Antibacterial Nanocellulose-TiO2/Polyester Fabric for the Recyclable Photocatalytic Degradation of Dyes
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Alkali Reduction Pretreatment of Fabric and Preparation of NC
2.3. Preparation of NC-TiO2/PET
2.4. Characterization
2.5. Photocatalytic Performance
2.6. Antibacterial Performance
3. Results and Discussion
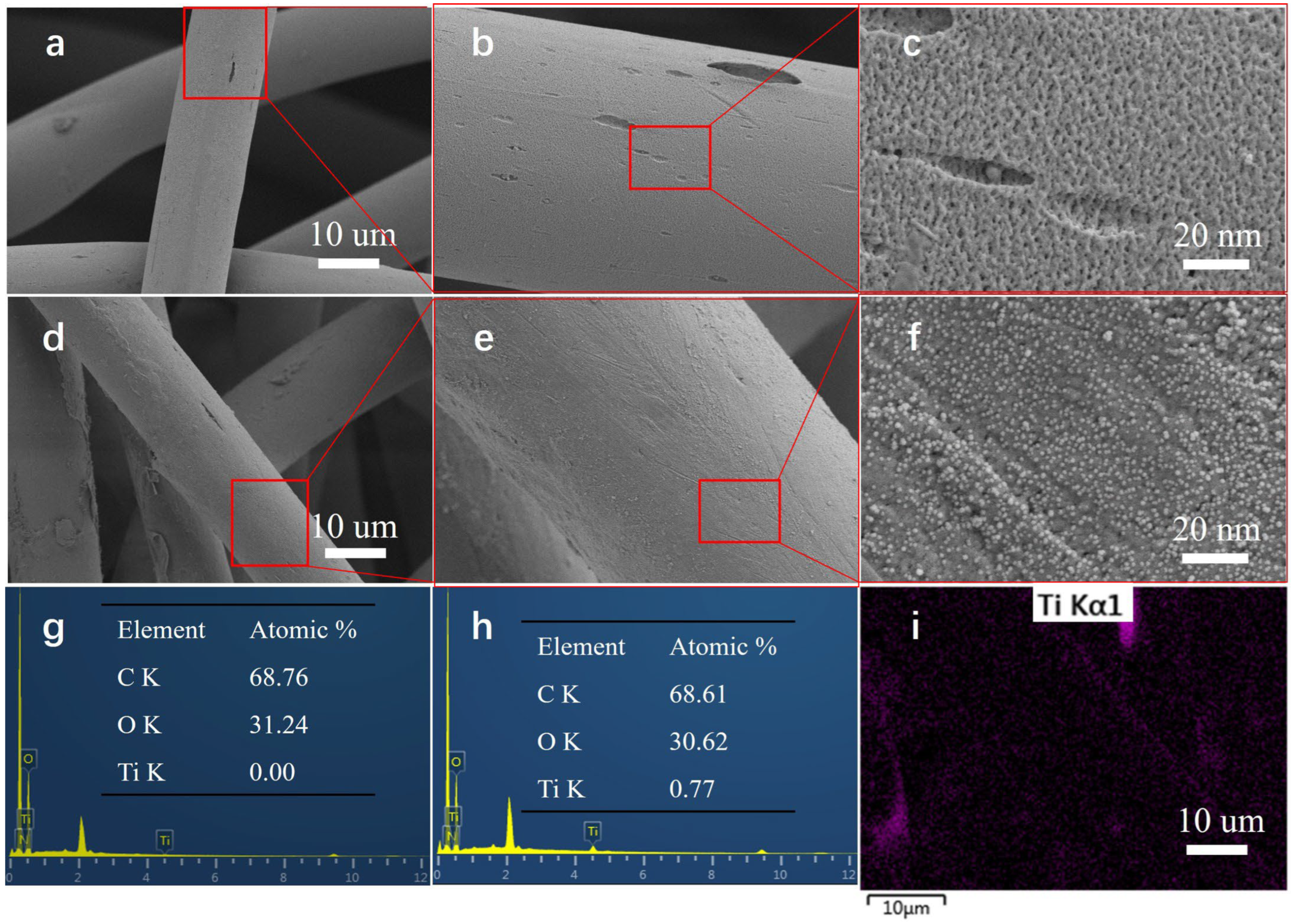
3.1. Microscopic Surface Structures
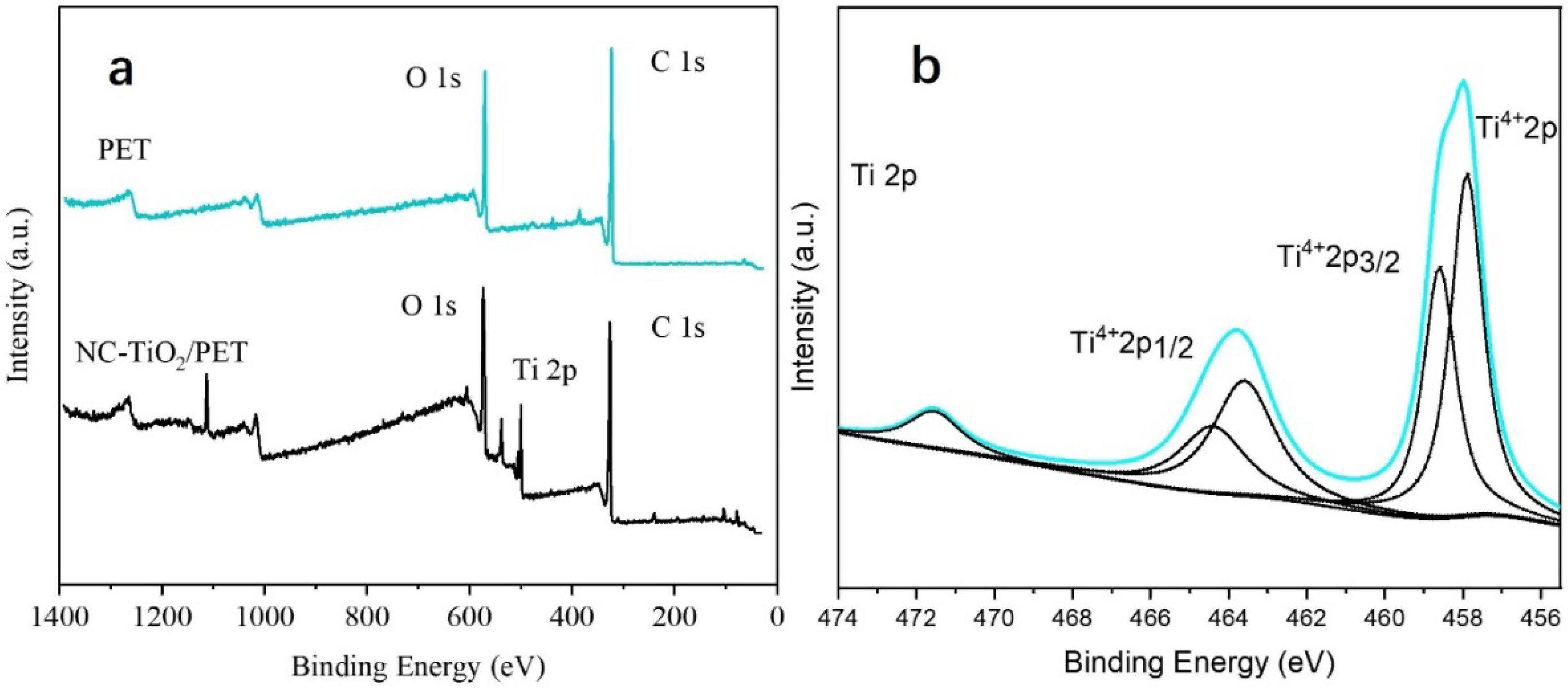
3.2. Chemical Characterizations
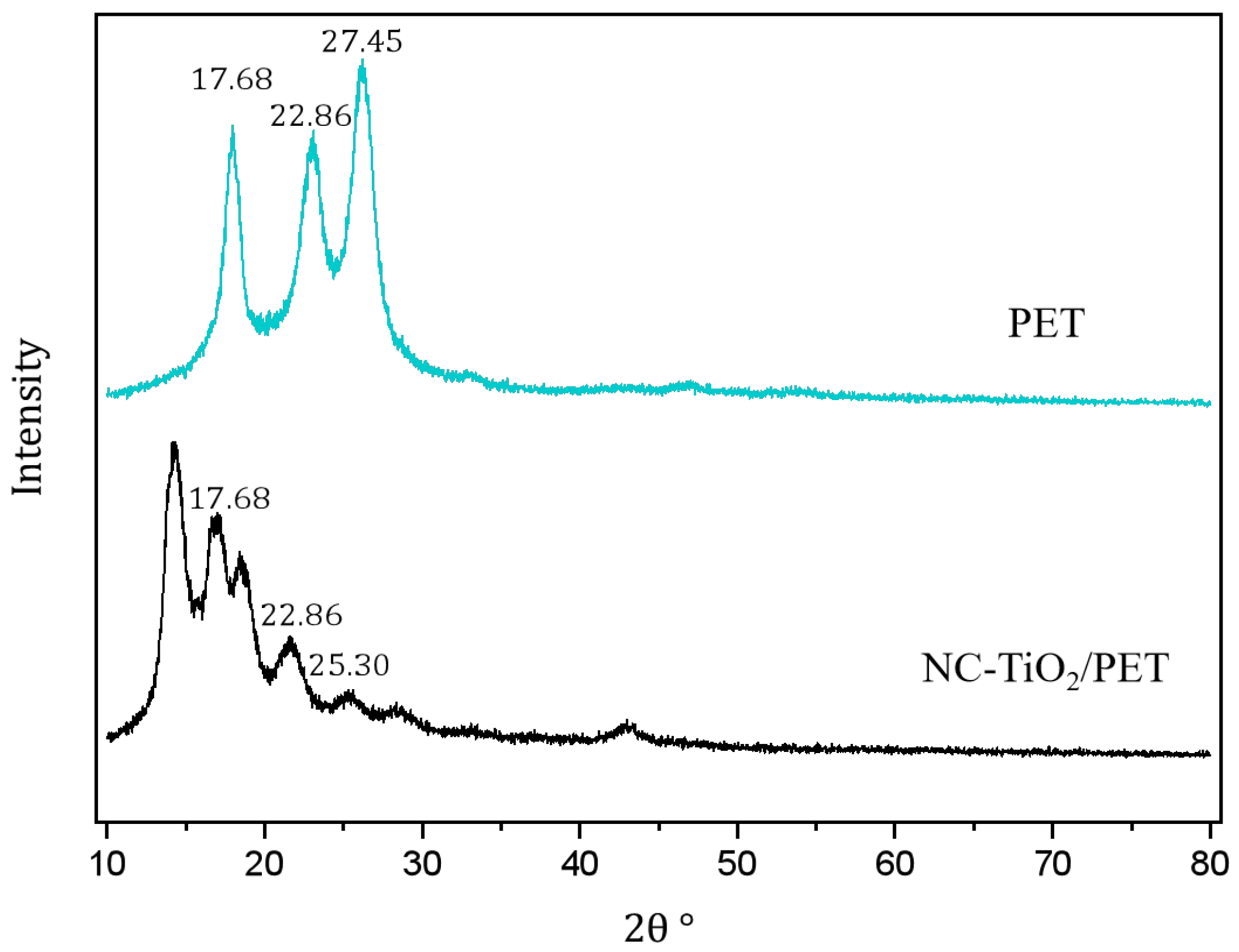
3.3. Crystal Phase Structure Analysis
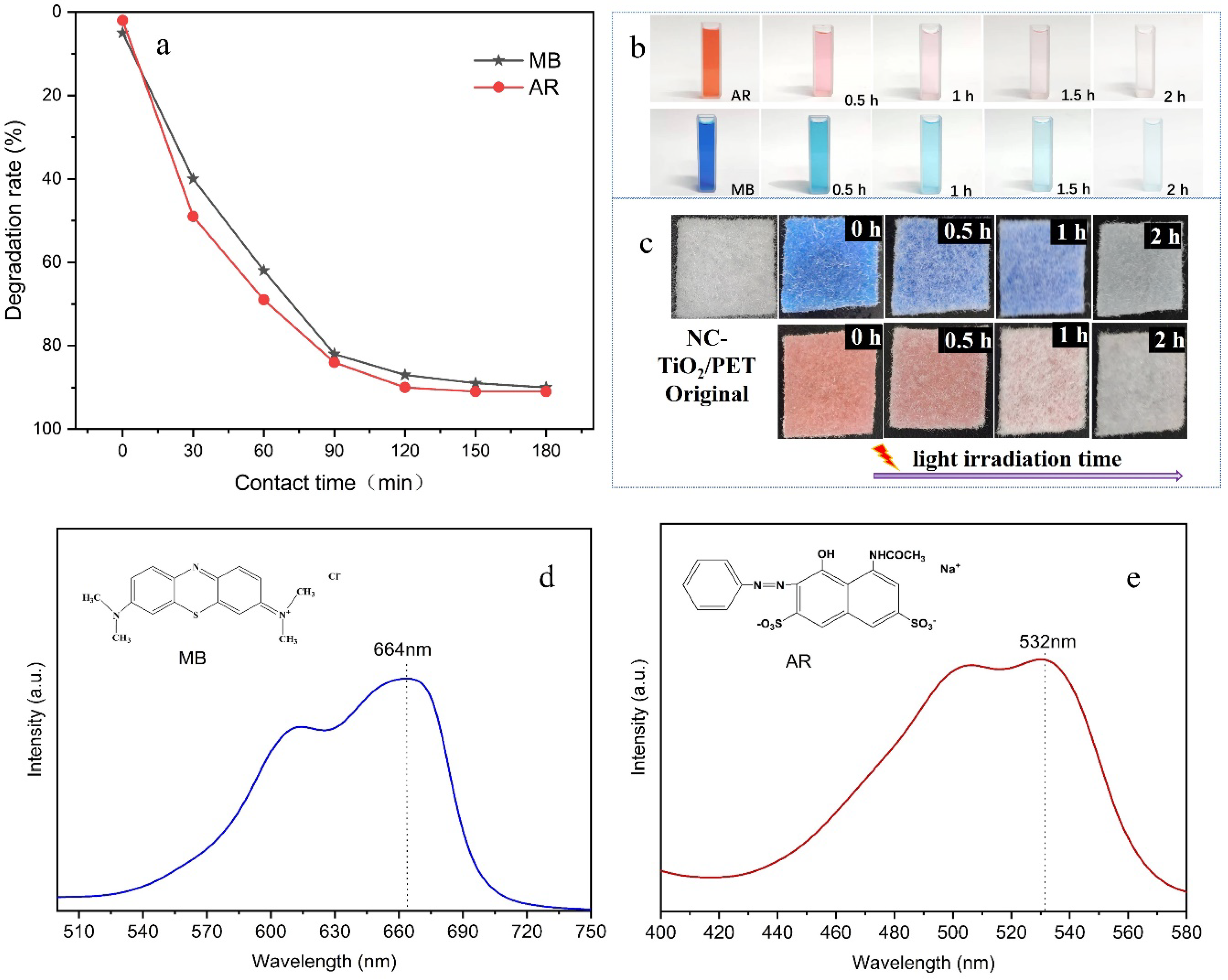
3.4. Performance of Photocatalytic Degradation
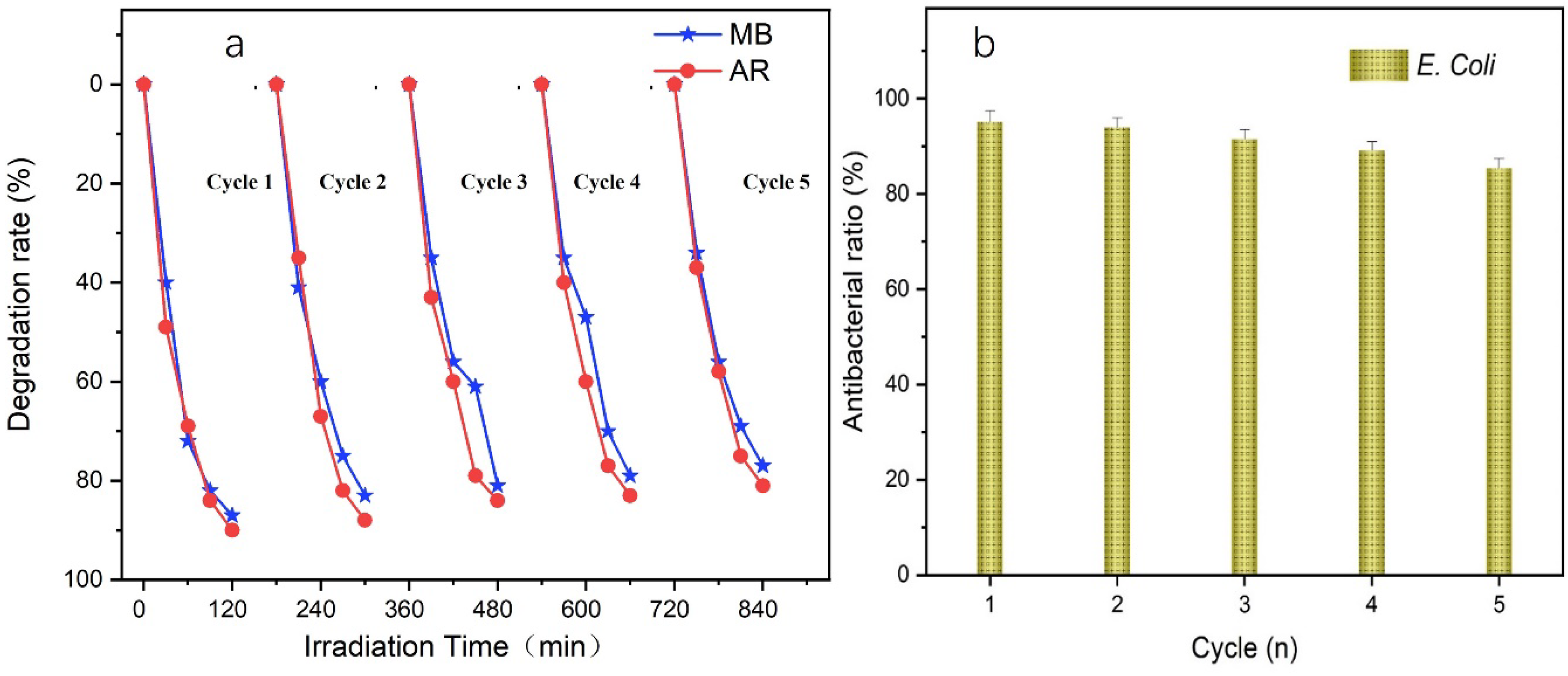
3.5. Recycling Performance and Stability
3.6. Antibacterial Properties and Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aldalbahi, A.; El-Naggar, M.E.; El-Newehy, M.H.; Rahaman, M.; Hatshan, M.R.; Khattab, T.A. Effects of Technical Textiles and Synthetic Nanofibers on Environmental Pollution. Polymers 2021, 13, 155. [Google Scholar] [CrossRef] [PubMed]
- Pendergraft, M.A.; Belda-Ferre, P.; Petras, D.; Morris, C.K.; Mitts, B.A.; Aron, A.T.; Bryant, M.; Schwartz, T.; Ackermann, G.; Humphrey, G.; et al. Bacterial and Chemical Evidence of Coastal Water Pollution from the Tijuana River in Sea Spray Aerosol. Environ. Sci. Technol. 2023, 57, 4071–4081. [Google Scholar] [CrossRef] [PubMed]
- Mathew, M.M.; Khatana, K.; Vats, V.; Dhanker, R.; Dahms, H.U.; Shwang, J. Biological approaches integrating algae and bacteria for the degradation of wastewater contaminants—A Review. Front. Microbiol. 2022, 12, 801051. [Google Scholar] [CrossRef] [PubMed]
- Bogdanovsky, G.A.; Vidovich, G.L.; Kultin, D.Y.; Lebedeva, O.K.; Zakharov, A.N. Chemical model for dioxin destruction in aqueous medium—Electrocatalytic oxidation of dioxin-like substance simulating natural pollutant. Appl. Catal. A General 2002, 232, 137–145. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, P.; An, W.; Liu, L.; Liang, Y.; Cui, W. In-situ Fe-doped g-C3N4 heterogeneous catalyst via photocatalysis-Fenton reaction with enriched photocatalytic performance for removal of complex wastewater. Appl. Catal. B Environ. 2019, 245, 130–142. [Google Scholar] [CrossRef]
- Shi, W.; Ren, H.; Li, M.; Shu, K.; Xu, Y.; Yan, C.; Tang, Y. Tetracycline removal from aqueous solution by visible-light-driven photocatalytic degradation with low-cost red mud wastes. Chem. Eng. J. 2020, 382, 122876. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, M.; Liu, H.; Zhu, Y.; Wang, D. removal of dye and antibiotic from water with the functionalized zirconium-based metal-organic framework and graphene oxide composite nanomaterial Uio-66-(OH)2/GO. Appl. Surf. Sci. 2020, 525, 146614. [Google Scholar] [CrossRef]
- Zhao, D.W.; Quan, L.Y. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. J. Clean. Prod. 2020, 259, 120879. [Google Scholar] [CrossRef]
- Dong, J.C.; Yan, L.C.; Nan, Z.; Chen, P.; Wang, Y.P.; Li, K.; Huo, S.H.; Cheng, P.F.; Peng, P.; Zhang, R.C.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2021, 6, 30172–30864. [Google Scholar] [CrossRef]
- Wei, Z.D.; Liu, J.Y.; Shang, G.W.F. A review on photocatalysis in antibiotic wastewater: Pollutant degradation and hydrogen production. Chin. J. Catal. 2021, 41, 1440–1450. [Google Scholar] [CrossRef]
- Li, Y.; Liu, M.; Zhang, M.; Liu, Y.; Zhao, Q.; Li, X.; Zhou, Q.; Chen, Y.; Wang, S. Preparation of g-C3N4/TiO2 Heterojunction Composite Photocatalyst by NaCl Template Method and Its Photocatalytic Performance Enhancement. Nano 2023, 18, 2350009. [Google Scholar] [CrossRef]
- Alhalili, Z.; Romdhani, C.; Chemingui, H.; Smiri, M. Removal of dithioterethiol (DTT) from water by membranes of cellulose acetate (AC) and AC doped ZnO and TiO2 nanoparticles. J. Saudi Chem. Soc. 2021, 25, 101282. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Ding, X.; Zhang, P.; Wang, Q.; Zheng, K.; Chen, L.; Ding, J.J.; Tian, X.Y.; Zhang, X. Convenient and Recyclable TiO2/g-C3N4 Photocatalytic Coating: Layer-by-layer Self-assembly Construction on Cotton Fabrics Leading to Improved Catalytic Activity under Visible Light. Ind. Eng. Chem. Res. 2019, 58, 3978–3987. [Google Scholar] [CrossRef]
- Hussain, M.Z.; Zhu, X.Y.; Linden, B.V.D.; Zheng, H.; Jia, Q.L.; Cerrato, E.; Fischer, R.A.; Kapteijn, F.; Zhu, Y.Q.; Xia, Y.D. Surface functionalized N-C-TiO2/C nanocomposites derived from metal-organic framework in water vapour for enhanced photocatalytic H2 generation. J. Eng. Chem. 2021, 57, 485–495. [Google Scholar] [CrossRef]
- Mallakpour, S.; Mohammadi, N. Development of sodium alginate-pectin/TiO2 nanocomposites: Antibacterial and bioactivity investigations. Carbohydr. Polym. 2022, 285, 119226. [Google Scholar] [CrossRef] [PubMed]
- Rol, F.; Belgacem, M.N.; Gandini, A.; Bras, J. Recent advances in surface-modified cellulose nanofibrils. Prog. Polym. Sci. 2019, 88, 241–264. [Google Scholar] [CrossRef]
- Chen, W.; Feng, X.L.; Zhang, D.Y.; Lu, F.F.; Wang, H.R.; Tan, J.C.; Xu, Q.; Liu, Y.K.; Cao, Z.H.; Su, X.P. In situ synthesis of TiO2/NC on cotton fibers with antibacterial properties and recyclable photocatalytic degradation of dyes. RSC Adv. 2022, 12, 19974–19980. [Google Scholar] [CrossRef]
- Wang, D.C.; Yu, H.Y.; Qi, D.; Ramasamy, M.; Yao, J.M.; Tang, F.; Tam, K.C.; Ni, Q.Q. Supramolecular self-assembly of 3D conductive cellulose nanofiber aerogels for flexible supercapacitors and ultrasensitive sensors. ACS Appl. Mater. Interfaces 2019, 11, 24435–24446. [Google Scholar] [CrossRef]
- Juan, X.; Feng, S.; Xiu, D.; Xue, W.L.; Ye, W.; Wang, J.M.; Wang, C.; Wang, X.L.; Zhong, Y. Controlling Self-Assembly of Cellulose Nanocrystal to Synergistically Regulate (001) Reactive Facets and Hierarchical Pore Structure of Anatase Nano-TiO2 for High Photocatalytic Activity. ACS Sustain. Chem. Eng. 2019, 7, 1973–1979. [Google Scholar] [CrossRef]
- Xu, Q.B.; Wu, Y.H.; Zhang, Y.Y. Durable antibacterial cotton modified by silver nanoparticles and chitosan derivative binder. Fiber Polym. 2016, 17, 1782–1789. [Google Scholar] [CrossRef]
- Ghosh, A.; Saha, I.; Fujita, M.; Debnath, S.C.; Hazra, A.K.; Adak, M.K.; Hasanuzzaman, M. Photoactivated TiO2 Nanocomposite Delays the Postharvest Ripening Phenomenon through Ethylene Metabolism and Related Physiological Changes in Capsicum Fruit. Plants. 2022, 11, 513. [Google Scholar] [CrossRef] [PubMed]
- Toro, R.G.; Adel, A.M.; de Caro, T.; Brunetti, B.; Al-Shemy, M.T.; Caschera, D. A Facile One-Pot Approach to the Fabrication of Nanocellulose–Titanium Dioxide Nanocomposites with Promising Photocatalytic and Antimicrobial Activity. Materials 2022, 15, 5789. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.F.; Yu, X.H.; Dong, J.J.; Zhao, L.; Liu, L.L.; Liu, J.L.; Zhao, X.T.; Liu, L.F. Diatom-inspired TiO2-PANi-decorated bilayer photothermal foam for solar-driven clean water generation. ACS Appl. Mater. Interfaces 2021, 13, 58124–58133. [Google Scholar] [CrossRef] [PubMed]
- Ao, Y.; Xu, J.; Fu, D.; Shen, X.; Yuan, C. Low temperature preparation of anatase TiO2-activated carbon composite film. Appl. Surf. Sci. 2008, 254, 4001–4006. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, Y.; Li, H.; Liu, X.; Liu, W. Ag Deposition on N and B Co-doped TiO2 Nanoparticles: An Avenue for High-Efficiency Photocatalytic Degradation of Dye and Hydrocarbons in Oil-Contaminated Wastewater. Nano 2021, 16, 2150042. [Google Scholar] [CrossRef]
- Moon, J.W.; Yun, C.Y.; Chung, K.W.; Kang, M.S.; Yi, J.H. Photocatalytic activation of TiO2 under visible light using Acid Red 44. Catal. Today 2003, 87, 77–86. [Google Scholar] [CrossRef]
- Karimi, L.; Zohoori, S.; Amini, A. Multi-wall carbon nanotubes and nano titanium dioxide coated on cotton fabric for superior self-cleaning and UV blocking. New Carbon Mater. 2014, 29, 380–385. [Google Scholar] [CrossRef]
- Rodríguez-Rojas, A.; Kim, J.J.; Johnston, P.R.; Makarova, O.; Eravci, M.; Wei, S.C.; Heng, G.R.; Rolff, J. Non-lethal exposure to H2O2 boosts bacterial survival and evolvability against oxidative stress. PLoS Genet. 2020, 12, 16–19. [Google Scholar] [CrossRef]

| Sample | NC/TiO2 | Degradation Rate (%) |
|---|---|---|
| PET | 0 | 6.47 |
| TiO2/PET | 0 wt%/0.8 wt% | 89.79 |
| NC-TiO2/PET-0.4 | 0.2 wt%/0.4 wt% | 65.07 |
| NC-TiO2/PET-0.6 | 0.2 wt%/0.6 wt% | 79.37 |
| NC-TiO2/PET | 0.2 wt%/0.8 wt% | 90.02 |
| NC-TiO2/PET-1.0 | 0.2 wt%/1.0 wt% | 79.71 |
| Sample | TiO2 per NC-TiO2/PET (mg g−1) | Breaking Strength (N) | Antibacterial Properties against E. coli (CFU mL−1) |
|---|---|---|---|
| PET | -- | 470.21 ± 3.59 | 602 |
| NC-TiO2/PET | 10.11 ± 0.78 | 468.54 ± 3.44 | 142 |
| NC-TiO2/PET after Fifth cycle | 9.29 ± 0.86 | 448.17 ± 3.57 | 201 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, J.; Deng, H.; Lu, F.; Chen, W.; Su, X.; Wang, H. Antibacterial Nanocellulose-TiO2/Polyester Fabric for the Recyclable Photocatalytic Degradation of Dyes. Polymers 2023, 15, 4376. https://doi.org/10.3390/polym15224376
Tan J, Deng H, Lu F, Chen W, Su X, Wang H. Antibacterial Nanocellulose-TiO2/Polyester Fabric for the Recyclable Photocatalytic Degradation of Dyes. Polymers. 2023; 15(22):4376. https://doi.org/10.3390/polym15224376
Chicago/Turabian StyleTan, Jiacheng, Hangjun Deng, Fangfang Lu, Wei Chen, Xiuping Su, and Hairong Wang. 2023. "Antibacterial Nanocellulose-TiO2/Polyester Fabric for the Recyclable Photocatalytic Degradation of Dyes" Polymers 15, no. 22: 4376. https://doi.org/10.3390/polym15224376
APA StyleTan, J., Deng, H., Lu, F., Chen, W., Su, X., & Wang, H. (2023). Antibacterial Nanocellulose-TiO2/Polyester Fabric for the Recyclable Photocatalytic Degradation of Dyes. Polymers, 15(22), 4376. https://doi.org/10.3390/polym15224376






