Polycarbosilane/Divinylbenzene-Modified Magnesium Hydroxide to Enhance the Flame Retardancy of Ethylene–Vinyl Acetate Copolymer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PCS Cross-Linking Degree Test
2.3. Preparation of MH/PCS/DVB
2.4. Preparation of Composites
2.5. Characterization
3. Results and Discussion
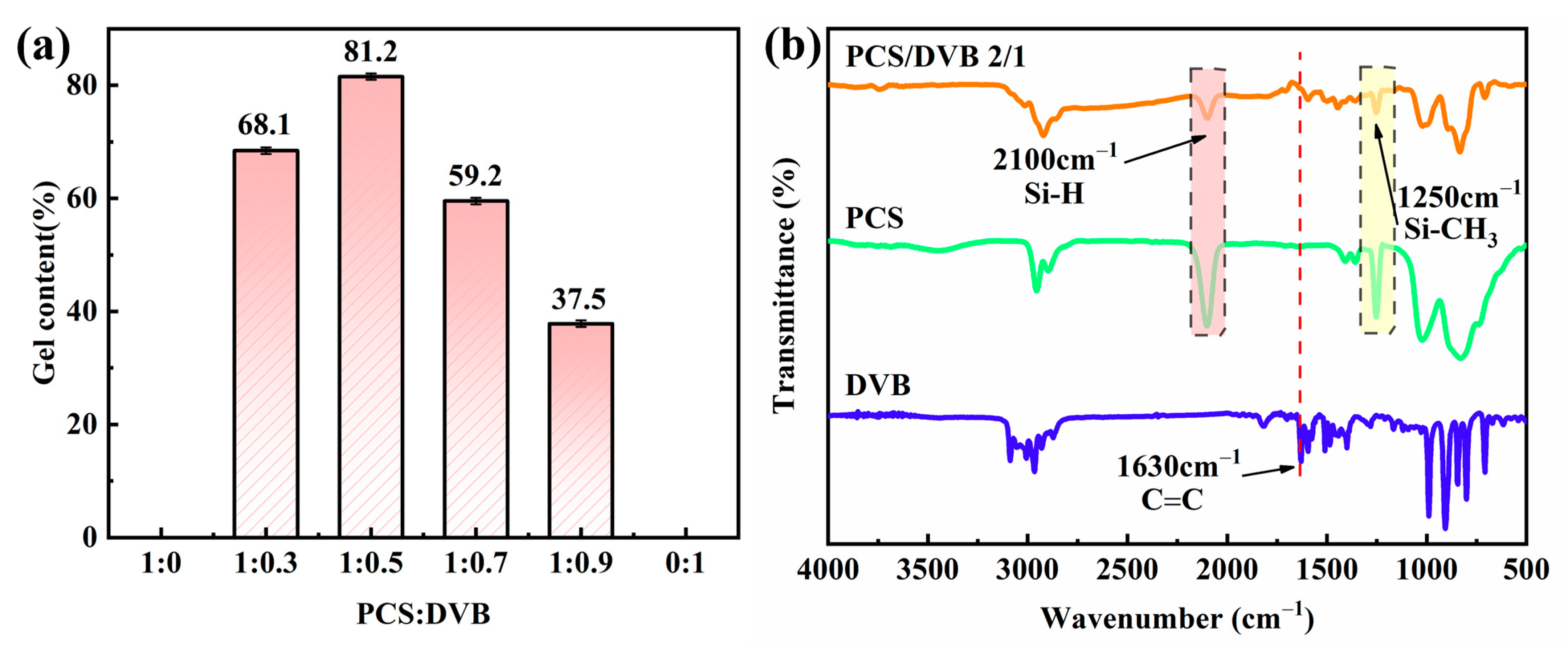
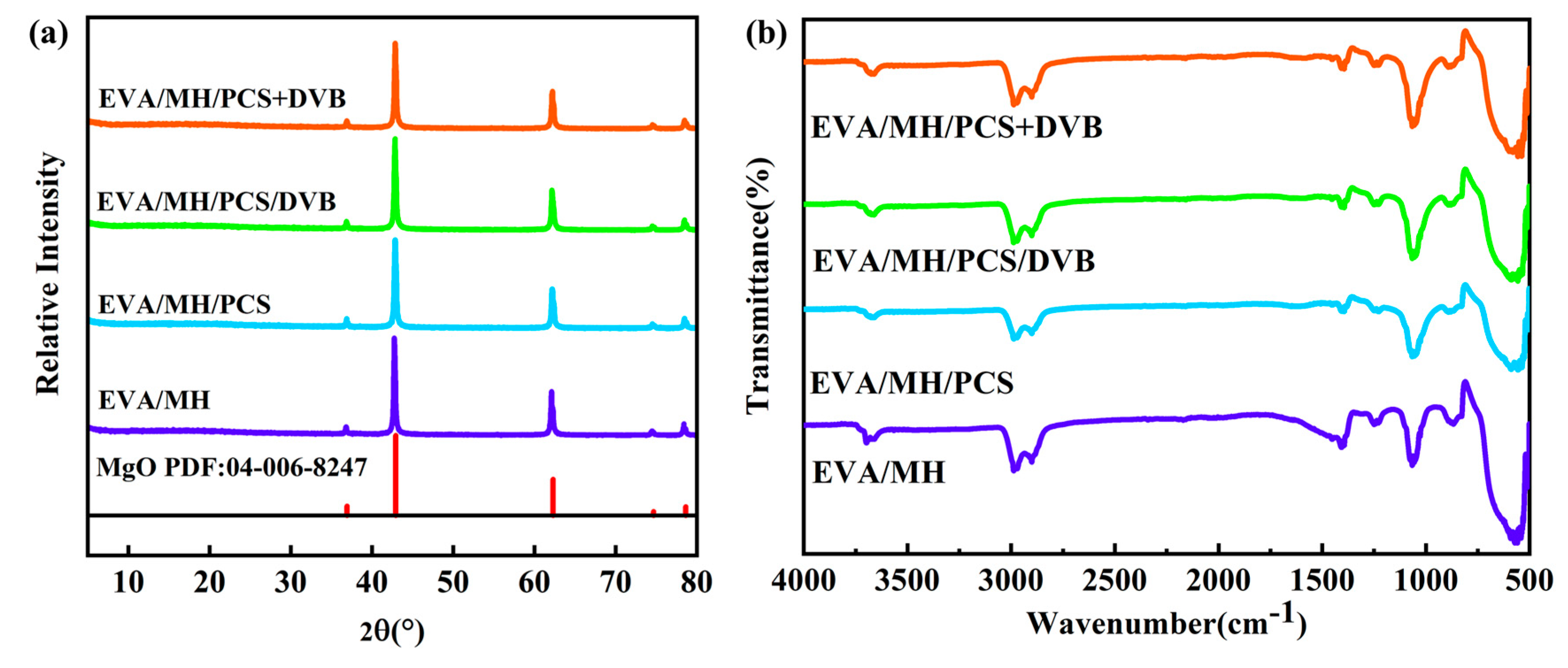
3.1. Cross-Linking Degree of PCS/DVB
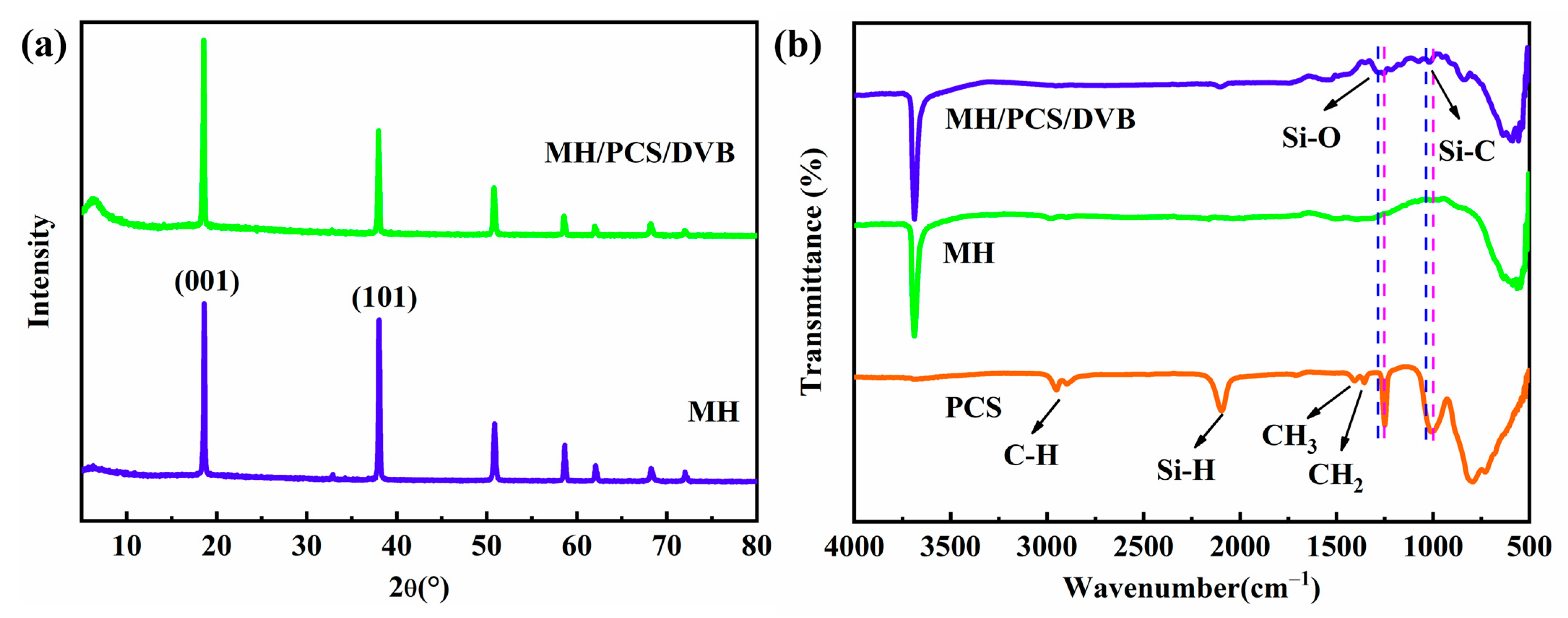
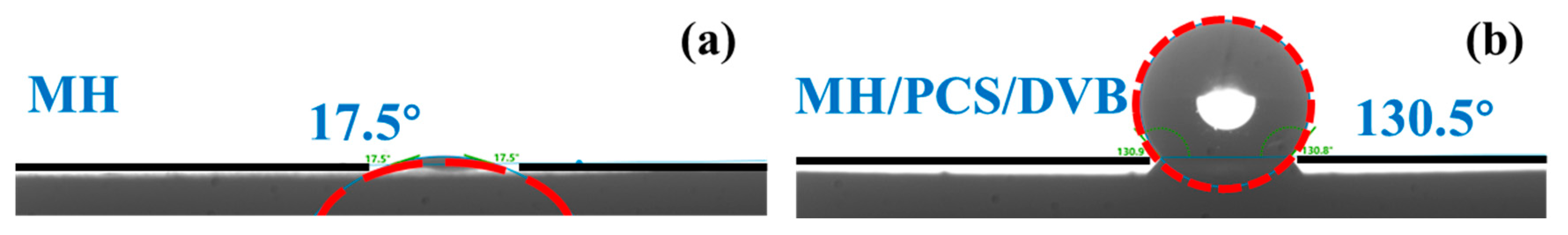
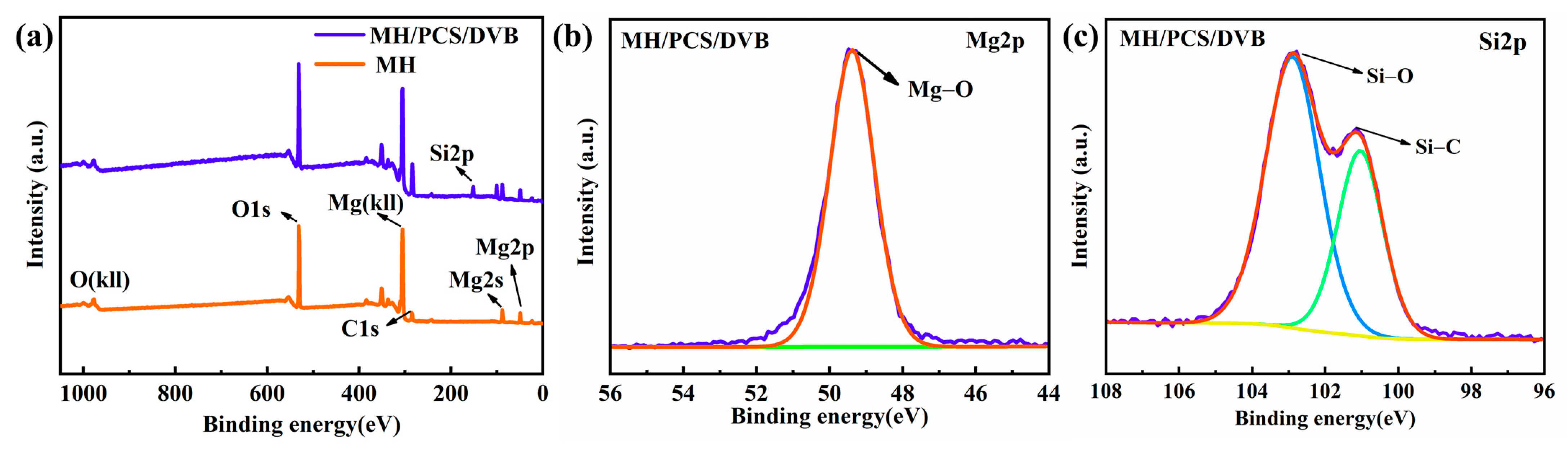
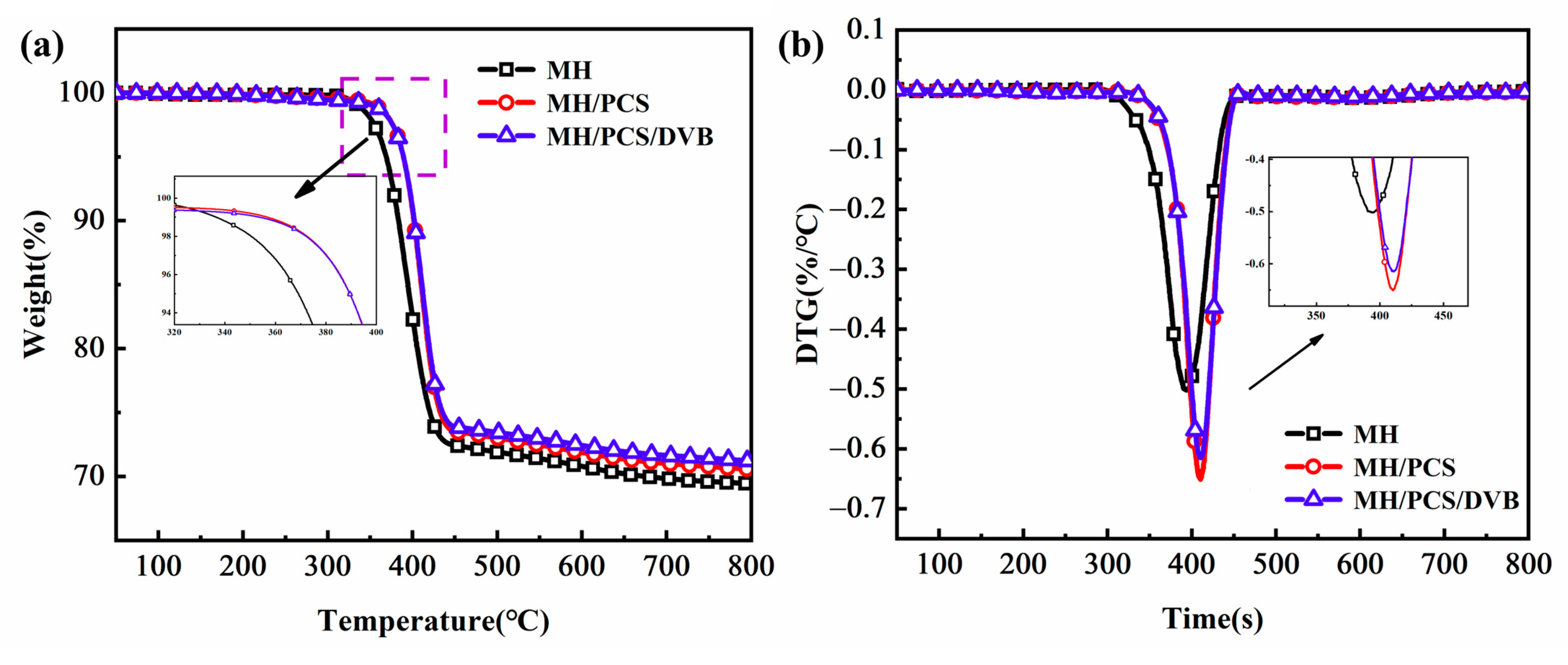
3.2. Properties of MH/PCS/DVB
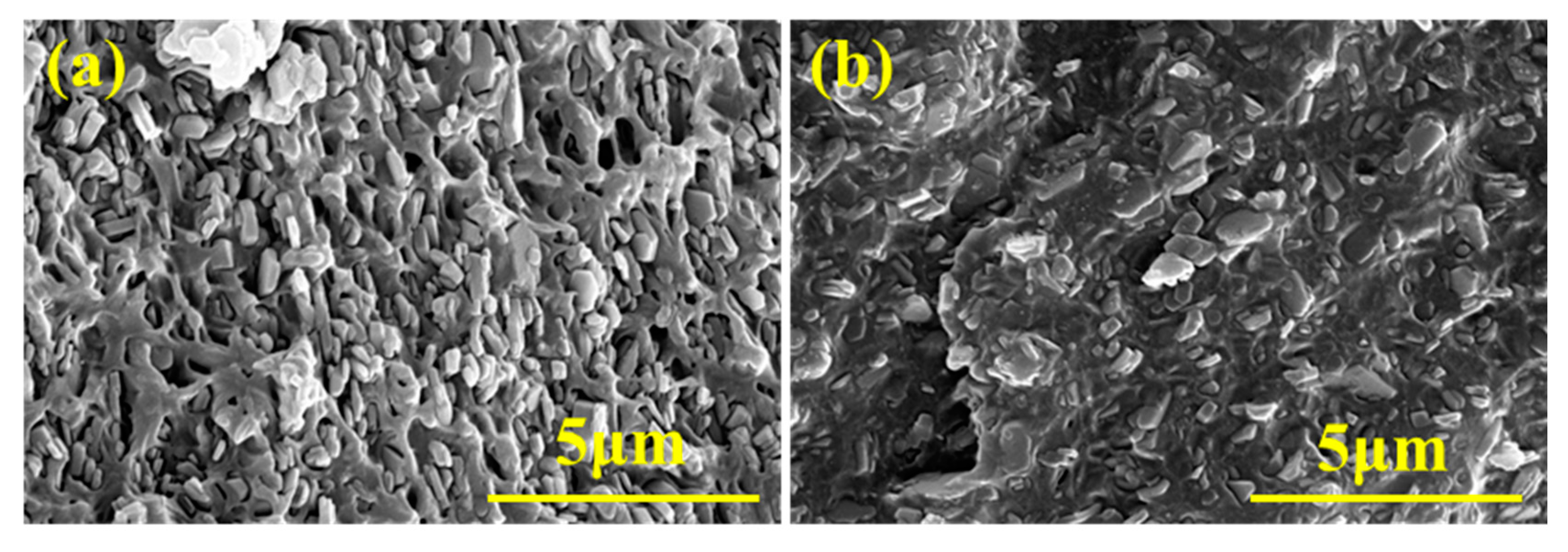
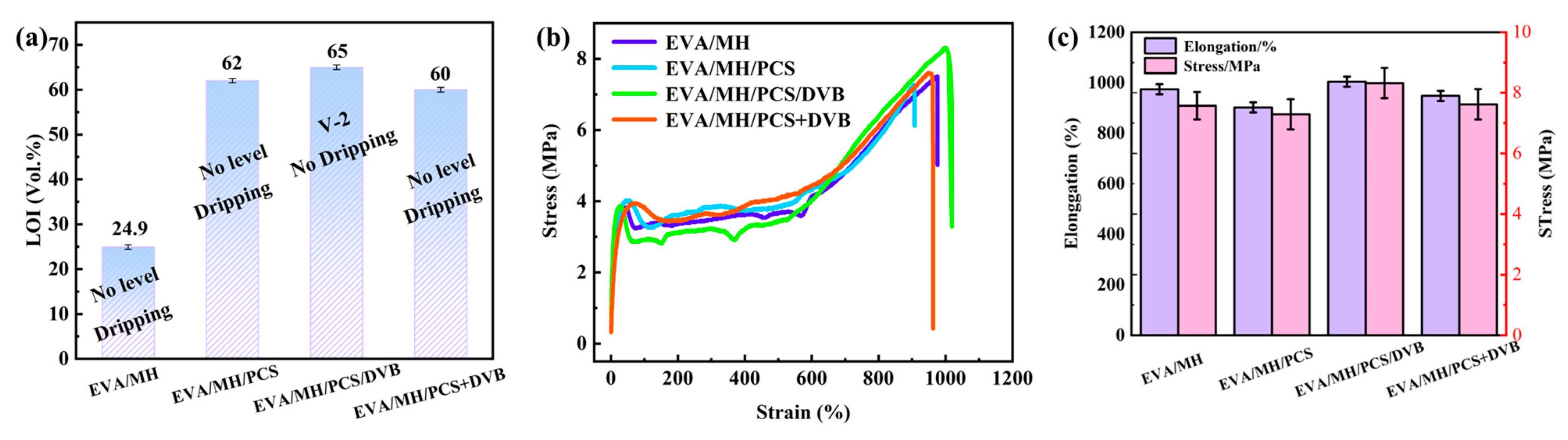
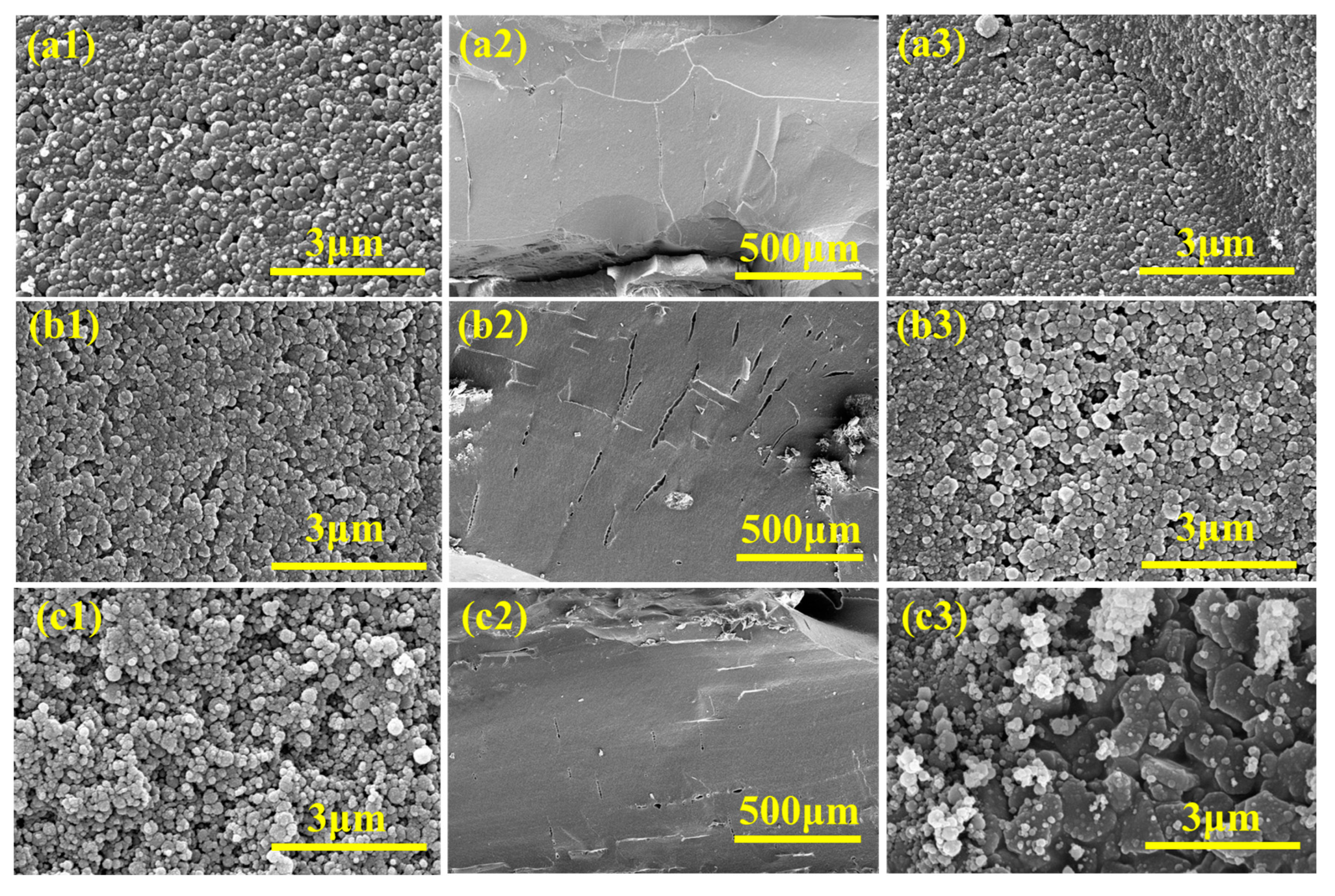
3.3. The Microstructure, Flame-Retardant Properties, and Mechanical Properties of EVA Composites
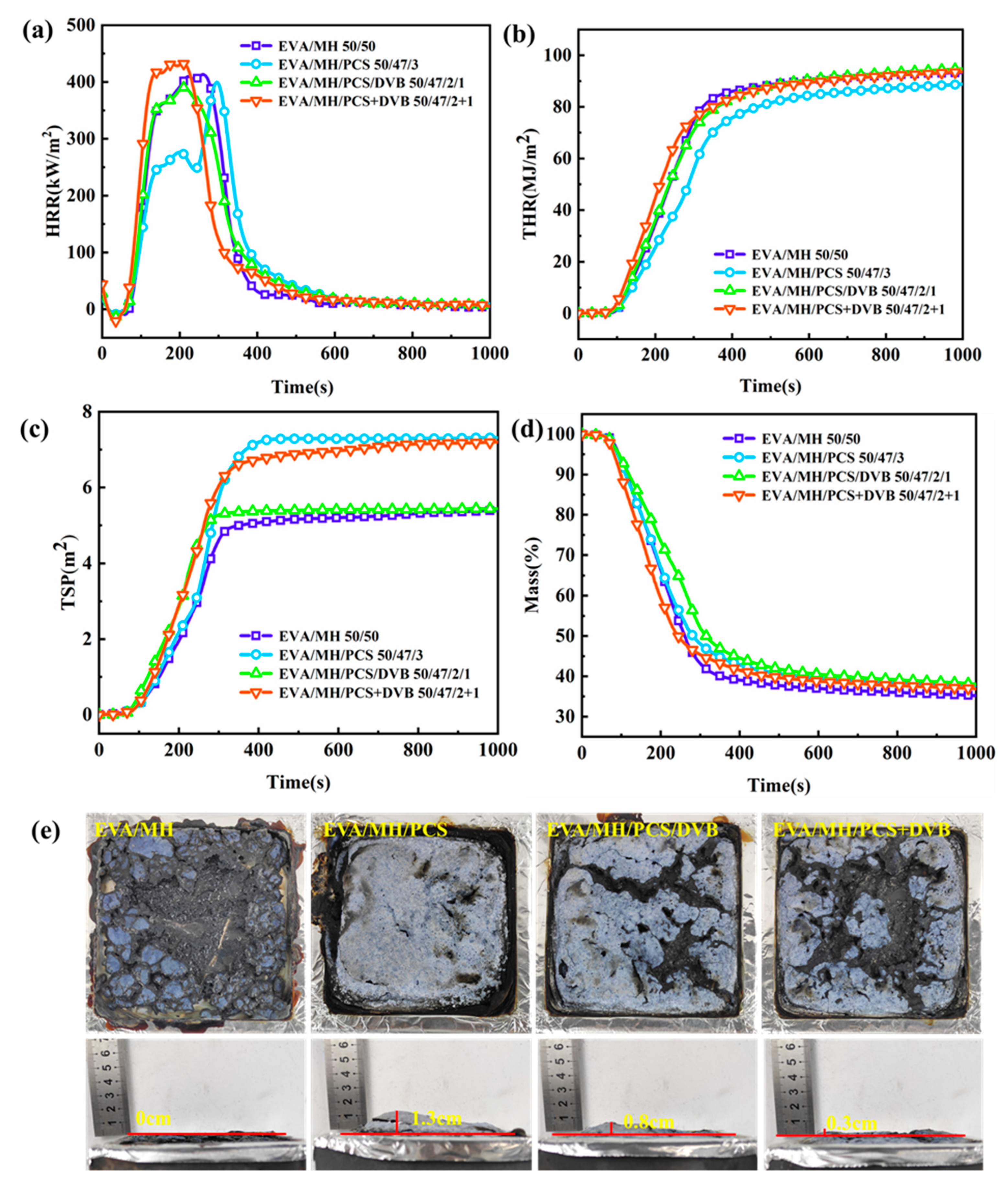
3.4. Combustion Behaviors of EVA Composites
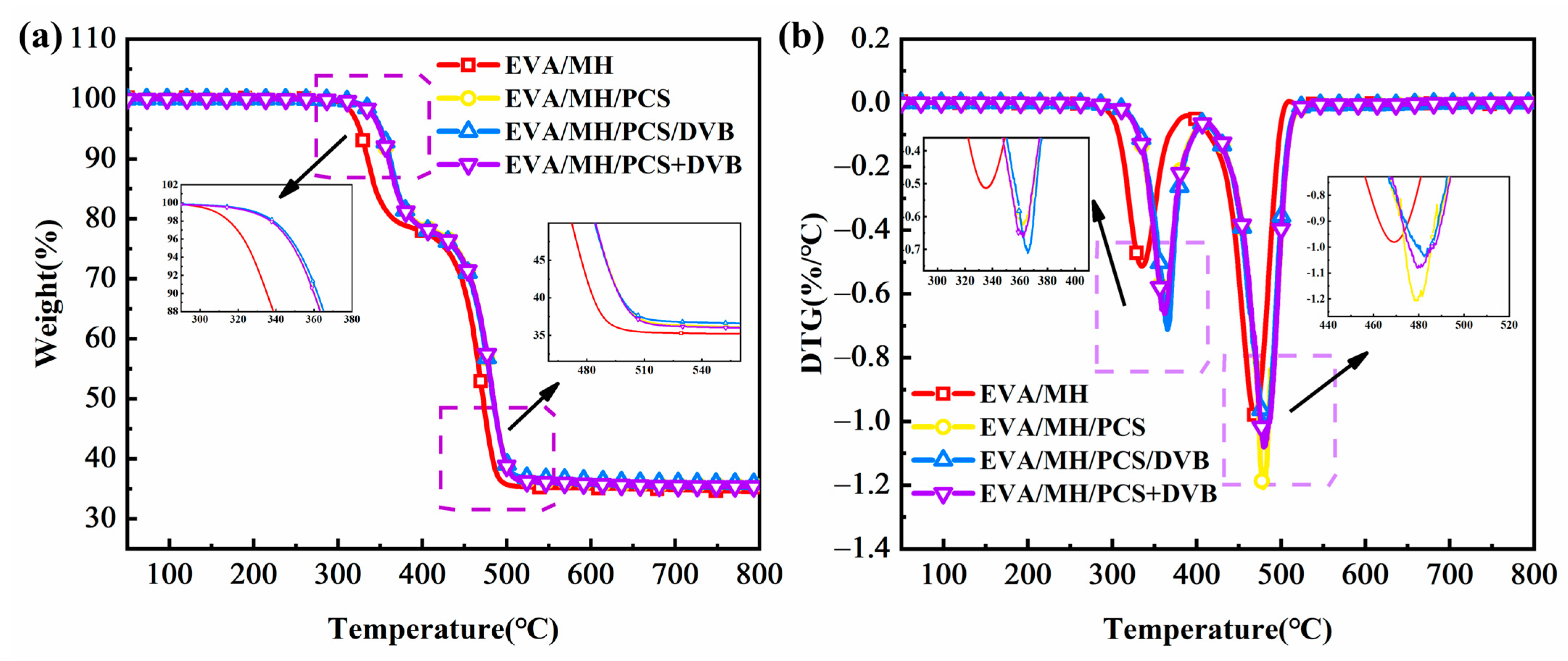
3.5. Thermal Degradation of EVA/MH/PCS Composites
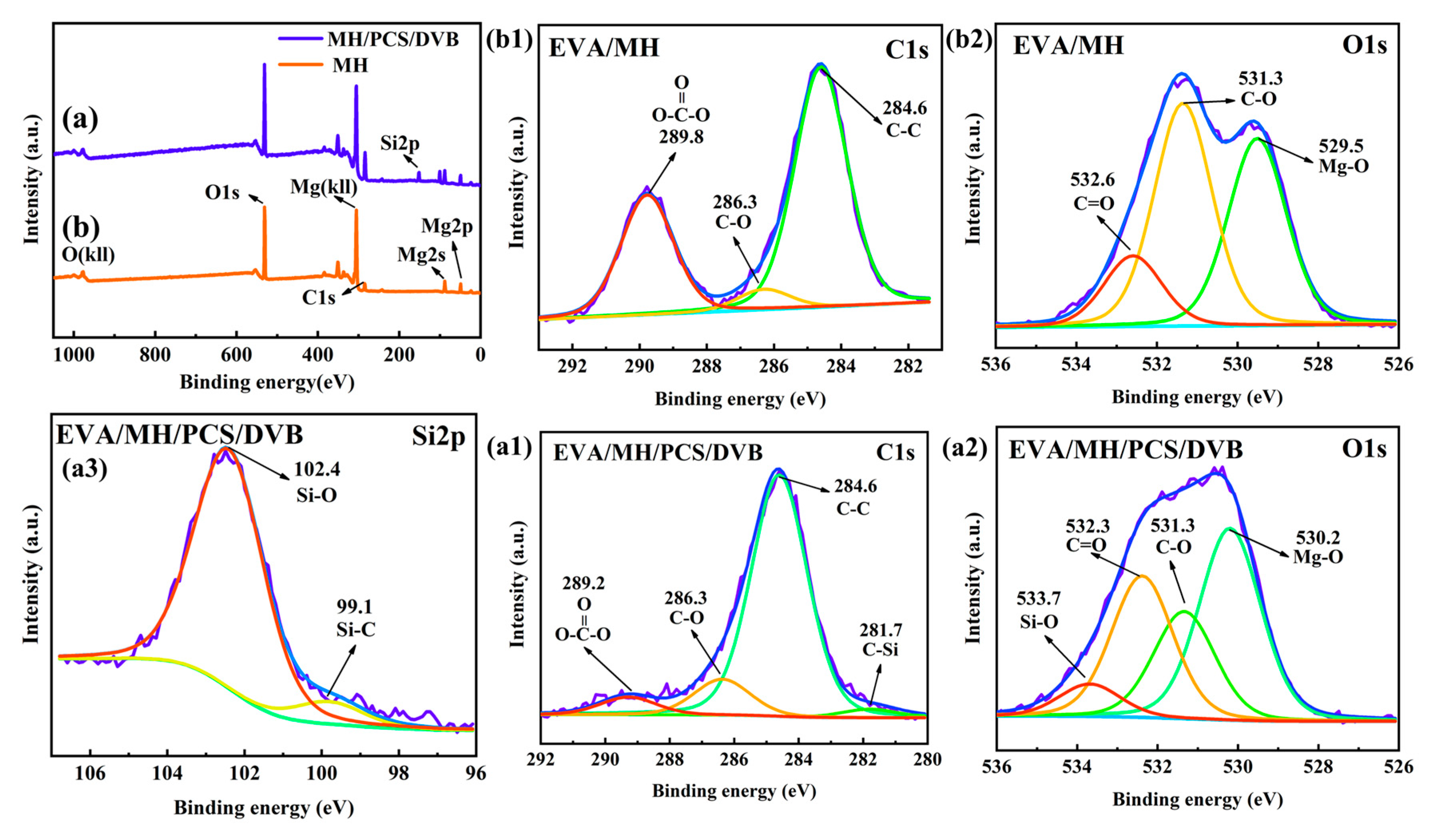
3.6. The Formation Mechanism of the Condensed Phase
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Heng, Z.; Chen, Y.; Zou, H.; Liang, M. Investigations of environmental stress cracking resistance of LDPE/UHMWPE and LDPE/EVA blends. Plast. Rubber Compos. 2015, 44, 218–225. [Google Scholar] [CrossRef]
- Gu, P.; Zhang, J. Vinyl acetate content influence on thermal, non-isothermal crystallization, and optical characteristics of ethylene–vinyl acetate copolymers. Iran. Polym. J. 2022, 31, 905–917. [Google Scholar] [CrossRef]
- Costache, M.C.J.; Wilkie, D.D.; Charles, A. Thermal degradation of ethylene–vinyl acetate coplymer nanocomposites. Polymer 2005, 46, 6947–6958. [Google Scholar] [CrossRef]
- Ye, L.; Miao, Y.; Yan, H.; Li, Z.; Zhou, Y.; Liu, J.; Liu, H. The synergistic effects of boroxo siloxanes with magnesium hydroxide in halogen-free flame retardant EVA/MH blends. Polym. Degrad. Stab. 2013, 98, 868–874. [Google Scholar] [CrossRef]
- Liu, Y.; Li, B.; Xu, M.; Wang, L. Highly Efficient Composite Flame Retardants for Improving the Flame Retardancy, Thermal Stability, Smoke Suppression, and Mechanical Properties of EVA. Materials 2020, 13, 1251. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-W.; Liang, X.-X.; Xu, X.-Q.; Lei, C.; He, X.-L.; Song, T.; Huo, W.-Y.; Ma, H.-C.; Lei, Z.-Q. PGS@B–N: An efficient flame retardant to improve simultaneously the interfacial interaction and the flame retardancy of EVA. RSC Adv. 2016, 6, 65921–65929. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, A.; Cheng, Y.; Li, M.; Cui, Y.; Li, Z. Recent advances in biomass phytic acid flame retardants. Polym. Test. 2023, 124, 108100. [Google Scholar] [CrossRef]
- Romanovskaia, N.; Minchenkov, K.; Gusev, S.; Klimova-Korsmik, O.; Safonov, A. Effects of Additives on the Mechanical and Fire Resistance Properties of Pultruded Composites. Polymers 2023, 15, 3581. [Google Scholar] [CrossRef]
- Liu, B.W.; Zhao, H.B.; Wang, Y.Z. Advanced Flame-Retardant Methods for Polymeric Materials. Adv. Mater. 2022, 34, e2107905. [Google Scholar] [CrossRef]
- Lan, S.; Li, L.; Xu, D.; Zhu, D.; Liu, Z.; Nie, F. Surface modification of magnesium hydroxide using vinyltriethoxysilane by dry process. Appl. Surf. Sci. 2016, 382, 56–62. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, C.; Wang, Y.; Qian, L.; Han, Z. Enhanced Flame Retardancy in Ethylene-Vinyl Acetate Copolymer/Magnesium Hydroxide/Polycarbosilane Blends. Polymers 2021, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.-L.; Zhao, P.-C.; Liu, Z.-Q.; Wu, Q.-S.; Yan, D.-Q.; Li, Y.-L.; Chen, Y.-N.; Li, J.-S. Preparation of Magnesium Hydroxide Flame Retardant from Hydromagnesite and Enhance the Flame Retardant Performance of EVA. Polymers 2022, 14, 1567. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zeng, X.-F.; Chen, J.-Y.; Wang, J.-X.; Zhang, L.-L.; Chen, J.-F. Magnesium hydroxide nanodispersion for polypropylene nanocomposites with high transparency and excellent fire-retardant properties. Polym. Degrad. Stab. 2017, 146, 327–333. [Google Scholar] [CrossRef]
- Zheng, T.; Xia, W.; Guo, J.; Liu, Y. Modified magnesium hydroxide encapsulated by melamine cyanurate in flame-retardant polyamide-6. J. Polym. Res. 2020, 27, 258. [Google Scholar] [CrossRef]
- Lv, X.; Fan, H.; Zeng, W.; Yang, Z.; Wang, Y.; Lei, Z. Novel nanocomposites based on epoxy resin and modified magnesium hydroxide: Focus on flame retardancy and mechanical properties. Polym. Adv. Technol. 2019, 30, 3026–3037. [Google Scholar] [CrossRef]
- Liu, L.; Xu, M.; Hu, Y.; Xu, Y.; Zhang, Z.; Li, B. Surface modification of magnesium hydroxide and its application in flame-retardant oil-extended styrene–ethylene–butadiene–styrene/polypropylene composites. J. Appl. Polym. Sci. 2018, 136, 47129. [Google Scholar] [CrossRef]
- Ai, L.; Chen, S.; Zeng, J.; Yang, L.; Liu, P. Synergistic Flame Retardant Effect of an Intumescent Flame Retardant Containing Boron and Magnesium Hydroxide. ACS Omega. 2019, 4, 3314–3321. [Google Scholar] [CrossRef]
- Cheng, L.; Ren, S.; Brosse, N.; Ju, Z.; Li, S.; Lu, X. Synergic flame-retardant effect of cellulose nanocrystals and magnesium hydroxide in polyurethane wood coating. J. Wood Chem. Technol. 2022, 42, 297–304. [Google Scholar] [CrossRef]
- Liang, S.; Liu, J.; Guo, Y.; Luo, J.; Liu, H.; Peng, S. Role of expandable graphite on flame retardancy, smoke suppression, and acid resistance of polypropylene/magnesium hydroxide composites. Polym. Eng. Sci. 2022, 62, 3168–3179. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, J.; Luo, J.; Liu, H.; Peng, S. Enhancement of thermal stability and flame retardancy of ethylene vinyl acetate/magnesium hydroxide composite by carbon black. Fire Mater. 2022, 47, 251–261. [Google Scholar] [CrossRef]
- Han, Y.; Xu, S.; Wang, A.; Cheng, P.; Li, J.; Shen, L.; Liu, H. Remarkable effects of silicone rubber on flame retardant property of high-density polyethylene/magnesium hydroxide composites. Polym. Degrad. Stab. 2022, 203, 110061. [Google Scholar] [CrossRef]
- Cheng, X.; Wu, J.; Yao, C.; Yang, G. Flame-retardant mechanism of zinc borate and magnesium hydroxide in aluminum hypophosphite–based combination for TPE-S composites. J. Fire Sci. 2019, 37, 273–300. [Google Scholar] [CrossRef]
- Lenża, J.; Merkel, K.; Rydarowski, H. Comparison of the effect of montmorillonite, magnesium hydroxide and a mixture of both on the flammability properties and mechanism of char formation of HDPE composites. Polym. Degrad. Stab. 2012, 97, 2581–2593. [Google Scholar] [CrossRef]
- Sonnier, R.; Viretto, A.; Dumazert, L.; Longerey, M.; Buonomo, S.; Gallard, B.; Longuet, C.; Cavodeau, F.; Lamy, R.; Freitag, A. Fire retardant benefits of combining aluminum hydroxide and silica in ethylene-vinyl acetate copolymer (EVA). Polym. Degrad. Stab. 2016, 128, 228–236. [Google Scholar] [CrossRef]
- Guo, Y.; Xue, Y.; Zuo, X.; Zhang, L.; Yang, Z.; Zhou, Y.; Marmorat, C.; He, S.; Rafailovich, M. Capitalizing on the molybdenum disulfide/graphene synergy to produce mechanical enhanced flame retardant ethylene-vinyl acetate composites with low aluminum hydroxide loading. Polym. Degrad. Stab. 2017, 144, 155–166. [Google Scholar] [CrossRef]
- Ye, L.; Wu, Q.; Qu, B. Synergistic effects and mechanism of multiwalled carbon nanotubes with magnesium hydroxide in halogen-free flame retardant EVA/MH/MWNT nanocomposites. Polym. Degrad. Stab. 2009, 94, 751–756. [Google Scholar] [CrossRef]
- Liu, L.; Hu, J.; Zhuo, J.; Jiao, C.; Chen, X.; Li, S. Synergistic flame retardant effects between hollow glass microspheres and magnesium hydroxide in ethylene-vinyl acetate composites. Polym. Degrad. Stab. 2014, 104, 87–94. [Google Scholar] [CrossRef]
- Chen, H.; Wang, T.; Wen, Y.; Wen, X.; Gao, D.; Yu, R.; Chen, X.; Mijowska, E.; Tang, T. Expanded graphite assistant construction of gradient-structured char layer in PBS/Mg(OH)2 composites for improving flame retardancy, thermal stability and mechanical properties. Compos. B Eng. 2019, 177, 107402. [Google Scholar] [CrossRef]
- Piao, J.; Lai, Y.; Ren, J.; Wang, Y.; Feng, T.; Wang, Y.; Liu, W.; Dong, H.; Chen, W.; Jiao, C.; et al. Zn-doped carbon microspheres as synergist in intumescent flame-retardant thermoplastic polyurethane composites: Mechanism of char residues layer regulation. Compos. Commun. 2022, 32, 101173. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Cheng, X.; Yang, J. Aggregation Structure of Polycarbosilane. Macromolecules 2019, 52, 7847–7857. [Google Scholar] [CrossRef]
- Wang, C.; Liu, J.; Wang, Y.; Han, Z. Enhanced flame retardance in polyethylene/magnesium hydroxide/polycarbosilane blends. Mater. Chem. Phys. 2020, 253, 123373. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Han, Z. Enhanced flame retardancy of polyethylene/magnesium hydroxide with polycarbosilane. Sci. Rep. 2018, 8, 14494. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Zhou, K.; Wu, N.; Luo, H.; Wang, X.; Zhou, X.; Yan, Z.; Abrahams, I.; Zhang, D. Porous SiC ceramics with dendritic pore structures by freeze casting from chemical cross-linked polycarbosilane. Ceram. Int. 2018, 44, 6293–6299. [Google Scholar] [CrossRef]
- ASTM D2863-97; Standard Test Method for Measuring the Minimum Oxygen Concentration to Support Candle-Like Combustion of Plastics (Oxygen Index). ASTM: West Conshohocken, PA, USA, 1997.
- ASTM D3801; Standard Test Method for Measuring the Comparative Burning Characteristics of Solid Plastics in a Vertical Position. ASTM: West Conshohocken, PA, USA, 2020.
- ISO 5660-1:2015; Reaction-to-Fire Tests—Heat Release, Smoke Production and Mass Loss Rate—Part 1: Heat Release Rate (Cone Calorimeter Method) and Smoke Production Rate (Dynamic Measurement). ISO: Geneva, Switzerland, 2015.
- Zhou, L.; Cheng, Y.; Zhang, W.; Zhang, W.; Liu, W.; Li, Y.; Wang, M. Improving the ceramic yield of polycarbosilane by radiation cross-linking in the presence of multifunctional monomers. Int. J. Appl. Ceram. Technol. 2018, 15, 1510–1517. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Chen, Q.; Wang, J.; Zhou, T. Experimental study on combustion and yield characteristics of dimethyl carbonate/n-heptane blends in the cone calorimeter. J. Therm. Anal. Calorim. 2020, 143, 3057–3064. [Google Scholar] [CrossRef]
- Marcilla, A.; Gómez, A.; Menargues, S. TG/FTIR study of the thermal pyrolysis of EVA copolymers. J. Anal. Appl. Pyrolysis 2005, 74, 224–230. [Google Scholar] [CrossRef]
- Barth, R.L.G.; Bryson, C. Advances in Charge Neutralization for XPS Measurements of Nonconducting Materials. Surf. Interface Anal. 1988, 11, 307–311. [Google Scholar] [CrossRef]
- Sosulnikov, M.I.; Teterin, Y.A. X-ray photoelectron studies of Ca, Sr and Ba and their oxides and carbonates. J. Electron. Spectrosc. 1992, 59, 111–126. [Google Scholar] [CrossRef]




| Samples | EVA (wt.%) | MH (wt.%) | PCS (wt.%) | DVB (wt.%) |
|---|---|---|---|---|
| EVA/MH | 50 | 50 | 0 | 0 |
| EVA/MH/PCS | 50 | 47 | 3 | 0 |
| EVA/MH/PCS/DVB | 50 | 47 | 2 | 1 |
| EVA/MH/PCS+DVB | 50 | 47 | 2 | 1 |
| Bond | Vibration Mode | Wave Number (cm−1) | ||
|---|---|---|---|---|
| PCS | DVB | PCS/DVB | ||
| C-H in benzene | Stretching | -- | 3087, 3058, 3006 | 3015 |
| C-H in -CH2- | Stretching | 2955, 2895 | 2966, 2930, 2873 | 2922 |
| Si-H | Stretching | 2100 | -- | 2100 |
| C=C in -CH=CH2 | Stretching | -- | 1630 | -- |
| C=C in benzene | Skeleton vibration | -- | 1595, 1577, 1510,1480 | 1595,1500, 1445 |
| C-H | Bending in plane | 1410,1359 | 1400 | 1410, 1359 |
| Si-CH3 | Deformation | 1250 | -- | 1250 |
| Si-O | Stretching | 1020 | -- | 1020 |
| C-H in -CH=CH2 | Bending out of plane | -- | 989 | 1000 |
| C-H in benzene | Bending out of plane | -- | 906, 844, 800, 707 | 890, 709 |
| Si-C in Si-CH2-Si | Stretching | 830 | -- | 830 |
| Element | MH (Atomic %) | MH/PCS/DVB (Atomic %) |
|---|---|---|
| Mg | 22.78 | 7.76 |
| O | 59.03 | 40.98 |
| C | 18.20 | 39.99 |
| Si | 11.27 |
| Samples | MH | MH/PCS | MH/PCS/DVB |
|---|---|---|---|
| T5 (°C) | 369 | 389 | 389 |
| T10 (°C) | 383 | 399 | 402 |
| Tmax1 (°C) | 388 | 404 | 413 |
| Rmax1 (°C/min) | −0.66 | −0.91 | −0.73 |
| Residue600°C (%) | 70.0 | 71.9 | 72.4 |
| Sample | EVA/MH 50/50 | EVA/MH/PCS 50/47/3 | EVA/MH/PCS/DVB 50/47/2/1 | EVA/MH/PCS+DVB 50/47/2+1 |
|---|---|---|---|---|
| TTI (s) | 74 | 71 | 68 | 60 |
| tpHRR1 (s) | 280 | 201 | 219 | 195 |
| pHRR1 (kW/m2) | 438.8 | 291.1 | 396.7 | 450.6 |
| tpHRR2 (s) | - | 296 | - | - |
| pHRR2 (kW/m2) | - | 426.6 | - | - |
| THR6min (MJ/m2) | 90 | 84 | 90 | 89 |
| TSP6min (m2) | 5.2 | 7.3 | 5.4 | 6.9 |
| Residue6min (%) | 36.9 | 38.7 | 40.5 | 39.5 |
| FPI (s·m2/kW) | 0.17 | 0.17 | 0.17 | 0.13 |
| FGI (kW/m2·s) | 1.24 | 1.16 | 1.38 | 1.77 |
| THRI6min (MJ/m2) | 1.94 | 1.89 | 1.93 | 1.92 |
| TSPI6min (m2) | 0.68 | 0.88 | 0.67 | 0.72 |
| Sample | EVA/MH 50/50 | EVA/MH/PCS 50/47/3 | EVA/MH/PCS/DVB 50/47/2/1 | EVA/MH/PCS+DVB 50/47/2+1 |
|---|---|---|---|---|
| T5 (°C) | 325 | 349 | 351 | 349 |
| T10 (°C) | 335 | 360 | 362 | 360 |
| T50 (°C) | 472 | 484 | 484 | 484 |
| Tmax1 (°C) | 336 | 365 | 369 | 366 |
| Tmax2 (°C) | 467 | 479 | 485 | 476 |
| Rmax1 (°C/min) | −0.93 | −0.74 | −0.93 | −0.95 |
| Rmax2 (°C/min) | −1.96 | −2.95 | −1.31 | −1.43 |
| Residue600s (%) | 35.1 | 35.9 | 36.4 | 35.8 |
| Element | EVA/MH (Atomic %) | EVA/MH/PCS/DVB (Atomic %) |
|---|---|---|
| Mg | 22.36 | 19.5 |
| O | 56.28 | 54.44 |
| C | 21.36 | 18.97 |
| Si | - | 7.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Wang, C.; Wang, G.; Wang, Y.; Han, Z. Polycarbosilane/Divinylbenzene-Modified Magnesium Hydroxide to Enhance the Flame Retardancy of Ethylene–Vinyl Acetate Copolymer. Polymers 2023, 15, 4440. https://doi.org/10.3390/polym15224440
Li S, Wang C, Wang G, Wang Y, Han Z. Polycarbosilane/Divinylbenzene-Modified Magnesium Hydroxide to Enhance the Flame Retardancy of Ethylene–Vinyl Acetate Copolymer. Polymers. 2023; 15(22):4440. https://doi.org/10.3390/polym15224440
Chicago/Turabian StyleLi, Siyuan, Chunfeng Wang, Guodong Wang, Yongliang Wang, and Zhidong Han. 2023. "Polycarbosilane/Divinylbenzene-Modified Magnesium Hydroxide to Enhance the Flame Retardancy of Ethylene–Vinyl Acetate Copolymer" Polymers 15, no. 22: 4440. https://doi.org/10.3390/polym15224440
APA StyleLi, S., Wang, C., Wang, G., Wang, Y., & Han, Z. (2023). Polycarbosilane/Divinylbenzene-Modified Magnesium Hydroxide to Enhance the Flame Retardancy of Ethylene–Vinyl Acetate Copolymer. Polymers, 15(22), 4440. https://doi.org/10.3390/polym15224440






