Mimicking Transmural Helical Cardiomyofibre Orientation Using Bouligand-like Pore Structures in Ice-Templated Collagen Scaffolds
Abstract
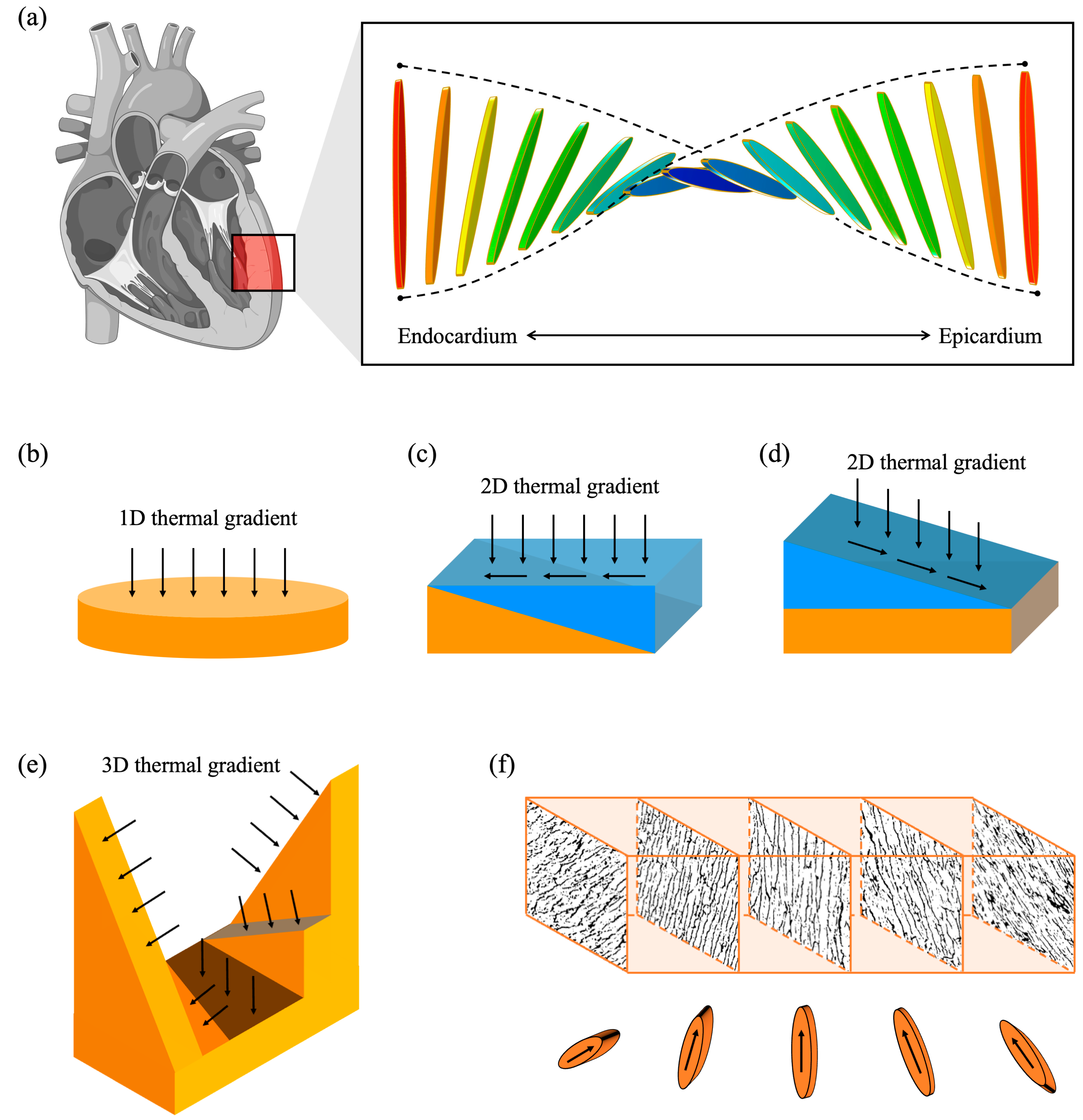
1. Introduction
2. Materials and Methods
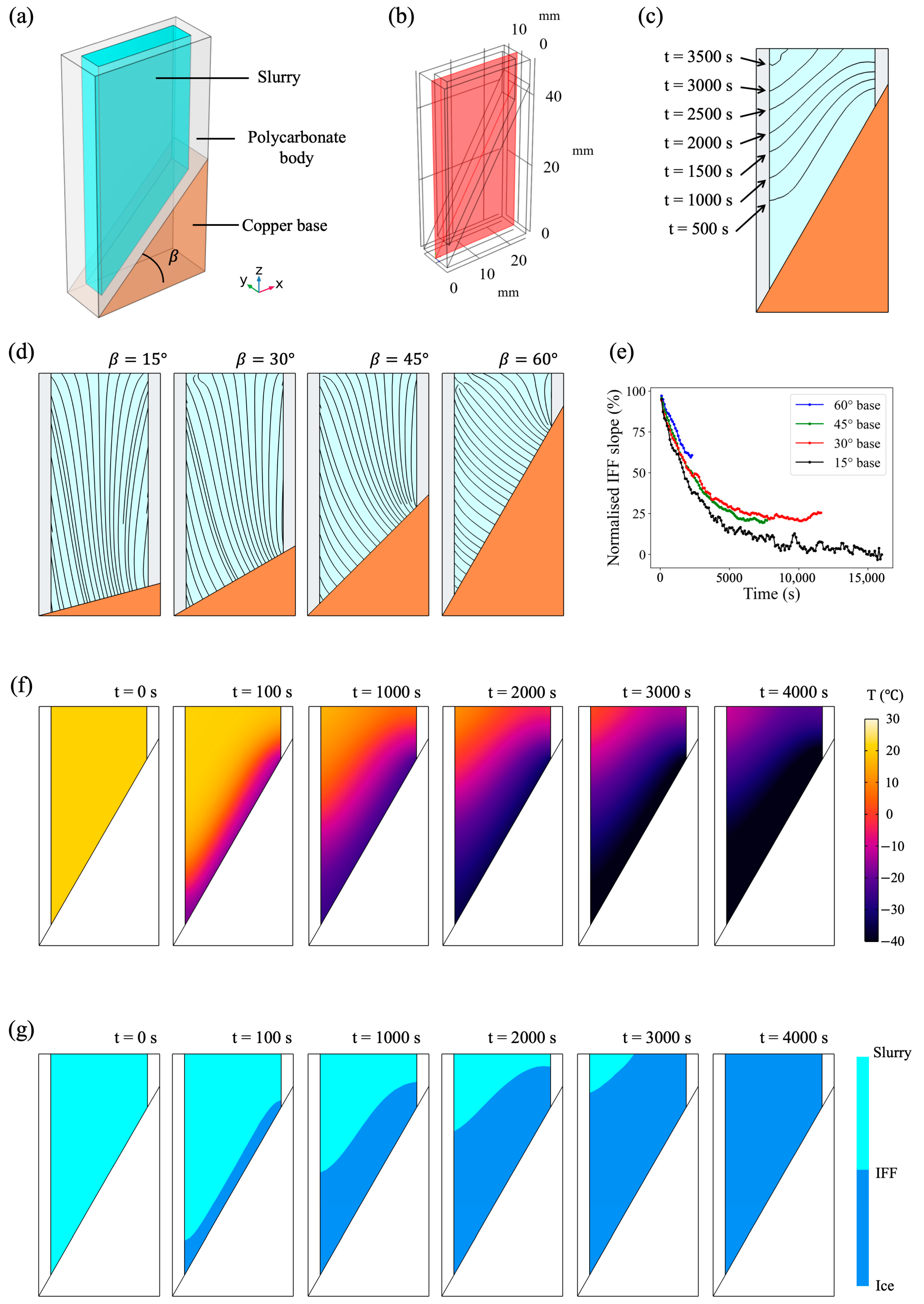
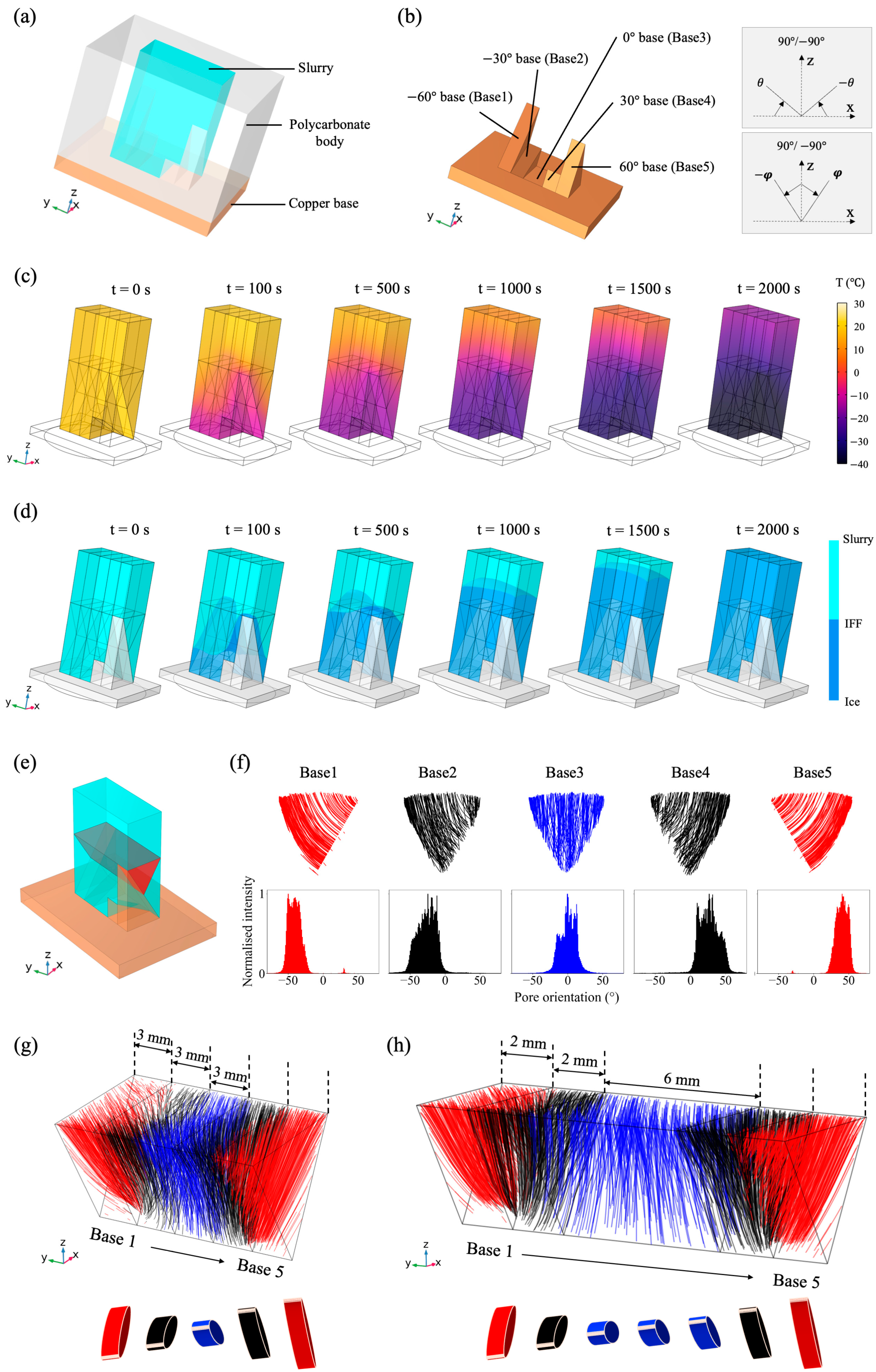
2.1. Finite Element Simulation
2.2. Scaffold Fabrication
2.3. Structural Characterisation
2.4. Statistical Analysis
3. Results
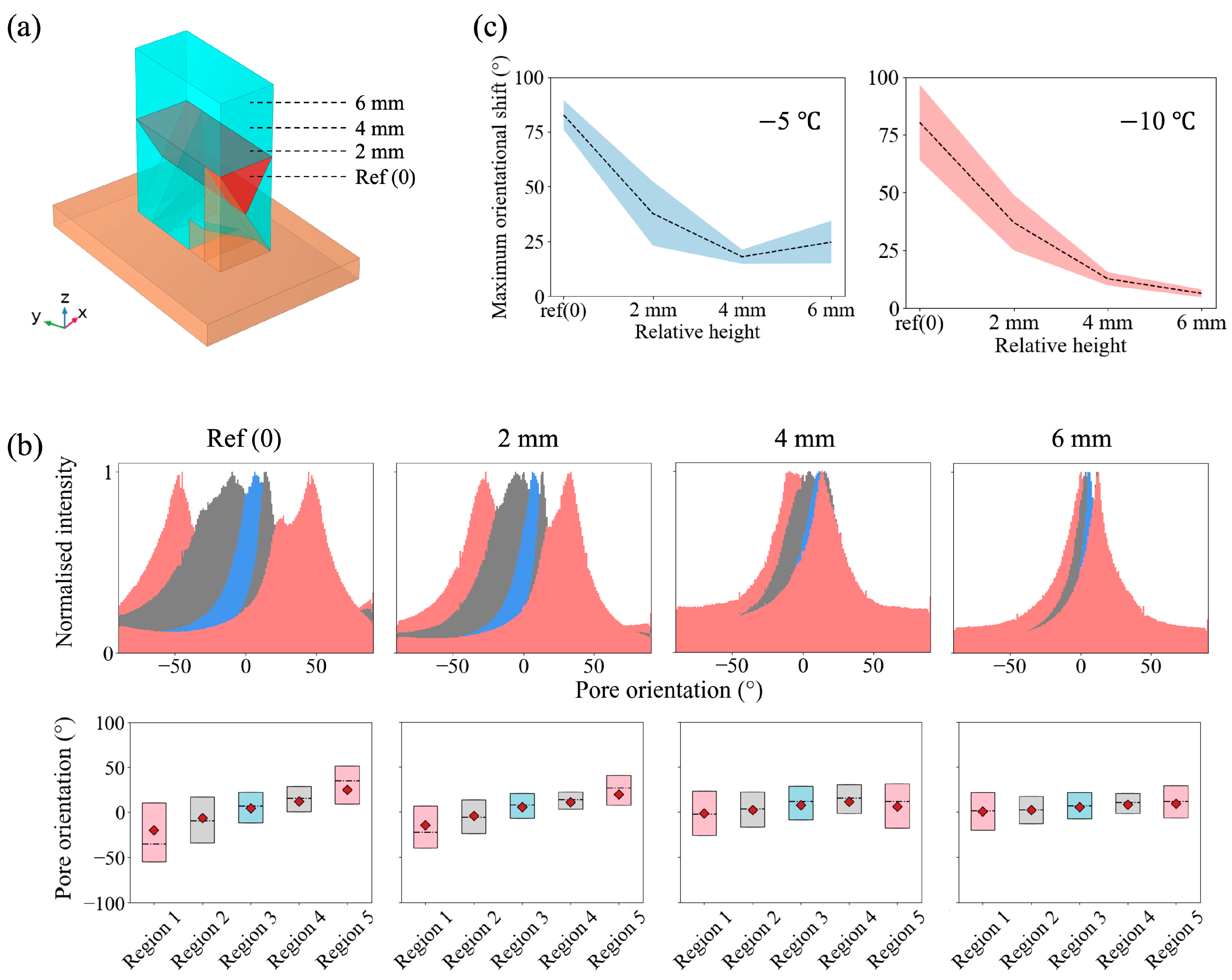
3.1. The Effects of Slope Angle in a Wedge Base
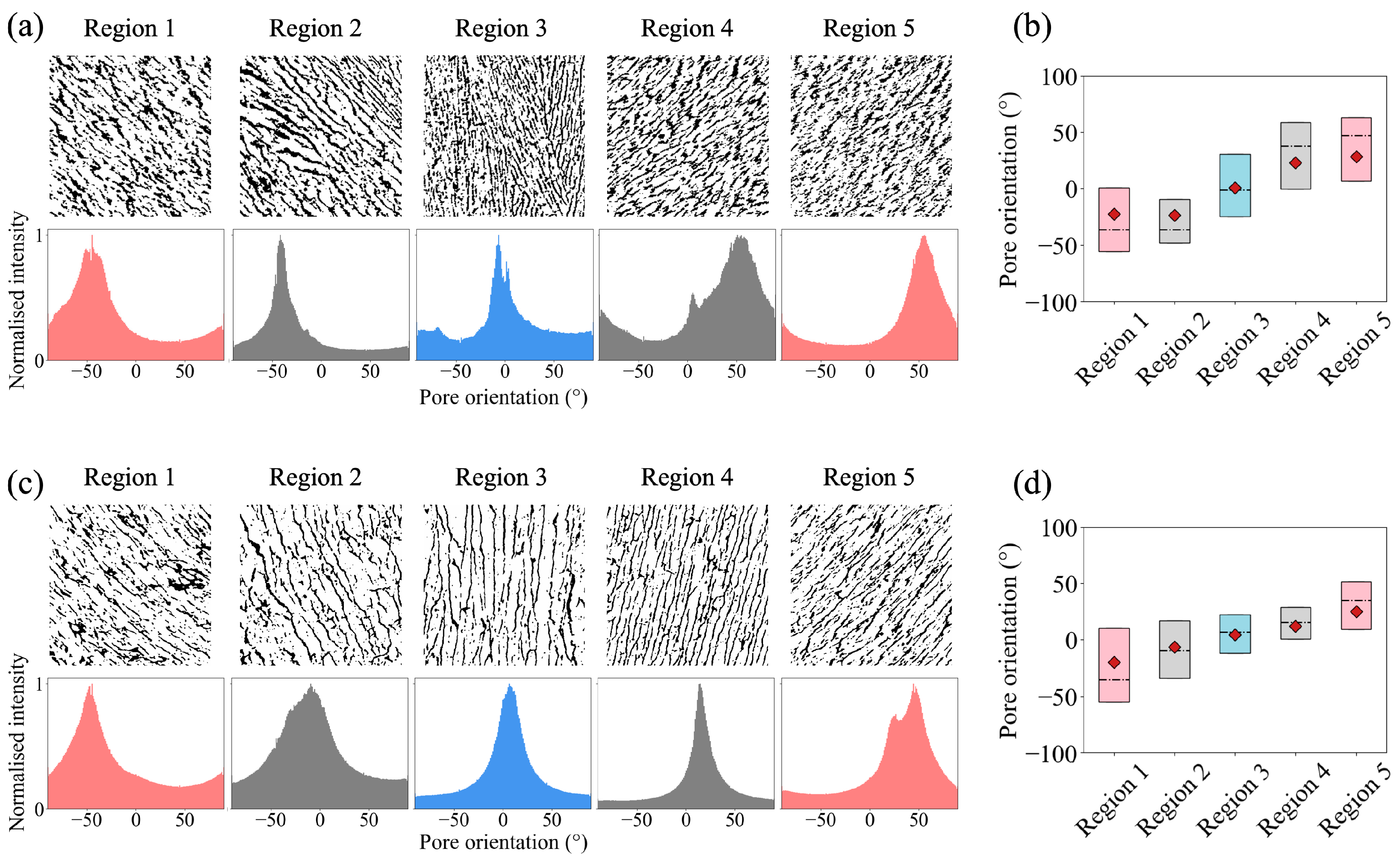
3.2. Bouligand-Like Orientation Design: Proof of Principle
3.3. Tuneable Orientation Change for Personalised Medicine
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Streeter, D.D.; Spotnitz, H.M.; Patel, D.P.; Ross, J.; Sonnenblick, E.H. Fiber Orientation in the Canine Left Ventricle during Diastole and Systole. Circ. Res. 1969, 24, 339–347. [Google Scholar] [CrossRef]
- Streeter, D.D.; Bassett, D.L. An Engineering Analysis of Myocardial Fiber Orientation in Pig’s Left Ventricle in Systole. Anat. Rec. 1966, 155, 503–511. [Google Scholar] [CrossRef]
- Nielles-Vallespin, S.; Khalique, Z.; Ferreira, P.F.; de Silva, R.; Scott, A.D.; Kilner, P.; McGill, L.A.; Giannakidis, A.; Gatehouse, P.D.; Ennis, D.; et al. Assessment of Myocardial Microstructural Dynamics by In Vivo Diffusion Tensor Cardiac Magnetic Resonance. J. Am. Coll. Cardiol. 2017, 69, 661–676. [Google Scholar] [CrossRef]
- Papadacci, C.; Finel, V.; Provost, J.; Villemain, O.; Bruneval, P.; Gennisson, J.L.; Tanter, M.; Fink, M.; Pernot, M. Imaging the Dynamics of Cardiac Fiber Orientation in Vivo Using 3D Ultrasound Backscatter Tensor Imaging. Sci. Rep. 2017, 7, 830. [Google Scholar] [CrossRef]
- Sengupta, P.P.; Tajik, A.J.; Chandrasekaran, K.; Khandheria, B.K. Twist Mechanics of the Left Ventricle. Principles and Application. JACC Cardiovasc. Imaging 2008, 1, 366–376. [Google Scholar] [CrossRef]
- Khalique, Z.; Ferreira, P.F.; Scott, A.D.; Nielles-Vallespin, S.; Firmin, D.N.; Pennell, D.J. Diffusion Tensor Cardiovascular Magnetic Resonance Imaging: A Clinical Perspective. JACC Cardiovasc. Imaging 2020, 13, 1235–1255. [Google Scholar] [CrossRef]
- Sallin, E.A. Fiber Orientation and Ejection Fraction in the Human Left Ventricle. Biophys. J. 1969, 9, 954–964. [Google Scholar] [CrossRef]
- Chang, H.; Liu, Q.; Zimmerman, J.F.; Lee, K.Y.; Jin, Q.; Peters, M.M.; Rosnach, M.; Choi, S.; Kim, S.L.; Ardoña, H.A.M.; et al. Recreating the Heart ’ s Helical Structure-Function Relationship with Focused Rotary Jet Spinning. Science 2022, 185, 180–185. [Google Scholar] [CrossRef]
- Kai, D.; Prabhakaran, M.P.; Jin, G.; Ramakrishna, S. Guided Orientation of Cardiomyocytes on Electrospun Aligned Nanofibers for Cardiac Tissue Engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 98 B, 379–386. [Google Scholar] [CrossRef]
- Orlova, Y.; Magome, N.; Liu, L.; Chen, Y.; Agladze, K. Electrospun Nanofibers as a Tool for Architecture Control in Engineered Cardiac Tissue. Biomaterials 2011, 32, 5615–5624. [Google Scholar] [CrossRef]
- Kharaziha, M.; Shin, S.R.; Nikkhah, M.; Topkaya, S.N.; Masoumi, N.; Annabi, N.; Dokmeci, M.R.; Khademhosseini, A. Tough and Flexible CNT-Polymeric Hybrid Scaffolds for Engineering Cardiac Constructs. Biomaterials 2014, 35, 7346–7354. [Google Scholar] [CrossRef] [PubMed]
- Deville, S.; Saiz, E.; Tomsia, A.P. Freeze Casting of Hydroxyapatite Scaffolds for Bone Tissue Engineering. Biomaterials 2006, 27, 5480–5489. [Google Scholar] [CrossRef] [PubMed]
- Davidenko, N.; Gibb, T.; Schuster, C.; Best, S.M.; Campbell, J.J.; Watson, C.J.; Cameron, R.E. Biomimetic Collagen Scaffolds with Anisotropic Pore Architecture. Acta Biomater. 2012, 8, 667–676. [Google Scholar] [CrossRef]
- Pawelec, K.M.; Husmann, A.; Best, S.M.; Cameron, R.E. Understanding Anisotropy and Architecture in Ice-Templated Biopolymer Scaffolds. Mater. Sci. Eng. C 2014, 37, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhou, C.; Wu, Y.; Liu, F.; Wu, Q. Self-Assembling Behavior of Cellulose Nanoparticles during Freeze-Drying: Effect of Suspension Concentration, Particle Size, Crystal Structure, and Surface Charge. Biomacromolecules 2013, 14, 1529–1540. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, H.B.; Luo, J.Q.; Wang, Q.W.; Xu, B.; Hong, S.; Yu, Z.Z. Highly Electrically Conductive Three-Dimensional Ti3C2Tx MXene/Reduced Graphene Oxide Hybrid Aerogels with Excellent Electromagnetic Interference Shielding Performances. ACS Nano 2018, 12, 11193–11202. [Google Scholar] [CrossRef]
- Cyr, J.A.; Colzani, M.; Bayraktar, S.; Graup, V.; Farndale, R.; Sinha, S.; Best, S.M.; Cameron, R.E. Unravelling Form and Function: Improved Function of Engineered Cardiac Tissue through Extra-Cellular Anisotropy. bioRxiv 2021. [Google Scholar] [CrossRef]
- Cyr, J.A.; Husmann, A.; Best, S.M.; Cameron, R.E. Complex Architectural Control of Ice-Templated Collagen Scaffolds Using a Predictive Model. Acta Biomater. 2022, 153, 260–272. [Google Scholar] [CrossRef]
- Basara, G.; Saeidi-Javash, M.; Ren, X.; Bahcecioglu, G.; Wyatt, B.C.; Anasori, B.; Zhang, Y.; Zorlutuna, P. Electrically Conductive 3D Printed Ti3C2Tx MXene-PEG Composite Constructs for Cardiac Tissue Engineering. Acta Biomater. 2022, 139, 179–189. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Z.; Song, X.; Zhang, Z.; Zhang, J.; Shung, K.K.; Zhou, Q.; Chen, Y. Biomimetic Anisotropic Reinforcement Architectures by Electrically Assisted Nanocomposite 3D Printing. Adv. Mater. 2017, 29, 1–8. [Google Scholar] [CrossRef]
- Jang, J.; Park, H.J.; Kim, S.W.; Kim, H.; Park, J.Y.; Na, S.J.; Kim, H.J.; Park, M.N.; Choi, S.H.; Park, S.H.; et al. 3D Printed Complex Tissue Construct Using Stem Cell-Laden Decellularized Extracellular Matrix Bioinks for Cardiac Repair. Biomaterials 2017, 112, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Kupfer, M.E.; Jung, J.P.; Yang, L.; Zhang, P.; Da Sie, Y.; Tran, Q.; Ajeti, V.; Freeman, B.T.; Fast, V.G.; et al. Myocardial Tissue Engineering with Cells Derived from Human-Induced Pluripotent Stem Cells and a Native-Like, High-Resolution, 3-Dimensionally Printed Scaffold. Circ. Res. 2017, 120, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Daghrery, A.; Ferreira, J.A.; Xu, J.; Golafshan, N.; Kaigler, D.; Bhaduri, S.B.; Malda, J.; Castilho, M.; Bottino, M.C. Tissue-Specific Melt Electrowritten Polymeric Scaffolds for Coordinated Regeneration of Soft and Hard Periodontal Tissues. Bioact. Mater. 2023, 19, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Lee, K.Y.; Kim, S.L.; MacQueen, L.A.; Chang, H.; Zimmerman, J.F.; Jin, Q.; Peters, M.M.; Ardoña, H.A.M.; Liu, X.; et al. Fibre-Infused Gel Scaffolds Guide Cardiomyocyte Alignment in 3D-Printed Ventricles. Nat. Mater. 2023, 22, 1039–1046. [Google Scholar] [CrossRef]
- Badrossamay, M.R.; McIlwee, H.A.; Goss, J.A.; Parker, K.K. Nanofiber Assembly by Rotary Jet-Spinning. Nano Lett. 2010, 10, 2257–2261. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, L.; Guo, B.; Ma, P.X. Interwoven Aligned Conductive Nanofiber Yarn/Hydrogel Composite Scaffolds for Engineered 3D Cardiac Anisotropy. ACS Nano 2017, 11, 5646–5659. [Google Scholar] [CrossRef]
- Asuncion, M.C.T.; Goh, J.C.H.; Toh, S.L. Anisotropic Silk Fibroin/Gelatin Scaffolds from Unidirectional Freezing. Mater. Sci. Eng. C 2016, 67, 646–656. [Google Scholar] [CrossRef]
- Lu, T.Y.; Lin, B.; Kim, J.; Sullivan, M.; Tobita, K.; Salama, G.; Yang, L. Repopulation of Decellularized Mouse Heart with Human Induced Pluripotent Stem Cell-Derived Cardiovascular Progenitor Cells. Nat. Commun. 2013, 4, 2307. [Google Scholar] [CrossRef]
- Guyette, J.P.; Charest, J.M.; Mills, R.W.; Jank, B.J.; Moser, P.T.; Gilpin, S.E.; Gershlak, J.R.; Okamoto, T.; Gonzalez, G.; Milan, D.J.; et al. Bioengineering Human Myocardium on Native Extracellular Matrix. Circ. Res. 2016, 118, 56–72. [Google Scholar] [CrossRef]
- Takahashi, H.; Shimizu, T.; Nakayama, M.; Yamato, M.; Okano, T. The Use of Anisotropic Cell Sheets to Control Orientation during the Self-Organization of 3D Muscle Tissue. Biomaterials 2013, 34, 7372–7380. [Google Scholar] [CrossRef]
- Fleischer, S.; Shapira, A.; Feiner, R.; Dvir, T. Modular Assembly of Thick Multifunctional Cardiac Patches. Proc. Natl. Acad. Sci. USA 2017, 114, 1898–1903. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Pikal, M.J. Design of Freeze-Drying Processes for Pharmaceuticals: Practical Advice. Pharm. Res. 2004, 21, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Deville, S. Freeze-Casting of Porous Ceramics: A Review of Current Achievements and Issues. Adv. Eng. Mater. 2008, 10, 155–169. [Google Scholar] [CrossRef]
- Fereshteh, Z. 7—Freeze-Drying Technologies for 3D Scaffold Engineering. In Functional 3D Tissue Engineering Scaffolds; Deng, Y., Kuiper, J., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 151–174. ISBN 978-0-08-100979-6. [Google Scholar]
- Deville, S.; Saiz, E.; Tomsia, A.P. Ice-Templated Porous Alumina Structures. Acta Mater. 2007, 55, 1965–1974. [Google Scholar] [CrossRef]
- Pot, M.W.; Faraj, K.A.; Adawy, A.; Van Enckevort, W.J.P.; Van Moerkerk, H.T.B.; Vlieg, E.; Daamen, W.F.; Van Kuppevelt, T.H. Versatile Wedge-Based System for the Construction of Unidirectional Collagen Scaffolds by Directional Freezing: Practical and Theoretical Considerations. ACS Appl. Mater. Interfaces 2015, 7, 8495–8505. [Google Scholar] [CrossRef]
- Bai, H.; Chen, Y.; Delattre, B.; Tomsia, A.P.; Ritchie, R.O. Bioinspired Large-Scale Aligned Porous Materials Assembled with Dual Temperature Gradients. Sci. Adv. 2015, 1, e1500849. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, X.; Xue, T.; Yang, F.; Fan, W.; Liu, T. Bidirectional Anisotropic Polyimide/Bacterial Cellulose Aerogels by Freeze-Drying for Super-Thermal Insulation. Chem. Eng. J. 2020, 385, 123963. [Google Scholar] [CrossRef]
- Su, F.Y.; Mok, J.R.; McKittrick, J. Radial-Concentric Freeze Casting Inspired by Porcupine Fish Spines. Ceramics 2019, 2, 161–179. [Google Scholar] [CrossRef]
- Park, C.H.; Kim, K.H.; Rios, H.F.; Lee, Y.M.; Giannobile, W.V.; Seol, Y.J. Spatiotemporally Controlled Microchannels of Periodontal Mimic Scaffolds. J. Dent. Res. 2014, 93, 1304–1312. [Google Scholar] [CrossRef]
- Nakagawa, K.; Hottot, A.; Vessot, S.; Andrieu, J. Modeling of Freezing Step during Freeze-Drying of Drugs in Vials. AIChE J. 2007, 53, 1362–1372. [Google Scholar] [CrossRef]
- Muzzio, C.R.; Dini, N.G. Simulation of Freezing Step in Vial Lyophilization Using Finite Element Method. Comput. Chem. Eng. 2011, 35, 2274–2283. [Google Scholar] [CrossRef]
- Husmann, A.; Pawelec, K.; Burdett, C.; Best, S.; Cameron, R. Numerical Simulations to Determine the Influence of Mould Design on Ice-Templated Scaffold Structures. J. Biomed. Eng. Inform. 2015, 1, 47. [Google Scholar] [CrossRef]
- Nelson, I.; Varga, J.; Wadsworth, P.; Mroz, M.; Kruzic, J.J.; Kingstedt, O.T.; Naleway, S.E. Helical and Bouligand Porous Scaffolds Fabricated by Dynamic Low Strength Magnetic Field Freeze Casting. JOM 2020, 72, 1498–1508. [Google Scholar] [CrossRef]
- Legrice, I.J.; Smaill, B.H.; Chai, L.Z.; Edgar, S.G.; Gavin, J.B.; Hunter, P.J. Laminar Structure of the Heart: Ventricular Myocyte Arrangement and Connective Tissue Architecture in the Dog. Am. J. Phys. 1995, 269, H571–H582. [Google Scholar] [CrossRef]
- Pawelec, K.M.; Husmann, A.; Best, S.M.; Cameron, R.E. A Design Protocol for Tailoring Ice-Templated Scaffold Structure. J. R. Soc. Interface 2014, 11, 20130958. [Google Scholar] [CrossRef]
- Offeddu, G.S.; Ashworth, J.C.; Cameron, R.E.; Oyen, M.L. Structural Determinants of Hydration, Mechanics and Fluid Flow in Freeze-Dried Collagen Scaffolds. Acta Biomater. 2016, 41, 193–203. [Google Scholar] [CrossRef]
- Nishitani, S.; Torii, N.; Imai, H.; Haraguchi, R.; Yamada, S.; Takakuwa, T. Development of Helical Myofiber Tracts in the Human Fetal Heart: Analysis of Myocardial Fiber Formation in the Left Ventricle from the Late Human Embryonic Period Using Diffusion Tensor Magnetic Resonance Imaging. J. Am. Heart Assoc. 2020, 9, e016422. [Google Scholar] [CrossRef]
- Davidenko, N.; Hamaia, S.; Bax, D.V.; Malcor, J.D.; Schuster, C.F.; Gullberg, D.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Selecting the Correct Cellular Model for Assessing of the Biological Response of Collagen-Based Biomaterials. Acta Biomater. 2018, 65, 88–101. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.L.; Sinha, S.; Cameron, R.E.; Best, S.M. Mimicking Transmural Helical Cardiomyofibre Orientation Using Bouligand-like Pore Structures in Ice-Templated Collagen Scaffolds. Polymers 2023, 15, 4420. https://doi.org/10.3390/polym15224420
Zhang HL, Sinha S, Cameron RE, Best SM. Mimicking Transmural Helical Cardiomyofibre Orientation Using Bouligand-like Pore Structures in Ice-Templated Collagen Scaffolds. Polymers. 2023; 15(22):4420. https://doi.org/10.3390/polym15224420
Chicago/Turabian StyleZhang, Huijie L., Sanjay Sinha, Ruth E. Cameron, and Serena M. Best. 2023. "Mimicking Transmural Helical Cardiomyofibre Orientation Using Bouligand-like Pore Structures in Ice-Templated Collagen Scaffolds" Polymers 15, no. 22: 4420. https://doi.org/10.3390/polym15224420
APA StyleZhang, H. L., Sinha, S., Cameron, R. E., & Best, S. M. (2023). Mimicking Transmural Helical Cardiomyofibre Orientation Using Bouligand-like Pore Structures in Ice-Templated Collagen Scaffolds. Polymers, 15(22), 4420. https://doi.org/10.3390/polym15224420








