Synthesis and Application of Hybrid Aluminum Dialkylphosphinates as Highly Efficient Flame Retardants for Polyamides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
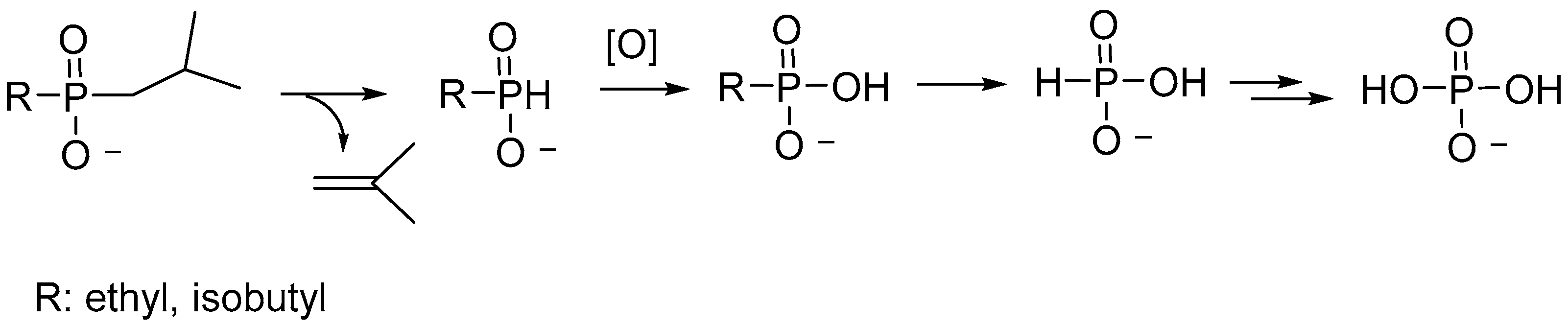
2.2. Synthesis of Hybrid Al Phosphinates with Mixed Ligands
2.3. Preparation of Polyamides/PFR Blends
2.4. Characterization
3. Results and Discussion
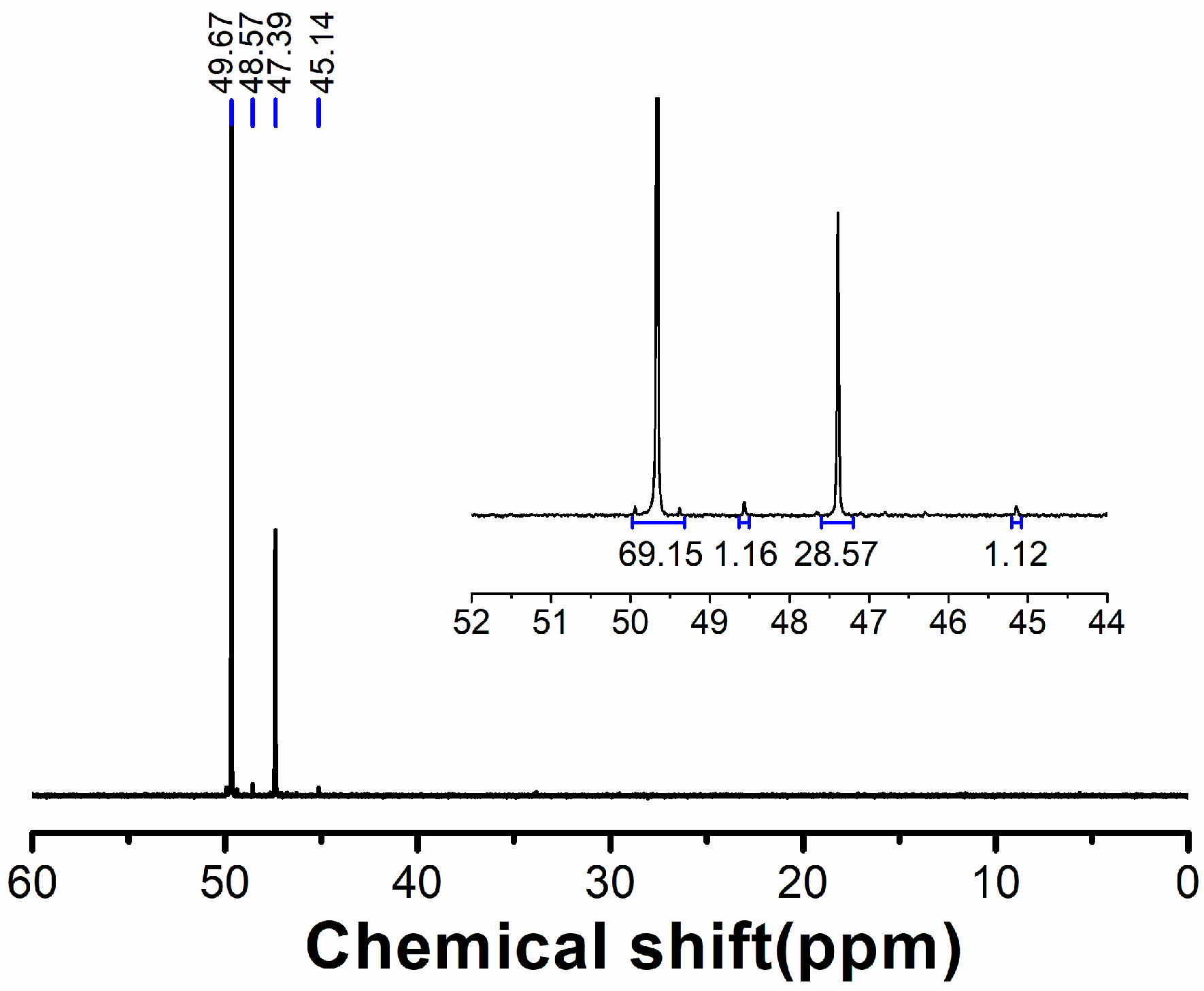
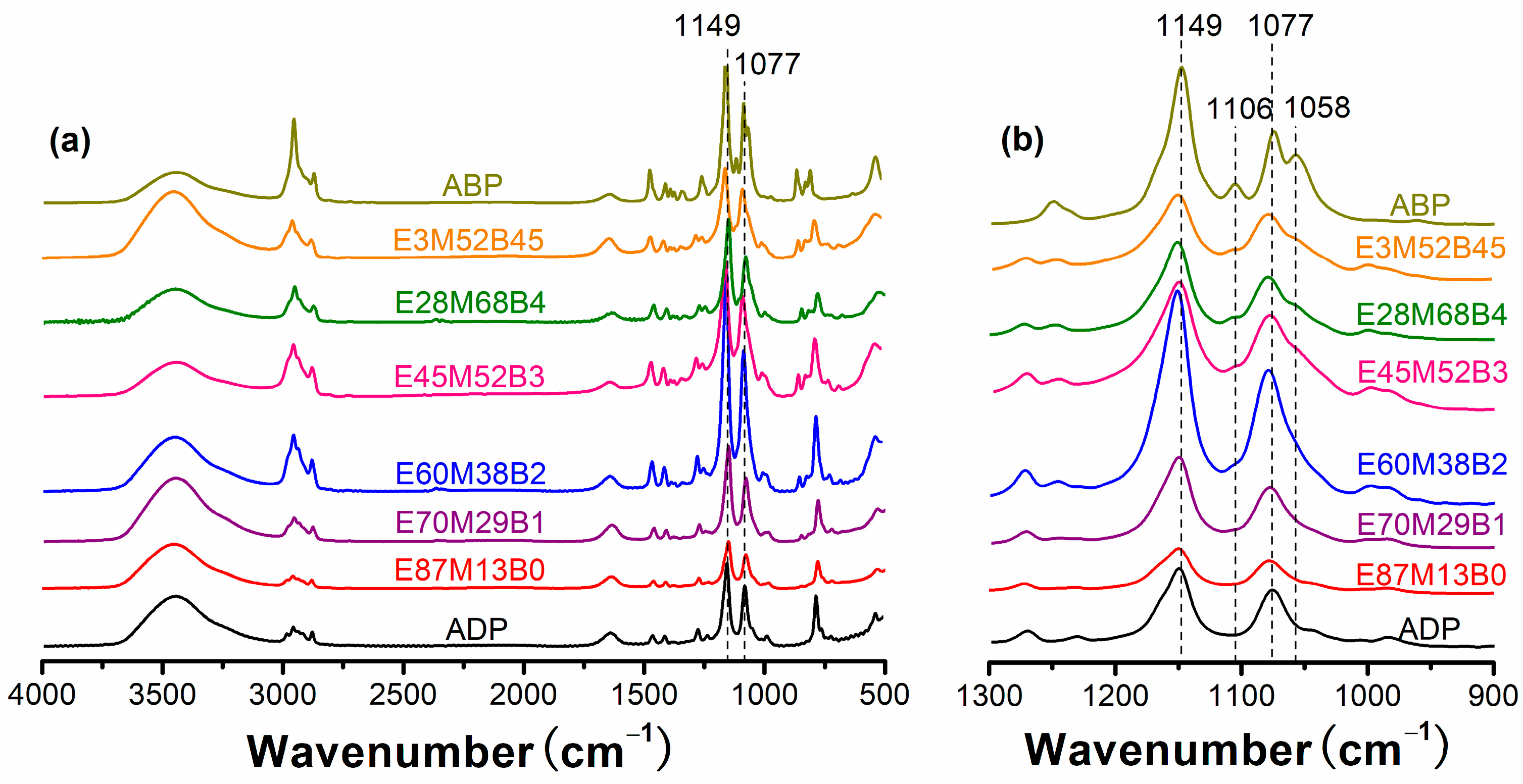
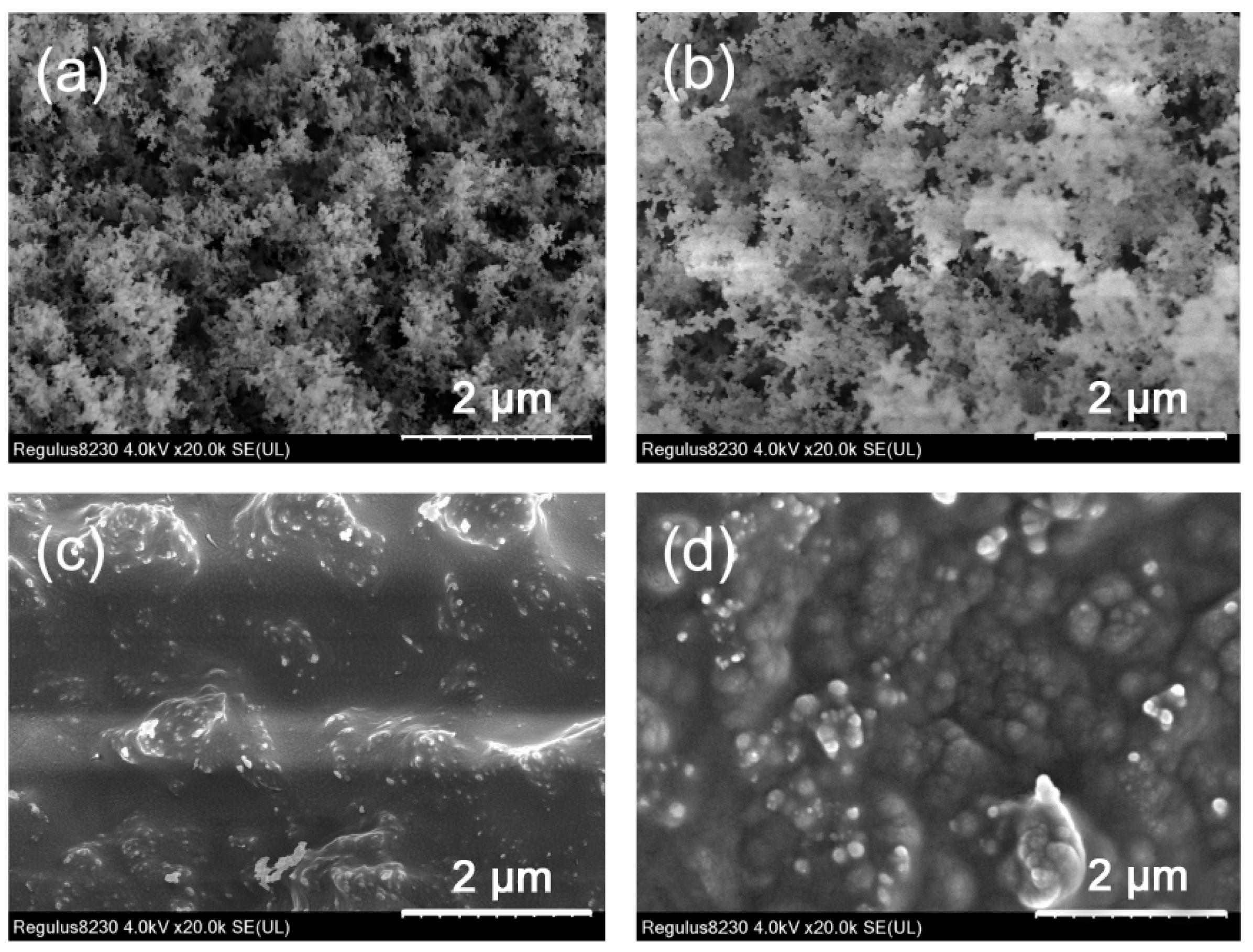
3.1. Characterizations of Hybrid Al Phosphinates
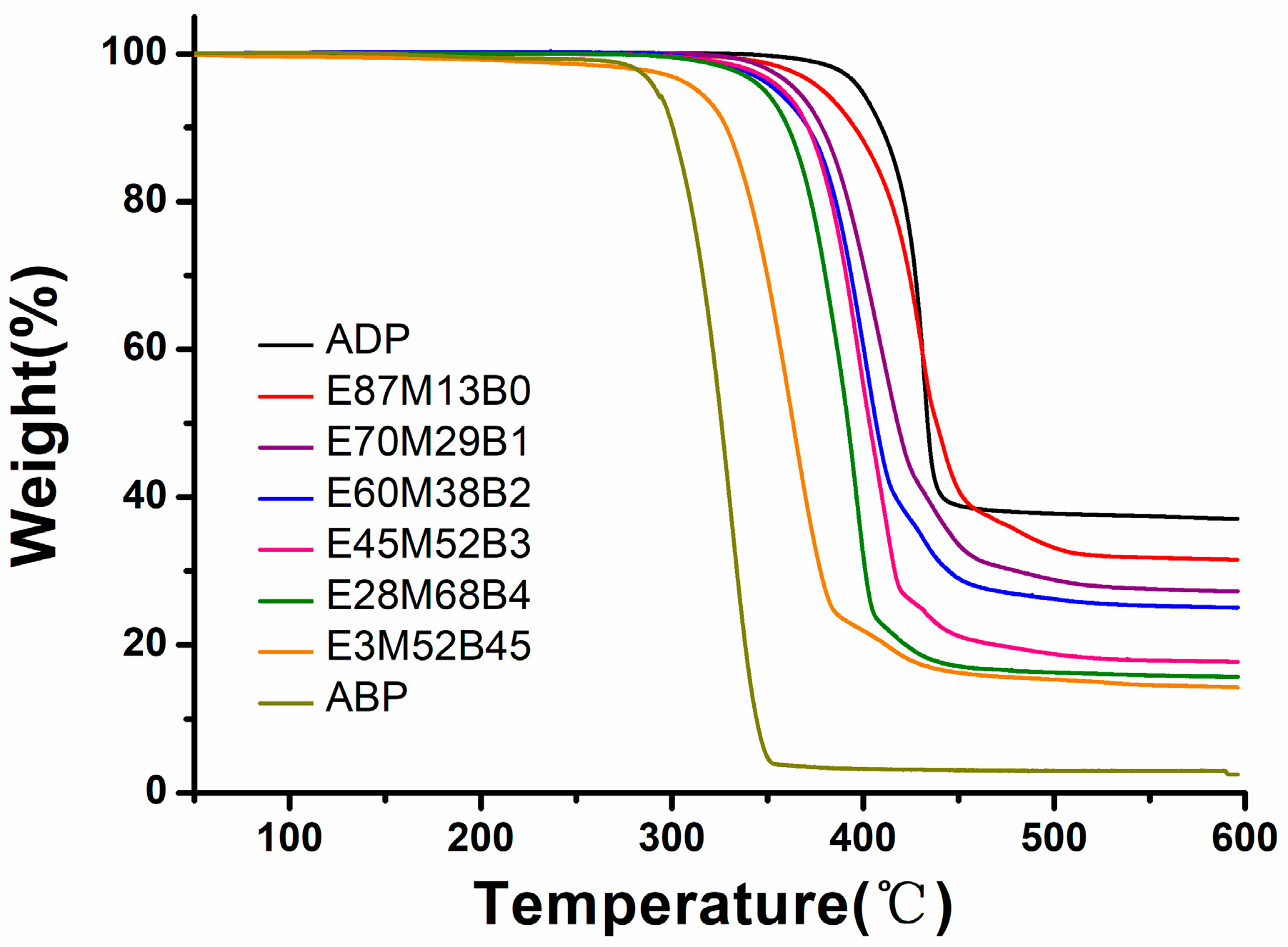
3.2. Thermal Stability of Al Phosphinates and Flame-Retardant Polyamides
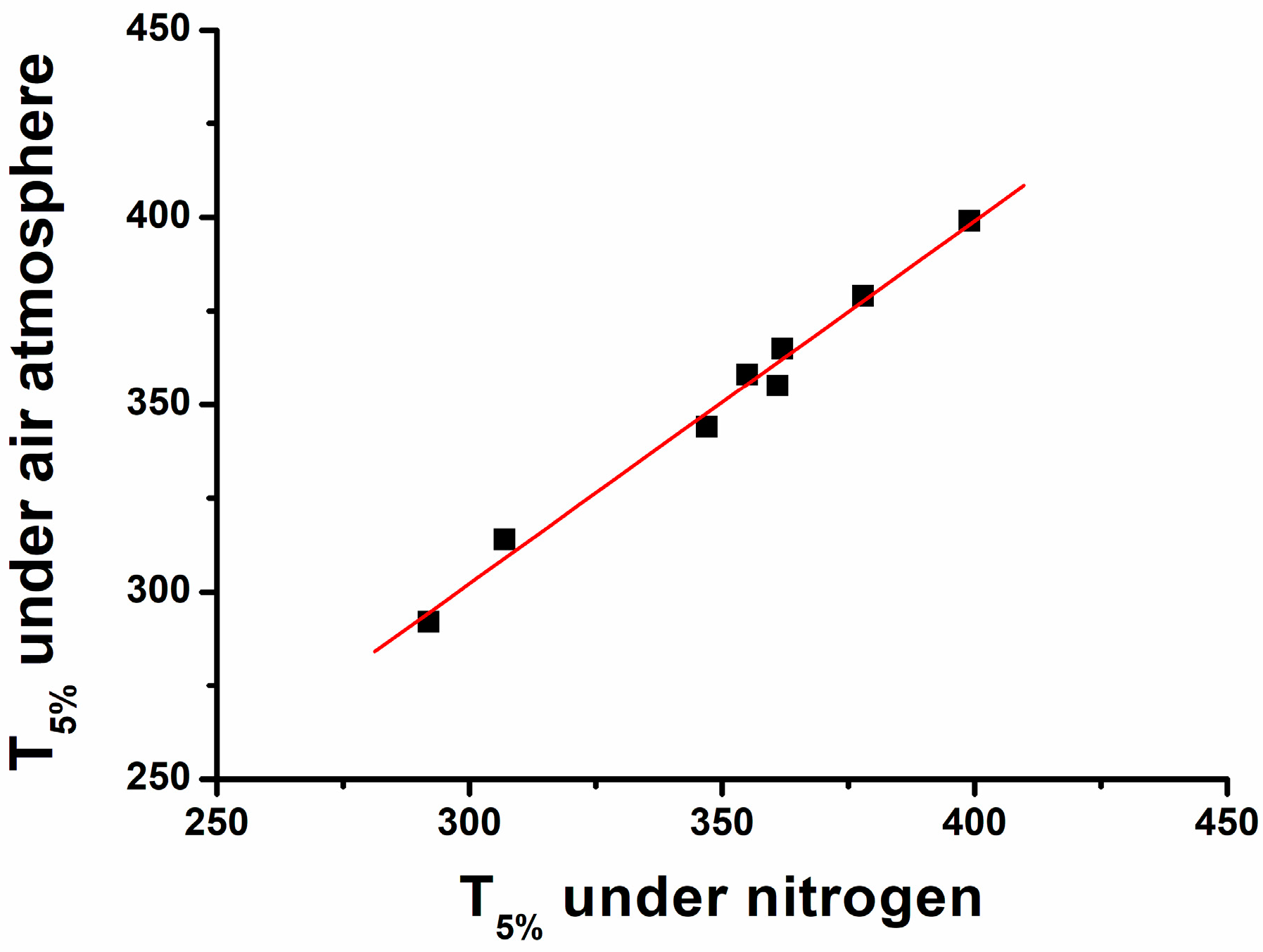
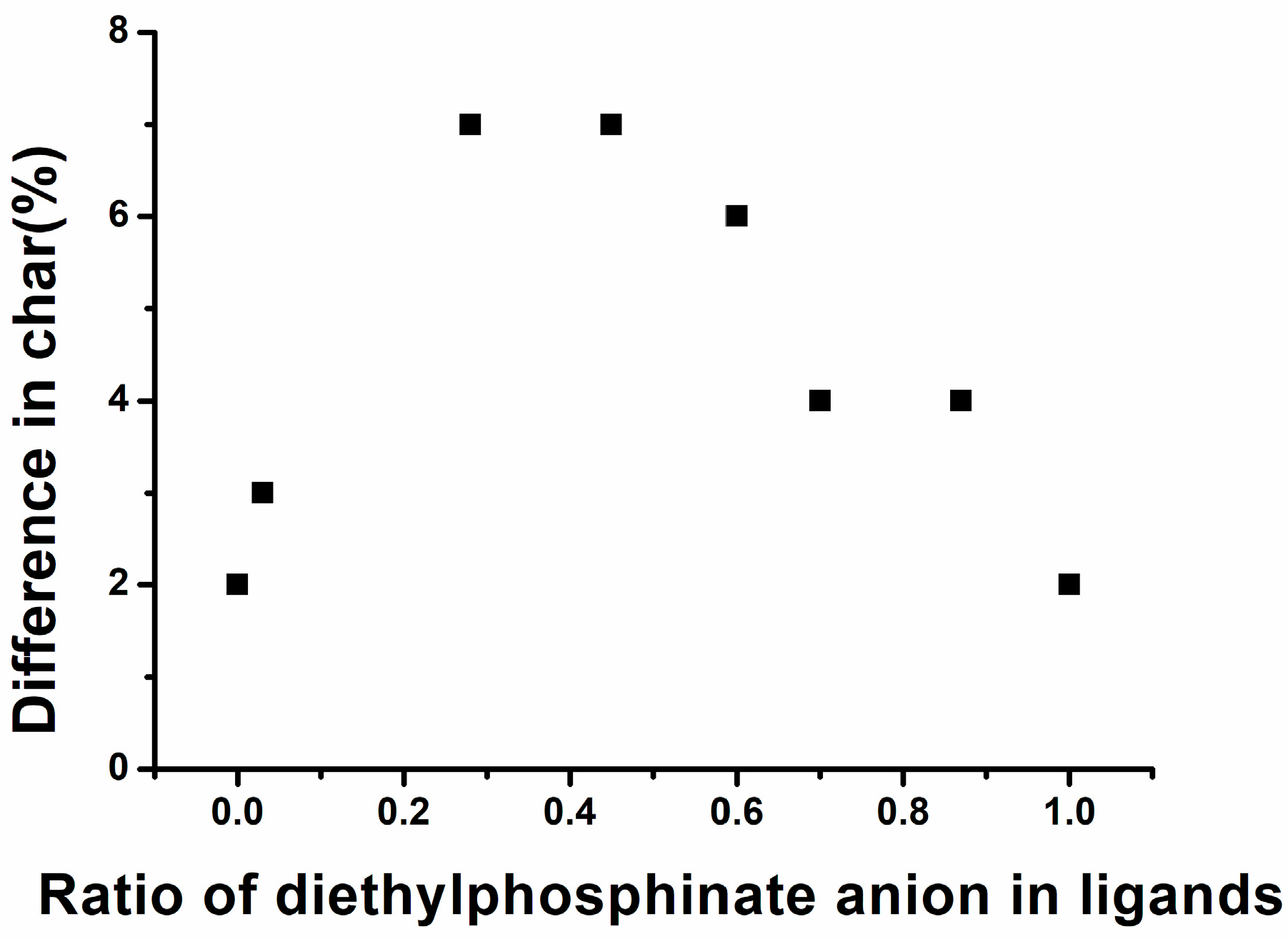
3.3. Thermoxidative Stability of Al Phosphinates and Flame-Retardant Polyamides
3.4. UL-94 Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Q.; Li, B.; Zhang, S.; Lin, M. Investigation on Effects of Aluminum and Magnesium Hypophosphites on Flame Retardancy and Thermal Degradation of Polyamide 6. J. Appl. Polym. Sci. 2012, 125, 1782–1789. [Google Scholar] [CrossRef]
- Costanzi, S.; Leonardi, M. Polyamide Compositions Flame Retarded with Aluminum. U.S. Patent 8178607B2, 15 May 2012. [Google Scholar]
- Zhao, B.; Chen, L.; Long, J.W.; Chen, H.B.; Wang, Y.Z. Aluminum Hypophosphite Versus Alkyl-substituted Phosphinate in Polyamide 6: Flame Retardance, Thermal Degradation, and Pyrolysis Behavior. Ind. Eng. Chem. Res. 2013, 52, 2875–2886. [Google Scholar] [CrossRef]
- Kleiner, H.J.; Budzinsky, W.; Kirsch, G. Low-Flammability Polyamide Molding Materials. U.S. Patent 5773556A, 30 June 1998. [Google Scholar]
- Braun, U.; Schartel, B.; Fichera, M.A.; Jager, C. Flame Retardancy Mechanisms of Aluminium Phosphinate in Combination with Melamine Polyphosphate and Zinc Borate in Glass-fibre Reinforced Polyamide 6,6. Polym. Degrad. Stab. 2007, 92, 1528–1545. [Google Scholar] [CrossRef]
- Braun, U.; Bahr, H.; Schartel, B. Fire Retardancy Effect of Aluminium Phosphinate and Melamine Polyphosphate in Glass Fibre Reinforced Polyamide 6. e-Polymer 2010, 10, 041. [Google Scholar] [CrossRef]
- Wu, W.; Lv, S.; Liu, X.; Qu, H.; Zhang, H.; Xu, J. Using TG–FTIR and TG–MS to Study Thermal Degradation of Metal Hypophosphites. J. Therm. Anal. Calorim. 2014, 118, 1569–1575. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Pan, Y.T.; Xu, X.; Song, Y.; Yang, R. Mitigation the Release of Toxic PH3 and the Fire Hazard of PA6/AHP Composite by MOFs. J. Hazardous Mat. 2020, 395, 122604. [Google Scholar] [CrossRef] [PubMed]
- Nass, B.; Wlfgan, W. Synergistic Flameproofing Combination for Polymers. U.S. Patent 6 207 736 B1, 27 March 2001. [Google Scholar]
- Yao, Q.; Levchik, S.; Alessio, G. Phosphorus-Containing Flame Retardant for Thermoplastic Polymers. U.S. Patent 7807737B2, 5 October 2010. [Google Scholar]
- Yao, Q.; Levchik, S. Process for the Alkylation of Phosphorus-Containing Compounds. U.S. Patent 8097747B2, 17 January 2012. [Google Scholar]
- He, W.; Zhu, H.; Xiang, Y.; Long, L.; Qin, S.; Yu, J. Enhancement of Flame Retardancy and Mechanical Properties of Polyamide 6 by Incorporating an Aluminum Salt of Diisobutylphosphinic Combined with Organoclay. Polym. Degrad. Stab. 2017, 144, 442–453. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, L.; Long, J.W.; Jian, R.K.; Wang, Y.Z. Synergistic Effect between Aluminum Hypophosphite and Alkyl-Substituted Phosphinate in Flame-Retarded Polyamide 6. Ind. Eng. Chem. Res. 2013, 52, 17162–17170. [Google Scholar] [CrossRef]
- Rose, S.H.; Block, B.P. Inorganic coordination polymers. VIII. Cobalt (II) and Zinc (II) Phosphinate Polymers and Copolymers. J. Polym. Sci. Part A-1 1966, 4, 583–592. [Google Scholar] [CrossRef]
- Rose, S.H.; Block, B.P. Zinc(II) and Cobalt(II) Phosphinate Polymers with Low-Temperature Flexibility. J. Amer. Chem. Soc. 1965, 87, 2076–2077. [Google Scholar] [CrossRef]
- Schmidt, D.L.; Flagg, E.E. Inorganic Aluminum-Oxygen-Phosphorus Bond Polymers. U.S. Patent 3538136A, 3 November 1970. [Google Scholar]
- Giancotti, V.; Giordano, F.; Randaccio, L.; Riapmonti, A. X-ray Study of Zinc(II) Di-n-alkylphosphinate Copolymers. J. Chem. Soc. A 1968, 757–763. [Google Scholar] [CrossRef]
- ASTM D3801-10; Standard Test Method for Measuring the Comparative Burning Characteristics of Solid Plastics in a Vertical Position. ASTM International: West Conshohocken, PA, USA, 2010.
- Gillman, H.D.; Eichelberger, J.L. Inorganic Coordination Polymers. XIX. Steric Effects of Hydrocarbon Side Groups on the Structure and Properties of Zinc(II), Cobalt(II), Manganese(II), Nickel(II), and Copper(II) Bis(phosphinates). Inorg. Chem. 1976, 15, 840–843. [Google Scholar] [CrossRef]
- Carson, I.; Healy, M.R.; Doidge, E.D.; Love, J.B.; Morrison, C.A.; Tasker, P.A. Metal-binding Motifs of Alkyl and Aryl Phosphinates; Versatile mono and polynucleating ligands. Coord. Chem. Rev. 2017, 335, 150–171. [Google Scholar] [CrossRef]
- Block, B.P.; Rose, S.H.; Schaumann, C.W.; Rote, E.S.; Simkin, J. Coordinaiton Polymers with Inorganic Backbones Formed by Double- Bridging of Tetrahedral Elements. J. Am. Chem. Soc. 1962, 84, 3200–3201. [Google Scholar] [CrossRef]
- Kaya, H.; Hacaloglu, J. Thermal degradation of Polylactide/Aluminium Diethylphosphinate. J. Anal. Appl. Pyro. 2014, 110, 155–162. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D.; Lewin, M. Thermal Decomposition of Aliphatic nylons. Polym. Int. 1999, 48, 532–557. [Google Scholar] [CrossRef]
- Li, R.; Hu, X. Study on Discoloration Mechanism of Polyamide 6 During Thermos-oxidative Degradation. Polym. Degrad. Stab. 1998, 62, 523–528. [Google Scholar] [CrossRef]
- Levchik, S.V.; Levchik, G.F.; Balabanovich, A.I.; Camino, G.; Costa, L. Mechanistic Study of Combustion Performance and Thermal Decomposition Behaviour of Nylon 6 with Added Halogen-free Fire Retardants. Polym. Degrad. Stab. 1996, 54, 217–222. [Google Scholar] [CrossRef]





 | ||||||
|---|---|---|---|---|---|---|
| Denotation | X | Y | Z | P-theoretical (%) | P-actual (%) | D50 (μm) |
| ADP | 1 | 0 | 0 | 23.8 | 23.5 | 30.0 |
| E87M13B0 | 0.87 | 0.13 | 0 | 23.2 | 23.3 | 8.6 |
| E70M29B1 | 0.70 | 0.29 | 0.01 | 22.3 | 21.9 | 12.9 |
| E60M38B2 | 0.60 | 0.38 | 0.02 | 21.8 | 21.6 | 128.0 |
| E45M52B3 | 0.45 | 0.52 | 0.03 | 21.2 | 21.2 | 133.4 |
| E28M68B4 | 0.28 | 0.68 | 0.04 | 20.5 | 20.7 | 30.8 |
| E3M52B45 | 0.03 | 0.52 | 0.45 | 18.2 | 18.0 | 5.7 |
| ABP | 0 | 0 | 1 | 16.6 | 16.5 | 1.7 |
| Sample | FR | FR-PA66 1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T5% (°C) | Char (%) | T5% (°C) | Tmax (°C) | Char (%) | |||||||||
| N2 | Air | N2 | air | N2 | Air | N2 | Air | N2 | Cal. 2 | Air | Cal. | Gain 3 | |
| PA66 | 401 | 377 | 448 | 438 | 32 | 32 | 32 | 32 | 0 | ||||
| ADP | 399 | 399 | 2 | 37 | 365 | 381 | 400 | 427 | 28 | 32 | 35 | 33 | 2 |
| E87M13B0 | 378 | 379 | 5 | 32 | 362 | 376 | 399 | 422 | 29 | 28 | 36 | 32 | 4 |
| E70M29B1 | 362 | 365 | 5 | 27 | 355 | 368 | 390 | 408 | 28 | 29 | 36 | 31 | 4 |
| E60M38B2 | 361 | 355 | 2 | 25 | 356 | 368 | 390 | 416 | 28 | 29 | 37 | 31 | 6 |
| E45M52B3 | 355 | 358 | 4 | 18 | 352 | 362 | 383 | 416 | 28 | 28 | 37 | 30 | 7 |
| E28M68B4 | 347 | 344 | 4 | 16 | 345 | 354 | 378 | 420 | 28 | 28 | 37 | 30 | 7 |
| E3M52B45 | 307 | 314 | 7 | 14 | 339 | 357 | 430 | 439 | 29 | 28 | 33 | 30 | 3 |
| ABP | 292 | 292 | 2 | 3 | 333 | 334 | 430 | 442 | 28 | 29 | 30 | 28 | 2 |
| FR | UL-94 of PA66 | |
|---|---|---|
| 20% FR | 12.5% FR | |
| ADP | N.R. a | N.R. a |
| E87M13B0 | V1 | N.R. |
| E70M29B1 | V0 | N.R. |
| E60M38B2 | V0 | V0 |
| E45M52B3 | V0 | V0 |
| E28M68B4 | V0 | V0 |
| E3M52B45 | V0 | V0 |
| ABP | V0 | V1 |
| E59M0B41 b | N.R. a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Q.; Cao, W.; Zhao, Y.; Tang, T. Synthesis and Application of Hybrid Aluminum Dialkylphosphinates as Highly Efficient Flame Retardants for Polyamides. Polymers 2023, 15, 4612. https://doi.org/10.3390/polym15234612
Yao Q, Cao W, Zhao Y, Tang T. Synthesis and Application of Hybrid Aluminum Dialkylphosphinates as Highly Efficient Flame Retardants for Polyamides. Polymers. 2023; 15(23):4612. https://doi.org/10.3390/polym15234612
Chicago/Turabian StyleYao, Qiang, Weihong Cao, Yueying Zhao, and Tianbo Tang. 2023. "Synthesis and Application of Hybrid Aluminum Dialkylphosphinates as Highly Efficient Flame Retardants for Polyamides" Polymers 15, no. 23: 4612. https://doi.org/10.3390/polym15234612
APA StyleYao, Q., Cao, W., Zhao, Y., & Tang, T. (2023). Synthesis and Application of Hybrid Aluminum Dialkylphosphinates as Highly Efficient Flame Retardants for Polyamides. Polymers, 15(23), 4612. https://doi.org/10.3390/polym15234612






