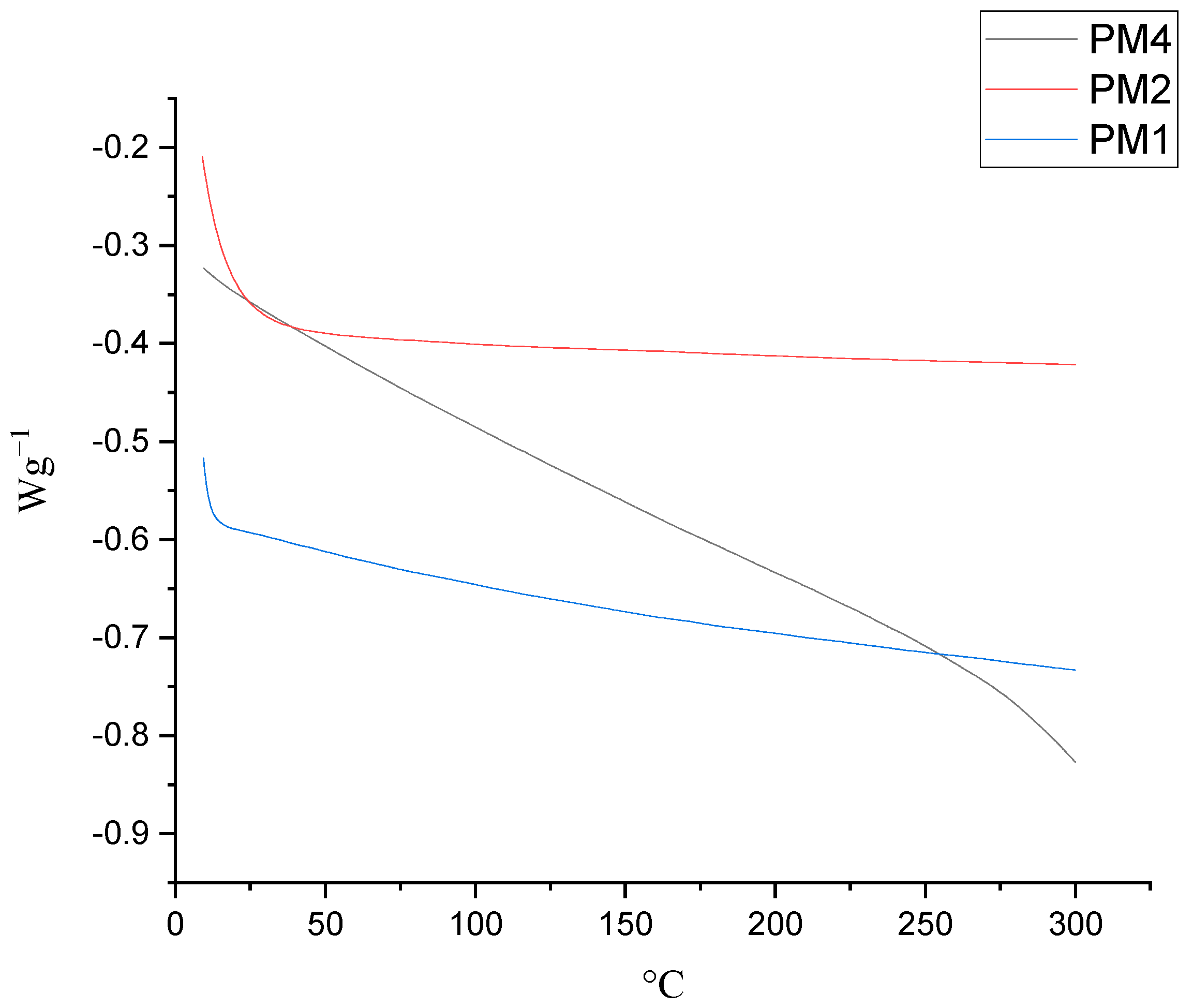
| PM1 | 1.538 | 13.8 | >Td |
| PM2 | 1.475 | 13.3 | 304 |
| PM4 | 1.357 | 14.7 | 291 |
| Polymer | Tensile Strength, σ, MPa | Elongation at Break, ε, % | Young’s Modulus, E, MPa |
|---|---|---|---|
| PM1 | 10 | 5 | 1237 |
| PM2 | 18 | 4 | 1008 |
| PM4 | 9 | 16 | 544 |
| Polymer | Td., a °C | |
|---|---|---|
| Ar | Air | |
| PM1 | 287 | 262 |
| PM2 | 333 | 271 |
| PM4 | 328 | 278 |
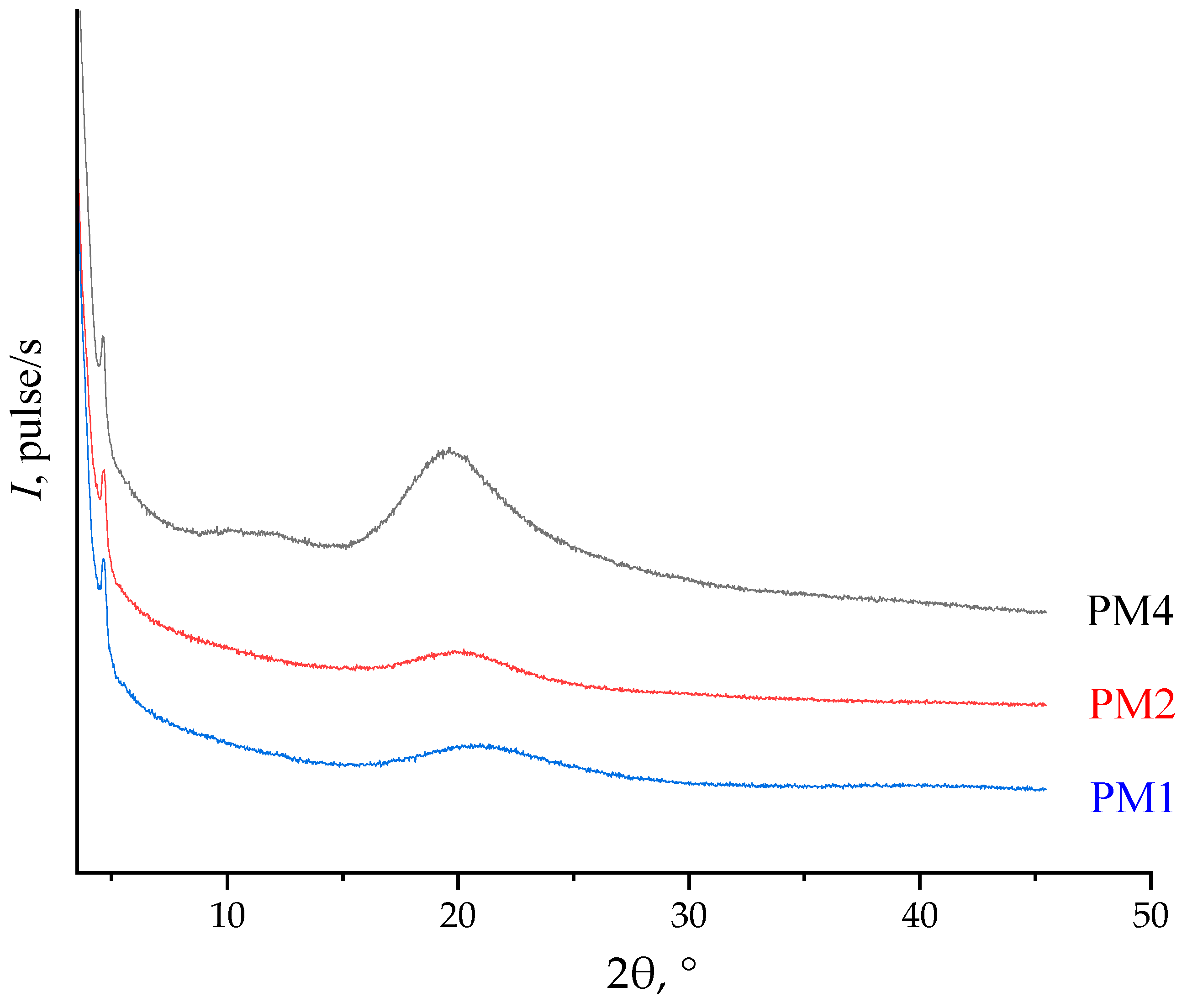
| Polymer | 2θ1, ° | d1, Å | 2θ2, ° | d2, Å | 2θ3, ° | d3, Å |
|---|---|---|---|---|---|---|
| PM1 | 21.6 | 4.1 | - | - | - | - |
| PM2 | 20.3 | 4.4 | - | - | - | - |
| PM4 | 11.2 | 7.9 | 19.5 | 4.6 | 23.3 | 3.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lunin, A.O.; Andreyanov, F.A.; Makarov, I.S.; Bermeshev, M.V. Vinyl-Addition Homopolymeization of Norbornenes with Bromoalkyl Groups. Polymers 2023, 15, 4444. https://doi.org/10.3390/polym15224444
Lunin AO, Andreyanov FA, Makarov IS, Bermeshev MV. Vinyl-Addition Homopolymeization of Norbornenes with Bromoalkyl Groups. Polymers. 2023; 15(22):4444. https://doi.org/10.3390/polym15224444
Chicago/Turabian StyleLunin, Artyom O., Fedor A. Andreyanov, Igor S. Makarov, and Maxim V. Bermeshev. 2023. "Vinyl-Addition Homopolymeization of Norbornenes with Bromoalkyl Groups" Polymers 15, no. 22: 4444. https://doi.org/10.3390/polym15224444
APA StyleLunin, A. O., Andreyanov, F. A., Makarov, I. S., & Bermeshev, M. V. (2023). Vinyl-Addition Homopolymeization of Norbornenes with Bromoalkyl Groups. Polymers, 15(22), 4444. https://doi.org/10.3390/polym15224444











